Abstract
Many risk factors can potentially influence sperm quality. Telomeres confer stability on the chromosome and their dysfunction has been implicated in conditions such as cancer, aging, and lifestyle. The impact of lifestyle on sperm cell telomeres is unclear. The objectives of this study were to evaluate the impact of lifestyle behaviors on telomere length in sperm and to follow the correlation with pregnancy outcomes in patients undergoing in vitro fertilization (IVF). In this prospective observational study, sperm was analyzed for telomere length (TL). Men were asked to report lifestyle behaviors including occupation (physical or sedentary), smoking duration and amount, physical activity, dietary habits, and where they keep their cellular phone (bag, pants, or shirt pocket). Correlations among semen analysis, TL, men’s habits, and embryo quality and pregnancy outcomes were evaluated. Among 34 patients recruited, 12 had longer TL and 13 shorter TL. Sperm motility was negatively correlated with TL (Pearson correlation = −.588, p = .002). Smoking adversely affected native sperm motility (53% motility in nonsmokers vs. 37% in smokers; p = .006). However, there was no significant impact on TL. The group with longer telomeres demonstrated significant association with healthy diet (10/12 vs. 6/13; p = .05) and a trend toward more sports activity, weekly (16/84 vs. 7/91; p = .04) compared with the shorter telomeres group. This study suggests that lifestyle, healthy diet, and sports activity are associated with long telomeres in sperm. Sperm quality is also influenced by patients’ habits. The study strongly recommends maintaining a healthy lifestyle to preserve general health and fertility.
Keywords: semen parameters, sperm telomeres, lifestyle, smoking fertility
Male infertility is a complex, multifactorial condition with a wide variety of phenotypic presentations, which affects 34% to 50% of infertile couples (Ilacqua et al., 2018; Odisho et al., 2014; Palermo et al., 1995). Studies have reported a progressive decline in sperm quality during the last few decades, worldwide (Lackner et al., 2005; Rolland et al., 2013). A wide variety of risk factors can affect sperm quality. Genetic and iatrogenic factors are the most well-accepted etiologies (Cerván-Martín et al., 2020; Oud et al., 2019), and many other factors are suggested but cannot always be explained. Of those that remain unexplained, lifestyle factors such as cigarette smoking, alcohol intake, use of illicit drugs, obesity, psychological stress, paternal age, diet, and caffeine intake are being investigated (Practice Committee of the American Society for Reproductive Medicine, 2015; Zilberlicht et al., 2015). Environmental factors such as global warming, air pollution, chemical toxins, and radiation (Zilberlicht et al., 2015) are other potential causes of male infertility.
Telomeres are noncoding DNA sequences located at the ends of the chromosomes of all somatic cells and gametes. Telomeres are composed of highly condensed nucleotide repeats (TTAGGG). The telomeres endow chromosomal stability and protect genomic resistance (Kimura et al., 2008). Their dysfunction has been implicated in different conditions, such as cancer and diseases related to aging telomere (Calado & Young, 2009). Telomeres undergo progressive shortening with each cell division, because of the inability of the normal DNA replication machinery to fully replicate at the 3′ end of chromosomes (Allsopp et al., 1995; Harley, 1997). When telomeres reach a critical minimum length, cells cannot divide. The cell enters cell-cycle arrest or undergoes apoptosis (Blackburn et al., 2006). Telomere length (TL) is maintained by telomerase, a ribonucleoprotein complex that is maximally expressed in highly proliferative cells, such as germ and neoplastic cells (Dolcetti & De Rossi, 2012). Studies evaluated TL in leukocytes and the effect of lifestyle factors on their integrity (Epel et al., 2004; Valdes et al., 2005). However, the impact of lifestyle on the TL of sperm cells and in vitro fertilization (IVF) outcomes has not been evaluated as extensively.
The accumulation of data regarding the association between TL and reproductive potential demonstrated that shorter sperm telomere length (STL) has been associated with various male infertility issues, indicating that the assessment of STL could be employed as a potential and valuable biomarker of male infertility including oligozoospermia and asthenospermia, and elevated DNA fragmentation rate, in the clinical set-up (Biron-Shental et al., 2018; Gomez et al., 2006; Lafuente et al., 2018; Rocca et al., 2016). The effect of TL in sperm cells on IVF outcomes was shown to be correlated. Ribas-Maynou et al. (2022) reported that lower embryo development up to blastocyst stage occurred in the shorter TL group in animal models. Sperm TL and the percentage of morulae at day 6 postfertilization were significantly correlated (r = .559; p = .047) (Ribas-Maynou et al., 2022).
This study was conducted to evaluate the connection between the impact of lifestyle on sperm cell telomeres and IVF. We evaluated the effect of lifestyle, including smoking and obesity on TL in sperm, and pregnancy outcomes in patients undergoing IVF.
Materials and Methods
This prospective, non-interventional cohort study was conducted at the hospital IVF unit from November 2019 to November 2020.
Participants
Men undergoing IVF with or without intracytoplasmic sperm injection (ICSI), ranging in age from 18 to 60 years, were eligible to participate in the study.
On the day of ovum pick-up (OPU), just after the partner provided the sperm for the fertilization in the laboratory, written consent was obtained to use the remaining sperm for further evaluation. Those who agreed to participate were asked to answer the following questions regarding their lifestyle: occupation (physical or sedentary), smoking duration and amount, whether they participate in regular physical activity, type of diet (Mediterranean, fast food, mainly carbohydrate intake), and where they keep their cellular phone (bag, pant pocket, shirt pocket, etc.) to examine the proximity of the cellular phone to their genitalia.
Semen was analyzed according to World Health Organization criteria (volume ≥1.5 mL, concentration ≥15 × 106/mL, progressive motility ≥32%, and ≥4% normal morphology). These are accepted normal values (Cooper et al., 2010). Patients with severe oligo-terato-asthenospermia were excluded from the study.
Embryo Grading
Either IVF or ICSI was performed following oocyte retrieval. Mature oocytes were placed on EmbryoSlides and incubated in the EmbryoScopeTM (Unisense FertiliTech, Aarhus, Denmark) for 3 to 5 days at 37°C in 5.8% CO2 and 5% O2 if ICSI was conducted. Images of the embryos were captured every 10 min in seven focal planes, from the time of second polar body extraction and up to 120 hr after fertilization, to determine the exact timing of the cell division. Embryos were graded according to the known implantation data (KID) score, the Alfa ESHRE score, and morphology. All available embryos were assessed for quality based on cell count, symmetry, granularity, type, percent fragmentation, multinucleate blastomere, and degree of compaction. A top-quality embryo was classified as one with 4 to 5 cells on day 2 or >6 equal-sized blastomeres on day 3, ≤20% fragmentation, and no multinucleate cells. To prevent interobserver error, all measurements were carried out by the same embryologist. No more than two embryos were transferred on day 3 or 5 of embryonic development; the remaining top-quality embryos were vitrified and used for the subsequent frozen embryo transfer, if pregnancy was not achieved.
Pregnancy Determination
Fourteen days after embryo transfer, beta-hCG level was assessed. Clinical pregnancy was confirmed when a gestational sac with a fetal heartbeat was detected by ultrasound at 7 weeks of gestation.
Sperm Cell Isolation and DNA Extraction
Residual semen samples obtained on OPU day were immediately transferred to the laboratory for further evaluation. Semen samples were allowed to liquefy for 30 min at 37°C. Total semen was centrifuged at 3,400 rpm for 5 min to remove supernatant. The pellets were re-suspended and washed twice with 1,000 µL of sterile phosphate-buffered saline (PBS, 0.5 M) at 3,400 rpm for 5 min to eliminate cellular and noncellular components before the addition of lysis buffer.
Semen DNA was extracted using the DNeasy Blood & Tissue Kit (QIAGEN, Ontario, Canada) according to the manufacturer’s instructions. DNA concentration was measured using a NanoDrop spectrophotometer (Thermo Scientific). DNA samples were stored at −20°C until further analysis.
Relative Telomere Length Measurement
Relative TL for each patient was determined by analyzing the ratio of telomere repeat copy number to single-copy number (T/S) utilizing quantitative polymerase chain reaction (qPCR). Two master mixtures were prepared, one containing the pair of telomere primers (T primer pair) and a second containing the pair of primers of the single gene 36B4 (S primer pair). The T and S qPCR reactions were performed separately. Each plate contained 1.68-fold serial dilution of reference DNA yielding the standard curve (Figure 1). A triplicate of 2 µL containing 5 ng DNA from each subject was loaded. Next, 18 µL of the T and S master mixes were added to the samples and to the standard curve wells (O’Callaghan & Fenech, 2011).
Figure 1.
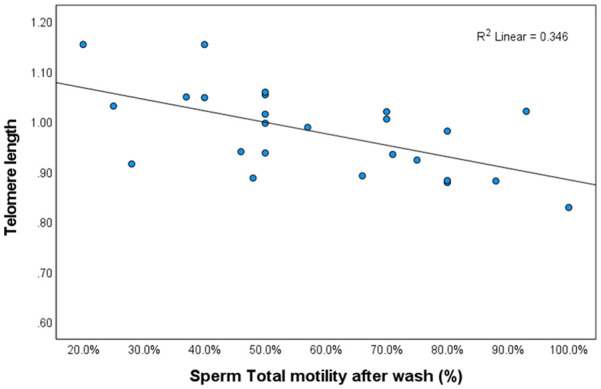
Correlation Between Telomere Length and Sperm Motility.
Note. p = .002.
The final concentrations of the primers were tel 1, 270 nM; tel 2, 900 nM; 36B4u, 300 nM; and 36B4d, 500 nM. The sequence of primers (5′ 3′) was tel 1, GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT; tel 2, TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC-CTA; 36B4f, CAGCAAGTGGGAAGGTGTAATCC; 36B4r, CCCATTCTATCATCAACGGGTACAA.
The analysis was performed using real-time polymerase chain reaction with a CFX96 detection amplifier (Bio-Rad, USA). The thermal cycle profiles of the two reactions were initiated with incubation at 95°C for activation of DNA polymerase for 5 min. For telomere PCR, 40 cycles consisting of 15 s at 95°C and 2 s at 54°C. For 36B4 PCR, 40 cycles consisting of 15 s at 95°C and 1 s at 58°C. Analyses were carried out automatically using the Bio-Rad CFX Manager 2.1 software package (Bio-Rad, USA) to produce the standard curve for each plate and to calculate T and S values. T/S ratio per sample was determined utilizing the standard curves. The qPCR run was approved if at least two of the triplicates for both the single gene and the telomere differed by <10% and the average of the two nearest T/S measurements was used. Otherwise, the sample was reanalyzed.
Statistical Analysis
Data were tested for normality using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Parametric variables that followed a normal distribution were analyzed by parametric tests; otherwise, nonparametric tests were applied. Descriptive statistics, mainly, mean ± standard deviation (SD), 95% confidence interval (CI), median, and interquartile range (IQR), were applied, as appropriate.
As this study was the first to evaluate the impact of lifestyle on the TL of sperm, a pilot study on 34 patients was conducted.
Results
A total of 34 patients consented to participate in the study. Their characteristics are presented in Table 1. TL of 25 patients was analyzed, as we were not able to detect DNA in nine samples. The results indicated that sperm motility was negatively correlated with TL: as telomeres became shorter, motility improved (Pearson correlation = −.588, p = .002) (Figure 1). The median TL was 0.9897 T/S ratio.
Table 1.
Men’s Characteristics According to Telomere Length.
| Characteristic | Total | Telomere length <0.98 (n = 13) |
Telomere length ≥0.98 (n = 12) | p-value |
|---|---|---|---|---|
| Age | 36.8 ± 7.5 | 4.7 ± 35.6 | 8.9 ± 38.0 | .41 |
| Occupation, n (%) | ||||
| Physical job | 14 (56) | 6 (46) | 8 (67) | .43 |
| Nonphysical/office | 11 (44) | 7 (54) | 4 (33) | |
| Body mass index | 5.15 ± 28.3 | 4.3 ± 28.5 | 6.2 ± 28.1 | .84 |
| Smoking, n (%) | ||||
| No | 8 (32) | 4 (31) | 4 (33) | 1.00 |
| Yes | 17 (68) | 9 (69) | 8 (67) | |
| Physical activity, n (%) | ||||
| 10 (77) | 7 (58) | .29 | ||
| No | 17 (68) | 3 (23) | 5 (42) | |
| Yes | 8 (32) | 7/91 | 16/84 | .07 |
| Eat “healthy food,” n (%) | ||||
| No | 9 (36) | 7 (54) | 2 (17) | .05 |
| Yes | 16 (64) | 6 (46) | 10 (83) | |
| Location of cellular phone, n (%) | ||||
| Adjacent to genitalia | 7 (28) | 10 (77) | 8 (67) | .67 |
| Far from genitalia | 18 (72) | 3 (23) | 4 (33) | |
| Sperm total motility before wash, % [range] | 48 [29–58] | 43 [29–52] | 35 [22.7–52.5] | .48 |
| Sperm concentration, % [range] | 30 [0.6–200] | 28 [6–55] | 37 [8.7–65 | .89 |
| Sperm total motility after wash, % [range] | 50 [40–71] | 71 [48–80] | 50 [37.7–65] | .046 |
| ICSI | 14 (56%) | 6 (46%) | 8 (67%) | .24 |
| IVF | 1 (4%) | 0 | 1 (8%) | |
| IVF + ICSI | 10 (40%) | 7 (54%) | 3 (25%) |
Note. ICSI = intracytoplasmic sperm injection; IVF = in vitro fertilization.
We divided our group above and below the median T/S ratio. A total of 12 patients had long telomeres and 13 had short telomeres. When telomeres were longer than the median, sperm motility was significantly slower compared with the group with shorter telomeres (Figure 2). Smoking adversely affected native sperm motility (53% motility nonsmokers vs. 37% in smokers; p = .006), but other parameters were not affected by smoking. All other behavioral characteristics examined did not affect sperm count.
Figure 2.
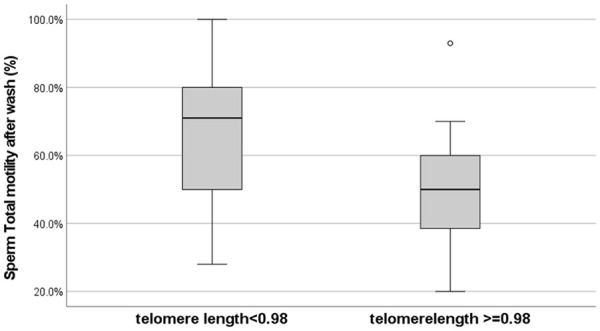
Longer Telomeres Were Related to Significantly Slower Sperm Motility.
Note. p = .046.
No correlation was identified between pregnancy outcomes and TL (Figure 3). Among couples who achieved pregnancy, body mass index (BMI) of the men was significantly lower compared with those who did not (26.9 ± 3.3 vs. 29.9 ± 5.2; p = .039; Figure 4).
Figure 3.
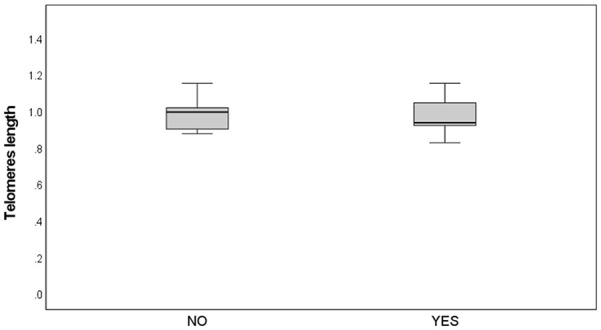
No Differences in Telomere Length Were Observed Between Patients Who Conceived and Those Who Did Not
Note. p = .86.
Figure 4.
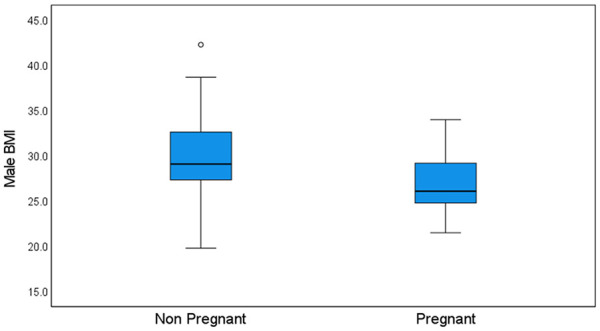
Higher Male BMI Was Related to Fewer Pregnancies
Note. p = .039.
Regarding lifestyle habits, the results indicated a correlation between consuming a healthy diet and TL: 10/12 patients in the long TL group consumed a healthy diet compared with 6/13 in the short TL group (p = .05). The group with longer telomeres also reported significantly more days per week of sports activity (16/84 vs. 7/91; p = .04) compared with the group with shorter telomeres. Notably, the lifestyle parameters did not have an impact on pregnancy outcomes.
Discussion
This study evaluated the impact of several lifestyle parameters on TL, sperm count, and treatment outcomes. Based on the knowledge that telomeres progressively shorten in somatic cells and are linked to biological aging and health problems, we evaluated TL in sperm cells. Our results indicate that sperm motility was negatively correlated with TL: as telomeres shortened, motility improved. However, longer telomeres were significantly correlated with diet and more physical activity compared with the group with shorter telomeres. No correlation was identified between TL and pregnancy outcomes.
Evaluating the effect of the different lifestyle parameters on sperm analysis, treatment outcomes, and pregnancy, the results indicated that smoking adversely affected native sperm motility (53% motility among nonsmokers vs. 37% in smokers; p = .006). In addition, higher paternal BMI was negatively associated with positive pregnancy results (pregnancy rate of 26.9 ± 3.3 in the higher BMI group vs. 29.9 ± 5.2 for the lower BMI group; p = .039).
Telomere Length and Sperm Parameters
Telomere Length and Semen Parameters
Studies have evaluated the correlation between semen parameters and TL. Sperm quality and infertility were linked to sperm TL; shorter telomeres have been associated with infertile men and poor IVF outcomes (Darmishonnejad et al., 2020). While Gentiluomo et al. (2021) did not find an association between sperm parameters and length of sperm telomeres or leukocytes, others reported shorter sperm TL in patients with male infertility and hypothesized that increased oxidative stress in spermatozoa can result in abnormal packing and damage to telomeres (Darmishonnejad et al., 2020; Tahamtan et al., 2019). The evaluation of telomeres could be considered a biomarker of spermatogenesis, sperm quality, and related male infertility. Balmori et al. (2022) reported statistically significant positive correlations between sperm count (p = .009) and motility (p = .007) and sperm TL only in younger male. They suggested that at younger ages, longer TL in spermatozoa have better seminal parameters.
As opposed to Balmori et al. (2022), the study by Ribas-Maynou et al. (2022) on pig’s sperm reported that TL was not correlated to sperm quality variables including viability, morphology, and DNA fragmentation. Moreover, they noted that regardless of the equivalent sperm parameters between TL groups, the blastulation rate was significantly better in the longer telomere group.
In agreement with Ribas-Maynou et al. (2022), our results indicated that longer telomeres were associated with relatively slower sperm motility. However, motility was in the normal range. This could be due to the higher molecular weight of sperm with longer telomeres and might explain the inverse correlation between TL and motility rate. However, total motility of both shorter and longer telomeres was within the range of good motility.
Lafuente et al. (2018) also reported that TL was directly correlated with the percentage of immotile sperm. In this sense, the telomere structure and mechanisms during the period from spermatogenesis until mature sperm cells are produced may be impaired and is not yet well understood.
Telomeres in mature spermatozoa are longer compared with all other human cells, probably due to delayed switching off of telomerase activity. The biological explanation for this phenomenon is to maintain intact telomeres for the future offspring (Achi et al., 2000; Rocca et al., 2019). In male gametogenesis, the development from the spermatogonia to the spermatozoa stage demonstrated elongation of telomeres, which was inversely correlated with telomerase activity (Achi et al., 2000).
Lifestyle and Telomere Length
Age and Telomere Length
While age-related telomere shortening in somatic cells is well accepted, it was shown that older men have longer telomeres in sperm relative to their younger counterparts and that telomeres in sperm are longer than those in leukocytes. For every additional year of paternal age, leukocyte TL in offspring increased. These data suggest that children of older men may inherit longer telomeres, which could affect their future health, age-related diseases, and lifespan (Kimura et al., 2008).
Although the role of sperm TL is not clearly known, the high activity of telomerase reverse transcriptase (TERT), the catalytic subunit of the telomerase in germ cells (Riou et al., 2005), and the death of sperm stem cells with shortened TL (Eisenberg et al., 2012) both enable the selection of a subset of sperm with longer telomeres.
Balmori et al. (2022) evaluated the difference in T/L between old and young men with normal and oligoasthenospermia. They reported a significantly positive correlation between sperm TL and sperm count and motility in younger men with normal sperm. They concluded that at younger ages, individuals with longer TL in spermatozoa have better seminal parameters. At older ages, the correlation between sperm TL and sperm count was not statistically significant.
In agreement with Balmori et al. (2022), our study evaluated the sperm from couples undergoing IVF. The mean age of the male partner was 36.8 ± 7.5 years. Therefore, we did not expect to see an effect of age on TL in this group.
Physical Activity and Telomere Length
The decline in male fertility associated with advancing age, the adverse impact of unhealthy lifestyles, and environmental factors have an important role in future human fertility. A few studies suggested that consistent, moderate intensity exercise may be more advantageous on oxidant/antioxidant markers in seminal plasma than in high-intensity continuous training and high-intensity interval training (Lalinde-Acevedo et al., 2017; Vaamonde et al., 2012) Exercise may help maintain leukocyte TL to attenuate premature biological aging and prevent age-related disease, yet the impact of exercise on telomere biology in germ cells is unknown (Ilacqua et al., 2018).
Our results demonstrated that the group with longer telomeres engaged in significantly more sports activity per week compared with the group with shorter telomeres. This supports the studies mentioned above that reported an advantage of sports activity on sperm count and quality.
Modification of lifestyle, educational programs to encourage physical exercise and healthier nutrition can have a positive impact on infertility and may help couples obtain better quality of life, as well as improve the possibility of spontaneous conception or chances of conception.
Smoking and Telomere Length
Various factors were identified as being involved in telomere shortening, including oxidative stress and lifestyle habits such as diet, tobacco use, obesity, or strenuous exercise, among others (Kawanishi & Oikawa, 2004; Thilagavathi et al., 2013). Smoking is among the modifiable risk factors of reproductive health. Approximately 37% of men of reproductive age smoke cigarettes (Sharma et al., 2016). Toxins from tobacco smoking have adverse effects on semen quality, including volume, sperm density, motility, viability, and normal morphology, thus contributing to male infertility (Sharma et al., 2016). In addition to its link with impaired male fertility, tobacco smoking is related to DNA injury, aneuploidies, and sperm mutations (Beal et al., 2017), as well as oxidative stress in spermatozoa, due to formation of excessive reactive oxygen species (ROS). In addition, decreased concentration of scavenging enzymes in the cytoplasm of spermatozoa decreases antioxidant capacity (Harlev et al., 2015). It is known that oxidative stress causes defective sperm function by affecting their viability, motility, DNA fragmentation, and membrane lipid peroxidation. Various enzymes were reported to have lower antioxidative effects, including superoxide dismutase, which is considered an important marker of male fertility and protects sperm from oxidative stress, lipid peroxidation, mitochondrial DNA impairment, and decreased motility (Murawski et al., 2007; Wagner et al., 2018). Glutathione peroxidase (GPX) was also identified as an essential enzyme for normal sperm function and integrity due to its defensive action against ROS (Schneider et al., 2009).
The current study did not detect any difference in sperm TL between smoking and nonsmoking patients, probably because the cohort was relatively small, most men were young and the knowledge that telomeres in sperm are maintained longer. However, in agreement with the above studies, our results revealed significantly slower sperm motility among patients who smoked. We encourage patients to stop smoking in general and especially those with abnormal sperm counts.
Male BMI and Pregnancy
Female obesity is a well-known factor in decreased fertility; however, male obesity as an isolated factor was also reported to adversely affect the time to conceive, as compared with normal weight men (Nguyen et al., 2007; Ramlau-Hansen et al., 2007). Obesity in males is associated with decreased pregnancy rates and an increase in pregnancy loss in couples undergoing assisted reproductive technology (ART). Various explanations were suggested including abnormal hormonal environment and the presence of anti-sperm antibodies (Hinz et al., 2010; Ramlau-Hansen et al., 2007). Male obesity has been correlated with lower sperm binding and fertilization rates during IVF and with reduced blastocyst development (Keltz et al., 2010). In agreement with the above, in our study, men’s BMI was significantly lower in the group of patients who conceived compared with those who did not.
Additional studies are needed on this topic, as limitations regarding sample size, cause of infertility, and other parameters to evaluate telomere performance were missing. We did not evaluate patients’ hormonal profiles, which might affect sperm performance and IVF outcomes. Neither did we investigate sperm morphology.
The strengths of this study are the homogeneity of the age group, which enabled us to control for the effects of age, which is considered one of the stronger factors influencing sperm parameters. In addition, evaluation of the treatment cycle until conception and the detailed questionnaire increased understanding of the effects of lifestyle habits on TL.
This study explored the impact of the lifestyle behaviors on sperm telomeres. The effects of diet, smoking, obesity, and physical activity as part of maintaining healthy habits are important and we strongly recommend maintaining a healthy lifestyle to preserve general health and future fertility.
Footnotes
Author contributions: Conceptualization—Einat Shalom-Paz, Shay Hantisteanu, Shilhav Meisel-Sharon; Data curation—Moamina Sharqawi, Shay Hantisteanu, Ofer Limonad, Shilhav Meisel-Sharon, Daniela Estrada; Formal analysis—Moamina Sharqawi, Shay Hantisteanu, Shilhav Meisel-Sharon, Einat Shalom-Paz; Investigation—Moamina Sharqawi, Einat Shalom-Paz, Shay Hantisteanu, Ofer Limonad; Methodology—Moamina Sharqawi, Einat Shalom-Paz; Writing—original draft—Moamina Sharqawi, Einat Shalom-Paz; Review and editing—Nardin Aslih, Yuval Atzmon, Asaf Bilgory, Yasmin Shibli, Abu-Raya, Daniela Estrada, Shay Hantisteanu, Shilhav Meisel-Sharon.
Availability of data and material: Upon request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: The local Ethics Committee of Hillel Yaffe Medical Center approved the study protocol, which was performed according to the Declaration of Helsinki, study number HYMC-0120-18, issued on March 2018 (NIH number NCT04158765).
Consent to participate: All participants provided written, informed consent to participate in the study. This prospective, non-interventional cohort study was conducted at the hospital IVF unit from November 2019 to November 2020.
ORCID iD: Einat Shalom-Paz  https://orcid.org/0000-0002-7785-4202
https://orcid.org/0000-0002-7785-4202
References
- Achi M. V., Ravindranath N., Dym M. (2000). Telomere length in male germ cells is inversely correlated with telomerase activity. Biology of Reproduction, 63(2), 591–598. 10.1095/biolreprod63.2.591 [DOI] [PubMed] [Google Scholar]
- Allsopp R. C., Chang E., Kashefi-Aazam M., Rogaev E. I., Piatyszek M. A., Shay J. W., Harley C. B. (1995). Telomere shortening is associated with cell division in vitro and in vivo. Experimental Cell Research, 220(1), 194–200. 10.1006/excr.1995.1306 [DOI] [PubMed] [Google Scholar]
- Balmori C., Cordova-Oriz I., De Alba G., Medrano M., Jiménez-Tormo L., Polonio A. M., Chico-Sordo L., Pacheco A., García-Velasco J. A., Varela E. (2022). Effects of age and oligoasthenozoospermia on telomeres of sperm and blood cells. Reproductive Biomedicine Online, 44(6), 1090–1100. 10.1016/j.rbmo.2021.10.010 [DOI] [PubMed] [Google Scholar]
- Beal M. A., Yauk C. L., Marchetti F. (2017). From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutation Research/Reviews in Mutation Research, 773, 26–50. 10.1016/j.mrrev.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Biron-Shental T., Wiser A., Hershko-Klement A., Markovitch O., Amiel A., Berkovitch A. (2018). Sub-fertile sperm cells exemplify telomere dysfunction. Journal of Assisted Reproduction and Genetics, 35(1), 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H., Greider C. W., Szostak J. W. (2006). Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nature Medicine, 12(10), 1133–1138. 10.1038/nm1006-1133 [DOI] [PubMed] [Google Scholar]
- Calado R. T., Young N. S. (2009). Telomere diseases. The New England Journal of Medicine, 361(24), 2353–2365. 10.1056/NEJMra0903373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerván-Martín M., Castilla J. A., Palomino-Morales R. J., Carmona F. D. (2020). Genetic landscape of nonobstructive azoospermia and new perspectives for the clinic. Journal of Clinical Medicine, 9(2), 300. 10.3390/jcm9020300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Noonan E., von Eckardstein S., Auger J., Baker H. W. G., Behre H. M., Haugen T. B., Kruger T., Wang C., Mbizno M. T., Vogelsong K. M. (2010). World Health Organization reference values for human semen characteristics. Human Reproduction Update, 16(3), 231–245. 10.1093/humupd/dmp048 [DOI] [PubMed] [Google Scholar]
- Darmishonnejad Z., Zarei-Kheirabadi F., Tavalaee M., Zarei-Kheirabadi M., Zohrabi D., Nasr-Esfahani M. H. (2020). Relationship between sperm telomere length and sperm quality in infertile men. Andrologia, 52(5), e13546. 10.1111/and.13546 [DOI] [PubMed] [Google Scholar]
- Dolcetti R., De Rossi A. (2012). Telomere/telomerase interplay in virus-driven and virus-independent lymphomagenesis: Pathogenic and clinical implications. Medicinal Research Reviews, 32(2), 233–253. 10.1002/med.20211 [DOI] [PubMed] [Google Scholar]
- Eisenberg D. T. A., Hayes M. G., Kuzawa C. W. (2012). Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proceedings of the National Academy of Sciences of the United States of America, 109(26), 10251–10256. 10.1073/pnas.1202092109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., Cawthon R. M. (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101(49), 17312–17315. 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentiluomo M., Luddi A., Cingolani A., Fornili M., Governini L., Lucenteforte E., Baglietto L., Piomboni P., Campa D. (2021). Telomere length and male fertility. International Journal of Molecular Sciences, 22(8), 3959. 10.3390/ijms22083959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M., Wu J., Schreiber V., Dunlap J., Dantzer F., Wang Y., Liu Y. (2006). PARP1 Is a TRF2-associated poly(ADP-ribose)polymerase and protects eroded telomeres. Molecular Biology of the Cell, 17(4), 1686–1696. 10.1091/mbc.e05-07-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlev A., Agarwal A., Gunes S. O., Shetty A., du Plessis S. S. (2015). Smoking and male infertility: An evidence-based review. The World Journal of Men’s Health, 33(3), 143–160. 10.5534/wjmh.2015.33.3.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B. (1997). Human ageing and telomeres. Ciba Foundation Symposium, 211, 129–139; discussion 139. [DOI] [PubMed] [Google Scholar]
- Hinz S., Rais-Bahrami S., Kempkensteffen C., Weiske W. H., Miller K., Magheli A. (2010). Effect of obesity on sex hormone levels, antisperm antibodies, and fertility after vasectomy reversal. Urology, 76(4), 851–856. 10.1016/j.urology.2010.01.055 [DOI] [PubMed] [Google Scholar]
- Ilacqua A., Izzo G., Emerenziani G. P., Baldari C., Aversa A. (2018). Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reproductive Biology and Endocrinology, 16(1), 115. 10.1186/s12958-018-0436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi S., Oikawa S. (2004). Mechanism of telomere shortening by oxidative stress. Annals of the New York Academy of Sciences, 1019, 278–284. 10.1196/annals.1297.047 [DOI] [PubMed] [Google Scholar]
- Keltz J., Zapantis A., Jindal S. K., Lieman H. J., Santoro N., Polotsky A. J. (2010). Overweight men: Clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. Journal of Assisted Reproduction and Genetics, 27(9–10), 539–544. 10.1007/s10815-010-9439-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Cherkas L. F., Kato B. S., Demissie S., Hjelmborg J. B., Brimacombe M., Cupples A., Hunkin J. L., Gardner J. P., Lu X., Cao X., Sastrasinh M., Province M. A., Hunt S. C., Christensen K., Levy D., Spector T. D., Aviv A. (2008). Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLOS GENETICS, 4(2), Article e37. 10.1371/journal.pgen.0040037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner J., Schatzl G., Waldhör T., Resch K., Kratzik C., Marberger M. (2005). Constant decline in sperm concentration in infertile males in an urban population: Experience over 18 years. Fertility and Sterility, 84(6), 1657–1661. 10.1016/j.fertnstert.2005.05.049 [DOI] [PubMed] [Google Scholar]
- Lafuente R., Bosch-Rue E., Ribas-Maynou J., Alvarez J., Brassesco C., Amengual M. J., Benet J., Garcia-Peiro A., Brassesco M. (2018). Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia, 50(2), e12840. 10.1111/and.12840 [DOI] [PubMed] [Google Scholar]
- Lalinde-Acevedo P. C., Mayorga-Torres B. J. M., Agarwal A., du Plessis S. S., Ahmad G., Cadavid Á. P., Cardona Maya W. D. (2017). Physically active men show better semen parameters than their sedentary counterparts. International Journal of Fertility & Sterility, 11(3), 156–165. 10.22074/ijfs.2017.4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski M., Saczko J., Marcinkowska A., Chwiłkowska A., Gryboś M., Banaś T. (2007). Evaluation of superoxide dismutase activity and its impact on semen quality parameters of infertile men. Folia Histochemica et Cytobiologica, 45(Suppl. 1), S123–S136. [PubMed] [Google Scholar]
- Nguyen R. H. N., Wilcox A. J., Skjaerven R., Baird D. D. (2007). Men’s body mass index and infertility. Human Reproduction, 22(9), 2488–2493. 10.1093/humrep/dem139 [DOI] [PubMed] [Google Scholar]
- O’Callaghan N. J., Fenech M. (2011). A quantitative PCR method for measuring absolute telomere length. Biological Procedures Online, 13, 3. 10.1186/1480-9222-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odisho A. Y., Nangia A. K., Katz P. P., Smith J. F. (2014). Temporal and geospatial trends in male factor infertility with assisted reproductive technology in the United States from 1999-2010. Fertility and Sterility, 102(2), 469–475. 10.1016/j.fertnstert.2014.05.006 [DOI] [PubMed] [Google Scholar]
- Oud M. S., Volozonoka L., Smits R. M., Vissers L. E. L. M., Ramos L., Veltman J. A. (2019). A systematic review and standardized clinical validity assessment of male infertility genes. Human Reproduction, 34(5), 932–941. 10.1093/humrep/dez022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo G. D., Cohen J., Alikani M., Adler A., Rosenwaks Z. (1995). Intracytoplasmic sperm injection: A novel treatment for all forms of male factor infertility. Fertility and Sterility, 63(6), 1231–1240. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. (2015). Obesity and reproduction: A committee opinion. Fertility and Sterility, 104(5), 1116–1126. 10.1016/j.fertnstert.2015.08.018 [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen C. H., Thulstrup A. M., Nohr E. A., Bonde J. P., Sørensen T. I. A., Olsen J. (2007). Subfecundity in overweight and obese couples. Human Reproduction, 22(6), 1634–1637. 10.1093/humrep/dem035 [DOI] [PubMed] [Google Scholar]
- Ribas-Maynou J., Mateo-Otero Y., Sanchez-Quijada M., Recuero S., Delgado-Bermúdez A., Llavanera M., Yeste M. (2022). Telomere length in pig sperm is related to in vitro embryo development outcomes. Animals: An Open Access Journal From MDPI, 12(2), 204. 10.3390/ani12020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou L., Bastos H., Lassalle B., Coureuil M., Testart J., Boussin F. D., Allemand I., Fouchet P. (2005). The telomerase activity of adult mouse testis resides in the spermatogonial alpha6-integrin-positive side population enriched in germinal stem cells. Endocrinology, 146(9), 3926–3932. 10.1210/en.2005-0502 [DOI] [PubMed] [Google Scholar]
- Rocca M. S., Foresta C., Ferlin A. (2019). Telomere length: Lights and shadows on their role in human reproduction. Biology of Reproduction, 100(2), 305–317. 10.1093/biolre/ioy208 [DOI] [PubMed] [Google Scholar]
- Rocca M. S., Speltra E., Menegazzo M., Garolla A., Foresta C., Ferlin A. (2016). Sperm telomere length as a parameter of sperm quality in normozoospermic men. Human Reproduction, 31(6), 1158–1163. 10.1093/humrep/dew061 [DOI] [PubMed] [Google Scholar]
- Rolland M., Le Moal J., Wagner V., Royère D., De Mouzon J. (2013). Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Human Reproduction, 28(2), 462–470. 10.1093/humrep/des415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Förster H., Boersma A., Seiler A., Wehnes H., Sinowatz F., Neumüller C., Deutsch M. J., Walch A., Hrabe de Angelis M., Wurst W., Ursini F., Roveri A., Maleszewski M., Maiorino M., Conrad M. (2009). Mitochondrial glutathione peroxidase 4 disruption causes male infertility. The FASEB Journal, 23(9), 3233–3242. 10.1096/fj.09-132795 [DOI] [PubMed] [Google Scholar]
- Sharma R., Harlev A., Agarwal A., Esteves S. C. (2016). Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. European Urology, 70(4), 635–645. 10.1016/j.eururo.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Tahamtan S., Tavalaee M., Izadi T., Barikrow N., Zakeri Z., Lockshin R. A., Abbasi H., Nasr-Esfahani M. H. (2019). Reduced sperm telomere length in individuals with varicocele is associated with reduced genomic integrity. Scientific Reports, 9(1), 4336. 10.1038/s41598-019-40707-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilagavathi J., Venkatesh S., Dada R. (2013). Telomere length in reproduction. Andrologia, 45(5), 289–304. 10.1111/and.12008 [DOI] [PubMed] [Google Scholar]
- Vaamonde D., Da Silva-Grigoletto M. E., García-Manso J. M., Barrera N., Vaamonde-Lemos R. (2012). Physically active men show better semen parameters and hormone values than sedentary men. European Journal of Applied Physiology, 112(9), 3267–3273. 10.1007/s00421-011-2304-6 [DOI] [PubMed] [Google Scholar]
- Valdes A. M., Andrew T., Gardner J. P., Kimura M., Oelsner E., Cherkas L. F., Aviv A., Spector T. D. (2005). Obesity, cigarette smoking, and telomere length in women. The Lancet, 366(9486), 662–664. 10.1016/S0140-6736(05)66630-5 [DOI] [PubMed] [Google Scholar]
- Wagner H., Cheng J. W., Ko E. Y. (2018). Role of reactive oxygen species in male infertility: An updated review of literature. Arab Journal of Urology, 16(1), 35–43. 10.1016/j.aju.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberlicht A., Wiener-Megnazi Z., Sheinfeld Y., Grach B., Lahav-Baratz S., Dirnfeld M. (2015). Habits of cell phone usage and sperm quality–Does it warrant attention? Reproductive Biomedicine Online, 31(3), 421–426. 10.1016/j.rbmo.2015.06.006 [DOI] [PubMed] [Google Scholar]


