Abstract
Acute Graft versus host disease (aGvHD) is an immune mediated reaction that can occur after hematopoietic stem cell transplantation in which donor T-cells recognize the host antigens as foreign, destroying host tissues. Establishment of a tolerogenic immune environment while preserving immune response to infectious agents is required for successful bone marrow transplantation. Pregnancy specific glycoprotein 1 (PSG1) is secreted by the human placenta into the maternal circulation throughout pregnancy. PSG1 likely plays a role in maintaining immunotolerance to prevent rejection of the fetus by the maternal immune system. We have previously shown that PSG1 activates the latent form of transforming growth factor-β1 (TGF-β), a cytokine essential for the differentiation of tolerance inducing CD4+FoxP3+ regulatory T-cells (Tregs). Consistant with this observation, treatment of naïve murine T-cells with PSG1 resulted in a significant increase in FoxP3+ cells that was blocked by a TGF-β receptor-I inhibitor. We also show here that PSG1 can increase the availability of active TGF-β in vivo. As the role of CD4+ FoxP3+ cells in the prevention of aGvHD is well established, we tested whether PSG1 has beneficial effects in a murine aGHVD transpant model. PSG1-treated mice had reduced numbers of tissue-infiltrating inflammatory CD3+ T-cells and had increased expression of FoxP3 in T-cells when compared to vehicle-treated mice. In addition, administration of PSG1 significantly inhibited aGVHD-associated weight loss and mortality. On the other hand, administration of PSG1 was less effective at managing aGvHD in the presence of an alloimmune reaction against a malignancy in a graf-versus-leukemia experimental model. Combined, this data strongly suggests that PSG1 could be a promising treatmet option for patients suffering aGVHD following bone marrow transplantation for non-malignant conditions such as autoimmune disorders and genetic immunodeficiencies.
Keywords: GvHD, Pregnancy specific glycoprotein, TGF-β1, immunotolerance, regulatory T-cells
INTRODUCTION
Hematopoietic stem cell transplantation is curative for many disorders including autoimmune disorders, genetic immunodeficiencies and malignancies; however, it can be associated with significant morbidity and mortality, often as a result of graft versus host disease (GvHD) 1. GvHD is characterized by an immune reaction of donor cells to host tissues and occurs in approximately 40% of allogeneic stem cell transplantation patients2. Currently, GvHD treatment and prevention involves the use of immunosuppressants, a treatment shown to be effective in only about 50% of patients and that can cause significant side effects 1.
Pregnancy specific glycoprotein 1, also known as pregnancy-specific β−1 glycoprotein 1 (PSG1), is the the most highly expressed protein of the family of 10 closely related pregnancy specific glycoproteins 3, with PSGs reaching concentrations of ~ 200 μg/ml in the serum of pregnant women at term 4,5. During pregnancy, changes in the immune system are required to maintain the fetal semi-allograft, resulting in a general reduction in clinical disease activity of several autoimmune-based pathologies, including multiple sclerosis, rheumatoid arthritis, and uveitis 6–11. These tolerogenic changes are believed to be mediated, at least in part, by factors secreted by the placenta, including PSGs 12,13.
PSG1 activates latent transforming growth factor beta-1 (TGF-β1), a cytokine essential for the differentiation of tolerance-inducing CD4+CD25+FoxP3+ regulatory T-cells (Tregs) and suppression of inflammatory T-cells 14–16. Because PSG1 has previously been shown to have the potential to induce immune tolerance and has a significant effect on activation of TGF-β1, we hypothesize that PSG1 might function as a treatment option for patients suffering from GvHD. Our results in an acute GvHD (aGvHD) mouse model suggest that PSG1 is effective in preventing acute GvHD and has the therapeutic potential for patients suffering from this disease.
METHODS
Mice
C57BL/6J (H2-Kb); B6.PL-Thy1a/CyJ (H2-Kb) and B6D2F1/J (H2-Kb/d) were purchased from The Jackson Laboratory (Bar Harbor, ME). FoxP3-IRES-GFP knock-in mice were provided by Dr. Strober (National Institutes of Health). All animal studies were approved by the National Institute of Allergy and Infectious Disease Animal Care and Use Committee.
Pregnancy-Specific Glycoprotein-1 Production and Purification
PSG1-Fc and the control FLAG-Fc were generated as previously described and were utilized at equimolar concentrations 17. For most in vivo experiments we utilized PSG1-His (R&D Systems, Minneapolis, MN).
Murine Transplant Models
Male and female 8–12 week old mice were used as transplant recipients and as cell donors. Bone marrow cells were collected from the femurs and tibia of donor mice and depleted of T-cells using mouse pan-t (Thy1.2) Dynabeads (ThermoFisher Scientific, Grand Island, NY) to produce CD3+ T-cell depleted bone marrow (TCDBM). Spleen CD3+ T-cells were purified from donor mice using mouse CD3+ T-cell enrichment columns (R&D Systems).
C57BL/6J or B6.PL-Thy1a/CyJ donor cells were transplanted into B6D2F1/J recipients (H2-Kb into H20Kb/d). Mice were first irradiated with 850 rads on Day −1. Twenty-four hours later on Day 0, mice were transplanted with donor TCD-BM (1×107 cells) and CD3+ T lymphocytes (1 × 107 cells) via tail vein injection to induce GvHD. A negative control group of B6D2F1/J mice received TCD-BM only from B6D2F1/J donor mice (designated BM only).
Mice were intraperitoneally injected every other day with 100 μg of PSG1, vehicle (PBS) or rapamycin (Sigma-Aldrich) beginning on Day −2 and continuing through to Day +14 or Day +26 as indicated. This dosage was chosen based on previous experiments15. Weights were monitored daily and any mice exceeding 20% weight loss from starting weight or exhibiting prostrated posture matching high GvHD clinical score were sacrificed. Clinical scores were given with the criteria described in Naserian et al. 18
To study the effects of PSG1 on T cell activation against leukemic cells (graft versus leukemia), 8–12 week old female mice were used as transplant recipients and cell donors. C57BL/6 donor cells were transplanted into B6D2F1 recipients. Mice were irradiated Day −1 as previously described and twenty four hours later were transplanted with donor TCD-BM (1×107 cells), CD3+ T lymphocytes (1×107 cells) and E2A PBX BL6 leukemia cells (5×104 cells) via tail vein injection 19. A positive control group of B6D2F1 mice received TCD-BM only from B6D2F1 donor mice along with E2A PBX BL6 leukemia cells. Mice were injected intraperitoneally every other day with 100 μg of PSG1, rapamycin or vehicle (PBS) beginning on Day −2. Weights were monitored as previously stated.
Flow Cytometric Analysis
For in vitro FoxP3 expression analysis, cells were washed and resuspended in serum and azide free PBS seventy-two hours after plating and stained for viability with eFluor 780 viability dye (Affymetrix, Santa Clara, CA) for 30 minutes at 4°C followed by a wash and incubation with anti-mouse CD4-FITC (RM4–5) and CD25-APC (PC61.5) or with anti-human CD4-FITC (OKT4) (eBioscience, San Diego, CA) and CD25-PE (BD Biosciences, San Jose, CA). Cells were fixed and permeabilized using the FoxP3 Staining Buffer Set (eBioscience), and then stained with anti-mouse FoxP3-PE (FJK-16s) or anti-human FoxP3-APC (236A/E7) (eBioscience).
SMAD2/3 phosphorylation was determined in cells treated with 100 μg/ml of recombinant proteins for 1 hour at 37° C prior to fixation in BD Phosflow Lyse/Fix Buffer (BD Biosciences). Cells were then permeabilized using Perm Buffer III (BD Biosciences) and stained with pSMAD2/3-PE (BD Biosciences).
For the Nrp1 studies, flow cytometric analysis was performed in spleen cell suspensions and peripheral blood collected from tail vein following removal of red blood cells with the ACK lysis buffer (Quality Biological, Gaithersburg, MD). Cells were then incubated with anti-mouse CD4-FITC (RM4–5), CD25-APC (PC61.5) or NrpI-PE-Cy7 (3DS304M) (Affymetrix), followed by fixation and permeabilization with the FoxP3 Staining Buffer Set and addition of anti-mouse Foxp3-PE (FJK-16s) (Affymetrix).
Isotype-matched control antibodies were used in all experiments. All samples were run on a benchtop BD FACSCanto flow cytometer and analysis was performed using FlowJo FACS analysis software (FlowJo LLC, Ashland, OR).
Cytokine and Chemokine Measurements
CD4+ T-cells were isolated from C57BL/6 mouse spleens using CD4 T-cell biotin antibody cocktail and anti-biotin microbeads (Miltenyi, Gaithersburg, MD). Cells were plated in 96-well plates at a concentration of 1×105 cells/well and activated with mouse CD3/CD28 T-cell activator Dynabeads (ThermoFisher Scientific). IL-2 secretion was exmined as previously described 20.
Cytokines were measured using Luminex cytokine/chemokine multiplex immunoassay kits (R&D Systems) with the BioPlex System (Bio-Rad, Hercules, CA) in serum collected from mouse peripheral blood after induction of aGvHD by adoptive transfer of T-cells. Serum was collected on Day 27 to determine cytokine expression after cessation of treatment on Day 14.
Mouse and Human Cell Culture
Spleens were collected from 12-week-old FoxP3-IRES-GFP knock-in mice on C57BL/6 background or from 12 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). Cell suspensions were prepared from spleens following treatment with ACK lysing buffer. CD4+CD25− and CD4+CD25+ splenic T-cells were isolated using a mouse CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi, Gaithersburg, MD). For FoxP3 expression studies, cells were cultured in 24 well plates (3×105 cells per well), stimulated with CD3/CD28 T-cell activator Dynabeads and incubated with 50 ng/ml of recombinant human IL-2 (Peprotech, Rocky Hill, NJ) in IMDM (Life Technologies) with 10% fetal bovine serum (FBS) (Atlanta Biologicals) and 100 μg/ml Penicillin/Streptomycin (Quality Biological) in the presence of the indicated recombinant proteins. To study the ability of PSG1 to phosphorylate SMAD2/3, cells were cultured in 24 well plates (1×106 cells per well) and incubated overnight in IMDM with no added supplements.
Human peripheral blood was collected from healthy volunteers after informed consent on an IRB approved protocol. Isolation of peripheral blood mononuclear cells (PBMC) was performed by gradient centrifugation using lymphocyte separation medium (MP Biomedicals, Solon, OH), followed by removal of red blood cells. CD4+CD25− T-cells were isolated from PBMCs using the human CD4+CD25+ Regulatory T-cell Isolation Kit (Miltenyi). Cells were cultured in 24 well plates (3×105 cells per well) in RPMI (Life Technologies) with 10% FBS and 100 μg/ml Penicillin/Streptomycin. Cells were treated with 50 ng/ml of human IL-2 (Peprotech) and stimulated with CD3/CD28 T-cell activator Dynabeads in the presence of the indicated proteins.
In vivo Bioluminescence Imaging
Bioluminescence emitted from the SBE-luc mice was detected with the In Vivo Imaging System (IVIS Spectrum; PerkinElmer, Waltham, MA). Mice were injected intraperitoneally with 150 mg/kg D-luciferin 10 min before imaging and were anesthetized during imaging. Images were obtained from the same mice in dorsal and ventral positions at 24 h before PSG1 or control protein (FLAG-Fc) injection (as baseline), and 6 h and 24 h after injection. For bioluminescence signal quantification, regions of interest (ROI) were manually selected over the head, abdomen and back areas on the surface of the mice, and photons emitted from each ROI were acquired as photons/s/cm2/steridian (sr) using LIVINGIMAGE software (version 4.0). For data analysis, bioluminescence was expressed as fold induction over baseline for each mouse.
Histology and Immunostaining
Colon and small intestine were fixed in 4% paraformaldehyde and embedded in paraffin. Some sections were stained with hematoxylin and eosin. The number of CD3+ intraepithelial lymphocytes were determined after staining with anti-CD3 Ab (2GV6)(VENTANA medical systems, Tucson, AZ) by counting three high power fields per section per mouse (n=5) per treatment. We also stained the formalin-fixed colon tissues of the negative control, positive control and PSG treatment groups with Trichrome II Blue (Ventana Medical Systems) under the manufacturer’s recommendations. This stain is specific for collagen fibers and is routinely utilized as an indicator of fibrosis, which was scored by a pathologist blinded to the treatments.
FoxP3 expression was determined in deparaffinized slides followed by heat induced epitope retrieval using sodium citrate buffer. After blocking with 10% normal serum, 1% BSA and 0.025% Triton X-100, slides were incubated overnight at 4°C with FoxP3-FITC (FJK-16s, eBioscience). Coverslips were applied with DAPI mounting medium (Sigma Aldrich). Blinded cell counts were performed over three areas chosen randomly in at least two different tissue sections each from a minimum of five mice. To assess presence or absence of GvHD and/or leukemia, slides of femur, spleen, colon and large intestine from all animals were reviewed by a pathologist.
Statistics
Prism software (GraphPad Software, La Jolla, CA) was used for all statistical analyses. Student t test with Welch’s Correction or Mann Whitney, one-way ANOVA with Bonferroni or two-way ANOVA with Tukey’s multiple comparisons test and Fisher’s exact test were used to compare groups.
RESULTS
PSG1 Induces the Differentiation of FoxP3+ Regulatory T-cells and Inhibits the Secretion of IL-2 in vitro in a TGF-β1 Dependent Manner
FoxP3 regulatory T-cells are important for immune tolerance and are induced by the presence of active TGF-β121, 22. Because PSG1 is involved in the activation of latent TGF-β1, we tested whether PSG1 was able to convert CD4+ naïve mouse T-cells into Tregs. Cells were collected from C57BL/6 mouse spleens, CD4+CD25− (CD4+ naïve) cells were isolated and activated with anti-CD3/CD28 Abs in the presence of PSG1 or vehicle (0.2% DMSO). Cells treated with PSG1 showed an increase in FoxP3 expression by Day 3, with even higher expression by Day 7 (Figure 1A). When cells were treated with PSG1 in conjunction with 5 μM of the TGF-β receptor I (ALK5) inhibitor SB431542, there was no increase in FoxP3 expression (Figure 1A). To determine whether PSG1 has the same effect on human cells, naïve CD4+ T-cells were isolated from peripheral blood and activated with anti-CD3/CD28 Abs. We observed an increase in the percentage of cells expressing FoxP3 upon PSG1 treatment (Figure 1B). As observed for mouse cells, human T-cells treated with PSG1 and 5 μM of SB431542 showed a reduction in FoxP3 expression down to untreated levels (Figure 1B, Right). These results indicate that PSG1 induces the differentiation of mouse and human naïve CD4+ T cells into regulatory T-cells and that this process requires a functional TGF-β receptor on the cell surface.
Figure 1: Conversion of mouse and human naïve T-cells into FoxP3 Regulatory T-cells by PSG1 is dependent on TGF-β.
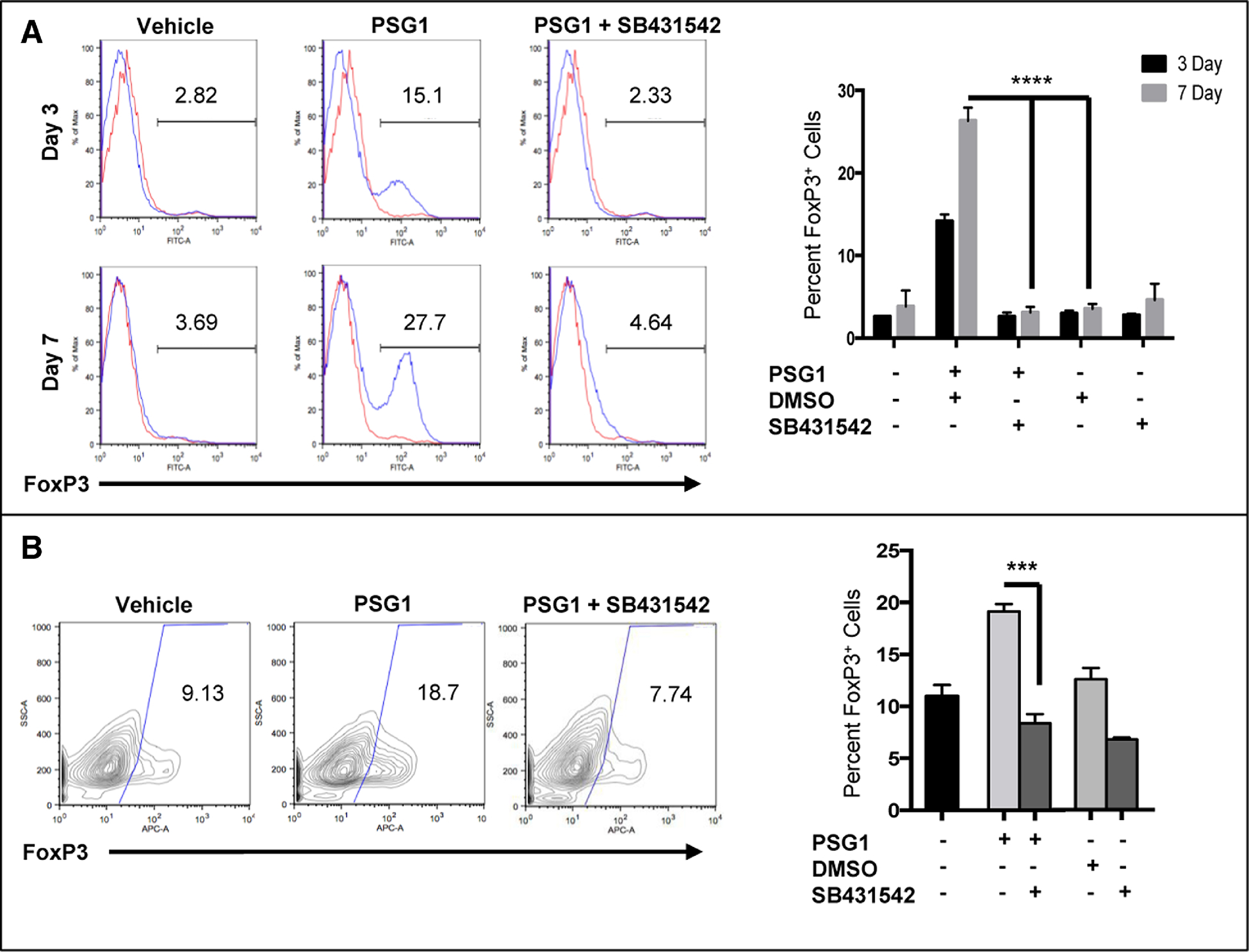
(A) Naïve CD4+ mouse T-cells isolated from the spleens of FoxP3-GFP transgenic mice were stimulated with T-cell activator Dynabeads in the presence of IL-2 and treated with PSG1 (100 ug/ml), 0.2% DMSO vehicle control or PSG1 plus 5 μM of SB431542. Cells were analyzed on Day 3 and Day 7 for FoxP3-GFP expression. Left, representative flow cytometry data of FoxP3 expression in murine cells on Day 3 and Day 7. (Red – isotype control, Blue – treated cells)Right, graphical representation of the flow cytometry data (****p<0.0001). (B) Naïve CD4+ human T-cells isolated from peripheral blood were stimulated with T-cell activator Dynabeads in the presence of IL-2 and treated with PSG1 (100ug/ml), vehicle (PBS) or PSG1 plus SB431542 (5μM). Cells were analyzed on Day 3. Left, representative flow cytometry data for FoxP3 expression in human cells. Cell populations were gated on viable CD4+CD25+ cells and were stained with APC-labeled anti-human FoxP3 antibody. Right, graphical representation of flow cytometry data (***p<0.0005). The data shown are representative of at least three independent experiments performed in triplicate. Mean ±SEM are shown and P-values were calculated using one-way ANOVA.
TGF-β1 plays a significant role in immune regulation by inducing the phosphorylation of Smad2/3, resulting in increased expression of FoxP3 and inhibition of IL-2 secretion in T-cells 23,24. PSG1 treatment of murine naïve CD4+ T-cells inhibited IL-2 secretion without affecting T-cell proliferation measured at 72 hours post-stimulation (Figure 2A and data not shown). This inhibition of IL-2 was reversed when cells were treated with the ALK5 inhibitor (Figure 2B). In addition, treatment with PSG1 increased the level of Smad2/3 phosphorylation in these cells (Figure 2C). To study Smad2/3-dependent signaling in vivo as a result of PSG1 administration, we used transgenic reporter mice that express luciferase in response to activation of Smad2/3 (SBE-luc mice)25. After SBE-luc mice were injected with a single dose of PSG1 and a luciferase substrate, we observed significant increases in luciferase expression in a temporal manner in the abdomen, head and back (Figure 2D). Increased luciferase expression over the vehicle injected mice was most evident at 6 h and could be still observed at 24 hours post-PSG1 injection, albeit at lower levels (Figure 2E). These results support the role of the TGFβ-SMAD pathway in the mouse immune response to PSG1.
Figure 2: Treatment of naïve mouse CD4+ T-cells with PSG1 inhibits IL-2 expression and activation of the pSMAD2/3 pathway by PSG1 in vitro and in vivo.
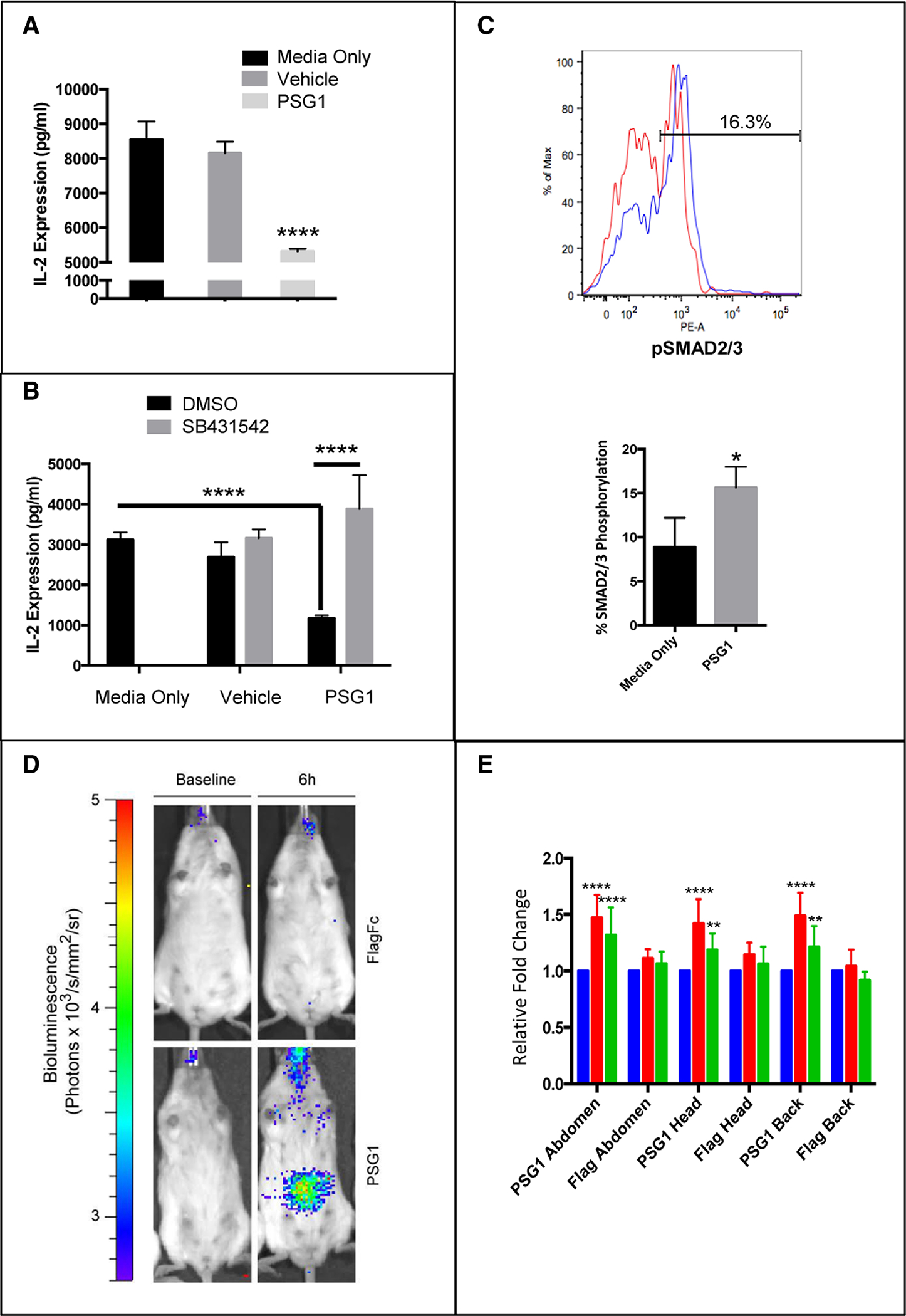
(A) Naïve CD4+ T-cells were purified from C57BL/6 mouse spleens and activated with T-cell activator Dynabeads. Cells were treated with vehicle (PBS) or (40 μg/ml) PSG1-Fc. IL-2 levels were determined by ELISA 72h after activation (****p<0.0001). (B) Cells were purified as previously stated and treated as in A in the presence of 5 μM of SB431542 or vehicle (0.2% DMSO). IL-2 levels were determined by ELISA 72h after activation (****p<0.0001). (C) CD4+CD25− T-cells were treated with 100 μg/ml PSG1 for one hour and stained with pSMAD2/3-PE. Top, representative flow cytometry of pSMAD2/3 expression (Red-Media Only, Blue-PSG1). Bottom, graphical representation of pSMAD2/3 expression (*p<0.05). All treatments were performed in triplicate and three independent experiments were completed. Mean ±SEM are shown, and all P-values were obtained using Student’s t-test (A, C) and two-way ANOVA (B). (D) Luciferase expression by bioluminescence imaging in the abdomen, head and back of SBE-luc mice 6 hours after intraperitoneal injection with a single dose of PSG1 (100 μg). (E) Luciferase expression in the head, neck and back of SBE-luc mice at 0 (baseline), 6 and 24 hours after PSG1 or Flag control injection (**p<0.005, ****p<0.0001). Mean ±SEM are shown and all P-values were obtained using two-way ANOVA. Blue = Baseline, Red = 6 hours after injection, Green = 24 hours after injection (n=8 mice, 4 female and 4 male)
Administration of PSG1 in a GvHD Mouse Model Ameliorates GvHD Severity
PSG1 has been shown to have a protective effect in DDS-induced colitis and in collagen-induced arthritis 26,27. Due to the protective effect of Tregs in aGvHD we hypothesized that PSG1 could ameliorate aGvHD and constitute a novel therapeutic strategy. Acute GvHD was induced in a MHC mismatch mouse model and PSG1 or vehicle (PBS) were administered as outlined in Figure 3A. A third group of mice, BM only, served as a control for the induction of aGvHD by adoptive transfer of T-cells. Briefly, beginning on Day −2, mice were injected with PSG1 24 hours before irradiation. Twenty-four hours after irradiation (Day 0), aGvHD was induced by administration of T-cell depleted bone marrow cells (TCD-BM) and T cells from the spleen. Mice were injected every other day from Day −2 to Day 14. On day 27, expression of FoxP3was analyzed in CD4+ cells isolated from the spleen and colon. There was a significant difference in survival between PBS-treated and PSG1-treated mice (P = 0.0002). While both vehicle-treated and PSG1-treated mice lost weight (Figure 3B), 50% of mice in the PBS-treated control group died or had to be euthanized before day 27. Mice in the PBS-treated GvHD group had clinical GvHD scores of between 4–5 and were hunched, moribund and suffering from diarrhea. Conversely, mice in the PSG1-treated GvHD group were alert and had clinical GvHD scores of between 1–2. BM only mice had clinical GvHD scores of 0.
Figure 3: PSG1 treatment reduces mortality and increases FoxP3 regulatory T-cell expression in an acute GvHD model.
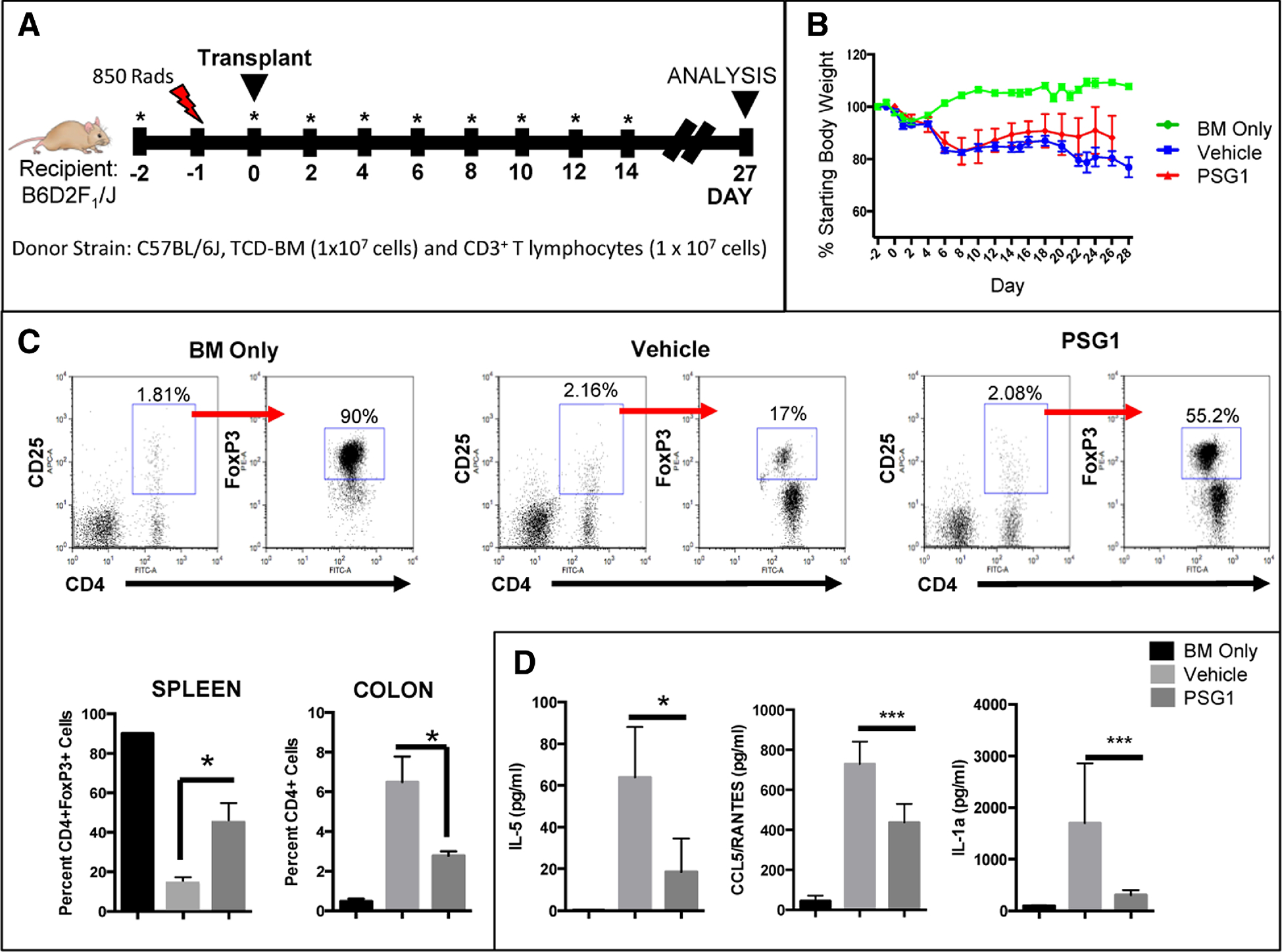
(A) aGvHD mice were injected with 100 μg of PSG1 or PBS on Day −2. On Day −1, mice were irradiated with 850 rads (red bolt) and GvHD was induced 24 hours later on Day 0 by tail vein injection of donor TCD-BM (1×107 cells) and CD3+ T lymphocytes (1 × 107 cells). PSG1 or vehicle (PBS) injections were repeated on Day 0 and every other day until Day 14 (as indicated by the stars). On Day 27, mice were analyzed. (B) Weight loss of mice from Day −2 to Day 27 indicated as a percentage of total starting body weight. (C) Representative flow cytometry showing the percentage of BM only cells and of PBS vehicle-treated or PSG1-treated cells expressing FoxP3 following the induction of aGvHD by adoptive transfer of T-cells. Splenic cells were gated on CD4 and CD25 expressing cells, followed by FoxP3 expression. Bottom left, graphical representation of the flow cytometry data for CD4+ spleen cells expressing Foxp3. Bottom right, graphical representation of colon cells expressing CD4 cells (*p<0.05). (D) IL-5, CCL5/RANTES and IL-1a cytokine analysis of mouse serums collected on Day 27. Cytokines were measured using multiplex immunoassay kits with the Bioplex system. (*p<0.05, ***p<0.0005) All experiments were performed in triplicate in at least two independent experiments (n=4–5 mice per treatment group for each experiment). Mean ±SEM are shown and all P-values were obtained using one way ANOVA.
Flow cytometric analysis showed an increase in the percentage of cells expressing FoxP3 in the spleen of PSG1 treated mice, with an average of 45 (±10) % of T-cells expressing FoxP3, while only 14 (±3.6) % of T-cells from vehicle-treated mice showed FoxP3 expression. This is in comparison to 90 (±0.1) % expression in healthy BM only controls. There was also a reduction in the percentage of total viable CD4+ helper T cells in the colon in the PSG1-treated mice compared to vehicle only(Figure 3C). We did not observe a significant difference in fibrosis in the colon or PSG1-treated mice when compared to vehicle-treated mice as determined by staining of collagen fibers (data not shown).
Analysis of cytokine expression in serum was performed on day 27 and results indicate that PSG1 is able to inhibit the expression of some inflammatory cytokines. IL-5, an inflammatory cytokine known to trigger activated B-cells for terminal differentiation into antibody-secreting plasma cells 28, was found to be significantly reduced in PSG1-treated mice when compared to vehicle-treated mice (Figure 3D). Two additional potent inflammatory chemokines, CCR5/RANTES and IL-1α, were also found to be significantly reduced in mice treated with PSG1. No differences in expression of TNF-α, IL-10, CCL3, CCL11, CXCL1, IL-4, IL-13, G-CSF and IL-9 were observed between the PSG1 and vehicle-treated mice and the expression of GM-CSF, CCL2, IL-2, IL-17a, CCL4, IL-12p70, IL-1β, IL-6 and IFN-γ were below the level of detection (data not shown).
Small intestine and colon, two important aGvHD target organs, were collected from all mice for analysis29 (Figure 4A). There was significant tissue erosion in the intestine from vehicle-injected mice, while mice treated with PSG1 resembled healthy BM only controls. Quantitation of the intraepithelial T-cells in the colon showed a significant decrease in CD3+ cells in mice treated with PSG1 (Figure 4B). Finally, we observed a significant increase in FoxP3+ cells in the colon of PSG1-treated mice over vehicle-treated control mice (Figure 4C). Overall, physiological and molecular data indicates that PSG1 is able to prevent inflammatory responses and tissue damage in a mouse model of aGvHD.
Figure 4: Beneficial effects of PSG1 administration are observed in the colon and small intestine of mice receiving TCD-BM plus T cells.
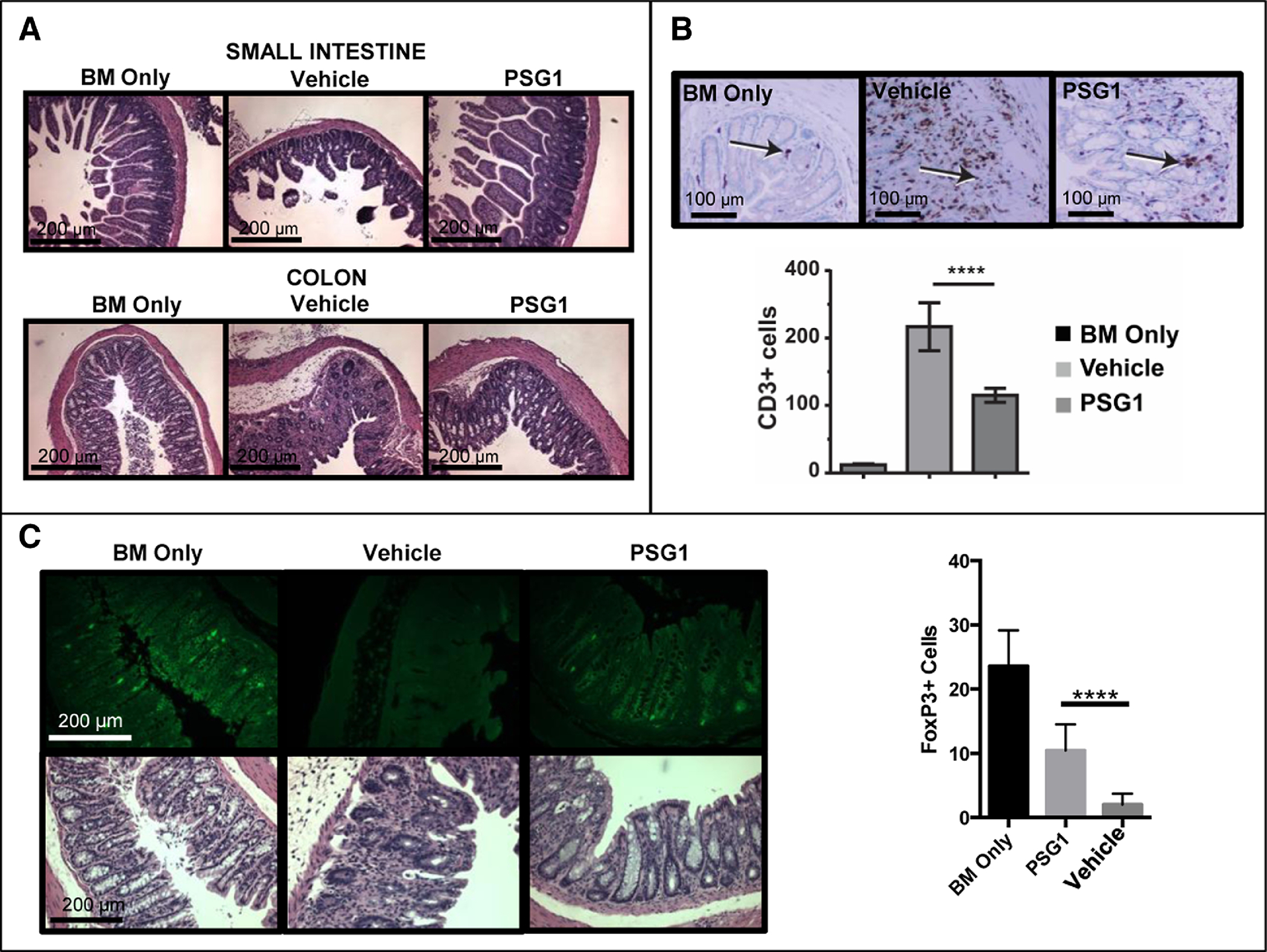
(A) Colon and small intestine were collected from BM Only control mice, PBS vehicle-treated GvHD mice and PSG1-treated GvHD mice on Day 27 for analysis. Hematoxylin and eosin stained sections from these tissues were photographed on a Zeiss AXIO Imager Z1 microscope with Zeiss 1026.548 PI 10X/23 lens and Plan-APOCHROMAT 10X/0.45 (1063–139) aperture and representative images from each treatment group are shown. (B) Colon sections were stained with an anti-CD3 mAb and intraepithelial cells expressing CD3 (indicated with an arrow) were enumerated as described in the methods section. (****p<0.0001) (C) Colon sections were stained with anti-FoxP3-FITC mAb and expression was quantified as indicated in the methods section (****p<0.0001). All images were taken on either the AxioCam ICC1 white light camera or the AxioCamMRm fluorescent camera with the AxioVision Rel.4.8 imaging software at the same exposure and magnification. All counts were performed in triplicate in at least three independent experiments (n=4–5 mice per treatment group for each experiment). Mean ±SEM are shown and all P-values were obtained using one way ANOVA.
Extended Administration of PSG1 Results in a More Favorabe Outcome Following Bone Marrow Transpant
Although PSG1 was able to improve the signs of aGvHD when administered for 14 days, mice lost weight when compared to the BM only group (Figure 3B). To determine if an extended dosage would further improve outcome, mice were treated with PSG1 from Day −2 until the termination of the experiment on Day 26. In addition, PSG1 efficacy was compared to rapamycin (sirolimus), a current standard of care immunosuppressant for the treatment of aGvHD that has significant side effects 30,31. As PSG1 is a natural human protein, the likelihood for side effects or toxicity is lower than for rapamycin.
Mice treated with an extended dosage of PSG1 showed marked improvement in weight gain over vehicle-treated mice (Figure 5A). This weight gain was comparable to that observed in mice receiving rapamycin. As we observed in the short duration treatment, there were marked differences in clinical score and survival between mice treated with vehicle (scores 4–5) and PSG1 treated mice (scores 0–1) (P= 0.003). When performing these experiments, Nutrigel was added in cages at day 0 in an attempt to prolong survival of the PBS-treated mice 32. Though early death in these experiments was not observed, by day 26, all vehicle treated mice had to be sacrificed as these mice were moribund and showed 20% body weight loss. PBS-treated mice showed severe hunching, diarrhea, ruffled fur and lethargy while PSG1 and rapamycin treated mice were active and alert, with no obvious signs of distress.
Figure 5: Extende dosage of PSG1 in aGvHD increases FoxP3 expression, decreases weight loss andinduces comparable or improved outcomes versus GvHD mice treated with rapamycin.
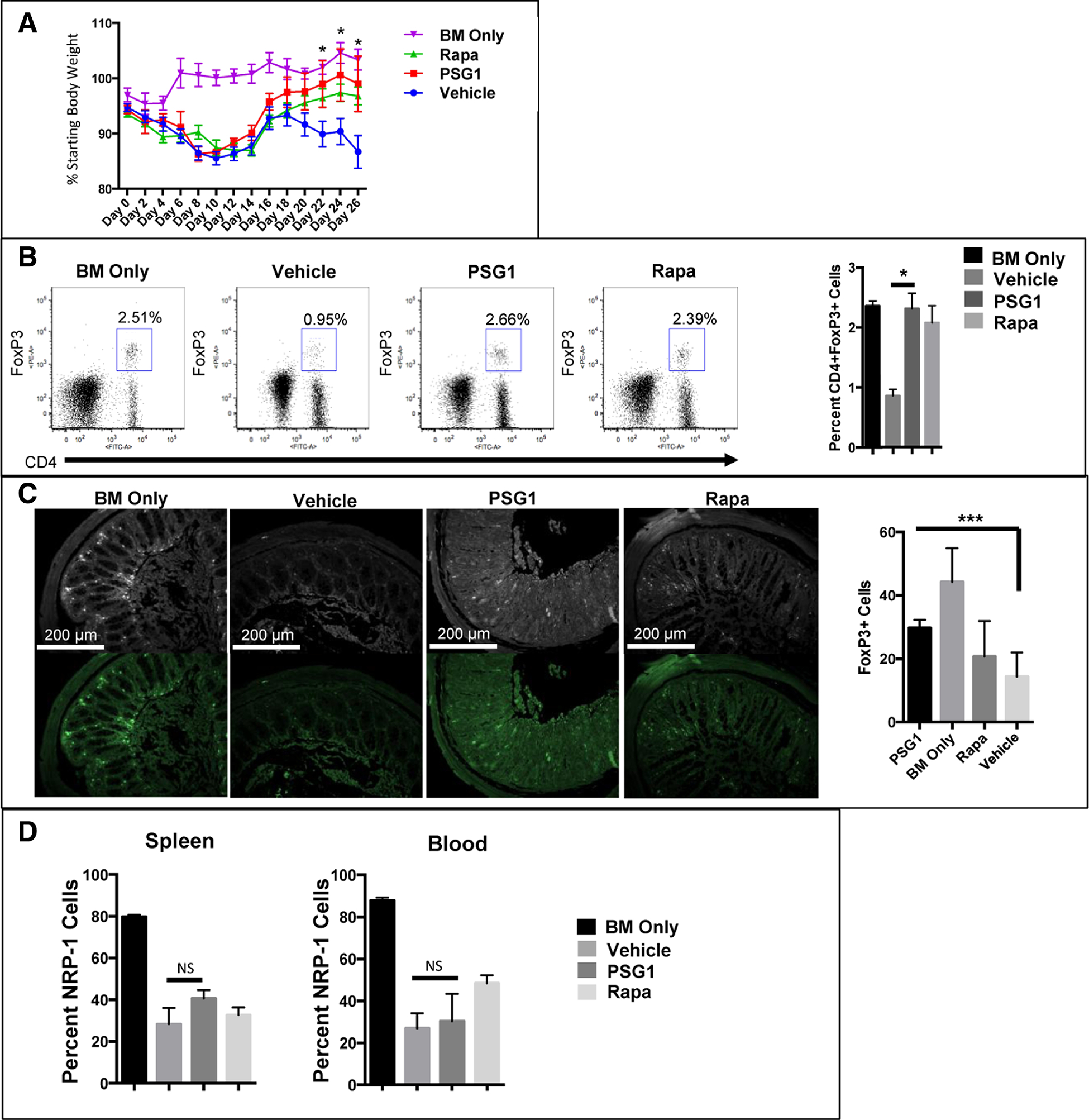
(A) aGvHD mice were injected with 100 μg of PSG1, PBS or rapamycin every other day from day −2 to day 26. The percentage of total starting body weight from day 0 to day 26 is shown. Rapa denotes rapamycin treated GvHD mice. (*p<0.05) (B) Representative flow cytometry data showing FoxP3 expression in splenic CD4+ T-cells from BM only, vehicle (PBS) treated GvHD mice, PSG1 treated GvHD mice and rapamycin treated GvHD mice. (*p<0.05) (C) Colon sections from all experimental mouse groups were stained for FoxP3 expression (***p<0.0005) and imaged on a Zeiss AXIO Imager Z1 microscope with Zeiss 1026.548 PI 10X/23 lens and Plan-APOCHROMAT 10X/0.45 (1063–139) aperture. All images were taken on either the AxioCam ICC1 white light camera or the AxioCamMRm fluorescent camera with the AxioVision Rel.4.8 imaging software at the same exposure and magnification. All counts were performed in triplicate in at least two independent experiments (n=4–5 mice per treatment group for each experiment). Mean ±SEM are shown and all p-values were obtained using two way ANOVA (A) or one way ANOVA (B,C). (D) FoxP3+ T-cells were collected from the spleen and blood of all experimental groups on Day 27. Nrp-1 expression was analyzed by flow cytometry.
Both PSG1 and rapamycin were able to induce FoxP3 expression to the levels observed in the BM only control mice, something that was only partially achieved with a shorter duration of PSG1-treatment (Figure 5B). Sections of colon from all treatment groups were analyzed for cells expressing FoxP3 and it was observed that PSG1-treated mice had significantly more FoxP3+ cells in the colon than the rapamycin and vehicle treated groups (Figure 5C). Based on these results, we conclude that PSG1 is at least as effective for GvHD prevention as other immunosuppressants.
Neuropilin-1 (Nrp-1) is expressed at high levels on natural Tregs and can be used to distinguish natural Tregs that arise in the thymus from Tregs induced in the periphery 33. To determine the origin of the FoxP3+ cells found in PSG1 and rapamycin-treated mice, we analyzed Nrp-1 expression on cells from the spleen and circulation (Figure 5D). We found that FoxP3+ cells in PSG1 and rapamycin-treated mice did not have significantly higher Nrp-1 expression over vehicle treated mice, indicating that these cells are induced in the periphery rather than originating in the thymus.
Regulatory T-Cells in Mice Treated with PSG1 are of Donor Origin
To determine the origin of the FoxP3+ Tregs observed following PSG1 treatment, we substituted B6 donor mouse cells with donor cells from B6-Thy1.1 mice. These Thy1.1+ donor cells allow us to determine the origin of FoxP3+ Tregs in the recipient by tracking expression of Thy1.1 on Tregs appearing in transplanted mice. This B6-Thy1.1 into B6-D2 mouse model was successful in replicating aGvHD conditions observed in the B6 into B6-D2 mouse model and responded equally well to PSG1 treatment. Analysis of the Thy1.1 donor marker versus the Thy1.2 recipient marker showed that in the BM only control group, all Tregs are of recipient origin, which was expected since donor T-cells were depleted. FoxP3+ Tregs in mice treated with PSG1 were found to be mostly Thy1.1 positive, indicating that Tregs arising in PSG1-treated mice are almost all of donor origin (Figure 6). This data supports the requirement for a successful donor graft in order for functional Tregs to develop and provide protection against aGvHD.
Figure 6: GvHD mice treated with PSG1 have an increase in regulatory T-cells of donor origin.
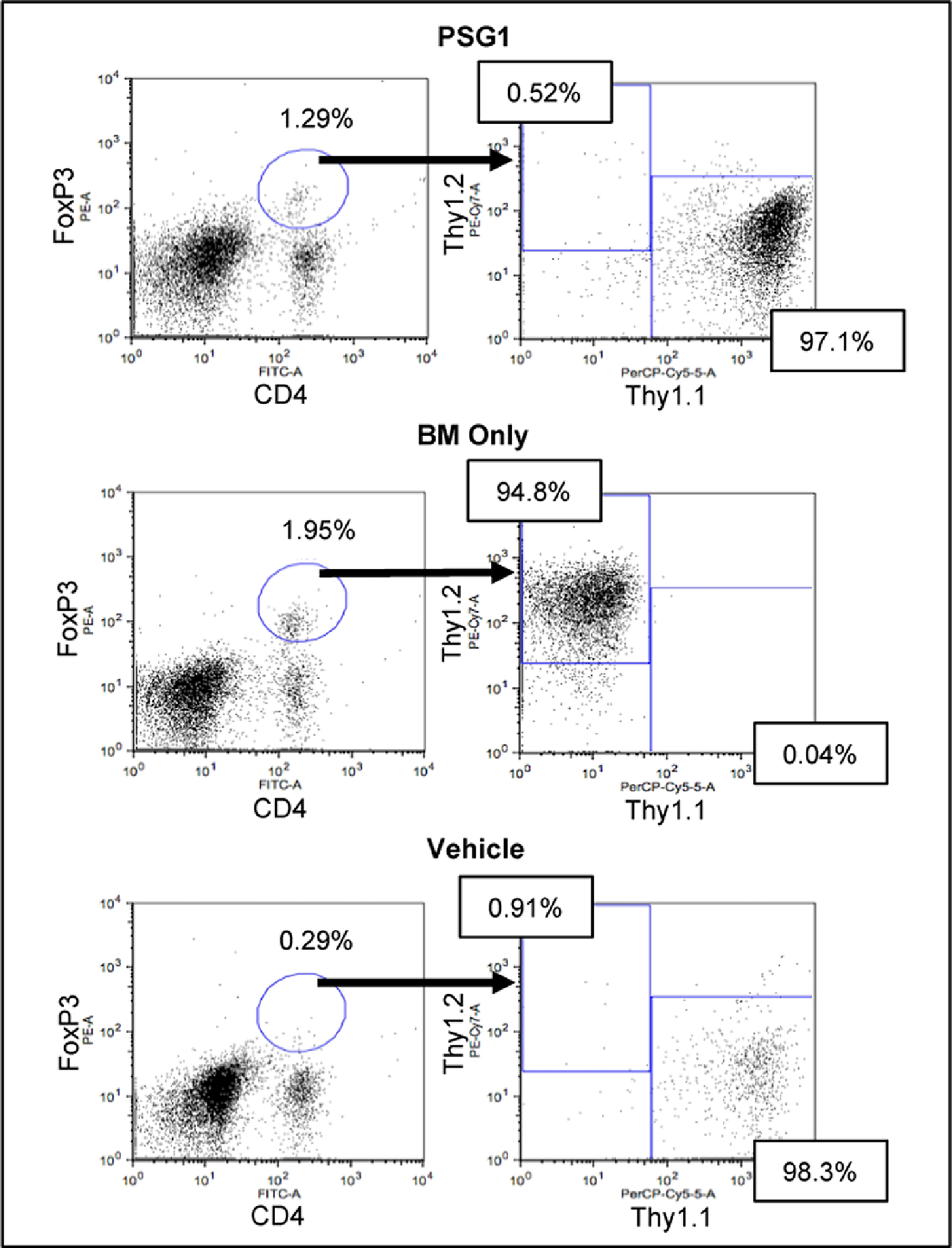
Shown are the percentages of CD4, FoxP3 positive cells found in B6-D2 recipient spleens after administration of cells from B6-Thy1.1 donors and treated with either vehicle (PBS) or PSG1. BM Only micerecieved B6-D2 donor cells without T cells. Flow cytometry was performed on cells from 5 mice per treatment group.
The Graft-versus-Leukemia (GvL) Effect is Impaired Following PSG1 Treatment
To determine if PSG1 is able to ameliorate aGvHD and maintain the GvL effect, we treated aGvHD leukemia mice with PSG1 19,34. Mice received PSG1, rapamycin or vehicle (PBS) every other day for 14 days. After treatment, mice were subjected to pathological evaluation for presence of leukemic cells and signs of aGvHD. As expected, control mice treated with leukemic cells and bone marrow but no T cells showed evidence of diffuse leukemia as seen by infiltration of the leukemic cells and in some cases, disruption of splenic architecture (Arrows, Figure 7). Rapamaycin treated mice had evidence of leukemia but no aGvHD and those treated with PSG1 showed only focal leukemia in addition to aGvHD. These findings suggest that PSG1 does not completely abrogate the GvL effect of the T cells, but is less effective at managing GvHD in the presence of an alloimmune reaction against a malignancy.
Figure 7: Signs of GvHD and Leukemia in mice treated with PSG1 or Rapamycin.
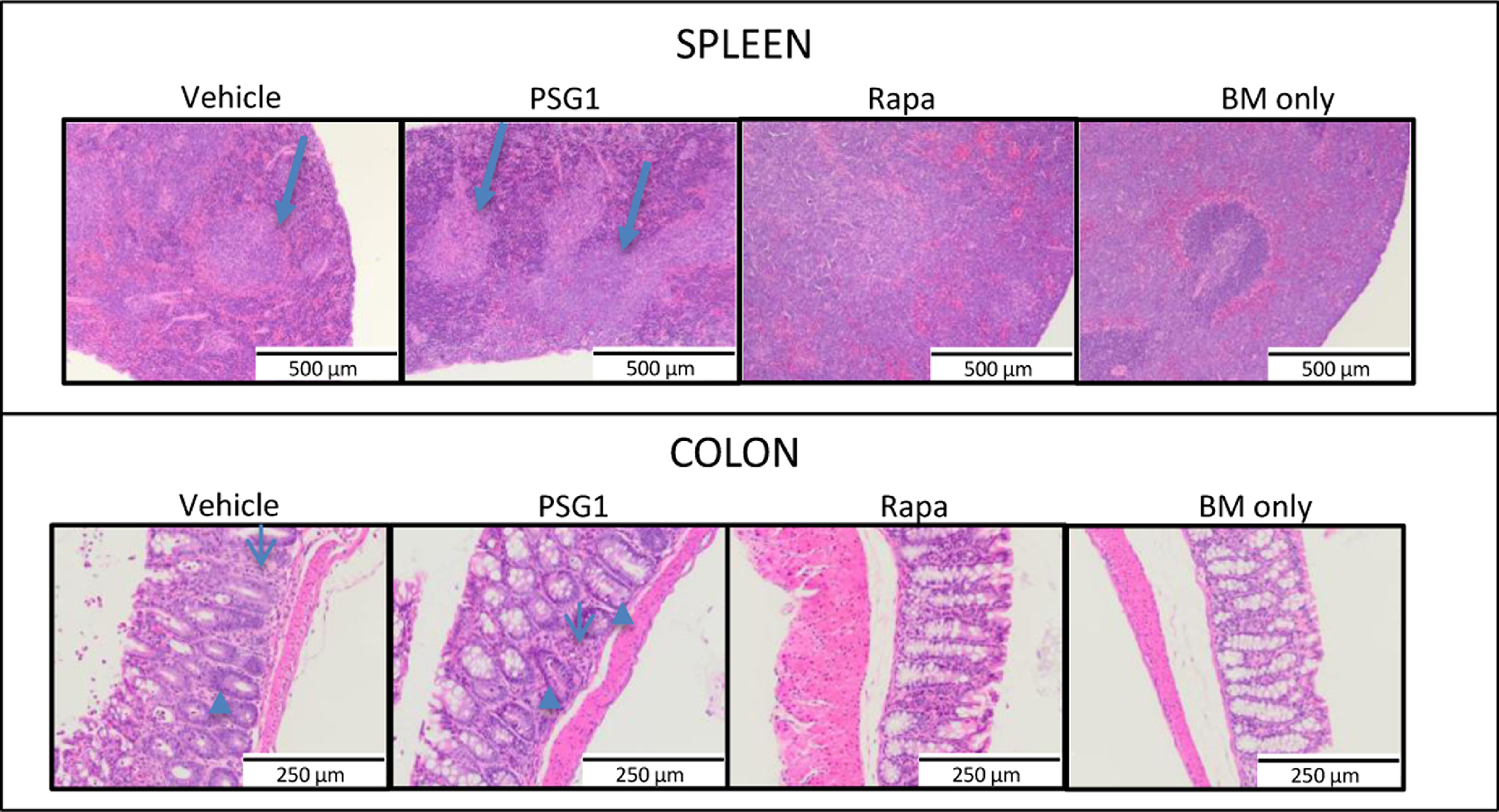
Colons and spleens were collected from mice infused with bone marrow and T cells along with E2A PBX BL6 leukemic cells and then treated with PBS, PSG1, or rapamycin. Mice receiving bone marrow and leukemia cells without T cells (BM only) were also analyzed. Hematoxylin and eosin stained sections from mouse colon and spleen were reviewed by the pathologist for evidence of GvHD (particularly apoptotic cells (marked by arrowheads) and disruption of crypt archictecture and T cell infiltration (marked by thin arrows), presence of leukemia (foci of leukemic cells or diffusely spread cell groups (marked by arrows), and disruption of splenic architecture. Representative slides were imaged on a Zeiss AXIO Imager Z1 microscope with Zeiss 1026.548 PI 10X/23 lens and Plan-APOCHROMAT 20X/0.75 440649(1101–957) aperture. All images were taken on the AxioCam ICC1 white light camera with the AxioVision Rel.4.8 imaging software at the same exposure and 20x magnification.
DISCUSSION
Allogeneic transplantation is currently the only curative option for a number of diseases, both malignant and non malignant35–42. However, bone marrow transplantation is associated with GvHD, a disorder that is the leading cause of the most significant adverse effects associated with this procedure 43. Current treatments for aGvHD involve the use of immunosuppressants, including corticosteroids, increasing the susceptibility of patients to opportunistic infections 44,45. In addition, a small subset of GvHD patients are steroid refractory and have a very poor prognosis46. Because of the limitations of current treatment options for GvHD patients, there is a need for novel therapeutic strategies to treat this disease.
PSG1 differs in its mechanism of action from all available therapies currently used for prevention or treatment of aGvHD and likely lacks the toxicity and immunogenicity seen with other treatments, a hypothesis supported by the high levels of this protein found during human pregnancy and its expression in the normal epithelium of the colon and esophagus 47. The experiments presented here have been performed in both male and female mice, indicating that PSG1 will likely be effective in humans of both sexes.
Treatment of mouse naïve and human T-cells with PSG1 resulted in a significant increase in their expression of FoxP3. We further observed both in vitro and in vivo an increase in pSMAD2/3 after treatment with PSG1, an important mediator of TGF-β signaling 48. The results presented here support the potential benefits of PSG1 to treat aGvHD. We found that upon administration of PSG1 in an aGvHD model, tissue damage and molecular changes associated with aGvHD were minimized or prevented entirely. In addition, PSG1-treated mice showed a reduction in some pro-inflammatory cytokines with no increased signs of fibrosis.
When the PSG1 dosage was extended, the percentage of weight loss in mice treated with PSG1 improved by day 26, almost reaching the levels of healthy controls. PSG1 and rapamycin treated mice showed an increased number of Foxp3 expressing cells in the spleen, but only PSG1 was able to increase FoxP3 expression in the colon, which is in agreement with previous results from our laboratory 15.
PSG1 has been shown to induce the expression of an alternative phenotype in macrophages marked by induction of arginase I and to affect the maturation of dendritic cells 49, 50. Whether the effects of PSG1 in the phenotype of these cells is related to the increase in availability of active TGF-β, which has been shown to induce arginase I and affect DC maturation, has not been explored 51,52. As such, additional mechanisms of action in aGvHD protection by PSG1 independent of TGF-β or increased FoxP3 expression in T-cells, cannot be ruled out.
We have previously showed that mice receiving i.p. injections of PSG1 are protected from dextran sulfate-induced colitis15. In these mice, mesenteric lymph node cells from PSG1-treated mice secreted less IL-6, TNF-α and IFN-γ when stimulated with anti-CD3/anti CD28 Abs when compared to controls. These same cellsexpressed increased IL-10 mRNA levels while showing reduced expression of TNF-α and IFN-γ and IL-17 mRNA in the colon. In addition and in agreement with these results, splenocytes from mice infected with a vaccinia virus expressing PSG1 expressed lower levels of IL-2 and IFN-γ when challenged with mitogens (Con A), anti-CD3 or specific antigen (OVA)50. Motran et al. have also shown that PSG1 does not inhibit the proliferation of purified CD3+ murine T-cells when treated with PMA and ionomycin or anti-CD3/CD28 antibodies50. In agrrement with this report, while we observed an inhibition of IL-2 secretion, we did not observe a significant effect on the proliferation (measured at 72 hs) of murine CD4+ T cells in response to anti-CD3/CD28 Ab stimulation (data not shown). While our group and others have previously measured cytokines in cells isolated from PSG1-treated mice, this is the first time that a change in serum cytokines following i.p. injection of PSG1 has been examined. Our results show a reduction in the concentration of RANTES and IL-1α in the PSG1-treated mice. Changes in the production of other chemokines, which may result from the proposed regulation of monocyte/macrophage phenotype by PSG1, should be further explored as many of the chemokines we tested were below the level of detection of the Luminex assay.
We also explored whether PSG1 could be employed for treatment or prevention of aGvHD when HSCT is performed for the treatment of hematological malignancies. The main mediators of the beneficial graft-versus-leukemia activity of allogeneic bone marrow transplantation are T cells within the donor grafts, though NK cells have also been shown to play a role 53. Tregs may be detrimental in cancer and it is predicted that reducing Treg function in cancer patients should augment antitumor immune responses 54. The partial anti-leukemic effect observed in PSG1-treated mice compared to mice receiving vehicle (PBS) may be explained by the inhibitory effects of PSG1 on NK cells, which have a role in antileukemic effects as well as engraftment 53, and this pathway should be explored in the future. As stated above, PSG1 regulates the function of other cells such as antigen presenting cells which play a role in aGvHD initiation but not in GvL 55.
The data collected in this study strongly suggests that PSG1 could be a promising treatment option for patients suffering aGVHD. Though no significant GvL effect was observed, parameters such as tumor burden and the characteristics of the malignant cells used in the study likely determine whether GvL activity islost upon PSG1 treatment, and these factors will be explored in future studies.
However, our preclinical studies do support the use of PSG1 to prevent aGVHD when bone marrow transplantation is utilized for treatment of nonmalignant monogeneic hematopoietic disorders, such as primary immunodeficiencies, where graft-versus-tumor effect is not a consideration 56.
Highlights.
Pregnancy specific glycoprotein 1(PSG1), working through the TGFβ-SMAD pathway, induces differentiation of FoxP3+ Regulatory T-cells
Administration of PSG1 to a GvHD mouse model decreases or eliminates GvHD occurrence and severity.
ACKNOWLEDGEMENTS
This work was supported in part by the NIH AI1290918 and Henry M. Jackson training and innovation awards to G. Dveksler. This research was also supported n part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases under Intramural Project No. 1-ZAI-AI000989. E2A PBX BL6 leukemia cells were a kind gift of Dr. Terry Fry at the National Cancer Institute, National Institutes of Health (NIH) and FoxP3-IRES-GFP knock-in mice on C57BL/6 background were provided by Dr. Warren Strober, NIH, Bethesda, USA. We would also like to thank Dr. Victoria Hoffmann (NIH), for reviewing the slides.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors declare no competing financial interests.
The opinions expressed here are those of the authors and should not be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hill L, Alousi A, Kebriaei P, Mehta R, Rezvani K, Shpall E. New and emerging therapies for acute and chronic graft versus host disease. Ther Adv Hematol. 2018;9(1):21–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobsohn DA, Vogelsang GB. Acute graft versus host disease. Orphanet J Rare Dis. 2007;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore T, Dveksler GS. Pregnancy-specific glycoproteins: complex gene families regulating maternal-fetal interactions. Int J Dev Biol. 2014;58(2–4):273–280. [DOI] [PubMed] [Google Scholar]
- 4.Towler CM, Horne CH, Jandial V, Campbell DM, MacGillivray I. Plasma levels of pregnancy-specific beta1-glycoprotein in normal pregnancy. Br J Obstet Gynaecol. 1976;83(10):775–779. [DOI] [PubMed] [Google Scholar]
- 5.Camolotto S, Racca A, Rena V, et al. Expression and transcriptional regulation of individual pregnancy-specific glycoprotein genes in differentiating trophoblast cells. Placenta. 2010;31(4):312–319. [DOI] [PubMed] [Google Scholar]
- 6.Gatson NN, Williams JL, Powell ND, et al. Induction of pregnancy during established EAE halts progression of CNS autoimmune injury via pregnancy-specific serum factors. J Neuroimmunol. 2011;230(1–2):105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patas K, Engler JB, Friese MA, Gold SM. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J Reprod Immunol. 2013;97(1):140–146. [DOI] [PubMed] [Google Scholar]
- 8.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–291. [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri SP, Navare T, Gross J, Raychaudhuri SK. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42(7):518–520. [DOI] [PubMed] [Google Scholar]
- 10.Ostensen M, Villiger PM. Immunology of pregnancy-pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl Immunol. 2002;9(2–4):155–160. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal RK, Chan CC, Wiggert B, Caspi RR. Pregnancy ameliorates induction and expression of experimental autoimmune uveitis. J Immunol. 1999;162(5):2648–2654. [PubMed] [Google Scholar]
- 12.Langer-Gould A, Garren H, Slansky A, Ruiz PJ, Steinman L. Late pregnancy suppresses relapses in experimental autoimmune encephalomyelitis: evidence for a suppressive pregnancy-related serum factor. J Immunol. 2002;169(2):1084–1091. [DOI] [PubMed] [Google Scholar]
- 13.Svensson-Arvelund J, Mehta RB, Lindau R, et al. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J Immunol. 2015;194(4):1534–1544. [DOI] [PubMed] [Google Scholar]
- 14.Snyder SK, Wessner DH, Wessells JL, et al. Pregnancy-specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-beta1 by human monocytes. Am J Reprod Immunol. 2001;45(4):205–216. [DOI] [PubMed] [Google Scholar]
- 15.Blois SM, Sulkowski G, Tirado-Gonzalez I, et al. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-beta and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol. 2014;7(2):3448–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ha CT, Wu JA, Irmak S, et al. Human Pregnancy Specific Beta-1-Glycoprotein 1 (PSG1) Has a Potential Role in Placental Vascular Morphogenesis. Biol Reprod. 2010;83(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naserian S, Leclerc M, Thiolat A, et al. Simple, Reproducible, and Efficient Clinical Grading System for Murine Models of Acute Graft-versus-Host Disease. Front Immunol. 2018;9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bijl J, Sauvageau M, Thompson A, Sauvageau G. High incidence of proviral integrations in the Hoxa locus in a new model of E2a-PBX1-induced B-cell leukemia. Genes Dev. 2005;19(2):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones K, Ballesteros A, Mentink-Kane M, et al. PSG9 Stimulates Increase in FoxP3+ Regulatory T-Cells through the TGF-beta1 Pathway. PLoS One. 2016;11(7):e0158050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187(5):2061–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejaco C, Duftner C, Grubeck-Loebenstein B, Schirmer M. Imbalance of regulatory T cells in human autoimmune diseases. Immunology. 2006;117(3):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, Tamiya T, Takada I, et al. Histone 3 lysine 9 (H3K9) methyltransferase recruitment to the interleukin-2 (IL-2) promoter is a mechanism of suppression of IL-2 transcription by the transforming growth factor-beta-Smad pathway. J Biol Chem. 2011;286(41):35456–35465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takimoto T, Wakabayashi Y, Sekiya T, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185(2):842–855. [DOI] [PubMed] [Google Scholar]
- 25.Luo J, Wyss-Coray T. Bioluminescence analysis of Smad-dependent TGF-beta signaling in live mice. Methods Mol Biol. 2009;574:193–202. [DOI] [PubMed] [Google Scholar]
- 26.Blois SM, Sulkowski G, Tirado-Gonzalez I, et al. Pregnancy-specific glycoprotein 1 (PSG1) activates TGF-beta and prevents dextran sodium sulfate (DSS)-induced colitis in mice. Mucosal Immunol. 2014;7(2):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcon CR, Martinez FF, Carranza F, Cervi L, Motran CC. In Vivo Expression of Recombinant Pregnancy-Specific Glycoprotein 1a Inhibits the Symptoms of Collagen-Induced Arthritis. Am J Reprod Immunol. 2014;72(6):527–533. [DOI] [PubMed] [Google Scholar]
- 28.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–1309. [DOI] [PubMed] [Google Scholar]
- 29.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler C, Antin JH. Sirolimus for GVHD prophylaxis in allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34(6):471–476. [DOI] [PubMed] [Google Scholar]
- 31.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72(12):1924–1929. [DOI] [PubMed] [Google Scholar]
- 32.Moccia KD, Olsen CH, Mitchell JM, Landauer MR. Evaluation of hydration and nutritional gels as supportive care after total-body irradiation in mice (Mus musculus). J Am Assoc Lab Anim Sci. 2010;49(3):323–328. [PMC free article] [PubMed] [Google Scholar]
- 33.Singh K, Hjort M, Thorvaldson L, Sandler S. Concomitant analysis of Helios and Neuropilin-1 as a marker to detect thymic derived regulatory T cells in naive mice. Sci Rep. 2015;5:7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacoby E, Yang Y, Qin H, Chien CD, Kochenderfer JN, Fry TJ. Murine allogeneic CD19 CAR T cells harbor potent antileukemic activity but have the potential to mediate lethal GVHD. Blood. 2016;127(10):1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battiwalla M, Barrett J. Allogeneic transplantation using non-myeloablative transplant regimens. Best Pract Res Clin Haematol. 2001;14(4):701–722. [DOI] [PubMed] [Google Scholar]
- 36.Chiesa R, Veys P. Reduced-intensity conditioning for allogeneic stem cell transplant in primary immune deficiencies. Expert Rev Clin Immunol. 2012;8(3):255–266; quiz 267. [DOI] [PubMed] [Google Scholar]
- 37.Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in Philadelphia-positive acute lymphoblastic leukaemia. Bone Marrow Transplant. 2008;41(5):447–453. [DOI] [PubMed] [Google Scholar]
- 38.Hale GA, Arora M, Ahn KW, et al. Allogeneic hematopoietic cell transplantation for neuroblastoma: the CIBMTR experience. Bone Marrow Transplant. 2013;48(8):1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan A, Booth C, Brightwell A, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–3624; quiz 3626. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312(1):48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messer M, Steinzen A, Vervolgyi E, et al. Unrelated and alternative donor allogeneic stem cell transplant in patients with relapsed or refractory Hodgkin lymphoma: a systematic review. Leuk Lymphoma. 2014;55(2):296–306. [DOI] [PubMed] [Google Scholar]
- 42.Milano F, Appelbaum FR, Delaney C. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med. 2016;375(22):2204–2205. [DOI] [PubMed] [Google Scholar]
- 43.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garnett C, Apperley JF, Pavlu J. Treatment and management of graft--host disease: improving response and survival. Ther Adv Hematol. 2013;4(6):366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deeg HJ. How I treat refractory acute GVHD. Blood. 2007;109(10):4119–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houston A, Williams JM, Rovis TL, et al. Pregnancy-specific glycoprotein expression in normal gastrointestinal tract and in tumors detected with novel monoclonal antibodies. MAbs. 2016;8(3):491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. [DOI] [PubMed] [Google Scholar]
- 49.Martinez FF, Knubel CP, Sanchez MC, Cervi L, Motran CC. Pregnancy-specific glycoprotein 1a activates dendritic cells to provide signals for Th17-, Th2-, and Treg-cell polarization. Eur J Immunol. 2012;42(6):1573–1584. [DOI] [PubMed] [Google Scholar]
- 50.Motran CC, Diaz FL, Montes CL, Bocco JL, Gruppi A. In vivo expression of recombinant pregnancy-specific glycoprotein 1a induces alternative activation of monocytes and enhances Th2-type immune response. Eur J Immunol.2003;33(11):3007–3016. [DOI] [PubMed] [Google Scholar]
- 51.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol. 1995;155(4):2077–2084. [PubMed] [Google Scholar]
- 52.Sanjabi S, Oh SA, Li MO. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb Perspect Biol. 2017;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. [DOI] [PubMed] [Google Scholar]
- 54.Lienart S, Stockis J, Dedobbeleer O, Lucas S. Targeting immunosuppression by Tregs with monoclonal antibodies against GARP. Oncoimmunology. 2016;5(3):e1074379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10(9):987–992. [DOI] [PubMed] [Google Scholar]
- 56.Marciano BE, Holland SM. Primary Immunodeficiency Diseases: Current and Emerging Therapeutics. Front Immunol. 2017;8:937. [DOI] [PMC free article] [PubMed] [Google Scholar]


