Abstract
Background
To evaluate changes in the vessel density (VD) of the optic nerve head (ONH) microvasculature in thyroid eye disease (TED) using optical coherence tomography angiography (OCTA). This study aimed to applicate the OCTA as a noninvasive modality in screening TED patients to assess sub-clinical changes.
Methods
In a cross-sectional study, the control group patients were healthy individuals with no ocular abnormalities and were euthyroid. All patients with TED had clinical features of the disease. We divided them into two groups using the clinical activity score (CAS). Patients with CAS scores 0–2 were categorized as group A, and scores three or more as group B. All vessels (AV) and small vessels (SV) VD inside disc and radial peripapillary capillary network were measured using the ONH-OCTA.
Results
We evaluated 29 patients with TED and 28 healthy controls. The mean whole image AV VD (mean ± SD: 56.33 ± 2.56, p-value = 0.17) and the mean whole image SV VD (mean ± SD: 49.94 ± 2.56, p-value = 0.16) in the TED group had no statically significant difference compared with the control group (AV mean ± SD: 57.20 ± 20.22, SV mean ± SD: 50.84 ± 2.23). We found a non-significant decrease in AV and SV radial peripapillary capillary VD in the TED group. There was a significant decrease in the mean whole image AV VD (mean ± SD: 54.83 ± 3.07, p-value = 0.005) and the mean whole image SV VD (mean ± SD: 48.60 ± 3.18, p-value = 0.013) in CAS group B compared to group A (AV mean ± SD: 57.45 ± 1.33, SV mean ± SD: 50.95 ± 1.37).
Conclusion
Our study showed non-significant ONH vascular alterations in patients with TED, including reduced VD of ONH in the radial peripapillary capillary. Patients with higher CAS scores had a more noticeable decrease in ONH microvasculature.
Keywords: Thyroid eye disease, Optical coherence tomography angiography, Optic nerve head, Radial peripapillary capillary network
Background
Thyroid eye disease (TED) is one of the main extra-thyroidal manifestations of Graves' disease [1]. Although it often develops in patients with hyperthyroidism, it can also occur in association with euthyroidism or hypothyroidism [2]. Subclinical eye involvement in Graves' disease is prevalent, and half of Graves' disease patients present with the spectrum of TED. In these patients, orbital fibroblasts and adipocytes are targeted by auto-immune reactions. It leads to edema and inflammation of extraocular muscles and increases physical pressure on the orbital connective tissue and fat [3]. Changes in TED are usually documented in the orbital and periocular tissues, and the activity of the disease is usually characterized by clinical findings [3]. Optic nerve compression is the result of increased pressure in the orbital cavity. It leads to ischemia and nerve damage in the affected eye [4]. However, in some cases, optic nerve involvement occurs without prominent orbital involvement [4].
The retinal microvascular network was previously evaluated using fluorescein angiography. It is an invasive and time-consuming procedure with two-dimensional images [4]. Optical coherence tomography angiography (OCTA) is a non-invasive technique that gives us high-resolution three-dimensional maps of the retinal and choroidal microvasculature. It also makes it possible to quantify the superficial and deep retinal capillary plexus in the fovea and radial peripapillary capillary network in the peripapillary area [5]. In small case series of patients with TED, significant optic nerve head (ONH) changes have been reported using optical coherence tomography [6–8]. In this study, we aimed to evaluate the density of the ONH microvasculature in TED using OCTA imaging. We may use OCTA to screen patients with TED for sub-clinical compressive optic neuropathy.
Methods
Study population
In this cross-sectional case–control study, hyperthyroid patients were enrolled in the study. The diagnosis of hyperthyroidism was confirmed by laboratory tests and endocrinologists. All patients in the TED group had clinical features of TED and documented laboratory test confirmation of hyperthyroidism. The control group patients were healthy individuals with no ocular abnormalities and were euthyroid according to clinical examination and laboratory test results. Cases were selected consecutively in a convenient sampling manner. Detailed ocular and systemic histories were obtained. Exclusion criteria included a history of diabetes mellitus, current pregnancy and breastfeeding, migraine, auto-immune disease, and any intraocular surgeries. Additional exclusion criteria considered as more than five diopters absolute spherical and greater than two diopters cylindrical refractive errors, best-corrected visual acuity less than 20/20, glaucoma, smoking, clinically apparent retinal disease, and other ocular diseases. Patients with a history of dysthyroid optic neuropathy were excluded. Moreover, signs of dysthyroid optic neuropathy like a positive relative afferent pupillary defect, decreased visual acuity, and decreased color vision were checked, and patients with any positive sign for optic neuropathy were excluded from study.
Retinal evaluation performed by a vitreoretinal sub-specialist. Palpebral fissure measurement, exophthalmometry, TED clinical activity score (CAS), and corneal condition evaluated by an oculoplastic sub-specialist.
Assessment of severity and activity of TED
The severity of TED was scored with a modified NOSPECS classification, and the TED activity was evaluated with a 7-point scale CAS index based on Mourits et al. presented criteria [9]. Each item has one point. CAS is the sum of individual scores, and ranging from 0 (no activity) to 7 (maximal activity). Seven parameters are spontaneous retrobulbar pain, painful eye movements, eyelid erythema, conjunctiva injection, chemosis, caruncle swelling, and edema or fullness of the eyelid. Patients with CAS 0–2 were categorized as group A and scored three or more as group B.
Imaging procedures
Imaging was performed in the imaging clinic of Khatam al Anbia Eye Hospital from December 2018 to September 2019. The spectral-domain instrument used for optical coherence tomography and OCTA images (AngioVue) is based on the Optovue RTVue XR Avanti technology to obtain optical coherence tomography images with a wavelength of 840 nm and an A-scan rate of 70,000 scans per second. The radial peripapillary capillary network evaluations were acquired using the default automated segmentation with the preset settings. Using optical coherence tomography 3D volume set at 4.5 × 4.5 mm, the AngioDisc 4.5 × 4.5 mm HD scan (400 lines × 400 A-scans) protocols with AngioVue 3D Projection Artifact Removal were applied.
All images centered on the optic disc and scan quality indexes were 7/10 or better. Low-quality images were discarded and reacquired. All images were carefully reviewed for sufficient quality and resolution. Images with significant motion artifacts that interfere with vessel density (VD) analysis were excluded. For all participants, the analysis used eye data with better image quality.
The radial peripapillary capillary network was evaluated using a slab between the outer edge of the retinal nerve fiber layer and the internal limiting membrane. All images were checked for segmentation errors and were adjusted manually before testing the VD. The machine software evaluated all vessels (AV) and small vessels (SV) VD separately in the RPC network. The analyses for the radial peripapillary capillary SV VD were reported as the whole image, inside disc area, whole peripapillary, superior and inferior hemifields, and eight segments. For the evaluation of AV VD, we divided the whole image into nine (three by three) grid-based sections, and it was reported separately in all sections. Moreover, the AV VD was reported for the whole image, inside the disc area, whole peripapillary, and peripapillary superior and inferior hemifields.
Statistical analysis
The variables' normal distribution was examined using the Shapiro–Wilk test and variances normality plots, and Levene's test determined homogeneity. The independent-samples t-test, paired t-tests, or Mann–Whitney U test was used for comparisons based on data distribution and type. The statistical significance level was set at 0.05. P-values were corrected for multiple comparisons. The SPSS program version 16 for Windows was used for all statistical analyses (IBM SPSS Statistics, IBM Corporation, Chicago, IL, USA).
Ethical considerations
The study protocol adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent, and the Regional Committee on Medical Ethics approved the ethical aspects of the study at Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.MEDICAL.REC.1397.629).
Results
We enrolled 29 patients with TED (19 females, 65.5%) (29 eyes) and 28 normal healthy controls (18 females, 64.2%) (28 eyes) in the study. The mean ages of the TED and control groups were 42.5 ± 10.7 and 39.7 ± 6.3 years, respectively. The difference in mean ages (p-value: 0.146) and the participant's gender (p-value: 0.462) between the two groups were not statistically significant. The TED and control groups mean scan quality were 8.11 ± 0.68 and 8.42 ± 0.66, respectively (p-value: 0.178) (Fig. 1). Among patients with TED, eighteen were grouped in CAS group A and eleven in group B.
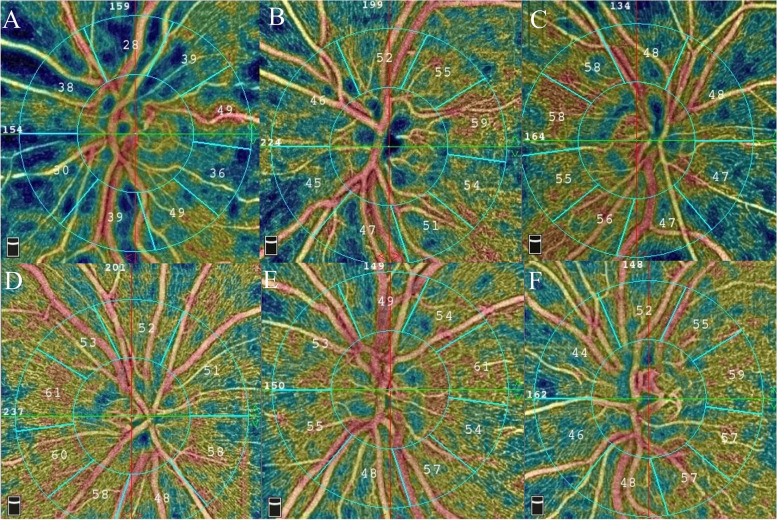
Fig. 1.
En-face optical coherence tomography angiograms (OCTA) segmented at the level of the radial peripapillary capillary network from three patients with thyroid eye disease (TED) (A-C) versus three age-matched normal controls (D-F). Note the remarkable flow deficits present in the en-face angiograms from the TED cases
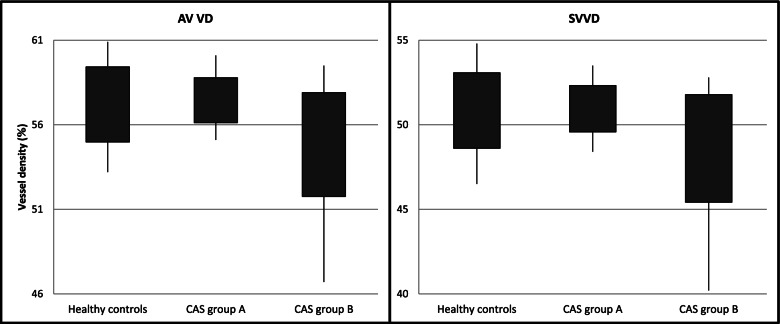
The TED group mean 4.5 × 4.5 mm whole image SV VD (49.94 ± 2.56) had no statistically significant difference compared to the control group (50.84 ± 2.23) (p-value: 0.16) (Table 1). Also, the TED group mean 4.5 × 4.5 mm whole image AV VD (56.33 ± 2.56) had no statistically significant difference compared to the control group (57.20 ± 20.22) (p-value: 0.17); however, there was a tendency for lower values in the TED group (Table 2). In addition, the TED group had non-significant lower means for VD in AV and SV VD in all other parameters compared to the control group (Fig. 2).
Table 1.
Comparison of the mean small vessels (SV) vessel density (VD) between patients with thyroid eye disease (TED) and normal controls eyes. (SV: small vessels; VD: vessel density; SD: Standard Deviation)
| Healthy controls (28 eyes) Mean ± SD (Range) |
TED patients (29 eyes) Mean ± SD (Range) |
P-value | |
|---|---|---|---|
| Whole image SV VD |
50.84 ± 2.23 (46.5,54.8) |
49.94 ± 2.56 (40.20,53.70) |
0.16 |
| Inside disc SV VD |
49.39 ± 4.76 (40.40,57.70) |
48.44 ± 5.18 (37.50,60.00) |
0.47 |
| Whole peripapillary SV VD |
53.75 ± 2.65 (48.00,59.30) |
52.80 ± 3.38 (38.20,55.90) |
0.24 |
| Peripapillary superior hemifield SV VD |
54.36 ± 2.59 (48.50,60.00) |
53.13 ± 3.38 (38.60,56.50) |
0.12 |
| Peripapillary inferior hemifield SV VD |
53.06 ± 3.09 (47.00,58.60) |
52.42 ± 3.70 (37.70,56.20) |
0.48 |
| Peripapillary nasal superior SV VD |
51.38 ± 2.88 (43.80,58.00) |
50.25 ± 4.68 (38.30, 60.00) |
0.27 |
| Peripapillary nasal inferior SV VD |
48.98 ± 5.22 (33.00,56.30) |
48.15 ± 4.45 (35.90,58.00) |
0.51 |
| Peripapillary inferior nasal SV VD |
51.95 ± 4.37 (41.40,61.20) |
51.41 ± 5.12 (35.90,59.90) |
0.66 |
| Peripapillary inferior temporal SV VD |
59.03 ± 3.10 (53.10,64.60) |
58.26 ± 3.97 (49.80,64.80) |
0.41 |
| Peripapillary temporal inferior SV VD |
54.94 ± 4.77 (45.5,64.00) |
53.40 ± 5.29 (40.60,62.00) |
0.24 |
| Peripapillary temporal superior SV VD |
57.24 ± 3.48 (51.00,63.40) |
55.70 ± 4.19 (46.80,63.40) |
0.13 |
| Peripapillary superior temporal SV VD |
57.78 ± 2.50 (52.30,62.90) |
55.94 ± 4.77 (43.00,62.60) |
0.07 |
| Peripapillary superior nasal SV VD |
51.55 ± 4.17 (44.20,64.40) |
50.55 ± 5.07 (34.90,61.50) |
0.41 |
Table 2.
Comparison of all vessels (AV), including both small and large vessels, vessel density (VD) of patients with thyroid eye disease (TED) versus normal controls eyes. (AV: all vessels; VD: vessel density; SD: Standard Deviation)
| Healthy controls (28 eyes) Mean ± SD (Range) |
TED patients (29 eyes) Mean ± SD (Range) |
P-value | |
|---|---|---|---|
| Whole image AV VD |
57.20 ± 2.22 (53.20,60.90) |
56.33 ± 2.56 (46.70,61.10) |
0.17 |
| Inside disc AV VD |
59.28 ± 3.86 (50.10,64.60) |
57.97 ± 4.38 (45.50,67.80) |
0.23 |
| Whole peripapillary AV VD |
59.88 ± 2.47 (55.10,64.20) |
59.03 ± 3.14 (45.70,62.00) |
0.26 |
| Peripapillary superior hemifield AV VD |
60.52 ± 2.41 (55.40,65.00) |
59.68 ± 3.08 (46.70,62.80) |
0.25 |
| Peripapillary inferior hemifield AV VD |
59.18 ± 2.83 (52.50,63.40) |
58.32 ± 3.37 (44.70,62.30) |
0.29 |
| Grid based superotemporal AV VD |
59.86 ± 2.22 (56.20,64.70) |
57.94 ± 4.44 (44.00,64.60) |
0.04 |
| Grid based temporal AV VD |
57.74 ± 3.19 (51.70,63.20) |
56.62 ± 3.63 (46.40,62.60) |
0.21 |
| Grid based inferotemporal AV VD |
59.91 ± 2.64 (54.90,64.00) |
58.46 ± 4.34 (45.60,66.30) |
0.13 |
| Grid based superior AV VD |
58.60 ± 3.38 (51.40,64.20) |
57.03 ± 5.30 (43.00,63.00) |
0.18 |
| Grid based central AV VD |
60.04 ± 4.07 (49.90,65.90) |
59.62 ± 3.94 (50.30,67.40) |
0.69 |
| Grid based inferior AV VD |
62.55 ± 3.04 (54.50,67.40) |
61.73 ± 3.02 (54.30,66.10) |
0.30 |
| Grid based superonasal AV VD |
52.75 ± 3.96 (45.30,60.00) |
50.95 ± 5.14 (38.90,61.60) |
0.14 |
| Grid based nasal AV VD |
54.49 ± 4.56 (42.80,64.10) |
53.11 ± 4.35 (42.40,59.80) |
0.24 |
| Grid based inferonasal AV VD |
50.49 ± 4.50 (36.10,57.90) |
49.23 ± 4.64 (36.80,60.10) |
0.29 |
Fig. 2.
Comparison between different groups all vessel and small vessel vessel density. (AV: All vessel, SV: Small vessel, VD: Vessel density)
There was a significant difference in comparison of the mean 4.5 × 4.5 mm whole image SV VD between the CAS group A (50.95 ± 1.37) and group B patients (48.60 ± 3.18) (p-value: 0.013) (Table 3). Also, the mean 4.5 × 4.5 mm whole image AV VD in the group A (57.45 ± 1.33) was significantly higher than in the group B (54.83 ± 3.07) (p-value: 0.005) (Table 4).
Table 3.
Comparison of SV VD of CAS group A versus CAS group B patients. Patients with CAS 0–2 were categorized as group A and scored three or more as group B. (SV: small vessels; VD: vessel density; SD: Standard Deviation; TED: thyroid eye disease; CAS: Clinical Activity Score)
| CAS group A (n:18) Mean ± SD (Range) |
CAS group B (n:11) Mean ± SD (Range) |
P-value | |
|---|---|---|---|
| Whole image SV VD |
50.95 ± 1.37 (48.40–53.50) |
48.60 ± 3.18 (40.20–52.80) |
0.013 |
| Inside disc SV VD |
49.83 ± 3.88 (43.80–55.80) |
46.59 ± 6.24 (37.50–60) |
0.103 |
| Whole peripapillary SV VD |
53.88 ± 1.38 (51.40–55.90) |
51.35 ± 4.63 (38.20–55) |
0.092 |
| Peripapillary superior hemifield SV VD |
54.12 ± 1.61 (51.40–56.50) |
51.80 ± 4.59 (38.60–55.70) |
0.072 |
| Peripapillary inferior hemifield SV VD |
53.58 ± 2.04 (49.60–56.20) |
50.87 ± 4.84 (37.70–55) |
0.045 |
| Peripapillary nasal superior SV VD |
50.55 ± 3.26 (41.20–54.20) |
49.81 ± 6.31 (38.30–60) |
0.682 |
| Peripapillary nasal inferior SV VD |
49.15 ± 3.12 (43.50–54.40) |
46.74 ± 5.71 (35.90–58.00) |
0.155 |
| Peripapillary inferior nasal SV VD |
52.05 ± 3.73 (46.50–58.30) |
50.51 ± 6.71 (35.90–59.90) |
0.437 |
| Peripapillary inferior temporal SV VD |
58.97 ± 3.65 (49.80–62.30) |
57.67 ± 4.53 (51.70–64.80) |
0.647 |
| Peripapillary temporal inferior SV VD |
54.88 ± 3.80 (48.70–61.10) |
51.30 ± 6.48 (40.60–62.00) |
0.073 |
| Peripapillary temporal superior SV VD |
56.29 ± 3.14 (49.10–60.50) |
54.85 ± 5.38 (46.80–63.40) |
0.373 |
| Peripapillary superior temporal SV VD |
57.17 ± 2.94 (51.30–62.60) |
54.20 ± 6.30 (43.00–61.50) |
0.152 |
| Peripapillary superior nasal SV VD |
51.28 ± 3.33 (42.10–56.20) |
49.53 ± 6.89 (34.90–61.50) |
0.371 |
Table 4.
Comparison of AV VD of CAS group A versus CAS group B patients. Patients with CAS 0–2 were categorized as group A and scored three or more as group B. (AV: all vessels; VD: vessel density; SD: Standard Deviation; TED: thyroid eye disease; CAS: Clinical Activity Score)
| CAS group A (n:18) Mean ± SD (Range) |
CAS group B (n:11) Mean ± SD (Range) |
P Value | |
|---|---|---|---|
| Whole image AV VD |
57.45 ± 1.33 (55.10–60.10) |
54.83 ± 3.07 (46.70–59.50) |
0.005 |
| Inside disc AV VD |
59.20 ± 2.81 (55.20–64.40) |
56.33 ± 5.59 (45.50–67.80) |
0.087 |
| Whole peripapillary AV VD |
60.31 ± 1.25 (57.60–62) |
57.33 ± 4.08 (45.70–61.10) |
0.010 |
| Peripapillary superior hemifield AV VD |
60.86 ± 1.30 (57.90–62.80) |
58.10 ± 4.03 (46.70–61.60) |
0.016 |
| Peripapillary inferior hemifield AV VD |
59.70 ± 1.66 (56.80–62.30) |
56.49 ± 4.21 (44.70–60.60) |
0.010 |
| Grid based superotemporal AV VD |
58.57 ± 3.27 (49.80–63.30) |
57.05 ± 5.77 (44.00–64.60) |
0.375 |
| Grid based temporal AV VD |
56.90 ± 3.17 (52.60–62.60) |
56.21 ± 4.31 (46.40–61.70) |
0.623 |
| Grid based inferotemporal AV VD |
58.91 ± 3.46 (50.70–62.70) |
57.84 ± 5.46 (45.60–66.30) |
0.523 |
| Grid based superior AV VD |
57.89 ± 5.82 (43–63) |
55.80 ± 4.41 (47.30–61.70) |
0.306 |
| Grid based central AV VD |
60.40 ± 2.51 (56.10–65.50) |
58.52 ± 5.29 (50.30–67.40) |
0.272 |
| Grid based inferior AV VD |
61.81 ± 2.37 (57.80–65.30) |
61.61 ± 3.87 (54.30–66.10) |
0.875 |
| Grid based superonasal AV VD |
52.01 ± 5.03 (40.60–61.60) |
49.45 ± 5.13 (38.90–57.60) |
0.193 |
| Grid based nasal AV VD |
55.06 ± 3.06 (48.90–59.80) |
50.35 ± 4.50 (42.40–57.10) |
0.002 |
| Grid based inferonasal AV VD |
50.97 ± 4.32 (43.20–60.10) |
46.77 ± 4.06 (38.60–52.10) |
0.014 |
Discussion
Twenty-nine TED patients were compared with twenty-eight healthy controls to evaluate OCTA VD changes. According to the results, patients with TED were not different from normal subjects in VD, either in SV or AV VD. This study evaluated the OCTA as a noninvasive modality in screening patients with TED for sub-clinical compressive optic neuropathy.
The concern of ONH changes in patients with TED has been considered recently. Increased intra-orbital pressure and extraocular muscle volume could lead to compressive optic neuropathy [10]. It has been reported that optic disc function may be affected before the clinical complaint of decreased vision, and therefore the diagnosis of optic nerve damage initiation could be beneficial in preventing significant visual loss. Therefore, follow-up of patients with hyperthyroidism, especially in visual function, is recommended in the literature [11, 12]. Using diffusion-tensor imaging in computed tomography, the ONH was affected by TED before the development of dysthyroid optic neuropathy compared to controls and between active and inactive stages of TED [4].
Few studies evaluated the ONH parameters in patients with TED compared to healthy subjects. A case–control study by Mihailovic et al. on 29 patients with TED and 29 healthy controls using OCTA, reported that ONH parameters were significantly lower in TED group compared to controls [7]. In another study, patients with TED had thinner, inferior retinal nerve fiber layer thickness. Also, the disc area and cup/disc ratio were higher in patients with TED compared to the control group [6]. The results of our study did not fully support Mihailovic and Sayin's conclusions since we report that the radial peripapillary capillary or disc parameters were not significantly different in TED compared to controls, although the trend was toward decreasing VD. It may be due to the limitation of our study to patients with 20/20 vision, and in Mihailovic et al. study, visual acuity limitation was not considered. Moreover, in that study, only inactive TED cases were enrolled, and they concluded that CAS is not associated with VD.
In our study we have included different severity groups of patients with TED (CAS groups A and B) and we found a decrement in VD with the increase in activity of the TED. The findings of our study showed that in contrast to the anticipation of increasing VD due to engorgement resulting from orbital inflammation, there is decreased vascularity. This finding may present another justification for nerve damage during TED.
It has been proposed that nearly 90% of dysthyroid optic neuropathy cases are associated with nerve compression; the remainder is proposed to be associated with stretching of the optic nerve without compression [13]. A comparative case series reported that although the optic nerve stretching is essential in dysthyroid optic neuropathy, ONH involvement is most probably due to ONH microvascular ischemia secondary to orbital apex changes [13, 14]. Moreover, the main risk factors for dysthyroid optic neuropathy include advancing age, smoking, and diabetes mellitus, which are associated with microvascular changes in OCTA [5, 13]. In a case report, a patient with worsening TED complained of complete vision loss in the left eye on up-gaze, considered gaze-evoked amaurosis [15]. The mechanism of vision loss in this patient was presumed optic nerve ischemia due to elevation in intraocular pressure in a congested optic disc. An increase in choroidal thickness in patients with TED indicates vascular tissue involvement in Graves’ disease. These findings support the vasculogenic pathogenesis of dysthyroid optic neuropathy in TED.
In a study by Ceylanoglu et al. they demonstrate that smoker patients with inactive TED had significantly lower ONH VD compared to healthy controls [16]. So, we aimed to exclude smoker subjects in our study to evaluate the sole effects of TED on ONH VD more specifically.
Yu et al. evaluated the difference in retinal nerve fiber layer thickness, choroidal thickness, and VD between patients with different severities of TED and healthy controls. They divided patients into three groups (active TED, inactive TED, and control groups). They found that VD had no significant relation with different clinical variables [17]. Jian et al. also found that the ONH VD was lower in patients with TED who had optic neuropathy [18]. In another study by Del Noce et al., there was a significant decrease in the peripapillary choriocapillaris and deep capillary plexus VD in patients with TED compared to healthy age and gender-matched controls. The study demonstrates a significant relationship between higher CAS scores and lower peripapillary choriocapillaris VD [19]. These studies showed significant alteration in ONH VD in patients with higher CAS scores like ours.
On the other hand, Pinhas et al., in a study on eight patients with TED, compared the non-capillary and capillary ONH VD with 133 normal eyes. They found that the non-capillary VD decreased significantly in the TED group, but the capillary VD did not show a significant difference. However, their study population was tiny [20].
Decreased vessel density in patients with higher CAS could support the theory that inflammation leads to atrophic changes; hence, perfusion of atrophic tissues is lower because of decreased consumption of oxygen and other nutrients [7]. In patients with active TED, Wu et al. found that the activity status and serum antibodies associated with TED were the relevant factors for reduced capillary density of the retina [8]. These findings correlate the activity of TED with a reduction in retinal or ONH VD, indicating retina lower demand in patients with active disease or retinal ischemia.
We enrolled a relatively small sample size of nearly thirty patients in our study, and this study limitation could improve by a larger-scale OCTA analysis performed in the active phase of the TED. Repeating imaging at fixed intervals in a longitudinal study could provide valuable information about the effects of TED on the ONH vascular system in the short and long term.
Conclusions
In conclusion, our study showed non-significant ONH vascular alterations in TED compared to healthy controls, including reduced VD of ONH in the radial peripapillary capillary. However, patients with higher CAS scores had a significant decrease in ONH microvasculature. TED's potential vascular involvement of the ONH warrants further study on a larger scale.
Acknowledgements
The authors would like to express their gratitude to Somayeh Ghassemi-Moghaddam, MSc and Masoomeh Ramoon BSc, optometrists; and Zahra Emaverdian MSc, Sepideh Nazari Noghabi MSc, Roghaye Kahani BSc, and Roya Gholamzadeh BSc, retinal imaging operators of Khatam-al-Anbia Eye Hospital, Mashhad, Iran. It is a pleasure for us to also acknowledge the kind supports of Capt. Hamid Reza Akef.
Abbreviations
- VD
Vessel density
- ONH
Optic nerve head
- TED
Thyroid eye disease
- OCTA
Optical coherence tomography angiography
- AV
All vessels
- SV
Small vessels
- CAS
Clinical activity score
Authors’ contributions
A.S., N.S, and M.A conceived and designed the study; Z.S. and H.J. assisted in sample collection; M.A. reviewed all images; M.A. and E.B. performed most of experiments and analyzed the data; T.S.R. and H.R.H. assisted in some experiments and analyzed the data; M.A. and H.R.H. wrote the paper; All authors read and approved the final version of the manuscript.
Funding
The authors would like to acknowledge the financial support of the Vice-Chancellor of Research of Mashhad University of Medical Sciences for this research project (code: 971241). The funding organization had no role in the design or conduct of this research.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol adhered to the tenets of the Declaration of Helsinki. All participants provided written informed consent before enrollment and the ethical aspects of the study were approved by the Regional Committee on Medical Ethics at Mashhad University of Medical Sciences, Mashhad, Iran (IR.MUMS.MEDICAL.REC.1397.629).
Consent for publication
Not applicable.
Competing interests
The authors declared no potential conflicts of interest for the research, authorship, and/or publication of this article.
Footnotes
This study has been presented in the “8th International Congress on OCT and OCT Angiography (ICOOR 2020) - Virtual” in December 2020, as an oral presentation.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Chiou SH, et al. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye (Lond) 2010;24(9):1520–1525. doi: 10.1038/eye.2010.31. [DOI] [PubMed] [Google Scholar]
- 2.Bartalena L, Baldeschi L, Boboridis K, Eckstein A, Kahaly GJ, Marcocci C, et al. The 2016 European thyroid association/European group on graves' Orbitopathy guidelines for the management of graves' Orbitopathy. Eur Thyroid J. 2016;5(1):9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, Graves' disease, and thyroid-associated ophthalmopathy. Thyroid. 2008;18(9):953–958. doi: 10.1089/thy.2007.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee H, Lee YH, Suh SI, Jeong EK, Baek S, Seo HS. Characterizing intraorbital optic nerve changes on diffusion tensor imaging in thyroid eye disease before Dysthyroid optic neuropathy. J Comput Assist Tomogr. 2018;42(2):293–298. doi: 10.1097/RCT.0000000000000680. [DOI] [PubMed] [Google Scholar]
- 5.Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sayın O, Yeter V, Arıtürk N. Optic disc, Macula, and retinal nerve fiber layer measurements obtained by OCT in thyroid-associated ophthalmopathy. J Ophthalmol. 2016;2016:9452687. doi: 10.1155/2016/9452687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihailovic N, Lahme L, Rosenberger F, Hirscheider M, Termühlen J, Heiduschka P, et al. Altered retinal perfusion in patients with inactive graves Ophthalmopathy using optical coherence tomography angiography. Endocr Pract. 2020;26(3):312–317. doi: 10.4158/EP-2019-0328. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Tu Y, Bao L, Wu C, Zheng J, Wang J, et al. Reduced retinal microvascular density related to activity status and serum antibodies in patients with graves' Ophthalmopathy. Curr Eye Res. 2020;45(5):576–584. doi: 10.1080/02713683.2019.1675177. [DOI] [PubMed] [Google Scholar]
- 9.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 1997;47(1):9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RL, Tweeten JP, Patrinely JR, Garland PE, Thiese SM. Dysthyroid optic neuropathy without extraocular muscle involvement. Ophthalmic Surg. 1989;20(8):568–574. [PubMed] [Google Scholar]
- 11.Beden Ü, Kaya S, Yeter V, Erkan D. Contrast sensitivity of thyroid associated ophthalmopathy patients without obvious optic neuropathy. ScientificWorldJournal. 2013;24(2013):943789. doi: 10.1155/2013/943789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennerdell JS, Rosenbaum AE, El-Hoshy MH. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol. 1981;99(5):807–809. doi: 10.1001/archopht.1981.03930010807002. [DOI] [PubMed] [Google Scholar]
- 13.Rose GE, Vahdani K. Optic nerve stretch is unlikely to be a significant causative factor in Dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2020;36(2):157–163. doi: 10.1097/IOP.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 14.Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest. 2021;44(3):421–429. doi: 10.1007/s40618-020-01361-y. [DOI] [PubMed] [Google Scholar]
- 15.Seery LS, Zaldívar RA, Garrity JA. Amaurosis and optic disc blanching during upgaze in graves ophthalmopathy. J Neuroophthalmol. 2009;29(3):219–222. doi: 10.1097/WNO.0b013e3181b2842b. [DOI] [PubMed] [Google Scholar]
- 16.Ceylanoglu KS, Sen EM, Doguizi S, Hondur G. Smoking effect on peripapillary and macular microvascular structure in inactive Graves' ophthalmopathy. Int Ophthalmol. 2021;41(10):3411–3417. doi: 10.1007/s10792-021-01904-z. [DOI] [PubMed] [Google Scholar]
- 17.Yu L, Jiao Q, Cheng Y, Zhu Y, Lin Z, Shen X. Evaluation of retinal and choroidal variations in thyroid-associated ophthalmopathy using optical coherence tomography angiography. BMC Ophthalmol. 2020;20(1):421. doi: 10.1186/s12886-020-01692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jian H, Wang Y, Ou L, He W. Altered peripapillary vessel density and nerve fiber layer thickness in thyroid-associated ophthalmopathy using optical coherence tomography angiography. Int Ophthalmol. 2022;42(3):855–862. doi: 10.1007/s10792-021-02051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Noce C, Roda M, Valsecchi N, et al. Evaluation of peripapillary vascular flow in patients with Thyroid-Associated Ophthalmopathy (TAO) by OCT Angiography. Graefes Arch Clin Exp Ophthalmol. 2022;260(8):2711–2716. doi: 10.1007/s00417-022-05551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinhas A, Andrade Romo JS, Lynch G, et al. A pilot study of subclinical non-capillary peripapillary perfusion changes in thyroid-related orbitopathy detected using optical coherence tomography angiography. Clin Ophthalmol. 2022;16:867–875. doi: 10.2147/OPTH.S356631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.