Abstract
Polycystic ovary syndrome (PCOS) is a common endocrine disorder, defined by reproductive and endocrine abnormalities, with metabolic dysregulation including obesity, insulin resistance and hepatic steatosis. Recently, it was found that skeletal muscle insulin sensitivity could be improved in obese, post-menopausal, pre-diabetic women through treatment with nicotinamide mononucleotide (NMN), a precursor to the prominent redox cofactor nicotinamide adenine dinucleotide (NAD+). Given that PCOS patients have a similar endocrine profile to these patients, we hypothesised that declining NAD levels in muscle might play a role in the pathogenesis of the metabolic syndrome associated with PCOS, and that this could be normalized through NMN treatment. Here, we tested the impact of NMN treatment on the metabolic syndrome of the dihydrotestosterone (DHT) induced mouse model of PCOS. We observed lower NAD levels in the muscle of PCOS mice, which was normalized by NMN treatment. PCOS mice were hyperinsulinaemic, resulting in increased adiposity and hepatic lipid deposition. Strikingly, NMN treatment completely normalized these aspects of metabolic dysfunction. We propose that addressing the decline in skeletal muscle NAD levels associated with PCOS can normalize insulin sensitivity, preventing compensatory hyperinsulinaemia, which drives obesity and hepatic lipid deposition, though we cannot discount an impact of NMN on other tissues to mediate these effects. These findings support further investigation into NMN treatment as a new therapy for normalizing the aberrant metabolic features of PCOS.
Keywords: NMN, Hyperandrogenism, Polycystic ovary syndrome (PCOS), Animal model
Highlights
-
•
Polycystic ovarian syndrome (PCOS) leads to declining NAD+ levels in skeletal muscle tissue.
-
•
Declining NAD+ in muscle can be rescued by treating with the metabolic precursor nicotinamide mononucleotide (NMN).
-
•
NMN treatment corrects compensatory hyperinsulinemia in a mouse model of PCOS.
-
•
Reductions in circulating insulin levels from NMN treatment moderates adipose expansion and hepatic lipid deposition.
1. Introduction
Polycystic ovary syndrome (PCOS) is a common and complex endocrine disorder which is diagnosed by a woman exhibiting at least two of the following three diagnostic criteria: clinical and/or biochemical hyperandrogenism, oligo-ovulation or anovulation and polycystic ovary morphology (PCOM) observed on ultrasound [67]. These patients also suffer from a wider range of secondary conditions, including reproductive, endocrine, metabolic and psychological features [21,67]. Adverse metabolic features, although not included as diagnostic criteria, are present in the majority of women suffering from PCOS [60] and include insulin resistance, obesity, dyslipidemia, liver steatosis and an increased risk of type 2 diabetes and cardiovascular disease [21,28,49,58].
A range of treatment options are available for the management of PCOS, ranging from lifestyle modifications (diet and exercise interventions) and pharmacological treatments to more invasive strategies such as laparoscopic ovarian drilling and bariatric surgery [67]. Despite the major health and economic impacts of PCOS, there are no regulatory approved drugs for the treatment of PCOS [56] and current management strategies are suboptimal as they rely on symptomatic treatment [67]. Excess body weight is present in 30–75% of women with PCOS [24,32], and obesity worsens the presentation of symptoms of PCOS [26,36,48]. Weight loss is effective in improving clinical outcomes associated with PCOS [32], and consequently the 2018 international evidence-based guideline for the assessment and management of PCOS recommends that lifestyle interventions should be applied to all women with PCOS [67]. Despite the reported beneficial effect of a modest weight loss of 5–15% on many key features of PCOS [50], weight management is particularly challenging for patients with PCOS, with a Cochrane review reporting attrition rates of up to 46% in women with PCOS undertaking lifestyle intervention studies [47]. Moreover, clinical data implies that weight management interventions may be less effective in women with PCOS than in women without PCOS [66], which is congruent with recent evidence demonstrating that a hyperandrogenic PCOS mouse model displayed a decreased capacity for its metabolic system to respond to variations in diet [57]. Elevated androgens represent a major feature of PCOS [37] and numerous lines of evidence support a role for androgens in the development of the PCOS characteristics [70]. Targeted suppression of androgen excess using anti-androgenic drugs can improve several features of PCOS [70], however there is also evidence that antiandrogens have unacceptable hepatotoxicity [18], limiting their use in PCOS. Therefore, while systemic androgen blockade is a logical approach for treating PCOS, new treatments are needed to address the impacts of PCOS.
Insulin resistance and metabolic dysfunction during PCOS is distinct from the metabolic syndrome classically observed in pre-diabetics. Hyperinsulinaemic euglycaemic clamp studies of PCOS patients demonstrate profound peripheral insulin resistance, regardless of obesity, however the suppression of hepatic glucose output remains insulin sensitive in lean patients [22]. Similarly, androgen administration severely impairs peripheral insulin resistance, but does not impact hepatic glucose output in females [19,54]. As expected during insulin resistance, PCOS patients have elevated insulin levels [40], accompanied by increased adiposity and hepatic steatosis which may be secondary to hyperinsulinaemia and insulin resistance [22]. Metformin is an insulin sensitising drug, commonly used in women with PCOS, however there are varying reports on its efficacy to ameliorate metabolic features of PCOS [67]. Given the distinct nature of insulin resistance in PCOS patients, interventions that enhance insulin sensitivity in muscle would be uniquely positioned to improve overall metabolic homeostasis in PCOS patients.
One approach to restoring insulin sensitivity is through elevation of the essential redox cofactor nicotinamide adenine dinucleotide (NAD+), levels of which decline during ageing and metabolic disease [39] and recently NAD levels were identified to be impaired in granulosa cells from PCOS ovaries [72]. NAD is required for energy metabolism, DNA repair and epigenetic maintenance, and its levels can be restored through treatment with its metabolic precursors nicotinamide mononucleotide (NMN) [76] and nicotinamide riboside (NR) [9]. Extensive preclinical studies have found that treatment with these precursors can ameliorate metabolic disease [76], sub-fertility [7,45,55], neurocognitive disorders [35] and overall late-life health [46,78]. Importantly, a recent placebo-controlled, double-blind clinical trial of NMN treatment in post-menopausal, obese, prediabetic women demonstrated that NMN restored insulin sensitivity in skeletal muscle [77]. Given that the endocrine and metabolic profile of this patient population of obese, prediabetic post-menopausal women partially resembles the profile observed in younger PCOS patients, we sought to test the hypothesis that insulin resistance in skeletal muscle induced during a hyperandrogenic PCOS environment was mediated by a decline in NAD levels. If true, this would support the therapeutic targeting of insulin resistance and metabolic dysfunction associated with PCOS through NMN treatment. Here, we find that development of a PCOS animal model by DHT exposure of peripubertal female mice, leads to a decline in muscle NAD levels, but restoring these levels using NMN treatment corrects hyperinsulinaemia, adiposity and hepatic steatosis.
2. Results
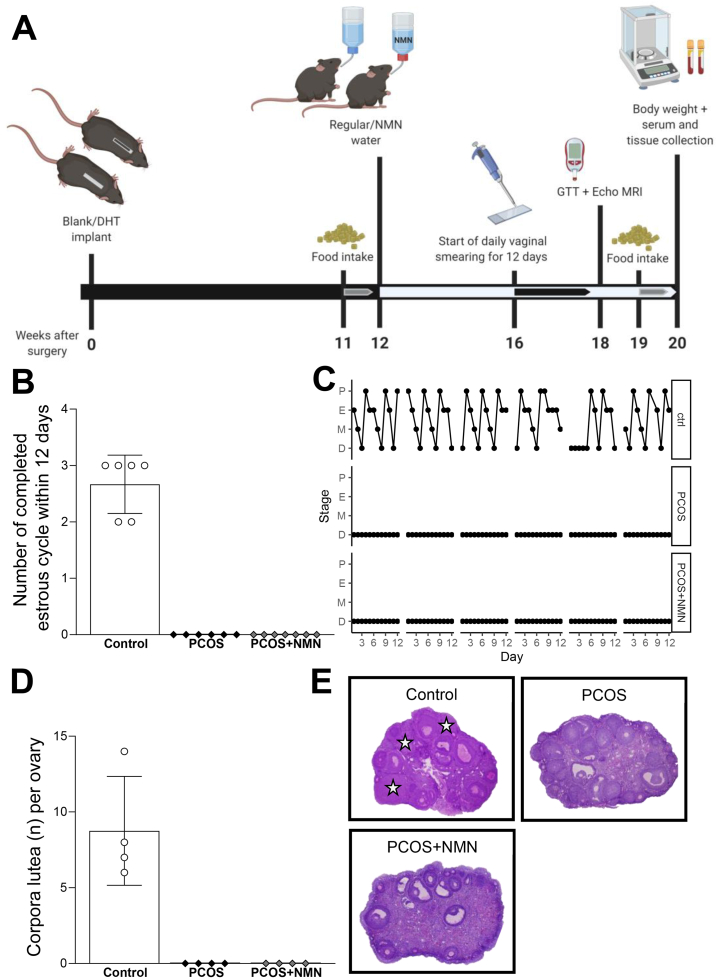
In this study, we used chronic dihydrotestosterone (DHT) treatment in peripubertal female mice, a well-established animal model of PCOS [65]. After 12 weeks of DHT treatment, animals also received NMN through its addition to their drinking water (2 g/L) for a further 8 weeks, as summarised in Figure 1A. DHT treatment recapitulated the reproductive features of PCOS, with a disruption of regular estrous cycling patterns (Figure 1B,C). In addition, disruption of ovulation, a defining characteristic of PCOS, was also recapitulated in this model, confirmed by the absence of corpora lutea in the ovaries of DHT exposed females (Figure 1D,E). These data confirm our ability to model the reproductive features of PCOS in a mouse model.
Figure 1.
Experimental design and validation.A. For this study, PCOS was induced in female mice by subcutaneous insertion of a dihydrotestosterone (DHT) implant in a peripubertal mice for 12 weeks. Control mice were implanted with a blank (empty) pellet. Mice were treated for 8 weeks with NMN (2 g/L) via drinking water or regular water from week 12. Food intake, estrous cycling, fasted blood glucose and insulin and glucose tolerance test (GTT) were assessed before collection of serum and tissues following 8 full weeks of NMN administration. B Number of completed cycles in a 12-day period, showing DHT-induced acyclicity in PCOS females and NMN had no influence on the number of completed cycles, n = 6–7 mice per experimental group. Data expressed as the mean ± SEM. C, Estrous cycle pattern in representative females. P, proestrus; E, estrus; M, metestrus; D, diestrus. D, Number of corpora lutea per ovary, showing DHT-induced anovulation in PCOS mice and there was no significant effect of NMN, n = 4 ovaries per experimental group. Data expressed as the mean ± SEM. E, Histological sections of representative ovaries from Control and DHT-induced PCOS mice treated with and without the NMN. Star, corpora lutea.
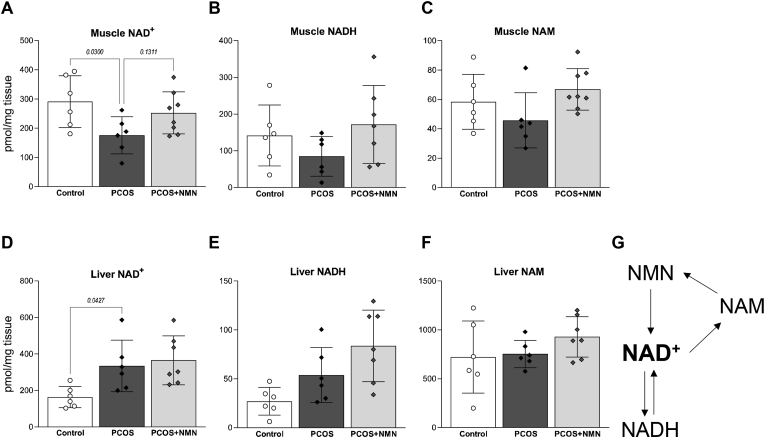
To investigate the hypothesis that insulin resistance associated with a hyperandrogenic PCOS environment is due to declining NAD levels in muscle, NAD metabolites were measured using mass spectrometry (Figure 2). Consistent with our hypothesis, PCOS mice exhibited a significant decline in NAD+ levels in muscle (Figure 2A, P < 0.05), but not liver (Fig. 2D), which also remains insulin sensitive in lean PCOS patients or females treated with androgens [19,22,54]. This decline in NAD levels in muscle was attenuated by treatment with NMN (Fig. 2A), which acts as an immediate precursor to NAD biosynthesis (Fig. 2G). A similar trend was observed for NADH, the reduced form of NAD, and nicotinamide, which is both a breakdown product and a precursor that is recycled back into NAD synthesis.
Figure 2.
PCOS depletes the muscle NAD + metabolome. Levels of NAD+, NADH and nicotinamide (NAM) in muscle (A–C) and liver (D–F) of PCOS mice treated with or without NMN. These metabolites form part of the recycling pathway for NAD+ synthesis, shown in G. Metabolites measured by mass spectrometry and normalised to tissue weight. n = 6–8 per group and data expressed as mean ± SD. Analysed by one-way ANOVA with a post-hoc Dunnett's multiple comparison test, p-value results as indicated on graphs.
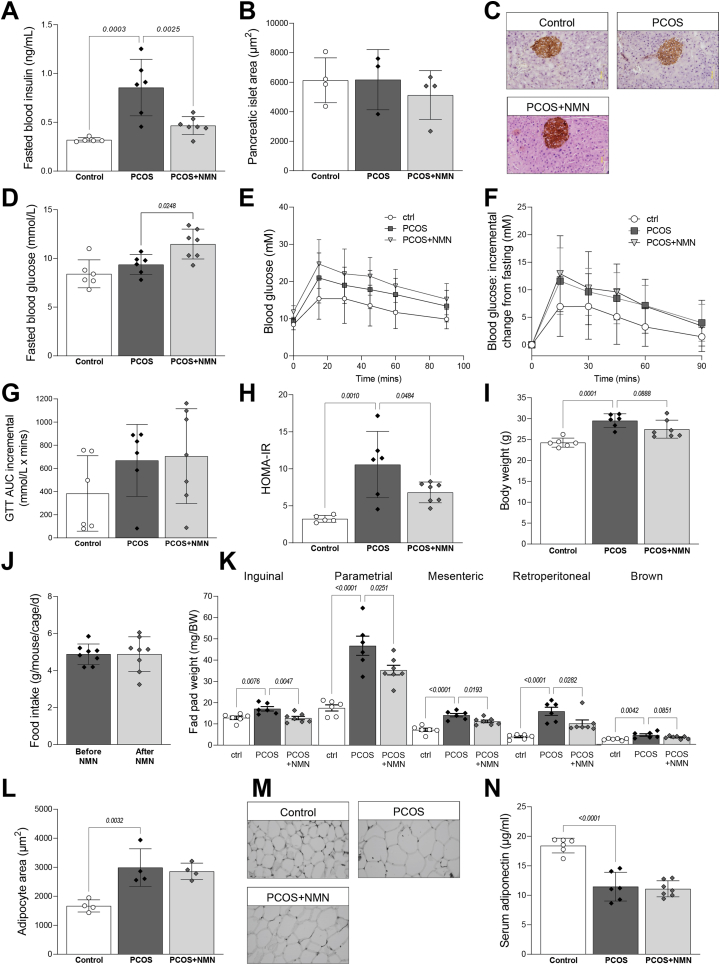
Given the recent finding that NMN can improve whole body glucose homeostasis and muscle insulin sensitivity in post-menopausal, obese, pre-diabetic women [77], we assessed the impact of NMN treatment on metabolic homeostasis in this PCOS model. Elevated fasting insulin levels have been reported in the majority of PCOS patients, most likely reflecting insulin resistance [60]. Consistent with this, DHT treatment significantly elevated fasting blood insulin, to levels that were more than doubled in PCOS mice compared to control mice (Figure 3A; P < 0.01). NMN co-treatment reduced fasting insulin almost to the levels of untreated controls, which could reflect reduced compensatory insulin secretion. Alternatively, these data could be consistent with a direct impact of NMN on insulin secretion from pancreatic β-cells, which is elevated in PCOS [19,31,40], although pancreatic islet mass was unchanged between treatments (Figure 3B,C), and previous work has failed to show an impact of chronic NMN treatment on β-cells function [63]. NMN treatment resulted in a slight increase in fasting blood glucose levels (Fig. 3D), however the homeostatic model of assessment for insulin resistance (HOMA-IR) demonstrated a strong reduction in insulin resistance from NMN co-treatment (Fig. 3E). No change in glucose tolerance was observed, as quantified by the incremental area under the curve (iAUC) of glucose tolerance tests (Fig. 3F).
Figure 3.
NMN ameliorates DHT-induced hyperinuslinaemia and obesity.A. Fasting blood insulin levels are increased with DHT treatment and reduced by NMN co-treatment, despite no change in B pancreatic islet area, as assessed by immunohistochemistry, with C representative images shown. D, Fasting blood glucose levels were obtained during glucose tolerance test (GTT), with E incremental area under the curve (iAUC) of GTTs shown. These fasting values were used to calculate F, homeostatic measure of insulin resistance (HOMA-IR), showing a significant increase in HOMA-IR in PCOS mice, but a partial amelioration in PCOS + NMN females. DHT treatment increased G body weight, with no impact of NMN treatment on H food intake in PCOS + NMN mice before and after treatment. PCOS mice were obese, with I increased fat pad weights in inguinal, parametrial, mesenteric, retroperitoneal and brown fat-pad weights, however these were reduced in animals co-treated with NMN. J, Histomorphometric assessment of adipocyte diameter in parametrial fat pads, with K, representative histology shown. Increased adipocyte dimeter was matched by F decreased serum levels of adiponectin. n = 6–7 per experimental group and data are expressed as the mean ± SD. Analysed by one-way ANOVA with a post-hoc Dunnett's multiple comparison test, p-value results as indicated on graphs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Hyperinsulinaemia can drive obesity through promoting adipocyte hypertrophy, adipocyte hyperplasia, glucose uptake and de novo lipogenesis [10,41,42,73]. Increased adiposity is also a well-known clinical feature of PCOS [49], which can be recapitulated in this hyperandrogenic PCOS animal model [1,13]. In agreement with this, DHT treated animals displayed a 20% increase in overall body weight (Fig. 3G) and a significant increase in fat mass, with an increase in weight observed in inguinal, parametrial, mesenteric, retroperitoneal and brown adipose depots (Fig. 3I). The increases in fat pad weights were ameliorated by NMN treatment for all adipose depots (P < 0.05), except brown fat pads. This reduction in fat mass weight in PCOS + NMN mice was likely unrelated to food intake, as this was unchanged before and after NMN treatment (Figure 3H). Histomorphometry analysis of parametrial fat pads revealed that DHT-induced PCOS resulted in adipocyte hypertrophy, with an ∼80% increase in area, compared to untreated control females (Figure 3J,K; P < 0.01), though unlike overall fat pad mass, this was not impacted by NMN treatment. This adipocyte hypertrophy was matched by a reduction in adiponectin levels (Figure 3L), which matches the known inverse relationship between adipocyte diameter and adiponectin levels [43], as well as the reductions in adiponectin levels observed in PCOS patients [3] and PCOS animal models [5,14].
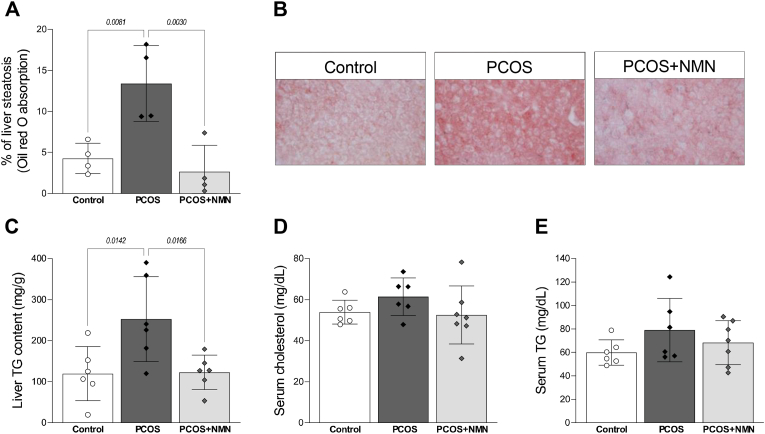
In addition to promoting adipose tissue expansion, hyperinsulinaemia can also drive ectopic lipid deposition in the liver through stimulating de novo lipogenesis [42,62], with non-alcoholic fatty liver disease (NAFLD) a common feature of PCOS [11]. In the current study, livers from PCOS mice displayed a 3-fold increase in oil red O staining, which indicates lipid droplets (Figure 4A; P < 0.01). Strikingly, this increase in lipid deposition in PCOS mice was almost completely abolished by NMN treatment (Figure 4A; P < 0.01 and Figure 4B). This was confirmed through measurements of total triglyceride content, which doubled in PCOS mice and was reduced to control levels in PCOS mice that received NMN (Figure 4C; P < 0.01). While dyslipidaemia has been reported in patients with PCOS [33], no difference in serum levels of cholesterol (Figure 4D) or triglycerides (Figure 4E) were identified between any of the treatment groups (P > 0.05).
Figure 4.
Resolution of hepatic steatosis with NMN treatment. Liver sections were subject to oil red O staining to assess hepatic lipid deposition, as assessed by A, area of oil red O staining in each group, with B representative sections shown. The pattern of oil red O staining was consistent with measurements of C liver triglyceride content, showing a DHT-induced increase in PCOS mice, and a beneficial impact of NMN administration. Unlike liver, serum levels of D cholesterol and E triglycerides showed no differences between experiment groups. n = 6–7 per experimental group and data are expressed as the mean ± SEM. Analysed by one-way ANOVA with a post-hoc Dunnett's multiple comparison test, p-value results as indicated on graphs. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
Metabolic traits associated with reduced NAD+ levels such as obesity, insulin resistance and hepatic steatosis are common in women with PCOS. Recent clinical evidence supports the ability of the NAD precursor nicotinamide mononucleotide (NMN) to restore insulin sensitivity in the muscle of obese, pre-diabetic post-menopausal women [77]. Given the similarities in endocrine and metabolic profile between this population and patients with PCOS, and the recent description of reduced NAD levels in the granulosa cells of PCOS patients [72], we hypothesised that NAD deficiency in muscle may play a role in the distinct profile of insulin resistance observed in women with PCOS.
Here, we showed that NAD levels in muscle are strongly reduced in an hyperandrogenized rodent model of PCOS, and that treatment with the NAD precursor NMN can correct this deficit. This restoration of NAD levels in muscle was accompanied by an improvement in a range of metabolic parameters induced by DHT treatment, including hyperinsulinaemia, obesity and hepatic lipid deposition. Increased fasting insulin levels are a widely recognised feature of PCOS, which occurs even in lean PCOS patients [31,40]. Hyperinsulinaemia is viewed as a compensatory response to peripheral insulin resistance, and can in fact both precede and drive subsequent obesity [73]. Attenuation of insulin signalling either through genetic deletion of its cognate receptor or of insulin itself can ameliorate adiposity and hepatic lipid deposition [8,41,42,52], again highlighting the importance of fasting hyperinsulinaemia in driving these conditions. In accordance with this, we found that the reduction in fasting insulin levels due to NMN treatment in PCOS mice was correlated with decreased adiposity and hepatic steatosis. Together, we propose chronic androgen receptor signalling in muscle lowers NAD levels, resulting in decreased insulin sensitivity and compensatory hyperinsulinaemia, which drives hepatic de novo lipogenesis and adipose tissue expansion. Correction of this NAD deficit in muscle using NMN treatment could therefore be used as a strategy to address the distinct metabolic syndrome observed during PCOS. It is important to note however that our data cannot exclude the possibility that NMN may impact these metabolic effects through its actions on other tissues. For example, our data would be consistent with a model where NMN could act on pancreatic β-cells to suppress insulin secretion, however previous in vitro work have not found this to be the case [16,63].
One alternative interpretation of these data is that NMN treatment could directly act on the liver, for example, through activating the mitochondrial unfolded protein response (mtUPR) [27,30,51], which corrects diet induced hepatic steatosis. Similarly, other groups have identified direct mechanisms whereby NAD precursors can reverse liver fibrosis and NAFLD via SIRT1 and SIRT3-dependent processes [2,27,71]. While previous work has shown that hepatic NAD+ levels are reduced in a high-fat high-sucrose diet mouse model of NAFLD [27], we did not identify a reduction in hepatic NAD levels in PCOS mice – unlike muscle, NAD levels in the liver were elevated above control animals. Given this, it is unlikely that a PCOS environment or NMN treatment impact hepatic steatosis through altering NAD levels in the liver. Although we and others have previously shown that NMN treatment reduces hepatic triglyceride content in diet and ageing models [46,68,69], we propose that the profound differences in liver triglycerides with NMN treatment in this PCOS model are secondary to a resolution of hyperinsulinaemia, which is itself driven by peripheral insulin resistance. This finding could provide an important insight into the distinct differences in the aetiology of the metabolic syndrome associated with PCOS, compared to that observed during diet induced obesity. In particular, this would match the finding from hyperinsulinaemic euglycaemic clamp studies where women with classified lean PCOS have profound peripheral insulin resistance, while changes to hepatic glucose output are unchanged, with subsequent hepatic insulin resistance only occurring during subsequent obesity [22]. Similarly, chronic treatment with exogenous androgens impacts peripheral, but not hepatic insulin sensitivity in female to male transexuals, and in females receiving acute treatment with methyltestosterone [19,54].
This reduction in liver triglyceride content has important implications for PCOS patients. NAFLD is characterised by lipid accumulation and is the most common chronic liver disease worldwide [38], and can proceed to non-alcoholic steatohepatitis (NASH), cirrhosis and an increased risk of hepatocellular carcinoma (HCC) [34]. Changes in hepatic lipid content during hyperinsulinemia are primarily due to increased de novo lipogenesis [62], and future work should aim to test whether the differences in hepatic lipid content observed here are indeed due to insulin-induced de novo lipogenesis.
Obesity plays a key role in the pathogenesis of PCOS and is present in 40–70% of patients with the condition [32,36]. Compared to control females, DHT-exposed females exhibited a significant increase in body weight and fat-pad weights which accords with clinical studies demonstrating that women with PCOS manifest global adiposity [4]. Strikingly, treatment with NMN overcame the effects of DHT on fat-pad weights in the present study, and these reductions were not associated with reduced food intake of the PCOS mice. Insulin signalling can both drive and is required for obesity [8,10,41,42,52], and as with the resolution of hepatic triglyceride content, we propose that the impact of NMN on adiposity observed here is due to a resolution of insulin resistance and compensatory hyperinsulinaemia. Our interpretation of these results is limited by the fact that we did not directly assess insulin sensitivity in muscle, for example using the hyperinsulinaemic euglycaemic clamp method. This method was used to identify an increase in skeletal muscle insulin sensitivity after NMN supplementation in post-menopausal, prediabetic women [77], hence it will be important to assess this in either a preclinical model of PCOS, or, given the clinical trials of NMN, in PCOS patients.
While we were inspired to complete this study based on the idea that NMN could restore metabolic health in PCOS due to impacts on muscle resulting in improved insulin sensitivity [77], an alternative explanation to our findings of reduced hepatic steatosis and adiposity with NMN treatment could instead be due to direct actions on pancreatic β-cells. In this alternate explanation, NMN would reduce insulin secretion, which would explain reductions in insulin driven adiposity, hepatic steatosis and the slight increase in fasting glucose levels. This idea would be supported by the slight increase in fasting blood glucose levels with NMN treatment (Fig. 3D). What is missing from our study was a direct measurement of insulin sensitivity in peripheral tissues, which should ideally be addressed using hyperinsulinaemic euglycaemic clamp experiments in future follow-up studies.
A key question from this work is how PCOS depresses NAD levels in muscle. One possibility is that declining NAD+ levels are a secondary impact from obesity, as has been described in diet-induced obesity models [17,27,76]. Importantly, this decline in NAD+ with obesity occurs in liver, but not in muscle – the opposite of our findings from the DHT model of PCOS (Figure 2A,D), where DHT treatment actually increased hepatic NAD, despite the presence of obesity – again highlighting the distinct metabolic profile of PCOS. Although this work does not provide a mechanism for the loss of NAD+ in the muscle of PCOS mice, we speculated that aberrant PARP activation due to androgen receptor signalling may be involved. The greatest consumers of NAD levels in the cell are members of the poly-ADP-ribose polymerase (PARP) family of enzymes, which are involved in DNA repair and epigenetic regulation. These enzymes consume NAD as a substrate to carry out ADP-ribosylation, hence PARP inhibition or activation profoundly impacts cellular NAD levels [6]. Androgen receptor signalling is ablated by PARP1 inhibition [59], the androgen receptor also interacts with PARP2 to regulate gene expression [29], and androgen receptor signalling induces the production of PARP7, which acts with PARP9 to mediate androgen receptor signalling [74]. The NAD+ dependent deacetylase SIRT1 deacetylates the ligand bound androgen receptor to dampen its signalling [25], however unlike the PARP enzymes it is unclear whether NAD consumption by members of the sirtuin family are sufficient to deplete overall NAD levels. While this idea was supported by our finding that DHT treatment induced PARylation in vitro, the absence of changes in NAD+ levels suggest other mechanisms are at play. These could include an increase in inflammation observed in PCOS [23], which could induce activity of the NAD+ glyochydrolase CD38, however the presence of inflammation in PCOS is subject to debate [20].
Induction of hyperandrogenism recapitulates a broad variety of endocrine, reproductive and metabolic PCOS traits in a range of animal models [64]. Consistent with this, in the present study DHT-exposed females were totally acyclic and exhibited ovulatory dysfunction. Previous studies in aged mice using NMN and NR observed enhancements in ovulation rate and oocyte quality [7,44,75]. Ovulation rates were not affected in the present study, suggesting that androgen-mediated reproductive PCOS-like traits are not due to dysregulated NAD+ metabolism.
PCOS is characterised by a distinct profile of metabolic dysregulation. Here, we show that PCOS is associated with deficiencies in the NAD+ metabolome in muscle but not in liver, matching the distinct pattern of insulin resistance observed in PCOS patients [22,54]. This decline can be corrected by treatment with NMN, resulting in a reduction in fasting insulin levels, decreased adiposity and reduced fatty liver. Given the ongoing clinical translation of NMN and the widespread availability of other precursors such as NR, this work may represent a promising strategy for treating the metabolic traits of PCOS in women.
4. Materials and methods
4.1. Mouse housing and experimental procedures
Female C57Bl6 mice were purchased from Australian Bio-Resources (ABR; Moss Vale, NSW, Australia). Mice were maintained under standard housing conditions (ad libitum access to food and water in a temperature- and humidity-controlled, 12-hour light/dark environment) at the Biological Resources Centre facility at UNSW (Sydney, Australia). Subcutaneous implantation surgeries and blood collection via cardiac puncture were performed under isoflurane inhalation anaesthesia. All procedures were approved by the Animal Care and Ethics committee of the University of New South Wales within National Health and Medical Research Council guidelines for animal experimentation.
4.2. Experiment design
To examine the effect of NMN on a range of metabolic and reproductive traits of PCOS, a DHT-induced PCOS mouse model was used. Based on our previous development of a PCOS mouse model [13], peripubertal (4 week-old) female mice were implanted s.c. with a DHT 1-cm Silastic implant (i.d., 1.47 mm; o.d., 1.95 mm; Dow Corning; 508–006) containing about 10 mg DHT. Controls were implanted with an empty implant. Silastic implants provide steady-state DHT release for at least 6 months [61]. After 12 weeks of implant exposure, NMN treatment (2 g/L) via drinking water was administrated to a subset of DHT-induced PCOS mice for 8 weeks. All water in this animal house is acidified to pH3 with HCl to decrease microbial growth. NMN (GeneHarbor Biotechnology, Hong Kong, China) was added to drinking water at 2 g/L as previously described [7]. Groups were identified as Control (n = 6), PCOS (n = 6), PCOS + NMN (n = 7). Pre-collection assessments of food intake, estrous cycle patterns, glucose tolerance test (GTT) and measurement of fasted circulating glucose and insulin were performed during the 8 weeks of NMN treatment (Figure 1). Food intake was assessed by daily measurements of chow pellet mass in each cage for five consecutive days. Terminal collections were performed at the diestrus stage of the estrous cycle, under 1–2% isoflurane inhalation anaesthesia (Henry Schein, Mascot, Australia). Mice were sacrificed and tissues collected 8 weeks post-NMN treatment (Figure 1). At the time of tissue collection, all implants were removed and checked to ensure they still contained DHT powder in them (which they did) and had not ruptured or leaked.
4.3. Adipose tissue morphometry
Dissected white inguinal (representing subcutaneous fat depot), parametrial, mesenteric, and retroperitoneal (all representing visceral fat depots) fat pads and interscapular brown fat pads were weighed. Parametrial fat pads were fixed in 4% (w/v) paraformaldehyde overnight at 4 °C and stored in 70% ethanol before histological processing. Fixed parametrial fat pads were embedded in paraffin, sectioned at 8 μm and stained with hematoxylin and eosin. To assess adipocyte cell size, five images from three distinct sections of each parametrial fat pad (at least 160 μm apart) were taken using an Olympus microscope (DP70). Adipocyte area was quantified using ImageJ version 1.51 software (NIH), as previously described [13]. All parametrial fat pad assessments were performed blinded.
4.4. Circulating adiponectin levels
A Quantikine ELISA Kit from R&D Systems (catalog no. MRP300) was used to determine serum concentrations of total full-length mouse adiponectin according to the manufacturer's instructions. The mouse adiponectin detection limit was 0.003 ng/mL, intra-assay coefficient of variation (CV) was 6.1% and inter-assay CV was 5.8%.
4.5. Fasted blood glucose and glucose tolerance test (GTT)
Fasting glucose levels were measured after 6 h of fasting. Glucose tolerance tests were performed by blood glucose measurement after an i.p. glucose injection as previously reported [13]. Briefly, after measuring the baseline blood glucose, mice were i.p. injected with glucose at 2 g/kg body weight (BW). Blood glucose was then measured at 15, 30, 60 and 90 min after the glucose injection. Blood was obtained from a tail prick, and blood glucose was measured with glucose strips and an Accu-Chek glucometer (Roche).
4.6. Fasting insulin and HOMA-IR
On the same day as the GTT and after a 6-hour fasting period, 5 μL of the blood from the tail prick was collected and insulin levels were measured using an Ultra Sensitive Mouse Insulin ELISA kit (Crystal Chem, Catalog #90080). Fasting glucose and fasting insulin levels were used to calculate the homeostasis model assessment of insulin resistance (HOMA-IR), using the following formula after the conversion of glucose unit from mmol/L to mg/dl: HOMA-IR = fasting glucose (mg/dL) X fasting insulin (μU/mL)/405 [53].
4.7. Assessment of hepatic steatosis
The right lateral lobe of the whole liver was isolated and fixed in 10% neutral buffered formalin overnight at 4 °C followed by immersion in 30% sucrose for 48-hours. From the isolated lobe, 1 cm × 1 cm sections were excised, embedded in OCT compound (TissueTek), cryosectioned at 10 μm and air-dried onto slides. Slides were stained with oil red O in 60% isopropanol to visualise lipid deposition, as previously described [15]. Three images from three distinct sections of liver (200 μm apart) were taken using an Olympus microscope (DP73). These images were used for quantification of the oil red O-positive staining using ImageJ version 1.51 software (NIH) to determine hepatic lipid deposition. All liver assessments were performed blinded.
4.8. Serum cholesterol and triglyceride levels and liver triglyceride content
Serum levels of total cholesterol and triglyceride were assayed enzymatically with commercial kits obtained from Wako (Cholesterol E Kit, 439–17501; Triglyceride Kit, 432–40201). Liver triglyceride content was determined by a triglyceride colorimetric quantification assay from Abcam (ab65336), according to manufacturer's instructions.
4.9. NAD+ and metabolite measurement
Tissue was crushed by A Cellcrusher tissue pulverizer (REF). ∼10 mg of the crushed tissue was transferred to an Eppendorf tube and snap-frozen. Tissue was placed in ice-cold 80% methanol, sonicated on ice for 2 × 30 s, incubated at −30 °C for 20 min, and then centrifuged at 14,000g for 10 min at 4 °C. Supernatants were collected and mixed with an equal volume of MTBE:Hexane (80:20), and shaken for 10 min. Ultrapure water (1:2 water to extract) was added, samples were shaken for 10 min, centrifuged at 14,000g for 3 min, and the lower phase was collected and dried down with a fixed volume of internal standard mixture (Nicotinamide-d4; β-NADH-d5 (d5-major) diammonium salt; β-NAD-d4 (major), from Toronto Research Chemicals, Canada) using vacuum centrifugation. Samples were reconstituted in 100 mM NH4OAc and filtered through a 0.22 μm filter. Metabolites were quantified by LC-MS/MS as described by [12] with minor modifications. Briefly, metabolites were separated on a Phenomenex NH2 column (150 mm × 2 mm x 3 μm) using a binary solvent gradient consisting of 5 mM NH4OAc (pH 9.5 adjusted with ammonia) and acetonitrile with a flow rate of 250 μl/min. Metabolites were measured using a using a TSQ Vantage mass spectrometer (ThermoFisher Scientific, Waltham, MA). Calibration curves of individual metabolites were constructed using the peak area ratios (peak area of the metabolite divided by peak area of the selected internal standard) of each calibrator versus its concentration. Selected internal standards were as follows: Nicotinamide-d4 for nicotinamide; β-NADH-d5 for NADH; β-NAD-d4 for NAD+. The concentrations of the endogenous metabolites in the tissue extracts were obtained from these calibration curves. Data were normalised to tissue weight. Spectra were processed and peak areas integrated using Xcalibur™ software (version 2.2, 2011, ThermoFisher Scientific). Data processing was performed using the LCquan feature of the software.
4.10. Assessment of estrous cyclicity
Four weeks post initiation of NMN treatment (16 weeks post-implantation surgery), vaginal epithelial cell smears were taken daily for 12 consecutive days and estrous cycle stage was determined by examination under light microscopy [13]. Estrous cycle stage classification was determined by the presence or absence of leukocytes, cornified epithelial, and nucleated epithelial cells as described previously [13]. An estrous cycle was defined as complete when a mouse exhibited all four stages of the estrous cycle in the following order: proestrus, estrus, metestrus and diestrus.
4.11. Ovarian morphometry and enumeration and health
Dissected ovaries were weighed, fixed in 4% (w/v) paraformaldehyde overnight at 4 °C and stored in 70% ethanol before histological processing. Ovaries were processed through graded alcohols and embedded in glycol methacrylate resin (Technovit 7100; Heraeus Kulzer). Serial sections of 20 μm were stained with periodic acid-Schiff and counterstained with hematoxylin. Corpora lutea were counted in every 3rd Corpora lutea (CL) were identified based on morphological properties consistent with luteinized follicles and by being observable throughout several serial sections. All ovaries were analysed blinded to treatment group.
4.12. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.4 software (GraphPad Software, San Diego, California) with statistical significance considered when P ≤ 0.05. Data that were not normally distributed were transformed before analysis using log transformation. Statistical differences were determined by t-test (food intake) or ordinary one-way ANOVA, with a post hoc test for comparisons between PCOS vs. control, and PCOS vs PCOS + NMN groups, using a Dunnett's multiple comparison test.
Acknowledgements
We thank Professor David Handelsman Andrology Laboratory, ANZAC Research Institute for access to the DHT implants. We thank Sonia Bustamante from the Mark Wainwright Analytical Centre (UNSW Sydney) for her technical guidance with the mass spectrometry.
Conflict of interest
AA, VRP, DR, MCE, BJC, WLL, RBG, MJB and KAW have nothing to disclose. LEW is a shareholder, director and advisor to Jumpstart Fertility and to Metro Biotech, which are developing NAD+ precursors for therapeutic use.
Funding
This work was supported by Australian National Health and Medical Research Council (NHMRC) Project Grants (APP1158540 and APP1139763) and NHMRC Fellowships to LEW and RBG (APP1127821 and APP1117538).
References
- 1.Aflatounian A., edwards M.C., Rodriguez Paris V., Bertoldo M.J., Desai R., Gilchrist R.B., et al. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. Journal of Endocrinology. 2020;245:381–395. doi: 10.1530/JOE-19-0530. [DOI] [PubMed] [Google Scholar]
- 2.Amano H., Chaudhury A., Rodriguez-Aguayo C., Lu L., Akhanov V., Catic A., et al. Telomere dysfunction induces sirtuin repression that drives telomere-dependent disease. Cell Metabolism. 2019;29:1274–1290 e9. doi: 10.1016/j.cmet.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldani D.P., Skrgatic L., Kasum M., Zlopasa G., Kralik Oguic S., Herman M. Altered leptin, adiponectin, resistin and ghrelin secretion may represent an intrinsic polycystic ovary syndrome abnormality. Gynecological Endocrinology. 2019:1–5. doi: 10.1080/09513590.2018.1534096. [DOI] [PubMed] [Google Scholar]
- 4.Barber T.M., Hanson P., Weickert M.O., Franks S. Obesity and polycystic ovary syndrome: implications for pathogenesis and novel management strategies. Clinical Medicine Insights: Reproductive Health. 2019;13 doi: 10.1177/1179558119874042. 1179558119874042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benrick A., Chanclon B., Micallef P., Wu Y., Hadi L., Shelton J.M., et al. Adiponectin protects against development of metabolic disturbances in a PCOS mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E7187–E7196. doi: 10.1073/pnas.1708854114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger N.A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiation Research. 1985;101:4–15. [PubMed] [Google Scholar]
- 7.Bertoldo M.J., Listijono D.R., Ho W.-H.J., Riepsamen A.H., Goss D.M., Richani D., et al. NAD+ repletion rescues female fertility during reproductive aging. Cell Reports. 2020;30:1670–1681. doi: 10.1016/j.celrep.2020.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biddinger S.B., Hernandez-Ono A., Rask-Madsen C., Haas J.T., Aleman J.O., Suzuki R., et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metabolism. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 10.Bluher M., Michael M.D., Peroni O.D., Ueki K., Carter N., Kahn B.B., et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Developmental Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 11.Brzozowska M.M., Ostapowicz G., Weltman M.D. An association between non-alcoholic fatty liver disease and polycystic ovarian syndrome. Journal of Gastroenterology and Hepatology. 2009;24:243–247. doi: 10.1111/j.1440-1746.2008.05740.x. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante S., Jayasena T., Richani D., Gilchrist R.B., Wu L.E., Sinclair D.A., et al. Quantifying the cellular NAD plus metabolome using a tandem liquid chromatography mass spectrometry approach. Metabolomics. 2018;14 doi: 10.1007/s11306-017-1310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caldwell A.S., Middleton L.J., Jimenez M., Desai R., Mcmahon A.C., Allan C.M., et al. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology. 2014;155:3146–3159. doi: 10.1210/en.2014-1196. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell A.S.L., Edwards M.C., Desai R., Jimenez M., Gilchrist R.B., Handelsman D.J., et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E3334–E3343. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell A.S.L., Edwards M.C., Desai R., Jimenez M., Gilchrist R.B., Handelsman D.J., et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proceedings of the National Academy of Sciences. 2017;114:E3334. doi: 10.1073/pnas.1616467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caton P.W., Kieswich J., Yaqoob M.M., Holness M.J., Sugden M.C. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia. 2011;54:3083–3092. doi: 10.1007/s00125-011-2288-0. [DOI] [PubMed] [Google Scholar]
- 17.Choi S.E., Fu T., Seok S., Kim D.H., Yu E., Lee K.W., et al. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway G., Dewailly D., Diamanti-Kandarakis E., Escobar-Morreale H.F., Franks S., Gambineri A., et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. European Journal of Endocrinology. 2014;171:1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 19.Diamond M.P., Grainger D., Diamond M.C., Sherwin R.S., Defronzo R.A. Effects of methyltestosterone on insulin secretion and sensitivity in women. Journal of Clinical Endocrinology and Metabolism. 1998;83:4420–4425. doi: 10.1210/jcem.83.12.5333. [DOI] [PubMed] [Google Scholar]
- 20.Duleba A.J., Dokras A. Is PCOS an inflammatory process? Fertility and Sterility. 2012;97:7–12. doi: 10.1016/j.fertnstert.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumesic D.A., Oberfield S.E., Stener-Victorin E., Marshall J.C., Laven J.S., Legro R.S. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocrine Reviews. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunaif A., Segal K.R., Shelley D.R., Green G., Dobrjansky A., Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–1266. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 23.Escobar-Morreale H.F., Luque-Ramirez M., Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertility and Sterility. 2011;95:1048–10458. doi: 10.1016/j.fertnstert.2010.11.036. e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escobar-Morreale H.F., Millán J.L.S. Abdominal adiposity and the polycystic ovary syndrome. Trends in Endocrinology and Metabolism. 2007;18:266–272. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Fu M., Liu M., Sauve A.A., Jiao X., Zhang X., Wu X., et al. Hormonal control of androgen receptor function through SIRT1. Molecular and Cellular Biology. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gambineri A., Pelusi C., Vicennati V., Pagotto U., Pasquali R. Obesity and the polycystic ovary syndrome. International Journal of Obesity. 2002;26:883. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- 27.Gariani K., Menzies K.J., Ryu D., Wegner C.J., Wang X., Ropelle E.R., et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glintborg D., Rubin K.H., Nybo M., Abrahamsen B., Andersen M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovascular Diabetology. 2018;17:37. doi: 10.1186/s12933-018-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gui B., Gui F., Takai T., Feng C., Bai X., Fazli L., et al. Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proceedings of the National Academy of Sciences of the United States of America. 2019;116:14573–14582. doi: 10.1073/pnas.1908547116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houtkooper R.H., Mouchiroud L., Ryu D., Moullan N., Katsyuba E., Knott G., et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibanez L., Potau N., Zampolli M., Prat N., Virdis R., Vicens-Calvet E., et al. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. Journal of Clinical Endocrinology and Metabolism. 1996;81:1237–1243. doi: 10.1210/jcem.81.3.8772605. [DOI] [PubMed] [Google Scholar]
- 32.Kataoka J., Tassone E.C., Misso M., Joham A.E., Stener-Victorin E., Teede H., et al. Weight management interventions in women with and without PCOS: a systematic review. Nutrients. 2017;9:996. doi: 10.3390/nu9090996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.kim J.J., Choi Y.M. Dyslipidemia in women with polycystic ovary syndrome. Obstetrics & gynecology science. 2013;56:137–142. doi: 10.5468/ogs.2013.56.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C., Chalmers T.J., Wong A.S.A., Zhou M., Marinova M., Bertoldo M.J., et al. In: Advances in stem cells and their niches. NILSSON S., editor. Elselvier; 2020. Chapter Six - hepatic regeneration in aging: cell type plasticity and redundancies. [Google Scholar]
- 35.Li C., Wu L.E. Risks and rewards of targeting NAD(+) homeostasis in the brain. Mechanism of Ageing and Development. 2021;198 doi: 10.1016/j.mad.2021.111545. [DOI] [PubMed] [Google Scholar]
- 36.Lim S.S., Norman R.J., Davies M.J., Moran L.J. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obesity Reviews. 2013;14:95–109. doi: 10.1111/j.1467-789X.2012.01053.x. [DOI] [PubMed] [Google Scholar]
- 37.Livadas S., Pappas C., Karachalios A., Marinakis E., Tolia N., Drakou M., et al. Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine. 2014 doi: 10.1007/s12020-014-0200-7. [DOI] [PubMed] [Google Scholar]
- 38.Marjot T., Moolla A., Cobbold J.F., Hodson L., Tomlinson J.W. vol. 41. Endocr Rev; 2020. (Nonalcoholic fatty liver disease in adults: current concepts in etiology, outcomes, and management). [DOI] [PubMed] [Google Scholar]
- 39.Massudi H., Grant R., Braidy N., Guest J., Farnsworth B., Guillemin G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS One. 2012;7 doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mather K.J., Kwan F., Corenblum B. Hyperinsulinemia in polycystic ovary syndrome correlates with increased cardiovascular risk independent of obesity. Fertility and Sterility. 2000;73:150–156. doi: 10.1016/s0015-0282(99)00468-9. [DOI] [PubMed] [Google Scholar]
- 41.Mehran A.E., Templeman N.M., Brigidi G.S., Lim G.E., Chu K.Y., Hu X., et al. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metabolism. 2012;16:723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Merry T.L., Hedges C.P., Masson S.W., Laube B., Pohlmann D., Wueest S., et al. Partial impairment of insulin receptor expression mimics fasting to prevent diet-induced fatty liver disease. Nature Communications. 2020;11:2080. doi: 10.1038/s41467-020-15623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer L.K., Ciaraldi T.P., Henry R.R., Wittgrove A.C., Phillips S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013;2:217–226. doi: 10.4161/adip.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao Y., Cui Z., Gao Q., Rui R., Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Reports. 2020;32 doi: 10.1016/j.celrep.2020.107987. [DOI] [PubMed] [Google Scholar]
- 45.Miao Y., Cui Z., Gao Q., Rui R., Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Reports. 2020;32 doi: 10.1016/j.celrep.2020.107987. [DOI] [PubMed] [Google Scholar]
- 46.Mills K.F., Yoshida S., Stein L.R., Grozio A., Kubota S., Sasaki Y., et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metabolism. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran L.J., Hutchison S.K., Norman R.J., Teede H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database of Systematic Reviews. 2011 [Google Scholar]
- 48.Moran L.J., Ko H., Misso M., Marsh K., Noakes M., Talbot M., et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. Journal of the Academy of Nutrition and Dietetics. 2013;113:520–545. doi: 10.1016/j.jand.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Moran L.J., Norman R.J., Teede H.J. Metabolic risk in PCOS: phenotype and adiposity impact. Trends in Endocrinology and Metabolism. 2015;26:136–143. doi: 10.1016/j.tem.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Moran L.J., Pasquali R., Teede H.J., Hoeger K.M., Norman R.J. Treatment of obesity in polycystic ovary syndrome: a position statement of the androgen excess and polycystic ovary syndrome society. Fertility and Sterility. 2009;92:1966–1982. doi: 10.1016/j.fertnstert.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 51.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Canto C., et al. The NAD(+)/Sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page M.M., Skovso S., Cen H., Chiu A.P., Dionne D.A., Hutchinson D.F., et al. Reducing insulin via conditional partial gene ablation in adults reverses diet-induced weight gain. The FASEB Journal. 2018;32:1196–1206. doi: 10.1096/fj.201700518R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks B.W., Sallam T., Mehrabian M., Psychogios N., Hui S.T., Norheim F., et al. Genetic architecture of insulin resistance in the mouse. Cell Metabolism. 2015;21:334–347. doi: 10.1016/j.cmet.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polderman K.H., Gooren L.J., Asscheman H., Bakker A., Heine R.J. Induction of insulin resistance by androgens and estrogens. Journal of Clinical Endocrinology and Metabolism. 1994;79:265–271. doi: 10.1210/jcem.79.1.8027240. [DOI] [PubMed] [Google Scholar]
- 55.Riepsamen A., Wu L., Lau L., Listijono D., Ledger W., Sinclair D., et al. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rocca M.L., Venturella R., Mocciaro R., Di Cello A., Sacchinelli A., Russo V., et al. Polycystic ovary syndrome: chemical pharmacotherapy. Expert Opinion on Pharmacotherapy. 2015;16:1369–1393. doi: 10.1517/14656566.2015.1047344. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez Paris V., Solon-Biet S.M., Senior A.M., Edwards M.C., Desai R., Tedla N., et al. Defining the impact of dietary macronutrient balance on PCOS traits. Nature Communications. 2020;11:5262. doi: 10.1038/s41467-020-19003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin K.H., Glintborg D., Nybo M., Abrahamsen B., Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2017;102:3848–3857. doi: 10.1210/jc.2017-01354. [DOI] [PubMed] [Google Scholar]
- 59.Schiewer M.J., Goodwin J.F., Han S., Brenner J.C., Augello M.A., Dean J.L., et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discovery. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shorakae S., Boyle J., Teede H. Polycystic ovary syndrome: a common hormonal condition with major metabolic sequelae that physicians should know about. Internal Medicine Journal. 2014;44:720–726. doi: 10.1111/imj.12495. [DOI] [PubMed] [Google Scholar]
- 61.Singh J., O'neill C., Handelsman D.J. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995;136:5311–5321. doi: 10.1210/endo.136.12.7588276. [DOI] [PubMed] [Google Scholar]
- 62.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spinnler R., Gorski T., Stolz K., Schuster S., Garten A., Beck-Sickinger A.G., et al. The adipocytokine Nampt and its product NMN have no effect on beta-cell survival but potentiate glucose stimulated insulin secretion. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stener-Victorin E., Padmanabhan V., Walters K.A., Campbell R.E., Benrick A., Giacobini P., et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocrine Reviews. 2020 doi: 10.1210/endrev/bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stener-Victorin E., Padmanabhan V., Walters K.A., Campbell R.E., Benrick A., Giacobini P., et al. Animal models to understand the etiology and pathophysiology of polycystic ovary syndrome. Endocrine Reviews. 2020;41 doi: 10.1210/endrev/bnaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teede H.J., Joham A.E., Paul E., Moran L.J., Loxton D., Jolley D., et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity. 2013;21:1526–1532. doi: 10.1002/oby.20213. [DOI] [PubMed] [Google Scholar]
- 67.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Human Reproduction. 2018;33:1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uddin G.M., Youngson N.A., Doyle B.M., Sinclair D.A., Morris M.J. Nicotinamide mononucleotide (NMN) supplementation ameliorates the impact of maternal obesity in mice: comparison with exercise. Scientific Reports. 2017;7 doi: 10.1038/s41598-017-14866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uddin G.M., Youngson N.A., Sinclair D.A., Morris M.J. Head to head comparison of short-term treatment with the NAD(+) precursor nicotinamide mononucleotide (NMN) and 6 Weeks of exercise in obese female mice. Frontiers in Pharmacology. 2016;7:258. doi: 10.3389/fphar.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters K.A., Rodriguez Paris V., aflatounian A., Handelsman D.J. Androgens and ovarian function: translation from basic discovery research to clinical impact. Journal of Endocrinology. 2019 doi: 10.1530/JOE-19-0096. [DOI] [PubMed] [Google Scholar]
- 71.Wang L.F., Wang X.N., Huang C.C., Hu L., Xiao Y.F., Guan X.H., et al. Inhibition of NAMPT aggravates high fat diet-induced hepatic steatosis in mice through regulating Sirt1/AMPKalpha/SREBP1 signaling pathway. Lipids in Health and Disease. 2017;16:82. doi: 10.1186/s12944-017-0464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y., Yang Q., Wang H., Zhu J., Cong L., Li H., et al. NAD+ deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndromedouble dagger. Biology of Reproduction. 2021;105:371–380. doi: 10.1093/biolre/ioab078. [DOI] [PubMed] [Google Scholar]
- 73.Wiebe N., Ye F., Crumley E.T., Bello A., Stenvinkel P., Tonelli M. Temporal associations among body mass index, fasting insulin, and systemic inflammation: a systematic review and meta-analysis. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C.S., Jividen K., Kamata T., Dworak N., Oostdyk L., Remlein B., et al. Androgen signaling uses a writer and a reader of ADP-ribosylation to regulate protein complex assembly. Nature Communications. 2021;12:2705. doi: 10.1038/s41467-021-23055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Q., Cong L., Wang Y., Luo X., Li H., Wang H., et al. Increasing ovarian NAD+ levels improve mitochondrial functions and reverse ovarian aging. Free Radical Biology and Medicine. 2020;156:1–10. doi: 10.1016/j.freeradbiomed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 76.Yoshino J., Mills K.F., Yoon M.J., Imai S.-I. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metabolism. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoshino M., Yoshino J., Kayser B.D., Patti G.J., Franczyk M.P., Mills K.F., et al. Nicotinamide mononucleotide increases muscle insulin sensitivity in prediabetic women. Science. 2021;372:1224–1229. doi: 10.1126/science.abe9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]