Abstract
The GafD lectin of the G (F17) fimbriae of diarrhea-associated Escherichia coli was overexpressed and purified from the periplasm of E. coli by affinity chromatography on GlcNAc-agarose. The predicted mature GafD peptide comprises 321 amino acids, but the predominant form of GafD recovered from the periplasm was 19,092 Da in size and corresponded to the 178 N-terminal amino acid residues, as judged by mass spectrometry and amino acid sequencing, and was named ΔGafD. Expression of gafD from the cloned gaf gene cluster in DegP-, Lon-, and OmpT-deficient recombinant strains did not significantly decrease the formation of ΔGafD. The peptide was also detected in the periplasm of the wild-type E. coli strain from which the gaf gene cluster originally was cloned. We expressed gafD fragments encoding C-terminally truncated peptides. Peptides GafD1-252, GafD1-224, GafD1-189, and the GafD1-178, isolated from the periplasm by affinity chromatography, had apparent sizes closely similar to that of ΔGafD. Only trace amounts of truncated forms with expected molecular sizes were detected in spheroplasts. In contrast, the shorter GafD1-157 peptide was detected in spheroplasts but not in the periplasm, indicating that it was poorly translocated or was degraded by periplasmic proteases. Pulse-chase assays using gafD indicated that ΔGafD was processed from GafD and is not a primary translation product. The ΔGafD peptide was soluble by biochemical criteria and exhibited specific binding to GlcNAc-agarose. Inhibition assays with mono- and oligosaccharides gave a similar inhibition pattern in the hemagglutination by the G-fimbria-expressing recombinant E. coli strain and in the binding of [14C]ΔGafD to GlcNAc-agarose. ΔGafD bound specifically to laminin, a previously described tissue target for the G fimbria. Our results show that a soluble, protease-resistant subdomain of GafD exhibits receptor-binding specificity similar to that for intact G fimbriae and that it is formed when gafD is expressed alone or from the gaf gene cluster.
Bacterial adhesion to epithelial and subepithelial surfaces is a prerequisite for colonization of mammalian tissues by pathogenic bacterial species as well as by members of the normal bacterial flora. Pathogenic and commensal strains of Escherichia coli express a variety of fimbrial types with characteristic receptor-binding specificities and serological properties. In the best-characterized fimbrial filament, the P fimbriae of uropathogenic E. coli, the globoside-binding PapG adhesin is a minor component of the filament and is located in the tip-associated fibrillum of the P fimbria (23). Although detailed studies on P-fimbrial biogenesis have increased our understanding of bacterial adhesive structures (reviewed in reference 48), it has remained uncertain how well the P fimbria serves as a structural model for other fimbrial types. For example, the mannoside-binding FimH lectin of the E. coli type 1 fimbria is located on specific sites along the fimbrial filament as well as at the fimbrial tip (1, 18, 22).
It has remained an open question whether the carbohydrate-binding specificity of fimbrial filaments is dictated by the lectin peptide alone or whether the other components of the fimbrial filament also influence binding. The mannoside-binding specificity of the FimH lectin in different enterobacterial species has been, by complementation assays, found to be influenced by the fimbrial shaft (31). On the other hand, variability in type 1 fimbria binding to mannosides and nonmannoside targets has been found to result solely from sequence variations in FimH (37, 45, 47), and purified, aggregated SfaS lectin has been shown to exhibit sialic acid-binding activity (32). PapG, FimH, and the G-fimbrial lectin GafD have been expressed as fusions to maltose-binding protein (14, 40, 54) and have been shown to exhibit the correct mono- or disaccharide binding. Hence, several reports strongly indicate that carbohydrate-binding specificity relies on the lectin.
The correct biosynthesis of P and type 1 fimbriae requires the periplasmic chaperones PapD and FimC, which form complexes with lectin proteins PapG and FimH, with the other minor fimbrial proteins, and with major fimbrial subunits PapA and FimA (reviewed in reference 48). PapD has been proposed to protect PapG from proteolytic cleavage, prevent misassembly of PapG in the periplasm, and facilitate import and folding of subunits in the periplasm (19, 49). PapD binds to a hydrophobic C-terminal motif in PapG to form a 1:1 complex, whereas the N terminus of PapG is important for receptor binding (14, 15, 24, 49). The X-ray structure of the FimC-FimH chaperone-adhesin complex, resolved recently by Choudhury and coworkers (8), showed that the FimH lectin consists of two domains connected by a short linker region. The N-terminal receptor-binding domain of FimH accommodates the carbohydrate-binding pocket at the distal tip of the domain, and the C-terminal fimbrillin-binding domain binds to the chaperone and subsequently anchors the adhesin to the fimbrial shaft. In the absence of specific chaperones, fimbrial subunits are proteolytically processed by the periplasmic DegP protease and other so far unidentified proteases (3, 51, 57). C-terminally truncated receptor-binding forms of fimbrial minor proteins have been detected in the periplasm of recombinant E. coli expressing the adhesin gene or chaperone-deficient fimbrial gene clusters; the coexpression of the chaperone significantly decreases the rate of proteolysis of fimbrial proteins in E. coli periplasm (15, 17–19, 26, 46, 57).
Due to aggregation, complex purification procedures, and sensitivity to proteolytic cleavage, few fimbrial adhesins have been purified for binding or structural analysis. Moch and coworkers (32) purified the sialic acid-binding SfaS adhesin from the S fimbria of E. coli; the lectin was, however, aggregated into complexes of an apparent molecular weight of >106. PapG and FimH have been affinity purified from the bacterial periplasm as a complex with their specific chaperones PapD and FimC (8, 15, 19, 54). Recently, two independent reports have shown that stable forms of FimH can be obtained in the periplasm of E. coli by fusing the C terminus of FimH with a polyhistidine tag or a fimbrillin-derived peptide; these fusion proteins apparently are resistant to proteolysis (4, 44).
The G fimbria belongs to the closely related F17 family of fimbriae that are present on bovine enteropathogenic and septicemic E. coli and that bind to the intestinal brush border as well as to the basement membrane (9, 28, 29, 40, 41, 43). In contrast to type 1 and P fimbriae, which are encoded by chromosomal gene clusters comprising 9 and 11 genes (15, 22), the fimbriae in the F17 family are encoded by only four chromosomal genes (27). Two of the f17 gene products (F17A and F17G) are components of the fimbrial filament, the major fimbrillin and the adhesin (28, 29). It has been suggested that F17C is an outer membrane usher needed for translocation of subunits across the outer membrane (27) and that the fourth protein, F17D, has amino acid sequence homology to the prototype chaperone PapD and hence should be considered a fimbrial chaperone (16, 24).
In this paper, we describe the construction and purification of a soluble, nonfusion form of the G-fimbrial GlcNAc-binding GafD lectin of E. coli in the absence of its chaperone. Such soluble fimbrial peptides may be useful in the analysis of structure-function relationships in fimbrial adhesins and as convenient antiadhesive vaccine antigens.
MATERIALS AND METHODS
Bacteria and plasmids.
The G-fimbriate E. coli strains IHE11165, IHE11088(pRR-5), and IHE11088(pHUB110) have been described earlier (38, 41, 56). Plasmid pRR-5 contains the complete gaf gene cluster from E. coli strain IHE11165 on a 7-kb DNA fragment in pACYC184 (38), and pHUB110 contains a 6-bp in-frame deletion within the coding region of gafD, resulting in G fimbriae lacking GlcNAc-binding capacity (40). pHUB113 (40) contains the gafD reading frame cloned into pUC19. Type 1 fimbria-expressing E. coli strain EH826 and DegP-deficient E. coli strain KS474 have been described (39, 50). E. coli strain BL21 λDE3 (ompT lon) and expression vector pET-22b(+) were from Novagen Inc. (Madison, Wis.). The bacteria were cultivated at 37°C in Luria broth (42) containing the appropriate antibiotics.
Expression and purification of the GafD constructs.
The DNA fragment encoding the entire GafD peptide (GafD1-321) on pHUB113 was cloned as a BamHI-HindIII fragment into pET-22b(+) to obtain plasmid pKJ1. The gene fragments encoding C-terminally truncated peptides GafD1-252, GafD1-224, GafD1-189, GafD1-178, and GafD1-157 were amplified by PCR using plasmid pHUB113 as a template. The universal pUC19 primer was used as the 5′ primer; the 3′ primers of each construct were designed on the basis of the nucleotide sequence of gafD (40) and contained a stop codon and a HindIII site. After restriction with BamHI and HindIII, the DNA fragments were ligated into BamHI- and HindIII-digested pET-22b(+) and transformed into E. coli BL21 λDE3. Restriction enzymes were used according to the manufacturers' instructions (New England Biolabs, Inc., Beverly, Mass.; Promega, Madison, Wis.), and routine methods were used for PCR, DNA ligation, and plasmid transformation (42). Expression of the gafD constructs in E. coli BL21 λDE3 was performed essentially as described by Studier and coworkers (52) and the manufacturer's instructions (Novagen Inc.). Briefly, bacteria were cultivated in 20 ml of Luria broth containing ampicillin (75 μg/ml) to an optical density at 600 nm (OD600) of 1.0, washed twice with M9 (42) salt solution, and starved for 1 h at 37°C in 10 ml of M9 solution containing 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG), 0.4% (vol/vol) glycerol, and ampicillin. In some experiments, a mixture of inhibitors of serine and cysteine proteases (Complete; Boehringer Mannheim) was added to the induction mixture as recommended by the manufacturer. Cells were collected and incubated further for 30 min at 37°C in 2 ml of M9 supplemented with 200 μg of rifampin/ml. The bacterial cells were then pulse-labeled by adding 100 μl of the 14C-amino acid mixture (Amersham Life Science, Buckinghamshire, United Kingdom) and by further incubation for 1 h. Samples for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and autoradiography were taken immediately and at 1- to 10-min intervals after the addition of 14C-amino acids. The cells were chased by adding 1 ml of Luria broth and by incubating them for 20 min at 37°C and then washed twice with M9 solution; periplasmic peptides were released by an osmotic shock procedure (7). For expression on a larger scale, the cells expressing gafD were grown in Luria broth without starvation, rifampin, and 14C labeling to an OD600 of 1.0, induced with 1 mM IPTG, and further incubated for 2 h at 37°C. Samples of the cells, spheroplasts, and of the corresponding periplasms were analyzed by SDS-PAGE in 15% (wt/vol) slab gels (25) and by autoradiography or Western blotting (55). In autoradiography, radioactivity at a level of 7,000 dpm/sample was loaded on the gel. Bacterial cells were converted into spheroplasts by treatment with lysozyme and EDTA as described earlier (5). Plasmolysis was achieved by resuspending the collected cells in ice-cold 20% glucose in 10 mM Tris-HCl, pH 8.0 (40). Lysozyme was then added to a concentration of 100 μg/ml followed by two volumes of ice-cold 1.5 mM EDTA, pH 7.5, within 10 min. The cells were kept on ice for 15 min, the formation of spheroplasts was confirmed microscopically, and then the cells were centrifuged. The spheroplasts were resuspended into 480 μl of M9 solution or 10 mM Tris-HCl, pH 7.5, and stored at −20°C. For affinity purification of the GafD and the GafD constructs, the periplasm was mixed with N-acetyl-d-glucosamine (GlcNAc)–agarose beads (Sigma, St. Louis, Mo.), the slurry was rocked slowly for 16 h at 4°C, and the periplasm-agarose mixture was packed into a column. Unbound material was removed by washing with phosphate-buffered saline (PBS), pH 7.1, and peptides bound to the GlcNAc-agarose particles were eluted in PBS containing 5% (wt/vol) GlcNAc (Sigma) (GlcNAc-PBS). Gel filtration through a PD10 column (Pharmacia) was used to remove the carbohydrate from the GafD peptide. For expression of gafD from the gaf gene cluster, plasmid pRR-5 was transformed into E. coli strains BL21 λDE3, KS474, and IHE11088 and bacteria were grown in Luria broth as described above, without IPTG induction. Expression of GlcNAc-binding G fimbriae in these strains was verified by agglutination of GlcNAc-agarose particles.
Immunological methods.
Antiserum against the purified GafD peptide was raised in rabbits using standard procedures. The antiserum raised against the purified G-fimbrial filaments of E. coli has been described elsewhere (38). Agglutination of bacterial cells in anti-GafD antisera was performed as described previously (40). For Western blotting, purified fimbriae available from previous work (39, 40), the purified GafD peptides, periplasmic peptides, whole cells, and spheroplasts were analyzed by SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, Calif.) using a semidry transfer apparatus (Pharmacia) at 0.9 mA/cm2 for 2 h. After the transfer, the membranes were quenched with 2% (wt/vol) bovine serum albumin (BSA) in PBS for 18 h at room temperature. Polypeptides were visualized by staining with anti-G fimbria or anti-GafD peptide antibodies (diluted 1/1,500 in PBS containing 1% BSA). Bound antibodies were visualized by alkaline phosphatase-conjugated secondary antibodies (Dakopatts, Glostrup, Denmark) diluted 1/2,000 in PBS-BSA and a phosphatase substrate containing nitroblue tetrazolium (Sigma) and 5-bromo-4-chloro-3-indolyl-1-phosphate (Sigma).
Binding and inhibition assays.
Hemagglutination and inhibition assays using endo-β-galactosidase-treated human erythrocytes were performed as previously described (38, 56). In the binding assays with the [14C]GafD constructs, 50 μl of the periplasm from pulse-labeled cells was incubated with 50 μl of the GlcNAc-agarose or amylose-agarose (New England Biolabs) particle suspension for 30 min in Eppendorf tubes over crushed ice. The particles were washed three times for 5 min in PBS, and the radioactivity remaining on the particles was determined in an LKB 1409 liquid scintillation counter (Wallac, Turku, Finland). In inhibition assays, the carbohydrates were added to the incubation buffer at a final concentration of 20 mM. The carbohydrates tested as inhibitors were from Sigma. The binding of the purified GafD peptide to laminin immobilized on microtiter plates was assessed by a modified enzyme-linked immunosorbent assay as described earlier (58). The microtiter plate was coated with laminin at a concentration of 25 μg/ml, the GafD peptide was used at a final concentration of 5 to 150 nM, the incubation time with the purified GafD peptide was 4 h, and the anti-GafD peptide serum was used at the dilution of 1/2,000 in PBS containing 0.05% (vol/vol) Tween 20. For a control, the binding of GafD to immobilized BSA (25 μg/ml) was assessed. The effect of carbohydrates on the binding of ΔGafD (the form of GafD corresponding to the N-terminal 178 amino acids; 75 nM) to laminin was tested at the 50 mM concentration.
Protein chemical methods.
For molecular weight determination, matrix-assisted laser desorption ionization–time of flight mass spectrometry was performed in the linear positive-ion mode with a BIFLEX mass spectrometer (Bruker-Franzen Analytik, Bremen, Germany), using a 337-nm nitrogen laser. One microliter of sample was mixed with 1 μl of matrix solution (saturated solution of sinapic acid [Fluka Chemika, Buchs, Switzerland] in 30% [wt/vol] acetonitrile–0.1% [wt/vol] trifluoroacetic acid) and dried with a stream of air. External calibration was performed with horse heart myoglobin (Sigma). For protein sequencing, an aliquot corresponding to approximately 50 pmol of protein was concentrated to 30 μl in a vacuum centrifuge and subjected to Edman degradation in a Procise 494A protein-sequencing system (Applied Biosystems, Perkin-Elmer, Foster City, Calif.). Protein concentration was estimated spectrophotometrically as described previously (36). Ultracentrifugation was performed for 100 min at 100,000 × g and 4°C in a 50 Ti rotor in an L8 ultracentrifuge (Beckman Instruments Inc., Palo Alto, Calif.).
Protein sequence analysis.
The amino acid sequences of GafD1-321 and GafD1-178 were sent to the ExPASy SWISS-MODEL automated protein modeling server (version 3.5; Swiss Institute of Bioinformatics [http://www.expasy.ch/swissmod]) (13) and modeled without user-defined templates as well as with FimH (1QUN.pdb; Structural Bioinformatics Protein Databank code 1QUN) of the FimC-FimH chaperone adhesin complex as the template (8).
Amino acid sequence accession numbers.
The amino acid sequences of GafD, F17-D, FimH, PapG, MrkD, and CooD are deposited at EMBL/GenBank under the accession no. AAA69514, AAC45720, S56545, AAA24290, AAA25098, and CAA54230, respectively (10, 11, 20, 27, 30, 37, 40).
RESULTS
Purification and chemical properties of ΔGafD.
We cloned the gafD gene on pHUB113 as a BamHI-HindIII fragment into expression plasmid pET-22b(+). In the plasmid obtained, pKJ1, gafD is expressed with its own Shine-Dalgarno and leader sequences, and, due to the stop codons upstream and downstream of the gafD reading frame, the peptide lacks the PelB leader sequence as well as the His tag sequence encoded by the vector. We first purified 14C-labeled GafD from the periplasm of rifampin-treated and pulse-labeled E. coli BL21 λDE3(pKJ1) cells using affinity chromatography on GlcNAc-agarose. We tested the binding of 14C-labeled periplasmic peptides to GlcNAc-agarose and, as a control, to amylose-agarose. With 50 μl of the labeled periplasm and 50 μl of the resin suspension, a radioactivity of 20,818 cpm was bound to the GlcNAc-agarose whereas only 329 cpm was bound to the amylose-agarose, indicating specificity for GlcNAc in the binding.
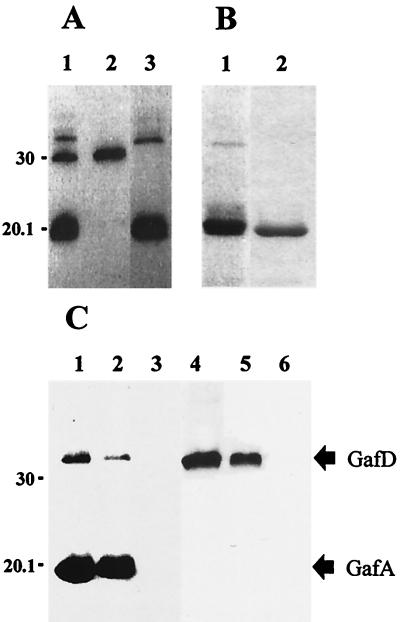
Autoradiographic analysis of the [14C]GafD binding to GlcNAc-agarose is shown in Fig. 1A, and SDS-PAGE analysis of purified GafD1-321 and GafD1-178 expressed in nonlabeled E. coli BL21 λDE3 is shown in Fig. 1B. After the pulse-labeling of rifampin-treated cells with 14C-amino acids, three radioactive peptides were detected in the periplasm of BL21 λDE3(pKJ1) (lane 1 in Fig. 1A). The minor peptide with the apparent molecular mass of 32 kDa was bound to the GlcNAc-agarose (lane 3) and eluted in GlcNAc-PBS, suggesting that it was GafD (calculated size, 33,889 Da). The peptide of 30 kDa not bound to the GlcNAc-agarose (lane 2) was considered to be β-lactamase encoded by the vector on the basis of its migration properties in SDS-PAGE gel and its lack of reactivity with GlcNAc-agarose (lane 3) and with anti-GafD antibodies (see below). The major radioactive peptide in the periplasm was a peptide with an apparent size of 20 kDa that bound to the GlcNAc-agarose (lane 3) and that was eluted in GlcNAc-PBS (lane 1 in Fig. 1B); the affinity-purified 32-kDa minor peptide was clearly visible only when expressed on a larger scale (lane 1 in Fig. 1B). For comparison, the affinity-purified proteolytically resistant peptide GafD1-178 is shown in lane 2. The addition of Complete, a cocktail of various inhibitors of serine and cysteine proteases, to the induction mixture did not prevent the degradation (data not shown). The sequencing of the 20-kDa peptide gave a single N-terminal sequence, AVSFIG, which perfectly matches the N-terminal sequence of the mature GafD (40). The peptide was therefore named ΔGafD. Mass spectrometric analysis of the ΔGafD peptide gave a mass of 19,092 +/− 20 Da, which indicates that the C-terminal residue in ΔGafD is Thr-178 (calculated molecular mass, 19,074 Da). An aliquot of the affinity-purified [14C]ΔGafD was gel filtered to remove excess GlcNAc, diluted 1/2 in PBS, and ultracentrifuged for 100 min at 100,000 × g; the radioactivity in the supernatant was 29,740 cpm/ml before and 28,410 cpm/ml after the ultracentrifugation. This indicates that [14C]ΔGafD was soluble and not sedimented.
FIG. 1.
Purification of GafD from E. coli BL21 λDE3(pKJ1) periplasm by affinity chromatography and analysis of the reactivities of fimbrial preparations with anti-G-fimbria and anti-GafD antibodies. (A) Binding of 14C-labeled GafD to GlcNAc-agarose. Lane 1, pulse-labeled periplasmic peptides from rifampin-treated E. coli BL21 λDE3(pKJ1); lane 2, peptides not bound to GlcNAc-agarose; lane 3, peptides bound to GlcNAc-agarose. (B) Coomassie blue-stained SDS-PAGE gel of GafD peptides eluted with 5% (wt/vol) GlcNAc from the GlcNAc-agarose column. The peptides were prepared from periplasm of nonlabeled E. coli BL21 λDE3(pKJ1) (lane 1) and E. coli BL21 λDE3 expressing the truncated GafD1-178 (lane 2). (C) Western blot of fimbrial preparations. Lanes 1 to 3, reactivities of fimbrial peptides with anti-G-fimbria antibodies; lanes 4 to 6, reactivities with anti-GafD antibodies. The fimbrial preparations were the G fimbria isolated from E. coli HB101(pRR-5) (lanes 1 and 4), the mutated G fimbria without lectin activity isolated from E. coli HB101(pHUB110) (lanes 2 and 5), and the type 1 fimbria from E. coli EH826 (lanes 3 and 6). The migration distances of molecular weight markers are indicated on the left; those of the GafD and the GafA peptides are indicated on the right.
Production and specificity of the anti-GafD antibodies.
We immunized rabbits with the affinity-purified ΔGafD and assessed the specificity of the obtained antibodies by Western blotting (Fig. 1C). The antiserum raised against purified G fimbriae detected the GafA fimbrillin as well as GafD in the G fimbriae purified from E. coli HB101(pRR-5) and HB101(pHUB110) (lanes 1 and 2 in Fig. 1C) but did not react with the type 1 fimbrial peptides tested as the control (lane 3 in Fig. 1C). Plasmid pRR-5 expresses the complete G-fimbrial gene cluster, whereas the 6-bp in-frame deletion within the coding region of gafD in plasmid pHUB110 results in nonfunctional G-fimbrial filaments. The antibodies raised against the purified GafD detected the full-length GafD peptide in the two G-fimbrial preparations. The truncated 20-kDa ΔGafD form was not detected in G-fimbrial preparations (lanes 4 and 5 in Fig. 1C).
Expression of gafD in pulse-labeled cells.
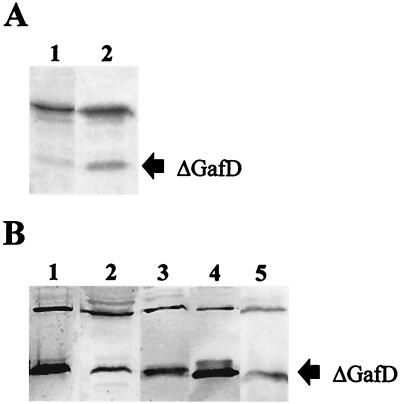
To determine whether ΔGafD is a posttranslational processing product from GafD or a primary translation product, we analyzed by SDS-PAGE and autoradiography pulse-labeled cells of E. coli BL21 λDE3(pKJ1). Samples were taken immediately after the addition of 14C-labeled amino acids and at short intervals up to 1 h 30 min after the addition of the label. Autoradiographic analysis of peptides expressed in the pulse-labeled cells are shown in Fig. 2A. A major polypeptide of 32 kDa corresponding to intact GafD and trace amounts of 20- and 30-kDa peptides corresponding to ΔGafD and vector-encoded β-lactamase, respectively, were detected immediately after addition of the label (lane 1 in Fig. 2A). The amount of ΔGafD increased over time, and the peptide was clearly visible 1 h 30 min after addition of the 14C label (lane 2 in Fig. 2A).
FIG. 2.
Expression of gafD peptides in rifampin-treated pulse-labeled E. coli cells and in the periplasm of G fimbria-expressing E. coli strains. (A) Autoradiographic analysis of 14C-labeled peptides of E. coli BL21 λDE3(pKJ1) immediately after addition of 14C-amino acids (lane 1) and 1 h 30 min later (lane 2). (B) Western blotting with anti-GafD antibodies of periplasmic peptides prepared from recombinant E. coli strains BL21 λDE3(pRR-5), KS474(pRR-5), IHE11088(pRR-5), BL21 λDE3(pKJ1) (lanes 1 to 4, respectively) and from wild-type E. coli strain IHE11165 (lane 5). Plasmid pRR-5 contains the entire gaf gene cluster, and pKJ1 encodes GafD1-321. The migration distance of the ΔGafD peptide is indicated on the right.
Expression of gafD from the gaf gene cluster in various host strains and in wild-type strain E. coli IHE11165.
To analyze whether ΔGafD was formed in other host backgrounds, plasmid pRR-5 was introduced into the following E. coli host strains: BL21 λDE3, which is deficient in cytoplasmic protease Lon and outer membrane protease OmpT, KS474, which lacks periplasmic protease DegP, and IHE11088, which is a clinical isolate previously shown to support fimbrial synthesis from plasmid pRR-5 (41). A Western blot of periplasmic extracts from these strains is shown in Fig. 2B, which also shows the blot obtained from G-fimbriated clinical isolate E. coli IHE11165, which expresses gafD from the chromosomal gaf gene cluster and which was used as the source for cloning the gaf gene cluster (38). GafD appeared as a minor 32-kDa peptide and ΔGafD appeared as the major form in the periplasmic extracts of all the strains tested (lanes 1 to 3 in Fig. 2B), including strain IHE11165 (lane 5). The periplasmic forms of GafD expressed from E. coli BL21 λDE3(pKJ1) are shown for comparison in lane 4. As judged by bacterial agglutination in anti-GafD antiserum and of GlcNAc-agarose particles, the strains tested expressed GafD lectin on their surfaces (data not shown). The results show that ΔGafD was accumulated in the periplasm concomitantly with the expression of functional G fimbrial filaments in the clinical isolate of E. coli as well as in the recombinant strains.
Expression and characterization of C-terminally truncated GafD constructs.
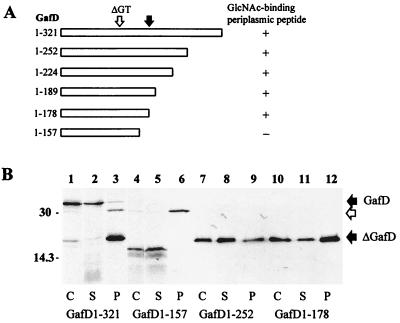
To analyze the size requirements of ΔGafD expression and binding activity in more detail, we constructed deletions in the 3′ region of gafD in pKJ1 and analyzed the peptide products in rifampin-treated, starved, and pulse-labeled E. coli BL21 λDE3. The constructs are schematically shown in Fig. 3A, and examples of autoradiographic analysis of their expression in E. coli cells, spheroplasts, and periplasm are shown in Fig. 3B. In whole cells and in spheroplasts, the complete GafD molecule ([14C]GafD1-321) migrated mainly as a 32-kDa peptide but appeared in the periplasm mainly as a 20-kDa peptide (lanes 1 through 3 in Fig. 3B). [14C]GafD1-157 (calculated size, 16,825 Da) was detected in the cells and spheroplasts as a 15-kDa peptide and was not found in the periplasm of pulse-labeled cells (lanes 4 through 6 in Fig. 3B). The GafD1-252 (26,348 Da), GafD1-224 (23,544 Da), GafD1-189 (20,216 Da), and GafD1-178 (19,074 Da) peptides were detected in cells, spheroplasts, and periplasm mainly as peptides with apparent sizes of 20 kDa; GafD1-252 and GafD1-178 are shown in lanes 7 through 12 in Fig. 3B. GafD1-178 was subjected to mass spectrometric analysis, and the result gave a mass of 19,113 Da, which is close to its calculated molecular mass. The 32-, 20-, and 15-kDa peptides present in whole-cell samples reacted in Western blot analysis with anti-GafD antibodies (data not shown), indicating that these peptides indeed were different forms of GafD.
FIG. 3.
Expression in E. coli BL21 λDE3 of gafD constructs. (A) Schematic presentation of GafD and the truncated GafD peptides and their recovery from the periplasm by affinity chromatography. Black arrow, position of the cleavage site in ΔGafD; ΔGT, position of the in-frame deletion abolishing binding activity. (B) Autoradiographic analysis of pulse-labeled peptides in the whole cells (C; lanes 1, 4, 7, and 10), spheroplasts (S; lanes 2, 5, 8, and 11), and the periplasm (P; lanes 3, 6, 9, and 12) of E. coli BL21 λDE3 expressing gafD constructs. The DNA fragments encoded full-length GafD1-321 (lanes 1 through 3) and the truncated forms GafD1-157 (lanes 4 through 6), GafD1-252 (lanes 7 through 9), and GafD1-178 (lanes 10 through 12). The migration distances of molecular weight markers are indicated on the left; those of the GafD and the ΔGafD peptides are indicated on the right. Open arrow, migration distance of β-lactamase.
Binding characteristics of ΔGafD.
We then assessed the carbohydrate sensitivity of the binding of ΔGafD to GlcNAc-agarose. d-Glucose, 2-deoxyglucose, d-glucosamine, and d-mannose did not inhibit binding, whereas N-acetyl-d-mannosamine was weakly inhibitory and N-acetyl-d-glucosamine was more efficient. Oligosaccharides N,N′-d-diacetylchitobiose, N,N′,N′"-triacetylchitotriose, N,N′,N",N′"-d-tetraacetylchitotetraose, and N,N′-d-diacetylchitobiose were slightly more efficient inhibitors than was N-acetyl-d-glucosamine (Table 1). These carbohydrates exhibited a similar inhibition pattern in the hemagglutination by G-fimbriate E. coli cells of erythrocytes with terminal GlcNAc residues on their surfaces (Table 1).
TABLE 1.
Inhibition of the hemagglutination by the G-fimbriate E. coli IHE11088(pRR-5) and of the binding of ΔGafD to GlcNAc-agarose
| Inhibitor | Inhibition of:
|
|
|---|---|---|
| Hemagglutination by bacteria (MICa [mM]) | Binding of [14C]ΔGafD to GlcNAc-agarose (%)b | |
| d-Glucose | 7.5 | 0 |
| 2-Deoxyglucose | 7.5 | 0 |
| d-Glucosamine | >7.5c | 0 |
| N-Acetyl-d-galactosamine | >7.5c | 0 |
| d-Mannose | 7.5 | 0 |
| N-Acetyl-d-mannosamine | 3.8 | 11 |
| N-Acetyl-d-glucosamine | 0.5 | 35 |
| N,N′-d-Diacetylchitobiose | 0.5 | 54 |
| p-Nitrophenyl-β-N,N′-d-diacetylchitobiose | 0.2 | 65 |
| N,N′,N"-d-Triacetylchitotriose | 0.1 | 62 |
| N,N′,N",N′"-d-Tetraacetylchitotetraose | 0.2 | 56 |
MIC of the carbohydrate required to prevent hemagglutination of endo-β-galactosidase-treated human erythrocytes (56).
The inhibitory effect was tested at a 20 mM concentration and is given as the percent inhibition compared to the binding in the absence of added carbohydrate.
No inhibition at the highest test concentration.
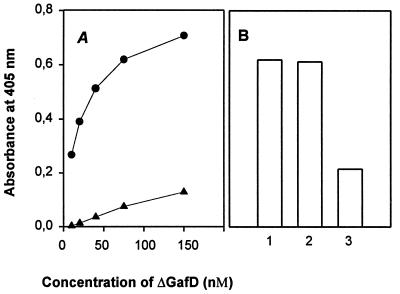
The basement membrane glycoprotein laminin was recently identified as a tissue target for the G fimbria (41). We therefore assessed by enzyme-linked immunosorbent assay methodology whether ΔGafD binds to laminin immobilized on microtiter plates (Fig. 4). A dose-dependent binding to laminin was evident; no significant binding to BSA was detected (Fig. 4A). The binding to laminin was inhibited by 50 mM N-acetyl-d-glucosamine but not by 50 mM N-acetyl-d-galactosamine (Fig. 4B).
FIG. 4.
Binding of ΔGafD to proteins immobilized on microtiter plates. (A) Binding to immobilized laminin (●) and BSA (▴). (B) Binding to immobilized laminin in the absence of carbohydrates (bar 1), in the presence of 50 mM GalNAc (bar 2), and in the presence of 50 mM GlcNAc (bar 3).
DISCUSSION
We describe here a soluble, C-terminally truncated form of the GafD fimbrial lectin that was named ΔGafD. The truncated 20-kDa form was present in the periplasm of G-fimbria-expressing E. coli strains but was not incorporated into the G-fimbrial filament. Pulse-chase experiments indicated that ΔGafD is a proteolytic product of GafD rather than a primary translation product. ΔGafD shows receptor-binding characteristics similar to those shown by the complete fimbrial filament, and our results support the concept that the carbohydrate-binding specificity of a gram-negative fimbria is dictated by the lectin subunit alone (14, 37, 45, 47). The receptor-binding region of GafD is apparently located at the N terminus of the molecule. This paper is the first to report on the expression and purification on a larger scale of a soluble nonfusion form of a fimbrial lectin. The biotechnological application potential of soluble adhesin forms is high: they could be used in receptor identification or isolation, in production of antiadhesive vaccines, and in structural studies.
The GafD peptide was not processed in the cytoplasm but appeared in the periplasm as ΔGafD. These findings indicated that the C terminus of GafD is resistant to cytoplasmic proteases but is processed during translocation into or in the periplasm. Overexpressed GafD1-178, GafD1-189, GafD1-224, and GafD1-252 peptides appeared in the cellular, spheroplastic, and periplasmic compartments mainly as the ΔGafD form, which suggests that the peptides were proteolytically processed to the stable 20-kDa structure already in the cytoplasm. GlcNAc-binding peptides of 20 and 32 kDa in apparent size were detected in Western blots of the periplasm of E. coli BL21 λDE3 cells overexpressing GafD. The peptides were also detected when GafD was expressed from the entire gaf gene cluster in plasmid pRR-5 in host strains deficient in proteases DegP, Lon, and OmpT as well as in clinical isolate E. coli IHE11088, previously shown to support fimbrial synthesis from plasmid pRR-5 (41). In addition, we detected ΔGafD in the periplasm of wild-type clinical isolate E. coli IHE11165, from which the gaf gene cluster was originally cloned, which suggests that the ΔGafD form may be a natural product of the gaf gene cluster. All strains with the gaf gene cluster also expressed functional G fimbriae on their surfaces, further indicating that ΔGafD is not a result of the overexpression technology. However, G-fimbrial filaments contained full-size GafD but not ΔGafD, which indicated that the C-terminally truncated ΔGafD is not polymerized into the fimbrial filaments.
Coexpression of GafD1-321 with G-fimbrial putative chaperone GafB (16, 24) or P-fimbrial chaperone PapD in E. coli BL21 λDE3, according to procedures used for expression of the PapG-PapD complex (46), did not prevent C-terminal degradation of GafD (data not shown). In this respect GafD differs from PapG, FimH, and the MrkD adhesin of type 3 fimbriae of Klebsiella pneumoniae, which can form complexes with and utilize PapD in assembly into the fimbrial tip (11, 17, 19, 46, 53). The results suggest that the secretion and folding of ΔGafD to the functionally correct conformation in the periplasm are not dependent on GafB function in the expression system.
We detected ΔGafD in host cells lacking proteases DegP, Lon, and OmpT, which indicates that the C-terminal degradation of GafD is mediated by some other, so far unidentified proteases. Very little data on the target sequences of E. coli proteases exist; they seem to recognize a target sequence in combination with a target protein conformation (reviewed in reference 12). We found no significant homology of the GafD region around residue Thr-178 to target sequences of E. coli proteases (12). The exact cleavage sites were not determined for the periplasmic C-terminally truncated forms of FimH, PapG, and CooD previously observed (15, 18, 57), which makes it more difficult to infer why GafD is cleaved up to residue 178 but not more extensively to the N-terminal region of this site. The X-ray structure of the FimC-FimH complex, recently presented by Choudhury and coworkers (8), reveals that FimH is composed of two domains, a mannose-binding lectin domain that comprises the 156 N-terminal residues and a chaperone-binding C-terminal domain that comprises residues 160 to 279. Recently, Schembri and coworkers expressed in the periplasm of E. coli the 156-mer receptor-binding domain of FimH fused to a polyhistidine tail (44). In another recent study, the 13 N-terminal residues of FimG were fused to the C terminus of FimH and the modified FimH was expressed in the periplasm (4). These reports suggest that protection of the C terminus of FimH facilitates proper folding and renders the peptide proteolytically resistant in the periplasm. In the primary sequence alignment of GafD and FimH, the Thr-178 of GafD is located at the position of the flexible linker region in FimH. However, ΔGafD shows only 21.5% identity to the N-terminal domain of FimH, which does not facilitate the modeling of the three-dimensional structure of ΔGafD on the basis of the FimH structure. We anticipate that GafD, like FimH, has two domains, an N-terminal protease-resistant receptor-binding domain and a C-terminal protease-sensitive one, but that the fine structures of the two lectins differ. The explanation why ΔGafD is protease resistant and soluble requires the crystal structure of ΔGafD, which we currently are solving.
The receptor-binding region of the GafD lectin is apparently located within the 178 N-terminal amino acids of the mature protein. GafD resembles PapG, DraE, and the FimH fimbrial adhesin of E. coli in that the N-terminal part of the adhesin molecule is important for the receptor-binding activity (6, 8, 14, 21, 54). Such an arrangement, however, is not shared by all fimbrial adhesins (35). In the sialic acid-binding SfaS minor protein of the S fimbriae, the receptor-binding region was mapped to the C-terminal part of the SfaS molecule (33). We have previously described in pHUB110 an in-frame deletion of the codons for amino acid residues Gly-94 and Thr-95, which abolishes GlcNAc binding by GafD (40). This mutated GafD peptide was detected as a component of purified G fimbriae, indicating that the nonbinding phenotype resulted from destruction of binding activity rather than from lack of GafD incorporation into the fimbriae.
ΔGafD specifically bound to GlcNAc-agarose, and the binding was inhibited by GlcNAc but not by GalNAc. We also observed that purified ΔGafD binds to laminin, a glycosylated basement membrane protein that we recently identified as a tissue receptor for the G fimbriae using isolated fimbriae as well as recombinant E. coli (41). These results confirm the role of GafD as the GlcNAc-binding fimbrial lectin. The inhibition of ΔGafD binding to GlcNAc-agarose by carbohydrates exhibited a pattern closely similar to that seen for inhibition of hemagglutination by G-fimbriate E. coli (38, 56). The GlcNAc-di-, tri-, and -tetrasaccharides diacetylchitobiose [2-acetamido-2-deoxy-4-O-(2-acetamido-2-deoxy)-β-d-glucopyranosyl)-d-glucopyranose], triacetylchito-triose, and tetraacetylchitotetraose were only slightly more efficient inhibitors of ΔGafD binding than was GlcNAc-monosaccharide, which indicates that the β1–4-linked GlcNAc chains are not well recognized by the receptor-binding region of GafD. Indeed, in laminin, which is recognized by GafD, the terminal GlcNAc residues are β1–3 linked to N-acetyllactosamine residues (2). Similar receptor preference has been observed with the G lectin of the F17 fimbriae. Inhibition by glycoproteins of hemagglutination by purified F17 fimbriae or E. coli cells expressing F17 fimbriae has shown that the F17 fimbrial lectin preferentially recognizes oligosaccharides carrying β1–3 linked GlcNAc chains and binds only poorly to those having 1–4 and 1–6 linkages (34). As far as it is possible to conclude from these inhibition assays, the carbohydrate-binding specificity of ΔGafD and that of the G fimbriae are closely similar. We are currently analyzing the function of ΔGafD in the periplasm of E. coli.
ACKNOWLEDGMENTS
We thank Raili Lameranta for skilled technical assistance.
This study was supported by the Academy of Finland (projects 42103, 42107, and 164916), the Helsinki Graduate School in Microbiology, and the University of Helsinki.
REFERENCES
- 1.Abraham S N, Goguen J D, Sun D, Klemm P, Beachey E H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fimgene products. J Bacteriol. 1987;169:5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arumugham R G, Hsieh T C-Y, Tanzer M L, Laine R A. Structures of the asparagine-linked sugar chains of laminin. Biochim Biophys Acta. 1986;883:112–126. doi: 10.1016/0304-4165(86)90142-x. [DOI] [PubMed] [Google Scholar]
- 3.Bakker D, Vader C E M, Roosendaal B, Mooi F R, Oudega B, de Graaf F K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991;5:867–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 4.Barnhart M M, Pinkner J S, Soto G E, Sauer F G, Langermann S, Waksman G, Frieden C, Hultgren S J. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc Natl Acad Sci USA. 2000;97:7709–7714. doi: 10.1073/pnas.130183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birdsell D C, Cota-Robles E H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplast of Escherichia coli. J Bacteriol. 1967;93:427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnoy C, Mosley S L. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coliadhesins. Mol Microbiol. 1997;23:365–379. doi: 10.1046/j.1365-2958.1997.2231590.x. [DOI] [PubMed] [Google Scholar]
- 7.Charm S E, Matteo C C. Scale-up of protein isolation. Methods Enzymol. 1971;XXII:476–556. [Google Scholar]
- 8.Choudhury D, Thompson A, Stojanoff V, Langermann S, Pinkner J, Hultgren S J, Knight S D. X-ray structure of the FimC-FimH chaperone-adhesin complex from uropathogenic Escherichia coli. Science. 1999;285:1061–1066. doi: 10.1126/science.285.5430.1061. [DOI] [PubMed] [Google Scholar]
- 9.El Mazouari K, Oswald E, Hernalsteens J-P, Lintermans P, De Greve H. F17-like fimbriae from an invasive Escherichia colistrain producing cytotoxic necrotizing factor type 2 toxin. Infect Immun. 1994;62:2633–2638. doi: 10.1128/iai.62.6.2633-2638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froehlich B J, Karakashian A, Melsen L R, Wakefield J C, Scott J R. CooC and CooD are required for assembly of CS1 pili. Mol Microbiol. 1994;12:387–401. doi: 10.1111/j.1365-2958.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 11.Gerlach G-F, Clegg S, Allen B L. Identification and characterization of the genes encoding the type 3 and the type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989;171:1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottesman S. Regulation by proteolysis: developmental switches. Curr Opin Microbiol. 1999;2:142–147. doi: 10.1016/S1369-5274(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 13.Guex N, Peitsch M C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 14.Haslam D B, Borén T, Falk P, Ilver D, Chou A, Xu Z, Normark S. The amino-terminal domain of the P-pilus adhesin determines receptor specificity. Mol Microbiol. 1994;14:399–409. doi: 10.1111/j.1365-2958.1994.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 15.Hultgren S J, Lindberg F, Magnusson G, Kihlberg J, Tennent J M, Normark S. The PapG adhesin of uropathogenic Escherichia colicontains separate regions for receptor binding and for the incorporation into the pilus. Proc Natl Acad Sci USA. 1989;86:4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung D, Knight S, Woods R M, Pinkner J S, Hultgren S J. Molecular basis of two subfamilies of immunoglobulin-like chaperones. EMBO J. 1996;15:3792–3805. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones C H, Pinkner J S, Nicholes A V, Slonim L N, Abraham S N, Hultgren S J. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci USA. 1993;90:8397–8401. doi: 10.1073/pnas.90.18.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholes A V, Abraham S N, Hultgren S J. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones C H, Danese P N, Pinkner J S, Silhavy T J, Hultgren S J. The chaperone-assisted membrane release and folding pathway is sensed by two signal-transduction systems. EMBO J. 1997;16:6394–6406. doi: 10.1093/emboj/16.21.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm P, Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia colitype 1 fimbriae. Mol Gen Genet. 1987;208:439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen T B, Klemm P. Probing the receptor binding recognition site of the FimH adhesin by fimbriae-displayed FimH-FocH hybrids. Microbiology. 1998;144:1919–1929. doi: 10.1099/00221287-144-7-1919. [DOI] [PubMed] [Google Scholar]
- 22.Krogfelt K, Bergmans H, Klemm P. Direct evidence that the FimH protein is the mannose-specific adhesin of Escherichia colitype 1 fimbriae. Infect Immun. 1990;58:1995–1998. doi: 10.1128/iai.58.6.1995-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuehn M J, Heuser J, Normark S, Hultgren S. P pili of uropathogenic Escherichia coliare composite fibers with distinct fibrillar adhesive tips. Nature. 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 24.Kuehn M J, Ogg D J, Kihlberg J, Slonim L N, Flemmer K, Bergfors T, Hultgren S. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993;262:1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones C H, Hultgren S J. Prevention of mucosal Escherichia coliinfection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 27.Lintermans P. Karakterizatie van de F17 en F111 fimbriae van Escherichia coli en genetische analyse van de F17 genkluster. Academic dissertation. Ghent, Belgium: Rijksuniversiteit Gent; 1990. [Google Scholar]
- 28.Lintermans P, Pohl P, Bertels A, Charlier G, Vanderkerckhove J, van Damme J, Schoup J, Schlicker C, Korhonen T, De Greve H, van Montagu M. Characterization and purification of F17 adhesin on the surface of bovine enteropathogenic and septicemic Escherichia coli. Am J Vet Res. 1988;49:1749–1759. [PubMed] [Google Scholar]
- 29.Lintermans P, Bertels F A, Schlicker C, Deboeck F, Charlier G, Pohl P, Norgren M, Normark S, van Montagu M, De Greve H. Identification, characterization, and nucleotide sequence of the F17-G gene, which determines receptor binding of Escherichia coliF17 fimbriae. J Bacteriol. 1991;173:3366–3373. doi: 10.1128/jb.173.11.3366-3373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lund B, Lindberg F, Normark S. Structure and antigenic properties of the tip-located P pilus proteins of uropathogenic Escherichia coli. J Bacteriol. 1988;170:1887–1894. doi: 10.1128/jb.170.4.1887-1894.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madison B, Ofek I, Clegg S, Abraham S N. Type 1 fimbrial shafts of Escherichia coli and Klebsiella pneumoniaeinfluence sugar-binding specificities of their FimH adhesins. Infect Immun. 1994;62:843–848. doi: 10.1128/iai.62.3.843-848.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moch T, Hoschützky H, Hacker J, Kröncke K-D, Jann K. Isolation and characterization of the α-sialyl-β-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci USA. 1987;84:3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morschhäuser J, Hoschützky H, Jann K, Hacker J. Functional analysis of the sialic acid binding adhesin SfaS of pathogenic Escherichia coliby site-specific mutagenesis. Infect Immun. 1990;58:2133–2138. doi: 10.1128/iai.58.7.2133-2138.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mouricout M, Milhavet M, Durié C, Grange P. Characterization of glycoprotein glycan receptors for Escherichia coliF17 fimbrial lectin. Microb Pathog. 1995;18:297–306. doi: 10.1016/s0882-4010(05)80006-3. [DOI] [PubMed] [Google Scholar]
- 35.Nagata H, Sharma A, Sojar H T, Amano A, Levine M J, Genco R J. Role of the carboxyl-terminal region of Porphyromonas gingivalisfimbrillin in binding to salivary proteins. Infect Immun. 1997;65:422–427. doi: 10.1128/iai.65.2.422-427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pace C N, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouttu R, Puustinen T, Virkola R, Hacker J, Klemm P, Korhonen T K. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia colito collagens. Mol Microbiol. 1999;31:1747–1757. doi: 10.1046/j.1365-2958.1999.01311.x. [DOI] [PubMed] [Google Scholar]
- 38.Rhen M, Klemm P, Korhonen T K. Identification of two new hemagglutinins of Escherichia coli, N-acetyl-d-glucosamine-specific fimbriae and a blood group M-specific agglutinin, by cloning the corresponding genes in Escherichia coliK-12. J Bacteriol. 1986;168:1234–1242. doi: 10.1128/jb.168.3.1234-1242.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhen M, Knowles J, Penttilä M E, Sarvas M, Korhonen T K. P fimbriae of Escherichia coli: molecular cloning of DNA fragments containing the structural genes. FEMS Microbiol Lett. 1983;19:119–123. [Google Scholar]
- 40.Saarela S, Taira S, Nurmiaho-Lassila E-L, Makkonen A, Rhen M. The Escherichia coli G-fimbrial lectin protein participates both in fimbrial biogenesis and in recognition of the receptor N-acetyl-d-glucosamine. J Bacteriol. 1995;177:1477–1484. doi: 10.1128/jb.177.6.1477-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saarela S, Westerlund-Wikström B, Rhen M, Korhonen T K. The G (F17) fimbrial complex confers adhesiveness of Escherichia colito laminin. Infect Immun. 1996;64:2857–2860. doi: 10.1128/iai.64.7.2857-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Sanchez R, Kanarek L, Koninkx J, Hendriks H, Lintermans P, Bertels A, Charlier G, van Driessche E. Inhibition of adhesion of enterotoxigenic Escherichia colicells expressing F17 fimbriae to small intestinal mucus and brush-border membranes of young calves. Microb Pathog. 1993;15:407–419. doi: 10.1006/mpat.1993.1090. [DOI] [PubMed] [Google Scholar]
- 44.Schembri M A, Hasman H, Klemm P. Expression and purification of the mannose recognition domain of the FimH adhesin. FEM Microbiol Lett. 2000;188:147–151. doi: 10.1111/j.1574-6968.2000.tb09186.x. [DOI] [PubMed] [Google Scholar]
- 45.Schembri M A, Sokurenko E V, Klemm P. Functional flexibility of the FimH adhesin: insights from a random mutant library. Infect Immun. 2000;68:2638–2646. doi: 10.1128/iai.68.5.2638-2646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slonim L N, Pinkner J S, Brändén C-I, Hultgren S J. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 1992;11:4747–4756. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokurenko E V, Courtney H S, Ohman D E, Klemm P, Hasty D. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimHgenes. J Bacteriol. 1994;176:748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soto G E, Hultgren S J. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999;181:1059–1071. doi: 10.1128/jb.181.4.1059-1071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soto G E, Dodson K W, Ogg D, Liu C, Heuser J, Knight S, Kihlberg J, Jones C H, Hultgren S J. Periplasmic chaperone recognition motif of subunits mediates quaternary interactions in the pilus. EMBO J. 1998;17:6155–6167. doi: 10.1093/emboj/17.21.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strauch K L, Johnson K, Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coliat high temperature. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Striker R, Jacob-Dubuisson F, Frieden C, Hultgren S J. Stable fiber-forming and non-fiber forming chaperone-subunit complexes in pilus biogenesis. J Biol Chem. 1994;269:12233–12239. [PubMed] [Google Scholar]
- 52.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 53.Tarkkanen A-M, Allen B L, Westerlund B, Holthöfer H, Kuusela P, Risteli L, Clegg S, Korhonen T K. Type V collagen as the target for type 3 fimbriae, enterobacterial adherence organelles. Mol Microbiol. 1990;4:1353–1361. doi: 10.1111/j.1365-2958.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 54.Thankavel K B, Madison M, Ikeda T, Malaviya R, Shah A H, Arumugham P M, Abraham S N. Localization of a domain in the FimH adhesin of Escherichia colitype 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Investig. 1997;100:1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Väisänen-Rhen V, Korhonen T K, Finne J. Novel cell-binding activity specific for N-acetyl-d-glucosamine in an Escherichia colistrain. FEBS Lett. 1983;159:233–236. doi: 10.1016/0014-5793(83)80453-0. [DOI] [PubMed] [Google Scholar]
- 57.Voegele K, Sakellaris H, Scott J R. CooB plays a chaperone-like role for the protein involved in formation of CS1 pili of enterotoxigenic Escherichia coli. Proc Natl Acad Sci USA. 1997;94:13257–13261. doi: 10.1073/pnas.94.24.13257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen T K. The O75X adhesin of uropathogenic Escherichia coliis a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]