Abstract
Purpose of Review
The “fourth trimester” concept, defined as the first 12 weeks after delivery (and beyond), is a critical window of time for clinicians to intervene to optimize women’s cardiovascular health after pregnancy. A timely and comprehensive postpartum cardiovascular assessment should be performed in all women following delivery in order to (1) follow up medical conditions present prior to conception, (2) evaluate symptoms and signs of common postpartum complications, and (3) identify risk factors and prevent future adverse cardiovascular outcomes. In this review, we aim to discuss major maternal cardiovascular risk factors such as hypertensive disorders of pregnancy, gestational diabetes mellitus, postpartum weight retention, and postpartum depression, as well as lactation as a potential protective risk modifying factor. Additionally, we will review effectiveness of outpatient interventions to enhance transitions in cardiovascular care during the fourth trimester.
Recent Findings
A seamless hand-off from obstetric to primary care, and potentially cardiology, is needed for early detection and management of hypertension, weight, glycemic control, stress and mood, and long-term cardiovascular risk. Additionally, the use of telemedicine, blood pressure self-monitoring, remote activity monitoring, and behavioral health coaches are potentially feasible modalities to augment clinic-based care for cardiovascular risk factors and weight management, but additional studies are needed to study their long-term effectiveness.
Summary
Development of a comprehensive postpartum care plan with careful consideration of each patient’s risk profile and access to resources is critical to improve maternal morbidity and mortality, reduce health disparities, and achieve long-term cardiovascular health for women. Supporting postpartum well-being of women during this transition period requires a multidisciplinary approach, especially primary care engagement, and planning should start before delivery.
Keywords: Fourth trimester, Adverse pregnancy outcomes, Cardiovascular disease, Prevention, Blood pressure control, Weight management
Introduction
The fourth trimester concept, defined as the first 12 weeks after delivery (and beyond), has been proposed as a critical window of time for clinicians to intervene to optimize women’s cardiovascular health after pregnancy. Cardiovascular disease (CVD) remains a leading cause of maternal death in the USA. The number of maternal deaths has been increasing in the USA and approximately half of pregnancy-related deaths are due to cardiomyopathy, thrombotic pulmonary or other embolism, cerebrovascular accidents (CVA), hypertensive disorders of pregnancy (HDP), and other cardiovascular complications [1, 2]. Data from the Centers for Disease Control have shown that 18% of maternal deaths occur between 1 and 6 days postpartum, 21% between 7 and 41 days postpartum, and 13% after 42 days [3]. The American College of Obstetricians and Gynecologists (ACOG) recommends an initial postpartum evaluation within the first 3 weeks after delivery and a complete biopsychosocial evaluation within the first 3 months to optimize interconception and long-term health [4]. It is important for primary care clinicians, obstetricians, and cardiologists to have a systematic approach to comprehensive cardiovascular evaluation during this transition period.
Postpartum visit attendance rates are low in general due to new mothers’ competing priorities, but particularly for those with low-income [5]. Racial disparities are also apparent in the care of postpartum women. Black, indigenous, and other women of color have the highest risk of pregnancy-related mortality and morbidity and they also have higher risk of getting fragmented postpartum medical care [6,7,8••] There is an urgent need to direct attention to these women who will benefit most from carefully coordinated transition of care after delivery.
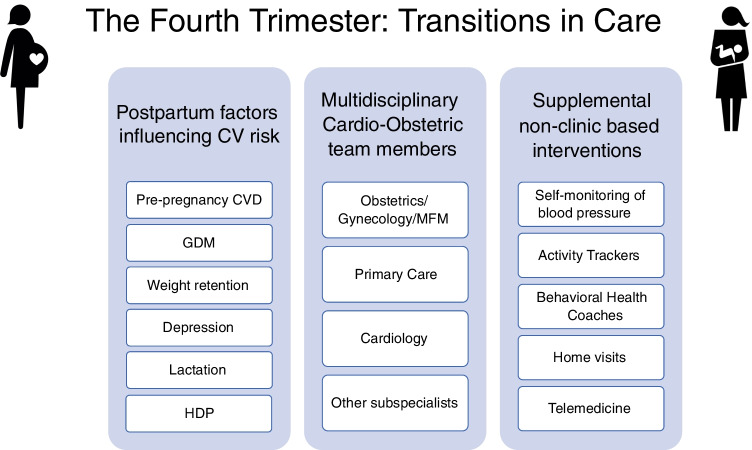
A timely and comprehensive postpartum cardiovascular assessment should be performed in all women in order to [1] follow up pre-existing medical conditions, [2] evaluate symptoms and signs for common postpartum complications, and [3] identify risk factors and prevent future adverse cardiovascular outcomes. In this review, we aim to discuss major maternal cardiovascular risk factors such as HDP, gestational diabetes mellitus (GDM), postpartum weight retention, postpartum depression, and lactation. Additionally, we will review different outpatient interventions used for improvement of transitions in cardiovascular care during the fourth trimester (Fig. 1).
Fig. 1.
Snapshot of notable postpartum risk factors, multidisciplinary team members, and supplemental non-clinic interventions to be considered in postpartum cardiovascular care
We refer to “women” throughout this review based on presumed female sex assigned at birth, as the majority of prior studies focused on “women” and did not define how gender was ascertained or assess other gender identities. However, we acknowledge that not all birthing persons self-identify as women. Unfortunately, insufficient evidence exists regarding maternal outcomes for individuals who are transgender, non-binary, or other, which is an area of needed research.
Addressing Maternal Cardiovascular Risk Profile in the Fourth Trimester
Hypertensive Disorders of Pregnancy
Hypertensive disorders of pregnancy (HDP) complicate up to 20% of all pregnancies and include preeclampsia, eclampsia, chronic hypertension of any etiology, and preeclampsia superimposed on chronic hypertension and gestational hypertension [9]. The incidence of HDP is on the rise in the USA and a recent scientific statement by the American Heart Association (AHA) emphasizes the importance of recognizing the contribution of HDP to maternal morbidity and mortality as well as long-term cardiovascular health [3, 10, 11]. HDP, especially preeclampsia, has been associated with cardiovascular complications such as myocardial infarction including spontaneous coronary artery dissection, CVA, and peripartum cardiomyopathy [12–16]. HDP increases the risk of developing chronic HTN twofold [17, 18], as well as the long-term cardiovascular outcomes such as CVA, ischemic heart disease, heart failure, and atrial fibrillation [19–21].
HDPs often persist following delivery and sometimes develop after delivery [22]. HDP complications such as preeclampsia and eclampsia also occur during the postpartum period even in mothers with uncomplicated pregnancies and births. In fact, one third of eclampsia occurs postpartum with half within 48 h of delivery [23, 24]. The National Institute for Health and Care Excellence (NICE) recommends frequent blood pressure monitoring for women with both preeclampsia (every 1–2 days for 2 weeks) and gestational hypertension (at least once between 3 and 5 days) [25].
In pregnant patients, hypertension is defined internationally as a blood pressure ≥ 140/90 mmHg, but the threshold for pharmacotherapy varies [10]. The ACOG had previously recommended a blood pressure threshold of systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 110 mmHg for initiation of antihypertensive therapy in women with acute and chronic hypertension [26, 27]. A recent multicenter randomized study (the Chronic Hypertension And Pregnancy [CHAP] trial) showed that targeting a blood pressure < 140/90 mmHg was associated with better pregnancy outcomes without an increase in small-for-gestational-age (SGA) infants in women with chronic hypertension [28••]. Following the publication of this trial, ACOG now recommends using a lower blood pressure threshold of ≥ 140/90 for initiation or titration of medical therapy for women with chronic hypertension in pregnancy [29••]. An optimal blood pressure target for women with HDP in the absence of chronic hypertension remains unknown.
Labetalol and nifedipine extended release are generally accepted as the first-line antihypertensive therapy during pregnancy [10]. There is no clear evidence that one antihypertensive drug is superior to another according to a Cochrane review of 29 randomized trials [30]. However, a recent meta-analysis demonstrated that atenolol is associated with a significantly increased risk for SGA infants when used for chronic hypertension in pregnancy and is thus generally avoided [31]. A recent randomized trial comparing a 5-day postpartum course of low dose furosemide (20 mg daily) versus placebo in 384 women with gestational hypertension and preeclampsia (with and without severe features) showed faster resolution of hypertension in the treatment group, predominantly in those without severe features [32•]. Medical comorbidities, side effect profile and patient preference should be considered when choosing an antihypertensive agent.
Severe preeclampsia/eclampsia can manifest in the early postpartum period. For women with HDP, it is important to have continued heightened surveillance for these “red-flags” that hallmark preeclampsia with severe features such as pulmonary edema, new onset headache, visual disturbances, kidney or liver dysfunction, and/or thrombocytopenia [33•]. Magnesium sulfate is used to prevent seizures in women with postpartum preeclampsia with severe features [34]. ACOG recommends treatment with magnesium for 24 h postpartum, but acknowledges that duration of postpartum magnesium may need to be individualized based on benefit vs harm of treatment [35]. Studies of shorter (6–12 h) duration have been done which showed no increased risk of seizures but were likely underpowered given few events.
There are more antihypertensive options available during the postpartum period as there is no concern for adverse fetal outcomes. Some of the antihypertensive medications that are preferred in breastfeeding women include calcium channel blockers (nifedipine, amlodipine, diltiazem, verapamil), beta blockers (labetalol, metoprolol, propranolol), angiotensin-converting enzyme (ACE) inhibitors (captopril, enalapril, benazepril), diuretics (hydrochlorothiazide, spironolactone), and methyldopa [36]. It is important to follow women longitudinally if there is new initiation of antihypertensive medications during pregnancy as the majority of these women have resolution of hypertension by 3 months [37].
Gestational Diabetes Mellitus
Gestational diabetes mellitus (GDM) is diagnosed based on detection of new hyperglycemia at 24 to 28 weeks of gestation [38]. GDM not only increases the risk of maternal complications but also affects the health of the offspring by increasing the risk of larger birth size, obesity, and diabetes mellitus [38, 39]. In the USA, women who are non-white, low-income, and obese are disproportionately affected by the increase in the prevalence of GDM [40]. A systemic review has found that postpartum (3 months to 3 years post-delivery) glucose monitoring follow up rates are low (3 months to 3 years post-delivery), ranging between 5.7 and 57.9% (median 21.8%) among women with GDM [41]. Unfortunately, women who are most at risk for type 2 diabetes, such as Black and Hispanic women, those with lower education, and those with comorbidities, have lower rates of postpartum glucose testing [41]. Although GDM pharmacotherapy generally can be stopped after delivery, the American Diabetes Association recommends regular glucose monitoring starting a few days after delivery, at 2–6 months postpartum and every 1–3 years thereafter [42]. Open-ended interviews conducted with women with GDM revealed that many women considered GDM as an acute, resolvable condition that does not require further surveillance and worry and only a small minority considered it a wake-up call for continuing healthy lifestyle [43]. Understanding this narrative can help clinicians better communicate with affected women to improve transition in care after delivery.
The estimated risk of developing type 2 diabetes after GDM is approximately 20% at 10 years and further increases with time [44]. Intensive lifestyle modifications and medical therapy with metformin can prevent progression to long-term diabetes mellitus [45, 46]. Women with GDM should be advised to maintain regular physical activity and healthy dietary habits with a focus on avoiding excessive weight gain [39, 47]. Importantly, exclusivity and longer duration of breastfeeding has also been shown to reduce the risk of GDM progression to type 2 diabetes [48]. Newer oral hypoglycemic agents such as sodium glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP1-RA) hold promise for preventing the development of type 2 diabetes and future CVD in women with a history of GDM and obesity, who are not currently pregnant or lactating [49–51], but further study is needed in this population. Most importantly, women should be screened for additional CVD risk factors given the increasing evidence of the association of GDM with future coronary artery calcification, heart failure, and cardiovascular events that occur even in the absence of postpartum diabetes mellitus [52–54].
Excess Gestational Weight Gain and Postpartum Weight Retention
Excessive weight gain during pregnancy is a modifiable risk factor for future obesity [55, 56] At 6 months postpartum, women weigh about 11.8 pounds (5.4 kg) more than their pre-pregnancy body weight [57]. Factors associated with postpartum weight retention include higher body weight gain during pregnancy, Black race, and lower socioeconomic status [57]. Over the past two decades, there have been more women entering pregnancy with higher body mass index [55]. It is suggested that women with pre-existing obesity tend to accumulate visceral fat deposit during pregnancy and in turn have higher rates of postpartum metabolic complications [58, 59]. The relationship between gestational weight gain and postpartum weight retention has been described as a vicious cycle as retained excess weight gain from first pregnancy can affect the next pregnancy [55]. Similar to other cardiovascular risk factors, postpartum weight retention increases future metabolic risk including development of type 2 diabetes mellitus [60], and thus postpartum weight management should be a target for preventive efforts.
Postpartum Depression
Up to 10% of women experience postpartum depression during the first year after delivery and regular screening has been recommended by the US Preventive Services Task Force, ACOG, and American Academy of Pediatrics [61–63]. It has been found that nearly 1 in 3 women with a history of peripartum cardiomyopathy report symptoms of clinical depression and have poor attendance at medical follow-up visits [64]. Therefore, recognition of postpartum depression is particularly important in women with preexisting medical conditions and cardiovascular risk factors to improve clinical outcomes. Screening tools such as Patient Health Questionnaire-2, Patient Health Questionnaire-9, and Edinburgh Postpartum Depression Scale can be used for initial assessment.
Lactation
Pregnancy is associated with significant cardiometabolic changes including weight gain, elevated circulating lipids, insulin resistance, vasomotor sympathetic activity, and renin–angiotensin–aldosterone activation [65, 66]. In the short-term, these changes support fetal growth. In the long-term, they may confer an increased risk of CVD. Breastfeeding is posited to lead to more rapid reversal of the cardiometabolic changes of pregnancy, and possibly to a reduced risk of both cardiovascular risk factors and CVD later in life [67]. Breastfeeding has long been associated with a decreased risk of breast and ovarian cancers [68]. More recently, it has been shown to reduce the risk of hypertension and diabetes mellitus [69]. In a recent cross-sectional analysis, Yu et al. studied the short-term impact of lactation on specific CVD risks in women whose pregnancies were complicated by GDM, intrauterine growth restriction, abruption, HDP, or preterm birth [70]. In this study, women who had breastfed for greater than or equal to 6 months had significantly lower triglycerides, fasting serum glucose, body mass index, waist circumference, systolic blood pressure, and higher high-density lipoprotein compared to women who never breastfed or those who breastfed for less than 6 months. Parity in this study ranged from 2 to 3.1 with a weighted mean of 2.3 [70]. In a recent systematic review and meta-analysis, it was found that women who breastfed had a relative risk reduction of 11% (95% CI, 5–17%) for incident CVD, 17% for fatal CVD events (8–24%), 14% for coronary heart disease (5–22%), and 12% (1–21%) for stroke. There was a lower risk for all of those incident events for longer breastfeeding duration up to 12 months, with a relative plateau thereafter [71]. Despite these benefits, only 25% of women in the USA breastfeed exclusively at 6 months and the prevalence of breastfeeding is < 20% at 12 months in most high-income countries [72, 73]. The ACOG recommends exclusive breastfeeding for an infant’s first 6 months of life with continued breastfeeding as foods are introduced during the first year of life and up to 2 years.
Postpartum Cardiovascular Complications
Pregnancy causes dynamic cardiometabolic and physiological changes to meet the demands of fetal growth and development. These include up to 45% increase in cardiac output, 20–25% increase in the heart rate, peripheral vasodilation leading to 35–40% decrease in peripheral vascular resistance and subsequent decrease in arterial blood pressure [66]. The physiologic stress on the cardiovascular system during pregnancy and labor reveals underlying subclinical pathology. Women with underlying cardiovascular conditions may also have difficulty adapting to postpartum fluid shifts and “normalization” of hemodynamic changes after delivery but can persist in both normal and abnormal states.
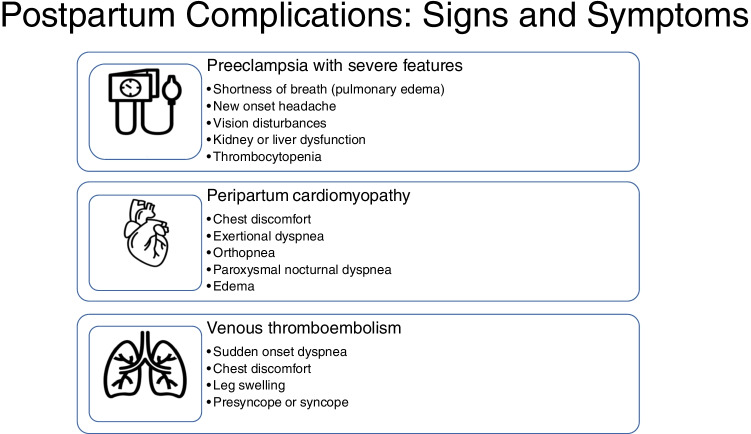
The postpartum period is a time for critical monitoring of maternal cardiovascular morbidity and mortality for women, especially those with heart failure, pulmonary hypertension, or valvular heart disease. In women with established CVD, readmissions within the first 7 weeks postpartum are common [74]. Women at high risk should thus be monitored inpatient for 72 h or greater and be followed up in clinic within 3 to 6 days post-discharge [33•]. To ensure maternal safety, patients should also receive appropriate guidance on self-monitoring and identification of high-risk features that should prompt seeking medical care. A list of concerning symptoms has been made available by the American College of Cardiology (ACC) Cardiovascular Disease in Women Committee and the Cardio-Obstetrics Work Group and should be shared with these high-risk groups [33•]. These symptoms include chest pain, dyspnea, orthopnea, cough, edema, tachycardia, non-vagal syncope, headache, visual changes, hypotension, and hypertension. More specifically, warning symptoms for peripartum cardiomyopathy include exertional dyspnea, orthopnea, paroxysmal nocturnal dyspnea, edema, and chest tightness. Women with postpartum preeclampsia or eclampsia may present with severe headache, visual changes, abdominal pain, altered mental status, and/or dyspnea [27]. Sudden onset dyspnea should prompt evaluation for pulmonary embolism as the incidence of venous thromboembolism is approximately five times higher during the postpartum period as compared to during pregnancy and the highest during the first 3 weeks after delivery [75, 76]. Risk factors associated with higher incidence of postpartum venous thromboembolism include cesarean delivery, preeclampsia, hemorrhage, and postpartum infection [76]. Finally, women should be counseled to seek care for chest pain or discomfort, which could be representative of coronary or aortic dissection, as well as for changes in sensation or strength, which may represent postpartum CVA [33•]. Examples of serious cardiovascular complications and associated symptoms are shown in Fig. 2.
Fig. 2.
Signs and symptoms of serious postpartum complications
A Time for Transitions in Care: Models for Improvement
Team-Based Postpartum Care
The fourth trimester is a time for care transitions for most women, especially those with known CVD, adverse pregnancy outcomes (APOs), and elevated cardiovascular risk. It is important to plan a seamless hand-off from obstetric care to primary care, and potentially also to outpatient cardiology care if at particularly elevated CVD risk, for early detection and management of hypertension, glycemic control, and long-term cardiovascular risk. The responsibility of identifying, counseling, and managing long-term risks should be shared by a multidisciplinary Cardio-Obstetrics team [33•, 77]. A co-located, co-timed maternal postpartum and newborn preventive care visit (“mommy-baby visit”) has been proposed to improve postpartum visit attendance rates [78]. In a randomized controlled trial comparing mommy-baby visits and enhanced usual care (staff assisted scheduling of postpartum visit before hospital discharge) in 116 postpartum women with limited English proficiency, postpartum visit attendance rates were high (> 90%) in both groups [78]. This study demonstrated feasibility and acceptability of a co-located, co-timed maternal-newborn visits.
Home Based Postpartum Care
Approximately 40% of women do not attend postpartum visits due to stress, fatigue, caring for a newborn, lack of social support, lack of transport, or a language barrier [33•]. Telemedicine is an alternative solution for individuals unable to attend in-person visits during and outside the COVID-19 pandemic. Telehealth visits have been shown to be effective in various aspects of peripartum care such as blood pressure management, tobacco cessation counseling, postpartum depression and postpartum weight management [79]. Access to telehealth also allows effective self-monitoring and management of blood pressure. The SNAP-HT Trial was a small study (n = 61) which randomized women with gestational hypertension or preeclampsia to home blood pressure monitoring and automated medication adjustment via telemonitoring versus usual care via general practitioners and showed that self-monitoring/management is feasible and effective in reducing blood pressure at 6 months [80]. Long-term follow up study of the SNAP-HT group confirmed that self-monitoring/management of blood pressure after a hypertensive pregnancy results in a persistent reduction in blood pressure at 3–4 years postpartum [81]. Unfortunately, the recent, larger BUMP2 trial (n = 850) investigating the use of blood pressure self-monitoring vs usual care in pregnant individuals with chronic hypertension or gestational hypertension did not find improved clinic-based blood pressure control between the 2 groups [82]. However, given reports from this trial that approximately 50% of pregnant individuals with hypertension already self-monitor their blood pressure, this may have diluted any effect of the assigned intervention [82]. According to the recent joint policy statement from the AHA and American Medical Association on self-measured home blood pressure monitoring, there is sufficient evidence to support the efficacy of self-monitoring when combined with individualized in-person or tele-counseling [83].
A Cochrane review of 16 randomized trials and 12,080 women studying the effect of early postpartum home visits did not draw a conclusion on their effect on maternal and fetal mortality, however, suggested that home visits may improve depression scores, exclusive breastfeeding, and infant healthcare utilization [84]. Community wide services such as the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) which provides supplemental food and education for pregnant women and children under the age of 5 may also encourage healthy dietary habits.
Lifestyle Interventions
Team-based postpartum care extends outside of the usual clinic setting and can be useful for implementing lifestyle interventions such as postpartum weight management [85]. Involvement of behavioral health coaches for postpartum weight reduction has been found to be feasible and well accepted [86]. In addition, a prior trial showed that an internet-based weight loss program may be feasible and effective in postpartum women of lower income as part of supplement to the WIC program [87]. Another trial of women with GDM found that an intervention of 13 telephone sessions postpartum to deliver a Diabetes Prevention Program (DPP)-derived lifestyle intervention had modest benefits in reducing postpartum weight retention and increasing physical activity [88]. Remote monitoring intervention using wearable activity trackers has been shown to be effective in increasing daily physical activity during the postpartum period. Lewey et al. studied the role of gamified digital health intervention (involving text messages providing feedback, social incentives, and points for meeting goals) in increasing daily step counts in women diagnosed with a hypertensive disorder during a recent pregnancy and found the intervention effective in increasing physical activity over the 12-week study period [89]. Additional studies are needed to study the cost-effectiveness of these interventions for weight reduction and activity promotion.
Next Steps: Optimization of Inter-Pregnancy Maternal Health
It is important for women with cardiovascular risk factors to have an opportunity to optimize their health before another pregnancy. The AHA recommends all women with a history of HDP and other APOs to have contraception counseling [90]. Careful planning of future pregnancy is particularly imperative for women with pre-existing cardiac conditions that require teratogenic pharmacotherapy such as warfarin or ACE inhibitors. Shared decision-making regarding the method and duration of contraception should take place between the patient and her cardio-obstetrics team during the fourth trimester and regularly thereafter [91].
Many women report significant perinatal anxiety and stress due to inadequate social support, poor healthcare experiences, and health-related concerns [92]. There is some evidence that stress reduction techniques such as meditation and faith-based strategies can be effective in reducing blood pressure among Black women [93, 94]. The postpartum period provides an opportunity to intervene with both pharmacotherapy and behavioral therapy to affect women’s overall well-being.
After the Fourth Trimester: Long-Term Cardiovascular Risk for Women
For many women, their increased cardiovascular risk does not end after delivery. Maternal and fetal complications such as HDP, GDM, pre-term birth, and SGA newborns have been associated with future CVD risk development [77, 95, 96, 97••, 98]. These conditions, known as adverse pregnancy outcomes (APOs), are important in identifying a population that should be targeted for preventive efforts. A meta-analysis including 6.4 million women over a 40-year span illustrated a 71% increased risk of CVD mortality, 2.5-fold increased risk in coronary artery disease, and fourfold increased risk of heart failure in women with a history of preeclampsia compared to those without pre-eclampsia [99]. Additionally, women with GDM have been shown to have a 30% increased risk of CVD and 41% increased risk of coronary artery disease compared to those without GDM during pregnancy [100]. Women with SGA infants are around twice as likely to experience future CVD risk [101] and 2.5 fold increase in death from a cardiovascular cause [102]. Preterm delivery prior to 37 weeks gestational age is associated with 38% increased risk of ischemic heart disease and 71% increased risk of stroke, with twofold overall risk of CVD [103].
It is clear given the long-term risk factors described above that APOs enhance CVD risk for women later in life, well after pregnancy and delivery. Therefore, the role of prevention is paramount in mitigating future risk and improving health outcomes. Women are often diagnosed and treated for CVD at lower rates than men [104, 105], and this is a prime opportunity for early intervention and addressing this systemic gap. The 2019 ACC/AHA guideline for the primary prevention of CVD and the 2021 AHA scientific statement on APOs both endorse that a detailed history of APOs is important for comprehensive CVD risk assessment in women [96, 106]. Counseling women on lifestyle changes including diets rich in vegetables/fruits, 150 min/week of moderate intensity aerobic activity during pregnancy and postpartum, and smoking cessation during could mitigate the risks of women who have experienced APOs. Although guidelines provide preventive recommendations for the general population, specific CVD preventive recommendations for women with history of APO specifically have not yet been written in guidelines [77, 96, 106]. In terms of pharmacologic therapy, it is not clear whether long-term use of aspirin, statins, and metformin have a special role in CVD risk prevention after APOs. However, per the 2019 ACC/AHA primary prevention guidelines, APOs should be considered as risk enhancers when considering risk-based decisions for statin initiation [96, 106]. Given the long-term effects of APOs as described above, legislation to continue coverage farther into the postpartum period especially for vulnerable populations could better provide preventative care for high risk populations [96]. Additional studies to create more concrete guidelines and provide tailored postpartum preventive care for women with APOs are needed.
Conclusion
Women face major physiologic and emotional changes during the postpartum period. Development of a comprehensive postpartum care plan with careful consideration of each patient’s risk profile and access to resources is critical in improving maternal morbidity and mortality as well as improving the long-term cardiovascular health for women. Supporting postpartum wellbeing of women during this transition period requires a multidisciplinary approach and planning should start before delivery.
Funding
Dr. Michos is supported by the Amato Fund for Women’s Cardiovascular Health research at Johns Hopkins University. Dr. Minhas is supported by the Lou and Nancy Grasmick Research Fellowship and the Marie-Josee and Henry R. Kravis Endowed Fellowship at Johns Hopkins University. Drs. Bennett and Michos are supported by NIH/NIDDK grant R01DK127222. Dr. Bennett is supported by NIH/NIDDK grant R18DK122416. Dr. Lewey is supported by a grant from the NIH (K23 HL153667).
Declarations
Conflict of Interest
Dr. Michos reports Advisory Board participation for AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Novartis, Novo Nordisk, and Pfizer. No other authors report any disclosures.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Women and Heart Disease
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •Of importance •• Of major importance
- 1.Centers for Disease Control and Prevention. Pregnancy-Related Deaths in the United States https://www.cdc.gov/hearher/pregnancy-related-deaths/index.html#:~:text=Almost%20two%20thirds%20of%20pregnancy,quality%20care%20can%20save%20lives. [Accessed February 11, 2022].
- 2.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. 2017. https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm# [Accessed July 10, 2022].
- 3.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366. doi: 10.1097/AOG.0000000000002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinney J, Keyser L, Clinton S, Pagliano C. ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;132:784–785. doi: 10.1097/AOG.0000000000002849. [DOI] [PubMed] [Google Scholar]
- 5.Thiel de Bocanegra H, Braughton M, Bradsberry M, Howell M, Logan J, Schwarz EB. Racial and ethnic disparities in postpartum care and contraception in California’s Medicaid program. Am J Obstet Gynecol. 2017;217(47):e1–47. doi: 10.1016/j.ajog.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 6.Essien UR, Molina RL, Lasser KE. Strengthening the postpartum transition of care to address racial disparities in maternal health. J Natl Med Assoc. 2019;111:349–351. doi: 10.1016/j.jnma.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Wouk K, Morgan I, Johnson J, et al. A systematic review of patient-, provider-, and health system-level predictors of postpartum health care use by people of color and low-income and/or uninsured populations in the United States. J Womens Health. 2021;30:1127–1159. doi: 10.1089/jwh.2020.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••.McCloskey L, Bernstein J, The Bridging The Chasm C et al. Bridging the chasm between pregnancy and health over the life course: a national agenda for research and action. Womens Health Issues. 2021;31:204–218. doi: 10.1016/j.whi.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts J, August P, Bakris G, et al. American College of Obstetricians and Gynecologists; task force on hypertension in pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists´ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 10.Garovic VD, Dechend R, Easterling T, et al. Hypertension in pregnancy: diagnosis, blood pressure goals, and pharmacotherapy: a scientific statement from the American Heart Association. Hypertension. 2022;79:e21–e41. doi: 10.1161/HYP.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita AT, Joseph K. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension. 2019;74:1089–1095. doi: 10.1161/HYPERTENSIONAHA.119.12968. [DOI] [PubMed] [Google Scholar]
- 12.Wu P, Jordan KP, Chew-Graham CA, et al. Temporal trends in pregnancy-associated stroke and its outcomes among women with hypertensive disorders of pregnancy. J Am Heart Assoc. 2020;9:e016182. doi: 10.1161/JAHA.120.016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Chan W-S, Ray JG, Kramer MS, Joseph K, System CPS. Stroke and cerebrovascular disease in pregnancy: incidence, temporal trends, and risk factors. Stroke. 2019;50:13–20. doi: 10.1161/STROKEAHA.118.023118. [DOI] [Google Scholar]
- 14.Afana M, Brinjikji W, Kao D, et al. Characteristics and in-hospital outcomes of peripartum cardiomyopathy diagnosed during delivery in the United States from the Nationwide Inpatient Sample (NIS) database. J Cardiac Fail. 2016;22:512–519. doi: 10.1016/j.cardfail.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Tweet MS, Hayes SN, Codsi E, Gulati R, Rose CH, Best PJ. Spontaneous coronary artery dissection associated with pregnancy. J Am Coll Cardiol. 2017;70:426–435. doi: 10.1016/j.jacc.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 16.Minhas AS, Ogunwole SM, Vaught AJ, et al. Racial disparities in cardiovascular complications with pregnancy-induced hypertension in the United States. Hypertension. 2021;78:480–488. doi: 10.1161/HYPERTENSIONAHA.121.17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine LD, Ky B, Chirinos JA, et al. Prospective evaluation of cardiovascular risk 10 years after a hypertensive disorder of pregnancy. J Am Coll Cardiol. 2022;79:2401–2411. doi: 10.1016/j.jacc.2022.03.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens I, Basit S, Melbye M, et al. Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. BMJ. 2017;358:j3078. doi: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garovic VD, White WM, Vaughan L, et al. Incidence and long-term outcomes of hypertensive disorders of pregnancy. J Am Coll Cardiol. 2020;75:2323–2334. doi: 10.1016/j.jacc.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haug EB, Horn J, Markovitz AR, et al. Association of conventional cardiovascular risk factors with cardiovascular disease after hypertensive disorders of pregnancy: analysis of the Nord-Trøndelag Health Study. JAMA Cardiol. 2019;4:628–635. doi: 10.1001/jamacardio.2019.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon LJ, McCarthy FP, Direk K, et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records: a CALIBER study. Circulation. 2019;140:1050–1060. doi: 10.1161/CIRCULATIONAHA.118.038080. [DOI] [PubMed] [Google Scholar]
- 22.Cairns AE, Pealing L, Duffy JMN, et al. Postpartum management of hypertensive disorders of pregnancy: a systematic review. BMJ Open. 2017;7:e018696. doi: 10.1136/bmjopen-2017-018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186:1174–1177. doi: 10.1067/mob.2002.123824. [DOI] [PubMed] [Google Scholar]
- 24.Kayem G, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. Maternal and obstetric factors associated with delayed postpartum eclampsia: a national study population. Acta Obstet Gynecol Scand. 2011;90:1017–1023. doi: 10.1111/j.1600-0412.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 25.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207. doi: 10.1136/bmj.c2207. [DOI] [PubMed] [Google Scholar]
- 26.Obstetricians ACo, Gynecologists. ACOG Practice Bulletin No. 203: chronic hypertension in pregnancy. Obstetrics and gynecology 2019;133:e26-e50. [DOI] [PubMed]
- 27.Obstetricians ACo, Gynecologists. Gestational hypertension and preeclampsia: ACOG Practice Bulletin, number 222. Obstet Gynecol 2020;135:e237-e260. [DOI] [PubMed]
- 28.••.Tita AT, Szychowski JM, Boggess K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022;386:1781–1792. doi: 10.1056/NEJMoa2201295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.••.ACOG. Clinical Guidance for the Integration of the Findings of the Chronic Hypertension and Pregnancy (CHAP) Study. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/04/clinical-guidance-for-the-integration-of-the-findings-of-the-chronic-hypertension-and-pregnancy-chap-study. Accessed 18 Jun 2022. A recent update to the ACOG clinical guidance on management of blood pressure in pregnant women with chronic hypertension with new data from the CHAP trial.
- 30.Abalos E, Duley L, Steyn DW, Gialdini C. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2018;10(10):CD002252. doi: 10.1002/14651858.CD002252.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellos I, Pergialiotis V, Papapanagiotou A, Loutradis D, Daskalakis G. Comparative efficacy and safety of oral antihypertensive agents in pregnant women with chronic hypertension: a network metaanalysis. Am J Obstet Gynecol. 2020;223:525–537. doi: 10.1016/j.ajog.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 32.•.Lopes Perdigao J, Lewey J, Hirshberg A, et al. Furosemide for accelerated recovery of blood pressure postpartum in women with a hypertensive disorder of pregnancy: a randomized controlled trial. Hypertension. 2021;77:1517–1524. doi: 10.1161/HYPERTENSIONAHA.120.16133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.•.Davis MB, Arendt K, Bello NA, et al. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum: JACC focus seminar 1/5. J Am Coll Cardiol. 2021;77:1763–1777. doi: 10.1016/j.jacc.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/S0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 35.Gestational Hypertension and Preeclampsia ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;135:1492–1495. doi: 10.1097/AOG.0000000000003892. [DOI] [PubMed] [Google Scholar]
- 36.Park K, BaireyMerz CN, Bello NA, et al. Management of women with acquired cardiovascular disease from pre-conception through pregnancy and postpartum: JACC focus seminar 3/5. J Am Coll Cardiol. 2021;77:1799–1812. doi: 10.1016/j.jacc.2021.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Podymow T, August P. Postpartum course of gestational hypertension and preeclampsia. Hypertens Pregnancy. 2010;29:294–300. doi: 10.3109/10641950902777747. [DOI] [PubMed] [Google Scholar]
- 38.Bulletins—Obstetrics CoP ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131:e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 39.McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:1–19. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 40.International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org [Accessed July 5, 2022].
- 41.Jones EJ, Hernandez TL, Edmonds JK, Ferranti EP. Continued disparities in postpartum follow-up and screening among women with gestational diabetes and hypertensive disorders of pregnancy: a systematic review. J Perinat Neonatal Nurs. 2019;33:136–148. doi: 10.1097/JPN.0000000000000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S137-S143. [DOI] [PubMed]
- 43.Gunn C, Bernstein J, Bokhour B, McCloskey L. Narratives of gestational diabetes provide a lens to tailor postpartum prevention and monitoring counseling. J Midwifery Womens Health. 2020;65:681–687. doi: 10.1111/jmwh.13122. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Cheng Y, Wang D, et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review and meta-analysis of 170,139 women. J Diabetes Res. 2020;2020:3076463. doi: 10.1155/2020/3076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratner RE, Christophi CA, Metzger BE, et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aroda VR, Christophi CA, Edelstein SL, et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the Diabetes Prevention Program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100:1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrara A, Hedderson MM, Albright CL, et al. A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BMC Pregnancy Childbirth. 2014;14:1–10. doi: 10.1186/1471-2393-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ley SH, Chavarro JE, Li M, et al. Lactation duration and long-term risk for incident type 2 diabetes in women with a history of gestational diabetes mellitus. Diabetes Care. 2020;43:793–798. doi: 10.2337/dc19-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elkind-Hirsch KE, Seidemann E, Harris R. A randomized trial of dapagliflozin and metformin, alone and combined, in overweight women after gestational diabetes mellitus. Am J Obstet Gynecol MFM. 2020;2:100139. doi: 10.1016/j.ajogmf.2020.100139. [DOI] [PubMed] [Google Scholar]
- 50.Elkind-Hirsch KE, Shaler D, Harris R. Postpartum treatment with liraglutide in combination with metformin versus metformin monotherapy to improve metabolic status and reduce body weight in overweight/obese women with recent gestational diabetes: a double-blind, randomized, placebo-controlled study. J Diabetes Complications. 2020;34:107548. doi: 10.1016/j.jdiacomp.2020.107548. [DOI] [PubMed] [Google Scholar]
- 51.Chen C, Huang Y, Dong G, Zeng Y, Zhou Z. The effect of dipeptidyl peptidase-4 inhibitor and glucagon-like peptide-1 receptor agonist in gestational diabetes mellitus: a systematic review. Gynecol Endocrinol. 2020;36:375–380. doi: 10.1080/09513590.2019.1703943. [DOI] [PubMed] [Google Scholar]
- 52.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 53.Echouffo-Tcheugui JB, Guan J, Retnakaran R, Shah BR. Gestational diabetes and incident heart failure: a cohort study. Diabetes Care. 2021;44:2346–2352. doi: 10.2337/dc21-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gunderson EP, Sun B, Catov JM, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the cardia study. Circulation. 2021;143:974–987. doi: 10.1161/CIRCULATIONAHA.120.047320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilmore LA, Klempel-Donchenko M, Redman LM. Pregnancy as a window to future health: excessive gestational weight gain and obesity. Sem Perinatol. 2015;39:296–303. doi: 10.1053/j.semperi.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317:2207–2225. doi: 10.1001/jama.2017.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paladine HL, Blenning CE, Strangas Y. Postpartum care: an approach to the fourth trimester. Am Fam Physician. 2019;100:485–491. [PubMed] [Google Scholar]
- 58.Ehrenberg HM, Huston-Presley L, Catalano PM. The influence of obesity and gestational diabetes mellitus on accretion and the distribution of adipose tissue in pregnancy. Am J Obstet Gynecol. 2003;189:944–948. doi: 10.1067/S0002-9378(03)00761-0. [DOI] [PubMed] [Google Scholar]
- 59.Luoto R, Mannisto S, Raitanen J. Ten-year change in the association between obesity and parity: results from the National FINRISK Population Study. Gend Med. 2011;8:399–406. doi: 10.1016/j.genm.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 60.In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC), 2009. [PubMed]
- 61.Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for depression in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:380–387. doi: 10.1001/jama.2015.18392. [DOI] [PubMed] [Google Scholar]
- 62.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 757: screening for perinatal depression. Obstet Gynecol 2018;132:e208-e212. [DOI] [PubMed]
- 63.Earls MF, Yogman MW, Mattson G, et al. Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics. 2019;143(1):e20183259. doi: 10.1542/peds.2018-3259. [DOI] [PubMed] [Google Scholar]
- 64.Rosman L, Salmoirago-Blotcher E, Cahill J, Wuensch KL, Sears SF. Depression and health behaviors in women with peripartum cardiomyopathy. Heart Lung. 2017;46:363–368. doi: 10.1016/j.hrtlng.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50:938–948. doi: 10.1097/GRF.0b013e31815a5494. [DOI] [PubMed] [Google Scholar]
- 66.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen B, Jin K, Ding D. Breastfeeding and maternal cardiovascular risk factors and outcomes: a systematic review. PLoS ONE. 2017;12:e0187923. doi: 10.1371/journal.pone.0187923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhury R, Sinha B, Sankar MJ, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104:96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunderson EP, Lewis CE, Lin Y, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA study. JAMA Intern Med. 2018;178:328–337. doi: 10.1001/jamainternmed.2017.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu J, Pudwell J, Dayan N, Smith GN. Postpartum breastfeeding and cardiovascular risk assessment in women following pregnancy complications. J Womens Health. 2020;29:627–635. doi: 10.1089/jwh.2019.7894. [DOI] [PubMed] [Google Scholar]
- 71.Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O'Keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta-analysis involving data from 8 studies and 1 192 700 parous women. J Am Heart Assoc. 2022;11:e022746. doi: 10.1161/JAHA.121.022746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 73.American College of Obstetricians and Gynecologists. Breastfeeding Challenges: ACOG Committee Opinion, Number 820. Obstet Gynecol. 2021;137:e42–53. [DOI] [PubMed]
- 74.Lima F, Nie L, Yang J, et al. Postpartum cardiovascular outcomes among women with heart disease from a nationwide study. Am J Cardiol. 2019;123:2006–2014. doi: 10.1016/j.amjcard.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ., III Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006. [DOI] [PubMed] [Google Scholar]
- 76.Tepper NK, Boulet SL, Whiteman MK, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol. 2014;123:987–996. doi: 10.1097/AOG.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 77.Jowell AR, Sarma AA, Gulati M, et al. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: a review. JAMA Cardiol. 2022;7:346–355. doi: 10.1001/jamacardio.2021.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Polk S, Edwardson J, Lawson S, et al. Bridging the postpartum gap: a randomized controlled trial to improve postpartum visit attendance among low-income women with limited English proficiency. Womens Health Rep. 2021;2:381–388. doi: 10.1089/whr.2020.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown HL, DeNicola N. Telehealth in maternity care. Obstet Gynecol Clin North Am. 2020;47:497–502. doi: 10.1016/j.ogc.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 80.Cairns AE, Tucker KL, Leeson P, et al. Self-management of postnatal hypertension: the SNAP-HT trial. Hypertension. 2018;72:425–432. doi: 10.1161/HYPERTENSIONAHA.118.10911. [DOI] [PubMed] [Google Scholar]
- 81.Kitt JA, Fox RL, Cairns AE, et al. Short-term postpartum blood pressure self-management and long-term blood pressure control: a randomized controlled trial. Hypertension. 2021;78:469–479. doi: 10.1161/HYPERTENSIONAHA.120.17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chappell LC, Tucker KL, Galal U, et al. Effect of self-monitoring of blood pressure on blood pressure control in pregnant individuals with chronic or gestational hypertension: the BUMP 2 randomized clinical trial. JAMA. 2022;327:1666–1678. doi: 10.1001/jama.2022.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimbo D, Artinian NT, Basile JN, et al. Self-measured blood pressure monitoring at home: a joint policy statement from the American Heart Association and American Medical Association. Circulation. 2020;142:e42–e63. doi: 10.1161/CIR.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 84.Yonemoto N, Nagai S, Mori R. Schedules for home visits in the early postpartum period. Cochrane Database Syst Rev. 2021;7:CD009326. doi: 10.1002/14651858.CD009326.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dodd JM, Deussen AR, O'Brien CM, et al. Targeting the postpartum period to promote weight loss: a systematic review and meta-analysis. Nutr Rev. 2018;76:639–654. doi: 10.1093/nutrit/nuy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coughlin JW, Martin LM, Henderson J, et al. Feasibility and acceptability of a remotely-delivered behavioural health coaching intervention to limit gestational weight gain. Obes Sci Pract. 2020;6:484–493. doi: 10.1002/osp4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phelan S, Hagobian T, Brannen A, et al. Effect of an internet-based program on weight loss for low-income postpartum women: a randomized clinical trial. JAMA. 2017;317:2381–2391. doi: 10.1001/jama.2017.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrara A, Hedderson MM, Brown SD, et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care. 2016;39:65–74. doi: 10.2337/dc15-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lewey J, Murphy S, Zhang D, et al. Effectiveness of a text-based gamification intervention to improve physical activity among postpartum individuals with hypertensive disorders of pregnancy: a randomized clinical trial. JAMA Cardiol. 2022;7:591–599. doi: 10.1001/jamacardio.2022.0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehta LS, Warnes CA, Bradley E, et al. Cardiovascular considerations in caring for pregnant patients: a scientific statement from the American Heart Association. Circulation. 2020;141:e884–e903. doi: 10.1161/CIR.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 91.O'Kelly AC, Michos ED, Shufelt CL, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. 2022;130:652–672. doi: 10.1161/CIRCRESAHA.121.319895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCarthy M, Houghton C, Matvienko-Sikar K. Women’s experiences and perceptions of anxiety and stress during the perinatal period: a systematic review and qualitative evidence synthesis. BMC Pregnancy Childbirth. 2021;21:811. doi: 10.1186/s12884-021-04271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Collins SV, Hines AL. Stress reduction to decrease hypertension for black women: a scoping review of trials and interventions. J Racial Ethn Health Disparities 2021. [DOI] [PubMed]
- 94.Levine GN, Lange RA, Bairey-Merz CN, et al. Meditation and cardiovascular risk reduction: a scientific statement from the American Heart Association. J Am Heart Assoc. 2017;6:e002218. doi: 10.1161/JAHA.117.002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol. 2018;41:239–246. doi: 10.1002/clc.22887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021;143:e902–e916. doi: 10.1161/CIR.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 97.••.Lane-Cordova AD, Khan SS, Grobman WA, Greenland P, Shah SJ. Long-term cardiovascular risks associated with adverse pregnancy outcomes: JACC review topic of the week. J Am Coll Cardiol. 2019;73:2106–2116. doi: 10.1016/j.jacc.2018.12.092. [DOI] [PubMed] [Google Scholar]
- 98.Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7:e009382. doi: 10.1161/JAHA.118.009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 100.Retnakaran R, Shah BR. Role of type 2 diabetes in determining retinal, renal, and cardiovascular outcomes in women with previous gestational diabetes mellitus. Diabetes Care. 2017;40:101–108. doi: 10.2337/dc16-1400. [DOI] [PubMed] [Google Scholar]
- 101.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846. doi: 10.1161/CIRCULATIONAHA.111.034884. [DOI] [PubMed] [Google Scholar]
- 102.Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr Perinat Epidemiol. 2010;24:323–330. doi: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 103.Heida KY, Velthuis BK, Oudijk MA, et al. Cardiovascular disease risk in women with a history of spontaneous preterm delivery: a systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23:253–263. doi: 10.1177/2047487314566758. [DOI] [PubMed] [Google Scholar]
- 104.Elder P, Sharma G, Gulati M, Michos ED. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am J Prev Cardiol. 2020;2:100028. doi: 10.1016/j.ajpc.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thakkar A, Agarwala A, Michos ED. Secondary prevention of cardiovascular disease in women: closing the gap. Eur Cardiol. 2021;16:e41. doi: 10.15420/ecr.2021.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]