Abstract
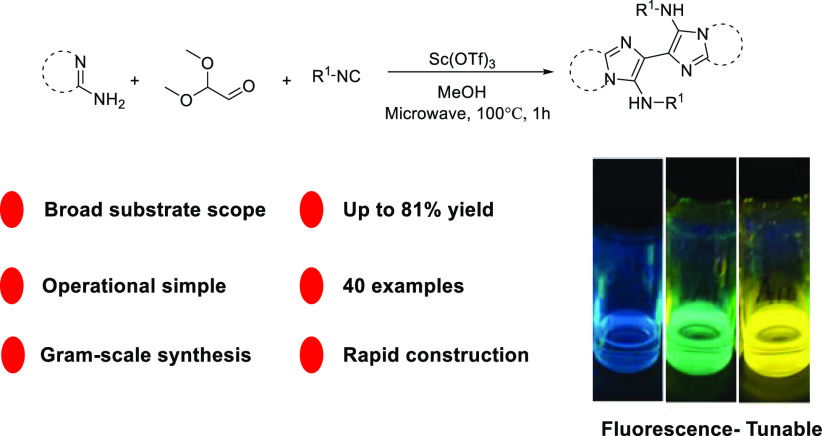
A short, concise, and one-pot synthesis of imidazo-fused heterocycle dimers with tunable fluorescent properties has been developed. By the first time use of glyoxal dimethyl acetal in the Groebke–Blackburn–Bienaymé (GBB) three-component reaction (3CR), the innovation features a new series of fluorescence-tunable imidazo-fused heterocycle dimers exhibiting a broad substrate scope with good yields. Luminescence studies demonstrate that these GBB-dimers possess color-tunable properties, and their emission colors can be successively changed from blue to green and yellow by easy substituent control.
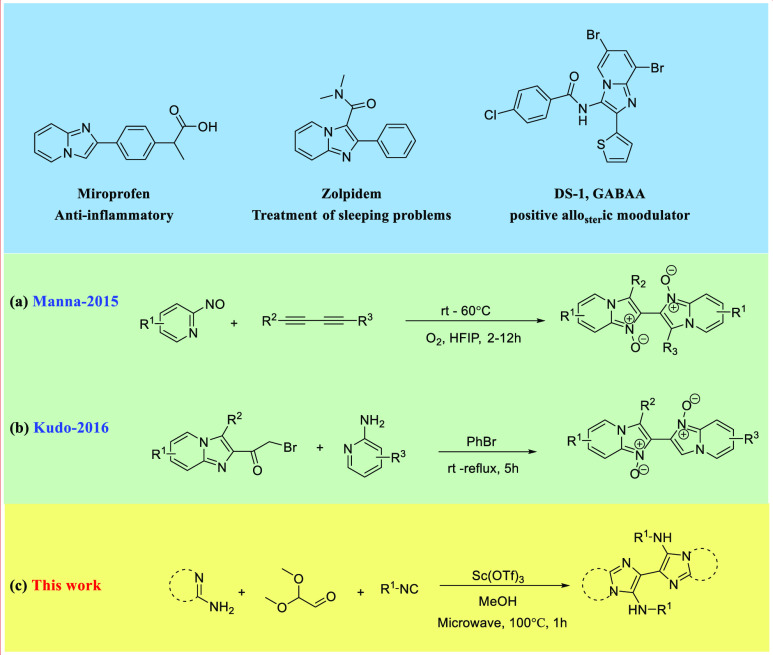
There are many natural and synthetic symmetrical small molecule dimers with potential biological activities, such as anticancer,1 antimalarial,2 antibacterial,3 and opioid antagonist activities.4,5 One reason to synthetically aim for symmetrical compounds are homodimeric symmetrical receptors, such as that for PD-L1.6 The imidazo[1,2-a]heterocyclic scaffold7 accessible by the Groebke–Blackburn–Bienaymé (GBB) multicomponent reaction (MCR) is well-known in many FDA-approved drugs, such as miroprofen,8 zolpidem,9 and DS-1.10 The first imidazo[1,2-a]pyridine dimer was reported in 2015 by Manna et al. and was produced by the annulation of nitrosopyridine with alkynes (Figure 1).11 In 2016, Kudo disclosed the synthesis of noxious organism control agents exploiting the condensation of 2-aminopyridine and imidazo-bromomethyl ketone.12 The GBB MCR reaction is an efficient way to get the important imidazo[1,2-a]heterocycle scaffold with various aldehydes.13 Based on our ongoing interest in MCR chemistry, we described the fast construction of a series of symmetric imidazo[1,2-a]heterocycle dimers by one-pot GBB reaction. Importantly, it is the first time that glyoxal dimethyl acetal, which acts as an orthogonal bifunctional monoprotected building block to achieve a new series of fluorescent imidazo[1,2-a]heterocycle dimers, was used in the GBB-3CR.
Figure 1.
Biological GBB scaffold drugs and previous and current scope of work.
We first selected 1a and 2a as model substrates and employed various molar ratios, solvents, and catalysts, and glyoxal dimethyl acetal (60% in H2O) for condition optimization (Table 1). First, we screened the ratio of 1a, glyoxal dimethyl acetal, and 2a (entries 1–4). We used scandium triflate (Sc(OTf)3) as catalyst and methanol as solvent because they are the most often used conditions for GBB-3CR. The reaction with ratio 1a/glyoxal dimethyl acetal/2a at 1:1:1 yielded product in 24% yield, and those with ratios 2:1:2, 2.2:1:2.2, and 1.3:1:1.3 gave 74%, 64%, and 34%, respectively. With the best ratio 2:1:2, we tried to find best solvent for this reaction. The product 3au was generated in 45% yield when we used trifluoroethanol (TFE) as solvent (entry 5). Water is also a widely used solvent in GBB-3CR; however, it only gave very low yield of 11% (entry 6). The reaction with acetonitrile as solvent gave 3au in 36% yield, while only 21% and 17% yield were achieved with solvent free reaction and PEG40, respectively (entries 7–9). Subsequently, we surveyed the effects of using different catalysts. When 4-methylbenzenesulfonic acid (PTSA) was employed, 64% yield of product was obtained (entry 5). Inorganic acids such as perchloric acid and acetic acid gave product in 43% and 48% yield, respectively, whereas ammonium chloride could generate 3au in 51% yield. Zirconium tetrachloride (ZrCl4) as Lewis acid could afford the desired product 3a in 63% yield. Using glyoxal (40 wt % in H2O) as the dialdehyde source gave a lower 40% yield. In addition, we also heated this reaction at 80 °C for 12 h, resulting in 54% yield.
Table 1. Optimization of Conditionsa.
| entry | ratio | solvent | catalyst | yieldb (%) |
|---|---|---|---|---|
| 1 | 1:1:1 | MeOH | Sc(OTf)3 | 24 |
| 2 | 2:1:2 | MeOH | Sc(OTf)3 | 74 |
| 3 | 2.2:1:2.2 | MeOH | Sc(OTf)3 | 64 |
| 4 | 1.3:1:1.3 | MeOH | Sc(OTf)3 | 34 |
| 5 | 2:1:2 | TFE | Sc(OTf)3 | 45 |
| 6 | 2:1:2 | water | Sc(OTf)3 | 11 |
| 7 | 2:1:2 | MeCN | Sc(OTf)3 | 36 |
| 8 | 2:1:2 | solvent free | Sc(OTf)3 | 21 |
| 9 | 2:1:2 | PEG40 | Sc(OTf)3 | 17 |
| 10 | 2:1:2 | MeOH | PTSA | 64 |
| 11 | 2:1:2 | MeOH | perchloric acid | 43 |
| 12 | 2:1:2 | MeOH | acetic acid | 48 |
| 13 | 2:1:2 | MeOH | NH4Cl | 51 |
| 14 | 2:1:2 | MeOH | ZrCl4 | 63 |
| 15c | 2:1:2 | MeOH | Sc(OTf)3 | 40 |
| 16d | 2:1:2 | MeOH | Sc(OTf)3 | 54 |
Reaction conditions: unless otherwise stated, all the reactions were performed with 1a (1 mmol), glyoxal dimethyl acetal (60% in H2O, 0.5 mmol), 2a (1 mmol), and catalyst (20 mol %) in solvent (0.5 mL) at 100 °C under microwave radiation for 1 h. PTSA = 4-methylbenzenesulfonic acid; TFE = trifluoroethanol.
Isolated yield.
Glyoxal (40 wt % in H2O) was used as dialdehyde source.
Heated at 80 °C for 12 h in sealed vial using aluminum heating blocks.
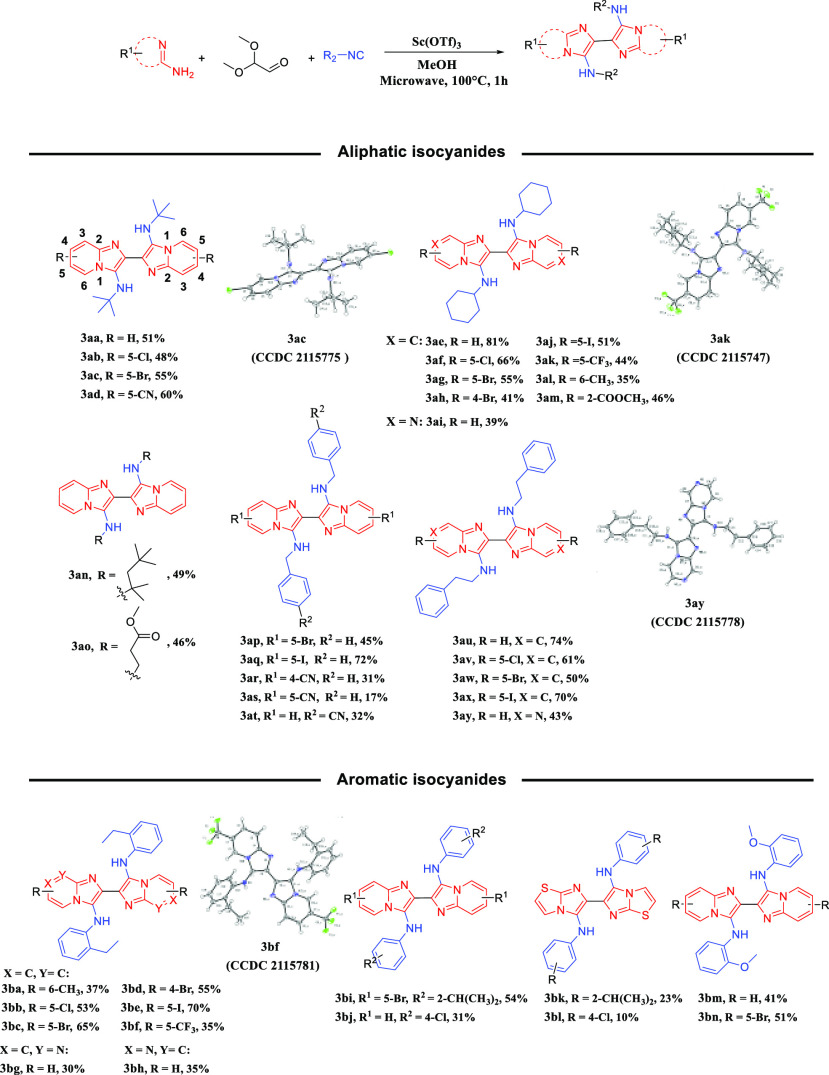
Having identified the optimal reaction conditions (Table 1, entry 2), we evaluated the substrate scope of the newly developed synthetic protocol (Scheme 1). When tert-butyl isocyanide reacted with substituted 2-aminopyridine with different electron-withdrawing groups, desired products were finally obtained in relatively good yields (3aa–3ad). Gratifyingly, cyclohexyl isocyanide was able to afford desired product 3ae in 81% yield. The use of various substituents on the 2-aminopyridine regardless of their electronic characteristics, such as chloro (3af), bromo (3ag, 3ah), iodo (3aj), trifluoromethyl (3ak), methyl (3al), and methyl formate (3am), afforded the corresponding products in 35–66% yields.
Scheme 1. Scope of the Substrates.
We also tried pyrimidin-2-amine to add more diversity of the central imidazo-bicycle to the GBB dimer scaffold, and the product was generated in 39% yield (3ai). In addition, some other isocyanides such as 2-isocyano-2,4,4-trimethylpentane and methyl 3-isocyanopropionate could also provide products in moderate yields, 49% and 46%, respectively (3an–3ao). Interestingly, benzyl isocyanide was also tolerated under the current conditions when reacted with bromo or iodo substituted 2-aminopyridine (3ap, 3aq), whereas it only furnished the corresponding products in reduced yields when reacted with cyano substituted 2-aminopyridine (3ar, 3as).
When 4-(isocyanomethyl)benzonitrile was used to react with 2-aminopyridine, product 3at was afforded only in 32% yield. When phenylethyl isocyanide reacted with substituted 2-aminopyridine with different halogen atoms, the desired products were obtained in good yields (3au–3ay). Subsequently, we explored the applicability of the reaction conditions to different aromatic isocyanides. Satisfactorily, when phenylethyl isocyanide reacted with substituted 2-aminopyridine with different halogen atoms, desired products were finally obtained in relatively good yields (3ba–3bf). However, pyrimidin-2-amine and pyrazin-2-amine could only afford the corresponding products with relatively low yields, 30% and 35%, respectively (3bg, 3bh). For aromatic isocyanides such as 1-isocyano-2-isopropylbenzene and 1-chloro-4-isocyanobenzene, compounds 3bi and 3bj were obtained in 54% and 31% yield, respectively. To further explore the scope, we used thiazol-2-amine to obtain a unique 6,6′-biimidazo[2,1-b]thiazole scaffold of 3ai and 3aj with somewhat diminished yield, 23% and 10%, respectively (3bk, 3bl). In addition, 2-methoxyphenyl isocyanide, which was also a suitable substrate, generated corresponding products in moderate to good yields (3bm, 3bn). Significantly, we successfully obtained crystal structures of 3ab, 3ac, 3ag, 3ak, 3aw, 3ay, and 3bf by X-ray crystallography analysis (Figure S1). Worthwhile to mention, the exocyclic NH of one imidazo ring forms an intramolecular hydrogen bond to the imidazo-N of the next ring, providing a rather rigid fully coplanar hexacyclic ring system, belonging to the symmetry point group C2h, with an inversion center, a 2-fold rotation axis, and a horizontal plane.
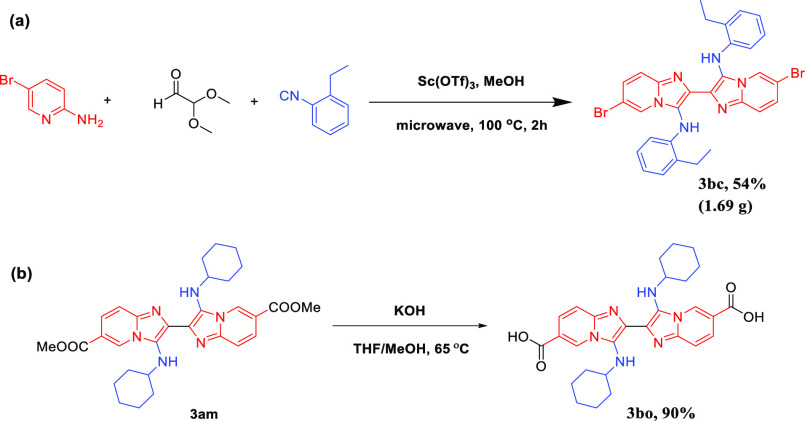
To verify the synthetic practicality of this simple workup reaction, we carried out a gram-scale experiment in which the model reaction was performed on a 5 mmol scale; compound 3bc was isolated in 54% yield (1.69 g) by prolonging the reaction time to 2 h (Scheme 2a). Further application was demonstrated by the hydrolysis of compound 3am (Scheme 2b). The hydrolysis of 3am proceeded smoothly, giving the corresponding 3,3′-bis(cyclohexylamino)-[2,2′-biimidazo[1,2-a]pyridine]-6,6′-dicarboxylic acid 3bo with 90% yield.14
Scheme 2. Gram-Scale Synthesis and Applications.
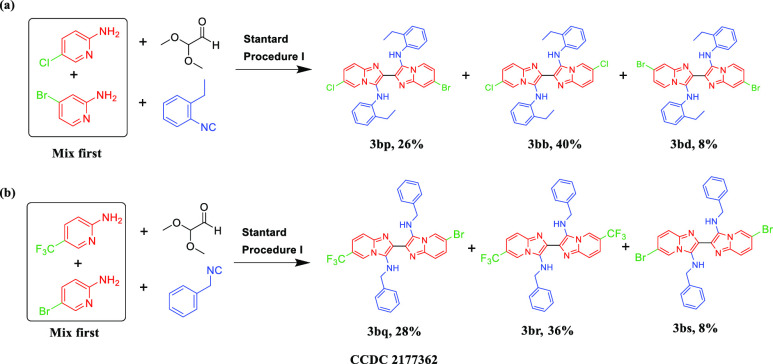
In addition to synthesis of symmetric GBB dimers, we also explored unsymmetric synthesis, incorporating two different imidazo heterocycles. As shown in Scheme 3, 5-chloropyridin-2-amine and 4-bromopyridin-2-amine were employed at the same time to afford unsymmetric compound 3bp in 26% yield, while the two homodimers 3bb and 3bd were generated in 40% and 8% yield, respectively. Likewise, another example produced unsymmetric 3bq in 28% yield and symmetric 3br and 3bs in 36% and 8% yield. We obtained the crystal structure of 3bp (Figure S1).
Scheme 3. Unsymmetric Synthesis.
Then, we investigated the luminescence properties of these GBB dimers. First, we determined a suitable wavelength for the photoluminescence assay by UV/vis spectroscopy. The UV/vis absorption spectrum of 3ae recorded in different solvents showed that THF is the best solvent to give a strong absorption band centered at 390 nm with another maxima (280 nm) in the ultraviolet region (Figure S2A). Next, we recorded the UV/vis absorption spectra of 3bj, 3bm, 3aa, 3al, 3ai, 3ag, and 3ak in THF and obtained their maximum absorption wavelengths (Figure S1B). Due to the weak fluorescence intensities achieved from the λmax in the ultraviolet region of these dimers, we chose another λmax in the visible region and tested the luminescence activity of 3ae with an excitation at 390 nm in different solvents, and THF turned out to be the best solvent, showing the highest luminescence intensity (Figure 2A). Then, we carried out luminescence photophysical studies on further GBB-dimer compounds. With excitation wavelengths at 365 or 370 nm, 3bj and 3bm, both containing aromatic amine moieties, emitted a blue luminescence at 455 nm in THF at 25 °C. When arylamino substituents were changed to alkylamino substituents like tert-butylamino or cyclohexylamino groups, the emission wavelengths of 3aa and 3ae were red-shifted to 460 nm (light blue) or 500 nm (green). Interestingly, when an electron-donating methyl group was introduced on the pyridine ring, compound 3al could achieved a slight blue shift to 490 nm compared to that of 3ae (green). The electron-withdrawing nitrogen atom (3ai, 520 nm), 5-Br (3ag, 530 nm), or 5-CF3 (3ak, 545 nm) employed with the pyridine resulted in the emission band being red-shifted to the yellow color range. Notably, 3ag showed a significantly weak fluorescence intensity, suggesting that 5-Br could be used to reduce luminescence activity. The relative fluorescence quantum yields (Φ) of compounds in Figure 2 are also summarized in Table S3 (see Supporting Information).
Figure 2.
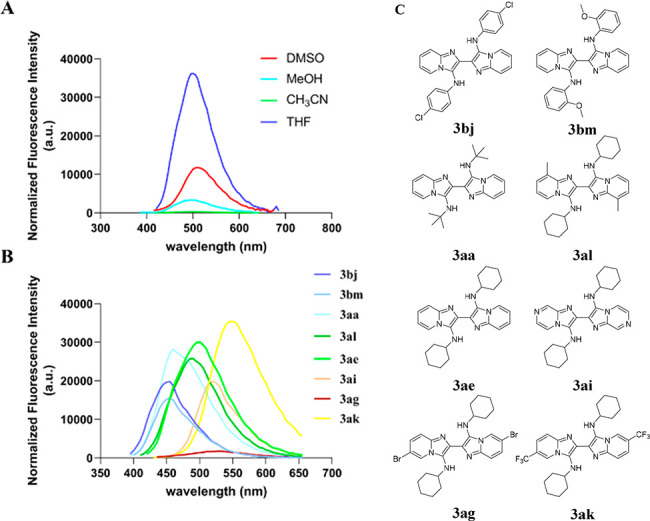
(A) Fluorescence spectra of 3ae (10 μM) in different solvents at 25 °C with an excitation at 390 nm. (B) Fluoroscence intensity of 3bj, 3bm, 3aa, 3ae, 3al, 3ai, 3ag, and 3ak (10 μM) in THF at 25 °C at corresponding excitation. (C) Structures of compounds.
In summary, we have reported a multicomponent reaction of isocyanide, amidine, and glyoxal dimethyl acetal leading to various tunable fluorescence imidazole-fused heterocycle dimers. This method features high synthetic efficiency, mild conditions, operational simplicity, and broad substrate scope. A plausible mechanism has also been proposed in the Supporting Information. Furthermore, these compounds possess color fine-tunable luminescence properties, and we can achieve a sequentially change in the emission colors of these GBB-dimers from blue to green and yellow by introducing corresponding 2-amino pyridines or isocyanides.
Acknowledgments
We thank Marcel de Vries (University of Groningen) for his help in HRMS analysis. Qiang Zheng and Xin Li acknowledge the China Scholarship Council for support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c01642.
Experimental procedures, 1H and 13C{1H} NMR spectra for all compounds, and X-ray crystallographic data for 3ab, 3ac, 3ag, 3ak, 3aw, 3ay, 3bf, and 3bq (PDF)
Accession Codes
CCDC 2115747, 2115775–2115776, 2115778–2115781, and 2177362 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Author Contributions
Qiang Zheng and Xin Li contributed equally. Qiang Zheng, Xin Li, and Alexander Dömling conceptualized the study and designed the methodology. Qiang Zheng and Xin Li performed the experiments and gathered the data. Katarzyna Kurpiewska performed the crystallographic studies. Qiang Zheng, Xin Li, and Alexander Dömling wrote the manuscript. All authors approved the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Gamage S. A.; Spicer J. A.; Atwell G. J.; Finlay G. J.; Baguley B. C.; Denny W. A. Structure–activity relationships for substituted bis (acridine-4-carboxamides): a new class of anticancer agents. J. Med. Chem. 1999, 42 (13), 2383–2393. 10.1021/jm980687m. [DOI] [PubMed] [Google Scholar]
- Jeyadevan J. P.; Bray P. G.; Chadwick J.; Mercer A. E.; Byrne A.; Ward S. A.; Park B. K.; Williams D. P.; Cosstick R.; Davies J.; et al. Antimalarial and antitumor evaluation of novel C-10 non-acetal dimers of 10β-(2-hydroxyethyl) deoxoartemisinin. J. Med. Chem. 2004, 47 (5), 1290–1298. 10.1021/jm030974c. [DOI] [PubMed] [Google Scholar]
- Seth P. P.; Jefferson E. A.; Risen L. M.; Osgood S. A. Identification of 2-aminobenzimidazole dimers as antibacterial agents. Bioorg. Med. Chem. Lett. 2003, 13 (10), 1669–1672. 10.1016/S0960-894X(03)00245-2. [DOI] [PubMed] [Google Scholar]
- Neumeyer J. L.; Zhang A.; Xiong W.; Gu X.-H.; Hilbert J. E.; Knapp B. I.; Negus S. S.; Mello N. K.; Bidlack J. M. Design and synthesis of novel dimeric morphinan ligands for κ and μ opioid receptors. J. Med. Chem. 2003, 46 (24), 5162–5170. 10.1021/jm030139v. [DOI] [PubMed] [Google Scholar]
- Bérubé G. Natural and synthetic biologically active dimeric molecules: anticancer agents, anti-HIV agents, steroid derivatives and opioid antagonists. Curr. Med. Chem. 2006, 13 (2), 131–154. 10.2174/092986706775197908. [DOI] [PubMed] [Google Scholar]
- Zak K. M.; Grudnik P.; Guzik K.; Zieba B. J.; Musielak B.; Dömling A.; Dubin G.; Holak T. A. J. O. Structural basis for small molecule targeting of the programmed death ligand 1 (PD-L1). Oncotarget. 2016, 7 (21), 30323. 10.18632/oncotarget.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rao C.; Mai S.; Song Q. Cu-Catalyzed Synthesis of 3-Formyl Imidazo[1,2-a]Pyridines and Imidazo[1,2-a]Pyrimidines by Employing Ethyl Tertiary Amines as Carbon Sources. Org. Lett. 2017, 19 (18), 4726–4729. 10.1021/acs.orglett.7b02015. [DOI] [PubMed] [Google Scholar]; b Balwe S. G.; Jeong Y. T. An approach towards the synthesis of novel fused nitrogen tricyclic heterocyclic scaffolds via GBB reaction. Org. Biomol. Chem. 2018, 16, 1287–1296. 10.1039/C7OB02933K. [DOI] [PubMed] [Google Scholar]; c Devi N.; Rawal R. K.; Singh V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: a review. Tetrahedron. 2015, 71, 183–232. 10.1016/j.tet.2014.10.032. [DOI] [Google Scholar]; d Konstantinidou M.; Boiarska Z.; Butera R.; Neochoritis C. G.; Kurpiewska K.; Kalinowska-Tłuscik J.; Dömling A. Diaminoimidazopyrimidines: Access via the Groebke–Blackburn–Bienaymé Reaction and Structural Data Mining. Eur. J. Org. Chem. 2020, 2020, 5601–5605. 10.1002/ejoc.202000933. [DOI] [Google Scholar]; e Zhi S.; Ma X.; Zhang W. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019, 17, 7632–7650. 10.1039/C9OB00772E. [DOI] [PubMed] [Google Scholar]
- Maruyama Y.; Anami K.; Terasawa M.; Goto K.; Imayoshi T.; Kadobe Y.; Mizushima Y. Anti-inflammatory activity of an imidazopyridine derivative (miroprofen). Arzneimforsch. 1981, 31 (7), 1111–1118. [PubMed] [Google Scholar]
- Swainston Harrison T.; Keating G. M Zolpidem. CNS drugs. 2005, 19 (1), 65–89. 10.2165/00023210-200519010-00008. [DOI] [PubMed] [Google Scholar]
- Wafford K.; Van Niel M.; Ma Q.; Horridge E.; Herd M.; Peden D.; Belelli D.; Lambert J. Novel compounds selectively enhance δ subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009, 56 (1), 182–189. 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Manna S.; Narayan R.; Golz C.; Strohmann C.; Antonchick A. P. Regioselective annulation of nitrosopyridine with alkynes: straightforward synthesis of N-oxide-imidazopyridines. Chem Commun. 2015, 51 (28), 6119–6122. 10.1039/C5CC00533G. [DOI] [PubMed] [Google Scholar]
- Kudo T.; Maizuru Y.; Tanaka A.; Noto K.; Matsui H.; Kobayashi M.. Preparation of condensed heterocyclic compounds as noxious organism control agents. International Patent WO2016129684, 2016.
- Boltjes A.; Dömling A. The Groebke-Blackburn-Bienaymé Reaction. Eur. J. Org. Chem. 2019, 2019 (42), 7007–7049. 10.1002/ejoc.201901124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X.; Wang Y.; Liu Y.; Wang F.; Shi L.; Lee K.-H.; Lin Z.; Lv H.; Zhang X. Highly efficient tetradentate ruthenium catalyst for ester reduction: especially for hydrogenation of fatty acid esters. Org. Lett. 2015, 17 (3), 454–457. 10.1021/ol503456j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.