Abstract
Background
Data are limited regarding the safety of and antibody response to the BNT162b2 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger ribonucleic acid vaccine in adolescents and young adults with underlying disease.
Methods
This prospective observational study enrolled patients age 12–25 years with chronic underlying disease who received 2 doses of BNT162b2. A 18-item questionnaire was used to assess adverse events within 7 days post-vaccination, and data regarding severe adverse events were collected from electronic medical records. An antibody titer for the receptor-binding domain of the spike protein in SARS-CoV-2 was used to assess antibody response after the second vaccine dose.
Results
Study participants were 429 patients (241 [56.2%] age 12–15 years; 188 [43.8%] age 16–25 years). The most common underlying diseases were genetic or chromosomal abnormalities and/or congenital anomalies, followed by endocrine or metabolic diseases; 32% of participants were immunocompromised. Severe adverse events were observed after the second dose in 1 (0.4%) patient age 12–15 years and in 2 (1.1%) patients age 16–25 years; all patients recovered. Seropositivity after the second vaccine dose was 99.0%. The geometric mean antibody titer was higher in patients age 12–15 years versus 16–25 years (1603.3 [1321.8–1944.7] U/mL vs. 949.4 [744.2–1211.0] U/mL). Compared with immunocompetent patients, immunocompromised patients had a lower antibody titer (2106.8 [1917.5–2314.7] U/mL vs. 467.9 [324.4–674.8] U/mL).
Conclusions
Vaccination with BNT162b2 was acceptably safe and immunogenic for adolescents and young adults with underlying disease.
Keywords: SARS-CoV-2, mRNA, Immunization
1. Introduction
Since December 2019, infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in more than 580 million cases of coronavirus disease 2019 (COVID-19) with 6.4 million deaths worldwide as of August 2022 [1]. To counter this global pandemic, several types of COVID-19 vaccines have been developed and distributed.
The COVID-19 vaccine BNT162b2 (BioNTech, Pfizer) is one of the most common vaccines used for this disease [2]. It contains messenger ribonucleic acid (mRNA) that encodes the spike glycoprotein of SARS-CoV-2. A multinational placebo-controlled trial of BNT162b2 in adults showed favorable outcomes with 95% efficacy for COVID-19 prevention and a low incidence of serious adverse events [3]. A subsequent clinical trial evaluated this vaccine for healthy adolescents and young adults age 12–25 years [4]. Results showed 100% efficacy for COVID-19 prevention, higher immunogenicity in patients age 12–15 years versus 16–25 years, and an acceptable safety profile with minimum serious adverse events [4].
Based on these data, several countries, including Japan, expanded the indication for BNT162b2 to include the young population age 12 years and older. In the clinical trial; however, participants were mainly healthy patients, and few had stable preexisting disease, including hepatitis B, hepatitis C, or human immunodeficiency virus infection [4]. Therefore, safety and antibody response data are still lacking for adolescents and young adults with underlying disease, including immunocompromised conditions. In Japan, use of BNT162b2 was initiated for adolescents with chronic underlying disease in June 2021. Our current study investigated the safety and antibody response for use of BNT162b2 in this population.
2. Patients and methods
2.1. Study design, participants and objectives
This prospective, observational, single-center study investigated the safety of and antibody response to BNT162b2 in adolescents and young adults with underlying disease. Vaccination was administered as 2 doses (30 μg/dose), typically more than 21 days apart, as part of general clinical practice between July and October 2021 at a tertiary children's hospital, the National Center for Child Health and Development in Tokyo, Japan. Patients age 12–25 years with chronic underlying medical conditions and regular hospital visits were eligible for the study. The underlying medical conditions eligible for the study are listed in Table S1. Immunocompromised status was defined by these criteria: receipt of at least one immunosuppressive agent, chemotherapy within 6 months, primary immunodeficiencies or hematologic/oncologic diseases, and hematopoietic cell transplantation within 2 years. The primary objective of the study was to assess the safety of BNT162b2, and the secondary objective was to evaluate the antibody response to BNT162b in this population.
2.2. Safety of BNT162b2
Participants were requested to complete a paper or web-based 18-item questionnaire regarding the local and systemic reactogenicity events that occurred within 7 days after each vaccination (Table S2). In addition, electronic medical record data were collected regarding severe adverse events within 3 weeks after the first dose (dose 1) and 4 weeks after the second dose (dose 2). Severe adverse events were defined as cases that required hospitalization for any reasons that were difficult to exclude in association with the vaccination. Patients who did not answer the questionnaire were excluded from the safety analysis. Absence of an answer regarding a particular symptom on the questionnaire was interpreted as a lack of this symptom for the participant.
2.3. Antibody response to BNT162b2
The SARS-CoV-2 antibody levels were measured by the electrochemiluminescence immunoassay (Elecsys®, Anti-SARS-CoV-2 S, Roche). This quantitative immunoassay is validated for determination of antibodies to the SARS-CoV-2 spike (S) protein receptor-binding domain (RBD) in human serum and plasma [5]. A cut-off value ≥ 0.8 U/mL was interpreted as a positive [6]. The SARS-CoV-2 antibody levels were measured before vaccination (only if the residual sample was available) and 2 weeks–4 months after dose 2. Patients with a history of COVID-19 before vaccination and patients who did not complete both vaccination doses were excluded from the antibody response analysis. Both seropositivity rate and quantitative antibody titer were compared between younger and older patients (age 12–15 years and 16–25 years) [4] and between patients with and without immunocompromised conditions.
2.4. Statistical analysis
The total sample size was not based on statistical hypothesis testing. The frequency and proportion of adverse events and antibody titer after the second dose were collected from electronic medical records and compared between the two age groups (12–15 years, 16–25 years) and between immunocompromised status (presence or absence of immunocompromise). The Bayesian approach was used to calculate the probability that the posterior distribution of the proportion of cases of fever after dose 2 is greater than the beta distribution, beta (200, 800). The beta (200, 800), which corresponds to the threshold of 20%, was set based on the reference of Frenck et al. (2021) [4]. To use the beta-binomial model, we also set the conjugate prior distribution, beta (2, 8). Antibody titer was described by the geometric mean antibody titer and its 95% confidence intervals (CIs). Values below the limit of quantification were substituted with a titer corresponding to the cut-off value of 0.4 U/mL. Interim analysis was planned to be performed when every 100 participants were enrolled; however, this analysis was not performed because of the unexpectedly rapid participant enrollment. All statistical analysis was performed using SAS software, version 9.4 (SAS Institute).
2.5. Ethical considerations
The study was conducted with the approval of the Ethics Committee of the National Center for Child Health and Development, Tokyo, Japan (2021-060). Written informed consent was obtained from the participants or their guardians.
3. Results
3.1. Patient background
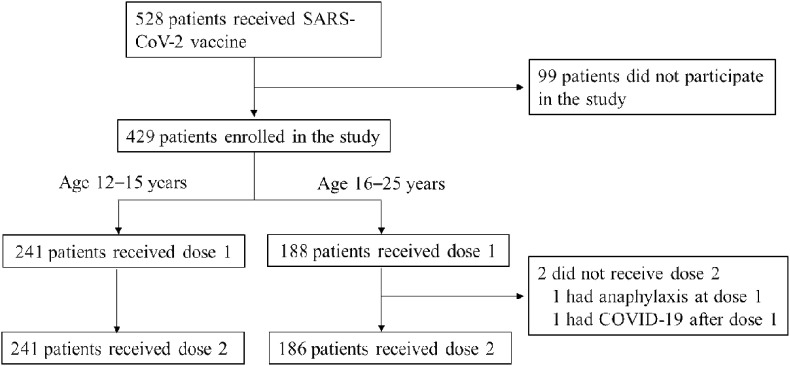
During the study period, 528 patients age 12–25 years with underlying medical conditions received at least 1 dose of BNT162b2. Among these 528 patients, 429 (81%) were enrolled into the study (Fig. 1 ). Characteristics of patients who received at least 1 dose of BNT162b2 are summarized in Table 1 . Median age was 15.0 years, and the number of male patients was 204 (47.6%). Genetic or chromosomal abnormalities and/or congenital anomalies were most common underlying medical conditions (67 patients, 15.6%), followed by endocrine or metabolic diseases (55 patients, 12.8%) and neurologic diseases (47 patients, 11.0%). Approximately 32% of patients had immunocompromised conditions, and 43 (10%) and 12 (2.8%) patients had undergone solid organ and hematopoietic cell transplantation, respectively. Only 3 patients had a history of COVID-19 infection before vaccination. The pre-vaccination SARS-CoV-2 antibody in 91 (21.2%) patients showed no evidence of pre-vaccine COVID-19, and only 1 patient showed a positive value near the cut-off. The age groups were 241 (56.2%) patients age 12–15 years and 188 (43.8%) patients age 16–25 years. The background characteristics, including the percentage of patients with immunocompromised conditions, were comparable between groups.
Fig. 1.
Patients selection flow.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Characteristics of patients who received at least 1 dose of BNT162b2.
| Variables | Subcategories | Total | 12–15 years | 16–25 years |
|---|---|---|---|---|
| Case number | 429 | 241 | 188 | |
| Age (years), median (IQR) | 15.0 (13.0–18.0) | 13.0 (13.0–15.0) | 18.0 (16.0–20.0) | |
| Male sex, number (%) | 204 (47.6) | 118 (49.0) | 86 (45.7) | |
| Underlying diseasea, number (%) | Genetic or chromosomal abnormalities, congenital anomalies Endocrine/Metabolic diseases Neurological diseases Liver diseases Gastrointestinal diseases Allergic diseases Hematologic/Oncologic diseases Renal/urinary diseases Cardiovascular diseases Collagen/Inflammatory diseases Other diseasesb |
67 (15.6) 55 (12.8) 47 (11.0) 43 (10.0) 40 (9.3) 34 (7.9) 34 (7.9) 33 (7.7) 32 (7.5) 25 (5.8) 19 (4.4) |
43 (17.8) 36 (14.9) 29 (12.0) 24 (10.0) 16 (6.6) 22 (9.1) 17 (7.1) 17 (7.1) 14 (5.8) 17 (7.1) 6 (2.5) |
24 (12.8) 19 (10.1) 18 (9.6) 19 (10.1) 24 (12.8) 12 (6.4) 17 (9.0) 16 (8.5) 18 (9.6) 8 (4.3) 13 (6.9) |
| Immunosuppressive conditionc, number (%) | 138 (32.2) | 75 (31.1) | 63 (33.5) | |
| Immunosuppressive agents, number (%) | Any Single agent Multiple agents |
124 (28.9) 71 (16.6) 53 (12.4) |
67 (27.8) 41 (17.0) 26 (10.8) |
57 (30.3) 30 (16.0) 27 (14.4) |
| Steroids, number (%) | 33 (7.7) | 19 (7.9) | 14 (7.5) | |
| Biologic agents | 38 (8.9) | 19 (7.9) | 19 (10.1) | |
| Transplantation | Any transplantation Solid organ transplantation Hematopoietic transplantation |
55 (12.8) 43 (10.0) 12 (2.8) |
32 (13.3) 27 (11.2) 5 (2.1) |
23 (12.2) 16 (8.5) 7 (3.7) |
| COVID-19 infection Pre-vaccination, number (%) | 3 (0.7) | 2 (0.8) | 1 (0.5) |
COVID-19, coronavirus disease 2019.
If patients had multiple comorbidities, the primary investigator selected the most relevant comorbidity.
Musculoskeletal diseases: 6, Primary immunodeficiencies: 6, Psychiatry diseases: 4, Blood vessel or lymphatic abnormalities: 2, Respiratory diseases: 1.
Immunocompromised status was defined by these criteria: previous receipt of at least one immunosuppressive agent, chemotherapy within 6 months to treat primary immunodeficiencies or hematologic/oncologic diseases, and hematopoietic cell transplantation within 2 years.
3.2. Safety and adverse events
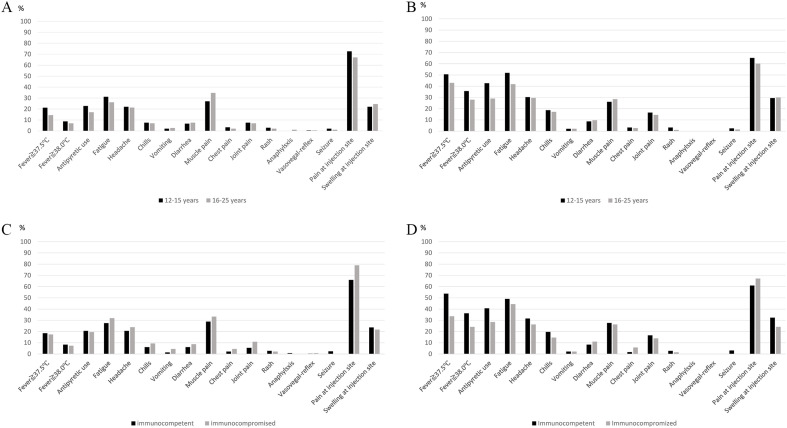
The frequency of local and systemic adverse events within 1 week after each dose of the vaccine are described in Fig. 2 and Fig. S1. The questionnaire collection rate for dose 1 and dose 2 was 92.5% and 80.6%, respectively. Most variables, including fever, were higher after dose 2 than after dose 1. In fact, 138 (32.3%, 95% Bayesian credible interval, 27.8–36.5) patients experienced fever ≥38.0 °C after dose 2. The probability of the hypothesis of a >20% proportion of fever after dose 2 was >0.999. Fever was more common in the younger age group (12–15 years) than in the older age group (16–25 years) and in immunocompetent than immunocompromised patients. Anaphylaxis was observed in 2 patients age 16–25 years after dose 1.
Fig. 2.
Incidence of adverse events. A: After dose 1 in different age groups.
B: After dose 2 in different age groups. C: After dose 1 in immunocompetent and immunocompromised patients. D: After dose 2 in immunocompetent and immunocompromised patients. After dose 1 and dose 2, 397/429 (92.5%) and 344/427(80.6%) of patients answered the questionnaire, respectively. Two patients did not receive dose 2.
Severe adverse events were observed in no (0.0%) patients age after dose 1 and were observed in 1 (0.4%) patient age 12–15 years and 2 patients (1.1%) age 16–25 years after dose 2. Serious adverse events potentially related to the vaccination were as follows: fever requiring hospitalization, facial pain and flushing, and bloody diarrhea. All patients fully recovered from those events. No patients developed myocarditis after the vaccination; however, chest pain was observed in 2.8% and 3.0% of patients after dose 1 and dose 2, respectively.
3.3. Antibody response
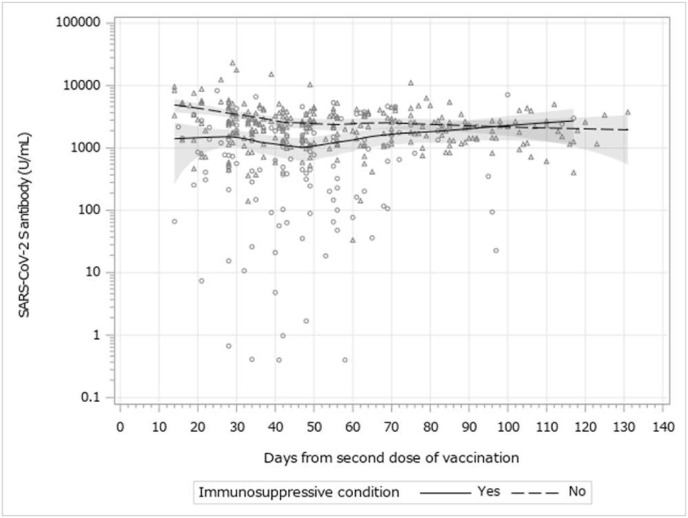
The SARS-CoV-2 S protein RBD antibody titer was measured in 397 patients. The median time interval from receipt of the vaccine to antibody measurement (interquartile range) from dose 2 was 42 (30–55) days. The seroconversion rate after the dose 2 was 99.0% (Table 2 ). However, 4 immunocompromised patients did not develop measurable antibodies; their primary diagnosis were agammaglobulinemia, severe combined immunodeficiency, and liver or kidney transplant recipient. The geometric mean antibody titer (95% CIs) was higher in the younger age group (12–15 years) than in the older group (16–25 years) (1603.3 [1321.8–1944.7] U/mL vs. 949.4 [744.2–1211.0] U/mL). Compared with immunocompetent patients, patients with immunocompromised conditions showed significantly lower antibody titers (2106.8 [1917.5–2314.7] U/mL vs. 467.9 [324.4–674.8] U/mL). The details of the antibody in each category are shown in Fig.S2. Among patients with immunocompromised conditions, patients taking multiple immunosuppressive agents, such as steroids or biologic agents tended to show lower antibody titers. The antibody titer and time after dose 2 is described in Fig. 3 . The antibody titers in immunocompetent patients were higher, but waned over time.
Table 2.
SARS-CoV-2 S antibody positivity and titers after the second vaccination.
| Patients, number | Antibody positive, number (%) | Geometric mean antibody titer (95% CI, U/mL) | |
|---|---|---|---|
| Total | 397 | 393 (99.0) | 1272.6 (1091.3–1484.0) |
| Immunosuppressive condition (+) | 133 | 129 (97.0) | 467.9 (324.4–674.8) |
| Immunosuppressive condition (−) | 264 | 264 (100) | 2106.8 (1917.5–2314.7) |
| Age group | |||
| 12–15 years | 222 | 221 (99.5) | 1603.3 (1321.8–1944.7) |
| Immunosuppressive condition (+) | 74 | 73 (98.6) | 707.6 (438.6–1141.7) |
| Immunosuppressive condition (−) | 148 | 148 (100) | 2413.3 (2132.5–2731.2) |
| 16–25 years | 175 | 172 (98.3) | 949.4 (744.2–1211.0) |
| Immunosuppressive condition (+) | 59 | 56 (94.9) | 278.5 (160.0–484.6) |
| Immunosuppressive condition (−) | 116 | 116 (100) | 1771.6 (1539.2–2038.9) |
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 3.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor-binding domain antibody titer and duration after vaccination dose 2.
Open triangle represents immunocompetent patients. Open circle represents immnocompromised patients. Dotted line represents local polynomial regression (LOESS) curve of immunocompetent patients. Solid line represents LOESS curve of immunocompromised patients.
4. Discussion
This study revealed a high seroconversion rate and acceptable safety with the use of BNT162b2 in adolescents and young adults age 12–25 years with underlying disease, although the antibody titer was lower in immunocompromised patients.
Safety and adverse reactions of BNT162b2 in healthy adolescents and young adults has been investigated [4]. Pain at injection site, fatigue, headache, chills, muscle pain, and fever were common adverse reactions and more frequently reported after dose 2. A similar trend was observed in our patient population. Regarding fever, 36% of patients age 12–15 years had a body temperature higher than 38 °C after dose 2, and 43% of the patients required antipyretics. This finding suggests that healthcare providers should provide patients and parents or guardians with guidance for the likelihood of adverse reactions, especially after dose 2, and the management of these events. Furthermore, fever post-vaccination was more common in immunocompetent versus immunocompromised patients. This finding may be due to the weak immune reaction after the vaccination in immunocompromised patients.
It is known that serious adverse events rarely occur after BNT162b2 in adolescents and young adults. A previous study showed 0.6% and 1.7% of patients age 12–15years and age 16–25 years who received BNT162b2 experienced serious adverse events [4]. In our study, serious adverse events were observed only in 3 (0.7%) patients after dose 2. Despite the presence of underlying disease, all patients fully recovered. This finding suggest that BNT162b2 is acceptably safe, even in the patients with underlying medical conditions.
Myocarditis is rare but important adverse event after receipt of the SARS-CoV-2 mRNA vaccine and mainly occur in male adolescents and young adults. In a review of the Vaccine Adverse Event Reporting System in the United States, the rate of myocarditis was highest in male adolescents age 12–17 years with 62.8 cases/million after dose 2 of the mRNA vaccine [7]. A similar result was reported from Israel, with the highest rate in male adolescents age 16–19 years (15.1 cases/100,000 after dose 2 of BNT162b) [8]. In our population, including cardiovascular or collagen diseases, none of the patients were diagnosed with myocarditis, although the sample size was relatively small. However, 3% of patients reported chest pain, which may be a sign of myocarditis, and clinicians should be aware of the possible risk of myocarditis after SARS-CoV-2 mRNA vaccination in this population.
To assess the immunogenicity of BNT162b2, we measured the SARS-CoV-2 S protein RBD antibody by the electrochemiluminescence immunoassay as an alternative to the neutralizing antibody assay. This assay is highly sensitive (97.92%) and specific (99.95%) [9] and commonly used in research on both COVID-19 infection [10] and the SARS-CoV-2 vaccine antibody response [11]. The optimal cut-off value of this antibody titer for preventing SARS-CoV-2 infection remains unclear; however, previous reports showed that this assay was highly correlated with those measuring neutralizing antibodies [12], which is directly associated with infection prevention. Therefore, we believe that the SARS-CoV-2 S protein RBD antibody may be a surrogate marker of the neutralizing antibody and vaccine effectiveness.
This study revealed that SARS-CoV-2 antibody titer was higher in the younger population (age 12–15 years) than the older population (age 16–25 years). Similar results were shown in the phase 3 trial of BNT162b2 in healthy adolescents [4]. The study showed the geometric mean antibody titer ratio of BNT162b2 neutralizing antibody in the younger age group (12–15 years) was 1.76 times higher than that in the older group (16–25 years). Furthermore, another phase 2/3 trial of BNT162b2 showed that the geometric mean antibody titer of BNT162b2 in children age 5–11 years who received 10 μg of BNT162b2 was similar to that in patients age 16–25 years who received 30 μg of BNT162b2 [13]. In that study, the BNT162b2 dose was decreased from 30 μg to 10 μg, given the high frequency of adverse reactions, including fever, in the 30-μg group. In our study, a high incidence of fever and high antibody titer were observed in the younger age group (12–15 years). This finding suggests that it may be worthwhile to consider performing clinical trials to investigate the option of dose reduction in this patient population.
Several studies have investigated the efficacy and immune reaction after SARS-CoV-2 mRNA vaccination in special circumstances, especially in the immunocompromised adult population. The vaccine effectiveness of BNT162b2 in immunocompromised patients is low compared with that in the general population [14,15]. It is also known that antibody response to SARS-CoV-2 mRNA vaccine is low in immunocompromised patients, including solid organ transplant recipients [16] and in those with leukemia [17] and cancer [18]. Thus, the Centers for Disease Control and Prevention recommend a booster dose of mRNA vaccine in these populations [19].
Our data showed a lower antibody titer in immunocompromised patients, including solid and hematopoietic cell transplant recipients and those taking immunosuppressive agents. This finding may indicate the need for a booster dose of the SARS-CoV-2 mRNA vaccine in adolescents and young adults, especially for those with immunocompromised conditions.
This study has several limitations. First, the number of study participants was too small to assess rare vaccine-related adverse events. For example, myocarditis is a known rare adverse event associated with the SARS-CoV-2 mRNA vaccine and occurs most often in young male adolescents (age 12–17 years); however, its frequency remains low, with approximately 1 cases/16,000 vaccinations [20]. If patients feel chest pain after receiving SARS-CoV-2 mRNA vaccine, they should visit the hospital and be assessed promptly. Second, we were unable to assess the clinical effectiveness of the vaccination because of the small number of children with COVID-19 in Japan. During the study period, Japan was experiencing an epidemic of COVID-19; however, the number of reported cases was about 0.8 million [21], which is only 0.6% of total population in Japan. In fact, only one case of COVID-19 after dose 1 was recognized in this cohort. Lastly, pre-vaccinated SARS-CoV-2 S protein RBD antibody was confirmed in only 21% of the study population. Therefore, it is difficult to exclude the possibility that unrecognized patients with COVID-19 may have influenced post-vaccine seropositivity. On the other hand, almost all patients who had undergone SARS-CoV-2 antibody testing before receiving the vaccine were seronegative, and none had a history of COVID-19.
In conclusion, BNT162b2 was acceptably safe and immunogenic in adolescents and young adult patients with underlying disease. Further large-scale studies are warranted to address assess this population for rare adverse events after vaccination with BNT162b2.
Authorship statement
KS contributed to designing and conceptualizing the study and drafted the manuscript. TF and MY contributed to data collection, statistical analysis, and revising of the manuscript. MM contributed to data collection, statistical analysis, and revising of the manuscript. KM, SU, CT, SM, HA, TM, CO contributed to data collection and the revised the manuscript. HK and IM contributed to supervise the study and revised the manuscript. All authors meet the ICMJE authorship criteria. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of competing interest
K. Shoji received payment for lectures from Biomelieux Japan, Gilead and VIATRIS. All the other authors have indicated they have no conflict of interests directly associated with this work.
Acknowledgments
This work was supported by a grant from National Center for Child Health and Development [NCCHD 30-E1 awarded to IM].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.09.013.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization Weekly epidemiological update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---10-august-2022 Available from:
- 2.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. PMID 33626250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. PMID 33301246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frenck R.W., Klein N.P., Kitchin N., et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. PMID 34043894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lou B., Li T.D., Zheng S.F., et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur Respir J. 2020;56 doi: 10.1183/13993003.00763-2020. PMID 32430429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roche diagnostics. Elecsys Anti-SARS-CoV-2 S cobas® Package insert. https://www.fda.gov/media/144037/download Available from:
- 7.Gargano J.W., Wallace M., Hadler S.C., et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. PMID 34237049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mevorach D., Anis E., Cedar N., et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. PMID 34614328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riester E., Findeisen P., Hegel J.K., et al. J Virol Methods. 2021;297 doi: 10.1016/j.jviromet.2021.114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stout R.L., Rigatti S.J. Seroprevalence of SARS-CoV-2 antibodies in the US adult asymptomatic population as of September 30, 2020. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.1552. PMID 33724387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paal M., Arend F.M., Lau T., et al. Antibody response to mRNA SARS-CoV-2 vaccines in haemodialysis patients. Clin Kidney J. 2021;14:2234–2238. doi: 10.1093/ckj/sfab127. PMID 34603700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong S., Lee N., Lee S.K., et al. Comparing results of five SARS-CoV-2 antibody assays before and after the first dose of ChAdOx1 nCoV-19 vaccine among health care workers. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01105-21. PMID 34191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter E.B., Talaat K.R., Sabharwal C., et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. doi: 10.1056/NEJMoa2116298. PMID 34752019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chodick G., Tene L., Rotem R.S., et al. The effectiveness of the two-DOSE BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. PMID 33999127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brosh-Nissimov T., Orenbuch-Harroch E., Chowers M., et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021;27:1652–1657. doi: 10.1016/j.cmi.2021.06.036. PMID 34245907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. PMID 33950155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herishanu Y., Avivi I., Aharon A., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165–3173. doi: 10.1182/blood.2021011568. PMID 33861303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22:765–778. doi: 10.1016/S1470-2045(21)00213-8. PMID 33930323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Center for Disease Control and Prevention COVID-19 vaccines for moderately or severely immunocompromised people. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html Available from:
- 20.Center for Disease Control and Prevention COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf
- 21.Ministry of Health LaW. newly_confirmed_cases_datail_weekly. Accessed. Available from. https://covid19.mhlw.go.jp/extensions/public/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.