ABSTRACT.
Infection of HIV is associated with an increased diabetes risk, which also increases tuberculosis risk. It is unknown if similar associations exist with gestational diabetes (GDM). We screened pregnant women living with and without HIV for GDM using oral glucose tolerance testing. In a subgroup of women with latent tuberculosis (positive interferon-gamma [IFN-γ] release assay), we used supernatants from tuberculosis antigen tubes to compare cytokine levels from women with and without GDM, matched by age and HIV status. Of 234 women, 21 (9%) had GDM, 13.9% living with HIV, and 6.5% without HIV (P = 0.06). Compared with women without GDM, women with GDM had lower median IFN-γ (19.1 versus 141.9 pg/mL, P = 0.03) and interleukin-2 (18.7 versus 249 pg/mL, P < 0.01). Our study suggests that HIV infection is associated with an increased risk of GDM, which is associated with decreased Mycobacterium tuberculosis immune responses. Gestational diabetes screening should be prioritized in tuberculosis-endemic countries, especially in women living with HIV.
INTRODUCTION
Gestational diabetes (GDM) affects 16% of women globally, resulting in adverse outcomes, including maternal infections and future type 2 diabetes.1,2 In nonpregnant populations, HIV infection is associated with an increased risk of type 2 diabetes, likely from direct effects of antiretroviral drugs and HIV-induced inflammation.3,4 It is unknown, however, if HIV infection is associated with an increased risk of GDM. This is especially important to understand because HIV infection and diabetes are both independent risk factors for tuberculosis (TB), a leading cause of maternal mortality in TB-endemic countries.4,5
There are few studies on GDM epidemiology in women living with HIV, and none on GDM and TB immunology. A meta-analysis identified only six studies of GDM and HIV infection that included women living with and without HIV, thereby concluding there was insufficient data to determine an association.6 Notably, none of the studies were from Asia, the region with the highest GDM prevalence among women living with HIV.7 India is an optimal place to study the intersection of GDM, HIV infection, and TB because it has the largest burden of TB, the second largest burden of diabetes, and the third largest burden of HIV infection globally.8
Our objectives were to 1) define the prevalence of GDM in pregnant Indian women living with and without HIV, and 2) explore the effect of GDM on the host immune response to Mycobacterium tuberculosis to optimize GDM and maternal TB prevention strategies.
MATERIALS AND METHODS
We analyzed cross-sectional data collected from women enrolled in the Pregnancy-Associated Changes in Tuberculosis immunology (PRACHITi) study. It was a prospective observational cohort study that characterized the effects of pregnancy on the immune response to M. tuberculosis.9 Women with and without latent TB were enrolled in a 2:1 ratio in an attempt to enroll equal numbers of women living with and without HIV. Participants were recruited from the antenatal clinic at Sassoon Government Hospital in Pune, India. Relevant enrollment criteria included gestational age 13–34 weeks and no active TB in the last 2 years.
All participants underwent latent TB testing with the interferon-gamma (IFN-γ) release assay (IGRA; Quantiferon-TB Gold or Gold-Plus).10 Women with latent TB underwent symptom screening, chest radiograph, and Gene Xpert to rule out active TB. Enrolled women were tested for GDM with the 75 g oral glucose tolerance test at 24–34 weeks’ gestation with results interpreted per the International Association of Diabetes and Pregnancy Study Groups criteria.11 Demographics and risk factors were collected, including CD4 count, HIV viral load, and antiretroviral therapy (women living with HIV only). We used cutoffs for Asian-Indians to categorize body mass index (BMI; underweight/normal weight: < 23 kg/m2, or overweight: ≥ 23 kg/m2).12
We used univariable logistic regression to assess risk factors for GDM and stratified by HIV status to identify unique GDM risk factors for this population. Variables with P < 0.1 were included in the multivariate model. The PRACHITi was powered to delineate how HIV impacted the immune response to M. tuberculosis. With 234 participants and 21 cases of GDM, the study had 80% power to detect a 26% difference in GDM prevalence between women living with and without HIV with a 1% margin of error.
To explore the effect of GDM on TB immunology, we performed a nested case-control study of IGRA+ women with GDM (cases) and without GDM (controls), matched on age and HIV status. In this subset, we performed enzyme-linked immunoassays on IGRA supernatant from the TB antigen tube (TB-1 for Quantiferon Plus) to measure and compare IFN-γ, interleukin-10 (IL-10), IL-4, TNF-α, IL-2, IL-6, and TGF-β levels between cases and controls using the Mann–Whitney U test.
Each woman provided written informed consent. We obtained ethical approval from Byramjee Jeejeebhoy Government Medical College in India, and Weill Cornell Medicine and Johns Hopkins University in the United States.
RESULTS
Of the 234 women, 79 (33.8%) were living with HIV and 165 (70.5%) had latent TB. The median age was 23 years (interquartile range [IQR] 21–26), gestational age was 28.6 weeks (IQR 28.3–30.5), and BMI was 21.4 kg/m2 (IQR 19.3–24.7). Two (0.9%) women had GDM in a prior pregnancy; none had diabetes before pregnancy. Women living with HIV had a median CD4 count of 467 cells/mm3 (IQR 344–675) and were all on antiretroviral therapy: 11 (13.9%) on a protease inhibitor–based regimen and 68 (86.1%) on a non-nucleoside reverse transcriptase inhibitor–based regimen. Fifty-four (68.4%) had an undetectable HIV-1 viral load (< 100 copies/mL) at entry. The remaining sociodemographic and clinical characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the cohort
| Characteristic | Total cohort (N = 234) |
|---|---|
| Median age, years (IQR) | 23 (21–26) |
| Median gestational age at time of GDM screening, weeks (IQR) | 28.6 (28.3–30.5) |
| Lower and lower-middle socioeconomic classes* | 223 (95.3%) |
| History of active TB | 5 (2.1%) |
| Type 2 diabetes before pregnancy | 0 (0%) |
| Gestational diabetes in prior pregnancy | 2 (0.9%) |
| Anthropometrics | |
| Third trimester weight, kg (IQR) | 54 (52–67) |
| Median height, cm (IQR) | 152 (148–156) |
| Median prepregnancy BMI†, kg/m2 (IQR) | 19.4 (16.9–21.7) |
| Median BMI at entry, kg/m2 (IQR) | 21.4 (19.3–24.7) |
| Underweight/Normal weight (< 23 kg/m2) | 148 (63.3%) |
| Overweight (> 23 kg/m2) | 86 (36.8%) |
| Comorbidities | |
| HIV infection | 79 (33.8%) |
| Median CD4 count, cells/mm3 (IQR) | 467 (344–675) |
| Median ART duration, days (IQR) | 743.5 (123–2012) |
| Protease inhibitor-based ART | 11 (13.9%) |
| Undetectable viral load (< 100 copies/mL) | 54 (68.4%) |
| Median viral load among detectable (N = 25), copies/mL (IQR) | 647 (163, 1845) |
| Latent TB infection | 153 (65.4%) |
| Quantiferon Gold | 147 (96.1%) |
| Quantiferon Gold-Plus | 6 (3.9%) |
ART = antiretroviral therapy; BMI = body mass index; GDM = gestational diabetes; IQR = interquartile range; PI = protease inhibitor; TB = tuberculosis.
Kuppuswamy socioeconomic status scale.
Available for 50 women.
Overall GDM prevalence was 9.0% (95% CI 5.6, 13.4%, N = 21). In women living with HIV, GDM prevalence was 13.9% (95% CI 7.2, 23.5%, N = 11) versus 6.5% (95% CI 3.1, 11.5%, N = 10) in women living without HIV (P = 0.06). On univariable analysis, odds of GDM increased if overweight (OR 6.54, 95% CI 2.30–18.57, P < 0.01), with age (OR 1.12, 95% CI 1.03–1.22, P = 0.01), and with HIV infection (OR 2.35, 95% CI 0.95–5.79, P = 0.06). After adjusting for age and BMI, odds ratio of GDM with HIV infection was 2.16 (95% CI 0.82, 5.69, P = 0.12).
Stratified by HIV status, overweight BMI was a risk factor for GDM in both groups, while age was a risk factor only in women living with HIV (OR 1.21 95% CI 1.06–1.39, P = 0.01). There was a trend toward the interaction between age and HIV infection (OR 1.20 95% CI 0.98, 1.47, P = 0.07). (Table 2)
Table 2.
Univariate and multivariate logistic regression for the association of risk factors with GDM
| Characteristic | GDM N = 21 | No GDM N = 213 | OR (95% CI) | P value | aOR (95% CI) | P value |
|---|---|---|---|---|---|---|
| HIV infection, % | 11 (52.4%) | 68 (31.9%) | 2.35 (0.95, 5.79) | 0.06 | 2.16 (0.82, 5.69) | 0.12 |
| Protease inhibitor-based ART, % | 2 (18.2%) | 9 (13.2%) | 0.69 (0.13, 3.70) | 0.66 | – | – |
| Age, years (IQR) | 26 (25–30) | 23 (21–26) | 1.12 (1.03, 1.22) | 0.01 | 1.07 (0.98, 1.18) | 0.13 |
| With HIV | 30 (26–32) | 24 (21–27.5) | 1.21 (1.06, 1.39) | 0.01 | – | – |
| Without HIV | 24.5 (21–26) | 22 (20–26) | 1.01 (0.87, 1.17) | 0.90 | – | – |
| Age* HIV | – | – | 1.20 (0.98, 1.47) | 0.07 | – | – |
| BMI at entry, kg/m2 (IQR) | 25.0 (23.0–28.8) | 21.2 (19.1–24.2) | 6.54 (2.30, 18.57) | < 0.01 | 5.87 (2.01, 17.15) | < 0.01 |
| With HIV | 25.1 (22.9–31.8) | 21.1 (19.3–23.8) | 5.38 (1.40, 20.68) | 0.01 | – | – |
| Without HIV | 24.2 (23.0–26.3) | 21.2 (18.9–24.6) | 3.96 (1.14, 13.77) | 0.03 | – | – |
| BMI* HIV | – | – | 0.76 (0.09, 6.42) | 0.80 | – | – |
aOR = adjusted odds ratio; ART = antiretroviral therapy; BMI = body mass index; GDM = gestational diabetes; IQR = interquartile range; OR = odds ratio.
Indicates the interaction term of HIV and the other variable.
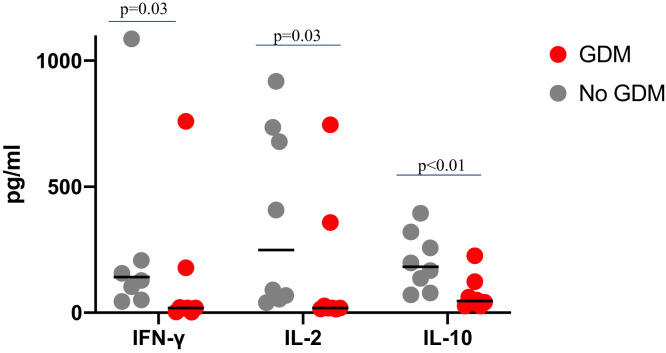
By design, 165 (70%) women had latent TB. Among women with latent TB, IGRA supernatant from pregnant women with GDM had significantly lower median IFN-γ (19.1 versus 141.9 pg/mL, P = 0.03), IL-2 (18.7 versus 249 pg/mL, P < 0.01), and IL-10 (45.8 versus 182 pg/mL, P = 0.03) compared with women without GDM (Figure 1). There were no significant differences in other cytokines by GDM status.
Figure 1.
Interferon -gamma (IFN-γ), IL-2, and IL-10 levels (pg/mL) after stimulation with Mycobacterium tuberculosis antigens in women with and without gestational diabetes (GDM) during pregnancy. (Testing was done on interferon gamma release assay supernatant from tuberculosis [TB] antigen tubes. For participants tested with Quantiferon Plus, the TB-1 tubes were used.) This figure appears in color at www.ajtmh.org.
DISCUSSION
We found that 9% of pregnant women in India with low socioeconomic status and low BMI had GDM, including 14% of pregnant women living with HIV. Furthermore, women with GDM and latent TB had a lower IFN-γ and IL-2 response to M. tuberculosis antigens compared with matched controls without GDM. Taken together, our data suggest that HIV may increase the prevalence of GDM, and GDM may decrease the immune response to M. tuberculosis.
Consistent with our findings, studies in nonpregnant populations also document that well-controlled HIV infection is associated with a 4-fold increased diabetes risk.4 While older antiretroviral medications directly disrupted glucose transporters, diabetes has been reported with newer medications as well.13,14 Current mechanistic theories focus on persistent inflammation from HIV infection, even when virally suppressed, as well as altered adipocyte metabolism.15 Inflammation increases insulin resistance, leading to impaired glucose tolerance,3 which may contribute to the greater prevalence of GDM observed in women living with HIV. We found age was significantly associated with GDM only among women living with (not without) HIV, suggesting that older age increases the strength of association between HIV infection and GDM.
The higher prevalence of GDM in pregnant women living with HIV, however, has not been reported in studies from the United States and Botswana, where GDM prevalence was similar in women living with and without HIV.16,17 Women in those studies had similar CD4 counts and antiretroviral therapy coverage as our cohort, but were mainly on protease or integrase inhibitor–based regimens. They also had higher BMIs and age than women in our cohort. Paradoxically, the lower BMI of our cohort may explain the higher prevalence of GDM in India, where more than half of people with diabetes are not overweight. Studies show that lean individuals, particularly Asians, have decreased ability to produce insulin because of decreased β-cell mass.18 In these individuals, GDM mainly results from an inability to increase insulin production in response to the hyperglycemia of pregnancy.19 For Indian women living with HIV, then, their risk of GDM may be higher because the insulin resistance from HIV-related inflammation accelerates β-cell exhaustion and further impairs insulin production.
Notably, GDM was associated with impaired IFN-γ, IL-2, and IL-10 responses to M. tuberculosis antigens. These cytokines are important in the host immune response to M. tuberculosis and have been found to be lower in IGRA+ nonpregnant adults with type 2 diabetes as well.20 Type 2 diabetes has been associated with a 3-fold increased risk of active TB, likely through impairing the function of T-cells and macrophages, which are important in killing M. tuberculosis.21 It remains unknown if GDM has a similar effect, but our findings suggest it is possible. Importantly, a lower IFN-γ response in GDM could lead to missed diagnoses of latent TB and missed opportunities to provide TB preventive therapy. Further study of the effect of GDM on TB risk is urgently needed.
A strength of this study was that we screened all women with gold standard oral glucose tolerance testing, allowing high confidence in our GDM diagnoses. Inclusion of a comparable cohort without HIV allowed us to isolate the effect of HIV infection on GDM from other sociodemographic risk factors. Few women were on protease inhibitors, however, limiting interpretation of a medication-related effect. Though women living with HIV were older than women without HIV infection in our cohort, the difference in age was not clinically significant. To our knowledge, this is the first study to investigate GDM in the context of HIV and TB.
In conclusion, our data suggest that pregnant women living with HIV are at increased risk of GDM, and GDM may impair the immune response to M. tuberculosis. In countries like India with a high burden of all three conditions, routine GDM screening should be considered, especially for pregnant women living with HIV. Larger studies are needed to validate our observations, controlling for interactions between HIV infection, age, and GDM.
REFERENCES
- 1. Guariguata L Linnenkamp U Beagley J Whiting DR Cho NH , 2014. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract 103: 176–185. [DOI] [PubMed] [Google Scholar]
- 2. Vounzoulaki E Khunti K Abner SC Tan BK Davies MJ Gillies CL , 2020. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369: m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kalra S Kalra B Agrawal N Unnikrishnan A , 2011. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown TT Cole SR Li X Kingsley LA Palella FJ Riddler SA Visscher BR Margolick JB Dobs AS , 2005. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 165: 1179–1184. [DOI] [PubMed] [Google Scholar]
- 5. Jeon CY Murray MB , 2008. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 5: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soepnel LM Norris SA Schrier VJMM Browne JL Rijken MJ Gray G Klipstein-Grobusch K , 2017. The association between HIV, antiretroviral therapy, and gestational diabetes mellitus. AIDS 31: 113–125. [DOI] [PubMed] [Google Scholar]
- 7. Biadgo B Ambachew S Abebe M Melku M , 2019. Gestational diabetes mellitus in HIV-infected pregnant women: a systematic review and meta-analysis. Diabetes Res Clin Pract 155: 107800. [DOI] [PubMed] [Google Scholar]
- 8. Alexander M, Gupta A, Mathad JS, 2019. Is there a connection between gestational diabetes mellitus, human immunodeficiency virus infection, and tuberculosis? Int J Tuberc Lung Dis 23: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramesh B Alexander M Deshpande P Kulkarni V Gupte N Gupta A Mathad J , 2021. Stages of pregnancy and HIV affect diagnosis of tuberculosis infection and Mycobacterium tuberculosis (MTB)-induced immune response: findings from PRACHITi, a cohort study in Pune, India. Int J Infect Dis 112: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. QuantiFERON-TB Gold Plus (QFT-Plus) Package Insert , 2019. Instruction manual in the Quantiferon test kit. Germantown, MD: Qiagen.
- 11. Library WRH , 2020. WHO Recommendation on the Diagnosis of Gestational Diabetes in Pregnancy. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 12. Misra A , 2015. Ethnic-specific criteria for classification of body mass index: a perspective for Asian Indians and American diabetes association position statement. Diabetes Technol Ther 17: 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murata H Hruz PW Mueckler M , 2000. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem 275: 20251–20254. [DOI] [PubMed] [Google Scholar]
- 14. Mynarcik DC McNurlan MA Steigbigel RT Fuhrer J Gelato MC , 2000. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr 25: 312–321. [DOI] [PubMed] [Google Scholar]
- 15. Mathad JS et al. 2016. Sex-related differences in inflammatory and immune activation markers before and after combined antiretroviral therapy initiation. J Acquir Immune Defic Syndr 73: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mmasa KN. et al. , 2021. Gestational diabetes in women living with HIV in Botswana: lower rates with dolutegravir- than with efavirenz-based antiretroviral therapy. HIV Med 22: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haeri S Shauer M Dale M Leslie J Baker AM Saddlemire S Boggess K , 2009. Obstetric and newborn infant outcomes in human immunodeficiency virus-infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol 201: 315.e1–315.e5. [DOI] [PubMed] [Google Scholar]
- 18. Staimez LR Deepa M Ali MK Mohan V Hanson RL Narayan KMV , 2019. Tale of two Indians: heterogeneity in type 2 diabetes pathophysiology. Diabetes Metab Res Rev 35: e3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Inaishi J Saisho Y , 2017. Ethnic similarities and differences in the relationship between beta cell mass and diabetes. J Clin Med 6: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Faurholt-Jepsen D et al. 2014. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand J Infect Dis 46: 384–391. [DOI] [PubMed] [Google Scholar]
- 21. Vallerskog T Martens GW Kornfeld H , 2010. Diabetic mice display a delayed adaptive immune response to Mycobacterium tuberculosis. J Immunol 184: 6275–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]