Summary
Despite considerable efforts to prevent and treat ischemic cardiomyopathy (ICM), effective therapies remain lacking, in part owing to the complexity of the underlying molecular mechanisms, which are not completely understood yet. It is now widely thought that mitochondria serve as “sentinel” organelles that are capable of detecting cellular injury and integrating multiple stress signals. These pathophysiological activities are temporally and spatially governed by the mitochondrial quality surveillance (MQS) system, involving mitochondrial dynamics, mitophagy, and biogenesis. Dysregulation of MQS is an early and critical process contributing to mitochondrial bioenergetic dysfunction and sublethal injury to cardiomyocytes during ICM. An improved understanding of the pathogenesis of ICM may enable the development of novel preventive and therapeutic strategies aimed at overcoming the challenge of myocardial ischemia and its cardiovascular sequelae. This review describes recent research on the protective effects of MQS in ICM and highlights promising therapeutic targets.
Keywords: Ischemic cardiomyopathy, Mitochondrial quality surveillance, Cardiomyocyte, Mitochondrial dynamics, Mitophagy, Mitochondrial biogenesis
Introduction
Ischemic cardiomyopathy (ICM) is mainly caused due to long-term ischemia/hypoxia in coronary atherosclerosis, leading to the impairment of cardiac systolic or diastolic function. This induces a series of phenotypic syndromes, including myocardial remodelling and heart failure.1 The pathophysiological mechanisms underlying ICM include a spectrum of metabolic, neurohumoral, and inflammatory alterations, such as cardiomyocyte hypertrophy, fibrosis, inflammation, oxidative stress, calcium mishandling, and cell death.2 The myocardium is abundant in mitochondria which generate ATP to support cardiomyocyte metabolism and function. There is increasing evidence that mitochondria serve as “sentinel” organelles that are not only capable of detecting cellular insults but are also involved in the integration of signals of oxidative stress, Ca2+ fluctuation, inflammation, post-transcriptional modification, protein synthesis, and cell death. Studies have highlighted the pivotal role of mitochondrial damage in the pathogenesis of ICM. In response to damage, the mitochondrial quality surveillance (MQS) system, involving mitochondrial dynamics, mitophagy, and mitochondrial biogenesis, is activated to restore homeostasis by inducing division, degradation, or regeneration. In turn, abnormalities in MQS, such as excessive mitochondrial fission,3 defective mitophagy,4 and delayed biogenesis,5 are associated with additional cardiomyocyte damage, thus serving as potential targets in ICM treatment. In this review, we briefly describe the molecular basis of MQS in the regulation of mitochondrial integrity and behaviour. The complex roles of MQS in the pathogenesis of ICM, including cardiomyocyte hypertrophy, fibrosis, inflammation, oxidative stress, calcium mishandling, and cell death, are comprehensively summarised and discussed. In addition, potential drugs and small-molecule compounds targeting MQS for the treatment of ICM are presented.
Mitochondrial quality surveillance (MQS)
Mitochondrial fission
Mitochondrial dynamics involve both fission and fusion, which are considered key processes for maintaining mitochondrial structure and cell viability. Dysregulation of mitochondrial fusion and fission leads to structural and functional alterations, commonly followed by oxidative stress and apoptosis. The core proteins that mediate mitochondrial fission and fusion are mechanochemical enzymes classified as GTPases (dynamin family) for membrane remodelling (Figure 1), including dynamin-related protein 1 (Drp1), mitofusin1/2 (Mfn1/2), and optic atrophy protein 1 (Opa1).
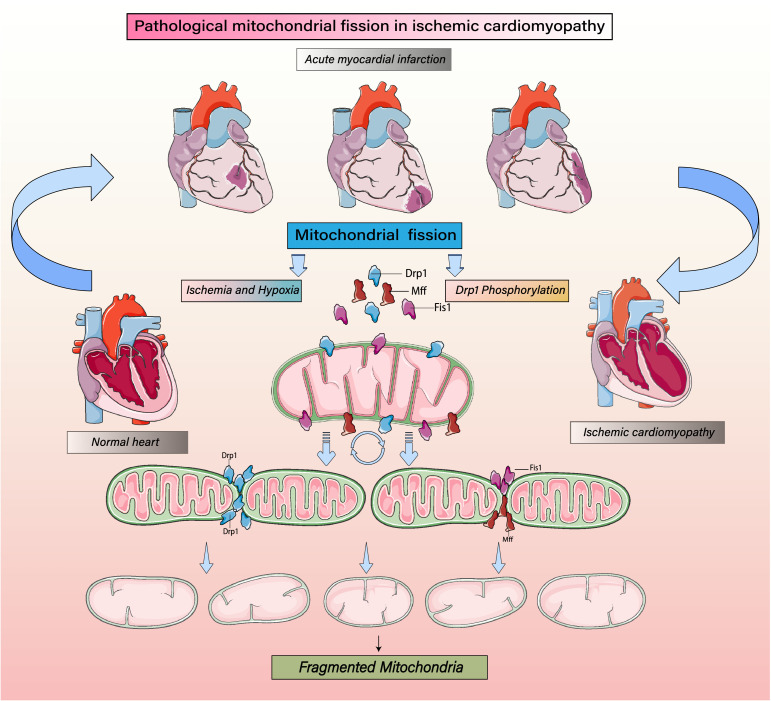
Figure 1.
Regulatory mechanism of mitochondrial fission on mitochondrial homeostasis. Mitochondrial fission induced by ICM. Ischemic/hypoxic stress increases phosphorylation of the mitochondrial fission protein Drp1 as well as the expression levels of the Drp1 receptor proteins fission protein 1(Fis1) and mitochondrial fission factor (Mff). Together these proteins mediate typical mitochondrial fission. Mitochondrial fission increases a precursor to mitophagy when cells are subjected to various stresses. This is related to the accumulation of various markers of mitochondrial autophagy. Drp1 phosphorylation induces mitochondrial fission by mediating its oligomerization around the mitochondrial outer membrane after recruitment and stabilization of Fis1 and Mff. Increasing levels of phosphorylated Drp1 increase mitochondrial fission, leading to their fragmentation, which will reduce both energy metabolism and cellular activity and stimulate mitophagy.
During fission, the mitochondrial fission 1 (Fis1) protein mediates the transfer of Drp1 from cytosol to outer membrane of the mitochondria. Subsequently, Drp1 accumulates at the fission site and binds to Fis1 to form a complex, altering the angle and distance between molecules via hydrolysed GTP. This process is followed by the cleavage of a mitochondrion into two or more independent mitochondria. Conversely, mitochondrial fusion is mediated by Mfn1/2 and Opa1. Mfn1/2 participates in the integration of the outer mitochondrial membrane (OMM), whereas Opa1 participates in the fusion of the inner mitochondrial membrane (IMM). During ICM development, ischemia contributes to alterations in Drp1 and Mfn1/2 expression and distribution, resulting in the formation of fragmented mitochondria with lower mitochondrial membrane potential, broken mitochondrial DNA (mtDNA), and misfolded proteins.3,6, 7, 8
In light of the important role of mitochondrial fission in aggravating ischemia-related myocardial damage, several drugs against mitochondrial division have shown promising effects on the ischemic myocardium via multiple mechanisms. Mdivi-1 is a pharmacological inhibitor of mitochondrial fission that suppresses the interaction between Drp1 and Fis1. Pre-treatment with Mdivi-1 before myocardial ischemia-reperfusion (I/R) injury is accompanied by a decrease in the number of fragmented mitochondria and a correlated decrease in cardiac troponin I levels.6,9 Mechanistically, Mdivi-1 prevents the opening of the mitochondrial permeability transition pore (mPTP),10 stabilises mitochondrial potential,11 increases the activity of antioxidant enzymes,9 and represses caspase-9-related cardiomyocyte apoptosis.8 Recently, two novel inhibitors, Drpitor1 and Drpitor1a, having higher specificity and potency than that of Mdivi-1 toward Drp1 GTPase activity, have been reported to maintain heart function during I/R injury.12 Other mitochondrial fission-targeted drugs with potentially beneficial effects on ICM are summarised in Table 1.
Table 1.
Compounds or drugs targeting mitochondrial quality surveillance in ischemic cardiomyopathy.
| Name | Target | Reference |
|---|---|---|
| Mdivi-1 | Mitochondrial fission (Drp1) | 6,9 |
| Drpitor1 | Mitochondrial fission (Drp1) | 12 |
| Cilnidipine | Mitochondrial fission (Drp1) | 54 |
| Salicylate | Mitochondrial fission (Drp1) | 41 |
| Propofol | Mitochondrial fission (Drp1) | 55 |
| Dapagliflozin | Mitochondrial fission (Drp1) | 56 |
| Dynasore | Mitochondrial fission (Drp1) | 57 |
| Isosteviol sodium (STVNa) | Mitochondrial fission (Drp1/Fis1) | 58 |
| P110 | Mitochondrial fission (Drp1/Fis1) | 59 |
| Pravastatin | Mitochondrial fusion (Mfn1) | 60 |
| Vildagliptin | Mitochondrial fusion (Mfn1/2) | 61 |
| SAMβA | Mitochondrial fusion (Mfn1) | 62 |
| M1 | Mitochondrial fusion (Mfn2/Opa1) | 18 |
| Urolithin A (UA) | Mitophagy (Parkin) | 27 |
| Melatonin | Mitophagy (Parkin) | 63 |
| Simvastatin | Mitophagy (Parkin) | 64 |
| Liraglutide | Mitophagy (Parkin) | 65 |
| Sevoflurane | Mitophagy (Parkin) | 66 |
| Curcumin | Mitophagy (Bnip3) | 67 |
| Ketone ester [KE] | Mitophagy (Parkin) | 68 |
| Bicarbonate | Mitophagy (Parkin) | 69 |
| Ellagic acid | Mitophagy (Bnip3) | 70 |
| Hydrogen-rich saline | Mitophagy (Parkin) | 71 |
| Tilianin and syringin | Mitochondrial biogenesis (PGC-1α) | 44 |
| Empagliflozin | Mitochondrial biogenesis (PGC-1α) | 72 |
| Metformin | Mitochondrial biogenesis (PGC-1α) | 73 |
| Resveratrol | Mitochondrial biogenesis (PGC-1α/Nrf1) | 74 |
| Polydatin | Mitochondrial biogenesis (Sirt3) | 75 |
Mitochondrial fusion
Mitochondrial fusion is accomplished by merging outer membrane lipid molecules between two mitochondria, resulting in the close arrangement of adjacent membrane structures and ensuring fusion specificity. OMM integration is regulated by Mfn1/2, whereas IMM fusion is modulated by Opa1 (Figure 2). During mitochondrial fusion, merging of membrane bilayers of the fusing mitochondria to form larger or reticular organelles occurs along with material and energy exchange.13 Although mitochondrial fusion has been regarded as a protective program favouring ATP production, the role of fusion-regulated proteins in ICM is controversial. Cardiomyocyte-specific Mfn1-knockout hearts show normal mitochondrial respiration and lack of sensitivity to hydrogen peroxide-mediated oxidative injury.14 Whereas, cardiomyocyte-specific Mfn2-knockout hearts show extensive mtDNA breaks and mitochondrial dysfunction.15 I/R-induced apoptosis is prominent in Mfn2-knockout cardiomyocytes.16 Furthermore, Mfn1/Mfn2 double deletion has been associated with the accumulation of dysfunctional mitochondria and the activation of mitochondrial unfolded protein response.17 Based on these findings, although Mfn1 and Mfn2 have similar roles in inducing mitochondrial fusion, their influence on the heart during ICM may differ. Thus, a small number of pharmacological experiments have focused on the identification of drugs that normalise Mfn1/2-dependent fusion. The mitochondrial fusion promoter M1 has been found to reduce the Drp1/Mfn2 ratio, thus reversing mitochondrial fusion and protecting the heart against myocardial ischemic injury.18 A subsequent study further confirmed that M1 is able to restore mitochondrial fusion in Mfn2-knockout cardiomyocytes,19 possibly via the increased expression of Opa1. Other pharmacological agents that improve mitochondrial fusion for cardioprotection are listed in Table 1.
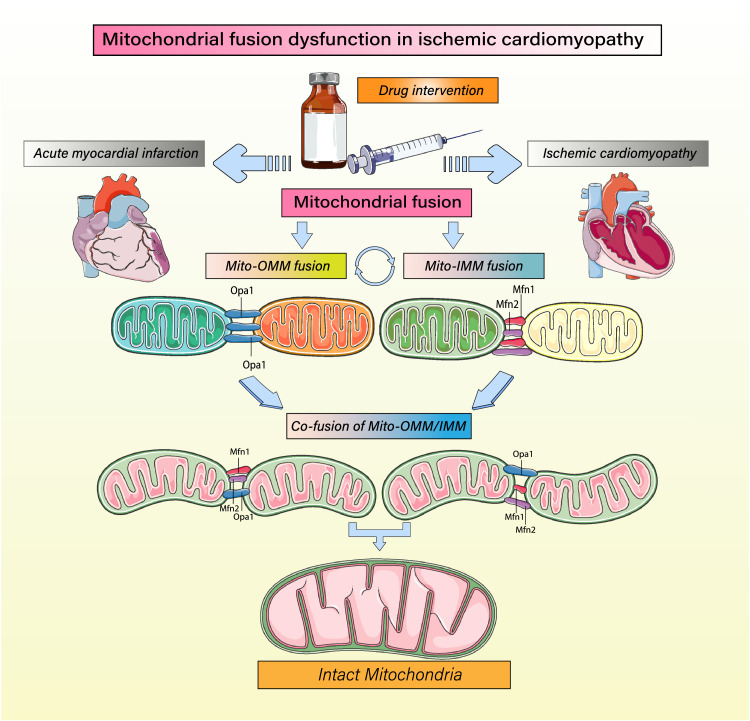
Figure 2.
Regulation of mitochondrial fusion and mitochondrial homeostasis. Mitochondrial fusion is highly important for maintaining mitochondrial homeostasis and the integrity and quality of mitochondrial structure to ensure that proper energy metabolism is maintained even during ischemia/hypoxia. Mitochondrial fusion is accomplished through the coordinated execution of separate mechanisms underlying fusion of the mitochondrial inner and outer membranes. Mitochondrial inner membrane fusion is mediated mainly by optic atrophy 1 (OPA1) protein, while outer membrane fusion is mediated mainly by mitofusin 1 (Mfn1) and Mfn2.
Mitophagy
Mitophagy is an evolutionarily conserved process in which excess or damaged mitochondria are selectively removed. It plays an important role in regulating the number of mitochondria in a cell and in maintaining the normal function and structure of mitochondria, especially in the ICM-affected myocardium.20 Receptor-dependent mitophagy mediates a group of mitophagy receptor proteins that bind directly to LC3 to transfer damaged mitochondria to autophagosomes (Figure 3).21 The receptor-independent mitophagy pathway is mainly driven by the stability of PTEN-induced kinase 1 (PINK1), a cytosolic kinase that translocates to the mitochondria upon a reduction in membrane potential and initiates receptor-independent mitophagy.22 Within the mitochondria, PINK1 recruits the E3 ubiquitin ligase Parkin, which induces the ubiquitination of multiple outer mitochondrial membrane proteins, enabling p62 recognition, LC3 binding, and autophagosome targeting.
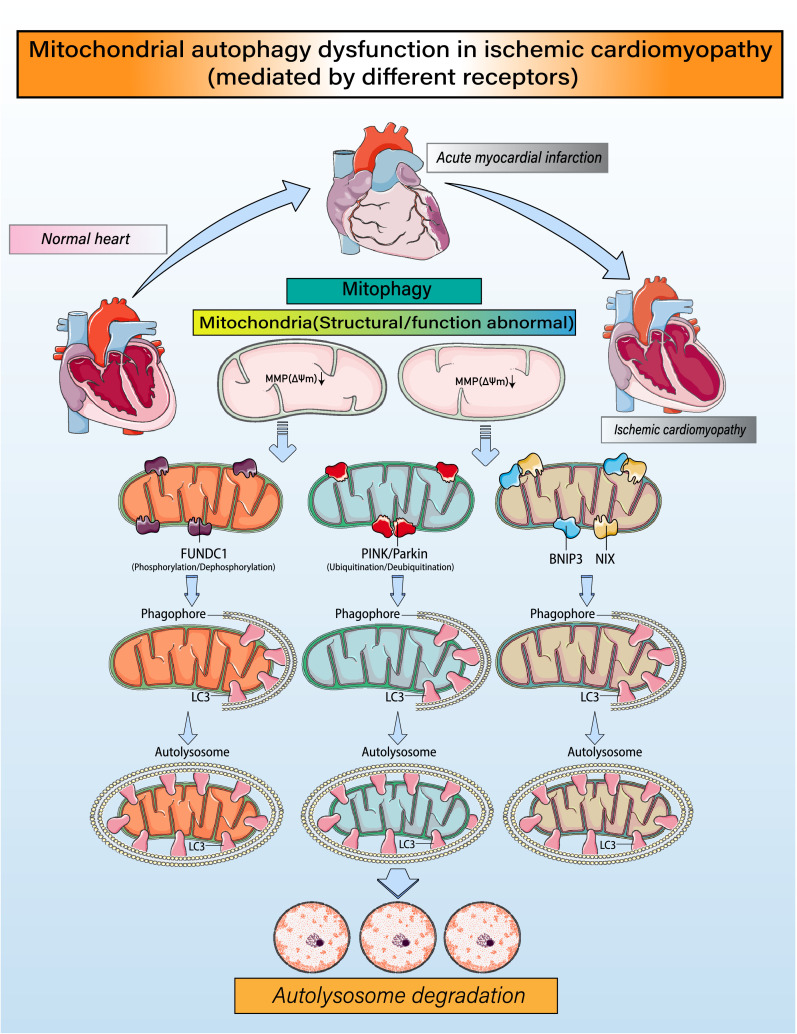
Figure 3.
Regulatory mechanisms governing mitophagy and mitochondrial homeostasis. Under ischemia/hypoxic conditions, fragmented mitochondria or mitochondria with structural damage caused by mitochondrial fission will experience a loss of mitochondrial membrane potential (ΔΨm). Mitochondria with low membrane potentials recruit FUN14 domain-containing 1(FUNDC1), which phosphorylates/dephosphorylates various proteins, leading to high expression of LC3 and formation of mitochondrial autophagic lysosomes. Phosphatase and tensin homolog-induced putative kinase 1 (PINK1) is also translocated to mitochondria, where it induces Parkin to ubiquitinate proteins in the mitochondrial outer membrane. The ubiquitinated mitochondria interact with LC3 on lysosomes to form autophagosomes. In addition, BCL-2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3) and NIX contain an LC3 interaction motif (LIR), which directly binds LC3 and promotes formation of autophagic lysosomes. Through these processes, the mitochondrial self-renewal and homeostasis necessary for cardiomyocyte activity are achieved.
Under stress conditions, such as nutrient deficiency and hypoxia, damaged mitochondria are often selectively cleared by mitophagy to maintain mitochondrial health and cellular homeostasis. In contrast, stress responses may also enhance mitophagy, resulting in excessive mitochondrial clearance and insufficient ATP production, in turn resulting in cell death.20 Therefore, excessive as well as insufficient mitophagy are linked to additional cell damage, and moderate mitophagy is essential for maintaining mitochondria. A clear example in which excessive mitophagy is harmful involves Bcl2/adenovirus E1B 19 kD interacting protein 3 (Bnip3)-induced mitophagy following cardiac I/R injury.23 Increased Bnip3-related mitophagy suppresses ATP synthesis, an alteration that is accompanied by depolarised mitochondrial potential and an extended mPTP opening rate.23 An example in which mitophagy has protective effects against myocardial ischemic stress is FUN14 domain-containing protein 1 (FUNDC1)-dependent mitophagy. Hypoxia or ischemia promotes FUNDC1 activation via post-transcriptional phosphorylation and this alteration reduces reperfusion-mediated myocardial dysfunction by neutralising oxidative stress, maintaining mitochondrial potential, promoting biogenesis, and suppressing cardiomyocyte death.24,25 Therefore, different mitophagy regulators may have distinct effects on cellular outcomes, ranging from cell survival to death. Based on this, several mitophagy-targeted drugs have been explored. In particular, urolithin A (UA) is promising for ICM treatment. UA is a gut microbiota-generated small metabolite derived from dietary pomegranate fruits. Supplementation of UA induces mitophagy and consequently attenuates myocardial fibrosis and inflammation in a rat diabetic cardiomyopathy model.26 Human clinical experiments have provided further evidence for the beneficial effects of UA on skeletal muscle health.27 Other compounds or drugs targeting mitophagy to prevent the progression of ICM are presented in Table 1.
Mitochondrial biogenesis
Mitochondrial biogenesis involves the synthesis of phospholipids comprising the inner and outer mitochondrial membranes and proteins encoded by both nuclear and mitochondrial DNA, as well as the replication of mtDNA and transfer of nuclear DNA-encoded proteins into the mitochondria.28 This dynamic process enables mitochondria to stably maintain their structure, function, and quantity to ensure normal cellular metabolism when the demand for energy increases, e.g., upon cellular stress or during increased activity or proliferation.29 The regulation of mitochondrial biogenesis can be divided into transcriptional and post-transcriptional components (Figure 4). These processes are activated by peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1 α), nuclear respiratory factors (Nrfs), mitochondrial transcription factor A (TFAM), and other signalling pathways.30 Nrf-1 is a positive regulator that stimulates the expression of components of the mitochondrial respiratory chain, including the cytochrome c promoter.31 It is also linked to the expression of essential genes encoded by mtDNA. Nrf-2 mainly acts on nuclear genes, including genes encoding respiratory subunits and TFAM, therefore promoting the transcription and replication of mtDNA. PGC-1α regulates the interaction between nuclear and mitochondrial genes by cooperating with Nrf-1/2, which finally drives the expression of TFAM, a key regulator of mtDNA transcription, and accelerates mitochondrial oxidative phosphorylation.32 Potential therapeutic strategies aimed at improving mitochondrial biogenesis to attenuate ICM are summarised in Table 1.
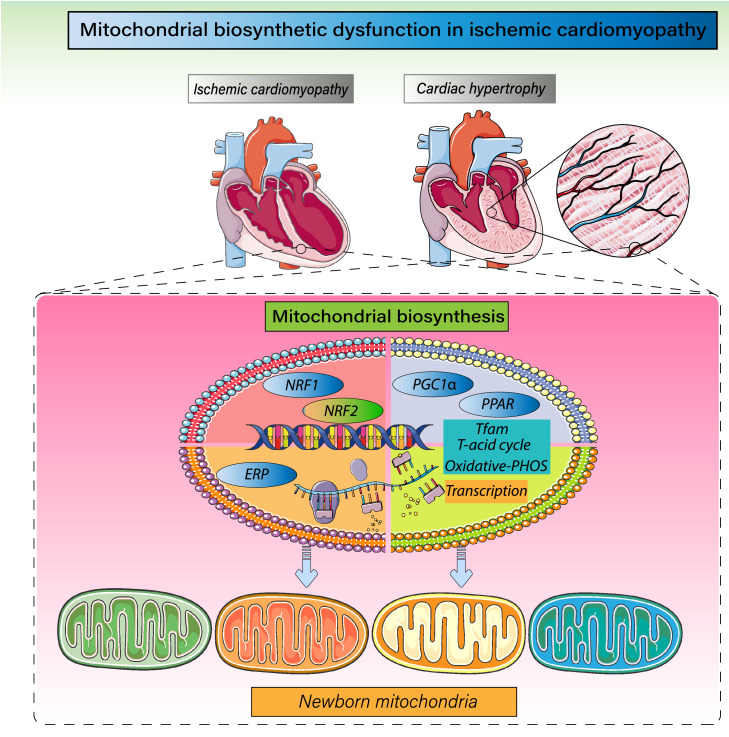
Figure 4.
Regulatory mechanism governing mitochondrial biogenesis and mitochondrial homeostasis. Mitochondrial biosynthesis mainly occurs in the late stage of mitochondrial fission and mitophagy. PGC1α is the primary regulatory factor controlling mitochondrial biogenesis and is situated upstream of the mitochondrial biosynthesis reticular regulatory system. It is the hub connecting external signals to internal functional regulation within mitochondria. PGC1α promotes transcription of mitochondrial transcription factor A (TFAM) by activating nuclear respiratory factors 1 and 2 (Nrf-1 and Nrf-2) and regulates the citric acid and tricarboxylic acid cycles and, in turn, oxidative phosphorylation. In addition, ERP and PPAR-γ are members of the nuclear receptor transcription factor superfamily that act to regulate expression of target genes and the levels of TFAM transcription and mitochondrial biosynthesis.
Role of MQS in ICM
MQS in myocardial hypertrophy and fibrosis
Drp1-induced mitochondrial fission causes mitophagic dysfunction, leading to cardiomyocyte hypertrophy.17 The absence of Mfn1 or Mfn2 expression in the myocardium of pregnant rats promotes cardiomyocyte hypertrophy and heart failure due to a disrupted mitochondrial structure.33 In a mouse model of cardiac hypertrophy, the expressions of mitochondrial biogenesis-related proteins (PGC-1α and Nrf-1) were significantly inhibited. Similarly, the levels of mitophagy proteins (PINK1 and FUNDC1) were significantly downregulated during myocardial hypertrophy.34 Conditional knockout of Drp1 in cardiomyocytes inhibited mitochondrial fission and induced mitochondrial enlargement. These data suggest that abnormal MQS may induce cardiomyocyte hypertrophy.
Excessive mitochondrial fission is thought to increase fibroblast proliferation and collagen production,27 possibly because mitochondrial division functions as a downstream effector of TGF-β1.35 The inhibition of mitochondrial fission via Mdivi-1 markedly alleviates fibroblast proliferation, collagen production, and fibrosis in the myocardial infarcted border zone.36 Unlike mitochondrial fission, the pharmacological activation of mitophagy is associated with decreased levels of angiotensin II and atrial natriuretic peptide, contributing to decreased cardiomyocyte apoptosis and myocardial fibrosis in a pressure overload-induced chronic cardiac dysfunction model.37 Lastly, mitochondrial biogenesis is evidently downregulated during myocardial fibrosis, but the underlying mechanism is undefined.38 Exercise has been regarded as a powerful stimulus for mitochondrial biogenesis, and post-infarction exercise training can decelerate cardiac remodelling and fibrosis by a mechanism involving PGC1α-regulated biogenesis.39
MQS in inflammation
Inflammatory signal transduction in cardiomyocytes usually starts as an early stress response to myocardial injury, which leads to the accumulation of mitochondrial ROS and, eventually, to the impairment of mitochondrial oxidative phosphorylation. In neonatal cardiomyocyte models of ischemia-like conditions, mitochondrial fission has been identified as an activator of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome.40 Mechanistically, Drp1-related mitochondrial fission promotes the release of mtDNA and mitochondrial ROS (mtROS) into the cytosol, which is followed by inflammasome formation within the myocardium and the elevated transcription of pro-inflammatory factors, such as caspase-3 and IL-18.40 In addition to cardiomyocytes, the endothelial inflammatory response is also affected by mitochondrial fission. Abnormal mitochondrial fission promotes endothelial cell membrane damage in hypoxic conditions, which is followed by the augmented adhesion of monocytes to endothelial cells.41 In light of the necessary role of mitophagy in suppressing mtDNA release and mtROS leakage, the anti-inflammatory properties of mitophagy have been reported in neurological diseases, although this concept has not been validated in ICM.42 Similarly, the inhibitory effects of PGC1α- or Nrf-2-dependent mitochondrial biogenesis on the NLRP3 inflammasome have been investigated in septic43 and diabetic44 cardiomyopathies; however, additional experiments are required to further demonstrate the beneficial effects of biogenesis on myocardial inflammation during ICM.
MQS in oxidative stress and calcium homeostasis
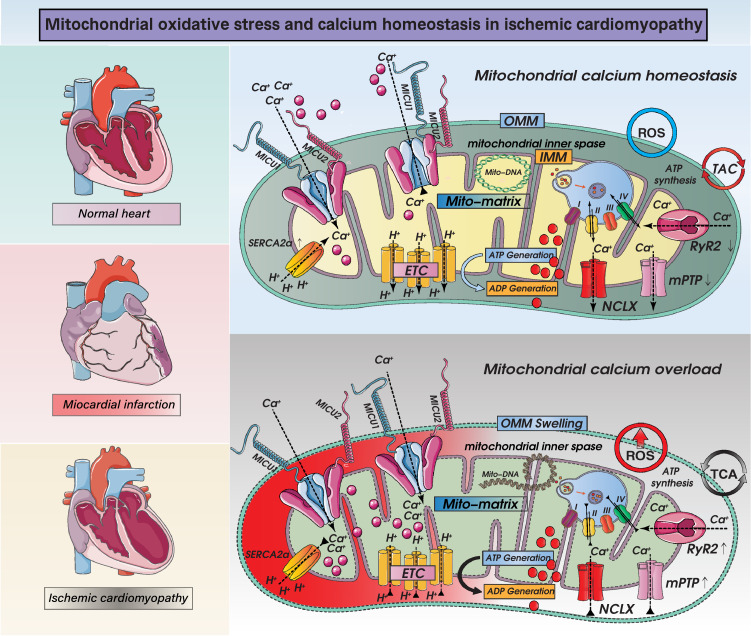
ROS are by-products of mitochondrial metabolism, and increased ROS production can induce oxidative stress injury, which in turn promotes mitochondrial lipid and protein oxidation. As the second calcium pool within cardiomyocytes, the mitochondria may act as a spatial Ca2+ buffer in response to fluctuations in intracellular calcium. Excessive accumulation of Ca2+ in the mitochondria is followed by disrupted mitochondrial oxidative phosphorylation and defective ATP synthesis, leading to impaired cardiomyocyte contraction/relaxation (Figure 5). Increased mitochondrial fission triggers oxidative stress in ischemic cardiomyocytes.8 Mechanistically, mtDNA is unevenly distributed in daughter mitochondria during mitochondrial division, and this can result in the decreased transcription of respiratory complex-related proteins45 and thereby in mtROS overproduction. Due to mtROS-mediated oxidative injury, a cysteine residue within the mitochondrial calcium uniporter (MCU) is S-glutathionylated, resulting in the assembly of MCU channels into higher-order complexes with persistent activity, which is associated with sustained mitochondrial calcium uptake. In addition, sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), a calcium channel that pumps calcium from the cytosol back to the sarco/endoplasmic reticulum, is prone to oxidation by the oxidative micro-environment at cysteine-674,46 resulting in intracellular calcium overload. The mitophagy-mediated removal of dysfunctional mitochondria substantially reduces mtROS production.47 Although there is a causal relationship between oxidative stress and intracellular/mitochondrial calcium overload, evidence supporting the regulatory effect of mitophagy on calcium handling is lacking. Similarly, although several studies have shown that moderate mitochondrial calcium accumulation promotes mitochondrial biogenesis, the influence of biogenesis on calcium handling merits further investigation. It is well known that mitochondrial biogenesis-related proteins, especially Nrf-1/2, act as transcription factors to augment the expression of mitochondrial antioxidant genes (including manganese superoxide dismutase, catalase, peroxiredoxin 3 and 5, uncoupling protein 2, thioredoxin 2, and thioredoxin reductase), highlighting the antioxidative property of mitochondrial biogenesis. However, additional data are required to validate the inhibitory effects of mitochondrial biogenesis on oxidative stress during ICM.
Figure 5.
Mechanisms of mitochondrial calcium homeostasis and ROS-mediated mitochondrial oxidative stress injury and mitochondrial dysfunction in ICM. Impact of ROS overproduction-activated oxidative stress injury in ICM on mitochondrial homeostasis. Intrinsic and extrinsic stress signals, such as hypoxia, ischemia, reperfusion, and inflammation, can mediate overproduction of ROS and can lead to mitochondrial oxidative stress damage. When mitochondria are damaged excessively, mitochondrial outer membrane permeabilization (OMM) is dysfunctional, and mitochondrial permeability transition pore (mPTP) is abnormally opened. On the other hand, mPTP opening induces necrosis or necroptosis, accompanied by oxidative phosphorylation dysfunction and dysregulation of the tricarboxylic acid cycle. A range of dysfunctions also induces abnormal calcium signaling, excess ROS production and mitochondrial respiratory chain dysfunction and related protein gene expression changes. Disruption of the mitochondrial calcium cycle has important linkages with mitochondrial uniporter complexes (consisting of MCU, EMRE, MICU1, MICU2, MICU3, MCUB and MCUR1), NCLX, LETM1. And calcium transport in mitochondria is also closely related to ryanodine receptors and mitochondrial permeability transition pore. The normal operation of the above mechanisms(mitochondrial calcium homeostasis and oxidative stress injury) directly or indirectly affects the normal operation of mitochondrial respiratory chain function and mitochondrial energy metabolism function.
MQS and cardiomyocyte fate regulation
MQS can participate in cardiomyocyte death. Increased Drp1-related mitochondrial fission induces the release of cytochrome c from the mitochondria into the cytoplasm, where caspase-9 is activated and cardiomyocyte apoptosis is subsequently induced in the presence of ischemia/hypoxia.47 In addition to apoptosis, cardiomyocyte necroptosis is also induced by mitochondrial fission during I/R injury.48 In comparison, the disruption of Drp1 mitochondrial translocation renders cells resistant to lethal hypoxia/reoxygenation injury.49 Ample data supports the functional importance of mitophagy in promoting cardiomyocyte survival during ischemic stress.4,50 Mechanistically, mitophagy prevents ROS accumulation, repairs mtDNA damage, promotes poor mitochondrial elimination, reduces the leakage of mitochondria-localised pro-apoptotic proteins, interrupts the assembly of mPTP, and restores the cardiomyocyte energy supply48,51, 52, 53 during myocardial ischemic challenge (Figure 6). Nevertheless, the mechanistic connection and functional relationship between mitochondrial biogenesis and cardiomyocyte fate regulation remain to be characterised.
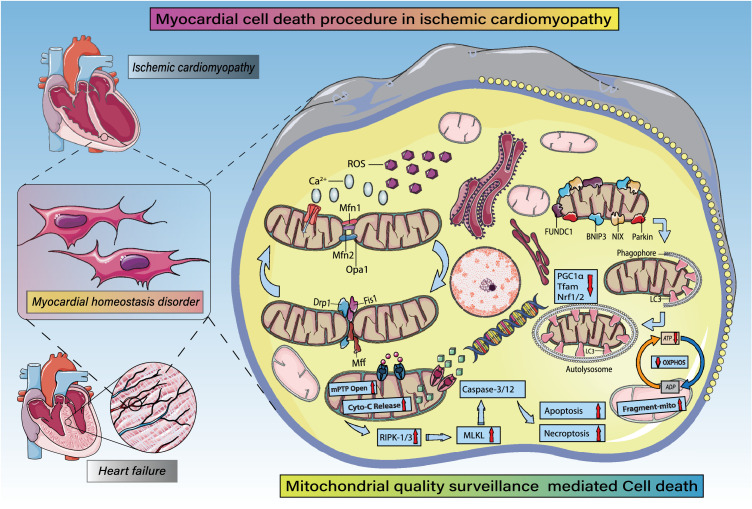
Figure 6.
MQS in cardiomyocyte fate regulation (apoptosis, necrosis, necroptosis, pyroptosis, autophagic cell death). MQS fully participates in apoptotic or necrotic cardiomyocyte death. Under ischemic/hypoxic stress, intracellular ROS are overproduced and accompanied by intracellular Ca2+ overload. Ischemic stress also breaks the balance between Drp1/FIS1/Mff-mediated mitochondrial fission and OPA1/Mfn1/Mfn2-mediated mitochondrial fusion, which leads to mitochondrial fragmentation and structurally abnormal mitochondria. Mitochondrial autophagy mediated by FUNDC1/Parkin /BNIP3 and NIX is enhanced, leading to PGC1α- and Nrf1/2-mediated decreases in mitochondrial biosynthesis, abnormal opening of mPTPs, and excessive release of Cyto-c into the cytosol. Dysregulation of the mitochondrial electron transport chain and tricarboxylic acid cycle leads to insufficient ATP production. Expression levels of RIPK3 and MLKL are increased, as is activation of caspase-3/-12 and levels of cardiomyocyte.
Future perspectives and translational issues
Despite extensive recent research on the contributions of disturbed MQS to the occurrence and development of ICM, the vast majority of results are based on animal experiments or cell-based studies. There are several unaddressed issues that may impede human clinical research and translating basic findings into clinical practice. First, serum biomarkers of impaired MQS are unavailable; therefore, it is difficult to evaluate or continually monitor the development of ICM. Additionally, complex interactive effects on MQS remain unknown and efforts to comprehensively describe relationships between upstream and/or downstream factors related to dynamics, mitophagy, and biogenesis are urgently needed. Finally, the cardioprotective effects of drugs or small molecular compounds that preserve mitochondrial function in cardiovascular diseases are well documented based on several clinical trials (Table 2). However, further attempts are required to search for safe and effective drugs or combined therapies targeting global MQS.
Table 2.
Clinical trials of compounds or drugs targeting mitochondria in cardiovascular diseases.
| Drug | Condition or disease | Targets | Title | Outcome measures | Results | Recruitment status | Clinical trial number |
|---|---|---|---|---|---|---|---|
| Pravastatin, carvedilol, perindopril | Heart failure | Mitochondrial metabolism | Evaluation of myocardial improvement in patients supported by ventricular assist device under optimal pharmacological therapy | Mitochondrial function | — | Completed | NCT00402376 |
| Cyclosporine A | Non shockable out of hospital cardiac arrest | Mitochondrial permeability transition pore | Cyclosporine A in cardiac arrest (CYRUS) | Sequential organ failure assessment score (SOFA) | In patients presenting with non-shockable cardiac rhythm after out-of-hospital cardiac arrest, cyclosporine does not prevent early multiple organ failure | Completed | NCT01595958 |
| Nitrate rich beetroot juice | Heart failure with normal ejection fraction | Mitochondrial oxidative capacity | Effect of inorganic nitrates (beetroot juice) on arterial hemodynamic and exercise capacity | Change in peak exercise efficiency during maximal effort supine-bicycle exercise and peak oxygen consumption (VO2) during a maximal effort supine-bicycle exercise test | Inorganic nitrate increased exercise capacity in heart failure with preserved ejection fraction by targeting peripheral abnormalities | Completed | NCT01919177 |
| Resveratrol | Type 1 diabetes | Mitochondrial metabolism and biogenesis | Type 1 diabetes, endothelin, and skeletal muscle mitochondrial dysfunction: the role of Sirtuin-1 (T-St1M) | Skeletal muscle mitochondrial function | — | Recruiting | NCT04449198 |
| Mitoquinol mesylate (mitoQ) | Dilated cardiomyopathy (DCM) | Mitochondrial oxidative phosphorylation | Examining the effects of mitochondrial oxidative Stress in DCM (MitoDCM) | Change in myocardial PCr:ATP | — | Recruiting | NCT05410873 |
| Nicotinamide riboside | Heart failure | Mitochondrial metabolism | The effect of nicotinamide riboside on skeletal muscle function in heart failure subjects | Number of participants who had enhancement of mitochondrial function in skeletal muscle | — | Recruiting | NCT03565328 |
| Pioglitazone | Type 2 diabetes, coronary heart disease | Mitochondrial function | Myocardial dysfunction in type 2 Diabetes mellitus (T2DM) | Myocardial glucose uptake | Pioglitazone improves whole-body and myocardial insulin sensitivity, left ventricular diastolic function, and systolic function in type 2 diabetes | Completed | NCT01588470 |
| Acipimox | Type 2 diabetes, dilated cardiomyopathy | Mitochondrial function | Cross-over study on effect of lipid lowering by acipimox on cardiac and skeletal muscle mitochondrial function (ACP) | Changes in mitochondrial function | A reduced basal ADP-stimulated and maximal mitochondrial respiratory capacity underlies the reduction in in vivo mitochondrial function, independent of mitochondrial content in diabetic patients. | Completed | NCT00943059 |
| Trimetazidine | Coronary artery disease | Mitochondrial respiration | Prospective observational study of trimetazidine influence on mitochondrial metabolism in human heart ventricle | Change in rate of myocardial oxygen consumption expressed in pmolO2/min/units of citrate synthase activity | — | Completed | NCT02152527 |
| Rosuvastatin | Cardiovascular disease | Mitochondrial oxidative phosphorylation | Effect of rosuvastatin on endothelial function | Change in flow mediated dilatation (FMD) of the brachial artery | — | Recruiting | NCT00986999 |
| Empagliflozin | Heart failure with preserved ejection fraction | Mitochondrial morphology and biogenesis | SGLT2i and KNO3 in HFpEF - The SAK HFpEF Trial | Submaximal exercise endurance | — | Recruiting | NCT05138575 |
| 14 N sodium nitrite | Heart failure | Mitochondrial function | Oral nitrite for older heart failure patients (ONTx+HF) | Skeletal muscle bioenergetics - mitochondrial function | — | Completed | NCT02457260 |
| Coenzyme Q10 | Cardiovascular disease | Mitochondrial function | Effects of CoQ10 on inflammatory response in cardiac surgery | Inflammatory and endothelial blood work biomarkers | — | Recruiting | NCT04444349 |
| Sitagliptin | Cardiovascular disease, type 2 diabetes | Mitochondrial function | Impact of sitagliptin on cardiovascular exercise performance in type 2 diabetes | Peak oxygen consumption (VO2 peak). | Three months of sitagliptin improved diastolic cardiac function, however, cardiorespiratory fitness did not change | Completed | NCT01951339 |
Outstanding questions
Despite significant advances in therapies, the mortality rate of ICM remains high at 102.6 deaths per 100,000 patients in the first year after diagnosis. The lifetime risk of ICM is 37.5% for men and 18.3% for women. The myocardium contains abundant mitochondria that generate ATP to support cardiomyocyte metabolism and performance. The role of mitochondria as “sentinel” organelles capable of detecting cellular insults as well as integrating multiple intracellular signals is increasingly recognised. MQS is an endogenous protective mechanism preserving mitochondrial integrity and function via mitochondrial dynamics, mitophagy, and mitochondrial biogenesis.
Mitochondrial dynamics, characterised by the coordinated actions of mitochondrial fission and fusion, fine tunes the mitochondrial population and has an irreplaceable impact on cardiomyocyte energy supply. Mitophagy and mitochondrial biogenesis account for mitochondrial degradation and regeneration, respectively, guaranteeing a healthy and appropriate mitochondrial population. Given the pathologically significant impact of MQS on myocardial ischemic events and ischemia-related aetiology, it is clinically important to map the upstream regulatory mechanisms underlying the MQS and downstream molecular events in the development of ICM.
Although much progress has been made in our understanding of the relationship between MQS and the pathogenesis of ICM, the detailed pathological basis of ICM is complex, involving various cell types and organelles, and is associated with numerous risk factors, such as atherosclerotic plaque formation, endothelial dysfunction, thrombogenesis, coronary artery spasm, and extracellular matrix metabolism. Consequently, investigations of the pathology of ICM must consider various perspectives, including genetic factors, smoking, diet, mental stress, obesity, and sedentary lifestyle. Other outstanding questions for further research include the development of more precise, personalised, and optimised therapeutics targeting MQS for patients with ICM.
Search strategy and selection criteria
Data for this review were obtained from PubMed and ClinicalTrials.gov using the key words “mitochondria quality control”, “mitochondrial quality”, “mitochondrial dynamics”, “mitophagy”, “mitochondrial biogenesis”, “mitochondria”, “ischemic cardiomyopathy”, “myocardial ischemia”, “cardiac ischemia”, “cardiomyocyte hypoxia”, and “myocardial hypoxia”. Articles published between 1980 and 2022 were included with particular emphasis on those published in the past five years.
Contributors
Xing Chang, Hao Zhou, and Ruibing Li collected and analysed all literature. Xing Chang and Hao Zhou wrote and revised the original manuscript. Sam Toan and Ruibing Li contributed to discussion and revision of the manuscript. All authors read and approved the final version of the manuscript.
Declaration of interests
The authors have no conflicts of interest to declare.
Acknowledgements
This study is supported by the NSFC (NO. 82270279, 82200296, 81900252, 82000537, and 82000243) and Young Talent Support Project in the Military Field (2020-JCJQQT-032). The funders had no role in paper design, data collection, data analysis, interpretation, and writing of the paper.
Contributor Information
Ruibing Li, Email: liruibing@plagh.org.
Hao Zhou, Email: zhouhao@plagh.org.
References
- 1.Hearse DJ. Myocardial ischaemia: can we agree on a definition for the 21st century? Cardiovasc Res. 1994;28(12):1737–1744. doi: 10.1093/cvr/28.12.1737. discussion 45-6. [DOI] [PubMed] [Google Scholar]
- 2.Cabac-Pogorevici I, Muk B, Rustamova Y, et al. Ischaemic cardiomyopathy. Pathophysiological insights, diagnostic management and the roles of revascularisation and device treatment. Gaps and dilemmas in the era of advanced technology. Eur J Heart Fail. 2020;22(5):789–799. doi: 10.1002/ejhf.1747. [DOI] [PubMed] [Google Scholar]
- 3.Kim H, Scimia MC, Wilkinson D, et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell. 2011;44(4):532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabinovich-Nikitin I, Rasouli M, Reitz CJ, et al. Mitochondrial autophagy and cell survival is regulated by the circadian clock gene in cardiac myocytes during ischemic stress. Autophagy. 2021;17(11):3794–3812. doi: 10.1080/15548627.2021.1938913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahuja P, Zhao P, Angelis E, et al. Myc controls transcriptional regulation of cardiac metabolism and mitochondrial biogenesis in response to pathological stress in mice. J Clin Invest. 2010;120(5):1494–1505. doi: 10.1172/JCI38331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ong SB, Subrayan S, Lim SY, et al. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno M, Kuno A, Yano T, et al. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6(12):e13741. doi: 10.14814/phy2.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, Hu S, Jin Q, et al. Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding M, Dong Q, Liu Z, et al. Inhibition of dynamin-related protein 1 protects against myocardial ischemia-reperfusion injury in diabetic mice. Cardiovasc Diabetol. 2017;16(1):19. doi: 10.1186/s12933-017-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu J, Maimaitili Y, Xie P, et al. High glucose concentration abrogates sevoflurane post-conditioning cardioprotection by advancing mitochondrial fission but dynamin-related protein 1 inhibitor restores these effects. Acta Physiol. 2017;220(1):83–98. doi: 10.1111/apha.12812. [DOI] [PubMed] [Google Scholar]
- 11.Maneechote C, Palee S, Kerdphoo S, et al. Differential temporal inhibition of mitochondrial fission by Mdivi-1 exerts effective cardioprotection in cardiac ischemia/reperfusion injury. Clin Sci. 2018;132(15):1669–1683. doi: 10.1042/CS20180510. [DOI] [PubMed] [Google Scholar]
- 12.Wu D, Dasgupta A, Chen KH, et al. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. Faseb J. 2020;34(1):1447–1464. doi: 10.1096/fj.201901467R. [DOI] [PubMed] [Google Scholar]
- 13.Bertholet AM, Delerue T, Millet AM, et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol Dis. 2016;90:3–19. doi: 10.1016/j.nbd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Papanicolaou KN, Ngoh GA, Dabkowski ER, et al. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. Am J Physiol Heart Circ Physiol. 2012;302(1):H167–H179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Sparks M, Bhandari P, et al. Mitochondrial genome linearization is a causative factor for cardiomyopathy in mice and drosophila. Antioxid Redox Signal. 2014;21(14):1949–1959. doi: 10.1089/ars.2013.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen T, Zheng M, Cao C, et al. Mitofusin-2 is a major determinant of oxidative stress-mediated heart muscle cell apoptosis. J Biol Chem. 2007;282(32):23354–23361. doi: 10.1074/jbc.M702657200. [DOI] [PubMed] [Google Scholar]
- 17.Song M, Mihara K, Chen Y, et al. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21(2):273–286. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maneechote C, Palee S, Kerdphoo S, et al. Balancing mitochondrial dynamics via increasing mitochondrial fusion attenuates infarct size and left ventricular dysfunction in rats with cardiac ischemia/reperfusion injury. Clin Sci. 2019;133(3):497–513. doi: 10.1042/CS20190014. [DOI] [PubMed] [Google Scholar]
- 19.Hu L, Ding M, Tang D, et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9(13):3687–3706. doi: 10.7150/thno.33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou H, Ren J, Toan S, et al. Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev. 2021;66 doi: 10.1016/j.arr.2020.101250. [DOI] [PubMed] [Google Scholar]
- 21.Lampert MA, Orogo AM, Najor RH, et al. BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy. 2019;15(7):1182–1198. doi: 10.1080/15548627.2019.1580095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narendra D, Tanaka A, Suen DF, et al. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5(5):706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- 23.Sun T, Ding W, Xu T, et al. Parkin regulates programmed necrosis and myocardial ischemia/reperfusion injury by targeting cyclophilin-D. Antioxid Redox Signal. 2019;31(16):1177–1193. doi: 10.1089/ars.2019.7734. [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Lu Q, Wang Q, et al. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation. 2017;136(23):2248–2266. doi: 10.1161/CIRCULATIONAHA.117.030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H, Zhu P, Wang J, et al. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25(6):1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savi M, Bocchi L, Mena P, et al. In vivo administration of urolithin A and B prevents the occurrence of cardiac dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2017;16(1):80. doi: 10.1186/s12933-017-0561-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreux PA, Blanco-Bose W, Ryu D, et al. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. 2019;1(6):595–603. doi: 10.1038/s42255-019-0073-4. [DOI] [PubMed] [Google Scholar]
- 28.Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–4899. doi: 10.1111/jcmm.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Toan S, Mui D, et al. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol. 2021;231(3):e13590. doi: 10.1111/apha.13590. [DOI] [PubMed] [Google Scholar]
- 30.Ploumi C, Daskalaki I, Tavernarakis N. Mitochondrial biogenesis and clearance: a balancing act. Febs J. 2017;284(2):183–195. doi: 10.1111/febs.13820. [DOI] [PubMed] [Google Scholar]
- 31.Gugneja S, Scarpulla RC. Serine phosphorylation within a concise amino-terminal domain in nuclear respiratory factor 1 enhances DNA binding. J Biol Chem. 1997;272(30):18732–18739. doi: 10.1074/jbc.272.30.18732. [DOI] [PubMed] [Google Scholar]
- 32.Riehle C, Abel ED. PGC-1 proteins and heart failure. Trends Cardiovasc Med. 2012;22(4):98–105. doi: 10.1016/j.tcm.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Liu Y, Dorn GW., 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109(12):1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu W, Ziemann M, Huynh K, et al. Activation of Hippo signaling pathway mediates mitochondria dysfunction and dilated cardiomyopathy in mice. Theranostics. 2021;11(18):8993–9008. doi: 10.7150/thno.62302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Lu M, Xiong L, et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020;11(1):29. doi: 10.1038/s41419-019-2218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J, Zhang Z, Li S, et al. Mdivi-1 alleviates cardiac fibrosis post myocardial infarction at infarcted border zone, possibly via inhibition of Drp1-activated mitochondrial fission and oxidative stress. Arch Biochem Biophys. 2022;718 doi: 10.1016/j.abb.2022.109147. [DOI] [PubMed] [Google Scholar]
- 37.An D, Zeng Q, Zhang P, et al. Alpha-ketoglutarate ameliorates pressure overload-induced chronic cardiac dysfunction in mice. Redox Biol. 2021;46 doi: 10.1016/j.redox.2021.102088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Tao H, Cao W, et al. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct Target Ther. 2021;6(1):54. doi: 10.1038/s41392-020-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia D, Hou L, Lv Y, et al. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J Cell Physiol. 2019;234(12):23705–23718. doi: 10.1002/jcp.28939. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z, Wang Z, Guan Q, et al. PEDF inhibits the activation of NLRP3 inflammasome in hypoxia cardiomyocytes through PEDF receptor/phospholipase A2. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrester SJ, Preston KJ, Cooper HA, et al. Mitochondrial fission mediates endothelial inflammation. Hypertension. 2020;76(1):267–276. doi: 10.1161/HYPERTENSIONAHA.120.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesharwani R, Sarmah D, Kaur H, et al. Interplay between mitophagy and inflammasomes in neurological disorders. ACS Chem Neurosci. 2019;10(5):2195–2208. doi: 10.1021/acschemneuro.9b00117. [DOI] [PubMed] [Google Scholar]
- 43.Rahim I, Sayed RK, Fernández-Ortiz M, et al. Melatonin alleviates sepsis-induced heart injury through activating the Nrf2 pathway and inhibiting the NLRP3 inflammasome. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(2):261–277. doi: 10.1007/s00210-020-01972-5. [DOI] [PubMed] [Google Scholar]
- 44.Yao J, Li Y, Jin Y, et al. Synergistic cardioptotection by tilianin and syringin in diabetic cardiomyopathy involves interaction of TLR4/NF-κB/NLRP3 and PGC1a/SIRT3 pathways. Int Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107728. [DOI] [PubMed] [Google Scholar]
- 45.Bradshaw E, Yoshida M, Ling F. Mitochondrial fission proteins Fis1 and Mdv1, but not Dnm1, play a role in maintenance of heteroplasmy in budding yeast. FEBS Lett. 2012;586(8):1245–1251. doi: 10.1016/j.febslet.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 46.Qin F, Siwik DA, Lancel S, et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc. 2013;2(4) doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Wang Y, Xu J, et al. Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/mitophagy and activating the AMPK-OPA1 signaling pathways. J Pineal Res. 2019;66(2):e12542. doi: 10.1111/jpi.12542. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Tan Y, Du W, et al. Phosphoglycerate mutase 5 exacerbates cardiac ischemia-reperfusion injury through disrupting mitochondrial quality control. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong Y, Undyala VVR, Przyklenk K. Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res Cardiol. 2016;111(5):59. doi: 10.1007/s00395-016-0578-x. [DOI] [PubMed] [Google Scholar]
- 50.Saito T, Nah J, Oka SI, et al. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J Clin Invest. 2019;129(2):802–819. doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan VP, Smith JM, Tu M, et al. Dissociation of mitochondrial HK-II elicits mitophagy and confers cardioprotection against ischemia. Cell Death Dis. 2019;10(10):730. doi: 10.1038/s41419-019-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H, Zhu P, Guo J, et al. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu H, Liu X, Zhou J, et al. Mitochondrial DNA is a vital driving force in ischemia-reperfusion injury in cardiovascular diseases. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/6235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura A, Shimauchi T, Tanaka T, et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission-associated myocardial senescence. Sci Signal. 2018;11(556) doi: 10.1126/scisignal.aat5185. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Zhuang J, Wang Y, et al. Propofol ameliorates H9c2 cells apoptosis induced by oxygen glucose deprivation and reperfusion injury via inhibiting high levels of mitochondrial fusion and fission. Front Pharmacol. 2019;10:61. doi: 10.3389/fphar.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, et al. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J Endocrinol. 2018;236(2):69–84. doi: 10.1530/JOE-17-0457. [DOI] [PubMed] [Google Scholar]
- 57.Gao D, Zhang L, Dhillon R, et al. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One. 2013;8(4):e60967. doi: 10.1371/journal.pone.0060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun X, Yang Y, Xie Y, et al. Protective role of STVNa in myocardial ischemia reperfusion injury by inhibiting mitochondrial fission. Oncotarget. 2018;9(2):1898–1905. doi: 10.18632/oncotarget.22969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Disatnik MH, Ferreira JC, Campos JC, et al. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2(5) doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oi M, Donner D, Peart J, et al. Pravastatin improves risk factors but not ischaemic tolerance in obese rats. Eur J Pharmacol. 2018;826:148–157. doi: 10.1016/j.ejphar.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 61.Pirzeh L, Babapour V, Badalzadeh R, et al. Pretreatment with vildagliptin boosts ischemic-postconditioning effects on cardioprotection and expression profile of genes regulating autophagy and mitochondrial fission/fusion in diabetic heart with reperfusion injury. Naunyn Schmiedebergs Arch Pharmacol. 2019;392(11):1371–1382. doi: 10.1007/s00210-019-01660-z. [DOI] [PubMed] [Google Scholar]
- 62.Ferreira JCB, Campos JC, Qvit N, et al. A selective inhibitor of mitofusin 1-βIIPKC association improves heart failure outcome in rats. Nat Commun. 2019;10(1):329. doi: 10.1038/s41467-018-08276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou H, Zhang Y, Hu S, et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. 2017;63(1) doi: 10.1111/jpi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andres AM, Hernandez G, Lee P, et al. Mitophagy is required for acute cardioprotection by simvastatin. Antioxid Redox Signal. 2014;21(14):1960–1973. doi: 10.1089/ars.2013.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiao H, Ren H, Du H, et al. Liraglutide repairs the infarcted heart: the role of the SIRT1/Parkin/mitophagy pathway. Mol Med Rep. 2018;17(3):3722–3734. doi: 10.3892/mmr.2018.8371. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Yu P, Zhang J, Yu S, et al. Protective effect of sevoflurane postconditioning against cardiac ischemia/reperfusion injury via ameliorating mitochondrial impairment, oxidative stress and rescuing autophagic clearance. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thompson JW, Wei J, Appau K, et al. Bnip3 binds and activates p300: possible role in cardiac transcription and myocyte morphology. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thai PN, Miller CV, King MT, et al. Ketone ester D-β-hydroxybutyrate-(R)-1,3 butanediol prevents decline in cardiac function in type 2 diabetic mice. J Am Heart Assoc. 2021;10(19) doi: 10.1161/JAHA.120.020729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Queliconi BB, Kowaltowski AJ, Gottlieb RA. Bicarbonate increases ischemia-reperfusion damage by inhibiting mitophagy. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0167678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhingra A, Jayas R, Afshar P, et al. Ellagic acid antagonizes Bnip3-mediated mitochondrial injury and necrotic cell death of cardiac myocytes. Free Radic Biol Med. 2017;112:411–422. doi: 10.1016/j.freeradbiomed.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 71.Yao L, Chen H, Wu Q, et al. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med. 2019;44(3):1048–1062. doi: 10.3892/ijmm.2019.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yurista SR, Silljé HHW, Oberdorf-Maass SU, et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. Eur J Heart Fail. 2019;21(7):862–873. doi: 10.1002/ejhf.1473. [DOI] [PubMed] [Google Scholar]
- 73.Emelyanova L, Bai X, Yan Y, et al. Biphasic effect of metformin on human cardiac energetics. Transl Res. 2021;229:5–23. doi: 10.1016/j.trsl.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma S, Feng J, Zhang R, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang M, Wang S, Cheng Z, et al. Polydatin ameliorates diabetic cardiomyopathy via Sirt3 activation. Biochem Biophys Res Commun. 2017;493(3):1280–1287. doi: 10.1016/j.bbrc.2017.09.151. [DOI] [PubMed] [Google Scholar]