Summary
A variety of axes between brain and abdominal organs have been reported, but the interaction between brain and visceral white adipose tissue (vWAT) remains unclear. In this review, we summarized human studies on the association between brain and vWAT, and generalized their interaction and the underlying mechanisms according to animal and cell experiments. On that basis, we come up with the concept of the brain-vWAT axis (BVA). Furthermore, we analyzed the potential mechanisms of involvement of BVA in the pathogenesis of Alzheimer's disease (AD), including vWAT-derived fatty acids, immunological properties of vWAT, vWAT-derived retinoic acid and vWAT-regulated insulin resistance. The proposal of BVA may expand our understanding to some extent of how the vWAT impacts on brain health and diseases, and provide a novel approach to study the pathogenesis and treatment strategies of neurodegenerative disorders.
Keywords: Brain, Visceral white adipose tissue, Alzheimer’ disease, Fatty acid, Retinoic acid
Introduction
Over the past few decades, the connections between central nervous system and other organs have always been the focus of research in the biomedical field. Currently, bidirectional communications and underlying mechanisms between brain and gut microbiota,1,2 brain and liver,3,4 and brain and pancreas5,6 have been extensively revealed, which greatly enriched our understanding of brain health and diseases.
The adipose tissue, also known as fat tissue or fatty tissue, is composed of multiple distinct cells and molecules (e.g. adipocytes, macrophages and other immune cells, stromal vascular fractions, nerve tissue, and various proteins in the extracellular matrix).7,8 Generally, it is divided into two types, white adipose tissue (WAT) and brown adipose tissue (BAT), depending on differences of location, function and cellular composition.9 In adults, BAT is located only in the interscapular region extending from the anterior neck to the thorax,9,10 while WAT is found throughout the whole body and conventionally organized into several anatomically distinct depots.11 The omental, mesenteric, retroperitoneal, gonadal and pericardial depots are the main locations of visceral WAT (vWAT),10,12 and subcutaneous WAT (sWAT) distributes primarily in back, abdominal wall and gluteofemoral regions which are considered as the subcutaneous depots.13 To date, methods for detection of vWAT mainly include waist circumference, computed tomography (CT), magnetic resonance imaging (MRI), three-dimensional body scanning technology, dual energy X-ray absorptiometry (DEXA), ultrasonography and bioelectrical impedance analysis (body composition analyzer).14 Waist circumference is the simplest and most economical among all methods. CT and MRI tests, which can directly measure the area and volume of vWAT, are considered as the standard methods for quantitative assessment of vWAT depots and have been the most widely used in previous studies.14, 15, 16
Up to now, studies on vWAT mainly focus on fat metabolism, inflammation, insulin resistance, and obesity17 in light of the secretory function of vWAT including leptin, adiponectin, resistin, apelin, visfatin, omentin, chemerin, lipocalin, and the cytokines such as tumor necrosis factor (TNF) -α and interleukin (IL) -618, 19, 20 (Table 1). However, the interaction between brain and vWAT and its underlying mechanisms have not been systematically discussed so far. Therefore, here we summarized human studies on the association between brain and vWAT and generalized their bidirectional communication and potential mechanisms according to animal and cell experiments. Based on this, we come up with a new concept of brain-vWAT axis (BVA). Given that many of brain changes mentioned in foregoing human studies are crucial clinical features of Alzheimer's disease (AD), we took AD as an example to further analyze the potential impacts of BVA on the pathogenesis of neurodegenerative disorders in this review.
Table 1.
The metabolic hormones secreted by adipocytes and their involvement in the pathogenesis of AD.
| Metabolic hormones secreted by adipocytes | Involved in AD pathology | References |
|---|---|---|
| Leptin | ↓ Aβ level | 181, 182, 183 |
| ↓ Phosphorylation of tau | 182,183,191,192 | |
| ↑ Hippocampal neurogenesis | 181,193,194 | |
| ↓ Neurodegeneration | 181,195 | |
| ↑ Synaptic function | 196, 198 | |
| ↓ Oxidative stress | 181,207 | |
| Resistin | ↓ Aβ induced oxidative stress | 182,184 |
| ↑ Neuroinflammation | 186 | |
| Adiponectin | ↑ Deposition of Aβ | 185, 187 |
| ↓ Neuroinflammation | 201,202 | |
| Chemerin | ↑ Clearance of Aβ42 | 188, 190 |
| Participating in regulate Tau seeding | 189 | |
| ↓ Tau phosphorylation level | ||
| ↓ Neuroinflammation | 203,204 | |
| Lipocalin 2 | ↓ Neuroinflammation | 205,206 |
| ↑ Synaptic toxicity | 199,200 | |
| ↑ Oxidative stress | 206,208 |
Abbreviations: AD, Alzheimer's disease; Aβ, Amyloid β.
Evidence from human studies on the association between brain and vWAT
Association between the general structure of brain and vWAT
Changes in the brain cortical thickness have been reported to be associated with the vWAT accumulation in neuroimaging studies at different ages (Table 2). In adolescents aged 15-18 years, the cortical thickness in several regions of the brain and the total cortical thickness measured by MRI were significantly positively correlated with the ratio of vWAT defined by dividing the area of intra-abdominal fat per the total area of the abdominal cavity.21 In subjects aged 19-50 years, both BMI and vWAT were independently correlated with the decrease of the cortical thickness in clusters comprising left lateral occipital region, left inferior temporal cortex, left precentral region and inferior parietal region.22 However, the cortical thickness of right insula, left fusiform gyrus and right inferior temporal region was only negatively correlated with the vWAT volume.22 In healthy middle-aged subjects, both the volume of sWAT and vWAT had remarkedly negative correlations with the cortical thickness measured by CT.23 And there was an even more significantly negative association between the increased volume of vWAT and the decreased cortical thickness independent of BMI and insulin resistance.23 In the elderly, the larger area of vWAT was accompanied with the reduced cortical thickness of global, parietal, temporal, cingulate and insula lobe.24
Table 2.
The main outcomes of human studies on the association between the general structure of brain and vWAT.
| Participants | Sample size | Outcomes | Correlation | Study design | References |
|---|---|---|---|---|---|
| Adolescents aged 15–18 years | 44 | The total cortical thickness vs. The ratio of vWAT to the total body fat | Positively | Cross-sectional study | 21 |
| Subjects aged 19–50 years | 72 | BMI and vWAT vs. The decrease of the cortical thickness in clusters comprising left lateral occipital area, left inferior temporal cortex, left precentral area and inferior parietal area | Negatively | Cross-sectional study | 22 |
| The cortical thickness of right insula, left fusiform gyrus and right inferior temporal area vs. The volume of vWAT | Negatively | ||||
| Healthy middle-aged subjects | 733 | The volume of sWAT and vWAT vs. The cortical thickness | Negatively | Prospective study | 23 |
| The increased volume of vWAT vs. The decreased cortical thickness | Negatively | ||||
| The elderly | 316 | The area of vWAT vs. The cortical thickness of global, parietal, temporal, cingulate and insula lobe | Negatively | Cross-sectional study | 24 |
| Community-dwelling healthy older population | 1209 | The volume ratio of the thigh muscle to vWAT vs. The volume of temporal lobe and cerebellum | Positively | Prospective study | 25 |
| The thigh muscle/vWAT volume ratio vs. The volume of left entorhinal cortex, right temporal pole and inferior temporal gyrus | Positively | ||||
| The thigh muscle/vWAT volume ratio vs. The volume of cerebellum and right globus pallidus | Positively | ||||
| The volume of vWAT vs. The temporal lobe volume | Negatively | ||||
| HIV-infected male patients | 226 | The volume of vWAT vs. The volume of bilateral posterior hippocampus, left mesial temporal lobe, temporal stem white matter, and the larger volume of cerebrospinal fluid | Negatively | Prospective study | 26 |
| Subjects from Southern Germany | 351 | The volume of the perihepatic adipose tissue vs. The volume of cingulate gyrus and hippocampal gray matter | Negatively | Prospective case-control study | 27 |
Abbreviations: vWAT, visceral white adipose tissue; AD, Alzheimer's disease; sWAT, subcutaneous white adipose tissue.
Also, vWAT has a close relationship with the volume of different brain regions (Table 2). The area of vWAT had a significant negative association with temporal lobe volume, and the volume ratio of the thigh muscle to vWAT was found to be markedly positively correlated with temporal lobe, gray matter of the temporal lobe, and cerebellum.25 Specifically, the thigh muscle/vWAT ratio was significantly positively correlated with right temporal pole, right inferior temporal cortex and left entorhinal cortex, all of which were thought to be related to cognition, and was markedly positively associated with right pallidum and bilateral cerebellar cortices, all of which were considered to be related to motor function.25 But, the vWAT volume had a negative correlation with the temporal lobe volume.25 Human immunodeficiency virus-infected male patients with the larger vWAT volume had the smaller volume of bilateral posterior hippocampus, left mesial temporal lobe and temporal stem white matter, as well as the larger volume of cerebrospinal fluid (CSF).26 Moreover, vWAT had a stronger correlation with the brain atrophy than age, BMI, hypertension and type 2 diabetes mellitus.26 Additionally, the volume of the perihepatic adipose tissue, rather than other obesity-related markers, was found to be significantly negatively correlated with the volume of cingulate gyrus and hippocampal gray matter, indicating that the association between the vWAT in different depots and the volume of distinct brain regions could be various.27
Association between the microstructure of brain and vWAT
The microstructure of brain has been found to be related to vWAT among all age groups (Table 3). In normal-weight children participating in physical activity, the reduction of vWAT was accompanied with faster performance in the Eriksen flanker task and increased amplitude of P3 event-related brain potential.28 In adolescents aged 12-18 years, the volume of vWAT was independently markedly positively associated with white/gray matter signal ratio, T1 weighted signal intensity in white matter, and standardized magnetization transfer ratio (MTR, a functional MRI index derived from MTON and MTOFF sequences and conventionally considered as an indicator of myelination and de-myelination) in both gray and white matter.29 In adolescents and middle-aged adults, vWAT was associated with serum C-reactive protein level, which was in turn correlated with changes in white matter microstructure and lower processing speed in cognitive testing.30 Glycerophosphocholines probably mediated the relationship between vWAT and both white matter microstructure and processing speed.30 The volume of vWAT was correlated negatively with gray matter density in both cerebellar hemispheres in young-to-mid-age subjects.31 Besides, the higher amount of vWAT was accompanied with the decreased eigenvector centrality (EC, a marker of functional brain architecture derived from the task-independent functional MRI data) in cerebellum, left posterior temporal and parietal lobe, and with an increased EC in the cingulate cortex.31 The MRI-T2 relaxation time in the mediobasal hypothalamus was positively correlated with the vWAT volume in healthy adult men.32 In the elderly, the area of vWAT was found to be negatively associated with the normalized MTR peak height in gray and white matter independent of sex, age, BMI, hypertension, current smoking, statin use and type 2 diabetes mellitus.33
Table 3.
The main outcomes of human studies on the association between the microstructure of brain and vWAT.
| Participants | Sample size | Outcomes | Correlations | Study design | References |
|---|---|---|---|---|---|
| Normal-weight children participating in physical activity | 206 | The area of vWAT vs. The task performance and the elevated amplitude of P3 event-related brain potential | Negatively | Prospective study | 28 |
| The adolescents | 970 | The volume of vWAT vs. The white/gray matter signal ratio, T1 weighted signal intensity of white matter, and the MTR of white and gray matter | Positively | Cross-sectional study | 29 |
| Adolescents and middle-aged adults | 872 | The volume of vWAT vs. The serum C-reactive protein level | Positively | Cross-sectional study | 30 |
| The volume of vWAT vs. The serum C-reactive protein level, the changes in white matter microstructure, and the processing speed in cognitive testing | Negatively | ||||
| Young subjects | 100 | The volume of vWAT vs. The structural regions of cerebellum involved in motor processing and cerebrum involved in cognitive and emotional processing | Negatively | Cross-sectional study | 31 |
| Healthy adult men | 41 | The vWAT volume vs. The MRI-T2 relaxation time in the mediobasal hypothalamus | Positively | Cross-sectional study | 32 |
| The elderly | 243 | The area of vWAT vs. The peak height of MTR in gray matter and white matter | Negatively | Cross-sectional study | 33 |
| The adults ranging in weight status (normal and excess weight) | 75 | The vWAT level vs. The functional connectivity of the middle-dorsal insula with a cluster involving the bed nucleus of stria terminalis and hypothalamus | Negatively | Cross-sectional study | 34 |
| The vWAT level vs. The functional connectivity of the rostral insula with the right amygdala, the middle-dorsal insula with the middle frontal gyri, and the middle-dorsal insula with the right intraparietal cortex | Positively | ||||
| Military helicopter pilots | 23 | The mass of vWAT vs. The white matter fractional anisotropy | Negatively | Cross-sectional study | 36 |
| High risk for type 2 diabetes participants | 300 | The brain insulin sensitivity vs. The volume of total body fat and vWAT | Negatively | Prospective study | 37 |
| The volume of vWAT vs. The insulin responsiveness of hypothalamus | Negatively | ||||
| Healthy lean and overweight obese participants | 48 | Cerebral blood flow in the hypothalamus vs. The volume of vWAT | Negatively | Prospective study | 38 |
Abbreviations: vWAT, visceral white adipose tissue; AD, Alzheimer's disease; MTR, magnetization transfer ratio; MRI, magnetic resonance imaging.
The vWAT level was negatively associated with the functional connectivity between dorsal-mid insula and a cluster encompassing lateral hypothalamus, preoptic nuclei and bed nucleus of the stria terminalis, and was positively correlated with the functional connectivity between dorsal-mid insula and bilateral middle frontal gyri, as well as the right intraparietal gyrus.34 A mutation near DHCR24 gene was identified to encode a cholesterol-synthesizing enzyme (rs588709), which was nominally associated with white matter microstructure, vWAT and circulating concentration of Omega-3 fatty acids in interaction with vWAT, respectively.35 Besides, the higher mass of vWAT assessed by DEXA fan-beam technology showed a significant correlation with the reduced fractional anisotropy (FA, a functional MRI indicator of white matter integrity) in a set of clusters in both hemispheres in normal-weight military pilots.36 The elevated baseline brain insulin sensitivity was correlated to the decreased volume of total body fat and vWAT after the lifestyle intervention.37 Notably, vWAT but not sWAT was closely associated with the strong insulin responsiveness of the hypothalamus.37 A significant decrease in hypothalamic cerebral blood flow was observed in subjects receiving intranasal insulin and the magnitude of this response was correlated with vWAT but not with other fat compartments.38 Brain insulin action was selectively impaired in prefrontal cortex and hypothalamus in subjects with the accumulation of vWAT.38
Association between lesions of brain and vWAT
Many brain parenchymal lesions have been proved to be associated with vWAT, especially cerebral small vessel diseases (CSVDs). The area of vWAT (≥ 100 cm2), as measured by CT, was linked to white matter lesions and silent lacunar infarction (SLI, defined as an asymptomatic, well-defined lesion with a diameter ≥ 3 mm with the same signal characteristics as the CSF) in participants without a history of stroke independent of age, BMI and other cardiovascular risk factors.39 Visceral obesity index (VAI) has been considered as a significant predictor of SLI because VAI had a positive dose-response relationship with the number of SLI lesions and had a more prominently close correlation with SLI in women than that in men.40 In neurologically healthy subjects, the higher VAI was associated with the higher prevalence and burden of SLI.40 Women with the higher VAI had the lower gray matter density in the caudal anterior cingulate cortex compared to those with the lower VAI.41 In subjects free of CSVDs, those with white matter hyperintensities (WMH) had more amount of vWAT area, BMI and waist circumference than those without WMH.42 The area of vWAT was found to be an independent risk factor for cerebral WMH after adjusting for age, sex, type 2 diabetes mellitus, hypertension, smoking and alcohol consumption.42 A higher vWAT/sWAT ratio was found to be an independent predictor of the cerebral microbleeds (CMBs) in neurologically healthy subjects.43 Similar results of comparison and regression analyses were also observed in the study of vWAT and lacunar infarction.42 In addition, the vWAT/sWAT ratio was also independently associated with the presence of ischemic changes, cerebral artery stenosis or occlusion, and cervical plaque in apparently healthy adults.44 After statistical adjustment of cardiovascular effects on WMH, the increase of vWAT had an independent negative effect on white matter in middle age prior to cognitive impairment.45 The percentage of vWAT in total body fat was significantly correlated with white matter lesion load, severity of atherosclerosis, ischemic encephalopathy, and the recognized markers of cerebrovascular diseases. The percentage of vWAT was also regarded as a risk factor for both CSVDs and cerebral atherosclerosis of large, medium and small arteries.46 Waist-to-hip ratio (WHR), a marker of visceral obesity, was proved to be related to higher deep WMH and higher deep-to-periventricular WMH ratio,47 in which the elevated IL-6 probably acted as a mediator shown by the mediation analysis.47 Besides, a study from Japan revealed that the vWAT area was larger in the white matter lesions-positive group than that in the white matter lesions-negative group, and both the larger vWAT area and insulin resistance were the independent predictors of white matter lesions in patients with type 2 diabetes mellitus.48
In summary, a wealth of human research evidence has proved that general structure, microstructure, and lesions of brain are closely related to vWAT at the phenotypic level, suggesting a certain interaction between brain and vWAT and its underlying mechanisms deserved further discussion.
Evidence from basic biomedical studies on the interaction between brain and vWAT
Influence of specific brain regions and related structure on the mass and function of vWAT
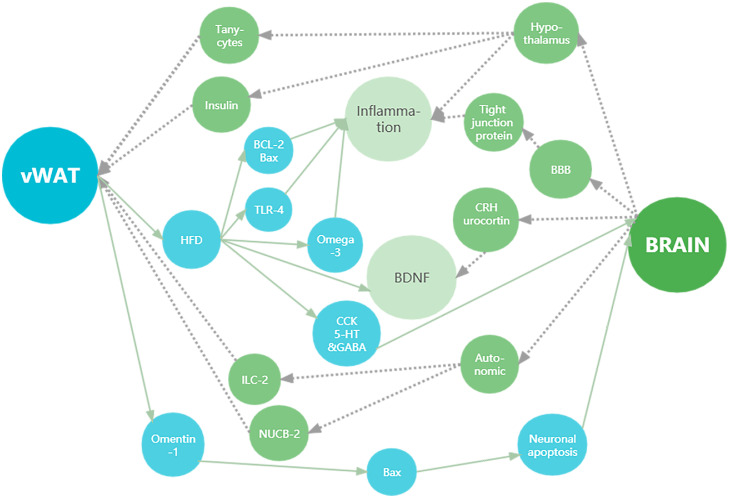
The hypothalamus and its related structure play a crucial role in regulating vWAT (Figure 1). The mesenteric vWAT in mice with the depletion of hypothalamic somatostatinergic neurons was resistant to lipolysis, as shown by a 50% reduction in isoproterenol-induced lipolysis compared to controls.49 The hypothalamic peptide oxytocin directly inhibited the inflammation in vWAT manifested by the decreased mRNA expression of IL-6 and TNF-α, and increased concentrations of anti-inflammatory adipokines.50 By means of an osmotic mini-pump, a long-term administration of brain-derived neurotrophic factor (BDNF) at the lateral ventricle or into the paraventricular nucleus of hypothalamus lowered food intake and body weight, decreased the weight of sWAT and vWAT (perirenal, mesenteric and epididymal depots), and reduced adipocyte size and serum concentration of triglyceride in mice.51 Corticotrophin-releasing hormone (CRH) and urocortin partially mediated long-term effects of BDNF to depress feeding and promote lipolysis.51 Compared with the controls, male mice undergoing selective genetic ablation of tanycytes in arcuate nucleus and median eminence of the hypothalamus had the higher mass of vWAT in perigonadal and retroperitoneal depots, the larger volume of perigonadal adipocytes, and the stronger insulin insensitivity, however, no significant differences in body weight or food intake were observed.52 A long-term intracerebroventricular infusion of adiponectin decreased the vWAT mass in the epididymal depot by increasing energy expenditure and fat oxidation via the activation of both insulin and leptin signaling pathways in the hypothalamus.53
Figure 1.
Schematic depicting the bidirectional communication between brain and vWAT.
Green circles show the involvement of vWAT in the regulation on the structure and function of brain. Blue circles display the influence of specific brain regions and related structure on the mass and function of vWAT.
Abbreviations: vWAT, visceral white adipose tissue; HFD, high-fat diet; ILC, innate lymphoid cell; NUCB, nesfatin/nucleobindin; BCL, B-cellymphoma; TLR, Toll-like receptor; CCK, cholecystokinin; 5-HT, 5-hydroxytryptamine, serotonin; GABA, γ-aminobutyric acid; BDNF, brain-derived neurotrophic factors; Bax, BCL associated X; CRH, corticotrophin-releasing hormone; BBB, blood-brain barrier.
The autonomic nerve also plays a key role in the regulation of brain on vWAT (Figure 1). A novel sympathetic aorticorenal circuit was identified in which the autonomic nerve plays a key bridging role.54 On one hand, sympathetic nerve endings downwards modulate the activity of innate lymphoid cell (ILC) group -2 and the expression of glial-derived neurotrophic factor in the gonadal vWAT depot via the β2-adrenergic signaling pathway, and on the other hand, the sympathetic nerve upwards connects to certain higher-order brain regions including the hypothalamic paraventricular nucleus.54 By inhibiting the sympathetic nerve activity in rats, the ventromedial hypothalamus injury significantly increased body weight, vWAT mass and sWAT mass, regulated the activity and balance of autonomic nervous system in WAT, and induced an increased expression of nesfatin/nucleobindin (NUCB)-2.55 Of note, NUCB-2 is the precursor of nesfatin-1, a kind of anorexia protein, overexpression of which can induce visceral obesity due to a high-fat diet (HFD) in mice.56 In addition, blockage of afferent signals from the common hepatic branch of the vagus by an injection of capsaicin promoted lard intake, leading to the selective deposition of vWAT in the mesenteric depot in a diabetic rats model.57
Involvement of vWAT in the regulation on the structure and function of brain
Increase of vWAT induced by dietary has been proved to participate in regulation on brain structure and function. In addition to elevated vWAT mass and body weight, the rats fed with a HFD showed worse performances in both open field test and Morris water maze test, increased production of mitochondrial reactive oxygen species and extent of mitochondrial swelling in the brain, decreased degree of electrical-induced long-term potentiation and density of dendritic spine in the hippocampus, and significant changes of anti-apoptotic protein B cell lymphoma (BCL)-2 and pro-apoptotic protein BCL-2 associated X (Bax) in the brain, all of which could be attenuated by an intraperitoneal injection of fibroblast growth factor –21.58 A long-term exercise on the treadmill reversed HFD-induced excessive gain of vWAT, enhanced the retention latency time in the passive avoidance task, revoked the increase of TNF-α, IL-1β and cyclooxygenase-2 in the hippocampus, attenuated the overexpression of both ionized calcium-binding adapter molecule (IBA) –1 and glial fibrillary acidic protein (GFAP) in the cerebral cortex and hippocampal dentate gyrus in the rats fed with a HFD.59 Intake of Omega-3 fatty acids reduced the vWAT mass, attenuated the inhibition on mitochondrial respiratory chain complex, and partially reversed the neuroinflammation and oxidative damage in rats fed with a HFD.60 The decreased sensitivity of intestinal sensory nerves to chemicals (cholecystokinin (CCK) and serotonin) released by the intestine at meals,61 and the decreased excitability of Gamma-aminobutyric acid (GABA) neurons in the ventral tegmental region of the midbrain62 were observed in rats fed with a HFD. The offspring of maternal HFD mice had the increase of vWAT mass, expression of tryptophan hydroxylase 2 and BDNF, and TNF-α mRNA level in the hippocampus.63 The diet could induce the changes in macrophage migration to the hypothalamus, adipose tissue and peritoneal cavity, especially in men.64 In addition, some hypothalamic macrophages were proved to originate from vWAT.64
Omentin-1 was confirmed to be a vWAT-specific secretory protein negatively associated with obesity and insulin resistance.65 Omentin-1 significantly reduced the expression of cocaine and amphetamine-regulated transcript and corticotrophin-releasing hormone, and stimulated the release of norepinephrine in hypothalamic synaptosomes.65 Omentin-1 significantly suppressed the expression level of Bax and attenuated H2O2-induced neuronal apoptosis by up-regulating miR-128-3p at its 3′-UTR.66 The increased permeability of blood-brain barrier (BBB) and decreased expression of tight junction protein were observed in vWAT-removed mice.67 After undergoing a transient middle cerebral artery occlusion, the ischemic cerebral infarction volume, BBB permeability and intracerebral pro-inflammatory cytokines were decreased in vWAT-removed rats with preprocessed surgical operation.67 Knockout of inflammasome protein cryopyrin in vWAT attenuated obesity-induced neuroinflammation and cognitive impairment through IL-1β.68 In addition, resveratrol reduced the levels of IL-1β, TNF-α and immunoglobulin in brain and vWAT, and decreased the adipocyte size in vWAT and immunoglobulin G extravasation in the brain.69
The evidence given by the aforementioned basic biomedical studies strongly suggests an obvious interaction between brain and vWAT with many different signaling pathways and molecular mechanisms involved in (Figure 1). It is worth mentioning that the connection between brain and vWAT is mutual, not unidirectional, even though they are physically far apart.
A new concept generalizing the bidirectional communication between brain and vWAT: brain-vWAT axis
So far, as an essential tissue mainly distributed in the abdominal cavity, vWAT has attracted increasing attention, and even have already been considered as an independent organ by some researchers.70 Previously, people summarized bidirectional communications and potential mechanisms between brain and certain abdominal organs, and have proposed a series of concepts of “axis” connecting brain and gut microbiota,1,2 brain and liver,3,4 as well as brain and pancreas.5,6 Likewise, both brain-gut-adipose tissue axis71, 72, 73 and brain-adipose axis74 have also been proposed before, nevertheless, there are still some important issues worth to be discussed. First, the elaboration of the bidirectional communication between brain and vWAT in the above literature has not been comprehensive enough. They either focused on how brain regulates food intake, energy metabolism, and obesity somehow (e.g., through insulin and leptin adiposity signaling pathway),72, 73, 74 or set changes of both brain and vWAT as the outcome of the independent variables (e.g., Chowiseung cheng-Tang, a herbal formulation) in the experimental design,71 but they both ignored the effects of vWAT on the brain. Second, as we concluded earlier in this review, the gut does not seem to be a required mediating factor between brain and vWAT. Third, and most importantly, considering differences in distribution, function, and cellular composition of vWAT and sWAT, and differences in the correlations between brain changes and vWAT as well as sWAT we summarized before, the current studies only focused on the association between adipose tissue and brain, which does not reflect the bidirectional communication between brain and vWAT.
As mentioned above, it should be concluded that there is a close connection between vWAT and the general structure, microstructure, and lesions of the brain according to human studies. Besides, people have preliminarily clarified the specific regions of the brain and their related structure which have the capacity to affect the mass, cellular composition, and function of vWAT, and also preliminarily revealed the regulatory effects of vWAT on the structure, cellular and molecular components of specific regions of the brain based on animal and cellular studies (Figure 1). Notably, the possible signaling pathways mediating the interaction between brain and vWAT are complex (Figure 1). Specifically, we summarized the potential mechanisms or mediating factors mainly including autonomic nervous system,49, 50, 51, 52, 53, 54, 55, 56, 57 neurotransmitters,51,54,61, 62, 63 inflammation,50,59,60, 67, 68, 69 hormones,52,54,65,66 fat metabolism51,56, 57, 58, 59,64 and glucose metabolism53,54,57 on the basis of current studies.
Taken together, here we come up with the concept of the BVA on the basis of all the findings we summarized in the preceding part of this review. Unlike other brain-related axes, the concept of BVA emphasizes more on the bidirectional communication between brain and vWAT rather than the unidirectional effects. Besides, in the naming process, we put “brain” in front of “vWAT”, which does not mean that vWAT is in a complete, passive, subordinate and downstream position. Obviously, as two extremely essential organs, the current human studies and mechanism research on the relationship between brain and vWAT have some apparent limitations in depth and breadth. Specifically, we believe that there are three issues that deserve further discussion: a, the regulation and signaling pathways of the body on BVA under physiological conditions; b, the intrinsic signaling pathways and mediating factors connecting brain and vWAT in BVA; and c, the role of BVA in the occurrence and progression of neurological diseases.
Potential involvement of brain-visceral white adipose tissue axis in the pathogenesis of AD
As discussed above, the proposed concept of BVA probably established a novel perspective to explore the relationship between vWAT with brain health and diseases. As neurological physicians, we further focused on whether vWAT influenced the occurrence and progression of central nervous system diseases through this axis. It is well known that the atrophy of specific brain regions,75 changes in brain microstructure76 and cerebral vascular dysfunction77 are crucial clinical features of AD. Thus, we take AD as an example to analyze the potential impacts of BVA on the pathogenesis of neurodegenerative disorders.
Characterized by the progressive decline of cognitive function, activities of daily living and social function,78 AD is an age-related neurodegenerative disorder that accounts for about 60%–80% of all dementia cases.79 Extracellular amyloid-β (Aβ) aggregates and intracellular tau neurofibrillary tangles are the two neuropathological hallmarks of AD,80 however, it is generally believed that the sporadic AD (accounting for more than 99% of all AD cases) is comprehensively caused by a combination of age, genetics, environment, metabolism, inflammation and other factors.81 Thus, its exact pathogenesis has not been fully elucidated and the treatment halting or reversing the disease progression has not been developed so far.82
Clinically, growing studies on the relationship between vWAT and AD have been reported.83 Several genetic mutation loci (such as APOM and APOA5) have been found to be co-associated with the genetic risk of late-onset AD and peripheral lipid metabolism.84 A meta-analysis showed that both midlife abdominal obesity and old age abdominal obesity were independent risk factors for AD (Evidence level I).85 The vWAT accumulation significantly increased the long-term risk of AD in normal elderly people over 60 years of age.86 Compared with age- and sex-matched controls, patients with mild-to-moderate AD had significantly higher vWAT mass, and the correlation was more pronounced in male AD patients.87 A prospective study of asymptomatic middle-aged adults with risk factors for AD showed that the vWAT accumulation was significantly associated with decline of brain Aβ burden, language learning and language memory.88 Using 18F-FDG uptake in vWAT on positron emission tomography (PET) images as a marker of vWAT metabolism, the subjects with higher vWAT metabolism exhibited significantly higher brain Aβ burden than those with lower vWAT metabolism.89 The 18F-FDG uptake in vWAT was significantly associated with brain Aβ burden independent of age, sex and WMH.89 In addition, the higher the vWAT index was, the higher the risk of mild cognitive impairment (MCI) would be in the elderly.90 The above studies indicate a close association between vWAT and the occurrence and progression of AD.
Also, there have been plenty of neuroimaging reports about the association between AD-related brain structure and vWAT. Adults with higher vWAT index had more pronounced memory loss, subcortical gray matter atrophy and hippocampal atrophy.91 And the strength of these correlations gradually increased with age.91 In healthy community dwelling elderly subjects without cognitive impairment, the volume of vWAT showed a positive association with the lateral ventricle volume and negative correlations with verbal episodic memory (assessed by the Rey Auditory Verbal Learning Test), attention (assessed by the Digit Span subtest from the Wechsler Memory Scale -III) and hippocampal volume.92 The thigh muscle/vWAT ratio in the elderly was positively correlated with the volume of right temporal pole and inferior temporal gyrus, but the vWAT volume was negatively correlated with the volume of temporal lobe.25 Using quantitative magnetization transfer and neurite orientation dispersion and density imaging techniques, the increased vWAT mass was found to be associated with the fornix myelin damage.93 The functional MRI studies also found significantly negative correlations between the vWAT volume and both memory and brain structural covariance network in adults,94 and a marked correlation between vWAT and the peak of the normalized MTR in the gray matter in the elderly.33 The above studies suggest a close association between vWAT and AD-related brain structure.
However, the underlying mechanisms of the association between vWAT and AD have not been fully elucidated so far. To this end, we have made several deductions and inferences based on the current literature (Figure 2).
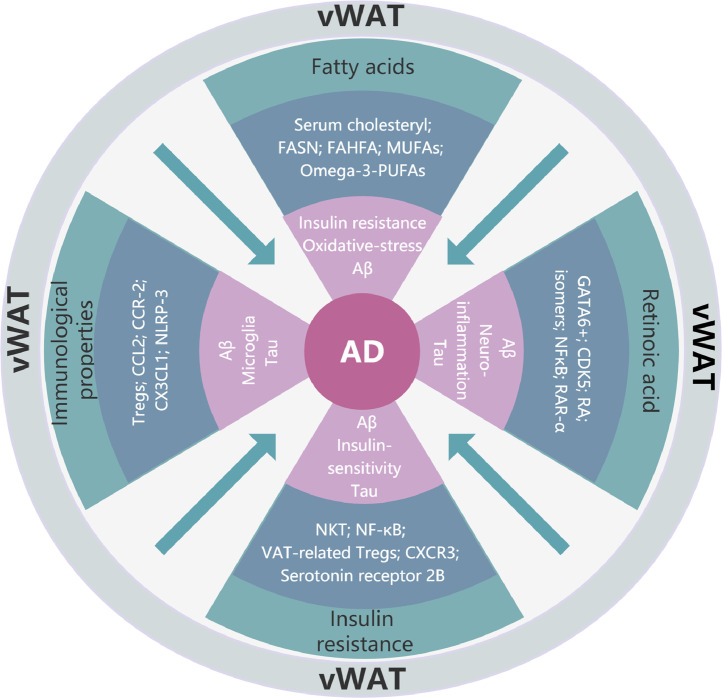
Figure 2.
Schematic depicting the potential vWAT-related pathways involved in the pathogenesis of AD.
The vWAT has the potential to influence Aβ metabolism, tau metabolism, microglial activation and neuroinflammation via several pathways including vWAT-derived fatty acids, immunological properties of vWAT, retinoic acid derived from vWAT, and vWAT-regulated insulin resistance.
Abbreviations: AD, Alzheimer's disease; Aβ, Amyloid β; SFAs, saturated fatty acids; PUFAs, polyunsaturated fatty acids; MUFAs, monounsaturated fatty acids; FAHFA, fatty acid esters of hydroxy fatty acids; FASN, fatty acid synthase; RA, retinoic acid; CDK, cyclin-dependent kinase; NF-κB, nuclear factor kappa B; RAR, retinoic acid receptor; Tregs, regulatory T cells; CCL, chemokine (CC-motif) ligand; CCR, CCL receptor; CX3CL1, C-X3-C motif chemokine ligand 1; NLRP, nucleotide binding domain leucine-rich repeats and Pyrin domain containing receptor; NKT, natural killer T; CXCR, chemokine (C-X-C motif) receptor.
Potential role of vWAT-derived fatty acids in the pathogenesis of AD
The fatty acid metabolism is closely related to vWAT. On one hand, the vWAT accumulation leads to the changes in the fatty acids profile.95 On the other hand, saturated fatty acids (SFAs) are capable to promote vWAT storage, while polyunsaturated fatty acids (PUFAs) have the capacity to prevent the vWAT accumulation.96
vWAT is linked to fatty acids in the blood. In the community-dwelling elderly, the palmitic acid in serum cholesteryl esters was correlated to the vWAT accumulation, while the inverse correlation between linoleic acid in serum phospholipids and vWAT remained significant.96 The healthy overweight subjects with the larger area of vWAT had higher levels of SFAs, myristic acid, palmitic acid, stearic acid, monounsaturated fatty acids (MUFAs), palmitoleic acid, oleic acid, n-6 PUFAs, linoleic acid, dihomo-Gamma-linolenic acid, arachidonic acid, n-3 PUFAs, docosapentaenoic acid and cis-10-heptadecenoic acid, and the higher activities of Delta 9- and Delta 6-desaturase, while a lower activity of Delta 5-desaturase.95,97 However, another study had found that the thickness of vWAT in overweight subjects was negatively correlated with serum levels of linoleic acid and PUFAs.97 In postmenopausal women, the area of vWAT was positively correlated with the plasma level of fatty acids, and negatively related to insulin resistance.98
Fatty acid metabolism in vWAT is associated with multiple factors. Compared with metabolically healthy obese subjects, metabolically abnormal obese subjects had the increased adipocyte volume and decreased levels of n-3 eicosapentaenoic acid and n-6 Gamma-linolenic acid in vWAT.99 Both palmitate tracer storage and calculated palmitate storage rates were higher in vWAT than those in sWAT and higher in women than those in men.100 Concentrations and activities of fatty acid storage enzymes were significantly increased in the vWAT of overweight individuals.100 In addition, overweight subjects had lower levels of phosphatidylcholine, triacylglycerol and diacylglycerol, and less phospholipid unsaturation in vWAT than in sWAT.101 Compared with non-obese subjects, morbidly obese subjects had significantly higher levels of palmitic acid, palmitoleic acid, PUFAs, Omega-6 and SFAs/PUFAs ratio, and a markedly lower arachidic acid concentration in vWAT.102 Among morbidly obese subjects, those with high insulin resistance had lower levels of lauric acid, myristic acid, docosahexaenoic acid (DHA), and Omega-3/Omega-6 ratio, and higher levels of arachidonic acid, eicosenoic acid, arachidonic acid, arachidonic acid/linoleic acid ratio than those with low insulin resistance.102 Uptake of fatty acids from plasma was increased by sWAT and vWAT but decreased by skeletal muscle in obese patients.103 The concentrations of fatty acid metabolites (heptanate, caprylate and gastrodiate) in vWAT in postmenopausal women were significantly higher than those in premenopausal women.104 The concentration of stearic acid in vWAT was negatively correlated with both vWAT area and serum triglyceride.105
The regulation of fatty acid metabolism is related to vWAT. Both in normal-weight and obese patients, the mRNA expression of the gene encoding fatty acid synthase (FASN) was increased in vWAT than that in sWAT.106 The level of p85α, a regulatory subunit of phosphatidylinositol 3 kinase (PI3K), was correlated with the levels of stearic acid and oleic acid in vWAT, and the level of p110β, a catalytic subunit of PI3K, was correlated with palmitic acid, DHA, linoleic acid and arachidonic acid in vWAT.107 The levels of SFAs (such as palmitate acid) in obese women's vWAT was related to the increased expression of 11-β-hydroxylidase 1.108 In obese women, the concentration of stearic acid in vWAT-derived large-diameter adipocytes was significantly lower than that in vWAT-derived small-diameter adipocytes.105 Additionally, levels of free fatty acids in vWAT-derived adipocytes were decreased in postabsorptive women.109
Animal experiments have shown that the removal of vWAT significantly restored plasma free fatty acid than the removal of sWAT in obese rats.110 In HFD-induced obese mice, the proportion of stearic acid and oleic acid in total fatty acids was lower and higher in smaller diameter vWAT-derived adipocytes than that in larger diameter vWAT-derived adipocytes, respectively.111 Deficiency of suppression of tumorigenicity 2 receptor in mice led to the elevated storage of SFAs and an increased expression of CD36 in vWAT, which aggravated the chronic inflammation of adipocytes and promoted the transformation of macrophagic polarization from M2 to M1 in vWAT.112 Fatty acid esters of hydroxy fatty acids (FAHFA) are a novel class of endogenous lipids with anti-inflammatory and anti-diabetic effects. A total of 51 FAHFA family members, including 301 regional isomers, have been detected in the vWAT of mice at all ages, and the number of FAHFA in vWAT were increased with age.113 Dehydroepiandrosterone-sulfate reduced total SFAs, n-6 PUFAs and n-6/n-3 PUFA ratio and increased MUFAs in human-derived vWAT.114 In addition, it was found that palmitic acid reduced the expression of insulin receptor substrate 1 in vWAT-derived adipocytes. Several fatty acids had the capacity to affect the regulation of vWAT-derived adipocytes on the expression of PI3K.107 Dehydroepiandrosterone-sulfate decreased the palmitic acid level, increased the isooleic acid concentration, and slightly elevated the estimated desaturase activity in vWAT.114
AD is closely associated with the composition and metabolic dysregulation of fatty acids.115 Palmitic acid significantly increased the levels of Aβ, β-site amyloid precursor protein cleaving enzyme-1 (BACE1) and cytokines such as TNF-α, IL-1β, and IL-6 in SH-SY5Y and HMC3 cells.116 Extracellular palmitic acid induced the expression of amyloid precursor protein (APP) and BACE1 and promoted the production of Aβ in SK-N-MC cells.117 Alpha-linoleic acid attenuated the learning and memory impairment of rats injected with Aβ42 intrahippocampally in the Morris water maze test, decreased the levels of malondialdehyde, nitric oxide, TNF-α, IL-1β, IL-6 and nuclear factor kappa B (NF-κB), and increased the content of glutathione and the activity of catalase in the CA1 region of the hippocampus.118 Conjugated linoleic acid reduced the co-localization of APP with BACE1 in late endosomes in mouse neurons, resulting in the reduced cleavage of APP by BACE1 and Aβ production.119 Conjugated linoleic acid-riched diet reduced the Aβ level, increased microglia numbers and the astrocytes expressing anti-inflammatory cytokines (IL-10 and IL-19) in the hippocampus of an AD mice model expressing human APP and bearing the Swedish and Indiana mutations (hAPPSwInd, J20).120 Supplementation with dietary oleic acid increased the Aβ40/Aβ42 ratio, decreased levels of BACE1, presenilin and amyloid plaques in the brain, and increased the sAPPα level in an early-onset AD transgenic mouse model expressing the double-mutant form of human APP, Swedish (K670N/M671L) and Indiana (V717F).121 In addition, Omega-3-PUFAs have been proved to reduce cerebral Aβ deposition, improve brain energy metabolism, lessen oxidative stress, and improve cognition and activities of daily living.115,122 DHA has the capacity of regulating fiber formation and aggregation of Aβ,123,124 redirecting the amyloid response of APP to the non-amyloid response, effectively reducing the production of Aβ,125 and decreasing the oligomeric Aβ-induced production of reactive oxygen species in mouse primary cultured microglia.126
Thus, considering the essential role of vWAT in regulating fatty acid metabolism and the roles of different fatty acids in Aβ metabolism and AD-like pathological features, it is reasonable to infer that vWAT may be involved in the pathogenesis of AD through fatty acids (Figure 2).
Potential role of immunological properties of vWAT in the pathogenesis of AD
A variety of immune cells exist in vWAT. For instance, as a unique structure of omentum, milky spots (MSs) are organized aggregates of leukocytes embedded between adipocytes just beneath the mesothelial cell layer that covers the omentum. MSs support a unique population of vWAT-related CD4+ regulatory T cells (Tregs) which express a variety of inflammatory cytokines and chemokines such as chemokine (CC-motif) ligand 2 (CCL2), C-X3-C motif chemokine ligand 1 (CX3CL1), IL-6 and TNF-α.127, 128, 129
Genetic studies have proved that the A-2518G polymorphism of CCL2 was associated with an elevated risk for AD and increased serum concentration of CCL2.130 The CSF level of CCL2 was positively correlated with the atrophy severity of medial temporal lobe structure and negatively correlated with Mini-Mental State Examination (MMSE) scores in AD patients.131 The plasma concentration of CCL2 was higher in AD patients compared with that in MCI patients and controls, and was positively correlated with the severity of cognitive impairment.132 The baseline level of CCL2 was markedly associated with changes in MMSE scores in the two-years follow-up.132 In prodromal AD, the CSF concentration of CCL2 was associated with a faster cognitive decline during follow-up.133 Besides, CCL2 combined with t-tau, p-tau and Aβ42 in CSF could predict the conversion of MCI to AD and the rate of cognitive decline.133 In addition, the genetic polymorphism of CCL2 receptor (CCR2) was associated with the risk of AD.134
Animal experiments showed that deficiency of CCL2 aggravated the impairment of learning and memory, reduced the microglial capacity to phagocytize Aβ, accelerated Aβ deposition and oligomer formation, and disrupted neurogenesis and differentiation in the subgranular zone of the hippocampal dentate gyrus in APP/PS1 transgenic mice.135 However, inhibition of CCL2 synthesis was proved to prevent the neurotoxicity of Aβ.136 Besides, overexpression of CCL2 in the brain exacerbated the accumulation of microglia and the deposition of diffuse Aβ in APP transgenic mice.137 Overexpression of CCL2 increased the hyperphosphorylation of tau protein, promoted the conversion of soluble tau polymers to insoluble tau polymers, and enhanced the activation of glial cells in the brain of rTg4510 transgenic mice.138 In addition, deficiency of CCR2 exacerbated the learning and memory impairment, increased the intracellular accumulation of soluble oligomer Aβ in the frontal brain, and promoted the expression of transforming growth factor (TGF) -β 1, TGF-β receptor and CX3CR1 in plaques-associated microglia in APP/PS1 transgenic mice.139 Deficiency of CCR2 accelerated early disease progression and markedly impaired microglial accumulation in Tg2576 transgenic mice which accumulated Aβ earlier and died prematurely in a manner that associated with CCR2 gene dosage.140 Transplantation of CCR2-deficient bone marrow cells (BMCs) enhanced memory deficits and increased the amount of soluble Aβ in APP/PS1 transgenic mice.141 In contrast, transplantation of wild-type BMCs restored memory and reduced soluble Aβ accumulation in APP/PS1/CCR2⁻/⁻ transgenic mice.141 Overexpressing of CCR2 in the brain was found to prevent cognitive decline and restore the function of monocytes in APP/PS1/CCR2⁻/⁻mice.141
Similarly, human studies have found that the CSF level of CX3CL1 in AD patients was remarkably lower than that of the control group.142 Autopsy showed that the expression of receptor of CX3CL1 (CX3CR1) in the cerebral cortex of AD patients was significantly increased and positively correlated with the severity of AD symptoms.143 Animal experiments have found that deficiency of CX3CR1 aggravated AD-related neuronal and behavioral deficits which were associated with the cytokine production rather than the Aβ plaque deposition.144 Ablation of CX3CR1 worsened the memory retention in passive avoidance and novel object recognition tests, enhanced tau pathology, and exacerbated the consumption of calcium-binding proteins in the hippocampal dentate gyrus in mice overexpressing human APP.144 In addition, overexpression of CX3CL1 promoted neurogenesis, attenuated tau pathology, improved cognitive function, and prolonged the survival time of PS19 tau transgenic mice.145 Neurogenesis enhanced by overexpression of CX3CL1 could be attenuated by interfering with the mothers against decapentaplegic homolog 2 (Smad2) signaling pathway independent of CX3CR1-CX3CL1 interaction.145 Overexpressing of the membrane-anchored C-terminal fragment of CX3CL1 significantly reduced Aβ deposition and neuronal loss,146 and overexpressing of CX3CL1-intracellular C-terminal domain reversed the neuronal loss through TGFβ2/3-Smad2/3 signaling pathway and some other genes important for neurogenesis in 5xFAD transgenic mice.146
In addition, vWAT-induced nucleotide binding domain leucine-rich repeats and Pyrin domain containing receptor (NLRP-3) was found to impair learning and memory through IL-1-mediated microglial activation in mice with dietary obesity.68 Administration of the conditioned medium derived from adipose tissue mesenchymal stem cells obtained from vWAT attenuated the deficits of spatial and recognition memory, decreased Aβ plaques formation, enhanced neuron survival, and reduced the levels of IL-1β, TNF-α, Toll like receptor (TLR)-2 and TLR-4 in an AD rats model.147
Therefore, in view of the immunological properties of vWAT and the roles of different immune molecules in Aβ metabolism, tau metabolism and AD-like pathological features, it is reasonable to speculate that vWAT may participate in the pathogenesis of AD (Figure 2).
Potential role of retinoic acid derived from vWAT in the pathogenesis of AD
The vWAT has the capacity to synthesize a large amount of retinoic acid (RA) and this synthesis can be regulated by the HFD.148 Resident macrophages (GATA6+) in MSs of omentum produce high level of retinaldehyde dehydrogenase (RALDH)-2,70,149 which is a key enzyme generating RA in vivo. RA is also generated from retinaldehyde in vWAT by the aldehyde dehydrogenase-1 family of enzymes (ALDH1).150 The enzyme ALDH1-A is involved in all-trans RA biosynthesis in a depot-specific manner in human vWAT.151
It was concluded that there was a very close relationship between RA and AD.152 Impaired RA signaling pathway has been generally thought to contribute to neuroinflammation, oxidative stress, mitochondrial dysfunction and neurodegeneration, further leading to the occurrence and progress of AD.153 A high dietary intake of RA was found to be associated with a reduced incidence of AD.154
All-trans RA was proved to prevent the accumulation of Aβ plaques, alleviate the hyperphosphorylation of tau protein through the inhibition of cyclin-dependent kinase (CDK) 5, inhibit the activation of microglia and astrocytes, attenuate neuron degeneration and death, and improve learning and memory in APP/PS1 transgenic mice.155 RA isomers (all-trans RA, 9-cis RA and 13-cis RA) were proved to protect primary cultured hippocampal neurons from Aβ-induced neurodegeneration and apoptosis.156 RA isoforms promoted production and lipidation of astrocytic apolipoprotein (Apo) E through the retinoid X receptor (RXR)/retinoic acid receptor (RAR) signaling pathway.157 9-cis-RA binding to ApoA-1 significantly reduced the stability of the C-terminal fragment of APP and decreased the production of Aβ.158 RA-induced RARα/RXRα signaling had the capacity to reduce Aβ production by directly inhibiting Gamma-secretase-mediated cleavage of APP.159 All-trans RA reduced the expression of BACE1 by regulating the NF-κB signaling pathway under inflammatory conditions.160 RA was found to have an anti-oligomerization effect on Aβ in vitro.161 In addition, RA induced apoptosis in tau model cells (tau/P19 cells) during neural differentiation in which Ca2+/calmodulin-dependent protein kinase II played an important mediating role.162
The RAR-α system was proved to be crucial in the homeostatic modulation of synaptic plasticity which is essential for memory function in adults.163 Administration of RAR agonist activated the RA signaling pathway, increased the synthesis of insulin-degrading enzyme (IDE) and neprilysin (NEP), accelerated the clearance of Aβ, reduced plaques and oligomers of Aβ, inhibited the phosphorylation of tau protein, and improved cognitive function in Tg2576 transgenic mice.163 RA and RAR agonist repaired Aβ-induced DNA double-strand breaks (DSBs) in SH-SY5Y cells and astrocytes derived from the cortical tissue of mice.164 RA was found to prevent DNA DSBs through RARα/β/γ and PPARβ/δ receptors.165 Activation of the RAR-α signaling pathway increased the level of α-hydrolase (ADAM10), antagonized the intracellular and extracellular production of Aβ, and prevented neuronal death induced by Aβ.166 In addition, overexpression of miR-138 activated glycogen synthase kinase (GSK) -3β and increased phosphorylation of tau protein in HEK293/tau cells. As a direct binding site of miR-138, RAR-α could significantly reduce the activity of GSK-3β and miR-138-induced tau phosphorylation.167 Additionally, an exogenous supplementation of RAR improved learning and memory abilities, increased the expression of IDE, reduced the brain Aβ burden, and decreased the levels of inflammatory factors in the hippocampus in APP23 transgenic mice.168
As mentioned above, given the regulatory role of vWAT on RA and the involvement of RA signaling pathway in Aβ- and tau- related pathologies, the deduction that vWAT is probably involved in the pathogenesis of AD should be valid (Figure 2).
Potential role of vWAT-regulated insulin resistance in the pathogenesis of AD
The vWAT is a key in regulating insulin resistance.169 Obesity-induced insulin resistance is thought to be caused by a combination of a local decrease in immunomodulatory cells and an increase in pro-inflammatory immune cells in vWAT.170 It has been found that insulin resistance of peripheral adipose tissue was able to change lipid composition and hippocampal synaptic function.171 Higher frequencies of natural killer T (NKT) cells were contained in vWAT (e.g. MSs of omentum) than those in other conventional lymphoid tissues.70 Several studies revealed that mice lacking NKT cells significantly gained higher body weight, reduced insulin sensitivity and an elevated number of inflammatory macrophages in vWAT after a HFD treatment.172,173 However, some animal experiments showed that the insulin sensitivity of vWAT could be enhanced when vWAT-related Tregs were consumed by blocking IL-33 receptors.174 Inhibition of serotonin signaling through serotonin receptor 2B attenuated insulin resistance by reducing lipolysis in vWAT-derived adipocytes.175 Rats lacking nuclear receptor subfamily 2 group E member 1 (NR2E1) had increased inflammatory cytokine secretion, pronounced hyperlipidemia, impaired insulin sensitivity and aberrant expression of insulin signaling pathway and NF-κB pathway-related molecules in vWAT after a HFD treatment.176 Removal of vWAT prevented obesity-induced insulin resistance and hyperinsulinemia, and significantly enhanced the phosphorylation insulin-stimulated AKT at serine 473 and threonine 308 sites in sWAT, liver and skeletal muscle.177 In men with abdominal obesity, the accumulation of NK cells in vWAT was associated with vWAT volume, low-grade inflammation, inflammatory macrophage polarization and insulin resistance.178 The proportion of ST2+ Tregs in vWAT was severely reduced in obese mice fed a high fat/sucrose diet.170 IL-33 treatment fully restored the level of ST2+ Tregs in vWAT and reduced vWAT inflammation, hyperinsulinemia and insulin resistance.170 Compared with wild type mice fed with a HFD, mice lacking chemokine (C-X-C motif) receptor 3 (CXCR3) had lower fasting blood glucose and improved glucose tolerance at a similar body weight.179 HFD-induced infiltration of vWAT innate and adaptive immune cells was significantly attenuated in CXCR3−/− mice.179
It is currently believed that insulin is extensively involved in the pathogenesis of AD.180 A growing body of evidence suggests that insulin has the capacity of protecting against the neurotoxicity of Aβ, regulating degradation of Aβ and phosphorylation of tau, enhancing dendritic spine formation and synaptic viability, and modulating neuroinflammation.180 Therefore, all the results above indicate that vWAT may be involved in the pathogenesis of AD by regulating insulin resistance (Figure 2).
From the above reasoning, we can find that vWAT has the potential to influence certain essential pathological features of AD including Aβ metabolism, tau metabolism, microglial activation and neuroinflammation via several pathways including vWAT-derived fatty acids, immunological properties of vWAT, vWAT-derived retinoic acid and vWAT-regulated insulin resistance, therefore, we speculate that vWAT is very likely to participate in the pathogenesis of AD through the four potential mechanisms (Figure 2). In addition, it must be pointed out that the above four aspects were only based on inference and deduction of the published literature. In fact, as a widely distributed connective tissue, the cellular composition of vWAT is extremely complex, suggesting that it should have a variety of different physiological functions. For example, vWAT contains plenty of adipocytes that are capable to secrete multiple hormones. Many of these hormones have been proved to participate in the pathogenesis of AD (Table 1), including metabolism of Aβ,181, 182, 183, 184, 185, 186, 187, 188, 189, 190 phosphorylation of tau,182,183,189,191,192 hippocampal neurogenesis,181,193,194 neurodegeneration,181,195 synaptic function,196, 197, 198, 199, 200 neuroinflammation186,201, 202, 203, 204, 205, 206 and oxidative stress.181, 182,184,206, 207, 208 All the findings indicate the vWAT-derived hormones could be a mediator between vWAT and AD. Nevertheless, to our knowledge, the direct research evidence on whether vWAT is involved in the pathogenesis of AD is still lacking so far.
Conclusions
In recent years, vWAT has increasingly become a hotspot in biomedical research, especially in immunity, inflammation, nervous system, etc. Emerging evidence from human and animal studies suggests there is a bidirectional communication between brain and vWAT. The human research evidence has shown their phenotypic association, and the basic experimental evidence has preliminary revealed the potential signaling pathways and underlying molecular mechanisms of this bidirectional communication. In addition, it is of scientific significance to investigate the pathogenesis of AD from the perspective of vWAT in the future.
Outstanding questions
We expect that the proposal of BVA may further emphasize their intrinsic relationship for researchers. Meanwhile, the proposal of BVA may expand our understanding of how systemic lipid metabolism impacts on brain health to some extent and provide a novel approach to investigate the pathogenesis and treatment strategies of neurodegenerative disorders. However, there are still many issues to be addressed.
First, the mechanisms of the bidirectional communication between vWAT and brain have been still lacking extensive and in-depth discussion. On one hand, the influence of the hypothalamus on vWAT has been studied more than other brain regions. It is still unknown what the effects of the other brain regions on vWAT are, and whether the other brain regions or the certain groups of brain cells have the capacity of regulating the location, cellular components, and function of vWAT. On the other hand, the effects of vWAT on brain structure and function, especially the signaling pathways between both, have not been adequately studied.
In addition, the effects of BVA on various brain-related diseases, especially neurodegenerative disorders, have still rarely been discussed. Although this review provides a preliminary overview of the possible involvement of vWAT in the pathogenesis of AD, the relationship between vWAT and other neurodegenerative disorders, such as Parkinson's disease and amyotrophic lateral sclerosis is still lacking, especially in clinical studies. We will follow research in these related areas to further explore the potential bidirectional communication between brain and vWAT, and that in turn might support the views we proposed in this review.
Search strategy and selection criteria
The articles cited in this review were collected from PubMed database after a deep research in the articles published from 2000 to 2022 with the following terms: “adipose tissue”, “visceral adipose tissue”, “visceral white adipose tissue”, “visceral fat”, “visceral obesity”, and the combinations of “visceral adipose tissue”/“visceral fat”/“visceral white adipose tissue” and “central nervous system”/“brain”, the combination of “brain” and “axis”, and the combinations of “visceral adipose tissue”/“visceral fat”/“visceral obesity”/“visceral white adipose tissue” and “Alzheimer's disease”/“Alzheimer”/“amyloid β”/“tau”.
Contributors
All authors contributed to the study conception and design. Conceptualization, Y.X. and Y.W.; resources, X.H. and Y.X.; data curation, X.H. and Y.X.; writing-original draft preparation, X.H. and Y.X.; writing-review and editing, Y.W. and Y.X.; project administration, Y.X. and Y.W.. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors confirm that there are no conflicts of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81601112, 81801090), Sichuan Science and Technology Program (2019YFS0213, 2020YFS0436, 2022NSFSC1374), Scientific Research Project of Sichuan Provincial Health Commission (21PJ086). The funders had no role in paper design, data collection, data analysis, interpretation or writing of the paper. We apologize to colleagues whose papers were not cited due to space limitations.
Contributor Information
Yan-Jiang Wang, Email: yanjiang_wang@tmmu.edu.cn.
Yang Xiang, Email: xiangyangttt@126.com.
References
- 1.Morais LH, HLt Schreiber, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 2.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10(11):735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth RF. The liver-brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10(9):522–528. doi: 10.1038/nrgastro.2013.99. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Ren Q, Zhang JC, Chen QX, Hashimoto K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: rethinking the brain-liver axis. Transl Psychiatry. 2017;7(5):e1128. doi: 10.1038/tp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai GS, Zheng C, Geetha T, et al. The pancreas-brain axis: insight into disrupted mechanisms associating type 2 diabetes and Alzheimer's disease. J Alzheimers Dis. 2014;42(2):347–356. doi: 10.3233/JAD-140018. [DOI] [PubMed] [Google Scholar]
- 6.Bruschetta G, Diano S. Brain-to-pancreas signalling axis links nicotine and diabetes. Nature. 2019;574(7778):336–337. doi: 10.1038/d41586-019-02975-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corvera S. Cellular heterogeneity in adipose tissues. Annu Rev Physiol. 2021;83:257–278. doi: 10.1146/annurev-physiol-031620-095446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cypess AM. Reassessing human adipose tissue. N Engl J Med. 2022;386(8):768–779. doi: 10.1056/NEJMra2032804. [DOI] [PubMed] [Google Scholar]
- 9.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwick RK, Guerrero-Juarez CF, Horsley V, Plikus MV. Anatomical, physiological, and functional diversity of adipose tissue. Cell Metab. 2018;27(1):68–83. doi: 10.1016/j.cmet.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vishvanath L, Gupta RK. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest. 2019;129(10):4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 13.Kwok KH, Lam KS, Xu A. Heterogeneity of white adipose tissue: molecular basis and clinical implications. Exp Mol Med. 2016;48:e215. doi: 10.1038/emm.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Curr Opin Clin Nutr Metab Care. 2018;21(5):360–365. doi: 10.1097/MCO.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neeland IJ, Marso SP, Ayers CR, et al. Effects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. 2021;9(9):595–605. doi: 10.1016/S2213-8587(21)00179-0. [DOI] [PubMed] [Google Scholar]
- 16.Neeland IJ, Ross R, Despres JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. doi: 10.1016/S2213-8587(19)30084-1. [DOI] [PubMed] [Google Scholar]
- 17.Wassink AM, van der Graaf Y, van Haeften TW, Spiering W, Soedamah-Muthu SS, Visseren FL. Waist circumference and metabolic risk factors have separate and additive effects on the risk of future type 2 diabetes in patients with vascular diseases. A cohort study. Diabetic Med. 2011;28(8):932–940. doi: 10.1111/j.1464-5491.2011.03318.x. [DOI] [PubMed] [Google Scholar]
- 18.Vasamsetti SB, Natarajan N, Sadaf S, Florentin J, Dutta P. Regulation of cardiovascular health and disease by visceral adipose tissue-derived metabolic hormones. J Physiol. 2022:1–22. doi: 10.1113/JP282728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trayhurn P. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2022;127(2):161–164. doi: 10.1017/S0007114521003962. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 21.Saute RL, Soder RB, Alves Filho JO, Baldisserotto M, Franco AR. Increased brain cortical thickness associated with visceral fat in adolescents. Pediatr Obes. 2018;13(1):74–77. doi: 10.1111/ijpo.12190. [DOI] [PubMed] [Google Scholar]
- 22.Veit R, Kullmann S, Heni M, et al. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307–311. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. 2010;68(2):136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho J, Seo S, Kim WR, Kim C, Noh Y. Association between visceral fat and brain cortical thickness in the elderly: a neuroimaging study. Front Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.694629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H, Seo HS, Kim REY, Lee SK, Lee YH, Shin C. Obesity and muscle may have synergic effect more than independent effects on brain volume in community-based elderly. Eur Radiol. 2021;31(5):2956–2966. doi: 10.1007/s00330-020-07407-2. [DOI] [PubMed] [Google Scholar]
- 26.Lake JE, Popov M, Post WS, et al. Visceral fat is associated with brain structure independent of human immunodeficiency virus infection status. J Neurovirol. 2017;23(3):385–393. doi: 10.1007/s13365-016-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beller E, Lorbeer R, Keeser D, et al. Hepatic fat is superior to BMI, visceral and pancreatic fat as a potential risk biomarker for neurodegenerative disease. Eur Radiol. 2019;29(12):6662–6670. doi: 10.1007/s00330-019-06276-8. [DOI] [PubMed] [Google Scholar]
- 28.Logan NE, Raine LB, Drollette ES, et al. The differential relationship of an afterschool physical activity intervention on brain function and cognition in children with obesity and their normal weight peers. Pediatr Obes. 2021;16(2):e12708. doi: 10.1111/ijpo.12708. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz DH, Dickie E, Pangelinan MM, et al. Adiposity is associated with structural properties of the adolescent brain. Neuroimage. 2014;103:192–201. doi: 10.1016/j.neuroimage.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Syme C, Pelletier S, Shin J, et al. Visceral fat-related systemic inflammation and the adolescent brain: a mediating role of circulating glycerophosphocholines. Int J Obes (Lond) 2019;43(6):1223–1230. doi: 10.1038/s41366-018-0202-2. [DOI] [PubMed] [Google Scholar]
- 31.Raschpichler M, Straatman K, Schroeter ML, et al. Abdominal fat distribution and its relationship to brain changes: the differential effects of age on cerebellar structure and function: a cross-sectional, exploratory study. BMJ Open. 2013;3(1):e001915. doi: 10.1136/bmjopen-2012-001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkseth KE, Rubinow KB, Melhorn SJ, et al. Hypothalamic gliosis by MRI and visceral fat mass negatively correlate with plasma testosterone concentrations in healthy men. Obesity (Silver Spring) 2018;26(12):1898–1904. doi: 10.1002/oby.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Widya RL, Kroft LJ, Altmann-Schneider I, van den Berg-Huysmans AA, van der Bijl N, de Roos A, et al. Visceral adipose tissue is associated with microstructural brain tissue damage. Obesity (Silver Spring) 2015;23(5):1092–1096. doi: 10.1002/oby.21048. [DOI] [PubMed] [Google Scholar]
- 34.Contreras-Rodriguez O, Cano M, Vilar-Lopez R, et al. Visceral adiposity and insular networks: associations with food craving. Int J Obes (Lond) 2019;43(3):503–511. doi: 10.1038/s41366-018-0173-3. [DOI] [PubMed] [Google Scholar]
- 35.Sliz E, Shin J, Syme C, et al. A variant near DHCR24 associates with microstructural properties of white matter and peripheral lipid metabolism in adolescents. Mol Psychiatry. 2021;26(8):3795–3805. doi: 10.1038/s41380-019-0640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardenas D, Madinabeitia I, Vera J, et al. Better brain connectivity is associated with higher total fat mass and lower visceral adipose tissue in military pilots. Sci Rep. 2020;10(1):610. doi: 10.1038/s41598-019-57345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullmann S, Valenta V, Wagner R, et al. Brain insulin sensitivity is linked to adiposity and body fat distribution. Nat Commun. 2020;11(1):1841. doi: 10.1038/s41467-020-15686-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kullmann S, Heni M, Veit R, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38(6):1044–1050. doi: 10.2337/dc14-2319. [DOI] [PubMed] [Google Scholar]
- 39.Yamashiro K, Tanaka R, Tanaka Y, et al. Visceral fat accumulation is associated with cerebral small vessel disease. Eur J Neurol. 2014;21(4):667–673. doi: 10.1111/ene.12374. [DOI] [PubMed] [Google Scholar]
- 40.Nam KW, Kwon HM, Jeong HY, et al. Visceral adiposity index is associated with silent brain infarct in a healthy population. Sci Rep. 2020;10(1):17271. doi: 10.1038/s41598-020-74454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iceta S, Dadar M, Daoust J, et al. Association between visceral adiposity index, binge eating behavior, and grey matter density in caudal anterior cingulate cortex in severe obesity. Brain Sci. 2021;11(9):1158. doi: 10.3390/brainsci11091158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KW, Seo H, Kwak MS, Kim D. Visceral obesity is associated with white matter hyperintensity and lacunar infarct. Int J Obes (Lond) 2017;41(5):683–688. doi: 10.1038/ijo.2017.13. [DOI] [PubMed] [Google Scholar]
- 43.Kwon HM, Park JH, Park JH, et al. Visceral fat is an independent predictor of cerebral microbleeds in neurologically healthy people. Cerebrovasc Dis. 2016;42(1-2):90–96. doi: 10.1159/000445300. [DOI] [PubMed] [Google Scholar]
- 44.Higuchi S, Kabeya Y, Kato K. Visceral-to-subcutaneous fat ratio is independently related to small and large cerebrovascular lesions even in healthy subjects. Atherosclerosis. 2017;259:41–45. doi: 10.1016/j.atherosclerosis.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Pasha EP, Birdsill A, Parker P, Elmenshawy A, Tanaka H, Haley AP. Visceral adiposity predicts subclinical white matter hyperintensities in middle-aged adults. Obesity Res Clin Practice. 2017;11(2):177–187. doi: 10.1016/j.orcp.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Karcher HS, Holzwarth R, Mueller HP, et al. Body fat distribution as a risk factor for cerebrovascular disease: an MRI-based body fat quantification study. Cerebrovasc Dis. 2013;35(4):341–348. doi: 10.1159/000348703. [DOI] [PubMed] [Google Scholar]
- 47.Lampe L, Zhang R, Beyer F, et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann Neurol. 2019;85(2):194–203. doi: 10.1002/ana.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anan F, Masaki T, Eto T, et al. Visceral fat accumulation is a significant risk factor for white matter lesions in Japanese type 2 diabetic patients. Eur J Clin Invest. 2009;39(5):368–374. doi: 10.1111/j.1365-2362.2009.02103.x. [DOI] [PubMed] [Google Scholar]
- 49.Huang C, Rosencrans RF, Bugescu R, et al. Depleting hypothalamic somatostatinergic neurons recapitulates diabetic phenotypes in mouse brain, bone marrow, adipose and retina. Diabetologia. 2021;64(11):2575–2588. doi: 10.1007/s00125-021-05549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerem L, Lawson EA. The effects of oxytocin on appetite regulation, food intake and metabolism in humans. Int J Mol Sci. 2021;22(14):7737. doi: 10.3390/ijms22147737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toriya M, Maekawa F, Maejima Y, et al. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2010;22(9):987–995. doi: 10.1111/j.1365-2826.2010.02039.x. [DOI] [PubMed] [Google Scholar]
- 52.Yoo S, Cha D, Kim S, et al. Tanycyte ablation in the arcuate nucleus and median eminence increases obesity susceptibility by increasing body fat content in male mice. Glia. 2020;68(10):1987–2000. doi: 10.1002/glia.23817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S, Kim DS, Kwon DY, Yang HJ. Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J Neuroendocrinol. 2011;23(8):687–698. doi: 10.1111/j.1365-2826.2011.02165.x. [DOI] [PubMed] [Google Scholar]
- 54.Cardoso F, Klein Wolterink RGJ, Godinho-Silva C, et al. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature. 2021;597(7876):410–414. doi: 10.1038/s41586-021-03830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Osaki A, Shimizu H, Ishizuka N, Suzuki Y, Mori M, Inoue S. Enhanced expression of nesfatin/nucleobindin-2 in white adipose tissue of ventromedial hypothalamus-lesioned rats. Neurosci Lett. 2012;521(1):46–51. doi: 10.1016/j.neulet.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 56.Shimizu H, Tanaka M, Osaki A. Transgenic mice overexpressing nesfatin/nucleobindin-2 are susceptible to high-fat diet-induced obesity. Nutrit Diabetes. 2016;6(3):e201. doi: 10.1038/nutd.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warne JP, Foster MT, Horneman HF, et al. Afferent signalling through the common hepatic branch of the vagus inhibits voluntary lard intake and modifies plasma metabolite levels in rats. J Physiol. 2007;583(Pt 2):455–467. doi: 10.1113/jphysiol.2007.135996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sa-Nguanmoo P, Tanajak P, Kerdphoo S, et al. FGF21 and DPP-4 inhibitor equally prevents cognitive decline in obese rats. Biomed Pharmacother. 2018;97:1663–1672. doi: 10.1016/j.biopha.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 59.Kang EB, Koo JH, Jang YC, et al. Neuroprotective effects of endurance exercise against high-fat diet-induced hippocampal neuroinflammation. J Neuroendocrinol. 2016;28(5) doi: 10.1111/jne.12385. [DOI] [PubMed] [Google Scholar]
- 60.de Mello AH, Schraiber RB, Goldim MPS, et al. Omega-3 fatty acids attenuate brain alterations in high-fat diet-induced obesity model. Mol Neurobiol. 2019;56(1):513–524. doi: 10.1007/s12035-018-1097-6. [DOI] [PubMed] [Google Scholar]
- 61.Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589(Pt 11):2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koyama S, Kawaharada M, Terai H, et al. Obesity decreases excitability of putative ventral tegmental area GABAergic neurons. Physiol Rep. 2013;1(5):e00126. doi: 10.1002/phy2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias CT, Curi HT, Payolla TB, et al. Maternal high-fat diet stimulates proinflammatory pathway and increases the expression of Tryptophan Hydroxylase 2 (TPH2) and brain-derived neurotrophic factor (BDNF) in adolescent mice hippocampus. Neurochem Int. 2020;139 doi: 10.1016/j.neuint.2020.104781. [DOI] [PubMed] [Google Scholar]
- 64.Chen KE, Lainez NM, Nair MG, Coss D. Visceral adipose tissue imparts peripheral macrophage influx into the hypothalamus. J Neuroinflammation. 2021;18(1):140. doi: 10.1186/s12974-021-02183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brunetti L, Orlando G, Ferrante C, et al. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides. 2013;44:66–74. doi: 10.1016/j.peptides.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Shi WZ, Li W, Cheng Y, et al. The cytoprotective role of omentin against oxidative stress-induced PC12 apoptosis. Artif Cells Nanomed Biotechnol. 2021;49(1):483–492. doi: 10.1080/21691401.2021.1892707. [DOI] [PubMed] [Google Scholar]
- 67.Shin JA, Jeong SI, Kim M, Yoon JC, Kim HS, Park EM. Visceral adipose tissue inflammation is associated with age-related brain changes and ischemic brain damage in aged mice. Brain Behav Immun. 2015;50:221–231. doi: 10.1016/j.bbi.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Guo DH, Yamamoto M, Hernandez CM, Khodadadi H, Baban B, Stranahan AM. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J Clin Invest. 2020;130(4):1961–1976. doi: 10.1172/JCI126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong SI, Shin JA, Cho S, et al. Resveratrol attenuates peripheral and brain inflammation and reduces ischemic brain injury in aged female mice. Neurobiol Aging. 2016;44:74–84. doi: 10.1016/j.neurobiolaging.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Meza-Perez S, TD Randall. Immunological functions of the omentum. Trends Immunol. 2017;38(7):526–536. doi: 10.1016/j.it.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ansari A, Bose S, Yadav MK, et al. CST, an herbal formula, exerts anti-obesity effects through brain-gut-adipose tissue axis modulation in high-fat diet fed mice. Molecules. 2016;21(11):1522. doi: 10.3390/molecules21111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 73.Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimizu H, Mori M. The brain-adipose axis: a review of involvement of molecules. Nutr Neurosci. 2005;8(1):7–20. doi: 10.1080/10284150500047245. [DOI] [PubMed] [Google Scholar]
- 75.Pini L, Pievani M, Bocchetta M, et al. Brain atrophy in Alzheimer's disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 76.Douaud G, Menke RA, Gass A, et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J Neurosci. 2013;33(5):2147–2155. doi: 10.1523/JNEUROSCI.4437-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction-the disregarded partner of Alzheimer's disease. Alzheimers Dement. 2019;15(1):158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.2021 Alzheimer’s disease facts and figures. Alzheimer’s dementia. 2021;17(3):327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 80.Villemagne VL, Dore V, Burnham SC, Masters CL, Rowe CC. Author correction: imaging tau and amyloid-beta proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol. 2018;14(7):446. doi: 10.1038/s41582-018-0021-z. [DOI] [PubMed] [Google Scholar]
- 81.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levey AI. Progress with treatments for Alzheimer's disease. N Engl J Med. 2021;384(18):1762–1763. doi: 10.1056/NEJMe2103722. [DOI] [PubMed] [Google Scholar]
- 83.Furiya Y, Ryo M, Kawahara M, Kiriyama T, Morikawa M, Ueno S. Renin-angiotensin system blockers affect cognitive decline and serum adipocytokines in Alzheimer's disease. Alzheimers Dement. 2013;9(5):512–518. doi: 10.1016/j.jalz.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 84.Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat Genet. 2019;51(3):414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu JT, Xu W, Tan CC, et al. Evidence-based prevention of Alzheimer's disease: systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J Neurol Neurosurg Psychiatry. 2020;91(11):1201–1209. doi: 10.1136/jnnp-2019-321913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim YJ, Kim SM, Jeong DH, Lee SK, Ahn ME, Ryu OH. Associations between metabolic syndrome and type of dementia: analysis based on the National Health Insurance Service database of Gangwon province in South Korea. Diabetol Metab Syndr. 2021;13(1):4. doi: 10.1186/s13098-020-00620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diehl-Wiesenecker E, von Armin CA, Dupuis L, Muller HP, Ludolph AC, Kassubek J. Adipose tissue distribution in patients with alzheimer's disease: a whole body MRI case-control study. J Alzheimer's Dis. 2015;48(3):825–832. doi: 10.3233/JAD-150426. [DOI] [PubMed] [Google Scholar]
- 88.Clark LR, Koscik RL, Allison SL, et al. Hypertension and obesity moderate the relationship between beta-amyloid and cognitive decline in midlife. Alzheimers Dement. 2019;15(3):418–428. doi: 10.1016/j.jalz.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim S, Yi HA, Won KS, Lee JS, Kim HW. Association between visceral adipose tissue metabolism and Alzheimer’s disease pathology. Metabolites. 2022;12(3):258. doi: 10.3390/metabo12030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papachristou E, Ramsay SE, Lennon LT, et al. The relationships between body composition characteristics and cognitive functioning in a population-based sample of older British men. BMC Geriatr. 2015;15:172. doi: 10.1186/s12877-015-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nyberg CK, Fjell AM, Walhovd KB. Level of body fat relates to memory decline and interacts with age in its association with hippocampal and subcortical atrophy. Neurobiol Aging. 2020;91:112–124. doi: 10.1016/j.neurobiolaging.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 92.Isaac V, Sim S, Zheng H, Zagorodnov V, Tai ES, Chee M. Adverse associations between visceral adiposity, brain structure, and cognitive performance in healthy elderly. Front Aging Neurosci. 2011;3:12. doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Metzler-Baddeley C, Mole JP, Leonaviciute E, et al. Sex-specific effects of central adiposity and inflammatory markers on limbic microstructure. Neuroimage. 2019;189:793–803. doi: 10.1016/j.neuroimage.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zsido RG, Heinrich M, Slavich GM, et al. Association of estradiol and visceral fat with structural brain networks and memory performance in adults. JAMA Netw Open. 2019;2(6) doi: 10.1001/jamanetworkopen.2019.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kang M, Lee A, Yoo HJ, et al. Association between increased visceral fat area and alterations in plasma fatty acid profile in overweight subjects: a cross-sectional study. Lipids Health Dis. 2017;16(1):248. doi: 10.1186/s12944-017-0642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosqvist F, Bjermo H, Kullberg J, et al. Fatty acid composition in serum cholesterol esters and phospholipids is linked to visceral and subcutaneous adipose tissue content in elderly individuals: a cross-sectional study. Lipids Health Dis. 2017;16(1):68. doi: 10.1186/s12944-017-0445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kishino T, Watanabe K, Urata T, et al. Visceral fat thickness in overweight men correlates with alterations in serum fatty acid composition. Clin Chim Acta. 2008;398(1-2):57–62. doi: 10.1016/j.cca.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 98.Lapointe A, Piche ME, Weisnagel SJ, Bergeron J, Lemieux S. Associations between circulating free fatty acids, visceral adipose tissue accumulation, and insulin sensitivity in postmenopausal women. Metabolism. 2009;58(2):180–185. doi: 10.1016/j.metabol.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 99.Torres-Castillo N, Martinez-Lopez E, Vizmanos-Lamotte B, Garaulet M. Healthy obese subjects differ in chronotype, sleep habits, and adipose tissue fatty acid composition from their non-healthy counterparts. Nutrients. 2020;13(1):119. doi: 10.3390/nu13010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ali AH, Koutsari C, Mundi M, et al. Free fatty acid storage in human visceral and subcutaneous adipose tissue: role of adipocyte proteins. Diabetes. 2011;60(9):2300–2307. doi: 10.2337/db11-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zacharia A, Saidemberg D, Mannully CT, et al. Distinct infrastructure of lipid networks in visceral and subcutaneous adipose tissues in overweight humans. Am J Clin Nutr. 2020;112(4):979–990. doi: 10.1093/ajcn/nqaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreno-Santos I, Garcia-Serrano S, Boughanem H, et al. The antagonist effect of arachidonic acid on GLUT4 gene expression by nuclear receptor type II regulation. Int J Mol Sci. 2019;20(4):963. doi: 10.3390/ijms20040963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dadson P, Ferrannini E, Landini L, et al. Fatty acid uptake and blood flow in adipose tissue compartments of morbidly obese subjects with or without type 2 diabetes: effects of bariatric surgery. Am J Physiol Endocrinol Metab. 2017;313(2):E175–E182. doi: 10.1152/ajpendo.00044.2017. [DOI] [PubMed] [Google Scholar]
- 104.Yamatani H, Takahashi K, Yoshida T, Soga T, Kurachi H. Differences in the fatty acid metabolism of visceral adipose tissue in postmenopausal women. Menopause. 2014;21(2):170–176. doi: 10.1097/GME.0b013e318296431a. [DOI] [PubMed] [Google Scholar]
- 105.Caron-Jobin M, Mauvoisin D, Michaud A, et al. Stearic acid content of abdominal adipose tissues in obese women. Nutr Diabetes. 2012;2:e23. doi: 10.1038/nutd.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berndt J, Kovacs P, Ruschke K, et al. Fatty acid synthase gene expression in human adipose tissue: association with obesity and type 2 diabetes. Diabetologia. 2007;50(7):1472–1480. doi: 10.1007/s00125-007-0689-x. [DOI] [PubMed] [Google Scholar]
- 107.Lopez-Gomez C, Santiago-Fernandez C, Garcia-Serrano S, et al. Oleic acid protects against insulin resistance by regulating the genes related to the PI3K signaling pathway. J Clin Med. 2020;9(8):2615. doi: 10.3390/jcm9082615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Petrus P, Rosqvist F, Edholm D, et al. Saturated fatty acids in human visceral adipose tissue are associated with increased 11- beta-hydroxysteroid-dehydrogenase type 1 expression. Lipids Health Dis. 2015;14:42. doi: 10.1186/s12944-015-0042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes. 2008;57(5):1186–1194. doi: 10.2337/db07-0664. [DOI] [PubMed] [Google Scholar]
- 110.Kim YW, Kim JY, Lee SK. Surgical removal of visceral fat decreases plasma free fatty acid and increases insulin sensitivity on liver and peripheral tissue in monosodium glutamate (MSG)-obese rats. J Korean Med Sci. 1999;14(5):539–545. doi: 10.3346/jkms.1999.14.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsubara Y, Kano K, Kondo D, Mugishima H, Matsumoto T. Differences in adipocytokines and fatty acid composition between two adipocyte fractions of small and large cells in high-fat diet-induced obese mice. Ann Nutr Metab. 2009;54(4):258–267. doi: 10.1159/000229506. [DOI] [PubMed] [Google Scholar]
- 112.Okamura T, Hashimoto Y, Mori J, et al. ILC2s improve glucose metabolism through the control of saturated fatty acid absorption within visceral fat. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.669629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu QF, Yan JW, Ni J, Feng YQ. FAHFA footprint in the visceral fat of mice across their lifespan. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865(5) doi: 10.1016/j.bbalip.2020.158639. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez-Morante JJ, Cerezo D, Cruz RM, Larque E, Zamora S, Garaulet M. Dehydroepiandrosterone-sulfate modifies human fatty acid composition of different adipose tissue depots. Obes Surg. 2011;21(1):102–111. doi: 10.1007/s11695-009-0064-8. [DOI] [PubMed] [Google Scholar]
- 115.Mett J. The impact of medium chain and polyunsaturated omega-3-fatty acids on amyloid-beta deposition, oxidative stress and metabolic dysfunction associated with Alzheimer’s disease. Antioxidants (Basel) 2021;10(12):1991. doi: 10.3390/antiox10121991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Phitthayaphong P, Kumfu S, Chattipakorn N, Chattipakorn SC. Blockage of Fc gamma receptors alleviates neuronal and microglial toxicity induced by palmitic acid. J Alzheimers Dis. 2021;82(3):1315–1332. doi: 10.3233/JAD-210417. [DOI] [PubMed] [Google Scholar]
- 117.Kim JY, Lee HJ, Lee SJ, et al. Palmitic Acid-BSA enhances amyloid-beta production through GPR40-mediated dual pathways in neuronal cells: Involvement of the Akt/mTOR/HIF-1alpha and Akt/NF-kappaB pathways. Sci Rep. 2017;7(1):4335. doi: 10.1038/s41598-017-04175-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tofighi N, Asle-Rousta M, Rahnema M, Amini R. Protective effect of alpha-linoleic acid on Abeta-induced oxidative stress, neuroinflammation, and memory impairment by alteration of alpha7 nAChR and NMDAR gene expression in the hippocampus of rats. Neurotoxicology. 2021;85:245–253. doi: 10.1016/j.neuro.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Hata S, Kano K, Kikuchi K, et al. Suppression of amyloid-beta secretion from neurons by cis-9, trans-11-octadecadienoic acid, an isomer of conjugated linoleic acid. J Neurochem. 2021;159(3):603–617. doi: 10.1111/jnc.15490. [DOI] [PubMed] [Google Scholar]
- 120.Fujita Y, Kano K, Kishino S, et al. Dietary cis-9, trans-11-conjugated linoleic acid reduces amyloid beta-protein accumulation and upregulates anti-inflammatory cytokines in an Alzheimer's disease mouse model. Sci Rep. 2021;11(1):9749. doi: 10.1038/s41598-021-88870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amtul Z, Westaway D, Cechetto DF, Rozmahel RF. Oleic acid ameliorates amyloidosis in cellular and mouse models of Alzheimer's disease. Brain Pathol. 2011;21(3):321–329. doi: 10.1111/j.1750-3639.2010.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fiala M, Lau YCC, Aghajani A, et al. Omega-3 fatty acids increase amyloid-beta immunity, energy, and circadian rhythm for cognitive protection of Alzheimer's disease patients beyond cholinesterase inhibitors. J Alzheimers Dis. 2020;75(3):993–1002. doi: 10.3233/JAD-200252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eto M, Hashimoto T, Shimizu T, Iwatsubo T. Characterization of the unique In Vitro effects of unsaturated fatty acids on the formation of amyloid beta fibrils. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee BY, Attwood SJ, Turnbull S, Leonenko Z. Effect of varying concentrations of docosahexaenoic acid on amyloid beta (1(-)42) aggregation: an atomic force microscopy study. Molecules. 2018;23(12):3089. doi: 10.3390/molecules23123089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grimm MO, Kuchenbecker J, Grosgen S, et al. Docosahexaenoic acid reduces amyloid beta production via multiple pleiotropic mechanisms. J Biol Chem. 2011;286(16):14028–14039. doi: 10.1074/jbc.M110.182329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geng X, Yang B, Li R, et al. Effects of docosahexaenoic acid and its peroxidation product on amyloid-beta peptide-stimulated microglia. Mol Neurobiol. 2020;57(2):1085–1098. doi: 10.1007/s12035-019-01805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Polyak A, Ferenczi S, Denes A, et al. The fractalkine/Cx3CR1 system is implicated in the development of metabolic visceral adipose tissue inflammation in obesity. Brain Behav Immun. 2014;38:25–35. doi: 10.1016/j.bbi.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 128.Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARgamma effects. Proc Nat Acad Sci USA. 2015;112(2):482–487. doi: 10.1073/pnas.1423486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15(8):930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu L, Dong Q, Xu L, Zou W, Li H. The MCP-1 A-2518G polymorphism increases the risk of Alzheimer's disease: a case-control study. Neurosci Lett. 2021;749 doi: 10.1016/j.neulet.2021.135710. [DOI] [PubMed] [Google Scholar]
- 131.Kimura A, Yoshikura N, Hayashi Y, Inuzuka T. Cerebrospinal fluid C-C motif chemokine ligand 2 correlates with brain atrophy and cognitive impairment in Alzheimer's disease. J Alzheimers Dis. 2018;61(2):581–588. doi: 10.3233/JAD-170519. [DOI] [PubMed] [Google Scholar]
- 132.Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL. Plasma MCP-1 and cognitive decline in patients with Alzheimer's disease and mild cognitive impairment: a two-year follow-up study. Sci Rep. 2018;8(1):1280. doi: 10.1038/s41598-018-19807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Westin K, Buchhave P, Nielsen H, Minthon L, Janciauskiene S, Hansson O. CCL2 is associated with a faster rate of cognitive decline during early stages of Alzheimer's disease. PLoS One. 2012;7(1):e30525. doi: 10.1371/journal.pone.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Galimberti D, Fenoglio C, Lovati C, et al. CCR2-64I polymorphism and CCR5Delta32 deletion in patients with Alzheimer's disease. J Neurol Sci. 2004;225(1-2):79–83. doi: 10.1016/j.jns.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 135.Kiyota T, Gendelman HE, Weir RA, Higgins EE, Zhang G, Jain M. CCL2 affects beta-amyloidosis and progressive neurocognitive dysfunction in a mouse model of Alzheimer's disease. Neurobiol Aging. 2013;34(4):1060–1068. doi: 10.1016/j.neurobiolaging.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Severini C, Passeri PP, Ciotti M, et al. Bindarit, inhibitor of CCL2 synthesis, protects neurons against amyloid-beta-induced toxicity. J Alzheimers Dis. 2014;38(2):281–293. doi: 10.3233/JAD-131070. [DOI] [PubMed] [Google Scholar]
- 137.Yamamoto M, Horiba M, Buescher JL, et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am J Pathol. 2005;166(5):1475–1485. doi: 10.1016/s0002-9440(10)62364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Joly-Amado A, Hunter J, Quadri Z, et al. CCL2 overexpression in the brain promotes glial activation and accelerates tau pathology in a mouse model of tauopathy. Front Immunol. 2020;11:997. doi: 10.3389/fimmu.2020.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J Neurosci. 2011;31(16):6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.El Khoury J, Toft M, Hickman SE, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13(4):432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 141.Naert G, Rivest S. Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. Mol Med. 2012;18:297–313. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perea JR, Lleo A, Alcolea D, Fortea J, Avila J, Bolos M. Decreased CX3CL1 levels in the cerebrospinal fluid of patients with Alzheimer's disease. Front Neurosci. 2018;12:609. doi: 10.3389/fnins.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gonzalez-Prieto M, Gutierrez IL, Garcia-Bueno B, et al. Microglial CX3CR1 production increases in Alzheimer's disease and is regulated by noradrenaline. Glia. 2021;69(1):73–90. doi: 10.1002/glia.23885. [DOI] [PubMed] [Google Scholar]
- 144.Cho SH, Sun B, Zhou Y, et al. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286(37):32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fan Q, He W, Gayen M, et al. Activated CX3CL1/Smad2 signals prevent neuronal loss and Alzheimer's tau pathology-mediated cognitive dysfunction. J Neurosci. 2020;40(5):1133–1144. doi: 10.1523/JNEUROSCI.1333-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fan Q, Gayen M, Singh N, et al. The intracellular domain of CX3CL1 regulates adult neurogenesis and Alzheimer's amyloid pathology. J Exp Med. 2019;216(8):1891–1903. doi: 10.1084/jem.20182238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mehrabadi S, Motevaseli E, Sadr SS, Moradbeygi K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer's disease rats. Behav Brain Res. 2020;379 doi: 10.1016/j.bbr.2019.112362. [DOI] [PubMed] [Google Scholar]
- 148.Perumal J, Sriram S, Lim HQ, Olivo M, Sugii S. Retinoic acid is abundantly detected in different depots of adipose tissue by SERS. Adipocyte. 2016;5(4):378–383. doi: 10.1080/21623945.2016.1245817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157(4):832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yasmeen R, Jeyakumar SM, Reichert B, Yang F, Ziouzenkova O. The contribution of vitamin A to autocrine regulation of fat depots. Biochim Biophys Acta. 2012;1821(1):190–197. doi: 10.1016/j.bbalip.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rubinow KB, Zhong G, Czuba LC, et al. Evidence of depot-specific regulation of all-trans-retinoic acid biosynthesis in human adipose tissue. Clin Transl Sci. 2022;15(6):1460–1471. doi: 10.1111/cts.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ono K, Yamada M. Vitamin A and Alzheimer's disease. Geriatr Gerontol Int. 2012;12(2):180–188. doi: 10.1111/j.1447-0594.2011.00786.x. [DOI] [PubMed] [Google Scholar]
- 153.Das BC, Dasgupta S, Ray SK. Potential therapeutic roles of retinoids for prevention of neuroinflammation and neurodegeneration in Alzheimer's disease. Neural Regen Res. 2019;14(11):1880–1892. doi: 10.4103/1673-5374.259604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Smith MA, Petot GJ, Perry G. Diet and oxidative stress: a novel synthesis of epidemiological data on Alzheimer's disease. J Alzheimers Dis. 1999;1(4-5):203–206. doi: 10.3233/jad-1999-14-502. [DOI] [PubMed] [Google Scholar]
- 155.Ding Y, Qiao A, Wang Z, et al. Retinoic acid attenuates beta-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. J Neurosci. 2008;28(45):11622–11634. doi: 10.1523/JNEUROSCI.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sahin M, Karauzum SB, Perry G, Smith MA, Aliciguzel Y. Retinoic acid isomers protect hippocampal neurons from amyloid-beta induced neurodegeneration. Neurotox Res. 2005;7(3):243–250. doi: 10.1007/BF03036453. [DOI] [PubMed] [Google Scholar]
- 157.Zhao J, Fu Y, Liu CC, et al. Retinoic acid isomers facilitate apolipoprotein E production and lipidation in astrocytes through the retinoid X receptor/retinoic acid receptor pathway. J Biol Chem. 2014;289(16):11282–11292. doi: 10.1074/jbc.M113.526095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Koldamova RP, Lefterov IM, Ikonomovic MD, et al. 22R-hydroxycholesterol and 9-cis-retinoic acid induce ATP-binding cassette transporter A1 expression and cholesterol efflux in brain cells and decrease amyloid beta secretion. J Biol Chem. 2003;278(15):13244–13256. doi: 10.1074/jbc.M300044200. [DOI] [PubMed] [Google Scholar]
- 159.Kapoor A, Wang BJ, Hsu WM, Chang MY, Liang SM, Liao YF. Retinoic acid-elicited RARalpha/RXRalpha signaling attenuates Abeta production by directly inhibiting gamma-secretase-mediated cleavage of amyloid precursor protein. ACS Chem Neurosci. 2013;4(7):1093–1100. doi: 10.1021/cn400039s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang R, Chen S, Liu Y, et al. All-trans-retinoic acid reduces BACE1 expression under inflammatory conditions via modulation of nuclear factor kappaB (NFkappaB) signaling. J Biol Chem. 2015;290(37):22532–22542. doi: 10.1074/jbc.M115.662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Takasaki J, Ono K, Yoshiike Y, et al. Vitamin A has anti-oligomerization effects on amyloid-beta in vitro. J Alzheimers Dis. 2011;27(2):271–280. doi: 10.3233/JAD-2011-110455. [DOI] [PubMed] [Google Scholar]
- 162.Tsukane M, Yamauchi T. Ca2+/calmodulin-dependent protein kinase II mediates apoptosis of P19 cells expressing human tau during neural differentiation with retinoic acid treatment. J Enzyme Inhib Med Chem. 2009;24(2):365–371. doi: 10.1080/14756360802187851. [DOI] [PubMed] [Google Scholar]
- 163.Goncalves MB, Clarke E, Hobbs C, et al. Amyloid beta inhibits retinoic acid synthesis exacerbating Alzheimer disease pathology which can be attenuated by an retinoic acid receptor alpha agonist. Eur J Neurosci. 2013;37(7):1182–1192. doi: 10.1111/ejn.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gruz-Gibelli E, Chessel N, Allioux C, et al. The vitamin A derivative all-trans retinoic acid repairs amyloid-beta-induced double-strand breaks in neural cells and in the murine neocortex. Neural Plast. 2016;2016 doi: 10.1155/2016/3707406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Colas J, Chessel N, Ouared A, et al. Neuroprotection against amyloid-beta-induced DNA double-strand breaks is mediated by multiple retinoic acid-dependent pathways. Neural Plast. 2020;2020 doi: 10.1155/2020/9369815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jarvis CI, Goncalves MB, Clarke E, et al. Retinoic acid receptor-alpha signalling antagonizes both intracellular and extracellular amyloid-beta production and prevents neuronal cell death caused by amyloid-beta. Eur J Neurosci. 2010;32(8):1246–1255. doi: 10.1111/j.1460-9568.2010.07426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Tan L, Lu Y, et al. MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Lett. 2015;589(6):726–729. doi: 10.1016/j.febslet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 168.Kawahara K, Suenobu M, Ohtsuka H, et al. Cooperative therapeutic action of retinoic acid receptor and retinoid x receptor agonists in a mouse model of Alzheimer's disease. J Alzheimer's Dis. 2014;42(2):587–605. doi: 10.3233/JAD-132720. [DOI] [PubMed] [Google Scholar]
- 169.Machann J, Stefan N, Wagner R, et al. Normalized indices derived from visceral adipose mass assessed by magnetic resonance imaging and their correlation with markers for insulin resistance and prediabetes. Nutrients. 2020;12(7):2064. doi: 10.3390/nu12072064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Han JM, Wu D, Denroche HC, Yao Y, Verchere CB, Levings MK. IL-33 reverses an obesity-induced deficit in visceral adipose tissue ST2+ T regulatory cells and ameliorates adipose tissue inflammation and insulin resistance. J Immunol. 2015;194(10):4777–4783. doi: 10.4049/jimmunol.1500020. [DOI] [PubMed] [Google Scholar]
- 171.Sallam HS, Tumurbaatar B, Zhang WR, et al. Peripheral adipose tissue insulin resistance alters lipid composition and function of hippocampal synapses. J Neurochem. 2015;133(1):125–133. doi: 10.1111/jnc.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37(3):574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124(9):3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528(7580):137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Choi WG, Choi W, Oh TJ, et al. Inhibiting serotonin signaling through HTR2B in visceral adipose tissue improves obesity-related insulin resistance. J Clin Invest. 2021;131(23):e145331. doi: 10.1172/JCI145331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.He G, Gu J, Wang H, et al. Nr2e1 deficiency aggravates insulin resistance and chronic inflammation of visceral adipose tissues in a diet-induced obese mice model. Life Sci. 2021;278 doi: 10.1016/j.lfs.2021.119562. [DOI] [PubMed] [Google Scholar]
- 177.Franczyk MP, He M, Yoshino J. Removal of epididymal visceral adipose tissue prevents obesity-induced multi-organ insulin resistance in male mice. J Endocr Soc. 2021;5(5):bvab024. doi: 10.1210/jendso/bvab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wouters K, Kusters Y, Bijnen M, et al. NK cells in human visceral adipose tissue contribute to obesity-associated insulin resistance through low-grade inflammation. Clin Transl Med. 2020;10(6):e192. doi: 10.1002/ctm2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Deiuliis JA, Oghumu S, Duggineni D, et al. CXCR3 modulates obesity-induced visceral adipose inflammation and systemic insulin resistance. Obesity (Silver Spring) 2014;22(5):1264–1274. doi: 10.1002/oby.20642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kellar D, Craft S. Brain insulin resistance in Alzheimer's disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–766. doi: 10.1016/S1474-4422(20)30231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Calió ML, Mosini AC, Marinho DS, et al. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2021;148 doi: 10.1016/j.nbd.2020.105219. [DOI] [PubMed] [Google Scholar]
- 182.Mooldijk SS, Ikram MK, Ikram MA. Adiponectin, leptin and resistin and the risk of dementia. J Gerontol Ser A, Biol Sci Med Sci. 2021;77(6):1245–1249. doi: 10.1093/gerona/glab267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Magalhães CA, Carvalho MG, Sousa LP, Caramelli P, Gomes KB. Leptin in Alzheimer's disease. Clin Chim Acta. 2015;450:162–168. doi: 10.1016/j.cca.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 184.Liu J, Chi N, Chen H, et al. Resistin protection against endogenous Aβ neuronal cytotoxicity from mitochondrial pathway. Brain Res. 2013;1523:77–84. doi: 10.1016/j.brainres.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 185.Letra L, Rodrigues T, Matafome P, Santana I, Seiça R. Adiponectin and sporadic Alzheimer's disease: clinical and molecular links. Front Neuroendocrinol. 2019;52:1–11. doi: 10.1016/j.yfrne.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 186.He K, Nie L, Ali T, et al. Adiponectin alleviated Alzheimer-like pathologies via autophagy-lysosomal activation. Aging Cell. 2021;20(12):e13514. doi: 10.1111/acel.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Waragai M, Ho G, Takamatsu Y, et al. Adiponectin paradox as a therapeutic target in Alzheimer's disease. J Alzheimer's Dis. 2020;76(4):1249–1253. doi: 10.3233/JAD-200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peng L, Yu Y, Liu J, et al. The chemerin receptor CMKLR1 is a functional receptor for amyloid-β peptide. J Alzheimer's Dis. 2015;43(1):227–242. doi: 10.3233/JAD-141227. [DOI] [PubMed] [Google Scholar]
- 189.Zhang H, Lin A, Gong P, et al. The chemokine-like receptor 1 deficiency improves cognitive deficits of AD mice and attenuates tau hyperphosphorylation via regulating tau seeding. J Neurosci. 2020;40(36):6991–7007. doi: 10.1523/JNEUROSCI.0455-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yu Y, Ye RD. Microglial Aβ receptors in Alzheimer's disease. Cell Mol Neurobiol. 2015;35(1):71–83. doi: 10.1007/s10571-014-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Greco SJ, Bryan KJ, Sarkar S, et al. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer's disease. J Alzheimer's Dis. 2010;19(4):1155–1167. doi: 10.3233/JAD-2010-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Greco SJ, Sarkar S, Casadesus G, et al. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009;455(3):191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mainardi M, Spinelli M, Scala F, et al. Loss of leptin-induced modulation of hippocampal synaptic trasmission and signal transduction in high-fat diet-fed mice. Front Cellular Neurosci. 2017;11:225. doi: 10.3389/fncel.2017.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pérez-González R, Antequera D, Vargas T, Spuch C, Bolós M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer's disease. J Alzheimer's Dis. 2011;24(suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- 195.Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci. 2018;12:930. doi: 10.3389/fnins.2018.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hamilton K, Harvey J. The neuronal actions of leptin and the implications for treating Alzheimer’s disease. Pharmaceuticals. 2021;14(1):52. doi: 10.3390/ph14010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McGregor G, Harvey J. Food for thought: Leptin regulation of hippocampal function and its role in Alzheimer's disease. Neuropharmacology. 2018;136(Pt B):298–306. doi: 10.1016/j.neuropharm.2017.09.038. [DOI] [PubMed] [Google Scholar]
- 198.McGregor G, Harvey J. Regulation of hippocampal synaptic function by the metabolic hormone, leptin: implications for health and neurodegenerative disease. Front Cellular Neurosci. 2018;12:340. doi: 10.3389/fncel.2018.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Staurenghi E, Cerrato V, Gamba P, et al. Oxysterols present in Alzheimer's disease brain induce synaptotoxicity by activating astrocytes: a major role for lipocalin-2. Redox Biol. 2021;39 doi: 10.1016/j.redox.2020.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maysinger D, Ji J, Moquin A, et al. Dendritic polyglycerol sulfates in the prevention of synaptic loss and mechanism of action on glia. ACS Chem Neurosci. 2018;9(2):260–271. doi: 10.1021/acschemneuro.7b00301. [DOI] [PubMed] [Google Scholar]
- 201.Bednarska-Makaruk M, Graban A, Wiśniewska A, et al. Association of adiponectin, leptin and resistin with inflammatory markers and obesity in dementia. Biogerontology. 2017;18(4):561–580. doi: 10.1007/s10522-017-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ng RC, Chan KH. Potential neuroprotective effects of adiponectin in Alzheimer's disease. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lei Z, Lu Y, Bai X, Jiang Z, Yu Q. Chemerin-9 peptide enhances memory and ameliorates Aβ(1-42)-induced object memory impairment in mice. Biol Pharm Bull. 2020;43(2):272–283. doi: 10.1248/bpb.b19-00510. [DOI] [PubMed] [Google Scholar]
- 204.Emre C, Hjorth E, Bharani K, Carroll S, Granholm AC, Schultzberg M. Receptors for pro-resolving mediators are increased in Alzheimer's disease brain. Brain Pathol. 2020;30(3):614–640. doi: 10.1111/bpa.12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Naudé PJ, Nyakas C, Eiden LE, et al. Lipocalin 2: novel component of proinflammatory signaling in Alzheimer's disease. FASEB J. 2012;26(7):2811–2823. doi: 10.1096/fj.11-202457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kang H, Shin HJ, An HS, et al. Role of lipocalin-2 in amyloid-beta oligomer-induced mouse model of Alzheimer's disease. Antioxidants. 2021;10(11) doi: 10.3390/antiox10111657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Grizzanti J, Lee HG, Camins A, Pallas M, Casadesus G. The therapeutic potential of metabolic hormones in the treatment of age-related cognitive decline and Alzheimer's disease. Nutr Res. 2016;36(12):1305–1315. doi: 10.1016/j.nutres.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dekens DW, Naudé PJW, Keijser JN, Boerema AS, De Deyn PP, Eisel ULM. Lipocalin 2 contributes to brain iron dysregulation but does not affect cognition, plaque load, and glial activation in the J20 Alzheimer mouse model. J Neuroinflammation. 2018;15(1):330. doi: 10.1186/s12974-018-1372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]