Abstract
Objectives
Review and assess cost-effectiveness studies of robotic-assisted radical prostatectomy (RARP) for localised prostate cancer compared with open radical prostatectomy (ORP) and laparoscopic radical prostatectomy (LRP).
Design
Systematic review.
Setting
PubMed, Embase, Scopus, International HTA database, the Centre for Reviews and Dissemination database and various HTA websites were searched (January 2005 to March 2021) to identify the eligible cost-effectiveness studies.
Participants
Cost-effectiveness, cost-utility, or cost-minimization analyses examining RARP versus ORP or LRP were included in this systematic review.
Interventions
Different surgical approaches to treat localized prostate cancer: RARP compared with ORP and LRP.
Primary and secondary outcome measures
A structured narrative synthesis was developed to summarize results of cost, effectiveness, and cost-effectiveness results (eg, incremental cost-effectiveness ratio [ICER]). Study quality was assessed using the Consensus on Health Economic Criteria Extended checklist. Application of medical device features were evaluated.
Results
Twelve studies met inclusion criteria, 11 of which were cost–utility analyses. Higher quality-adjusted life-years and higher costs were observed with RARP compared with ORP or LRP in 11 studies (91%). Among four studies comparing RARP with LRP, three reported RARP was dominant or cost-effective. Among ten studies comparing RARP with ORP, RARP was more cost-effective in five, not cost-effective in two, and inconclusive in three studies. Studies with longer time horizons tended to report favorable cost-effectiveness results for RARP. Nine studies (75%) were rated of moderate or good quality. Recommended medical device features were addressed to varying degrees within the literature as follows: capital investment included in most studies, dynamic pricing considered in about half, and learning curve and incremental innovation were poorly addressed.
Conclusions
Despite study heterogeneity, RARP was more costly and effective compared with ORP and LRP in most studies and likely to be more cost-effective, particularly over a multiple year or lifetime time horizon. Further cost-effectiveness analyses for RARP that more thoroughly consider medical device features and use an appropriate time horizon are needed.
PROSPERO registration number
CRD42021246811.
Keywords: health economics, surgery, urology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This review provided a comprehensive, systematic and transparent literature search strategy covering multiple databases as well as the grey literature for health technology assessment (HTA) reports. However, private or confidential HTAs containing cost-effectiveness analysis might still be missing.
The review conducted bias assessment using the Consensus on Health Economic Criteria Extended checklist, suitable for economic evaluation studies.
Four additional criteria unique to medical devices (organisational impact, learning curve, incremental innovation and dynamic pricing) were assessed for each included study.
Internal validity of the systematic review synthesis depends on the quality of the limited number of primary studies included.
Introduction
Prostate cancer is the second most frequent malignancy (after lung cancer) in men worldwide.1 For men diagnosed with clinically localised prostate cancer, radical prostatectomy is one of the primary treatment options. Radical prostatectomy can be performed with open radical prostatectomy (ORP) or minimally invasive techniques, including laparoscopic radical prostatectomy (LRP) and robotic-assisted radical prostatectomy (RARP). Despite being a less invasive approach, application of LRP is low,2–4 possibly due to its technical difficulty in performing complex procedures (eg, bilateral nerve-sparing dissection and construction of watertight urethrovesical anastomosis), steep learning curve, and limitations in dexterity and ergonomics.5–9 Robotic-assisted surgery (RAS) using the da Vinci surgical system (Intuitive Surgical Operations, Sunnyvale, California, USA) overcomes the technical challenges encountered by LRP by allowing for additional wrist movements and three-dimensional visualisation of the operative field.10 Surgeons perform RARP using a surgical system that translates the surgeon’s hand movement from the console in real time. This is achieved through the instrument’s 7 degrees of motion, with precision and tremor filtration. Globally, use of RARP has been increasing, and has become a common surgical approach in many countries.4 11
The clinical effectiveness of RARP has been well documented in literature. Compared with ORP, RARP has been shown to reduce postoperative complications (eg, blood loss and transfusion rate), reduce hospital length of stay and enable faster recovery.12 13 Compared with conventional LRP, RARP offers technical advantages (eg, the fully wristed dexterity, highly magnified three-dimensional high-resolution video) to overcome challenges from the complexity of radical prostatectomy and enables more patients to benefit from minimally invasive techniques.14 15
Despite the increased worldwide adoption of RARP, its economic value compared with ORP and LRP remains controversial, drawing attention from policy makers, payers and health technology assessment (HTA) agencies. Multiple cost-effectiveness analyses16–19 have been performed in different healthcare settings using different methodologies over the past decade and have come to diverse conclusions. A systematic assessment of previously conducted cost-effectiveness analyses is critical for researchers and decision-makers to understand the value of RARP and to reach consensus on the appropriate methodology to quantify its cost-effectiveness.
Moreover, medical devices, such as those used to perform robotic surgery, are characterised by distinctive features that are less frequently found in pharmaceuticals20; therefore, methods of conducting economic evaluations for medical devices need to consider additional attributes beyond those traditionally used in assessing drugs, such as the technology’s organisational impact, learning curve, incremental innovation and dynamic pricing.21 22 RAS is a good example to illustrate the need to evaluate medical device-specific features. First, substantial infrastructural investment in a robotic surgical systems triggers ‘organisational impact’. Second, a surgeon’s experience and proficiency with RAS impacts clinical outcomes and efficiency; in other words, a ‘learning curve’. Third, postlaunch innovation in robotic systems and instruments routinely occur over time and may be associated with changes in clinical outcomes, efficiency and cost. For example, since the first da Vinci surgery conducted in 2000, a total of four generations of da Vinci surgical systems have been launched with numerous instrument-level upgrades. Finally, the prices of medical devices typically decrease over time due to innovation, production scale, and market competition. This ‘dynamic pricing’ increases the level of uncertainty when assessing RAS. To our knowledge, no study in the existing cost-effectiveness literature for RARP has investigated these medical device features.
The aim of this systematic literature review is to assess the existing cost-effectiveness studies of RARP for localised prostate cancer, evaluate how medical device features have been considered in those studies, and provide insight for future cost-effectiveness studies in the field of robotic surgery.
Methods
The protocol for this systematic review was developed in advance and registered with PROSPERO (registration number CRD42021246811) in May 2021.
Search strategy and study selection
Multiple databases were searched to retrieve studies published from 1 January 2005 (before the earliest cost-effectiveness literature on RARP) to 1 March 2021 (search date). Specifically, the databases included PubMed, Embase, Scopus, International HTA database and the Centre for Reviews and Dissemination database, which includes the NHS Economic Evaluation database, Database of Abstracts and Reviews of Effects, and the Health Technology Assessment (HTA) database. The detailed search strategy is outlined in online supplemental appendix A.
bmjopen-2021-058394supp001.pdf (118.6KB, pdf)
In addition to these five databases, we also performed a targeted grey literature search of various HTA websites (eg, UK National Institute for Health and Care Excellence, US Agency for Healthcare Research and Quality Evidence-Based Reports, US Institute for Clinical and Economic Review, Canadian Agency for Drugs and Technologies in Health and Tufts Cost-Effectiveness Analysis Registry) and Google. Keywords used included robotic surgery, robot assist, da Vinci, prostatectomy and prostate cancer.
Studies were included in this review if they were cost-effectiveness analyses, cost–utility analyses (CUA) or cost–minimisation analyses, and contained da Vinci-assisted radical prostatectomy as an intervention of interest. Studies were excluded if they were not in English, did not include prostatectomy or RAS, only had cost data without cost-effectiveness outcomes, were based on duplicate patient populations, or reviews that only included primary studies already captured in our review. The inclusion and exclusion criteria specifications are detailed in online supplemental appendix B.
Two reviewers independently screened the literature and any inconsistencies in the identification of potentially relevant studies were discussed to reach a consensus. The results are reported according to Preferred Reporting Items of Systematic Reviews and Meta-Analyses guidelines.23
Data extraction
Data were extracted from included studies based on the study protocol. Extracted data elements included: study year, country, economic analysis type, comparator, perspective, time horizon, effectiveness measure and outcome value, cost measure and outcome value, incremental costs and incremental effectiveness, cost-effectiveness value (eg, incremental cost-effectiveness ratio [ICER] if applicable), discount rate, sensitivity analysis parameters and authors’ conclusion.
Data synthesis
The approach used for data synthesis followed steps consistent with the International Society for Pharmacoeconomics and Outcomes Research Good Practices for cost and cost-effectiveness systematic review.24 A meta-analysis of the findings was planned if feasible and appropriate for the available data. However, if the published literature contained substantial variability in clinical outcomes, healthcare setting, methodology, effects, costs and willingness-to-pay thresholds, a structured narrative synthesis approach would be used.
Study characteristics, such as type of analysis, patient population, perspective and methodological choices, were summarised. We reported incremental costs, incremental effectiveness and ICERs. Cost-effectiveness results across studies were displayed using scatterplots by plotting incremental quality-adjusted life-years (QALYs) and incremental costs on the x-axis and y-axis, respectively. All cost data were converted and reported in 2021 US dollars using purchasing power parities along with the original cost data.
Medical device features
The application of four distinctive features recommended for economic evaluations of medical devices was evaluated in the included studies, and each one was categorised using three levels: ‘adjustment made to model’, ‘acknowledged but no model adjustment’ or ‘not considered’. The study would be classified as ‘adjustment made to model’ if adjustments were made to the base case or sensitivity analyses. If a study only mentioned the features in its writing but no adjustment was made in modelling, it would be considered as ‘acknowledged but no model adjustment’.
Critical appraisal of risk of bias
Risk of bias of economic evaluations was assessed using the Consensus on Health Economic Criteria (CHEC)-Extended checklist. The CHEC-extended checklist contains guidelines for each criterion and scoring, and can be used to evaluate model-based and trial-based economic evaluations.25 26 A score of one point was assigned to each positive response, and zero to a negative response or for non-applicable items. The total score out of 20 items was converted to a score ranging from zero (low quality) to 100 (high quality). Included studies were categorised into four grades: low, moderate, good and excellent quality according to thresholds for the total score of ≤50, 51–75, 76–95 and >95, respectively.
Patient and public involvement
The systematic review did not involve animal or human subjects and did not use patient data. Patients and the public were not involved in the design and conduct of this systematic review since published studies were used to synthesise findings.
Results
We identified 930 articles from the initial literature search. On reviewing, nine full-text studies from the database search and three articles from the targeted grey literature search met the inclusion criteria and were included in the final analysis (figure 1). Five studies were derived from HTA reports. Studies that were excluded tended to be costing-only studies27 28 or had mixed RARP with other surgical modalities in an intervention group.29 30
Figure 1.
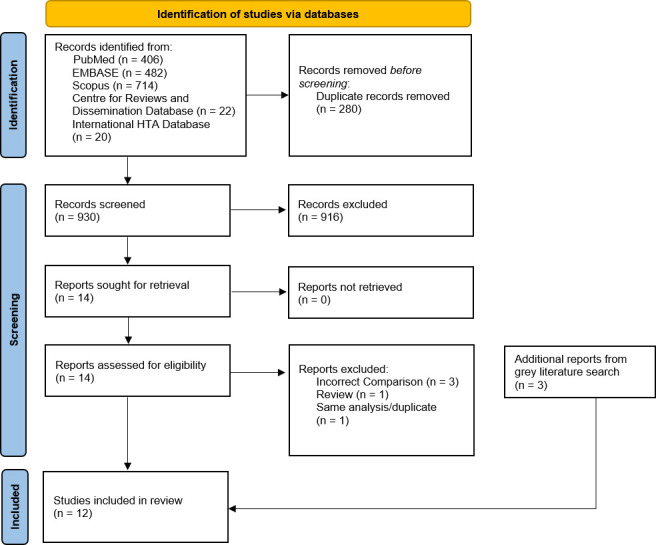
The PRISMA flow diagram of the literature search and selection process. PRISMA, Preferred Reporting Items of Systematic Reviews and Meta-Analyses.
Characteristics of included studies
Of the 12 studies16–19 31–38 included in this review, 11 are CUAs. The characteristics of all studies are presented in table 1. High variability across clinical and healthcare settings was observed. Among those studies, three18 33 37 were conducted in Canada, two35 36 in Australia, one17 in USA, one16 in UK, two32 38 in Ireland and three19 31 34 in other countries. Ten studies17–19 32–38 compared RARP with ORP, 4 studies16 17 31 32 compared RARP with LRP and 1 study38 compared RARP versus routine care of mixed ORP and LRP. Cooperberg et al17 and an HTA report by Alberta Health in Canada33 additionally included other non-surgical treatments as comparators.
Table 1.
Characteristics of included studies
| Study | Country | Type of literature | Population | Intervention | Comparator | Perspective | Time horizon | Type of analysis | Methods |
| MSAC 200635 | AUS | HTA report | localised PC | RARP | ORP | Societal | 10 years | CUA | Decision Tree |
| O'Malley 200736 | AUS | Journal article | PC | RARP | ORP | Payer* | * | CUA | Cohort based |
| Hohwü 201119 | DEN | Journal article | localised PC | RARP | ORP | Societal | 1 year | CEA | Cohort based |
| HIQA 201138 | IRE | HTA report | PC T2-T3 | RARP | Mix of ORP and LRP | Payer | 5 years | CUA | Markov Model |
| Close 201316 /Ramsay 201240 |
UK | Journal /HTA report |
localised PC | RARP | LRP | Payer | 10 years | CUA | Discrete event simulation |
| Cooperberg 201317 | USA | Journal article | localised PC | RARP | ORP, LRP+other† | Payer | Lifetime | CUA | Markov Model |
| Teljeur 201432 | IRE | Journal article | RP | RARP | ORP, LRP | Payer | Lifetime* | CUA | * |
| Ratchanon 201531 | THA | Journal article | localised PC | RARP | LRP | Health system | 10 years | CUA | Decision Tree |
| AHT 201733 | CAN | HTA report | localised PC | RARP | ORP+others‡ | Payer | 9 years | CUA | Markov Model |
| HQO 201718 | CAN | HTA report | localised PC | RARP | ORP | Payer | 1 year | CUA | Markov Model |
| Parackal 202037 | CAN | Journal article | localised PC | RARP | ORP | Payer | 10 years | CUA | Markov Model |
| de Oliveira 202134 | BRA | Journal article | localised PC | RARP | ORP | Hospital | 5 years | CUA | Cohort based |
*Not clearly stated
†IMRT, BT, 3DCRT, EBRT+BT.
‡Beam radiotherapy, brachytherapy and cryoablation.
AUS, Australia; BRA, Brazil; BT, brachytherapy; CAN, Canada; CEA, cost-effectiveness analysis; CUA, cost–utility analysis; 3DCRT, three-dimensional conformal radiation therapy; DEN, Denmark; EBRT, external beam radiation therapy; IMRT, Intensity-modulated radiation therapy; IRE, Ireland; LRP, local radical prostatectomy; ORP, open radical prostatectomy; PC, prostate cancer; RARP, robotic-assisted radical prostatectomy; RP, radical prostatectomy; THA, Thailand.
Most (8 out of 12) of the studies16–19 32 33 36 37 were conducted from the payer’s perspective, with 2 studies19 35 taking a societal perspective, 1 study31 from a healthcare system perspective and 1 study34 from a hospital perspective. Regarding the time horizon, lifetime horizon was considered in two studies,17 32 5–10 years was used in seven studies,16 31 33–35 37 38 short-term time horizon of 1 year was used in two studies18 19 and one study36 did not report the time horizon. Three studies19 34 36 are observational-based modelling studies, and the rest are simulation-based analyses. Among the nine simulation-based studies, eight studies clearly reported the model methods. Among these eight studies, Markov modelling was used in five studies,17 18 33 37 38 simple decision tree in two studies31 35 and discrete event simulation in one study.16
Narrative synthesis of study results
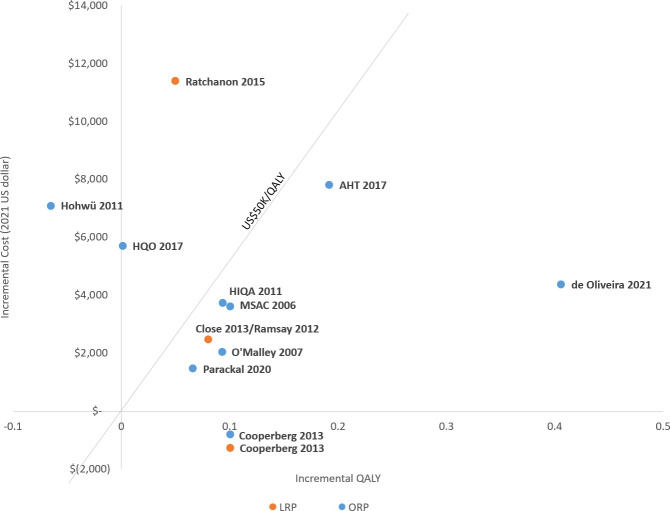
Based on the included studies, RARP was generally associated with higher effectiveness and higher cost. All studies showed RARP had higher QALYs than ORP or LRP across various time horizons, with one exception; Hohwü et al19 reported RARP had lower QALYs than ORP with a 1-year time horizon. The range of incremental QALYs gained for RARP varied from 0.05 to 0.1 when compared with LRP, and 0.001 to 0.41 when compared with ORP. In 11 of the 12 studies, RARP had higher costs relative to the comparators. The one exception was an analysis from the US payer perspective by Cooperberg et al,17 which demonstrated lower cost for RARP compared with ORP and LRP; however, capital cost was not considered in the analysis. Results from the CUAs, in the form of incremental costs (standardised to 2021 USD) and incremental QALYs, were plotted and presented in figure 2. The corresponding summary is presented in table 2.
Figure 2.
Incremental cost-effectiveness plane of the published study results. LRP, laparoscopic radical prostatectomy; ORP, open radical prostatectomy; QALY, quality-adjusted life-year.
Table 2.
The summary of cost-effectiveness literature
| Study | Incremental cost (in local unit) |
Incremental cost (in 2021 USD) |
Incremental QALY | ICER (Original) | ICER (in 2021 USD) | Willingness to pay | Conclusion | |
| RARP vs LRP | Ratchanon 201531 | 120 359 baht | US$11 385 | 0.05 | 2 407 180 baht/QALY | US$227K/QALY | 160K baht per QALY | RARP is not cost-effective |
| Close 2013/ Ramsay 201216 40 | GBP£1412 | US$2464 | 0.08 | GBP£18 329/QALY | US$31K/QALY | £30K per QALY | RARP is cost-effective if volume >150 | |
| Cooperberg 201317 | Low risk: US$−591 Intermediate risk: US$−1024 High risk: US$−104 |
Low risk: US$−732 Intermediate risk: US$−1268 High risk: US$−129 |
Low risk: 0 Intermediate risk: 0.1 High risk: 0 |
ICER not calculated | ICER not calculated | N/A | No difference in effectiveness And RARP lower cost |
|
| Teljeur 201432 | No reported | No reported | No reported | €26 643/QALY | Not reported | Not reported | Not reported | |
| RARP vs ORP | MSAC 200635 | A$3742 for ED; A$4502 for UI | US$3613 for ED; US$4348 for UI |
0.10 for ED; 0.01 for UI |
A$37K/QALY for ED, A$450K/QALY for UI |
US$36K/QALY for ED; US$435K/QALY for UI | Not reported | Lack of data |
| O'Malley 200736 | A$2264 | US$2049 | 0.09 | A$24 457/QALY | US$40K/QALY | Not reported | RARP cost-effective | |
| Hohwü 201119 | €4506 | US$7093 | −0.07 | €64 343/extra successful treatment | US$101K/extra successful treatment | N.A (main analysis CEA) | RARP not cost-effective | |
| HIQA 201138 | €2487 | US$3730 | 0.09 | €26 647/QALY | US$30K/QALY | No specified threshold | No specified threshold in Ireland | |
| Cooperberg 201317 | Low risk: $−344 Intermediate risk: $−572 High risk: $−1265 |
Low risk: US$−425.87 Intermediate risk: US$−708 High risk: US$−1566 |
Low risk: 0 Intermediate risk: 0.1 High risk: 0 |
ICER not calculated | ICER not calculated | N/A | No difference in effectiveness, RARP has lower cost | |
| Teljeur 201432 | No reported | No reported | No reported | €26 920/QALY | US$40K/QALY | Not reported | Not reported | |
| AHT 201733 | C$8541 | US$7813 | 0.19 | C$44 471/QALY | US$41K/QALY | C$50K per QALY | RARP cost-effective | |
| HQO 201718 | C$6234 | US$5702 | 0.001 | C$5.2M/QALY | US$5M/QALY | C$100K per QALY | RARP not cost-effective | |
| Parackal 202037 | C$1701 | US$1457 | 0.07 | C$25 704/QALY | US$22K/QALY | C$50K or 100K per QALY | RARP cost-effective | |
| de Oliveira 202134 | BRA R$9214 | US$4368 | 0.41 | R$22 690.83/QALY | US$11K/QALY | R$114 026.55 (3 times of GDP) | RARP cost-effective |
A$, Australian Dollar; BRA, Brazil; C$, Canadian Dollar; CEA, cost-effectiveness analysis; ED, erectile dysfunction; GBP, British pound sterling; GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; LRP, local radical prostatectomy; ORP, open radical prostatectomy; QALY, quality-adjusted life year; RARP, robotic-assisted radical prostatectomy; UI, urinary infection.
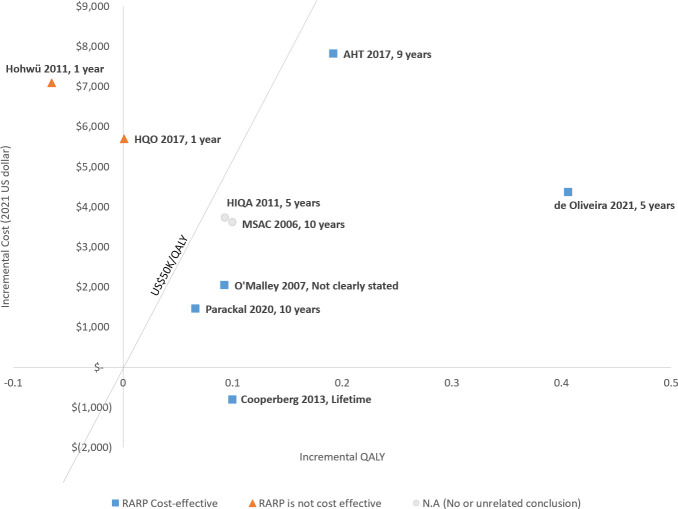
Most study results comparing RARP with ORP demonstrate RARP being cost-effective, although there is considerable heterogeneity across studies. As such, it is not appropriate to pool the cost-effectiveness results together. Five studies17 33 34 36 37 showed RARP to be more cost-effective than ORP, while two other studies18 19 showed RARP having higher ICERs that exceeded the willingness to pay (WTP) threshold value. Three studies32 35 38 were inconclusive on the cost-effectiveness of RARP due to insufficient comparative effectiveness data or an unspecified WTP threshold. Interestingly, the study time horizon was observed to correlate with study conclusions (figure 3). The only two studies18 19 that showed RARP was not cost-effective compared with ORP used a short-term time horizon (1 year), while the other five studies17 33 34 36 37 evaluated cost-effectiveness over 5, 9 and 10 years, or lifetime, all showed RARP to be more cost-effective than ORP.
Figure 3.
The cost-effectiveness and time horizon, RARP versus ORP. ORP, open radical prostatectomy; QALY, quality-adjusted life-year; RARP, robotic-assisted radical prostatectomy.
Cost-effectiveness results for RARP compared with LRP were inconclusive given the limited number of publications (four studies),16 17 31 32 but showed a tendency towards RARP being more cost-effective. One study17 showed RARP as the dominant surgical option (lower cost and more effective). Two studies,16 32 one from the UK and one from Ireland, demonstrated RARP to be cost-effective, while another study31 conducted in Thailand found the ICER of RARP to be much higher than the stated WTP threshold.
Systematic review on inclusion of medical device features
Distinctive medical device features were considered to various extents in the included studies. Capital investment, which is one aspect of organisation impact, was widely considered. Ten16 18 19 31–33 35–38 of 12 studies (83%) included capital investment, and one study17 (8.5%) justified why it was not included in the analysis. Capital equipment cost and procedure volume per system were considered as sensitive parameters in seven studies (58%).16 18 19 31 35 37 38 Dynamic pricing was reflected in five studies16 18 35 37 38 (42%) by evaluating the uncertainty of equipment or instrument prices in the sensitivity analyses. Although none of studies quantitatively evaluated the impact of surgeon experience on outcomes and efficiency, 7 of 12 studies16 18 33–35 37 38 (58%) mentioned ‘learning curve’. In terms of incremental innovation, two studies18 38 (17%) mentioned new generations of the surgical system and one study16 (8.5%) included different costs for the new generation system within the analysis.
Systematic review on risk of bias
Study quality for the 12 studies was evaluated using the CHEC-Extended checklist, and results are presented in online supplemental appendix C. Among the 12 studies, 1 study33 was classified as ‘excellent’, 8 studies16–19 31 34 37 38 as ‘good’, 1 study35 as ‘moderate’ and 2 studies32 36 as ‘low’ quality. The primary reason studies were classified as low or moderate quality is that they failed to appropriately measure, value or report cost and/or effectiveness. Among the 20 items on the checklist, the majority of the studies16–19 31 32 34–37 (10 out of 12) did not consider ethical and distributional issues, and 7 studies16–18 34 36–38 (58%) used utility data collected from different study populations.
Discussion
Our systematic literature review identified 12 studies that evaluated the cost-effectiveness of RARP for localised prostate cancer patients. Three-quarters of studies were of excellent or good quality based on the CHEC-Extended checklist. These studies vary widely in country/healthcare setting, comparators and time horizon. A majority of the studies found RARP to be more costly and more effective compared with ORP and LRP, although the cost-effectiveness conclusions (ie, the ICERs) varied and were dependent on the specific WTP thresholds used and influenced by time horizon. The four medical device features recommended to be included in economic evaluations were considered to varying degrees: capital investment in the surgical system (organisational impact) was widely considered, dynamic pricing was considered in about half of the studies, while learning curve and incremental innovation were poorly addressed in the included studies.
Cost, effectiveness and cost-effectiveness of RARP
Although the higher cost and effectiveness of RARP observed in this review were consistent with existing literature,4 11 33 39 40 the key question is whether the effectiveness gained is worth the increased cost. Although most of the CEAs comparing RARP with ORP or with LRP concluded RARP to be cost-effective in this systematic literature review, a definitive answer remains elusive given that cost-effectiveness thresholds varied across different countries and healthcare settings. Studies with longer time horizons tended to have more favourable cost-effectiveness results for RARP. Possible explanations for this correlation is that RARP incurs a high upfront capital, instrument and accessory cost in the perioperative time period, while improved patient outcomes observed after RARP including lower rates of positive surgical margins and better functional outcomes7 13 14 41 might lead to downstream healthcare cost savings11 and translate into better quality of life as well.
Medical device features in RARP
Despite recommendations in multiple authoritative methods publications,20–22 42 adoption of the four recommended special characteristics of medical devices within these cost-effectiveness evaluations was limited. Among most of the studies included in this review, the capital cost of acquiring a robotic system, one aspect of organisational impact, was considered in the cost calculation. The allocation of capital equipment cost per RARP case is challenging, given the complex financial allocation in different healthcare systems and sharing of the use of robotic systems across specialties. The capital cost calculation of robotic assisted surgery should reflect the actual cost allocation from the appropriate perspective and healthcare system. Only two included studies19 38 calculated the capital cost per RARP case by allocating the cost to multiple procedures across specialties to reflect real world practice. Additionally, if robotic capital cost is funded by a charitable donation, it is important to consider who actually paid for it and align cost calculations with the study perspective.
Learning curve, the second recommended medical device feature, is considered the most important characteristic associated with the use of a medical device.21 In this systematic review, while learning curve was mentioned among 58% of the studies, no action was taken to incorporate it within the analyses. Inclusion of a robotic surgical system’s learning curve could be considered on two fronts. First, surgeons need some practice to reach proficiency after adopting new technology, which could be accelerated by rigorous training. Second, higher surgical volume may not only reduce cost per procedure (economies of scale), but also improve patient outcomes and reduce the operative time per procedure as the surgeons become more skilled and their proficiency increases.43 44 Scenario analysis could be considered to further understand the uncertainty related to surgical volume consistent with a learning curve effect.
Incremental innovation, the third recommended medical device feature, is common in RARP. For example, four generations of da Vinci RAS systems and instruments, with numerous product innovations, have been launched in the past 20 years. However, incremental innovation was considered in only one study in this review, and it focused exclusively on the differential costs by generations of systems without considering changes in effectiveness. This is likely due to lack of clinical studies that differentiate effectiveness among various generations of surgical systems. Postmarket observational studies for newer RAS generations or subgroup analysis for the different system/product generations are needed to address this gap.
Lastly, medical device pricing is considered more dynamic than drugs, and launching new generations of technology often influences the price of existing devices.21 22 Five studies in this review empirically tested varying equipment prices. In addition to using updated pricing information, researchers could consider estimating a threshold price at which RAS provides a minimally acceptable value, which decision makers could consider in future purchasing or leasing decisions. For a healthcare system, the threshold price for a new technology is at the point of indifference between accepting and rejecting the technology, assuming all conditions for other options are equal.45 Analyses considering threshold price and technology generation were not found in this systematic review.
Suggestions for future RAS cost-effectiveness studies
Several opportunities to improve RAS economic evaluations were identified from this systematic literature review. First, the selection of time horizon should be long enough to capture the relevant differences in outcomes and costs to the various stakeholders. With emerging evidence demonstrating RAS’ long-term clinical benefits such as less positive surgical margin14 15 46 and better functional outcomes,7 13 14 41 researchers should consider applying appropriate time horizons consistent with the direct and, when relevant, indirect effects of the procedures on patient outcomes. Second, surgeon proficiency may affect patient outcomes and efficiency. Clinical studies might consider measuring and reporting the experience and proficiency of surgeons (eg, number of cases performed previously) when evaluating their surgical outcomes. Cost-effectiveness analyses could use clinical data from experienced surgeons who have passed the learning curve or consider stratified analyses by the performance of high-volume versus low-volume centres/surgeons to better examine the impact of surgeon proficiency. Moreover, with increasing numbers of new robotic surgical products and manufacturers, differentiation between products is critical for economic evaluations to inform decision-making, as it will be increasingly unlikely that all robotic surgery platforms are equivalent. Clinical studies that document clinical outcomes data by different brands and generations of devices are needed to enable the evaluation of incremental innovation of medical devices in cost-effectiveness analyses. Third, existing studies are primarily conducted from societal or payer perspectives. Future studies evaluating economic value of RAS should consider a healthcare systems perspective, given the purchasing decision is often made at this level. Researchers may need to carefully select cost and benefit components to align with the perspective of the study in the specific country. Fourth, the cost of infrastructure necessary to accommodate the device and any impact of the new device on procedure costs should be considered. This may include the cost of training, increase in surgical volume and conversion of procedure from inpatient to outpatient setting.47 Finally, the COVID-19 pandemic brings new challenges for constrained healthcare resources. The opportunity cost of using RAS to reduce downstream health resource use may be increasingly relevant in this environment.
Limitations of the systematic review
The current review is subject to several limitations. First, the literature search was limited to publicly available information. Private or confidential HTAs may contain cost-effectiveness analyses not included in this study, despite our effort to conduct a targeted grey literature search. Second, the internal validity of a systematic review synthesis depends on the quality of primary studies included. In our review, the general quality of included studies could be considered moderate to good, except for methods used to assess effectiveness. More than half of the studies did not use local utility data for prostate cancer. The lacking country-specific utility data increased the uncertainty of the published economic evaluations. Third, although studies with a cost-comparison design could provide insights on costs, they were excluded due to lack of effectiveness data. In addition, patient benefits that are not directly associated with clinical effectiveness measures, such as reduction of out-of-pocket cost48 and reduction on productivity loss,49 were not evaluated in most of the original studies in this review. Inclusion of these additional patient-focused outcomes may more accurately reflect value/cost. Finally, with the increasing use of non-surgical treatment for localised prostate cancer, such as high-intensity focused ultrasound,50 it is worthwhile to further investigate the cost-effectiveness across all treatment options.
Conclusions
To our knowledge, this is the first systematic literature review on the cost-effectiveness of RAS and evaluated the application of recommended medical device features. No conclusive cost-effectiveness result was identified in the literature due to study heterogeneity; however, RARP was found to be more costly and effective compared with ORP and LRP in most studies, providing a body of evidence supporting its cost-effectiveness. Analyses with longer time horizons showed more favourable cost-effectiveness results towards RARP. Further cost-effectiveness analyses for RARP that more thoroughly consider medical device features are needed to better understand and more appropriately estimate its economic value compared with other surgical and non-surgical treatments.
Supplementary Material
Acknowledgments
We thank Sadaf Saaber, April Hebert, Ana Yankovsky and Ben Forest for support with literature screening and Rachael Mann, from Labcorp Drug Development, for editorial support.
Footnotes
Contributors: CS, YL and UK were involved in the conception and design of the study. CS and LC were involved in the acquisition of data. All authors (CS, LC, UL, UK and SRS) made substantial contribution to the analysis and interpretation of the data, the drafting and critical revision of the manuscript. YL acts as the guarantor of this study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: LC, YL and UK are full-time employees of Intuitive Surgical. CS is a former full-time employee of Intuitive Surgical and a current full-time employee of Union Chimique Belge (UCB).
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethics approval was not required since the systematic review aggregated results from published studies.
References
- 1.Rawla P. Epidemiology of prostate cancer. World J Oncol 2019;10:63–89. 10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S-Y, Chang S-C, Chen C-I, et al. Latest comprehensive medical resource consumption in robot-assisted versus laparoscopic and traditional open radical prostatectomy: a nationwide population-based cohort study. Cancers 2021;13:1564. 10.3390/cancers13071564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis JW, Kreaden US, Gabbert J, et al. Learning curve assessment of robot-assisted radical prostatectomy compared with open-surgery controls from the premier perspective database. J Endourol 2014;28:560–6. 10.1089/end.2013.0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes D, Camp C, O'Hara J, et al. Health resource use after robot-assisted surgery vs open and conventional laparoscopic techniques in oncology: analysis of English secondary care data for radical prostatectomy and partial nephrectomy. BJU Int 2016;117:940–7. 10.1111/bju.13401 [DOI] [PubMed] [Google Scholar]
- 5.Cazzaniga W, Godtman RA, Carlsson S, et al. Population-Based, nationwide registration of prostatectomies in Sweden. J Surg Oncol 2019;120:803–12. 10.1002/jso.25643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter SC, Lipsitz S, Shih Y-CT, et al. Population-based determinants of radical prostatectomy operative time. BJU Int 2014;113:E112–8. 10.1111/bju.12451 [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Seo HJ, Lee NR, et al. Robot-Assisted radical prostatectomy has lower biochemical recurrence than laparoscopic radical prostatectomy: systematic review and meta-analysis. Investig Clin Urol 2017;58:152–63. 10.4111/icu.2017.58.3.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turpen R, Atalah H, Su L-M. Technical advances in robot-assisted laparoscopic radical prostatectomy. Ther Adv Urol 2009;1:251–8. 10.1177/1756287210364207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noël J, Moschovas MC, Sandri M, et al. Patient surgical satisfaction after da Vinci® single-port and multi-port robotic-assisted radical prostatectomy: propensity score-matched analysis. J Robot Surg 2022;16:473–81. 10.1007/s11701-021-01269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H-yin, Hevelone ND, Lipsitz SR, et al. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol 2012;187:1392–9. 10.1016/j.juro.2011.11.089 [DOI] [PubMed] [Google Scholar]
- 11.Okhawere KE, Shih I-F, Lee S-H, et al. Comparison of 1-year health care costs and use associated with open vs robotic-assisted radical prostatectomy. JAMA Netw Open 2021;4:e212265. 10.1001/jamanetworkopen.2021.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-Assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet 2016;388:1057–66. 10.1016/S0140-6736(16)30592-X [DOI] [PubMed] [Google Scholar]
- 13.Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-Assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol 2018;19:1051–60. 10.1016/S1470-2045(18)30357-7 [DOI] [PubMed] [Google Scholar]
- 14.Huang X, Wang L, Zheng X, et al. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: a systemic review and meta-analysis. Surg Endosc 2017;31:1045–60. 10.1007/s00464-016-5125-1 [DOI] [PubMed] [Google Scholar]
- 15.Porpiglia F, Fiori C, Bertolo R, et al. Five-Year outcomes for a prospective randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol Focus 2018;4:80–6. 10.1016/j.euf.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 16.Close A, Robertson C, Rushton S, et al. Comparative cost-effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of men with localised prostate cancer: a health technology assessment from the perspective of the UK National health service. Eur Urol 2013;64:361–9. 10.1016/j.eururo.2013.02.040 [DOI] [PubMed] [Google Scholar]
- 17.Cooperberg MR, Ramakrishna NR, Duff SB, et al. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int 2013;111:437–50. 10.1111/j.1464-410X.2012.11597.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health Quality Ontario . Robotic surgical system for radical prostatectomy: a health technology assessment. Ont Health Technol Assess Ser 2017;17:1–172. [PMC free article] [PubMed] [Google Scholar]
- 19.Hohwü L, Borre M, Ehlers L, et al. A short-term cost-effectiveness study comparing robot-assisted laparoscopic and open retropubic radical prostatectomy. J Med Econ 2011;14:403–9. 10.3111/13696998.2011.586621 [DOI] [PubMed] [Google Scholar]
- 20.Tarricone R, Torbica A, Drummond M. Challenges in the assessment of medical devices: the MedtecHTA project. Health Econ 2017;26 Suppl 1:5–12. 10.1002/hec.3469 [DOI] [PubMed] [Google Scholar]
- 21.Drummond M, Griffin A, Tarricone R. Economic evaluation for devices and drugs--same or different? Value Health 2009;12:402–4. 10.1111/j.1524-4733.2008.00476_1.x [DOI] [PubMed] [Google Scholar]
- 22.Taylor RS, Iglesias CP. Assessing the clinical and cost-effectiveness of medical devices and drugs: are they that different? Value Health 2009;12:404–6. 10.1111/j.1524-4733.2008.00476_2.x [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandrik OL, Severens JLH, Bardach A, et al. Critical appraisal of systematic reviews with costs and cost-effectiveness outcomes: an ISPOR good practices Task force report. Value Health 2021;24:463–72. 10.1016/j.jval.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 25.Sagili DKM, Nilgiriwala Malaisamy, Soli K. Consensus health economic criteria (CheC) extended checklist for quality assessment of the included studies. Plos One 2018. [Google Scholar]
- 26.Evers S, Goossens M, de Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. Int J Technol Assess Health Care 2005;21:240–5. 10.1017/S0266462305050324 [DOI] [PubMed] [Google Scholar]
- 27.Bhanvadia R, Ashbrook C, Gahan J, et al. Perioperative outcomes and cost of robotic vs open simple prostatectomy in the modern robotic era: results from the National inpatient sample. BJU Int 2021;128:168–77. 10.1111/bju.15258 [DOI] [PubMed] [Google Scholar]
- 28.Kang HW, Yun S-J, Chung JI, et al. National practice patterns and direct medical costs for prostate cancer in Korea across a 10 year period: a nationwide population-based study using a national health insurance database. BMC Health Serv Res 2019;19:408. 10.1186/s12913-019-4218-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal C, Aprikian A, Cury F, et al. Clinical management and burden of prostate cancer: a Markov Monte Carlo model. PLoS One 2014;9:e113432. 10.1371/journal.pone.0113432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu D, Lehmann HP, Frick KD, et al. Active surveillance versus surgery for low risk prostate cancer: a clinical decision analysis. J Urol 2012;187:1241–6. 10.1016/j.juro.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratchanon S, Apiwattanasawee P, Prasopsanti K. A cost-utility analysis of laparoscopic radical prostatectomy and robotic-assisted laparoscopic radical prostatectomy in men with localized prostate cancer in Thailand. J Med Assoc Thai 2015;98 Suppl 1:S14–20. [PubMed] [Google Scholar]
- 32.Teljeur C, O'Neill M, Moran P, et al. Using prediction intervals from random-effects meta-analyses in an economic model. Int J Technol Assess Health Care 2014;30:44–9. 10.1017/S0266462313000676 [DOI] [PubMed] [Google Scholar]
- 33.AlbertaHealth . robot-assisted laparoscopic prostatectomy (RALP) : final report. 2017. Available: https://open.alberta.ca/dataset/c48e8a3f-17b7-46f0-8aa5-5dbdd0973551/resource/bd3cd633-e7ad-4d5f-a896-dd8e3d81a50b/download/ahtdp-prostatectomy-ralp-2017.pdf [Accessed 29 Jul 2021].
- 34.de Oliveira RAR, Guimarães GC, Mourão TC, et al. Cost-Effectiveness analysis of robotic-assisted versus retropubic radical prostatectomy: a single cancer center experience. J Robot Surg 2021;15:859–68. 10.1007/s11701-020-01179-z [DOI] [PubMed] [Google Scholar]
- 35.MSAC . Laparoscopic remotely assisted radical prostatectomy, 2006. [Google Scholar]
- 36.O'Malley SP, Jordan E. Review of a decision by the medical services Advisory Committee based on health technology assessment of an emerging technology: the case for remotely assisted radical prostatectomy. Int J Technol Assess Health Care 2007;23:286–91. 10.1017/S0266462307070390 [DOI] [PubMed] [Google Scholar]
- 37.Parackal A, Tarride J-E, Xie F, et al. Economic evaluation of robot-assisted radical prostatectomy compared to open radical prostatectomy for prostate cancer treatment in Ontario, Canada. Can Urol Assoc J 2020;14:E350–7. 10.5489/cuaj.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.HIQA . Health technology assessment of robot-assisted surgery in selected surgical procedures, 2011. [Google Scholar]
- 39.Becerra V, Ávila M, Jimenez J, et al. Economic evaluation of treatments for patients with localized prostate cancer in Europe: a systematic review. BMC Health Serv Res 2016;16:541. 10.1186/s12913-016-1781-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess 2012;16:1–313. 10.3310/hta16410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalewski KF, Seifert L, Ali S, et al. Functional outcomes after laparoscopic versus robotic-assisted rectal resection: a systematic review and meta-analysis. Surg Endosc 2021;35:81–95. 10.1007/s00464-019-07361-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarricone R, Torbica A, Drummond M, et al. Key recommendations from the MedtecHTA project. Health Econ 2017;26 Suppl 1:145–52. 10.1002/hec.3468 [DOI] [PubMed] [Google Scholar]
- 43.Vonlanthen R, Lodge P, Barkun JS, et al. Toward a consensus on centralization in surgery. Ann Surg 2018;268:712–24. 10.1097/SLA.0000000000002965 [DOI] [PubMed] [Google Scholar]
- 44.Trinh Q-D, Bjartell A, Freedland SJ, et al. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol 2013;64:786–98. 10.1016/j.eururo.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothery C, Claxton K, Palmer S, et al. Characterising uncertainty in the assessment of medical devices and determining future research needs. Health Econ 2017;26 Suppl 1:109–23. 10.1002/hec.3467 [DOI] [PubMed] [Google Scholar]
- 46.Tewari A, Sooriakumaran P, Bloch DA, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 2012;62:1–15. 10.1016/j.eururo.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 47.Drummond M, Tarricone R, Torbica A. Economic evaluation of medical devices. Oxford: Oxford University Press, 2018. [Google Scholar]
- 48.Nabi J, Friedlander DF, Chen X, et al. Assessment of out-of-pocket costs for robotic cancer surgery in US adults. JAMA Netw Open 2020;3:e1919185. 10.1001/jamanetworkopen.2019.19185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pucheril D, Fletcher SA, Chen X, et al. Workplace absenteeism amongst patients undergoing open vs. robotic radical prostatectomy, hysterectomy, and partial colectomy. Surg Endosc 2021;35:1644–50. 10.1007/s00464-020-07547-y [DOI] [PubMed] [Google Scholar]
- 50.Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 2018;74:422–9. 10.1016/j.eururo.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058394supp001.pdf (118.6KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.