ABSTRACT.
Neonatal mortality is a major contributor to under-five mortality, and Nigeria has the second-highest number of neonatal deaths globally. The country has introduced evidence-based interventions to improve newborn care over the years. The aim of this study was to determine the current trends in neonatal morbidity and mortality at the Lagos University Teaching Hospital, monitor progress over time, and identify areas for improvement. The admission registers and case files of all the neonatal ward admissions were reviewed from January 2018 to April 2020; the age at admission, gestational age, sex, inborn or out-born status, diagnosis, and outcome were recorded and analyzed. Of the 2,959 admissions during the study period, 68.4% were out-born and 77.9% were term gestation infants. The most common diagnoses were neonatal jaundice (NNJ; 28.4%), infection (28.0%), prematurity with associated complications (22.1%), and hypoxic ischemic encephalopathy (HIE; 18.2%). The overall mortality rate was 17.6%. Prematurity with associated complications (39.2%), HIE (24.8%), congenital anomalies (CAs; 12.7%), and NNJ (11.5%) were the most common conditions associated with mortality. Of those who died, the most common diagnoses among term infants were HIE (40.7%), CAs (21.8%), NNJ (18.9%), and infection (15.5%); respiratory distress syndrome (52.4%), infection (31.8%), and CAs (7.8%) were the most common diagnoses in preterm infants. The high risk of mortality with HIE, jaundice, infections, and CAs in this cohort reflects the national figures and trends. Efforts to improve neonatal care, especially respiratory support and education of the populace on NNJ, should be intensified to reduce neonatal mortality in the country.
INTRODUCTION
Globally in 2019, 5.2 million children younger than 5 years old died, and 2.5 million (47%) of these deaths occurred during the neonatal period (< 28 days of life).1 As a result of improvements in the treatment of pneumonia, diarrheal diseases, malnutrition, and malaria, among other measures during the past two decades, there has been an appreciable reduction in the total number of under-five deaths from 12.5 million in 1990 to 5.2 million in 2019, amounting to a 59% reduction.1 The contribution of neonatal mortality to under-five mortality, however, increased from 40% in 1990 to 46% in 2019, as a result of the slower decline (52%) in neonatal mortality during this period.1 Therefore, it is important that efforts to reduce neonatal mortality are intensified to achieve the Sustainable Development Goal (SDG) of reducing neonatal mortality to 12 per 1,000 live births and reducing under-five mortality to at least as low as 25 per 1,000 live births by 2030 (goal 3, target 2).2
In sub-Saharan Africa, there is a significant burden of poor neonatal outcomes, with the region having the highest neonatal mortality rate of 27 deaths per 1,000 live births in 2019,1 and the majority of these deaths occur in the first week of life.3 Nigeria is a major contributor to neonatal mortality globally, with the second-highest number of neonatal deaths (270,000) in the world.4 There have been concerted efforts to reduce neonatal mortality throughout the years, using evidence-based interventions. These include the WHO recommendations for antenatal steroids, thermal care including the use of kangaroo mother care, and treatment of infections.5 The Essential Newborn Care Course (ENCC) package, which was adopted in Nigeria in 2008, encompasses practices and interventions that are provided to a newborn immediately after delivery, including thermal care, prevention of infection, initiation of breastfeeding within an hour after birth, and newborn resuscitation.6,7 The training of health-care workers in the ENCC has been harmonized in Nigeria by the Federal Ministry of Health, with assistance from partners including the American Academy of Pediatrics through the Helping 100,000 Babies Survive and Thrive Initiative,8 among others. Other interventions are care of the small baby, identification of small and sick babies, and determination of transport for further care.5 Despite these past improvements and evidence-based interventions, 53 countries including Nigeria risk missing the SDG targets for neonatal mortality because of the slow rate of reduction in these populations during the past two decades.1 It is therefore important to review regularly the trends in neonatal mortality to identify which interventions require increased focus and attention.9,10
The aim of this study was to determine the current trends in neonatal mortality at the Lagos University Teaching Hospital (LUTH) as well as common neonatal morbidities, to monitor progress over time, and to identify areas for improvement.
METHODS
This was a 28-month retrospective review of the hospital admission records— from January 2018 to April 2020—at Lagos University Teaching Hospital, a tertiary care center in Lagos, Nigeria. LUTH has one of the largest neonatal ward capacities in Nigeria, with 80 cot spaces for infants. There are two neonatal wards, one for infants born within the facility (inborn ward) and the other for infants born outside the facility who require further care (out-born ward). Patients admitted into the inborn ward come from the hospital’s labor ward, labor ward theater, and the postnatal wards. The out-born ward receives patients from the Children Emergency Center and outpatient clinics. The hospital has a blood bank, radiology, pharmacy, microbiology services, and chemical pathology laboratory services available 24 hours a day. The hospital has oxygen for respiratory support; however, there were no functioning mechanical ventilators at the time of the study. Continuous positive airway pressure (CPAP) was provided mainly by improvised bubble CPAP11 devices, which used 100% oxygen, because only a few commercial CPAP devices were available.
We reviewed the admission register and case files of all newborn admissions in the hospital for a 28-month period—January 2018 to April 2020. Information extracted included the infants’ biodata (age at admission, gestational age, and gender), inborn or out-born status, admitting diagnosis, and outcome (death or survival). Preterm neonates were categorized based on their gestational age at birth: extremely preterm, < 28 weeks; very preterm, 28 to < 32 weeks; moderately preterm, 32 to < 34 weeks; and late preterm, 34 to < 37 weeks.12,13 They were also classified based on their birthweight as extremely low birthweight (< 1,000 g), very low birthweight (1,000 to < 1,500 g), low birthweight (1,500 to < 2,500 g), and normal birthweight (2,500–4,000 g).14
Data extracted were entered into a Microsoft Excel version 16.20 spreadsheet (Microsoft Corporation, Redmond, WA), where it was cleaned and edited. The cleaned data were analyzed within Microsoft Excel. Analyzed data are presented as frequencies in tables and charts.
Ethical approval for the research was obtained from the Lagos University Teaching Hospital Health Research Ethics Committee (approval no. ADM/DCST/HREC/APP/4154). The extracted information was de-identified to protect and maintain patient confidentiality at the point of data abstraction.
RESULTS
Subjects.
A total of 2,959 neonatal patients (< 28 days of life on admission) were cared for at LUTH during the study period. Of the total number of patients, 2,024 (68.4%) were admitted to the out-born ward and 2,304 (77.9%) were born at term (gestational age, ≥ 37 weeks). The mean age of infants on admission was 4.4 days. The demographic characteristics of the study population are shown in Table 1.
Table 1.
Patient demographics
| Patient characteristic | Cases, n | Total admissions, % |
|---|---|---|
| Gender | ||
| Male | 1,843 | 62.3 |
| Female | 1,081 | 36.5 |
| Unspecified | 35 | 1.2 |
| Gestational age, weeks | ||
| Extremely preterm, < 28 | 85 | 2.8 |
| Very preterm, 28 to < 32 | 187 | 6.3 |
| Moderately preterm, 32 to < 34 | 168 | 5.7 |
| Late preterm, 34 to < 37 | 209 | 7.1 |
| Unspecified | 6 | 0.2 |
| Term, ≥ 37 | 2,304 | 77.9 |
| Birthweight, g | ||
| < 2,500 | 744 | 25.1 |
| 2,500–4,000 | 218 | 73.6 |
| > 4,000 | 37 | 1.3 |
| Place of admission | ||
| Inborn | 935 | 31.6 |
| Out-born | 2,024 | 68.4 |
Morbidity.
For all infants, neonatal jaundice was the most common diagnosis (28.4%, n = 840 [738 term and 102 preterm]), followed by neonatal infections (28.0%, n = 828 [549 term and 279 preterm]), prematurity (22.1%, n = 655), and hypoxic ischemic encephalopathy (HIE) (18.2%; n = 538 [529 term and 9 preterm]), as shown in Table 2. These totals include dual diagnoses (prematurity with jaundice, prematurity with infection, and so on); thus, the total numbers exceed the 2,959 infants in the study population. Analyzing only the population of term infants, jaundice remained the most common diagnosis (n = 738, 32.0%), followed by infection (n = 549, 23.8%), then HIE (n = 529, 23.0%). Of those born preterm, neonatal infections (n = 279, 42.6%), respiratory distress syndrome (RDS) (n = 174, 26.6%), and jaundice (n = 102, 15.6%) were the most common comorbidities.
Table 2.
Neonatal admissions by primary diagnosis
| Diagnosis | Cases, n | Total admissions, % |
|---|---|---|
| Jaundice | 738 | 25.0 |
| Prematurity and associated complications | 655 | 22.1 |
| With infection | (279) | (9.4) |
| With respiratory distress syndrome | (174) | (5.9) |
| With jaundice | (102) | (3.4) |
| With anomalies | (33) | (1.1) |
| With hypoxic ischemic encephalopathy | (9) | (0.3) |
| With other | (58) | (2.0) |
| Infection | 549 | 18.6 |
| Hypoxic ischemic encephalopathy | 529 | 17.9 |
| Congenital anomalies | 217 | 7.3 |
| Respiratory distress (meconium aspiration syndrome, transient tachypnea of the newborn) | 106 | 3.6 |
| Anemia | 34 | 1.1 |
| Macrosomia | 26* | 0.9 |
| Hypoglycemia | 15 | 0.5 |
| Other | 90 | 3.0 |
Eleven other macrosomic (birthweight, > 4,000 g) infants who were admitted for other reasons (nine with sepsis, one with jaundice, and one with hypoxic ischemic encephalopathy) were excluded.
Mortality.
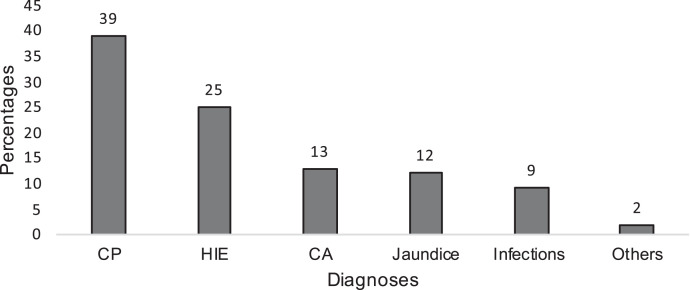
Of the total 2,959 patients included in the analysis, 521 died during the neonatal period, resulting in a mortality rate of 17.6% for the population studied. Among those who died, 337 were male (64.8%), 176 (33.8%) were female and eight were of unspecified gender. Of those who died, among both term and preterm populations, prematurity with associated complications was the most common condition associated with mortality during the neonatal period (n = 204, 39.2%), followed by HIE (n = 129, 24.8%), congenital anomalies (n = 69, 12.7%), and neonatal jaundice (n = 60, 11.5%; Figure 1). Of the 317 term infants who died, HIE was the most common diagnosis associated with mortality (n = 129, 40.7%), followed by congenital anomalies (n = 69, 21.8%), neonatal jaundice (n = 60, 18.9%), and infection (n = 49, 15.5%).
Figure 1.
Neonatal mortality by diagnosis. CA = congenital abnormalities; CP = complication of prematurity; HIE = hypoxic ischemic encephalopathy.
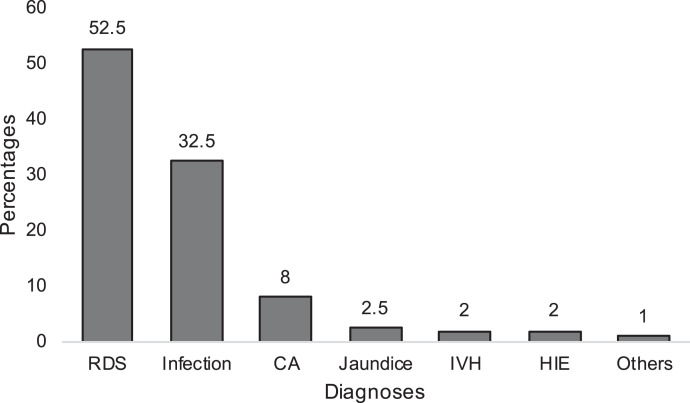
For the 204 preterm infants who died, RDS was the most common diagnosis associated with mortality (n = 107, 52.4%), followed by infection (n = 65, 31.8%), and congenital anomalies (n = 16, 7.8%), as shown in Figure 2. Seventy-five (88.2%) of the extremely preterm, 85 (45.2%) of the early preterm, 27 (16.1%) of the moderate preterm, and 16 (7.8%) of the late preterm infants died (Table 3). One hundred two infants (50.0%) were extremely low birthweight, and 70 (34.3%) and 31 (15.2%) were very low birthweight and low birthweight, respectively. Only one late preterm weighed 2,500 g at birth. Considering the place of birth, 381 (19.0%) of the out-born infants died compared with the 140 (15.0%) of the inborn infants. A greater proportion of infants with neonatal jaundice, infection, and HIE died in the out-born group compared with the inborn group (Table 4).
Figure 2.
Mortality according to complications associated with prematurity. CA = congenital abnormalities; HIE = hypoxic ischemic encephalopathy; IVH = intraventricular hemorrhage; RDS = respiratory distress syndrome.
Table 3.
Complications of prematurity by gestational age cohort
| Gestational age categories | Admissions, n | Deaths, n (% of subgroup by diagnosis) |
|---|---|---|
| Extremely preterm (< 28 weeks) | 85 | 75 (88.2) |
| Infection | 8 | 5 (62.5) |
| Respiratory distress syndrome | 77 | 70 (90.9) |
| Very preterm (28–31 weeks) | 187 | 85 (45.4) |
| Infection | 101 | 44 (44.4) |
| Respiratory distress syndrome | 68 | 36 (52.9 |
| Neonatal jaundice | 10 | 0 (0.0) |
| Congenital anomalies | 1 | 1 (100.0) |
| Congenital heart disease | 2 | 2 (100.0) |
| Intraventricular hemorrhage | 1 | 1 (100.0) |
| Hypoxic ischemic encephalopathy | 2 | 0 (0.0) |
| Other | 2 | 1 (50.0) |
| Moderately preterm (32–33 seeks) | 168 | 27 (16.1) |
| Infection | 100 | 6 (6.0) |
| Respiratory distress syndrome | 25 | 1 (4.0) |
| Neonatal jaundice | 16 | 0 (0.0) |
| Congenital anomalies | 13 | 12 (92.3) |
| Anemia | 5 | 0 (0.0) |
| Congenital heart disease | 0 | 0 (0.0) |
| Intraventricular hemorrhage | 2 | 2 (100.0) |
| Hypoxic ischemic encephalopathy | 2 | 2 (100.0) |
| Hypoglycemia | 1 | 0 (0.0) |
| Other | 4 | 4 (100.0) |
| Late preterm (34–36 weeks) | 209 | 16 (7.7) |
| Infection | 70 | 9 (12.9) |
| Respiratory distress syndrome | 4 | 0 (0.0) |
| Neonatal jaundice | 71 | 2 (2.8) |
| Congenital anomalies | 8 | 1 (12.5) |
| Anemia | 2 | 0 (0.0) |
| Congenital heart disease | 1 | 0 (0.0) |
| Intraventricular hemorrhage | 1 | 1 (100.0) |
| Hypoxic ischemic encephalopathy | 5 | 2 (40.0) |
| Hypoglycemia | 3 | 0 (0.0) |
| Other | 44 | 1 (2.3) |
| Unclassified | 6 | 1 (16.7) |
Table 4.
Admitting diagnosis and mortality by place of birth
| Primary diagnosis | Inborn | Out-born | ||
|---|---|---|---|---|
| Cases, n | Mortality, n (%) | Cases, n | Mortality, n (%) | |
| Neonatal jaundice | 186 | 1 (0.5) | 552 | 59 (10.7) |
| Infection | 84 | 1 (1.2) | 465 | 48 (10.3) |
| Prematurity with associated complications | 385 | 101 (26.2) | 270 | 103 (38.1) |
| Congenital anomalies | 48 | 21 (43.8) | 169 | 48 (28.4) |
| Hypoxic ischemic encephalopathy | 79 | 15 (19.0) | 450 | 114 (25.3) |
| Meconium aspiration syndrome/transient tachypnea of the newborn | 61 | 0 (0.0) | 45 | 3 (6.7) |
| Macrosomia | 21 | 0 (0.0) | 5 | 0 (0.0) |
| Hypoglycemia | 15 | 0 (0.0) | 0 | 0 (0.0) |
| Anemia | 6 | 0 (0.0) | 28 | 2 (7.1) |
| Other | 50 | 1 (2.0) | 40 | 4 (10.0) |
| Total | 935 | 140 (14.9) | 2024 | 381 (18.8) |
The current causes of mortality were compared with those reported about 20 years ago from the study center,9 as shown in Table 5. Prematurity, HIE, and congenital abnormalities were the leading conditions associated with mortality in our study, compared with asphyxia, jaundice, and infection in this earlier study.9
Table 5.
Trends in causes of neonatal mortality at the study center
| Ezeaka et al.9 (2003) | Current study (2021) | ||
|---|---|---|---|
| Disease condition | Contribution to overall mortality, % | Disease condition | Contribution to overall mortality, % |
| Asphyxia | 35.0 | Prematurity with associated complications | 39.2 |
| Jaundice | 23.3 | Hypoxic ischemic encephalopathy | 24.8 |
| Infection | 19.4 | Congenital abnormalities | 13.0 |
| Prematurity | 11.7 | Jaundice | 11.5 |
| Tetanus | 4.9 | Infection | 9.4 |
| Other | 5.7 | Other | 2.1 |
DISCUSSION
Neonatal mortality remains an important metric to follow globally because it reflects the general health and well-being of the population at large. The disparities in neonatal mortality generally reflect the inequalities in society.15 Sub-Saharan Africa—and Nigeria, in particular—have both shown great success in improving neonatal mortality, but need accelerated efforts in the future to work toward the WHO SDGs.1 The prevalence of neonatal mortality in our study was 17.6% of the study population. This has decreased somewhat from the 21.1% neonatal mortality reported by Ezeaka et al.9 from the same tertiary center. Globally, the three most common causes of neonatal mortality are prematurity with associated complications, asphyxia, and sepsis.4,16 In our study, however, after prematurity with associated complications and asphyxia, congenital anomalies and jaundice exceeded infection as the third and fourth most common diagnoses associated with neonatal mortality, respectively.
Prematurity has become more prevalent in sub-Saharan Africa, including Nigeria, in the past two decades. The WHO recommends interventions for improving outcomes for preterm infants, including the use of antenatal corticosteroids, surfactant, continuous bubble positive airway pressure ventilation, provision of warmth using kangaroo mother care, and prompt use of antibiotics to treat infections.5 When compared with the study by Ezeaka et al.9 from the same center in 2003, prematurity with associated complications ranked fourth in the cause of neonatal mortality; however, it is now the highest cause of mortality in our study. The most common causes of death among preterm infants in our study were RDS, and infection. This is not surprising because most of the infants were out-born and most likely had no benefit of antenatal steroids, which has been shown to improve outcome in preterm infants. The prohibitive cost of surfactant also limits its access to the very preterm and extremely preterm infants who require it. There were no functional neonatal ventilators for infants who required additional support beyond CPAP during the study period. In addition, during the study period, there were also insufficient commercial CPAP machines at the center. Therefore, most of the infants needing CPAP were placed on improvised CPAP devices,11 which may not be as effective as the standardized commercial devices. These factors, in addition to out-of-hospital deliveries, which reduce the possibility of antenatal corticosteroids for preterm births, further increase the risk of death from RDS.
The provision of improved neonatal care along with high-quality, low-cost commercial CPAP machines; ventilators; subsidized surfactant; and blended oxygen are urgently needed if we are to decrease neonatal morbidity and mortality significantly. This is important, especially for the extreme and early preterm neonates, with 88.2% and 45.4% mortality, respectively, and who jointly make up about 30% of the neonatal mortality in our study. The main causes of death in these two groups were RDS and sepsis.
Although majority of preterm admissions in our study were inborn infants, there was greater mortality (38.1%) among the out-born preterm admissions compared with inborn admissions (26.2%). The majority of these infants are usually transported to the hospital in suboptimal conditions,17,18 and are often hypothermic and in respiratory distress on arrival. They have also been exposed to conditions that increase the risk of sepsis, such as inappropriate cord care.17 These facts may explain the increased mortality in this group of neonates. Education of mothers and health-care providers on the importance of hospital delivery and early referral to facilities that are equipped to care for these vulnerable infants will reduce mortality in this group.19 The importance of appropriate means of transport with a prior notification of the receiving health-care facility is also very important to improve the outcome for these infants.19,20
Asphyxia, on the other hand, which was reported as the second highest morbidity 20 years ago, is now the fourth most common cause of morbidity in our study and also in a multicenter study on the burden of disease and risk factors for mortality among hospitalized newborns in Nigeria and Kenya.21 This is probably a reflection of the positive effect of increased training of health-care workers on neonatal resuscitation, step-down training to other health-care workers,22 and helping babies breathe training on newborn resuscitation. Possible reasons for the high number of deaths from asphyxia despite the reduced number of cases include the unavailability of facilities for therapeutic hypothermia and that only the more severe cases are referred whereas the other milder cases recover after resuscitation. It is also possible that the skills acquired by the trained health-care workers have depreciated over time. It is therefore important to review the areas from which these cases are being referred so that the health-care workers from facilities in these areas are trained in newborn resuscitation if they have not benefited from neonatal resuscitation training in the past. Other possible risk factors for the delivery of asphyxiated infants should also be explored for necessary intervention.
Congenital anomalies, which did not feature as a cause of death almost 20 years ago at the study center, was the third-highest cause of mortality. The improvement in antenatal care, delivery, neonatal care, diagnosis and treatment of infections may be responsible for the increasing number of congenital anomalies as the cause of neonatal deaths. Therefore, it is important to start measures to reduce congenital anomalies such as antenatal folic acid, focused antenatal care, and education of mothers on the need to avoid indiscriminate use of drugs, especially during early pregnancy, which may compromise the infant. The use of routine antenatal anomaly scans for all pregnant women will also aid in the early detection of anomalies and in prompt intervention for neonates with congenital anomalies.
Neonatal jaundice is common worldwide and is a common cause of readmission,23 but rarely as a cause of death or even significant morbidity.24 In stark contrast to high-income countries, where jaundice-related death is exceedingly rare, jaundice is often one of the leading causes of neonatal death in low- to middle-income countries (LMICs). This is especially true in Nigeria, where it is frequently one of the four most common causes of death. As noted in a systematic review and meta-analysis by Slusher et al.,24 as well as by others, including Ogunfowora in Nigeria,25 Eze in Yemen,26 and Jajoo in India,27 many infants still experience complications from severe neonatal jaundice and die of this preventable cause of morbidity and mortality. In a recent article by Olusanya et al.,28 neonatal jaundice ranked seventh as a cause of early neonatal death.
The reasons for the severe morbidity and mortality from neonatal jaundice in LMICs are multifactorial.29,30 They include the high rate of unsupervised home births, with more than 60% of deliveries occurring outside of hospitals31; lack of routine screening for neonatal jaundice before discharge for infants who are born in hospitals30; a high incidence of glucose-6-phosphate dehydrogenase deficiency combined with the use of hemolytic agents and sepsis.32,33 Additional factors include the high likelihood of ineffective phototherapy from sub-standard devices and erratic power supply; and the inability to perform emergent blood transfusions for several reasons, such as unavailability of blood.30,34 When jaundice develops, it is discovered late, and mothers present late to the hospital after trying various ineffective home remedies to no avail.35 Our finding that jaundice was the most common morbidity is consistent with reports from an earlier study at the center about two decades ago9 and a recent study of neonatal outcomes in secondary and tertiary facilities in Nigeria (study center included) and Kenya.21
There has been no widespread active training on the early recognition of neonatal jaundice over the years; however, this is now included in the essential care for every newborn (ENCC), but the effect of the addition of neonatal jaundice to these programs is yet to be seen both in term and preterm infants included in our study. One recent small study from Nigeria demonstrated that maternal education is effective in significantly reducing acute bilirubin encephalopathy.36 In addition, a packaged approach to prevention and treatment has been instituted in some LMICs and has been successful in reducing severe neonatal jaundice,37 and should be widely implemented in Nigeria.
The incidence of neonatal infections in our study is less than that reported in an earlier study by Lawn et al.,38 in which sepsis was estimated as a cause of 26% of all neonatal deaths worldwide and the 22% reported in Nigeria.39 Infections also ranked fifth in our study as a cause of mortality, compared with third in the 2003 study.9 Possible reasons for the decline in the contribution of infections to neonatal deaths in our study include the improved laboratory support for cultures, availability of life-saving antibiotics in the facility, the low threshold for treatment of neonatal sepsis, and the increase in infection control awareness and prevention.
When considering further the morbidities associated with mortality and place of birth, the percentage of mortality within the disease cohort was greater for all the conditions except congenital abnormalities in out-born infants. A plausible explanation for this is the referral nature of the study center, which encourages referrals of complicated cases, including those with in utero diagnosis of abnormality around the time of delivery. This precludes adequate preparation and counseling of the parents, especially in cases of anomalies that are not compatible with life.
The major limitations of our study include its retrospective nature, the single-center data source, and the resulting limited depth of analysis. That being said, LUTH is the largest teaching hospital in Nigeria, with an average of 1,200 births and 1,250 neonatal admissions (including out-born admissions) annually. Although a single center’s experience is not generalizable, given the location and the size of the neonatal unit at LUTH, we do believe our study findings are an important reflection of the current neonatal morbidity and mortality in urban centers in Nigeria. Future studies should include larger and more diverse populations, multiple centers, and more detailed analyses of patient characteristics to identify risks and possible strategies for improvement.
CONCLUSION
In conclusion, neonatal mortality is still high at LUTH and reflects the national figures and trends. Prematurity with associated complications, HIE, jaundice, infections, and congenital anomalies remain the leading causes of neonatal mortality. Efforts to improve antenatal care are crucial to prevent preterm births, and when this is inevitable, facilitation of delivery in or rapid appropriate transport to facilities staffed and equipped to handle these infants is imperative. Facilities for improved preterm care should be provided in secondary and tertiary hospitals. More awareness should be created on neonatal jaundice, with a packaged approach to prevention and effective treatment, including high-quality phototherapy at all levels of care, thus preventing severe neonatal jaundice and its sequelae. Training on neonatal resuscitation, essential newborn care, and care of small and sick infants should be intensified, in addition to the provision of low-cost medical devices40 to improve care. These efforts will help to reduce neonatal mortality and move the country closer to achieving SDG goal 3.2.
ACKNOWLEDGMENTS
We acknowledge Khadijah Oleolo-Ayodeji, who assisted in data collection.
REFERENCES
- 1. World Health Organization , 2020. Levels and Trends in Child Mortality: Report 2020. Available at: https://www.who.int/publications/m/item/levels-and-trends-in-child-mortality-report-2020. Accessed June 21, 2022.
- 2. United Nations General Assembly, 2015. Transforming our world: the 2030 Agenda for Sustainable Development. Available at: https://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E. Accessed July 5, 2022.
- 3. Oza S, Cousens S, Lawn J, 2014. Estimation of daily risk of neonatal death, including the day of birth, in 186 countries in 2013: a vital-registration and modelling-based study. Lancet Glob Health 2: e635–e644. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization , 2020. Newborns: Improving Survival and Well-being. Available at: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality. Accessed June 21, 2022.
- 5. World Health Organization , 2015. WHO Recommendations on Interventions to Improve Preterm Birth Outcomes. Available at: https://apps.who.int/iris/bitstream/handle/10665/183037/9789241508988_eng.pdf;jsessionid=6603B6C087D8A303D166E816DD9FA52F?sequence=1. Accessed July 5, 2021. [PubMed]
- 6. Federal Ministry of Health , 2016. Nigeria Every Newborn Action Plan: A Plan to End Preventable Newborn Deaths in Nigeria. Available at: https://www.healthynewbornnetwork.org/hnn-content/uploads/Nigeria-Every-Newborn-Action-Plan.pdf. Accessed February 4, 2021.
- 7. World Health Organization , 2010. Essential Newborn Care Course. Trainer’s guide Available at: https://www.who.int/publications/i/item/essential-newborn-care-course. Accessed July 5, 2022.
- 8. American Academy of Pediatrics , 2014. Solidarity bolstered in new global initiative to save 100,000 babies every year. AAP News 35: 30. [Google Scholar]
- 9. Ezeaka VC, Ogunbase AO, Awogbemi OT, Grange AO, 2003. Why our children die: a review of paediatric mortality in a tertiary centre in Lagos, Nigeria. Niger Q J Hosp Med 13: 17–21. [Google Scholar]
- 10. Fajolu IB, Egri-Okwaji MTC, 2011. Childhood mortality in children emergency centre of the Lagos University Teaching Hospital. Niger J Paediatr 38: 131–135. [Google Scholar]
- 11. Ezenwa B, Akintan P, Fajolu I, Ladele J, Ezeaka C, 2016. Bubble CPAP in the management of respiratory distress syndrome in resource constrained settings: the LUTH experience. Pediatr Oncall J. 13: 9–12. [Google Scholar]
- 12. March of Dimes, Partnership for Maternal Newborn and Child Heath Children, World Health Organization , 2012. Born Too Soon: The Global Action Report on Preterm Birth Born . Geneva, Switzerland: WHO. [Google Scholar]
- 13. Engle WA. et al. , 2007. “Late-preterm” infants: a population at risk. Pediatrics 120: 1390–1401. [DOI] [PubMed] [Google Scholar]
- 14. Cutland CL. et al. , 2017. Low birth weight: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 35: 6492–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hug L, Alexander M, You D, Alkema L, 2019. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health 7: e710–e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE, 2016. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388: 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdulraheem MA, Tongo OO, Orimadegun AE, Akinbami OF, 2016. Neonatal transport practices in Ibadan, Nigeria. Pan Afr Med J 24: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okonkwo IR, Abhulimhen-Iyoha BI, Okolo AA, 2020. Newborn transport practices: influence on newborn survival in Benin City, Nigeria. Am J Pediatr 6: 346–352. [Google Scholar]
- 19. Tette EMA, Nuertey BD, Akaateba D, Gandau NB, 2020. The transport and outcome of sick outborn neonates admitted to a regional and district hospital in the upper west region of Ghana: a cross-sectional study. Children (Basel) 7: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mohan KR, Kumar R, 2019. Study of indications, complications and outcomes of neonatal transport by a skilled team. Int J Contemp Pediatrics 6: 2402–2405. [Google Scholar]
- 21. Nabwera HM. et al. , 2021. Burden of disease and risk factors for mortality amongst hospitalized newborns in Nigeria and Kenya. PLoS One 16: e0244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Disu EA, Ferguson IC, Njokanma OF, Anga LA, Solarin AU, Olutekunbi AO, Ekure EN, Ezeaka VC, Esangbedo DO, Ogunlesi TA, 2015. National neonatal resuscitation training program in Nigeria (2008–2012): a preliminary report. Niger J Clin Pract 18: 102–109. [DOI] [PubMed] [Google Scholar]
- 23. Fowler TT, Fairbrother G, Owens P, Garro N, Pellegrini C, Simpson L, 2014. Trends in complicated newborn hospital stays and costs, 2002–2009: implications for the future. Medicare Medicaid Res Rev 4: mmrr2014-004-04-a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Slusher TM, Zamora TG, Appiah D, Stanke JU, Strand MA, Lee BW, Richardson SB, Keating EM, Siddappa AM, Olusanya BO, 2017. Burden of severe neonatal jaundice: a systematic review and meta-analysis. BMJ Paediatr Open 1: e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogunfowora OB, Ogunlesi TA, Ayeni VA, 2019. Factors associated with clinical outcomes among neonates admitted with acute bilirubin and hypoxic-ischaemic encephalopathies at a tertiary hospital in south-west Nigeria. S Afr Fam Pract 61: 177–183. [Google Scholar]
- 26. Eze P, Al-Maktari F, Alshehari AH, Lawani LO, 2020. Morbidities and outcomes of a neonatal intensive care unit in a complex humanitarian conflict setting, Hajjah Yemen: 2017–2018. Confl Health 14: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jajoo M, 2019. IDDF2019-ABS-0222 Exchange transfusion in neonatal hyperbilirubinemia and bilirubin encephalopathy: a long way to go. Gut 68: A149.
- 28. Olusanya BO, Teeple S, Kassebaum NJ, 2018. The contribution of neonatal jaundice to global child mortality: findings from the GBD 2016 study. Pediatrics 141: e20171471. [DOI] [PubMed] [Google Scholar]
- 29. Olusanya BO, Ogunlesi TA, Slusher TM, 2014. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch Dis Child Educ Pract Ed 99: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 30. Slusher TM, Day LT, Ogundele T, Woolfield N, Owa JA, 2017. Filtered sunlight, solar powered phototherapy and other strategies for managing neonatal jaundice in low-resource settings. Early Hum Dev 114: 11–15. [DOI] [PubMed] [Google Scholar]
- 31. UNICEF , 2019. The State of the World’s Children 2019: Statistical Tables. Available at: https://data.unicef.org/resources/dataset/sowc-2019-statistical-tables/. Accessed June 21, 2022.
- 32. Olusanya BO, Osibanjo FB, Mabogunje CA, Slusher TM, Olowe SA, 2016. The burden and management of neonatal jaundice in Nigeria: a scoping review of the literature. Niger J Clin Pract 19: 1–17. [DOI] [PubMed] [Google Scholar]
- 33. Olusanya BO, Emokpae AA, Zamora TG, Slusher TM, 2014. Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose-6-phosphate dehydrogenase deficiency. Acta Paediatr Int J Paediatr 103: 1102–1109. [DOI] [PubMed] [Google Scholar]
- 34. Cline BK, Vreman HJ, Faber K, Lou H, Donaldson KM, Amuabunosi E, Ofovwe G, Bhutani VK, Olusanya BO, Slusher TM, 2013. Phototherapy device effectiveness in Nigeria: irradiance assessment and potential for improvement. J Trop Pediatr 59: 321–325. [DOI] [PubMed] [Google Scholar]
- 35. Ezeaka VC, Ekure EN, Fajolu IB, Ezenwa BN, Akintan PE, 2016. Mothers’ perception of neonatal jaundice in Lagos, Nigeria: an urgent need for greater awareness. S Afr J Child Health 10: 227–230. [Google Scholar]
- 36. Wennberg RP. et al. , 2020. Maternal instruction about jaundice and the incidence of acute bilirubin encephalopathy in Nigeria. J Pediatr 221: 47–54.e4. [DOI] [PubMed] [Google Scholar]
- 37. Thielemans L. et al. , 2018. Indirect neonatal hyperbilirubinemia in hospitalized neonates on the Thai-Myanmar border: a review of neonatal medical records from 2009 to 2014. BMC Pediatr 18: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lawn JE, Cousens S, Zupan J, 2005. 4 Million neonatal deaths: When? Where? Why? Lancet 365: 891–900. [DOI] [PubMed] [Google Scholar]
- 39. Federal Ministry of Health , 2011. Saving Newborn Lives in Nigeria: Newborn Health in the Context of the Integrated Maternal, Newborn and Child Health Strategy. 2nd edition. Abuja: Federal Ministry of Health, Save the Children, Jhpiego.
- 40.Kirby R, Palamountain K, 2020. Target product profiles for newborn care in low-resource settings (v1..2). Consesus meeting report. Available at: https://www.unicef.org/supply/media/2556/file/TPP-newborn-care-final-report-v1-2.pdf. Accessed July 5, 2022.