Abstract
Introduction
A substantial proportion of intensive care unit (ICU) survivors develop psychological impairments after ICU treatment, part of the postintensive care syndrome, resulting in a decreased quality of life. Recent data suggest that an ICU-specific virtual reality intervention (ICU-VR) for post-ICU patients is feasible and safe, improves satisfaction with ICU aftercare, and might improve psychological sequelae. In the present trial, we firstly aim to determine whether ICU-VR is effective in mitigating post-traumatic stress disorder (PTSD)-related symptoms and secondly to determine the optimal timing for initiation with ICU-VR.
Methods and analysis
This international, multicentre, randomised controlled trial will be conducted in 10 hospitals. Between December 2021 and April 2023, we aim to include 300 patients who have been admitted to the ICU ≥72 hours and were mechanically ventilated ≥24 hours. Patients will be followed for 12 consecutive months. Patients will be randomised in a 1:1:1 ratio to the early ICU-VR group, the late ICU-VR group, or the usual care group. All patients will receive usual care, including a mandatory ICU follow-up clinic visit 3 months after ICU discharge. Patients in the early ICU-VR group will receive ICU-VR within 2 weeks after ICU discharge. Patients in the late VR group will receive ICU-VR during the post-ICU follow-up visit. The primary objective is to assess the effect of ICU-VR on PTSD-related symptoms. Secondary objectives are to determine optimal timing for ICU-VR, to assess the effects on anxiety-related and depression-related symptoms and health-related quality of life, and to assess patient satisfaction with ICU aftercare and perspectives on ICU-VR.
Ethics and dissemination
The Medical Ethics Committee United, Nieuwegein, the Netherlands, approved this study and local approval was obtained from each participating centre (NL78555.100.21). Our findings will be disseminated by presentation of the results at (inter)national conferences and publication in scientific, peer-reviewed journals.
Trial registration number
NL9812.
Keywords: Adult intensive & critical care, INTENSIVE & CRITICAL CARE, Adult intensive & critical care
Strengths and limitations of this study.
A randomised controlled trial examining the effects of intensive care unit-specific virtual reality (ICU-VR) on psychological well-being and health-related quality of life after ICU treatment.
ICU-VR is easy applicable and safe, and enables patients to be auditorily and visually exposed to the ICU environment traumatising them while receiving treatment-related information. However, the optimal timing of ICU-VR after critical illness is unknown.
Follow-up until 12 months after ICU discharge enables us to study long-term effects.
Blinding of patients or investigators is not possible due to the nature of the intervention.
ICU-VR content is hospital specific to expose patients to the actual ICU environment, but it limits the possibility of easily implementing the intervention in other hospitals.
Introduction
Because of improved survival after intensive care unit (ICU) treatment, a new challenge arises.1 2 A substantial proportion of ICU survivors suffers from psychological impairments, such as post-traumatic stress disorder (PTSD), anxiety, and depression.3–5 Along with cognitive and physical impairments, these sequelae are referred to as the post-intensive care syndrome (PICS). PICS is common, can last for years after ICU discharge, and has a profound impact on daily functioning and quality of life.6–8
Prevention and treatment of PICS have been recognised as a fundamental part of ICU care by the critical care community and recently it was demonstrated that the psychological component of PICS is the most important determinant of a decreased health-related quality of life (HRQoL) and impede a patients’ ability to rehabilitate.9–11 Although several interventions have been explored, such as keeping ICU diaries, organising ICU follow-up clinics, and offering psychosocial support, studies on their effectiveness in terms of psychological distress or quality of life have yielded unsatisfactory and ambiguous results.10 12–17 As such, evidence-based interventions to improve psychological recovery and HRQoL are lacking.
Post-ICU psychological impairments may be caused by amnesia during the early period of critical illness in combination with sensory overload and sensory deprivation. Amnesia can lead to loss of factual recall of their ICU stay and patients can instead create delusional and frightening memories.18 Moreover, the typical ICU environment is characterised by unpatterned exposure and frequent sensory input such as light, noise and tracheal tube aspiration. The exposure to these extremes initiates the development of PTSD and anxiety.19 We hypothesised that exposure to the factual ICU environment, and additionally receiving ICU-related treatment information, could enhance ICU treatment understanding and subsequently could decrease delusional memories and psychological impairments.10 20
Virtual reality (VR) allows users to fully immerse within a computer-generated three-dimensional environment. In psychiatry, exposure therapy using VR has been proven effective for the treatment of PTSD and anxiety, and thereby it addresses limitations of imaginal exposure.21–25 Also, VR can effectively and easily be used to deliver structured and uniform information to patients. VR could, thus, be a valuable adjunct to safely inform and expose post-ICU patients to the environment traumatising them and could enhance psychological recovery.26 27 In the current study, our primary aim is to assess the effect of an ICU-specific VR intervention for post-ICU patients (ICU-VR) on PTSD-related symptoms. Second, we want to determine optimal timing for initiation with ICU-VR, to assess the effects of ICU-VR on anxiety-related and depression-related symptoms and HRQoL, and to assess patient satisfaction with ICU aftercare and their perspectives on ICU-VR.
Methods and analysis
Study design and setting
A multicentre, randomised controlled trial will be conducted in ICUs of 10 hospitals in the Netherlands (Erasmus Medical Centre (university hospital), Franciscus Gasthuis & Vlietland hospital, Maasstad hospital, Ikazia hospital, IJsseland hospital, Groene Hart hospital, Van Weel-Bethesda hospital, Haaglanden Medical Centre and the Albert Schweitzer hospital) and Belgium (Cliniques universitaires de Bruxelles—Hôpital Erasme, Bruxelles) (table 1). The Medical Ethics Committee United (MEC-U), Nieuwegein, the Netherlands, approved this study (NL78555.100.21, approved 25 October 2021), and local approval was obtained from the institutional ethic review boards of each participating hospital. Inclusion will be conducted from December 2021 to October 2022, and patients will be followed for 12 months after ICU discharge. Any modifications to the study protocol, which may affect the conduct of the study or patient safety, including changes of the study objectives, study design, study population, sample size, study procedures or significant administrative aspects, will be sent for approval to the MEC-U and the institutional ethic review boards. Health authorities will be informed in accordance with local regulations.
Table 1.
ICU-related characteristics of study site
| Study site | Type of hospital | Type of ICU | Number of ICU beds |
| Erasmus Medical Centre, Rotterdam, The Netherlands |
Academic hospital | Mixed medical, surgical and cardiac ICU | 56 |
| Franciscus Gasthuis & Vlietland, Rotterdam, The Netherlands |
Community, teaching hospital | Mixed medical and surgical ICU | 19 |
| Maasstad Hospital, Rotterdam, The Netherlands |
Community, teaching hospital | Mixed medical and surgical ICU with an burn expertise centre | 25 |
| Ikazia Hospital, Rotterdam, The Netherlands |
Community hospital | Mixed medical and surgical ICU | 12 |
| IJsselland Hospital, Capelle a/d Ijssel, The Netherlands |
Community hospital | Mixed medical and surgical ICU | 8 |
| Groene Hart Hospital, Gouda, The Netherlands |
Community, teaching hospital | Mixed medical and surgical ICU | 12 |
| Van Weel-Bethesda Hospital, Dirksland, The Netherlands |
Community hospital | Mixed medical and surgical ICU | 6 |
| Haaglanden Medical Centre, The Hague, The Netherlands |
Community, teaching hospital | Mixed medical and surgical ICU | 22 |
| Albert Schweitzer Hospital, Dordrecht, The Netherlands |
Community, teaching hospital | Mixed medical and surgical ICU | 16 |
| Cliniques universitaires de Bruxelles—Hôpital Erasme, Bruxelles, Belgium | Academic hospital | Mixed medical and surgical ICU | 36 |
ICU, intensive care unit.
Study participants
We aim to include at least 300 patients. Patients admitted to the ICU for ≥72 hours, during which mechanically ventilated ≥24 hours, older than 17 years of age, and able to understand the Dutch language are eligible for inclusion. Patients admitted to the ICU with primary neurological impairment, a life expectancy <48 hours, or receiving palliative care, with documented active, established psychiatric disorders, a decreased cognitive function during inclusion (a telephone interview for cognitive status (TICS) score ≤27), with a new or active delirium during inclusion (defined as mentioning of a delirium in the daily status report of the treating physician or new administration of haloperidol), or without a formal home address will be excluded. Because the TICS is part of the study procedures, this will be assessed after inclusion and written informed consent. Patients with a TICS score ≤27 will be excluded after inclusion.
Randomisation and masking
Patients will be randomised in a 1:1:1 ratio to either the early ICU-VR group, the late ICU-VR group, or the usual care group. Randomisation will be according to a 1:1:1 ratio, stratified for study site, using a centralised internet-based randomisation procedure (Castor EDC, Amsterdam, The Netherlands). Due to the nature of the intervention, blinding of patients is not possible. Randomisation allocation will be coded in analysis with ‘0’ and ‘1’, and the analysist will as such be unaware of the randomisation allocation.
Intervention
ICU-VR for post-ICU patients is based on an uniform script that is designed by an interdisciplinary team and based on the several focus group meetings of this team. The content of the script is extensively described elsewhere and the content is found in online supplemental data file 1.26 27 We also have written a movie directors script to produce an uniform ICU-VR film in each participating centre.26 27 The ICU-VR film was produced for each centre, that is, hospital specific, to optimise immersiveness and to deliver relevant and truthful information regarding ICU stay and ICU treatment.27 28 The point of view for the camera is the field of vision of the mock patient lying in an ICU bed. ICU-VR will be watched using head-mounted display VR (Pico G2 VR All-In-One Headset) and a headset.
bmjopen-2022-061876supp001.pdf (316.2KB, pdf)
Study procedures
An oversight of the study procedures is presented in figure 1. Patients who are eligible for inclusion will be approached by an investigator of the research team or by a dedicated research nurse within 7 days after ICU discharge. A translation of the information for patients and the informed consent form are found in online supplemental data file 2.
Figure 1.
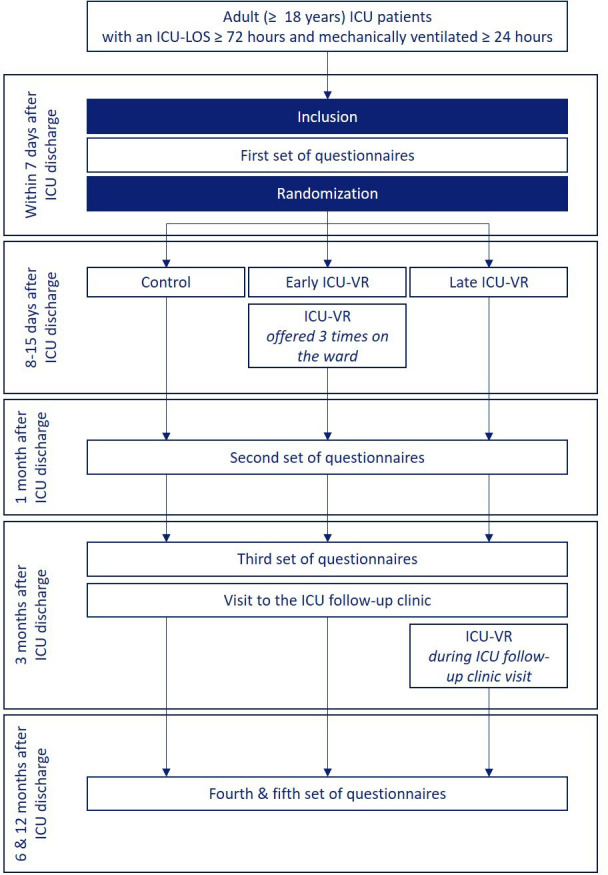
Flow diagram of the study. ICU, intensive care unit; ICU-LOS, ICU length of stay; ICU-VR, ICU-specific virtual reality.
bmjopen-2022-061876supp002.pdf (241.1KB, pdf)
After obtaining informed consent and completing the TICS assessment, patients will receive the first set of questionnaires (T0), consisting of a self-composed questionnaire regarding demographics and their history of mental health, the Impact of Event Scale-Revised (IES-R), the Hospital Anxiety and Depression Scale (HADS), the European Quality of life 5 dimensions (EQ-5D) and the short-form 36 (SF-36) (table 2). Patients are asked to fill in the HADS, EQ-5D, and SF-36 questionnaire both retrospectively and prospectively to obtain a baseline and over time measure of patient anxiety and depression levels and quality of life.
Table 2.
Questionnaires per follow-up time point
| Questionnaire | T0. inclusion | T1. 1 month after ICU discharge | T2. 3 months after ICU discharge | T3. 6 months after ICU discharge | T4. 12 months after ICU discharge |
| Demographics | X | X | X | X | X |
| Work resumption and financial decline | X | X | X | X | |
| History of mental illness | X | ||||
| IES-R (Post-Traumatic Stress Disorder) |
X | X | X | X | X |
| HADS (Anxiety and Depression) |
X (retrospectively and prospectively) | X | X | X | X |
| SF-36 Quality of Life |
X (retrospectively and prospectively) | X | X | X | X |
| EQ-5D Quality of life |
X (retrospectively and prospectively) | X | X | X | X |
| Satisfaction with ICU care | X | ||||
| Perspectives on ICU-VR | X (early ICU-VR) |
X (late ICU-VR) |
|||
| Visit to healthcare professionals | X | X | X | X |
EQ-5D, 5-Level European Quality of Life Questionnaire; HADS, Hospital Anxiety and Depression Scale; ICU, intensive care unit; ICU-VR, intensive care unit-specific virtual reality; IES-R, Impact of Event Scale-Revised; SF-36, Short-Form 36.
Patients randomised to the early ICU-VR group will receive ICU-VR between day 8 and day 15 after ICU discharge for a maximum of three times, unless the patient is discharged from the hospital ward sooner. The number of times ICU-VR is offered and accepted will be logged. Between 3 and 6 months after ICU discharge, all patients will visit the post-ICU follow-up clinic of the concurrent hospital. During this post-ICU follow-up visit, patients have a consultation with a dedicated ICU nurse and an intensivist. Patients randomised to the late ICU-VR group will receive ICU-VR once during their concurrent post-ICU follow-up clinic visit.
All patients will receive follow-up questionnaires at 1 month (T1), 3 months (T2), 6 months (T3) and 12 months (T4) after ICU discharge (table 2).
Outcomes and measurements
The primary outcome is the effect of ICU-VR on the severity of PTSD-related symptoms at 6 months after ICU discharge.
The severity of PTSD-related symptoms will be expressed as the sum score of the IES-R and an IES-R sum score ≥24 will be considered as clinically relevant PTSD.29 The IES-R comprises 22 items, assessing subjective distress caused by traumatic events, and has been used commonly in survivors of critical illness.30–32 The IES-R yields a total score (ranging from 0 to 88; higher scores indicate more severe symptoms) and subscale scores can be calculated for symptoms of intrusion, avoidance and hyper arousal.
Secondary outcomes are the effects of ICU-VR on the severity and prevalence of PTSD-related, anxiety-related and depression-related symptoms and on HRQoL throughout follow-up, the patient satisfaction with ICU aftercare, and patient perspectives on ICU-VR.
The severity of anxiety-related and depression-related symptoms will be expressed as the HADS anxiety and depression scores, and a HADS anxiety or depression score ≥8 will be considered as clinically relevant anxiety and depression, respectively. The HADS comprises of 14 items and is commonly used to determine the levels of anxiety and depression. Seven of the items relate to anxiety and seven relate to depression.33–35
HRQoL will be expressed as the overall HRQoL, which implies the time trade-of (TTO) score of the 5-level EQ-5D, and the mental HRQoL, which implies the mental component score of the SF-36. The EQ-5D measures HRQoL in five dimensions, that is, mobility, self-care, usual activities, pain/discomfort and anxiety/depression.36 By giving a certain weight to each answer option, the country-specific TTO score can be calculated, ranging from −0.446 (worst quality of life) to 1.000 (best quality of life).37 Also, patients score their subjective health state on a visual analogue scale (EQ-VAS), ranging from 0 (worst health imaginable) to 100 (best health imaginable). The SF-36 consists of 36 items, from which eight scaled scores can be calculated. These scores are the weighted sums of the questions in their section. Each scale is directly transformed to a scale ranging from 0 to 100 on the assumption that each question carries an equal weight. The eight sections are vitality, physical functioning, bodily pain, general health perception, physical role functioning, emotional role functioning, social role functioning and mental health.38 39 In addition, a mental and physical component scale, the MSC-36 and PCS-36, respectively, can be calculated as a reflection of physical and mental health.38–40
Patient satisfaction with ICU aftercare will be assessed using a novel questionnaire, based on the Patient Satisfaction Questionnaire and Family Satisfaction with ICU Care tools, altered to the needs of this study.41 42 Additional novel items were added to evaluate patient perspectives on the ICU-VR intervention.
We also explore feasibility and safety outcomes, and the cost-benefit ratio of ICU-VR. Feasibility will be expressed as the number of sessions patients in the early ICU-VR group will receive. Safety will be expressed as the number of ICU-VR sessions requiring interruption or termination due to side effects in terms of cybersickness, mainly experienced as nausea.26 27 For the cost-benefit ratio, costs will be expressed as, among others, development costs for ICU-VR, employments costs of ICU nurses offering the intervention, and the employment and organisational costs of the ICU follow-up clinic, and benefits will be expressed as the gain in quality-adjusted life years determined as the EQ-5D TTO score.
Demographics, such as age, gender, body weight, length, pre-existing comorbidities, previous ICU admissions and ICU readmissions, treatment-related characteristics, such as type of admission, ICU and hospital length of stay, mechanical ventilation-related characteristics, episodes of sedative coma and delirium during ICU treatment, assessed using the Richmond Agitation Sedation Scale and the Confusion Assessment Method for the ICU (CAM-ICU) scale, respectively, use of renal replacement therapy, infections and illness severity scores during ICU treatment, and 3-month, 6-month and 12-month mortality will be assessed using electronic patient records.43 44 Additionally, patients will be asked about their educational level, employment status prior to and after ICU treatment, financial decrease after ICU treatment, consultations with healthcare professionals, and their history of mental health in follow-up questionnaires.
Data management
Data will be uploaded, stored, and maintained using the electronic data capture (EDC) system of Castor (Castor EDC, www.castoredc.com, Amsterdam, the Netherlands). The study team will be responsible for data entry and quality control activities. Data will be checked by at least two persons from the study team and will be stored for at least 15 years on either the Castor EDC server or as a hardcopy in the ICU of the participating hospitals. Questionnaires will be sent digitally using Castor EDC or via hardcopy via postal mail whenever requested.
To maintain anonymity, data will be coded with a number and this number will be the only reference to patient identification. The principal investigator is the only one in possession of the translation key, making it impossible to link data to the patient.
Sample size calculation
Based on two previous studies yielding an ICU-VR Cohen’s d effect estimate of 0.56 (late intervention) to 0.88 (early intervention), the power calculation of the current study is based on a Cohen’s d of 0.56.26 45 We performed a G*Power analysis based on the Wilcoxon Mann-Whitney test, with no expectation about the underlying distribution of the outcome (parental distribution: ‘min ARE’). Using a two-sided alpha of 0.05, a power of 0.80 and a 1:1 allocation ratio, this resulted in a required sample size per group of 60 patients.46 We will use this required sample size for all three groups resulting in a total sample size of 180 patients. We anticipated a loss to follow-up rate of 40% for which we will anticipate in the current trial. We, therefore, aim to include a total of (3×60/0.60 =) 300 patients, with 100 patients per group.
Statistical analysis
All continuous data will be presented as medians (95% range). Categorical variables will be presented as absolute and relative frequency. Baseline demographics, treatment-related characteristics, and patient perspectives on ICU-VR will be summarised using descriptive statistics. Outcomes of mixed effects linear and logistic regression models will be presented as the coefficient of the model, which implies the estimated mean difference between groups, including its 95% CI, as the log of coefficient of the model, that is, the OR, including its 95% CI, respectively.
To analyse the effects of ICU-VR on the severity of PTSD-related, anxiety-related, and depression-related symptoms, on HRQoL, and on the prevalence of clinically relevant PTSD, anxiety, and depression at each follow-up time point, we will use mixed effects linear (for continuous outcomes) or logistic (for categorical outcomes) regression models. In these, the outcome at each follow-up time point will serve as dependent variable, the randomisation group, the retrospectively assessed pre-existent score/prevalence of the outcome of interest and a random intercept and/or slope for each study site will be used. The effect of ICU-VR on the course of (1) the severity of PTSD, anxiety-related and depression-related symptoms, (2) HRQoL and (3) the prevalence of clinically relevant PTSD, anxiety and depression throughout follow-up will be analysed using mixed effects linear (for continuous outcomes) and logistic (for categorical outcomes) regression models, in which the outcome/prevalence of interest of all follow-up time points will be used as dependent variable, the randomisation allocation, time, the retrospectively assessed pre-existent score/prevalence of interest will serve as independent variables and a random intercept and/or slope for each study site will be used.
To determine when ICU-VR is most effective, that is, early versus late, differences in psychological distress and HRQoL between the early ICU-VR group and late ICU-VR group at 6 and 12 months will be assessed. We will analyse these using mixed effects linear and logistic regression models. In these models, the score/prevalence of interest at either 6 months or 12 months after ICU discharge will be used as dependent variable, the randomisation allocation, the retrospectively assessed pre-existent score/prevalence of the outcome of interest will serve as independent variables and a random intercept and/or slope for each study site will be used. Differences in the course of the severity and prevalence of psychological distress and HRQoL between 6 and 12 months will be assessed using mixed effects linear and logistic regression models, in which the outcomes at 6 and 12 months will simultaneously be used as dependent variable, and time after discharge in months, randomisation allocation (early ICU-VR/late ICU-VR), the interaction between randomisation and time (randomisation×time), the pre-existent score of the outcome of interest will serve as independent variables and a random intercept and/or slope for each patient and each study site will be used.
We will analyse differences in the subscales of the SF-36, patient resumption to work, experienced financial decline and consultation with healthcare professionals using the abovementioned manners.
The main analysis will be an intention-to-treat analysis, in which all included patients will be included. Second, we will perform a per-protocol analysis, in which patients are included if (1) they are randomised to the control group, (2) they are randomised to the early ICU-VR group and received ICU-VR three times in the hospital ward and (3) they are randomised in the late ICU-VR group and received ICU-VR once during the ICU follow-up clinic visit. Thereafter, we will conduct a complete case analysis, in which all patients who have completed all assessment are included.
We will conduct the subanalyses in (1) patients who have been mechanically ventilated ≥72 hours, (2) patients who have been mechanically ventilated >7 days, (3) patients who have been treated in the ICU for >7 days, (4) patients who have been treated in the ICU for >14 days, (5) patients who had a delirium, as documented in the healthcare record, (6) per study site (study sites with <10 inclusions will be combined), (7) sepsis patients, to compare these results with our previously conducted pilot study
If the loss to follow-up at 6 months after ICU discharge will be higher than anticipated, we will impute missing data using both the last observation carried forward method and multiple imputation according to the Markov-chain Monte-Carlo.47
All data will be gathered using Castor EDC (Castor EDC, Amsterdam, the Netherlands). All analyses will be performed using SPSS (IBM SPSS Statistics for Windows, V.27.0; IBM Corporation, Armonk, New York) and R for Statistics (R Foundation for Statistical Computing, Vienna, Austria, 2015). A p value of ≤0.05 will be considered statistically significant.
Ethics and dissemination
This study will be conducted in accordance with the principles of the declaration of Helsinki (version October 2013; www.wma.net) and in accordance with the Medical Research involving human subjects act (WMO) and other guidelines, regulations and acts. We received approval from the MEC-U, Nieuwegein, and local approval has been obtained from the institutional ethic review boards of each participating hospital. If deviation from the protocol is necessary, it will not be implemented without the prior review and approval of the MEC-U and each participating hospital’s institutional ethic review board. Signed informed consent will be obtained from all patients prior to any study procedure. Previous research demonstrated that (ICU-)VR is safe, feasible and well accepted.25–27 48 Informed consent forms will be kept in a locked cabinet in a limited access room in the ICU of the participating study sites. Data will be archived for 15 years. The handling of personal data complies with the Dutch Law. On completion of the study, its findings will be published in peer-reviewed journals and presented at national and international scientific conferences to publicise the research to healthcare professionals, health services authorities and the public. A summary of results will be made available to the study patients if requested.
Patient and public involvement statement
Former ICU patients were involved in the development of the ICU-VR intervention. Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of the current research.
Supplementary Material
Footnotes
Contributors: JHV, JvB, E-JW, MVM, DG and MEvG conceived the study and initiated the study design. MEVG is the coordinating investigator and grant holder. DG is the principal investigator. TK provided statistical expertise in the clinical trial design, and JHV and TK wrote the statistical analysis plan. MVM provided expertise in the field of psychology, and JV and MVM determined what questionnaires are used. JvB, E-JW, FT, AFCS, JAML, JHE, AMTJR, AD and SA are the local principal investigators at each study site. All authors contributed to the refinement of the study protocol and approved the study protocol. JHV and AMTJR wrote the first draft of the manuscript, JvB, E-JW, TK, EK, MVM, DG, MEVG helped to further draft the manuscript. JHV and ALJ will be responsible for data collection. All authors approved the final version of the manuscript.
Funding: This study was supported by DSW (for the HORIZON-IC project; no grant number available), Stichting Theia (grant number: 2020286), Stichting SGS (grant number: 2020355) and BeterKeten (for the HORIZON-IC project; no grant number available). The funding sources had no role in the design of the study and collection, analysis, and interpretation of data nor in writing the manuscript.
Competing interests: DG has received speaker fees and travel expenses from Dräger, GE Healthcare (medical advisory board 2009–12), Maquet, and Novalung (medical advisory board 2015–18). TK has received speaker fees from Quidel, IBSA, Merck, Berlin Chemie, and Goodlife Healthcare. All other authors declare no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of the current research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Lilly CM, Swami S, Liu X, et al. Five-Year trends of critical care practice and outcomes. Chest 2017;152:723–35. 10.1016/j.chest.2017.06.050 [DOI] [PubMed] [Google Scholar]
- 2.Sakr Y, Jaschinski U, Wittebole X, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis 2018;5:ofy313.. 10.1093/ofid/ofy313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikayin S, Rabiee A, Hashem MD, et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry 2016;43:23–9. 10.1016/j.genhosppsych.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabiee A, Nikayin S, Hashem MD, et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med 2016;44:1744–53. 10.1097/CCM.0000000000001811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Righy C, Rosa RG, da Silva RTA, et al. Prevalence of post-traumatic stress disorder symptoms in adult critical care survivors: a systematic review and meta-analysis. Crit Care 2019;23:213. 10.1186/s13054-019-2489-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med 2012;40:502–9. 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 7.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med 2017;5:90–2. 10.1515/jtim-2016-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosinski S, Mohammad RA, Pitcher M, et al. What is Post-Intensive care syndrome (PICS)? Am J Respir Crit Care Med 2020;201:P15–16. 10.1164/rccm.2018P15 [DOI] [PubMed] [Google Scholar]
- 9.Sukantarat K, Greer S, Brett S, et al. Physical and psychological sequelae of critical illness. Br J Health Psychol 2007;12:65–74. 10.1348/135910706X94096 [DOI] [PubMed] [Google Scholar]
- 10.Vlake JH, van Genderen ME, Schut A, et al. Patients suffering from psychological impairments following critical illness are in need of information. J Intensive Care 2020;8:6. 10.1186/s40560-019-0422-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerckhoffs MC, Kosasi FFL, Soliman IW, et al. Determinants of self-reported unacceptable outcome of intensive care treatment 1 year after discharge. Intensive Care Med 2019;45:806–14. 10.1007/s00134-019-05583-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayde GE, Stefanescu A, Conrad E, et al. Implementing an intensive care unit (ICU) diary program at a large academic medical center: results from a randomized control trial evaluating psychological morbidity associated with critical illness. Gen Hosp Psychiatry 2020;66:96–102. 10.1016/j.genhosppsych.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrouste-Orgeas M, Flahault C, Vinatier I, et al. Effect of an ICU diary on posttraumatic stress disorder symptoms among patients receiving mechanical ventilation: a randomized clinical trial. JAMA 2019;322:229–39. 10.1001/jama.2019.9058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schofield-Robinson OJ, Lewis SR, Smith AF, et al. Follow-Up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Database Syst Rev 2018;11:CD012701. 10.1002/14651858.CD012701.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramnarain D, Aupers E, den Oudsten B, et al. Post intensive care syndrome (PICS): an overview of the definition, etiology, risk factors, and possible counseling and treatment strategies. Expert Rev Neurother 2021;21:1159–77. 10.1080/14737175.2021.1981289 [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson BH, Rattray J, Campbell MK, et al. The practical study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ 2009;339:b3723. 10.1136/bmj.b3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox CE, Hough CL, Carson SS, et al. Effects of a Telephone- and web-based coping skills training program compared with an education program for survivors of critical illness and their family members. A randomized clinical trial. Am J Respir Crit Care Med 2018;197:66–78. 10.1164/rccm.201704-0720OC [DOI] [PubMed] [Google Scholar]
- 18.Jones C, Griffiths RD, Humphris G, et al. Memory, delusions, and the development of acute posttraumatic stress disorder-related symptoms after intensive care. Crit Care Med 2001;29:573–80. 10.1097/00003246-200103000-00019 [DOI] [PubMed] [Google Scholar]
- 19.Granja C, Gomes E, Amaro A, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med 2008;36:2801–9. 10.1097/CCM.0b013e318186a3e7 [DOI] [PubMed] [Google Scholar]
- 20.Peri T, Gofman M. Narrative reconstruction: an integrative intervention module for intrusive symptoms in PTSD patients. Psychological Trauma: Theory, Research, Practice, and Policy 2014;6:176–83. 10.1037/a0031965 [DOI] [Google Scholar]
- 21.Freitas JRS, Velosa VHS, Abreu LTN, et al. Virtual reality exposure treatment in phobias: a systematic review. Psychiatr Q 2021;92:1685–710. 10.1007/s11126-021-09935-6 [DOI] [PubMed] [Google Scholar]
- 22.Kothgassner OD, Goreis A, Kafka JX, et al. Virtual reality exposure therapy for posttraumatic stress disorder (PTSD): a meta-analysis. Eur J Psychotraumatol 2019;10:1654782. 10.1080/20008198.2019.1654782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishkind MC, Norr AM, Katz AC, et al. Review of virtual reality treatment in psychiatry: evidence versus current diffusion and use. Curr Psychiatry Rep 2017;19:80. 10.1007/s11920-017-0836-0 [DOI] [PubMed] [Google Scholar]
- 24.Maples-Keller JL, Bunnell BE, Kim S-J, et al. The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harv Rev Psychiatry 2017;25:103–13. 10.1097/HRP.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekelis K, Calnan D, Simmons N, et al. Effect of an immersive preoperative virtual reality experience on patient reported outcomes: a randomized controlled trial. Ann Surg 2017;265:1068–73. 10.1097/SLA.0000000000002094 [DOI] [PubMed] [Google Scholar]
- 26.Vlake JH, Van Bommel J, Wils E-J, et al. Virtual reality to improve sequelae of the Postintensive care syndrome: a multicenter, randomized controlled feasibility study. Crit Care Explor 2021;3:e0538. 10.1097/CCE.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlake JH, Wils E-J, van Bommel J, et al. Virtual reality tailored to the needs of Post-ICU patients: a safety and Immersiveness study in healthy volunteers. Crit Care Explor 2021;3:e0388. 10.1097/CCE.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neo JRJ, Won AS, Shepley MM. Designing immersive virtual environments for human behavior research. Front Virtual Real 2021;2:603750. 10.3389/frvir.2021.603750 [DOI] [Google Scholar]
- 29.Creamer M, Bell R, Failla S. Psychometric properties of the Impact of Event Scale - Revised. Behav Res Ther 2003;41:1489–96. 10.1016/j.brat.2003.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Weiss DS. The impact of event scale: revised. Cross-cultural assessment of psychological trauma and PTSD: Springer, 2007: 219–38. [Google Scholar]
- 31.van der Ploeg E, Mooren TTM, Kleber RJ, et al. Construct validation of the Dutch version of the impact of event scale. Psychol Assess 2004;16:16–26. 10.1037/1040-3590.16.1.16 [DOI] [PubMed] [Google Scholar]
- 32.Bienvenu OJ, Williams JB, Yang A, et al. Posttraumatic stress disorder in survivors of acute lung injury: evaluating the impact of event Scale-Revised. Chest 2013;144:24–31. 10.1378/chest.12-0908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 34.Sukantarat KT, Williamson RCN, Brett SJ. Psychological assessment of ICU survivors: a comparison between the hospital anxiety and depression scale and the depression, anxiety and stress scale. Anaesthesia 2007;62:239–43. 10.1111/j.1365-2044.2006.04948.x [DOI] [PubMed] [Google Scholar]
- 35.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 36.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 37.M Versteegh M, M Vermeulen K, M A A Evers S, et al. Dutch tariff for the five-level version of EQ-5D. Value Health 2016;19:343–52. 10.1016/j.jval.2016.01.003 [DOI] [PubMed] [Google Scholar]
- 38.Hays RD, Sherbourne CD, Mazel RM. The Rand 36-Item health survey 1.0. Health Econ 1993;2:217–27. 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 39.Kosinski M, Keller SD, Hatoum HT, et al. The SF-36 health survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: tests of data quality, scaling assumptions and score reliability. Med Care 1999;37:MS10–22. 10.1097/00005650-199905001-00002 [DOI] [PubMed] [Google Scholar]
- 40.Ware JE, Kosinski M, Keller S. SF-36 physical and mental health summary scales. A user’s manual. Boston, MA: Health Assessment Lab, 1994. ISBN: 1-891810-00-6. [Google Scholar]
- 41.van Mol MMC, Bakker EC, Nijkamp MD, et al. Relatives' perspectives on the quality of care in an intensive care unit: the theoretical concept of a new tool. Patient Educ Couns 2014;95:406–13. 10.1016/j.pec.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 42.Marshall GN HR. The patient satisfaction questionnaire short-form (PSQ-18). Objective Analysis 1994. 10.7249/P7865 [DOI] [Google Scholar]
- 43.Ely EW, Truman B, Shintani A. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation scale (RASS). JAMA 2003;239:2983–91. 10.1001/jama.289.22.2983 [DOI] [PubMed] [Google Scholar]
- 44.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10. 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 45.Vlake JH, van Bommel J, Wils E-J, et al. Intensive care Unit–Specific virtual reality for critically ill patients with COVID-19: multicenter randomized controlled trial. J Med Internet Res 2021;24:e32368. 10.2196/32368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 47.Lacerda M, Ardington C, Leibbrandt M. Sequential regression multiple imputation for incomplete multivariate data using Markov chain Monte Carlo. A Southern Africa Labour and Development Research Unit Working Paper. 13. Southern Africa Labour and Development Research Unit, 2008. ISBN: 978-0-9814031-4-4. [Google Scholar]
- 48.Turon M, Fernandez-Gonzalo S, Jodar M, et al. Feasibility and safety of virtual-reality-based early neurocognitive stimulation in critically ill patients. Ann Intensive Care 2017;7:81. 10.1186/s13613-017-0303-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061876supp001.pdf (316.2KB, pdf)
bmjopen-2022-061876supp002.pdf (241.1KB, pdf)


