Abstract
Background
Fluid and vasopressor management in septic shock remains controversial. In this randomized controlled trial, we evaluated the efficacy of dynamic measures (stroke volume change during passive leg raise) to guide resuscitation and improve patient outcome.
Research Question
Will resuscitation that is guided by dynamic assessments of fluid responsiveness in patients with septic shock improve patient outcomes?
Study Design and Methods
We conducted a prospective, multicenter, randomized clinical trial at 13 hospitals in the United States and United Kingdom. Patients presented to EDs with sepsis that was associated hypotension and anticipated ICU admission. Intervention arm patients were assessed for fluid responsiveness before clinically driven fluid bolus or increase in vasopressors occurred. The protocol included reassessment and therapy as indicated by the passive leg raise result. The control arm received usual care. The primary clinical outcome was positive fluid balance at 72 hours or ICU discharge, whichever occurred first.
Results
In modified intent-to-treat analysis that included 83 intervention and 41 usual care eligible patients, fluid balance at 72 hours or ICU discharge was significantly lower (−1.37 L favoring the intervention arm; 0.65 ± 2.85 L intervention arm vs 2.02 ± 3.44 L usual care arm; P = .021. Fewer patients required renal replacement therapy (5.1% vs 17.5%; P = .04) or mechanical ventilation (17.7% vs 34.1%; P = .04) in the intervention arm compared with usual care. In the all-randomized intent-to-treat population (102 intervention, 48 usual care), there were no significant differences in safety signals.
Interpretation
Physiologically informed fluid and vasopressor resuscitation with the use of the passive leg raise-induced stroke volume change to guide management of septic shock is safe and demonstrated lower net fluid balance and reductions in the risk of renal and respiratory failure. Dynamic assessments to guide fluid administration may improve outcomes for patients with septic shock compared with usual care.
Clinical Trial Registration
Key Words: dynamic fluid response measure, hemodynamics, resuscitation, sepsis, shock
Abbreviations: CO, cardiac output; FR, fluid responsiveness; FRESH, fluid responsiveness evaluation in sepsis-associated hypotension; ITT, intent to treat; MACE, major adverse cardiac event; mITT, modified intent to treat; PLR, passive leg raise; qSOFA, quick sepsis-related organ failure assessment; RRT, renal replacement therapy; SIRS, systemic inflammatory response syndrome; SV, stroke volume
FOR EDITORIAL COMMENT, SEE PAGE 1319
Fluid resuscitation is a central component of septic shock treatment.1,2 Excessive fluid administration causes hypervolemia and is associated with tissue edema, organ dysfunction, increased ICU length of stay, prolonged ventilator dependence,3, 4, 5 and higher mortality rates.6, 7, 8, 9, 10 Guidelines recommend crystalloid fluid administration of at least 30 mL/kg for sepsis-induced tissue hypoperfusion or septic shock and suggest that “fluid administration beyond initial resuscitation requires careful assessment of the likelihood that the patient remains fluid responsive.”11 Robust evidence that supports this recommendation is lacking. Two-thirds of patients with septic shock demonstrate fluid overload on day 1, and fluid resuscitation often continues beyond the first day of care.9, 10, 11, 12, 13, 14, 15, 16 However, only one-half of septic patients will be fluid responsive and potentially benefit from fluid administration.17, 18, 19, 20 A propensity model analysis of 23,513 patients with sepsis demonstrated that day 1 fluid administration >5 L was associated with significantly increased risk of death.8
Take-home Points.
Study Question
Will resuscitation guided by dynamic assessments of fluid responsiveness in patients with septic shock improve patient outcomes?
Results
In this multicenter randomized controlled trial of 124 patients with septic shock, treatment that was guided by a dynamic assessment of fluid responsiveness (passive leg raise) compared with usual care resulted in a decreased fluid balance (0.65 L vs 2.02 L). Fewer patients required renal replacement therapy (5.1% vs 17.5%) or mechanical ventilation (17.7% vs 34.1%), and patients were more likely to be discharged home alive (63.9% compared with 43.9%).
Interpretation
Personalized, dynamic fluid responsiveness monitoring enhances appropriate resuscitation fluid and vasopressors administration and improves patient outcomes.
Traditional methods of the assessment of fluid responsiveness (FR) such as vital signs, physical examination,21 and static measurements of circulatory pressure do not reliably correlate with FR.16 In contrast, dynamic measurement of stroke volume (SV) after an IV fluid bolus or passive leg raise (PLR) is a safe and feasible method of rapidly assessing the effectiveness of fluid-induced augmentation of SV and cardiac output (CO).18,20,22, 23, 24 This approach has been associated with reduced length of stay, fewer postoperative complications, and earlier return to regular diet in surgical patients.25
A retrospective study of real-time, noninvasive bioreactance SV and cardiac performance measurements incorporated PLR to assess FR and guide resuscitation in patients with septic shock in an ICU.23 Bioreactance analyzes the relative phase shift of an oscillating current passing through the thoracic cavity.26, 27, 28, 29, 30 Dynamic SV-guided resuscitation was associated with decreased net fluid balance, reduced ICU length of stay, risk of mechanical ventilation, time on vasopressors, and risk of renal replacement therapy (RRT) initiation.23
Given the uncertainty surrounding the benefits of dynamic measure-guided fluid resuscitation, we designed the Fluid Responsiveness Evaluation in Sepsis-associated Hypotension (FRESH) Trial (NCT02837731). The primary objective was to determine if SV-guided dynamic assessment could guide the amount of IV fluid administered to patients with septic shock.
Methods
Study Design
We conducted a randomized unblinded clinical trial among adults with sepsis-associated hypotension to compare PLR-guided SV responsiveness as a guide for fluid management (intervention) vs usual care at 13 hospitals in the United States and the United Kingdom. A full description of how patients were assigned randomly is provided in the supplementary material (e-Appendix 1). We screened patients presenting to the ED with sepsis or septic shock (defined as ≥2 systemic inflammatory response syndrome (SIRS) criteria and a suspected or documented infection) and anticipated ICU admission. Other inclusion criteria included refractory hypotension, (mean arterial pressure ≤ 65 mm Hg after receiving ≥1 L and <3 L of fluid) and enrollment within 24 hours of hospital arrival (e-Appendix 2). Major exclusion criteria included infusion of >3 L of IV fluid before random assignment, active “do not resuscitate” order, hemodynamic instability due to active hemorrhage, transferred from another hospital, acute cerebral vascular event, acute coronary syndrome, acute pulmonary edema, status asthmaticus, major cardiac arrhythmia, drug overdose, injury from burn or trauma, status epilepticus, indication for immediate surgery, inability or contraindication to PLR, pregnancy, or being incarcerated. Patient race and ethnicity were included as demographic variables per standard study design and were determined for each patient by medical chart review. Randomization was in a 2:1 allocation of SV-guided to usual care.
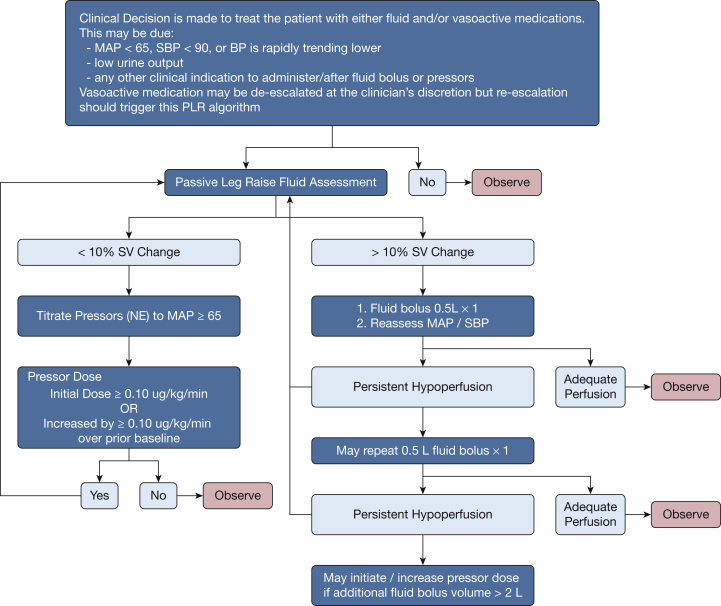
After enrollment and initial treatment in the ED, care was transferred to the ICU team per usual institutional practice. The remainder of sepsis care, including source control and antibiotic selection, was at the discretion of the treating clinicians. In the intervention arm, PLRs were performed before any treatment of hypoperfusion with either fluid bolus or vasopressors for the first 72 hours of ICU admission. SV-guided fluid and vasopressor management was used continuously during the intervention period (72 hours or ICU discharge, whichever occurred first). An increase in SV ≥10% was considered FR. If the patient demonstrated FR, protocol prompts were provided to administer a crystalloid fluid bolus (500 mL) for persistent hypotension, with repeat PLRs after every fluid bolus. If the patient was not FR, the initiation or up-titration of vasopressors was prompted with repeat PLRs after significant escalation (an increase of 1 μg/kg/min norepinephrine). In this manner, the protocol allowed for the physiologic titration of both fluid and vasopressors to treat hypoperfusion (Fig 1). Details of fluid volume collection and assessment are detailed in e-Appendix 3. This study was conducted in accordance with the amended Declaration of Helsinki. The study protocol was approved by site-specific institutional review boards. All patients or their surrogates provided written informed consent before enrollment and random assignment.
Figure 1.
Flow chart model of the algorithm used to guide treatment in the Fluid Responsiveness Evaluation in Sepsis-associated Hypotension study. MAP = mean arterial pressure; NE = norepinephrine; PLR = passive leg raise; SBP = systolic BP; SV = stroke volume.
Study End points
The primary end point was the difference in positive fluid balance at 72 hours or ICU discharge, whichever occurred first. Additional predefined secondary end points were a new requirement for RRT, a new requirement for mechanical ventilation, length of ICU stay, hours of ventilator use over a 30-day period, hours with vasopressor use, and change from baseline serum creatinine. Additional exploratory secondary end points included the incidence of adverse events, number of ICU readmissions, mortality rate, volume of treatment fluid, incidence of major cardiovascular end points (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke termed 3P- major adverse cardiac event [MACE]), discharge location, and mean difference in total fluid balance (including preenrollment) at 72 hours or ICU discharge.
Statistical Design
Sample size calculations are detailed in supplemental material, and statistical analyses were performed according to a prespecified Statistical Analysis Plan filed with an independent statistician (e-Appendix 4). The intent-to-treat (ITT) population included all patients who signed a consent form, who met study eligibility criteria, and who were assigned randomly. The modified intent-to-treat (mITT) population was predefined to include all patients who signed consent, met study eligibility criteria, who were assigned randomly, and who received monitoring for 72 hours or ICU discharge if earlier. After 90 evaluable patients had been enrolled, a predefined interim analysis was conducted by the independent statistician. A sample size reestimation was performed on the mITT population to determine promise for superiority in the key secondary end point with the option to increase the sample size of the trial to the maximum of 210 patients. The primary end point was not tested at interim analysis and was tested only at the planned final sample size of 120 patients. Under an assumption of an average treatment effect of −2 L with a SD of 3 L, the sample size of 120 evaluable subjects provided 92.7% power in a test of superiority of means for the primary effectiveness end point at a two-sided .05 level of significance. In the event of a sample size reestimation due to the key secondary end point, the primary end point was planned to be tested both at 120 patients and again at the final sample size. No alpha-adjustment was needed across this potential multiple testing, according to the methods of Mehta and Pocock.31 Multiple imputation was performed for missing variables as prespecified in the Statistical Analysis Plan. Multiple imputation for missing fluid balance at 72 hours or ICU discharge was conducted with the use of fully conditional specification with linear regression. The imputation model that was adjusted for baseline demographic variables included treatment group, age, sex, ethnicity, race, number of SIRS criteria exhibited, height, weight, and quick sepsis-related organ failure assessment (qSOFA). All efficacy analyses were performed on the mITT population, and safety analyses were performed on the ITT population. To minimize multiplicity risk, the predefined secondary end points were tested hierarchically in order on the mITT with each subsequent end points being tested only if the former demonstrated significance at a two-sided probability value of <.05. Analyses were performed using SAS software (version 9.4; Boston Biomedical Associates, LLC, Boston MA).
This study was conducted in accordance with the amended Declaration of Helsinki. All patients or their surrogates provided written informed consent before enrollment and random assignment (e-Appendix 5). The study protocol was approved by the site-specific institutional review boards (e-Appendix 6). All versions of the study protocol, statistics plan, and a summary of changes are detailed (e-Appendix 7).
Results
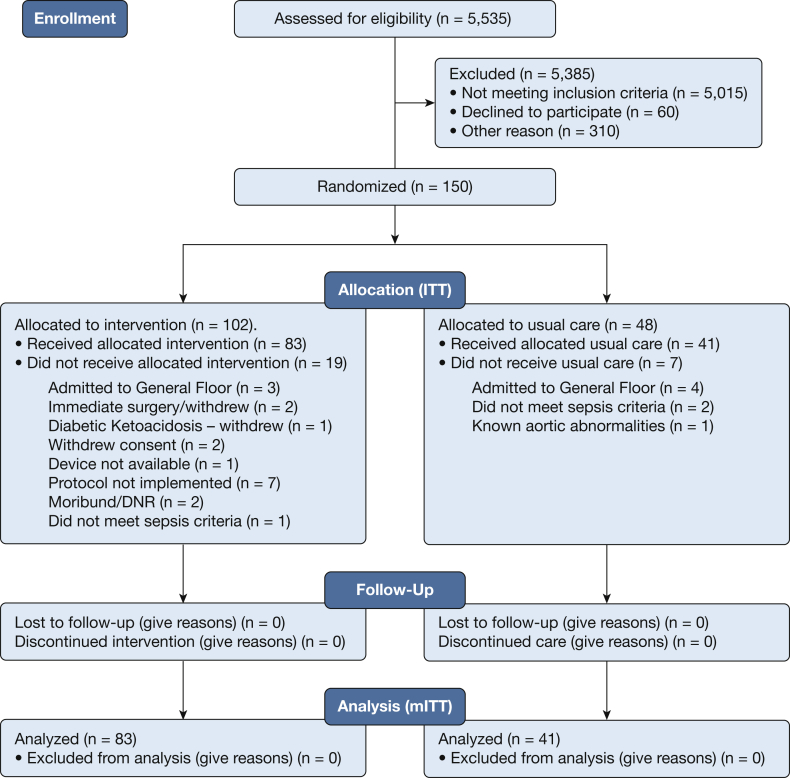
From October 2016 to February 2019, we enrolled and randomly assigned 150 patients in the study across 13 sites (Fig 2). No participants were lost to follow up. Based on the prespecified conditional power analysis on the mITT population that had been performed by an independent statistician at the 90 patient interim analysis, approval was granted to continue enrollment and to increase the sample size to a maximum of 210 patients. However, at the planned primary end point analysis at 120 patients, the primary end point had crossed the threshold for statistical significance, and enrollment was closed. An assessment of the equality of variances was performed before the statistical analysis and was found to be insignificant. A Student t-test assuming equal variance was used for the primary end point analysis.
Figure 2.
CONSORT patient flow chart diagram that tracks study participation and the number of patients whose condition was assessed for eligibility but could not be included in the study. DNR = do not resuscitate; ITT = intent to treat; mITT = modified intent to treat.
Primary End Point
One hundred twenty-four patients met the prespecified criteria for the mITT population. The mean patient age was 62.1 years (61.8 years in the intervention arm and 62.7 years in the usual care arm). Mean qSOFA score (intervention 1.9 ± 0.7 vs usual care 2.1 ± 0.7), number of SIRS criteria present on admission (intervention 2.7 ± 0.7 vs usual care 2.8 ± 0.8), and baseline comorbid medical conditions were similar between the two arms. There were relatively more women in the intervention arm than in the usual care arm (61.4% vs 31.7%). Race and ethnicity were balanced evenly between the two study arms (Table 1; e-Tables 1 and 2). Both arms received a similar volume of resuscitation fluid before enrollment (intervention arm 2.4 ± 0.6 L compared with usual care arm 2.2 ± 0.7 L) (Table 2). Positive fluid balance at 72 hours or ICU discharge was significantly less in the intervention arm (-1.37 L that favored the intervention arm, 0.65 ± 2.85 L [median, 0.53 L] vs the usual care arm, 2.02 ± 3.44 L [median, 1.22 L; P = .02) (Table 3).
Table 1.
Study Demographics
| Variablea | Modified Intent-to-Treat Population |
|
|---|---|---|
| Intervention (N = 83) | Usual care (N = 41) | |
| Age, y | ||
| Mean ± SD (No.) | 61.8 ± 16.9 (83) | 62.7 ± 15.0 (41) |
| Median (Q1, Q3) | 65.0 (48.0, 75.0) | 63.0 (55.0, 74.0) |
| Sex,b % (n/N) | ||
| Female | 61.4 (51/83) | 31.7 (13/41) |
| Male | 38.6 (32/83) | 68.3 (28/41) |
| Ethnicity, % (n/N) | ||
| Not Hispanic or Latino | 80.7 (67/83) | 85.4 (35/41) |
| Hispanic or Latino | 19.3 (16/83) | 12.2 (5/41) |
| Unknown | 0 | 2.4 (1/41) |
| Race, % (n/N) | ||
| White | 73.5 (61/83) | 75.6 (31/41) |
| Black | 20.5 (17/83) | 22.0 (9/41) |
| Asian | 3.6 (3/83) | 2.4 (1/41) |
| Native Hawaiian or Other Pacific Islander | 1.2 (1/83) | 0 |
| American Indian or Alaska Native | 0 | 0 |
| Other | 1.2 (1/83) | 0 |
| Unknown | 0 | 0 |
| Known or presumed infection, % (n/N) | 100.0 (83/83) | 100.0 (41/41) |
| Systemic inflammatory response syndrome criteria exhibitedc | ||
| Mean ± SD (No.) | 2.7 ± 0.7 (83) | 2.8 ± 0.8 (41) |
| Median (Q1, Q3) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) |
| Height, cm | ||
| Mean ± SD (No.) | 165.3 ± 10.1 (83) | 168.7 ± 11.7 (39) |
| Median (Q1, Q3) | 165.0 (158.8, 172.0) | 171.4 (163.8, 175.3) |
| Weight, kg | ||
| Mean ± SD (No.) | 73.7 ± 18.7 (83) | 73.6 ± 18.5 (41) |
| Median (Q1, Q3) | 73.1 (60.0, 85.0) | 70.2 (63.5, 81.7) |
| BMI, kg/m2 | ||
| Mean ± SD (No.) | 26.6 ± 5.7 (83) | 25.3 ± 6.0 (39) |
| Median (Q1, Q3) | 25.8 (22.4, 30.1) | 23.3 (22.0, 28.7) |
| Quick sepsis-related organ failure assessment | ||
| Mean ± SD (No.) | 1.9 ± 0.7 (82) | 2.1 ± 0.7 (40) |
| Median (Q1, Q3) | 2.0 (1.0, 2.0) | 2.0 (2.0, 3.0) |
| Sepsis diagnosis | ||
| Bacterial | 75.9 (63/83) | 80.5 (33/41) |
| Viral | 7.2 (6/83) | 4.9 (2/41) |
| Fungal | 1.2 (1/83) | 2.4 (1/41) |
| Other | 15.7 (13/83) | 12.2 (5/41) |
| Unknown | 0 | 0 |
| Baseline serum lactate | ||
| Mean ± SD (No.) | 3.6 ± 3.2 (66) | 3.8 ± 3.6 (33) |
| Median (Q1, Q3) | 2.5 (1.6, 3.8) | 2.0 (1.5, 5.7) |
| Baseline plasma lactate | ||
| Mean ± SD (No.) | 3.7 ± 3.2 (16) | 3.7 ± 3.3 (7) |
| Median (Q1, Q3) | 2.4 (1.7, 4.7) | 2.0 (1.4, 5.7) |
Q = quartile.
Subject demographics and baseline characteristics are summarized for all patients in the intent-to-treat group with available data, excluding 4 subjects with randomization error.
P = .001 for the intent-to-treat patients; there were no other statistically significant (P < .05) differences between study groups.
Subjects may meet >1 criteria.
Table 2.
Procedural Details
| Event | Modified Intent-to-Treat Population |
|
|---|---|---|
| Intervention (N = 83) | Usual care (N = 41) | |
| Time from hospital arrival to enrollment, h | ||
| Mean ± SD (No.) | 5.2 ± 4.2 (83) | 4.4 ± 2.8 (41) |
| Median (Q1,Q3) | 3.6 (2.8, 5.9) | 3.3 (2.5, 5.5) |
| Fluid: hospital arrival to enrollment, L | ||
| Mean ± SD (No.) | 2.4 ± 0.6 (83) | 2.2 ± 0.7 (41) |
| Median (Q1,Q3) | 2.5 (2.0, 2.8) | 2.2 (1.5, 2.5) |
| Time from hospital arrival to Starling monitor application, h | ||
| Mean ± SD (No.) | 6.8 ± 4.5 (83) | … |
| Median (Q1,Q3) | 5.6 (4.2, 7.7) | … |
| Total fluid assessments, No. | ||
| Mean ± SD (No.) | 6.3 ± 4.0 (83) | … |
| Median (Q1,Q3) | 5.0 (3.0, 8.0) | … |
| Fluid responsive PLR, % (n/N) | ||
| PLRs for treatment, No. | 382 | … |
| Within first 24 h | 67.3 (257/382) | … |
| Positive PLRs within first 24 h | 41.6 (107/257) | … |
| 24 to 48 h, % (n/N) | 24.1 (92/382) | … |
| Positive PLRs within 24 to 48 h | 43.5 (40/92) | … |
| 48 to 72 h | 6.5 (25/382) | … |
| Positive PLRs within 48 to 72 h | 60.0 (15/25) | … |
| PLRs for observation only, No. | 141 | … |
| Within first 24 h | 26.2 (37/141) | … |
| Positive PLRs within first 24 h | 37.8 (14/37) | … |
| 24 to 48 h | 39.0 (55/141) | … |
| Positive PLRs within 24 to 48 h | 45.5 (25/55) | … |
| 48 to 72 h | 27.7 (39/141) | … |
| Positive PLRs within 48 to 72 h | 33.3 (13/39) | … |
| Patients with a fluid status change during monitoring period | 69.9 (58/83) | … |
| Patients with positive first PLR assessment | 42.2 (35/83) | … |
| Patients fluid responsive for at least one PLR | 81.9 (68/83) | … |
| Patients fluid responsive at every measurement | 12.0 (10/83) | … |
| Patients never demonstrated fluid responsiveness | 18.1 (15/83) | … |
PLR = passive leg raise. See Table 1 legend for expansion of other abbreviation.
Table 3.
Key End points
| Parameter | Modified Intent-to-Treat Population |
|||
|---|---|---|---|---|
| Intervention (N = 83) | Usual Care (N = 41) | Treatment Difference in Mean or Percentage (95% CI) | P Valuea,b | |
| Primary efficacy end point | ||||
| Fluid balance at 72 h or ICU discharge, L | ||||
| Mean ± SD (No.) | 0.65 ± 2.85 (83) | 2.02 ± 3.44 (41) | −1.37 (−2.53 to −0.21) | .021 |
| Median (Q1, Q3) | 0.53 (−0.84, 2.53) | 1.22 (−0.03, 3.73) | … | … |
| Secondary end points for formal testing | ||||
| Requirement for renal replacement therapy,c % (n/N) | 5.1 (4/79) | 17.5 (7/40) | −12.4% (−0.27 to −0.01) | .042∗∗ |
| Requirement for ventilator use,d % (n/N) | 17.7 (14/79) | 34.1 (14/41) | −16.42% (−0.33 to 0.00) | .044∗∗ |
| Length of ICU stay,e d | ||||
| Mean ± SD (No.) | 3.31 ± 3.51 (74) | 6.22 ± 10.72 (35) | −2.91 (−6.67 to 0.85) | .113 |
| Median (Q1, Q3) | 2.09 (0.85, 3.75) | 2.90 (1.27, 3.80) | … | … |
| Ventilator use (30-day period),f h | ||||
| Mean ± SD (No.) | 46.99 ± 52.33 (14) | 119.42 ± 134.90 (14) | −72.43 (−154.08 to 9.22) | .079 |
| Median (Q1, Q3) | 20.47 (6.23, 59.04) | 75.60 (10.49, 213.63) | … | … |
| Vasopressor use,g h | ||||
| Mean ± SD (No.) | 40.74 ± 51.23 (55) | 55.64 ± 87.42 (26) | −14.91 (−52.50 to 22.68) | .426 |
| Median (Q1, Q3) | 20.98 (7.62, 45.27) | 30.85 (13.75, 47.60) | … | … |
| Changes in serum creatinine levels from baseline to 72 hh | ||||
| Mean ± SD (No.) | 0.13 ± 1.10 (79) | 0.04 ± 0.97 (34) | 0.09 (−0.34 to 0.52) | .453 |
| Median (Q1, Q3) | 0.00 (−0.19, 0.23) | −0.11 (−0.39, 0.12) | … | … |
| Exploratory end point: loop diuretic use, % (n/N) | ||||
| 0 to 24 h | 6.0 (5/83) | 9.8 (4/41) | −3.7% (−14.2% to 6.7%) | .451 |
| 24 to 48 h | 3.6 (3/83) | 12.2 (5/41) | −8.6% (−19.4% to 2.2%) | .067 |
| 48 to 72 h | 6.0 (5/83) | 7.3 (3/41) | −1.3% (−10.8% to 8.2%) | .783 |
| Discharge location, % (n/N) | ||||
| Home | 63.9 (53/83) | 43.9 (18/41) | 20.0% (1.6% to 38.3%) | .035 |
| Otheri | 36.1 (30/83) | 56.1 (23/41) | … | … |
See Table 1 legend for expansion of abbreviation.
Student t-test was used to compare the treatment groups.
Testing of the secondary end points followed a predefined hierarchic sequence. Because the the primary end point was met, secondary end points were tested in this manner. End points were tested at a two-sided alpha of .05. Formal testing under this predefined criterion is intended to account for multiple is intended to account for multiple comparisons and prevent the likelihood of a false finding. Significance is denoted with an asterisk; the successful passing of the hierarchic order denoted by a double asterisk.
Patients who entered the study on dialysis were excluded from the RRT end point, which was tested by Fisher exact test for proportions.
Patients who entered the study on ventilation were excluded from the end point, which was tested using a Chi-squared test for proportions.
Patients who died while in the ICU were censored from the analysis; the Wilcoxon Rank Sum test was used for the statistical analysis.
Patients who did not enter the study on ventilation, but required ventilator use during the study, were included in the analysis.
Patients who had vasopressors initiated throughout the trial are included in this analysis.
Change from baseline was defined as the change in serum creatinine from the earliest value collected to 72 hours after enrollment or ICU discharge; an analysis of variance that adjusted for baseline creatinine was used to compare groups.
Includes extended care facility, rehab facility, hospital, unknown, and other.
Secondary and exploratory end points were tested between the intervention and usual care arms (Table 3; e-Table 3). Fewer patients required RRT (5.1% vs 17.5%; P = .04) (Table 3), and fewer patients required mechanical ventilation in the intervention arm compared with the usual care arm (17.7% vs 34.1%; P = .04). ICU length of stay was similar in the two arms (2.9 days; intervention arm, 3.3 days [median, 2.09] vs usual care arm, 6.2 days [median, 2.90]; 95% CI, −6.7 to 0.9; P = .11). Additionally, among patients who required mechanical ventilation, there was no apparent difference in hours of ventilation (72.4 hour difference; intervention: 47.0 hours [median, 20.47 hours] vs usual care: 119.4 hours [median, 75.60 hours]; 95% CI, −154.1 to 9.2; P = .08). Notably, average hours of vasopressors were similar (intervention, 40.7 hours [median, 20.98 hours] vs usual care, 55.6 hours [median, 30.85 hours]; 95% CI, −52.5 to 22.7; P = .43) and change in serum creatinine level from baseline to 72 hours was similar (intervention, 0.13 ± 1.10 mg/dL [median, 0.00 mg/dL] vs usual care 0.04 ± 0.97 mg/dL [median, −0.11 mg/dL]). Intervention arm patients received less fluid over 72 hours. This difference in administered fluid remained significant when the preenrollment fluids were included. Intervention patients still exhibited a reduced positive fluid balance at 72 hours when the preenrollment fluids were included. Similar results in fluid balance were seen when patients on dialysis were included or excluded (e-Table 3). Hospital length of stay was comparable (1.2 day difference; intervention: 8.9 ± 8.1 days vs usual care: 10.2 ± 11.1 days; 95% CI, −4.70 to 2.25). More intervention patients were discharged home alive (63.9% compared with 43.9% usual care; 95% CI, 1.6% to 38.3%) (Table 3). There was no difference in overall 30-day mortality rate (6.3% difference intervention: 15.7% vs usual care: 22.0%; 95% CI, −21.2% to 8.6%) (e-Table 3) or 3P-MACE (6.2% difference; intervention: 6.0% vs usual care: 12.2%; 95% CI, −17.4% to 5.1%) (Figure 3, Figure 4, Figure 5, e-Table 3).
Figure 3.
Forest plots of study end points with clarification of 95% CI limits and mean difference. MACE = major adverse cardiac event; TEAE = treatment emergent adverse event.
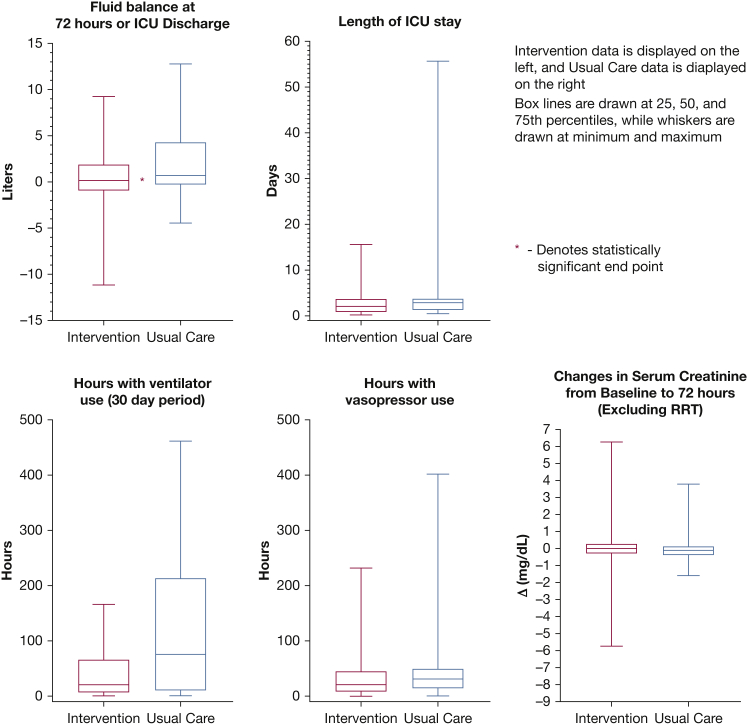
Figure 4.
Boxplots of continuous primary and secondary end points. RRT = renal replacement therapy.
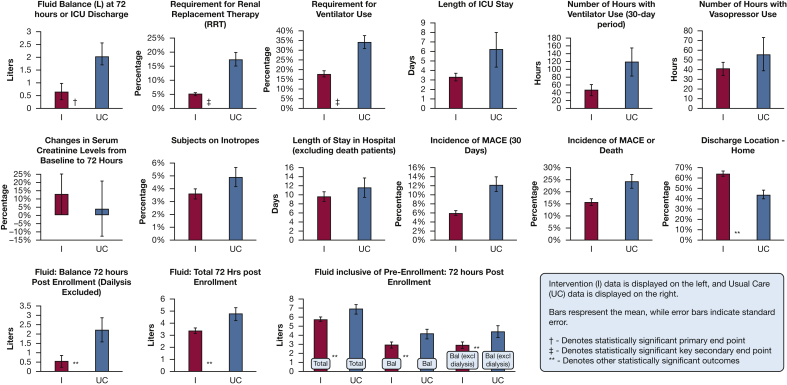
Figure 5.
Bar charts compare intervention to usual care for study end points. Bal = fluid balance; MACE = major adverse cardiac event.
Five hundred twenty-three PLRs were performed in 83 intervention patients in the first 72 hours or until ICU discharge (median, 5; interquartile range, 5 per patient) (Table 2); 35 patients (42.2%) were FR on initial PLR. Between 48 and 72 hours, 33.3% of PLRs were FR: 81.9% of the patients in the intervention arm were FR for at least one PLR; 10 patients (12.0%) were persistently FR at each measurement; and 15 patients (18.1%) never demonstrated FR (Table 2).
Safety End points
Safety end points were tested in the all randomized (ITT) population (e-Table 4). There was a similar rate of treatment emergent serious adverse events (intervention, 10 [10.2%] vs usual care, 7 [15.6%]; −5.4% difference; 95% CI, −17.5% to 6.8%). The 3P-MACE complications were similar between the intervention and usual care groups (intervention, 7.1% vs usual care, 11.1%; −4.0% difference; 95% CI, −14.5% to 6.5%) as was mortality rate (intervention, 19.6% vs usual care, 20.8%; −1.2% difference; 95% CI, −15.1% to 12.6% The rates of hospital discharge without ICU readmission were comparable (intervention, 94.9%; usual care, 97.8%).
Sensitivity Analysis
In separate sensitivity analyses, the impact of sex on the primary end point of positive fluid balance was assessed with the use of an analysis of variance model. Although the direction of effect was the same across sexes, the magnitude of treatment effect in female patients was higher. Secondary end point exploration by sex did not result in any significant differences across sexes.
A second sensitivity analysis was performed as delineated in the Statistical Analysis Plan that involved multiple imputation for missing fluid balance at 72 hours or ICU discharge with the use of a fully conditional specification with linear regression. Accounting for any fluid balance data for patients who were excluded after randomization did not have any effect on fluid balance at 72 hours or ICU discharge, (-0.48 L favoring intervention arm: intervention arem, 1.35 ± 3.76 L vs usual care arm, 1.84 ± 3.51 L; P = .4879) (e-Table 5).
Discussion
In this multicenter randomized trial, we evaluated the efficacy of dynamic measures to guide fluid and vasopressor administration in patients with sepsis-associated hypotension and shock. A strategy of PLR-guided resuscitation resulted in significantly lower net fluid balance and reduced renal and respiratory dysfunction at 72 hours. This finding supports the hypothesis that physiologically guided fluid administration in patients with sepsis-associated hypotension and shock is associated with lower fluid balance23 and improvements in vital end-organ function that are associated causally with sepsis-related death when treatment is coupled to the dynamic fluid management protocol.9,32
Neither systemic hypotension nor static endovascular pressures in the patient who is vasodilated with sepsis are reliable measures of circulatory effectiveness.16 Performing a PLR while monitoring SV response is a validated and reliable dynamic measure. PLR rapidly displaces approximately 300 cc venous blood to the chest, transiently increasing preload and identifying whether subsequent fluid administration is likely to enhance CO. If the SV increases by ≥10%, a subsequent 500-mL fluid bolus will increase CO by at least 15%.33 SV augmentation <10% is considered to be fluid nonresponsive because fluid will be very unlikely to increase CO and overall perfusion.17,20,33
We evaluated SV responses to PLR using noninvasive surface-electrode bioreactance technology. Average SV is assessed over a minute and is reliable even with strong respiratory variations and irregular heartbeat, including atrial fibrillation. The bioreactance-derived SV measurement has been validated against invasive flow-directed catheter-derived CO26,28, 29, 30 and echo-Doppler measurements.27,34
In the analysis, patients in the intervention arm who were treated with a protocol to direct FR-guided fluid or vasopressor administration had a net difference in fluid balance >72 hours of 1.37 L less than patients in the usual care arm. Notably, the lower 72-hour fluid balance in the intervention arm persisted, despite an increased use of diuretics in the patients in the usual care arm. Even mechanical fluid removal via dialysis or ultrafiltration did not remove the impact of PL-guided fluid management.
The volume of administered fluids at 72 hours was significantly less in the patients in the intervention arm than the usual care arm (e-Table 3). Separation between arms for 72-hour fluid balance and total amount of administered IV fluids were maintained even when preenrollment fluid was included in the analysis. Consistent with current guideline recommendations, patients received 2 to 3 L of IV fluids before randomization in both arms. These results indicate that PLR-guided protocol instructions during the first 72 hours of care accounted for the observed differences in fluid balance between arms.
Despite reduced fluid administration in the PLR-guided and vasopressor in the intervention arm, higher rates of new renal failure or serum creatinine elevation were not observed. There was a reduced need for RRT or invasive mechanical ventilation in the intervention arm. ICU length of stay was shorter by an average of 2.91 days in the intervention arm, although not statistically significant. Volume overload and elevated renal and central venous pressures increase renal interstitial edema, which in turn results in reduced filtration pressure, which translates into a fall in glomerular filtration.35 Similarly, excessive lung water is associated with worsening intrapulmonary shunting, progression to respiratory failure that necessitates intubation, potentially prolonged periods of mechanical ventilation, and death.36
FRESH provides a prospective validation of earlier observations that optimal real-time physiologic monitoring and individualized assessment of FR may inform treatment decisions and improve patient outcomes.17,23 In an earlier single center nonrandomized pre-post intervention assessment, a larger fluid difference (3.59 L) was reported between patients who were treated with PLR-guided strategy and usual care.23 We found a smaller difference in the mITT analysis of the FRESH study, which may be due to more restrictive fluid management in the usual care arm. However, the concordance in a lower risk for renal and respiratory failure between the two arms in the two studies suggests a consistent and strong clinical effect. Additionally, studies of dynamic measure-guided fluid administration in surgical patients have reported comparable overall fluid administration between dynamic measure guided treatment arms and usual care arms with greater preservation of renal and respiratory function in the intervention arm.25,37 These data suggest that resuscitation guided by dynamic assessments of cardiac performance is effective at the optimization of circulatory hemodynamics through the reduction of unnecessary and potentially harmful fluid and vasopressor administration.
Dynamic measure-guided fluid management in FRESH was associated with an improved likelihood of functional independence with discharge to home after hospitalization compared with discharge to a rehabilitation facility, an extended nursing care facility, or hospice facility. This may be attributable to the lower burden of organ failure in the intervention arm. Although there was a trend towards a reduction in both mortality rate and the composite MACE outcome, the study was underpowered to evaluate these outcomes definitively.
The rates of treatment-associated adverse events were similar between the arms. When combined with the unchanged serum creatinine levels and requirement for vasopressor support between study arms, physiologically guided resuscitation with the use of dynamic circulatory assessments appears to be safe.
Of note, it is interesting that a mean difference of only 1.37 L in fluid balance over 72 hours would lead to these clinical differences. The treatment effect may be more related to timing and dosing fluid to physiologic effect, rather than to fluid restriction. Because only approximately 50% of patients are FR, in usual practice we are often administering fluid when there is minimal to no perfusion benefit (increased CO). In our study, only 42% of patients were FR on presentation, but we noted that FR status was not static; patients would often have a change between FR and non-FR state during the 72 hours after enrollment. Administering fluid and increasing preload when there is no perfusion benefit (increased CO) may lead to increased renal edema and increased lung water. It is possible the treatment effect seen here resides in administering fluid when it is physiologically effective and avoiding the drug when there is no perfusion (increased CO) benefit. Further work must be done to understand the treatment effect.
This study has several notable limitations. We sought to minimize potential confounding by using a decision-support tool to guide fluid and vasopressors. Nevertheless, the inclusion of an unblinded usual care arm may have been insufficient to eliminate all sources of residual bias or potential for Hawthorne effect. Another potential limitation is the unequal sex distribution between the intervention and usual care arms, despite the 2:1 block-randomization schedule. The intervention and usual care arms were well matched on all other patient characteristics, including qSOFA and SIRS criteria. A sensitivity analysis did not support that there was significant confounding as a result of this imbalance; however, sex-differences in both sepsis disease severity and response to treatments cannot be discounted. BMI was similar between the arms and indicated that weight-guided preenrollment fluid volumes were also comparable between the arms, as confirmed by a nearly identical volume of preenrollment fluid between the two arms.
Although the study enrollment was sufficient to evaluate the prespecified primary outcome, it was not powered to detect differences in all sepsis-associated organ dysfunctions or patient deaths. Further research in larger patient populations, potentially including specific biomarker enrichment strategies, would be important to replicate the magnitude and direction of these results and to determine whether PLR-guided dynamic measure resuscitation results in improved sepsis survival.
In conclusion, physiologically informed fluid and pressor resuscitation with the use of PLR-induced SV change to guide personalized management of sepsis-associated hypotension and shock was safe. Among patients who met prespecified enrollment criteria and were treated according to protocol, dynamic measure-guided resuscitation was associated with lower net fluid balance and reductions in the risk of renal and respiratory failure. Functional evaluation for lack of FR adequately identifies a group of patients with sepsis-associated hypotension who should not have further IV fluids infused. Although PLR-guided fluid and vasopressor resuscitation did not improve survival in this study, the administration of IV fluids and vasopressors only when they were likely to improve CO did reduce 72-hour fluid balance and improve discharge to home.
Acknowledgments
Author contributions: I. S. D. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects; P. A., K. C., M. E., L. F., A. L. H., D. A. K., A. K., M. L., G. M., J. S., E. S., W. S., J. A. W., M. W. and D. M. H. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. A. S. and D. M. H. are employees of Cheetah Medical. None declared (P. M. A., K. A. C., I. S. D., M. C. E., L. G. F., A. L. H., D. A. K., A. K., M. M. L., G. S. M., E. S., W. H. S., J. A. W., M. W.).
Role of sponsors: Study sponsors were involved in study concept, design, and writing of the manuscript.
Other contributions: Boston Biomedical Associates, LLC, provided data analysis.
Additional information: The e-Appendixes and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This study was funded by Cheetah Medical.
Supplementary Data
References
- 1.Levy M.M., Evans L.E., Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000. doi: 10.1097/CCM.0000000000003119. [DOI] [PubMed] [Google Scholar]
- 2.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brotfain E., Koyfman L., Toledano R., et al. Positive fluid balance as a major predictor of clinical outcome of patients with sepsis/septic shock after ICU discharge. Am J Emerg Med. 2016;34(11):2122–2126. doi: 10.1016/j.ajem.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell K.H., Carlbom D., Caldwell E., et al. Volume overload: prevalence, risk factors, and functional outcome in survivors of septic shock. Ann Am Thorac Soc. 2015;12(12):1837–1844. doi: 10.1513/AnnalsATS.201504-187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiedemann H.P., Wheeler A.P., Bernard G.R., et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Tigabu B.M., Davari M., Kebriaeezadeh A., Mojtahedzadeh M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: a systematic review. J Crit Care. 2018;48:153–159. doi: 10.1016/j.jcrc.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald S.P.J., Taylor D.M., Keijzers G., et al. REstricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH): study protocol for a pilot randomised controlled trial. Trials. 2017;18(1):399. doi: 10.1186/s13063-017-2137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marik P.E., Linde-Zwirble W.T., Bittner E.A., Sahatjian J., Hansell D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 9.Boyd J.H., Forbes J., Nakada T.A., Walley K.R., Russell J.A. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39(2):259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 10.Vincent J.L., Sakr Y., Sprung C.L., et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 11.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 12.Kelm D.J., Perrin J.T., Cartin-Ceba R., et al. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43(1):68–73. doi: 10.1097/SHK.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sirvent J.M., Ferri C., Baro A., Murcia C., Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med. 2015;33(2):186–189. doi: 10.1016/j.ajem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Rivers E., Nguyen B., Havstad S., et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 15.Rowan K.M., Angus D.C., Bailey M., et al. Early, goal-directed therapy for septic shock - a patient-level meta-analysis. N Engl J Med. 2017;376(23):2223–2234. doi: 10.1056/NEJMoa1701380. [DOI] [PubMed] [Google Scholar]
- 16.Marik P.E., Cavallazzi R. Does the central venous pressure predict fluid responsiveness? an updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 17.Bentzer P., Griesdale D.E., Boyd J., et al. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298–1309. doi: 10.1001/jama.2016.12310. [DOI] [PubMed] [Google Scholar]
- 18.Marik P.E., Levitov A., Young A., Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364–370. doi: 10.1378/chest.12-1274. [DOI] [PubMed] [Google Scholar]
- 19.Michard F., Teboul J.L. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 20.Monnet X., Marik P., Teboul J.L. Passive leg raising for predicting fluid responsiveness: a systematic review and meta-analysis. Intensive Care Med. 2016;42(12):1935–1947. doi: 10.1007/s00134-015-4134-1. [DOI] [PubMed] [Google Scholar]
- 21.Wo C.C., Shoemaker W.C., Appel P.L., et al. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit Care Med. 1993;21(2):218–223. doi: 10.1097/00003246-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Toppen W, Aquije Montoya E, Ong S, et al. Passive leg raise: feasibility and safety of the maneuver in patients with undifferentiated shock. J Intensive Care Med. 2018;885066618820492. [DOI] [PMC free article] [PubMed]
- 23.Latham H.E., Bengtson C.D., Satterwhite L., et al. Stroke volume guided resuscitation in severe sepsis and septic shock improves outcomes. J Crit Care. 2017;42:42–46. doi: 10.1016/j.jcrc.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Krige A., Bland M., Fanshawe T. Fluid responsiveness prediction using Vigileo FloTrac measured cardiac output changes during passive leg raise test. J Intensive Care. 2016;4:63. doi: 10.1186/s40560-016-0188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calvo-Vecino J.M., Ripolles-Melchor J., Mythen M.G., et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low-moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial) Br J Anaesth. 2018;120(4):734–744. doi: 10.1016/j.bja.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Keren H., Burkhoff D., Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circ Physiol. 2007;293(1):H583–H589. doi: 10.1152/ajpheart.00195.2007. [DOI] [PubMed] [Google Scholar]
- 27.Heerdt P.M., Wagner C.L., DeMais M., Savarese J.J. Noninvasive cardiac output monitoring with bioreactance as an alternative to invasive instrumentation for preclinical drug evaluation in beagles. J Pharmacol Toxicol Methods. 2011;64(2):111–118. doi: 10.1016/j.vascn.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Rich J.D., Archer S.L., Rich S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J. 2013;42(1):125–133. doi: 10.1183/09031936.00102212. [DOI] [PubMed] [Google Scholar]
- 29.Raval N.Y., Squara P., Cleman M., et al. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008;22(2):113–119. doi: 10.1007/s10877-008-9112-5. [DOI] [PubMed] [Google Scholar]
- 30.Squara P., Denjean D., Estagnasie P., et al. Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med. 2007;33(7):1191–1194. doi: 10.1007/s00134-007-0640-0. [DOI] [PubMed] [Google Scholar]
- 31.Mehta C.R., Pocock S.J. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med. 2011;30(28):3267–3284. doi: 10.1002/sim.4102. [DOI] [PubMed] [Google Scholar]
- 32.Acheampong A., Vincent J.L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251. doi: 10.1186/s13054-015-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monnet X., Rienzo M., Osman D., et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402–1407. doi: 10.1097/01.CCM.0000215453.11735.06. [DOI] [PubMed] [Google Scholar]
- 34.Waldron N.H., Miller T.E., Thacker J.K., et al. A prospective comparison of a noninvasive cardiac output monitor versus esophageal Doppler monitor for goal-directed fluid therapy in colorectal surgery patients. Anesth Analg. 2014;118(5):966–975. doi: 10.1213/ANE.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 35.Prowle J.R., Echeverri J.E., Ligabo E.V., Ronco C., Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 36.Phillips C.R., Chesnutt M.S., Smith S.M. Extravascular lung water in sepsis-associated acute respiratory distress syndrome: indexing with predicted body weight improves correlation with severity of illness and survival. Crit Care Med. 2008;36(1):69–73. doi: 10.1097/01.CCM.0000295314.01232.BE. [DOI] [PubMed] [Google Scholar]
- 37.Pearse R.M., Harrison D.A., MacDonald N., et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311(21):2181–2190. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.