Abstract
The BvgA-BvgS two-component signal transduction system regulates expression of virulence factors in Bordetella pertussis. The BvgA response regulator activates transcription by binding to target promoters, which include those for the genes encoding filamentous hemagglutinin (fha) and pertussis toxin (ptx). We have previously shown that at both promoters the phosphorylated form of BvgA binds multiple high- and low-affinity sites. Specifically, at the fha promoter, we proposed that there may be high- and a low-affinity binding sites for the BvgA dimer. In our present investigation, we used DNA binding analyses and in vitro and in vivo assays of promoters with substitutions and deletions to support and extend this hypothesis. Our observations indicate that (i) binding of BvgA∼P to a primary (high-affinity) site and a secondary binding region (lower affinity) is cooperative, (ii) although both the primary binding site and the secondary binding region are required for full activity of the wild-type (undeleted) promoter, deletion of two helical turns within the secondary binding region can produce a fully active or hyperactive promoter, and (iii) BvgA binding to the secondary binding region shows limited DNA sequence specificity.
The establishment of infection by Bordetella pertussis, the causative agent of whooping cough, involves the expression of several virulence factors that include toxins (e.g., pertussis toxin, adenylate cyclase toxin, and dermonecrotic toxins) and adhesins (e.g., filamentous hemagglutinin, pertactin, and fimbriae). Expression of these virulence factors is mediated by the BvgA-BvgS two-component signal transduction system (1, 38, 39, 44; reviewed in reference 35). Changes in the environment are monitored by BvgS, a membrane-bound histidine kinase. BvgS undergoes a series of intramolecular and intermolecular phosphotransfers that ultimately result in the phosphorylation of its cognate response regulator, BvgA (40, 41). Unlike many other sensor kinase proteins, which phosphorylate their cognate response regulator only when specific environmental signals are encountered, BvgS is active under most conditions and is down-modulated only in the presence of modulators, such as MgSO4 and nicotinic acid, or at reduced temperature (15, 26, 27, 28). When phosphorylated, the cytosolic BvgA-phosphate protein (BvgA∼P) binds to and activates various target promoters that together constitute a positively controlled BvgA regulon. Based on a number of studies suggesting that BvgA dimerizes, it is presumed that the active form of BvgA is a dimer of BvgA∼P (2, 6, 28). BvgA also regulates the expression of a distinct set of genes (the vrg's) in a manner inverse to that in which the expression of those described above is regulated. This is achieved indirectly through the activation of the gene encoding BvgA-regulated repressor protein BvgR (19, 20).
Earlier studies established that the temporal patterns of induction of the genes encoding pertussis toxin (ptx) and filamentous hemagglutinin (fha) are different, with fha being expressed earlier following a temperature shift than ptx or at higher MgSO4 concentrations under steady-state conditions (26, 27, 28, 33). These observations are consistent with a difference in the sensitivities of the two promoters to the concentration of BvgA∼P. We have previously provided evidence to support the notion that architectural features of the different promoters dictate the mechanisms by which BvgA∼P effects the differential activation of virulence genes (5, 7). At the fha (early) promoter, high-affinity binding is associated with inverted heptanucleotide imperfect repeats abutting one another and centered at −88.5 relative to the transcription start site that forms the primary binding site for BvgA (25). Downstream of this is a secondary region, whose occupancy by BvgA∼P is correlated with transcription activation (7). At the ptx (late) promoter, primary binding by BvgA∼P occurs in a region previously shown by deletion analysis to be required for activation (2, 5, 11, 12). This region includes two inverted heptad repeats, matching the fha heptads at only five of seven positions, which are separated by 10 bp and which are centered farther upstream of the transcription start site at −136.5. DNA binding analyses have suggested that BvgA∼P progressively oligomerizes along the DNA downstream of this primary binding site at higher concentrations, and occupancy of this secondary binding region is considered necessary for transcriptional activation (5, 30, 45). The requirement for a higher concentration of BvgA∼P to activate the ptx promoter can be attributed to a primary binding site of lower affinity together with the requirement for more BvgA∼P molecules bound cooperatively to its longer secondary binding region.
The purpose of this study is to examine our initial proposal of a secondary binding region identified in the fha promoter and to investigate in molecular detail the contribution of each of the sites to this promoter's activity. A cursory examination of the secondary region of BvgA∼P interaction (−81 to −42) reveals no clear match to the consensus BvgA recognition sequence as previously proposed (14, 25, 45). We therefore undertook a systematic characterization of fha promoter derivatives by DNA binding and by in vitro and in vivo transcription analyses. Using an extensive set of defined promoter substitutions and deletions, we have shown that both the primary binding site and the secondary binding region are necessary for full activation and that binding to these regions occurs cooperatively. Results obtained with deletion derivatives suggest that the secondary site is occupied by a single BvgA∼P dimer. Additionally, by examining pools of promoters containing random substitutions, we have shown that DNA sequence specificity plays only a limited role in mediating the BvgA∼P-DNA interaction in the secondary region. We discuss the implications that these observations have on fha expression and transcription activation by bacterial response regulators in general.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Escherichia coli DH5α, which was used as a transformation recipient for all cloning steps, was obtained from Bethesda Research Laboratories. In vivo analyses of promoter activity were performed with B. pertussis strain BP536 (36). Vector pTE103 and derivatives were used for in vitro transcription assays as previously described (7). Plasmid pfhaP was used for preparation of DNA fragments for footprinting, as previously described (7). Plasmid pSS2809 was used for in vivo transcription analyses (see below). E. coli strains were grown on L agar or in L broth (21) supplemented with antibiotics as appropriate. B. pertussis strains were grown on Bordet-Gengou agar (BG) (Difco) containing 1% proteose peptone (Difco) and 15% defibrinated sheep blood. Concentrations of antibiotics were 10 μg of gentamicin and 100 μg of streptomycin/ml.
Mutageneses.
Plasmid pTE-FHA consists of a BamHI-SalI PCR fragment containing the fha promoter from −155 to +36 cloned between the BamHI and SalI sites of transcription assay vector pTE103 (9). This plasmid was used as the template for introduction of mutations in the fha promoter. The SUB1, SUB2, and SUB3 substitutions were introduced by performing inverse PCR using oligonucleotide pairs 5′-GCGCTCGAGCAGTGTCAAACCATCAAACCCTGT- 3′ and 5′-GCGCTCGAGTAGCCAAGTCTTGTATAAATATCC-3′, 5′-CGCG ATATCTCAATTCGCGATTATTCGCGACTTGTAGGAAATTTCTTA-3′ and 5′-CGCGATATCGGATCAGGCCTGACTGACGAAGTGCTGAG-3′, and 5′-CGCGATATCACGGGTCGCGACGGTTCGCGATGTAGGAAATTTCTTAGTCA-3′ and 5′-CGCGATATCGCGCGAGGCCTCTGACTGACGAAGTGCTGAG-3′, respectively. The linear PCR products were then digested with XhoI (SUB1) or EcoRV (SUB2 and SUB3) and circularized by ligation. Deletions ΔN/N, ΔN/R, ΔN/S, and ΔR/S were introduced into the SUB2- and SUB3-substituted promoters by digestion with NruI, NruI and EcoRV, NruI and StuI, or EcoRV and StuI, respectively, followed by religation of the blunt ends. Other deletions were introduced essentially as previously described (31) through the use of inverse PCR using primers, one of which spanned the desired deletion junction and contained 5′ BsaI sites. Following PCR the linear plasmid products were digested with class IIS restriction enzyme BsaI to remove the terminal portions containing the BsaI recognition sites and create cohesive ends, which upon religation regenerate the original DNA sequence spanning the junction. Promoters with plastic-DNA substitutions, PD1 to PD15, were created in a similar manner except that a sequence substitution was incorporated into one of the oligonucleotide primers, with an equal proportion of each of the four nucleotides (N) used at each position within the substituted sequence. For example, to create PD-14, primers 5′-CGCGGTCTCTTTGACNNNNNNNNNNNNNNCAAGTCTTGTATAAATATCCATTGA-3′ and 5′-CGCGGTCTCGTCAAACCATCAAACCCTGTCCGGC-3′ were used (BsaI sites underlined). Following BsaI digestion, religation, and transformation of the inverse PCR product, all of the resulting colonies (103 to 104) were pooled prior to further growth and plasmid DNA isolation prior to in vitro transcription and binding studies.
Preparation of protein samples.
BvgA was purified as detailed elsewhere (7) except that an additional column-chromatographic step was introduced to enhance purity and specific activity. After passage through the Q-Sepharose column, the flowthrough fraction containing BvgA was loaded directly onto an 8-ml SP-Sepharose Fast Flow (Pharmacia) column preequilibrated with column buffer. After loading, the column was washed with column buffer until baseline elution was achieved, and BvgA was eluted with column buffer containing 50 mM KCl. This fraction was then processed as detailed in the original protocol. E. coli ς70-saturated RNA polymerase (RNAP) was purchased from Pharmacia.
In vitro transcription assays.
Single-round transcription assays using E. coli-derived RNAP and purified BvgA phosphorylated in vitro with acetyl phosphate were conducted as described previously (7). The concentration of each component that was varied is detailed in the figure legends. The intensities of bands corresponding to terminated transcripts were assessed using a PhosphorImager (Molecular Dynamics). Relative transcription activities are expressed as percentages of that of the control promoter after normalization to the activity of the BvgA-independent trc promoter. Transcription templates were substitution or deletion derivatives of pTE-FHA and were purified by the alkaline lysis method (3) followed by CsCl-ethidium bromide equilibrium density centrifugation.
Construction of pSS2809.
In vivo transcription assay vector pSS2809 was constructed as follows. Plasmid pSS1910 is composed of a 250-bp BamHI-HindIII fragment containing oriT of RP4 cloned into pBR322 (37). An EcoRI-HindIII fragment containing a gene specifying gentamicin resistance, derived from pSS1673 (34), was cloned between the EcoRI and HindIII sites of pSS1910 to create pSS2658. The EcoRI site was destroyed, and a NotI site was added, by the addition of linker 5′-AATTGCGGCCGC-3′ to create pSS2659. The lac operon fusion vector pRS551 (29) was modified by the addition of complementary oligonucleotides 5′-AATTGTCTAGAGAATTCACTAGTA-3′ and 5′-GATCTACTAGTGAATTCTCTAGAC-3′ between the EcoRI and BamHI sites, resulting in the destruction of those sites and the addition of SpeI, EcoRI, and XbaI sites, to create pSS2661. Oligonucleotide 5′-AGCTGCGGCCGC-3′ was added at the HindIII site upstream of the transcription terminators in pSS2661, resulting in the destruction of that site and the addition of a NotI site, to create pSS2662. Oligonucleotide 5′-TCGAACTCGAGGCGGCCGCCTCGAGT-3′ was added at the SalI site downstream of the lac operon in pSS2662, resulting in the destruction of that SalI site and the introduction of a NotI site flanked by XhoI sites, to create pSS2686. The NotI fragment of pSS2686 containing the entire lac operon and upstream terminators was cloned into the NotI site of pSS2659 to create pSS2687. The HindIII site of pSS2687 was destroyed by the addition of oligonucleotide 5′-AGCTAGTTTAAACT-3′, resulting in the addition of a PmeI site and the creation of pSS2688. Oligonucleotide 5′-GATCATTTAAAT-3′ was added to destroy the BamHI site of pSS2688 and introduce an SwaI site, resulting in pSS2689. Oligonucleotide 5′-AATTGTTAATTAAGAATTCGGATCCCGGGATCCGAATTCTTAATTAAC-3′ was added at the EcoRI site of pSS2689, destroying that site, and resulting in the addition of a BamHI site flanked by EcoRI sites which are flanked by PacI sites, to create pSS2690. An I-SceI site was added by inserting oligonucleotides 5′-CTAGATAGGGATAACAGGGTAATT-3′ and 5′-CTAGAATTACCCTGTTATCCCTAT-3′ into the XbaI site of pSS2690, resulting in pSS2691. The NotI site upstream of the lac operon in pSS2691 was destroyed by the addition of oligonucleotide 5′-GGCCGTTTAAAC-3′, resulting in the addition of a PmeI site and the creation of pSS2735. A small deletion was introduced by digestion with SalI and ligation with oligonucleotide 5′-TCGAGATTTAAATC-3′, which added an SwaI site and destroyed the SalI site, resulting in pSS2737. A random NotI-SalI chromosomal fragment from B. pertussis TohamaI was cloned between the NotI and XhoI sites downstream of the lac operon of pSS2737, resulting in the destruction of the XhoI site, to create pSS2806. A SalI site was added upstream of the lac operon by addition of oligonucleotide 5′-CTAGAGTCGACT-3′ at the SpeI site, destroying that site and resulting in the creation of pSS2809.
In vivo transcription assays.
Wild-type or mutant fha promoter derivatives were cloned from their corresponding pTE-FHA derivatives as an EcoRI-SalI fragment between the EcoRI and SalI sites of pSS2809. This vector provides for the directional cloning of promoters upstream of the lac operon, with multiple tandem transcription terminators upstream of these promoters to minimize transcriptional readthrough. The resulting pSS2809 derivatives were transferred by conjugation to B. pertussis strain BP536 (36) and inserted in single copy into the B. pertussis chromosome at a site distant from the fha locus by triparental mating using pSS1827 as a helper plasmid (34). Selection for exconjugants was on BG media containing gentamicin and streptomycin. The resulting strains were restreaked onto BG containing gentamicin only and grown for 3 days prior to harvest for β-galactosidase assays performed as previously described (33). For statistical analysis, β-galactosidase assays were performed three times on different days with separate cultures. In vivo analysis of plastic DNA promoter substitution pool PD13 was performed by pooling all colonies arising from cloning the EcoRI-SalI promoter fragment pool into pSS2809 and transferring them en masse by conjugation into BP536. Individual colonies were picked, restreaked on BG media containing gentamicin, and assayed for β-galactosidase activity after growth for 3 days.
DNase I footprinting.
End labeling, protein binding, and DNase I treatment were conducted using fragments derived from mutant or wild-type promoter fragments after cloning into the pBluescript KS+ vector (Stratagene) as described previously (7). For footprinting PD13, a pool of substitutions was created in pfhaP as described above.
RESULTS
Cooperative binding of BvgA∼P to the fha promoter.
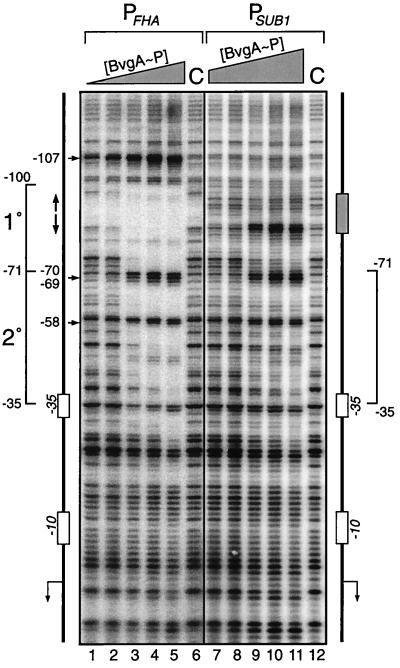
We have previously provided evidence for the binding of in vitro-phosphorylated BvgA (BvgA∼P) to a region just downstream of a primary binding site in the fha promoter (7). In a DNase I footprinting experiment, at low concentrations of BvgA∼P (200 nM) the observation of protection within the primary binding site, which encompasses the inverted heptanucleotide repeat centered at −88.5, and the appearance of a hypersensitive cleavage site at −107 indicate the interaction of BvgA∼P at this location (Fig. 1, lane 2) (7). It should be noted that there is very little DNase cleavage within the high-affinity binding region. In this regard the fha promoter is similar to the uhpT promoter, which binds and is activated by UhpA, a homologue of BvgA from E. coli (8). At higher concentrations (≥290 nM), an extended footprint is observed, up to −35, which represents a secondary region for binding by BvgA∼P. This secondary region contains no sequences obviously similar to the inverted heptads of the primary binding site but may contain a cryptic, low-affinity site(s) for BvgA∼P binding. We have previously observed that transcriptional activation of the fha promoter correlates with the binding of BvgA∼P to this region (7). To ascertain whether the secondary region alone is sufficient for BvgA∼P binding, we replaced the heptanucleotide repeat region within the primary binding site with an irrelevant DNA sequence (SUB1 in Fig. 2) and observed a reduction in binding to the secondary region by BvgA∼P (Fig. 1, right panel). Higher concentrations of BvgA∼P were required to show protection, and, even at the highest BvgA∼P concentration used (1.5 μM, lane 11), the protection seen was less complete than with the wild-type promoter. Both the pattern of nuclease protection and positions of nuclease-hypersensitive sites (at nucleotide positions −70, −69, and −58) strongly suggest that, within the secondary region of this mutant fha promoter (from −71 to −35), the manner in which BvgA∼P binds and induces DNA structural changes is similar to that observed with the wild-type promoter. The data also argue that the binding of BvgA∼P molecules along the fha promoter occurs cooperatively, as binding to the secondary region occurs more readily in the presence of an intact primary binding site.
FIG. 1.
DNase I footprinting analysis of the wild-type fha and SUB1 promoters. Binding reaction mixtures containing the wild-type promoter (lanes 1 to 6) or the SUB1 mutant promoter (lanes 7 to 12) were subjected to nuclease treatment in the absence of BvgA∼P (C) or increasing amounts of BvgA∼P (PFHA: 130, 200, 290, 440, and 660 nM; PSUB1: 290, 440, 660, 1,000, and 1,500 nM). Promoter DNA fragments used in the assay are graphically represented to the left (wild type) and right (SUB1). The regions of primary (1°) and secondary (2°) binding by BvgA∼P and the −10 and −35 RNAP recognition elements are shown. The upstream −35 element shown in Fig. 2 and 5 is indicated. Vertical arrows (wild-type promoter), BvgA primary binding site heptanucleotide repeat; shaded box (SUB1 promoter), absence of the heptanucleotide repeat; horizontal arrows and numbers, positions of major hypersensitive sites.
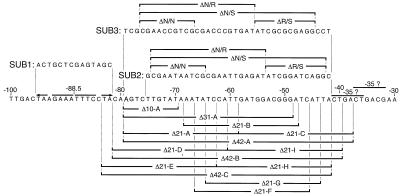
FIG. 2.
Summary of substitutions and deletions constructed in the fha promoter. The wild-type promoter spanning nucleotides −100 to −30 is shown along with the heptanucleotide repeat (arrows) and two potential −35 RNAP recognition elements. Each potential −35 region matches the TTGACA consensus sequence at (the same) 4 bp. The sequences of the SUB1, SUB2, and SUB3 substitutions and the segments deleted within each substitution are indicated (see text for details). The various 10-, 21-, 31-, and 42-bp deletions of the wild-type promoter are indicated below the wild-type sequence.
Variable activity of fha promoters with substitutions in the secondary binding region.
In order to assess the functional significance of secondary binding by BvgA∼P in vitro and in vivo, we created two promoters having substitutions with irrelevant DNA sequences within the secondary region (Fig. 2). SUB2 and SUB3 have 34- and 38-bp substitutions, respectively, that have a common 3′ limit just upstream of two potential −35 RNAP recognition elements. In order to determine the in vivo activities of these substituted promoters, promoter assay vector pSS2809 (Fig. 3) was constructed. This vector provides for the cloning of promoter fragments upstream of the lac operon, with multiple tandem transcription terminators upstream of the cloning sites to minimize transcriptional readthrough, and can be easily inserted in single copy in the B. pertussis chromosome at a site distant from the fha locus.
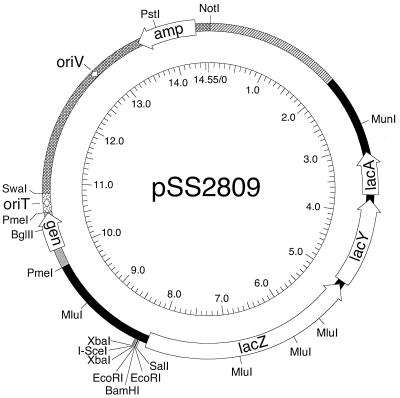
FIG. 3.
Schematic diagram of in vivo transcription assay vector pSS2809. Open arrows, open reading frames; arcs, intervening segments (pattern of fill indicates their derivation). Sources of segments (clockwise from NotI site) are as follows: B. pertussis chromosomal DNA (hatched), E. coli lac operon and upstream sequences from pRS551 (black) (29), pSK6 (gray) (32), RP4 (stippled) (43), and pBR322 (cross-hatched) (4). Diamonds, origin of conjugative transfer (oriT) and the vegetative origin of replication (oriV). The inner circular scale is in kilobases. Cleavage sites of relevant restriction enzymes are indicated.
As shown in Fig. 4, we observed that the substitution of the primary binding site (SUB1) resulted in reduction of β-galactosidase activity to levels comparable to the background level observed with no promoter. Surprisingly, although the two promoters with substitutions of the secondary region (SUB2 and SUB3) showed reduced promoter activity, the degree of reduction varied dramatically between the two. The SUB2 and SUB3 fha promoters were also examined for their BvgA∼P-dependent activities in an in vitro transcription assay (Fig. 5, left). Transcriptional activation of the fha promoter in this assay was clearly dependent on the presence of BvgA phosphorylated in vitro with acetyl phosphate (lanes 1 and 2). Activities of SUB1 (lane 13; 5% of wild type) and SUB2 (lane 3; 15% of wild type) were quite low, whereas the SUB3 promoter displayed significant in vitro activity (lane 8; 70% of wild type). While these results were in agreement with the data derived from the in vivo assays the reason for the difference in activity between the SUB2 and SUB3 promoters remained unclear.
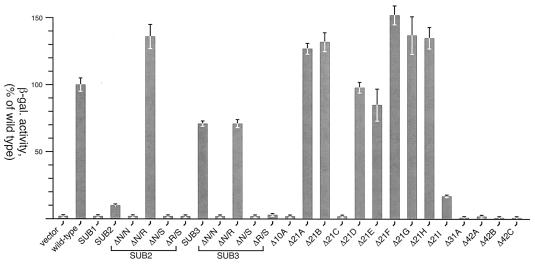
FIG. 4.
In vivo transcription assays. The levels of transcriptional activity are presented as percentages of wild-type β-galactosidase (β-gal.) activity and are the means of three independent assays. Error bars, standard deviations.
FIG. 5.
In vitro transcription analysis of mutant fha promoters. A single round of transcription was initiated in reaction mixtures containing 20 nM template plasmid, purified E. coli RNAP, and 440 nM BvgA∼P. Samples were electrophoresed on a 6% polyacrylamide sequencing gel alongside an RNA sizing ladder (not shown). Arrows, transcripts arising from the fha promoter and the trc control promoter. (Left) Transcription analysis of fha promoter substitutions; (right) a separate experiment analyzing the transcription activities of fha promoters with deletions. Although not shown here, basal transcription seen in the absence of BvgA∼P was determined for each promoter and was found to be comparable to that seen for the wild-type (wt) promoter shown in lane 1.
Deletion of 21 bp restores activity of substituted promoters.
The previous section provided evidence to suggest that binding of BvgA∼P to the secondary region is stabilized by high-affinity binding of an upstream BvgA∼P dimer. This model predicts that it may be possible to construct a strong, artificial fha promoter in which activation is dependent on a single BvgA∼P dimer bound to the primary binding site but positioned more closely upstream of the RNAP binding site. We therefore assessed the activity of modified SUB2 and SUB3 promoters which carry deletions within the secondary region (Fig. 2). These deletions were engineered so as to result in the deletion of one, two, or three full turns of the DNA helix (assuming 10.5 bp per turn of B DNA), in order to maintain the face-of-helix orientation between the upstream BvgA∼P dimer and RNAP. When tested in vivo, only those promoters containing a 21-bp deletion were active (Fig. 4). Deletion of 21 bp in SUB2 (SUB2-ΔN/R) resulted in the reacquisition of a promoter activity of 135% that of the wild type. In the SUB3 derivative (SUB3-ΔN/R), deletion of 21 bp resulted in no change in promoter activity (70% of wild-type activity). Results of in vitro transcription analyses qualitatively confirmed the in vivo data. The SUB2-ΔN/R promoter displayed 189% of wild-type activity (Fig. 5, lane 7), while the SUB3-ΔN/R promoters revealed 134% of wild-type activity (lane 12). All other deletions in both substituted-promoter backgrounds resulted in no significant activity.
Deletions of 21 bp within the secondary binding region of the wild-type promoter result in hyperactivation.
The 21-bp deletions that result in active promoters were constructed from the mutant SUB2 and SUB3 promoters and, although of similar size, are not located at the same site relative to the start of fha transcription and are in the context of a large substitution. In order to further validate the assertion of a 21-bp “unit” for binding by BvgA∼P dimers, we examined a more comprehensive set of deletions in the context of the wild-type fha promoter. A series of promoter derivatives which was composed primarily of promoters with 21-bp deletions clustered within and slightly beyond the secondary binding region (−42 to −81) was constructed (Fig. 2). Additionally, promoters with 10-, 31-, and 42-bp deletions were created. All deletions were tested in both in vivo and in vitro assays, which were in close agreement (Fig. 4 and 5, right). Deletions of 10, 31, and 42 bp were highly detrimental to promoter activity. However, consistent with the results obtained for the deletions of the SUB2 and SUB3 promoters, the promoters carrying 21-bp deletions were functional. Both assays revealed elevated transcription levels for most of the promoters with 21-bp deletions relative to that observed for the wild-type promoter (from 125 to 155% in vivo and greater than 200% in vitro). Only the in vivo data from the Δ21D and Δ21E deletions revealed levels equal to or slightly less than the wild-type level (99 and 85%, respectively). Interestingly, these two deletions impinge on the primary binding site, resulting in minor changes to the farthest-right 1 or 2 bp (Fig. 2). On the basis of a systematic mutational analysis of primary binding site mutations (unpublished data), these changes are not expected to drastically affect BvgA∼P binding to this site. The same is true of the Δ42C deletion. Therefore, the complete lack of activity observed in the Δ42C deletion derivative is apparently due to the amount of DNA deleted and not to any more-subtle effects on BvgA binding to the primary binding site. Deletions, regardless of size, that extend beyond the boundary defined by the furthest-upstream potential −35 RNAP recognition element (i.e., downstream from position −42) yield promoters that are defective (Δ21C, Δ21I, Δ42A, and Δ42B). Presumably, the reduced activity of Δ21C and Δ21I results from alteration of this sequence, which suggests that this may be the functional −35 region of the fha promoter.
Use of randomly substituted promoter pools to assess sequence specificity of secondary binding by BvgA∼P.
The discrepancies noted earlier between results for the SUB2 and SUB3 deletions show the inherent limitations of using defined substitutions to assess the contribution of specific DNA segments to promoter activity. That SUB3 retained a significant amount of activity in vivo and in vitro suggests that there may be little sequence specificity to the binding of BvgA∼P in this region. The absence of any discernible BvgA binding site in the secondary binding region of the wild-type promoter supports this possibility. On the other hand, the diminished activity of SUB2, which has a similar substitution, suggests that there may indeed be some sequence specificity within this secondary region that mediates the binding of an additional BvgA∼P dimer. In order to address this apparent incongruence, we searched for an appropriate method of removing sequence information from certain segments of promoter DNA without replacing them with any other specific DNA sequence. To this end, we developed a means for introducing random substitutions within defined segments. When several thousand of these substitutions are pooled and examined en masse, the net effect is of examining a promoter which has no specific DNA sequence within the substituted region. We refer to these substitutions as plastic-DNA substitutions.
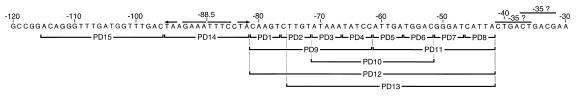
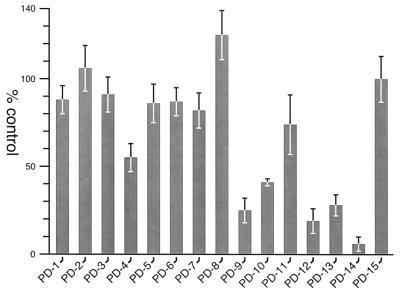
Using this technique, we created two plastic-DNA substitutions to serve as control templates in in vitro transcription assays (Fig. 6). Substitution of the heptanucleotide repeats within the primary binding site (PD14) and substitution of 20 bp immediately upstream of these repeats (PD15) served as negative and positive controls, respectively. Additionally, a series of eight 5-bp plastic-DNA substitutions within the secondary region between positions −41 and −82 was constructed. Plasmid DNA was purified from pools of bacterial transformants, and in vitro transcription analyses were conducted. As depicted in Fig. 7, in vitro analyses revealed that all but PD4 (55%) retained at least 82% of the activity of PD15. To assess the effects of larger substitutions within the secondary region, three 20-bp (PD9, PD10 and PD11) substitutions, a 40-bp (PD12) substitution, and a 34-bp (PD13) plastic DNA substitution were constructed (Fig. 6). The effect of the 20-bp substitutions was greater, although all three resulted in the maintenance of a significant level of transcriptional activity. The activities of the two that included the region affected by the PD4 substitution were the most reduced (25% of the remaining activity for PD9 and 41% for PD10). Surprisingly, promoters with substitutions of the entire secondary region still displayed a significant level of activity (19% for PD12 and 28% for PD13).
FIG. 6.
Summary of plastic DNA substitutions made at the fha promoter. The upstream sequence of the wild-type fha promoter (−30 to −120) is presented. As in Fig. 2, the heptanucleotide repeats and potential −35 elements are shown. The limits of the plastic DNA substitutions are indicated below the sequence.
FIG. 7.
Summary of in vitro transcription analyses of plastic DNA substitutions in the fha promoter. Single-round transcription assays were conducted as detailed in Materials and Methods, and samples were run on sequencing gels. The relative amounts of radiolabeled transcripts (normalized to the level of transcription from the BvgA-independent trc promoter) are presented as percentages of the PD15 positive control. Each plastic DNA pool was created twice independently, and the transcription assays were performed twice on each pool. The means and standard deviations from these four independent assays for each substitution are shown. Although not shown here, control experiments revealed the lack of measurable transcriptional activity in the absence of BvgA∼P.
These values for the composite activity of randomly substituted promoter pools give no information on how this activity is distributed among individual substituted sequences. To assess this more fully, the EcoRI-SalI promoter fragment population from the PD13 preparation was cloned into pSS2809 and returned en masse to the B. pertussis chromosome. From the resulting population of B. pertussis colonies, 200 were picked and β-galactosidase assays were performed. The distribution of their activities is shown in Fig. 8. Although these promoter activities ranged from 0 to 150%, the majority of these random substitutions were inactive, resulting in activities less than 15% that of the wild type. However, 20% of the promoters had activities greater than or equal to 50% of wild-type activity, and 10% had activities greater than or equal to 75% of wild-type activity. The average activity seen in this analysis was 27% that of the wild type, which agrees well with the in vitro value of 28%. Taken together with the in vitro transcription assays, these in vivo assays of randomly substituted promoters suggest that although binding by BvgA∼P to the secondary region correlates with fha promoter activity, significant activity can be observed even in the absence of extensive sequence-specific interaction. To further assess the possible contributions of DNA sequence to the activities of these randomly substituted PD13 variants, 16 having activities greater than or equal to that of the wild-type promoter were sequenced. The results failed to give any discernible indication of similarities in DNA sequence which could explain their activities (data not shown).
FIG. 8.
Distribution of in vivo promoter activities of individual sequences derived from the promoter pool with PD13 substitutions. The different ranges of activity, relative to that of the fha wild-type promoter, are presented as percentages of the 200 colonies so examined.
Protein-protein interactions permit sequence-independent secondary binding.
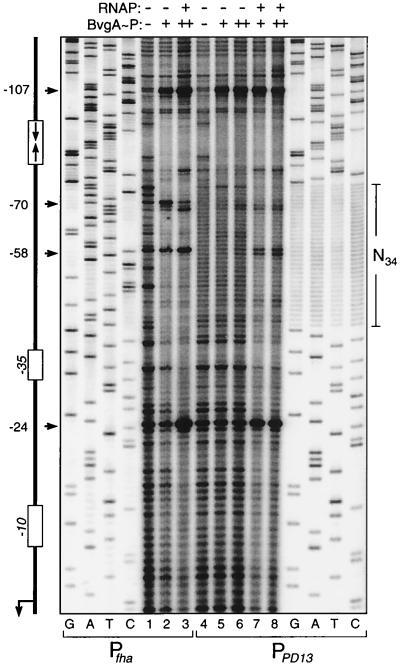
To examine the binding activity of BvgA∼P to a plastic-DNA-substituted fha promoter, the PD13 34-bp substitution was recreated in a context that allowed 32P end labeling of a fragment pool containing this substitution. The resulting PD13 pool and the wild-type fha promoter were subjected to DNase I footprinting analysis in the presence or absence of E. coli RNAP (Fig. 9). Our previous studies on the fha promoter support the use of E. coli-derived RNAP, as this enzyme and that derived from Bordetella spp. behave almost identically in in vitro DNA binding and transcription analyses (7). Products of sequencing reactions with the same DNA fragments were loaded alongside the footprinting samples, and results confirmed that all four possible nucleotides are equally represented at every position within the N34-substituted region. In the absence of RNAP, BvgA∼P protects a region just upstream of the plastic-DNA substitution in PD13 encompassing the heptanucleotide repeats and bounded by positions −100 and −75 (lanes 5 and 6). There is only a suggestion of binding to the substituted N34 region in the absence of RNAP, although the wild-type promoter shows strong protection of the secondary binding region. However, in the presence of RNAP, we noticed an enhanced protection of both the N34 region of plastic DNA and regions further downstream encompassing the RNAP binding site (lanes 7 and 8). Furthermore, the characteristic hypersensitive sites indicative of binding by both BvgA∼P and RNAP to the wild-type secondary region and beyond (at nucleotide positions −24, −58, and −70) were also observed in the PD13 promoter (compare lane 3 to lanes 7 and 8). There is no evidence of binding by RNAP alone (data not shown). From these data, we suggest that BvgA∼P is capable of binding to a secondary region within the fha promoter that contains no defined DNA sequence and that this binding is sufficient to mediate the cooperative and functional recruitment of RNAP.
FIG. 9.
DNase I footprinting analysis of the PD13 plastic-DNA substitution in the fha promoter. Binding reaction mixtures contained DNA fragments from either the fha (lanes 1 to 3) or PD13 (lanes 4 to 8) promoters. Samples contained BvgA∼P at 418 (lanes 2, 5, and 7) or 627 nM (lanes 3, 6, and 8) and E. coli RNAP at 150 nM (lanes 3, 7, and 8). Samples containing no added protein were loaded in lanes 1 and 4. The DNA fragment used is graphically represented on the left. Horizontal arrows, nucleotide positions of the DNase I-hypersensitive sites. Products of Sanger dideoxynucleotide sequencing reactions for each promoter are included in the gel for comparison. Primers for these sequencing reactions were chosen so that the 5′ ends of the labeled fragments would correspond to those in the footprinting reactions. The stretch of 34 N nucleotide residues (N34) in the substituted promoter is highlighted on the right.
DISCUSSION
Positive control of bacterial transcription is often effected by multiple molecules of a single activator protein. This type of regulation may have evolved to provide additional subtleties to the means by which a single protein species may control transcription initiation. For example, we have previously shown how multiple molecules of the BvgA activator may exert differential concentration-dependent control of two B. pertussis virulence factor genes (5, 7). At the ptx promoter, the phosphorylated form of BvgA (BvgA∼P) appears to initially bind to a high-affinity site far upstream of the transcription start site. At higher concentrations additional molecules (possibly three additional BvgA∼P dimers) progressively oligomerize downstream towards the RNAP binding site. This secondary binding may be mediated by multiple low-affinity degenerate binding sites and is presumed to involve cooperative interactions between the BvgA∼P dimers. Our present study supports our initial observations which suggested that activation at the fha promoter also involves an initial interaction at a high-affinity primary binding site and is followed by the cooperative binding of a single, additional dimer to a downstream low-affinity site. However, the fha promoter has a lower requirement for the concentration of BvgA∼P necessary for full activation than does the ptx promoter (7). Differences in the affinities of the primary binding sites and differences in the numbers and affinities of secondary binding sites would be expected to contribute to this means by which activation of an early promoter (fha) can be differentiated from that of a late promoter (ptx) by the level of intracellular BvgA∼P.
Several response regulators have been shown to interact with more than one site at cognate promoters. The OmpR response regulator binds to four sites in the ompF promoter and to three sites at the micF-ompC divergently transcribed promoters (13, 17, 23), while NarL binds to eight sites in the narG promoter (16, 42). Perhaps the system that most resembles the BvgA-regulated fha promoter is the UhpA-regulated uhpT promoter in E. coli. Interestingly, BvgA, UhpA, and NarL belong to the same subfamily of response regulators based on sequence similarity within their C-terminal output domains (22). Similar to BvgA, UhpA is able to bind to an upstream high-affinity site (centered at −64) in the unphosphorylated form, and phosphorylation appears to induce the binding to a lower-affinity downstream site (extending up to −32) (8).
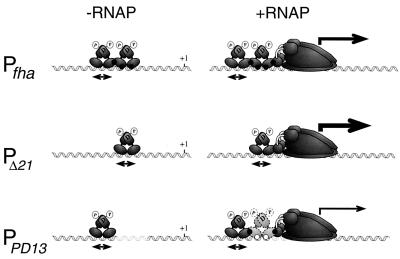
Based on the observations made from our deletion and substitution analyses using defined sequences, a simple model serves to illustrate the interplay of the different intermolecular interactions involved in fha promoter activation (Fig. 10, Pfha). The binding of a BvgA dimer to the primary high-affinity site in the wild-type promoter involves interaction with a consensus heptanucleotide repeat. Phosphorylation increases the affinity of BvgA for this site and also induces the cooperative binding of a second dimer to a site just downstream, a site from which the promoter-proximal dimer may recruit RNAP through a protein-protein interaction mediated, at least in part, by the C-terminal domain of the α subunit of RNAP (7). This simplified model predicts that the level of transcription activation might be augmented if the requirement for secondary binding were eliminated. Indeed, in our in vivo and in vitro transcription studies, we noticed that fha promoter derivatives with 21 bp deleted from the secondary region are hyperactivated as illustrated in Fig. 10 for PΔ21. In fact, among all of the promoters with deletions from the wild-type sequence examined, only those with two helical turns deleted maintained any transcriptional activity. A similar two-helical-turn dependence of ptx promoter activity on deletion size has been previously observed (18). Taken together with the accumulated in vitro and in vivo data that BvgA dimerizes (2, 6, 28), this implies that a dimer of BvgA∼P binds every 21 bp in situations where BvgA∼P oligomerizes along a DNA sequence. In this context, the complete lack of activity seen in promoters with 42-bp deletions suggests that only one dimer of BvgA∼P is bound in the secondary region. Indeed, deletion of 42 bp necessitates the overlap of the putative −35 region with the primary binding site of BvgA∼P, presumably prohibiting productive interaction with RNAP due to steric hindrance.
FIG. 10.
Model of BvgA∼P activity at fha and derivative promoters. The stoichiometries of binding by BvgA∼P in the absence and presence of RNAP are shown for three different promoters. Lightly shaded dimer, weaker BvgA∼P-DNA interaction made by the promoter-proximal dimer tethered by the neighboring BvgA∼P dimer and RNAP. Relative transcriptional activities are represented by thicknesses of arrows. Black dots, protein-protein or protein-DNA interactions involved in stabilizing BvgA binding in the secondary region (see text for details); white circles (PPD13), non-sequence-specific interaction of BvgA with DNA.
A cursory examination of the secondary region in the fha promoter reveals no sequence similar to the consensus recognition site as previously proposed (14, 45). In a more systematic attempt at identifying a secondary binding site, we utilized information derived from studies directed at identifying a true consensus sequence by examining the effects of a comprehensive set of point mutations within the heptanucleotide repeats of the primary binding site, and combinations thereof, on DNA binding and transcriptional activity (unpublished data). Using a simple algorithm based on the results of these studies, we found no sequence within the secondary region that would be predicted to be functional in binding BvgA∼P in the absence of other stabilizing protein-protein interactions. Thus, unlike many other response regulators, BvgA, it appears, binds to DNA lacking any apparent recognition site within the secondary binding region.
In an attempt to address experimentally the existence of sequences which might contribute to the cooperative binding of a second dimer of BvgA∼P, we systematically substituted different regions within the secondary region. In order to surmount the inherent biases and other limitations of using substitutions of the defined sequence, we opted to employ pools of DNA carrying randomized sequences within the region of the substitutions in in vitro transcription assays. Surprisingly, all but one, the PD4 substitution, of the eight 5-bp plastic-DNA substitutions so analyzed resulted in nearly fully active promoters, and even PD4 still retained significant activity (55%). Of the three promoters with 20-bp substitutions examined, PD9 and PD10, whose substitutions overlap the PD4 substitution, were the most affected, retaining 25 and 41%, respectively, of wild-type activity while PD11, whose substitution, does not overlap the PD4 substitution, retained 74% of the activity. Promoters with the 34- (PD13) and 40-bp (PD12) substitutions of the entire secondary region displayed a reduced, though significant, level of activity (28 and 19%, respectively). Taken together, these results suggest that what minimal sequence specificity may exist resides in the region of the PD4 substitution and upstream.
When the distribution of promoter activities within the pool with the PD13 substitution was examined, it was found that, while the majority of random sequence substitutions in the secondary region resulted in little or no promoter activity, approximately 10% of these substitutions resulted in nearly wild-type activity (75% or greater). The laws of probability dictate that the sequences of promoters with these active substitutions have little similarity to the wild-type sequence, a prediction upheld by the DNA sequence analysis of 16 of the most active variants arising within this pool of substituted promoters. This observation therefore supports the conclusion that, within the secondary binding region, DNA sequence requirements for promoter activity are very limited. Perhaps a better question would be why many of the substituted sequences are inactive. One possible explanation is that, although the requirements are minimal, these sequences lack the one or two specific nucleotides required at critical positions. Although these nucleotides were not identified in our sequence analysis of active PD13 variants, their presence could be obscured by functional redundancy (i.e., perhaps only one or two of several critical nucleotides need to be present). An alternative explanation is that individual substituted sequences which destroy promoter activity do so by imposing sequence-mandated structural constraints or conformations on the DNA in this region which result in an inability of BvgA∼P to interact successfully. Further experimentation is required to distinguish between these two possibilities.
While these data suggest that requirements for a specific sequence for functional interaction of BvgA∼P in the secondary binding region are limited, some specificity apparently resides in the wild-type sequence. This is evidenced by the DNAse I footprint analysis of BvgA∼P binding to the SUB1 promoter, which demonstrates binding to the secondary region even in the absence of the primary binding site, although only at higher BvgA∼P concentrations and to a lesser degree. It is also evident that the random substitutions present in the PD13 pool result in a reduction of BvgA∼P binding in the secondary region (Fig. 9, left). However, the majority of these substitutions do allow the binding of BvgA∼P to the secondary region in the presence of RNAP, as evidenced by the observation that the DNA footprint of PD13 in the presence of RNAP resembles that of the wild-type promoter in the secondary region. That a BvgA∼P dimer can bind to this region, which contains no specific DNA sequence, albeit in the presence of RNAP, is surprising. However, we have provided evidence that the association of the two BvgA∼P dimers on the fha promoter occurs cooperatively, suggesting that the two physically interact. Coupled with our earlier observation that BvgA contacts the α-CTD of RNAP (7), this leads us to propose that the promoter-proximal BvgA∼P dimer is “tethered” to the secondary region of PD13 DNA by specific protein-protein interactions with the promoter-distal BvgA∼P dimer and the downstream RNAP (Fig. 10).
Many promoters regulated by response regulator proteins of the BvgA family apparently involve occupation of low-affinity binding sites as a result of cooperative interactions with additional molecules bound upstream at higher-affinity sites. Such an arrangement has been described for the ComA regulator of Bacillus subtilis (24) and the FixJ activator of Rhizobium meliloti (10). Olekhnovich and Kadner (21a) have suggested that the role of the more loosely bound downstream regulator molecule, at least for UhpA, is to provide a greater responsiveness to environmental changes as represented intracellularly by changes in the state of the sensor kinase protein. By being more loosely bound to this site regulator molecules may have greater freedom to diffuse to the membrane-bound sensor kinase and have their activation states readjusted to suit the prevailing conditions. In such a scenario, the role of the promoter-distal BvgA∼P dimer bound to the primary site would be to assist the binding of the promoter-proximal dimer to the low-affinity downstream secondary site thus allowing it to recruit RNAP to initiate transcription. Recent observations in our laboratory suggest that BvgA∼P bound to the primary binding site of the fha promoter may play a more active role. We have observed that, although the fha promoter derivative in which the primary binding site has been destroyed (SUB1) is inactive, in vitro binding assays suggest that it is capable of recruiting RNAP to the promoter at levels approaching that of the wild-type promoter. This ternary initiation complex appears to be inactive primarily because it fails to isomerize to the open complex (unpublished data). Therefore, in the context of the wild-type fha promoter, the BvgA∼P dimer bound to the primary binding site may directly or indirectly exert an effect on a step(s) in transcription initiation subsequent to RNAP binding. Further experimentation is required to address this intriguing possibility.
ACKNOWLEDGMENTS
We thank Judith Kassis, Tod Merkel, and Michael Schmitt for critical reading of the manuscript.
Deanna M. Schmidt was supported under the Student and Teacher Program of the Howard Hughes Medical Institute together with Montgomery County Public Schools.
REFERENCES
- 1.Arico B, Miller J F, Roy C R, Stibitz S, Monack D M, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci USA. 1989;86:6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beier D, Schwarz B, Fuchs T M, Gross R. In vivo characterization of the unorthodox BvgS two-component sensor protein of Bordetella pertussis. J Mol Biol. 1995;248:596–610. doi: 10.1006/jmbi.1995.0245. [DOI] [PubMed] [Google Scholar]
- 3.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 5.Boucher P E, Stibitz S. Synergistic binding of RNA polymerase and BvgA-phosphate to the pertussis toxin promoter of Bordetella pertussis. J Bacteriol. 1995;177:6486–6491. doi: 10.1128/jb.177.22.6486-6491.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher P E, Menozzi F D, Locht C. The modular architecture of bacterial response regulators. J Mol Biol. 1994;241:363–377. doi: 10.1006/jmbi.1994.1513. [DOI] [PubMed] [Google Scholar]
- 7.Boucher P E, Murakami K, Ishihama A, Stibitz S. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J Bacteriol. 1997;179:1755–1763. doi: 10.1128/jb.179.5.1755-1763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahl J L, Wei B-Y, Kadner R J. Protein phosphorylation affects binding of the Escherichia coli transcription activator UhpA to the uhpT promoter. J Biol Chem. 1997;272:1910–1919. doi: 10.1074/jbc.272.3.1910. [DOI] [PubMed] [Google Scholar]
- 9.Elliott T, Geiduscheck E P. Defining a bacteriophage T4 late promoter: absence of a “−35” region. Cell. 1984;36:211–219. doi: 10.1016/0092-8674(84)90091-6. [DOI] [PubMed] [Google Scholar]
- 10.Galinier A, Garnerone A M, Reyrat J M, Kahn D, Batut J, Boistard P. Phosphorylation of the Rhizobium meliloti FixJ protein induces its binding to a compound regulatory region at the fixK promoter. J Biol Chem. 1994;269:23784–23789. [PubMed] [Google Scholar]
- 11.Gross R, Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci USA. 1988;85:3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross R, Carbonetti N H, Rossi R, Rappouli R. Functional analysis of the pertussis toxin promoter. Res Microbiol. 1992;143:671–681. doi: 10.1016/0923-2508(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 13.Huang K J, Schieberl J L, Igo M M. A distant upstream site involved in the negative regulation of the Escherichia coli ompF gene. J Bacteriol. 1994;176:1309–1315. doi: 10.1128/jb.176.5.1309-1315.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karimova G, Ullmann A. Characterization of DNA binding sites for the BvgA protein of Bordetella pertussis. J Bacteriol. 1997;179:3790–3792. doi: 10.1128/jb.179.11.3790-3792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacey B W. Antigenic modulation of Bordetella pertussis. J Hyg. 1960;31:423–434. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S F, DeMoss J A. Location of sequences in the nar promoter of Escherichia coli required for regulation by Fnr and NarL. J Biol Chem. 1988;263:13700–13705. [PubMed] [Google Scholar]
- 17.Maeda S, Mizuno T. Evidence for multiple OmpR-binding sites in the upstream activation sequence of the ompC promoter in Escherichia coli: a single OmpR-binding site is capable of activating the promoter. J Bacteriol. 1990;172:501–503. doi: 10.1128/jb.172.1.501-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques R R, Carbonetti N H. Genetic analysis of pertussis toxin promoter activation in Bordetella pertussis. Mol Microbiol. 1997;24:1215–1224. doi: 10.1046/j.1365-2958.1997.4371792.x. [DOI] [PubMed] [Google Scholar]
- 19.Merkel T J, Stibitz S. Identification of a locus required for the regulation of bvg-repressed genes in Bordetella pertussis. J Bacteriol. 1995;177:2727–2736. doi: 10.1128/jb.177.10.2727-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merkel T J, Barros C, Stibitz S. Characterization of the bvgR locus of Bordetella pertussis. J Bacteriol. 1998;180:1682–1690. doi: 10.1128/jb.180.7.1682-1690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 21a.Olekhnovich I N, Kadner R J. RNA polymerase α and ς70 subunits participate in transcription of the Escherichia coli uhpT promoter. J Bacteriol. 1999;181:7266–7273. doi: 10.1128/jb.181.23.7266-7273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson J S, Kofoid E C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 23.Rampersaud A, Harlocker S L, Inouye M. The OmpR protein of Escherichia coli binds to sites in the ompF promoter region in a hierarchical manner determined by its degree of phosphorylation. J Biol Chem. 1994;269:12559–12566. [PubMed] [Google Scholar]
- 24.Roggiani M, Dubnau D. ComA, a phosphorylated response regulator protein of Bacillus subtilis, binds to the promoter region of srfA. J Bacteriol. 1993;175:3182–3187. doi: 10.1128/jb.175.10.3182-3187.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy C R, Falkow S. Identification of Bordetella pertussis regulatory sequences required for transcriptional activation of the bvgAS operon. J Bacteriol. 1991;173:2385–2392. doi: 10.1128/jb.173.7.2385-2392.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarlato V, Rappuoli R. Differential response of the bvg virulence regulon of Bordetella pertussis to MgSO4 modulation. J Bacteriol. 1991;173:7401–7404. doi: 10.1128/jb.173.22.7401-7404.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarlato V, Arico B, Prugnola A, Rappuoli R. Sequential activation and environmental regulation of virulence genes in Bordetella pertussis. EMBO J. 1991;10:3971–3975. doi: 10.1002/j.1460-2075.1991.tb04967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scarlato V, Prugnola A, Arico B, Rappuoli R. Positive transcriptional feedback at the bvg locus controls the expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci USA. 1990;87:6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 30.Steffen P, Goyard S, Ullmann A. Phosphorylated BvgA is sufficient for transcriptional activation of virulence-regulated genes in Bordetella pertussis. EMBO J. 1996;15:102–109. [PMC free article] [PubMed] [Google Scholar]
- 31.Stemmer W P, Morris S K. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis. BioTechniques. 1992;13:214–220. [PubMed] [Google Scholar]
- 32.Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol. 1994;235:458–465. doi: 10.1016/0076-6879(94)35161-9. [DOI] [PubMed] [Google Scholar]
- 33.Stibitz S. Mutations affecting the α subunit of Bordetella pertussis RNA polymerase suppress growth inhibition conferred by short C-terminal deletions of the response regulator BvgA. J Bacteriol. 1998;180:2484–2492. doi: 10.1128/jb.180.9.2484-2492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stibitz S, Carbonetti N H. Hfr mapping of mutations in Bordetella pertussis that define a genetic locus involved in virulence gene regulation. J Bacteriol. 1994;176:7260–7266. doi: 10.1128/jb.176.23.7260-7266.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stibitz S, Miller J F. Coordinate regulation of virulence in Bordetella pertussis mediated by the vir (bvg) locus. In: Miller V L, Kaper J B, Portnoy D A, Isberg R I, editors. Molecular genetics of bacterial pathogenesis. Washington, D.C.: American Society for Microbiology; 1994. pp. 407–422. [Google Scholar]
- 36.Stibitz S, Yang M-S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4286. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stibitz S, Yang M-S. Genomic fluidity of Bordetella pertussis assessed by a new method for chromosomal mapping. J Bacteriol. 1997;179:5820–5826. doi: 10.1128/jb.179.18.5820-5826.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 39.Uhl M A, Miller J F. Autophosphorylation and phosphotransfer in the Bordetella pertussis BvgAS signal transduction cascade. Proc Natl Acad Sci USA. 1994;91:1163–1167. doi: 10.1073/pnas.91.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhl M A, Miller J F. Central role of the BvgS receiver as a phosphorylated intermediate in a complex two-component phosphorelay. J Biol Chem. 1996;271:33176–33180. doi: 10.1074/jbc.271.52.33176. [DOI] [PubMed] [Google Scholar]
- 41.Uhl M A, Miller J F. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 1996;15:1028–1036. [PMC free article] [PubMed] [Google Scholar]
- 42.Walker M S, DeMoss J A. NarL-phosphate must bind to multiple upstream sites to activate transcription from the narG promoter of Escherichia coli. Mol Microbiol. 1994;14:633–641. doi: 10.1111/j.1365-2958.1994.tb01302.x. [DOI] [PubMed] [Google Scholar]
- 43.Waters V L, Hirata K H, Pansegrau W, Lanka E, Guiney D G. Sequence identity in the nick regions of IncP plasmid transfer origins and T-DNA borders of Agrobacterium Ti plasmids. Proc Natl Acad Sci USA. 1991;88:1456–1460. doi: 10.1073/pnas.88.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss A A, Hewlett E L, Myers G A, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42:33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zu T, Manetti R, Rappouli R, Scarlato V. Differential binding of BvgA to two classes of virulence genes of Bordetella pertussis directs promoter selectivity by RNA polymerase. Mol Microbiol. 1996;21:557–565. doi: 10.1111/j.1365-2958.1996.tb02564.x. [DOI] [PubMed] [Google Scholar]