Abstract
Objectives
Possible immunomodulatory effect of amantadine in patients treated in intensive care unit (ICU), mostly among patients with brain injuries or vascular diseases was observed in several studies. The potential antiviral effect of amantadine against SARS-CoV-2 was discarded in clinical trials; however, immunomodulatory potential was not studied. The aim of the study was to investigate the effect of immunomodulatory amantadine therapy on mortality in patients with respiratory insufficiency due to COVID-19 requiring mechanical ventilation in ICU.
Methods
Retrospective analysis of 241 cases of 141 (58.5%) receiving intravenous amantadine sulfate vs 100 (41.5%) controls on standard of care only was performed.
Results
Overall mortality was 72.6%, being notably lower among amantadine treated patients (59.5%, n = 84) compared with controls (91%, n = 91), P-value = 0.001. In multivariate models administration of amantadine was independently associated with lower mortality rate (hazard ratio: 0.220, CI: 0.146-0.333 P-value = 0.001). Furthermore, survival was improved in patients who received amantadine; late administration of amantadine after 5th day was independently associated with lower mortality (hazard ratio: 0.560, CI: 0.313-0.999, P-value = 0.050).
Conclusion
In patients treated in ICU with severe respiratory failure, administration of amantadine is associated with lower mortality, which may be associated with the potential anti-inflammatory and immunomodulatory effects of this agent.
Keywords: COVID-19, Mortality, ICU, Amantadine, ARDS
1. Introduction
The first reports on the possible uses of amantadine were published as early as 1960s (Monto and Arden, 1992). Following initial reports on antiviral properties described in 1963, amantadine obtained approval for prophylaxis and treatment of influenza type A viruses infections in 1976 (Hubsher et al., 2012); however, in the following decades it was withdrawn from influenza's treatment recommendations due to universal viral protein M2 resistance (Influenza Antiviral Drug Resistance, 2021).
Amantadine is currently classified as a neurostimulant and used for this purpose in neurologic indications. It passes the blood-brain barrier easily with adequate concentrations in the cerebrospinal fluid (Hubsher et al., 2012). As a neurostimulating agent, the drug modulates dopamine activity by increasing its extracellular release, blocking reuptake, and upregulating postsynaptic dopamine receptors (Gualtieri et al., 1989). Amantadine binds to and acts as a σ1 receptor agonist. Activation of the σ1 receptor is related to dopaminergic (DA) effects of amantadine at therapeutically relevant concentrations (Peeters et al., 2004). In addition, amantadine non-competitively inhibits the n-methyl-d-aspartate receptor and mediates stimulation of acetylcholine release (Stoof et al., 1992). Despite the lack of a complete understanding of the molecular mechanisms of DA activity, amantadine has been successfully administered in a wide variety of consciousness alterations, memory, and cognitive functions (Geisler et al., 1996; Kraus et al., 2005; Stelmaschuk et al., 2015). Amantadine increases dopamine levels, especially in the frontal lobe while exhibiting a moderate anti-inflammatory effect by inhibiting the secretion of pro-inflammatory factors within the microglia (Jiménez-Jiménez et al., 2020). The neuroprotective effect is aided by the stimulation of neurotrophic factor expression, namely brain-derived neurotrophic factor and glial cell-derived neurotrophic factor (Zhong et al., 2020).
According to data obtained from research on traumatic brain injury, neurostimulants such as amantadine promote wakefulness and may increase patient participation in early rehabilitation (Ghalaenovi et al., 2018). Available studies indicate a beneficial effect of amantadine therapy on the state of consciousness in intensive care unit (ICU)-hospitalized traumatic brain injury patients whose treatment was started shortly after the injury (Giacino et al., 2012; Shafiee et al., 2022). It was also shown that early amantadine treatment was associated with a mortality decrease among conservatively treated large hemisphere infarction patients (Li et al., 2021). Finally, recently it was suggested that rapid initiation of amantadine during early care of patients admitted with ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage is associated with improved wakefulness (Leclerc et al., 2021).
DA receptors are known to be involved as a gateway for the entry of various viruses (Gaskill et al., 2014; Simanjuntak et al., 2017). There are at least a few examples associating the potential effect of dopamine on viral infections in vitro. Elevated dopamine levels play a significant role in the early stage of Japanese encephalitis virus infection, increasing viral entry into dopamine D2 receptors-expressing cells through a signaling pathway mediated by phospholipase C (Simanjuntak et al., 2017). It was also shown that midbrain DA neurons are permissive to SARS-CoV-2 infection. SARS-CoV-2 triggers DA neuronal inflammatory and cellular senescence responses (Chen et al., 2021). In the studies published to date, the direct antiviral effect of amantadine in the SARS-CoV-2 infection treatment was discarded. Moreover, we wish to emphasize that in the current analysis antiviral effect of amantadine was not studied, and there is no substantive basis to pursue such an assumption further. However, the possible neurostimulatory and anti-inflammatory effect in patients treated in ICUs seems to be worth reporting also in patients with COVID-19. Therefore, the aim of the study was to investigate the effect of amantadine therapy on mortality in patients treated in ICU with COVID-19 and describe associated factors.
2. Methods
2.1. Patients and methods
Our database contains information about patients hospitalized at the Regional Hospital in Szczecin, Poland. Patients participating in the study were observed from March 4, 2020 to January 23, 2022 when the database was closed with a total of 4287 records. ICU data was collected between March 24, 2020 and May 29, 2021, and contained 241 entries. All data were fully anonymized before statistical analysis.
In this study, we retrospectively analyzed the dataset of patients with COVID-19 requiring mechanical ventilation, admitted to ICU. All patients included in this analysis presented with acute respiratory distress syndrome (ARDS) in the course of SARS-CoV-2 infection. Each time the final decision on admission to ICU was made by a consultant anesthesiologist. In every case, polymerase chain reaction for SARS-CoV-2 or antigen testing was performed using pharyngeal swabs confirming infection with this virus, and pneumonia was confirmed using chest computed tomography (CT). In the assessment of imaging examinations, the radiologist was supported by the artificial intelligence algorithm and analyzed the percentage of lung tissue affected by COVID-19. All laboratory tests were performed within 24 hours following hospital admittance.
The primary objective of this study was to determine the efficacy and safety of amantadine sulfate in patients with COVID-19 pneumonia treated in the ICU. A secondary goal of this study was to try to identify the risk factors associated with higher mortality. The tertiary aim of this study was to try to determine the correlation between amantadine and other immunomodulatory agent use. In order to answer the above questions, we established the following inclusion and exclusion criteria for the study:
Inclusion criteria for this study were as follows: (i) acute respiratory failure requiring mechanical ventilation; (ii) CT confirmed COVID-19-pneumonia; (iii) age >18 years; (iv) confirmed SARS-CoV-2 infection as an indication for in-hospital admittance.
Exclusion criteria: (i) active neoplastic disease.
Treatment was in line with the current knowledge, guidelines of the Polish Society of Epidemiologists and Infectious Diseases Specialists and product characteristics.
If given, the following concomitant medications were used: (i) the 5-day remdesivir course started within 7 days of onset of symptoms; if symptoms lasted longer, drug administration was not initiated. Remdesivir was administered intravenously (IV) once daily for 5 days, with a loading dose of 200 mg on day 1, followed by a maintenance dose of 100 mg; (ii) tocilizumab (TCZ) was administrated IV at a dose of 8 mg/kg (maximum dose: 800 mg) twice, 12 hours apart. It was only used in patients with biochemically confirmed cytokine release storm (interleukin [IL]-6 >100 pg/ml). Before administering TCZ, we ruled out acute viral infections including cytomegalovirus, hepatitis B and C as well as human immunodeficiency virus based on standard serological methods. Acute Toxoplasma gondii was also excluded prior to TCZ administration; (iii) supportive treatment was applied to each of the patients; (iv) chloroquine or lopinavir were not used.
The supportive treatment included: (i) antibiotic therapy (Ceftriaxon was drug of choice but could vary depending on the patient's condition and was modified in the course of treatment); (ii) oxygen therapy (Only mechanical ventilation was used. Extracorporeal membrane oxygenation was not used.); (iii) IV rehydration; (iv) dexamethasone administered IV in a dose of at least 6 mg per day; (v) low-molecular-weight or non-fractionated heparin in prophylactic or therapeutic doses.
Amantadine sulfate was administered IV in two doses of 200 mg per day (each in 500 ml). After initiating therapy with amantadine, the drug was administered until extubating (unless the fluid and electrolyte imbalance required modification). Infusion of amantadine was based on the European Medicines Evaluation Agency drug registration; therefore, no ethical consent for this procedure was needed.
2.2. Ethical issues
The retrospective analysis was approved by the Bioethical Committee of Pomeranian Medical University, Szczecin, Poland (approval number: KB-0012/92/2020); however, no additional consent was necessary from the patients. The study was conducted in accordance with principles of the Declaration of Helsinki.
2.3. Sampling and data collection methodology
In this study, we collected clinical data from medical records, including age, sex, comorbidities, treatment history, duration of in-hospital stay, duration of treatment in the ICU, survival statistics, baseline blood oxygenation levels, chest CT scan results, and selected laboratory parameters. Comorbidities were assessed in terms of their incidence.
2.4. Statistics
Clinical and baseline laboratory characteristics were calculated for parametric and nonparametric statistics separately. Statistical comparisons were performed using the Mann-Whitney U test for nonparametric statistics. Multi-way tables were constructed with consideration of the chi-square test. CIs and Interquartile ranges (IQRs) are indicated where appropriate. Kaplan-Meyer cumulative mortality was calculated with statistical significance of survival data analyzed using the log-rank test. Unadjusted and multivariate Cox proportional hazards models were used to assess the effect of the analyzed parameter on the risk of death and to calculate the hazard ratios (HRs); for the final model, the best fit based on Akaike's information criteria was selected. P-values of 0.05 were considered significant. Commercial software (Statistica 13.0 PL; Statasoft, Warsaw, Poland) was used for the statistical calculations.
3. Results
3.1. Clinical characteristics of patients with COVID-19 and factors associated with mortality
The final dataset included 241 adult patients with a median age of 65 (IQR 59-71) years. None of the patients analyzed in the study were vaccinated with the full dose of the SARS-CoV-2 vaccine. In 143 patients, we confirmed infection with the wild-type variant SARS-CoV-2, which accounted for 59% of all included in this analysis. The remaining 41% (n = 98) were infections with the Alpha (B.1.1.7) variant. The key biochemical parameters indicative of advanced inflammation were significantly elevated in the analyzed group. Namely, the median IL-6 was 104 pg/ml (IQR 51.80-167.00) with a concurrently low procalcitonin concentration [median 0.27ng/ml (IQR 0.150-0.50)]. Body mass index (BMI) was calculated for each patient; median of BMI was 29.4 (IQR 26.57-34.41). Male gender dominated in the study group (n = 153; 63.5%). The most common concomitant disease was arterial hypertension, which was present in 129 (53.5%) cases. Obesity and diabetes were found in 97 (40.2%) and 81 (33.6%) cases, respectively. Chronic circulatory failure was observed in 24 (9.9%) cases. Key concomitant COVID-19 medication included dexamethasone which was administered IV in a dose of at least 6 mg per day (n = 241; 100%) in all cases, remdesivir was administrated in 99 cases (41%), while TCZ was used in 127 patients (52.7%).
Overall mortality in our study was high (72.6%). (Table 1 .) There were no statistically significant differences between survived/died groups regarding gender, height, weight, BMI, initial laboratory tests, comorbidities, administration of remdesivir, or TCZ. Groups differed significantly regarding percentage of lung involvement (survived 32.47% [16.09-51.57%] vs died 47.20% [31.74-59.68%]). Surviving patients were notably younger, with median age of 62 (IQR 55-68) years, compared to those who died (median: 67 [IQR: 60-72] years, P-value = 0.005).
Table 1.
Characteristic of study groups by survival outcomes.
| Survived N = 66 (27.4%) | Died N = 175 (72.6%) | P-value | |
|---|---|---|---|
| Age, years, median (IQR) | 62 (55-68) | 67 (60-72) | 0.005 |
| Height, cm (IQR) | 172 (162-178) | 172 (164-178) | 0.666 |
| Weight, kg (IQR) | 90 (75-103) | 88 (79-100) | 0.950 |
| Body mass index, kg/m² (IQR) | 30.47 (26.12-34.41) | 29.38 (27.16-34.29) | 0.756 |
| Percentage of lung involvement, % (IQR) | 32.47 (16.09 - 51.57) | 47.20 (31.74 - 59.68) | 0.010 |
| White blodd cell, x103/ul (IQR) | 7.73 (5.45-12.26) | 7.67 (5.4-10.31) | 0.545 |
| Hemoglobin, g/dl (IQR) | 13.7 (12.3-15) | 13.55 (12-14.7) | 0.651 |
| Patelets, x103/ul (IQR) | 221 (184-275) | 199 (156-277) | 0.139 |
| C-reactive protein, mg/l (IQR) | 123.06 (60.93-215.51) | 126.54 (70.27-190.4) | 0.894 |
| Procalcitonin, ng/ml (IQR) | 0.22 (0.13-0.54) | 0.27 (0.16-0.48) | 0.341 |
| Interleukin-6, pg/ml (IQR) | 86.5 (38.6-157) | 107 (53.9-175) | 0.162 |
| Creatinine, mg/dl (IQR) | 1.08 (0.8-1.46) | 1.11 (0.86-1.52) | 0.262 |
| Alanine aminotransferase, U/l (IQR) | 36 (23-58) | 40 (23-62) | 0.497 |
| D-dimer, ug/l (IQR) | 0.6 (0.39-1.67) | 0.71 (0.35-1.37) | 0.769 |
| Gender, n (%) Male |
35 (57%) | 118 (66%) | 0.251 |
| Obesity, n(%) Yes |
28 (46%) | 69 (38%) | 0.297 |
| Diabetes, n(%) Yes |
16 (26%) | 65 (36%) | 0.157 |
| Hypertension, n(%) Yes |
35 (57%) | 94 (52%) | 0.485 |
| Heart failure, n(%) Yes |
5 (8%) | 19 (11%) | 0.594 |
| Remdesivir administration, n(%) Yes |
23 (38%) | 76 (42%) | 0.535 |
| Tocilizumab admission, n(%) Yes |
27 (44%) | 100 (56%) | 0.126 |
| Virus strain, n(%) Wild/Alpha |
47 (71%) | 96 (55%) | 0.021 |
Abbreviation: IQR, Interquartile range.
P-values for gender, obesity, diabetes, hypertension, heart failure, remdesivir and tocilizumab admission and virus strain were recived from Chi-square Pearson.
3.2. Clinical and laboratory data on amantadine treated vs control group
The study group included 141 (58.5%) patients who received amantadine, and 100 (41.5%) controls treated without this agent. There were no statistically significant differences between groups regarding gender, height, weight, BMI, percentage of lung involvement, white blood cells count, hemoglobin levels, platelets count, C-reactive protein (CRP), procalcitonin, IL-6, creatinine levels, alanine aminotransferase (ALT) activity (Table 2 ). Patients receiving amantadine were notably older, with median age of 67 (IQR: 60-71) years, compared to non-amantadine groups (median: 63 [IQR: 58-68] years, P-value = 0.032), with slightly higher platelet counts (amantadine group 219.5 × 103/ul [176.5-285.5] vs non-amantadine group 185 × 103/ul [148-258] P-value = 0.014), and D-dimer (amantadine group 0.806 ug/l [0.413-1.566] vs non-amantadine group 0.522 [0.281-1.193] P-value = 0.025), at baseline. Arterial hypertension was statistically more common in the group treated with amantadine (59.5% vs 45%, P-value = 0.025). Of the 141 patients who received amantadine, 99 (70%) were infected with wild-type SARS-CoV-2, and 51 (30%) were infected with the Alpha variant. Of the 100 patients who were treated without amantadine, 44 (44%) were infected with wild-type SARS-CoV-2, and 56 (56%) were infected with the Alpha variant.
Table 2.
Characteristic of groups by amantadine use.
| Amantadine N = 141 |
Non-amantadine N = 100 |
P-value | |
|---|---|---|---|
| Age, years | 67 (60-71) | 63 (58-68) | 0.032 |
| Height, cm | 172 (164-178) | 172 (165-178) | 0.898 |
| Weight, kg | 88.5 (75-100) | 88 (80-103) | 0.747 |
| Body mass index | 29.705 (27.34-32.87) | 29.38 (26.55-34.94) | 0.904 |
| Percentage of lung involvement, % | 44.205 (25.065-58.79) | 45.180 (25.230-56.410) | 0.934 |
| White blodd cell, x103/ul | 8.145 (5.94-11.365) | 7.34 (5.02-9.77) | 0.077 |
| Hemoglobin, g/dl | 13.6 (12.05-14.7) | 13.4 (11.8-15.2) | 0.736 |
| Patelets, x103/ul | 219.5 (176.5-285.5) | 185 (148-258) | 0.014 |
| C-reactive protein, mg/l | 121.285 (75.98-202.595) | 127.23 (61.33-176.035) | 0.199 |
| Procalcitonin, ng/ml | 0.285 (0.15-0.545) | 0.24 (0.14-0.43) | 0.183 |
| Interleukin-6, pg/ml | 109 (51.4-181) | 95.2 (51.8-151) | 0.313 |
| Creatinine, mg/dl | 1.05 (0.855-1.475) | 1.12 (0.81-1.59) | 0.426 |
| Alanine aminotransferase, U/l | 40 (25-62) | 36.5 (20.5-59) | 0.194 |
| D-dimer, ug/l | 0.806 (0.413-1.566) | 0.522 (0.281-1.193) | 0.025 |
| Gender, n (%) Male |
85 (60%) | 68 (68%) | 0.223 |
| Obesity, n(%) Yes |
61 (43.2%) | 36 (36%) | 0.275 |
| Diabetes, n(%) Yes |
47 (33.3%) | 34 (34%) | 0.914 |
| Hypertension, n(%) Yes |
84 (59.5%) | 45 (45%) | 0.025 |
| Heart failure, n(%) Yes |
15 (10.5%) | 9 (9%) | 0.675 |
| Remdesivir administration, n(%) Yes |
54 (38.3%) | 45 (45%) | 0.297 |
| Tocilizumab admission, n(%) Yes |
81 (57.5%) | 46 (46%) | 0.079 |
| Mortality, n(%) Died |
84 (59.5%) | 91 (91%) | 0.001 |
| Virus strain, n(%) Wild/Alpha |
99 (70%) | 44 (44%) | 0.001 |
P-values for gender, obesity, diabetes, hypertension, heart failure, remdesivir and tocilizumab admission and virus strain were recived from Chi-square Pearson.
3.3. Amantadine associated general outcomes
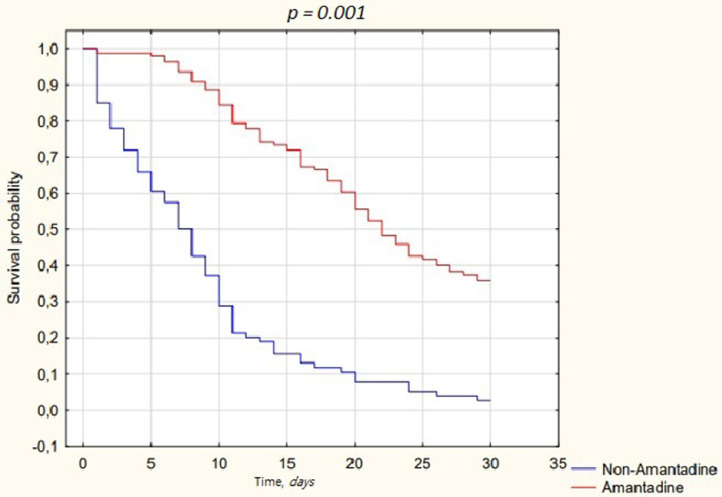
Overall mortality was high and reached 72.6% (n = 175). Mortality in the amantadine group was notably lower [59.5% (n = 84)] compared to non-amantadine group (91% (n = 91), P-value< 0.001). The median overall length of hospital stay was 12.7 (IQR 6.9-23.4) days, 19.9 (IQR 11.3-29.6) days for the amantadine group, and 6.6 (IQR 2.6-10.0) days for non-amantadine. For those who survived, the median length of treatment was 30 (IQR 21.9-30) days for the amantadine group, and 10.9 (IQR 3.9-13.9) days for the non-amantadine. For those who died, the median length of treatment was 15.8 (IQR 10.1-21.6) days for the amantadine group, and 6.5 (IQR 2.2-9.8) days for the non-amantadine (Figure 1 ).
Figure 1.
Kaplan-Meier curves displaying the estimated survival probability for amantadine related treatment.
We constructed multivariate Cox proportional hazards models for all statistically significant factors, administration of amantadine in line with the main goal of our study, comorbidities in line with the secondary goal of the study, and administration of remdesivir and TCZ in line with the tertiary goal. In multivariate proportional Cox hazards models (Table 3 ), administration of TCZ was associated with higher mortality (HR: 1.731,CI: 1.183-2.532, P-value = 0.005), while administration of amantadine associated with lower risk of death (HR: 0.220, CI: 0.146-0.333, P-value = 0.001). Virus strain was not a statistically significant factor in Cox proportional hazards model for mortality.
Table 3.
Cox proportional hazards model for mortality risk for all patients included in the study.
| Cox proportional hazards model for mortality |
||||
|---|---|---|---|---|
| P-value | HR | Lower 95% CI HR value | Upper 95% CI HR value | |
| Age, years | 0.275 | 1.011 | 0.991 | 1.031 |
| Platelets, x 103/ul | 0.174 | 0.999 | 0.996 | 1.001 |
| D-dimer, ug/l | 0.768 | 0.991 | 0.930 | 1.055 |
| Percentage of lung involvement, % | 0.395 | 1.004 | 0.995 | 1.013 |
| Tocilizumab, Yes (reference) |
0.005 | 1.731 | 1.183 | 2.532 |
| Remdesivir admission, Yes (reference) |
0.161 | 0.759 | 0.516 | 1.116 |
| Amantadine admission, Yes (reference) |
0.001 | 0.220 | 0.146 | 0.333 |
| Gender, Male (reference) |
0.114 | 1.368 | 0.927 | 2.019 |
| Hypertension, Yes (reference) |
0.269 | 1.248 | 0.843 | 1.847 |
| Heart failure, Yes (reference) |
0.671 | 1.139 | 0.624 | 2.079 |
| Diabetes, Yes (reference) |
0.978 | 1.006 | 0.683 | 1.481 |
| Obesity, Yes (reference) |
0.423 | 1.176 | 0.791 | 1.748 |
| Virus strain, Wild (reference) |
0.359 | 0.839 | 0.577 | 1.221 |
Abbreviation: HR, Hazard ratio.
3.4. Clinical characteristics of patients treated with tocilizumab depending on amantadine intake
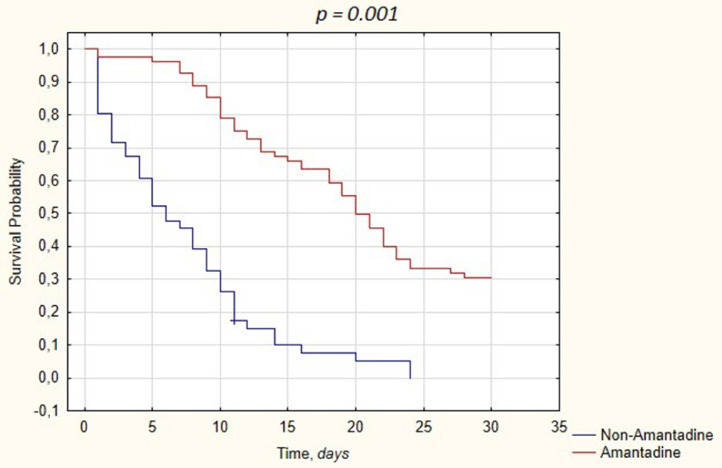
In line with the additional goals of this analysis, to reflect the combined effect of the agents with immunomodulatory potential, we analyzed the TCZ treatment-associated variables among 127 patients who received this agent in the dataset of ICU-treated cases. Subsequently, we have calculated the differences among patients who received TCZ by amantadine use, with the only significant difference between the groups being older age among patients receiving amantadine and administration of remdesivir. The multivariate Cox proportional hazards models constructed similarity to the statistical models of amantadine alone with administration of amantadine being the only factor associated with differences of mortality among patients receiving TCZ with severe COVID-19 (HR: 0.205 [CI: 0.131 - 0.322], P-value = 0.001) (Table 4 ). Kaplan-Meyer cumulative mortality was calculated with statistical significance of survival data (Figure 2 ).
Table 4.
Cox proportional hazards model for mortality risk for patients treated with tocilizumab.
| Cox proportional hazards model for mortality |
||||
|---|---|---|---|---|
| P-value | HR | Lower 95% CI HR value | Upper 95% CI HR value | |
| Age, years | 0.840 | 1.002 | 0.979 | 1.026 |
| White blood cell | 0.206 | 0.972 | 0.930 | 1.016 |
| Remdesivir administration, Yes |
0.405 | 1.207 | 0.775 | 1.878 |
| Amantadine admission, Yes |
0.001 | 0.205 | 0.131 | 0.322 |
| Gender, Male |
0.061 | 1.507 | 0.981 | 2.315 |
| Hypertension, Yes |
0.379 | 1.218 | 0.784 | 1.893 |
| Heart failure, Yes |
0.815 | 1.095 | 0.514 | 2.333 |
| Diabetes, Yes |
0.942 | 0.983 | 0.613 | 1.575 |
| Obesity, Yes |
0.504 | 1.159 | 0.752 | 1.788 |
Abbreviation: HR, Hazard ratio.
Figure 2.
Kaplan-Meier curves displaying the estimated survival probability for patients treated with TCZ divided into amantadine dependant groups.
3.5. Clinical characteristics of patients treated without tocilizumab depending on amantadine intake
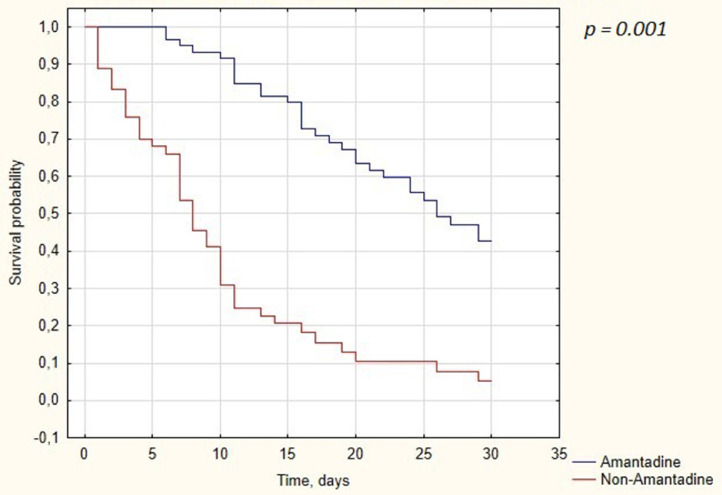
Of the 114 patients who did not receive TCZ, 60 (53%) were treated with amantadine and 54 (47%) without it. Groups were homogenous in terms of age, gender, height, weight, BMI, white blood cell, hemoglobin, CRP, procalcitonin, IL-6, creatinine, ALT, and D-dimer. Patients treated with amantadine had significantly higher levels of platelets (221 × 103/ul [184 -300] vs 182.5 × 103/ul [144 - 258], P-value = 0.04). Obesity and hypertension were more common in amantadine group (33 [55%] vs 18 [33.3%], P-value = 0.02) and (38 [63.3%] vs 21 [38.9%], P-value = 0.009), respectively. In the multivariate Cox proportional hazards models constructed identically to Section 3.4, administration of amantadine was the only factor associated with differences in mortality among patients with severe COVID-19 treated without TCZ (HR: 0.224 [CI: 0.133-0.375], P-value = 0.001). Kaplan-Meyer cumulative mortality was calculated with statistical significance of survival data (Figure 3 ).
Figure 3.
Kaplan-Meier curves displaying the estimated survival probability for patients treated without TCZ divided into amantadine dependant groups.
3.6. Characteristics of patients treated with amantadine divided into two groups based on time of admission
The median time from ICU admission to amantadine administration was 3 days (IQR 0-6 days). The decision to administer amantadine rested with the attending physician. The decisive issue in most cases was the patient's hydration status and temporary contraindications to the administration of amantadine, such as: severe decompensated heart failure (New York Heart Association grade IV), cardiomyopathy, myocarditis, second or third-degree atrioventricular block, bradycardia. To reflect possible bias related to the timing of administration, we divided patients treated with amantadine into those who received the first dose within 5 days of admission (n = 99 [70.2%]) to the ICU and those who received it after 5 days from intensive care initiation (42 [29.8%]). We set this limit as for majority of antiviral agents benefit beyond 5 days following COVID-19 symptom onset is not observed, and also to exclude the bias for early ICU mortality. The analyzed groups were homogeneous in terms of age, gender, BMI, laboratory results, comorbidities, and concomitant treatment but differed significantly in terms of mortality (34% points to the advantage of receiving amantadine after 5 days from admittance, P-value = 0.05). In multivariate proportional Cox hazards models (Table 5 ) administration of amantadine after 5th day was independently associated with lower mortality (HR: 0.560 [CI: 0.313-0.999], P-value = 0.050).
Table 5.
Cox proportional hazards model for mortality risk for patients treated with amantadine according to admission time groups.
| Cox proportional hazards model for mortality |
||||
|---|---|---|---|---|
| P-value | HR | Lower 95% CI HR value | Upper 95% CI HR value | |
| Remdesivir admission, Yes |
0.649 | 1.114 | 0.700 | 1.774 |
| Tocilizumab admission, Yes |
0.099 | 0.668 | 0.414 | 1.078 |
| Amantadine admission time, >5 day |
0.050 | 0.560 | 0.313 | 0.999 |
| Gender, Male |
0.309 | 1.275 | 0.799 | 2.036 |
| Hypertension, Yes |
0.717 | 1.089 | 0.686 | 1.730 |
| Heart failure, Yes |
0.405 | 1.377 | 0.649 | 2.924 |
| Diabetes, Yes |
0.322 | 0.783 | 0.482 | 1.271 |
| Obesity, Yes |
0.361 | 1.235 | 0.785 | 1.942 |
Abbreviation: HR, Hazard ratio.
3.7. Characteristic of patients treated in intensive care unit for at least 48 hours
In order to avoid a statistical error related to the enrollment of patients with immediate ICU mortality, we created an additional analysis excluding individuals with immediate (within 48 hours of ICU admission) mortality. The 48-hour period is based on the summary of product characteristics of amantadine, according to which amantadine achieves its full therapeutic effect after 48 hours (How long does it take for amantadine to start working? 2022). There were no statistically significant differences between particular groups regarding gender, age, height, weight, BMI, white blood cell count, hemoglobin levels, CRP, procalcitonin, IL-6, creatinine levels, and ALT activity. Patients receiving amantadine had slightly higher platelet counts and D-dimer at baseline. Arterial hypertension was statistically more common in the group treated with amantadine (59% vs 42%, P-value = 0.014).
In multivariate proportional Cox hazards models (Table 6 ), gender (HR: 1.526 [CI: 1.063-2.190], P-value = 0.023), and administration of TCZ was associated with higher mortality (HR: 1.543 [CI: 1.089-2.185], P-value = 0.015), while administration of amantadine associated with lower risk of death (HR: 0.242 [CI: 0.169-0.348], P-value = 0.001).
Table 6.
Cox proportional hazards model for mortality risk for patients who survived first 48 hours after admission to intensive care unit.
| Cox proportional hazards model for mortality |
||||
|---|---|---|---|---|
| P-value | HR | Lower 95% CI HR value | Upper 95% CI HR value | |
| Platelets, x 103/ul | 0.049 | 0.998 | 0.996 | 1.000 |
| D-dimer, ug/l | 0.410 | 1.026 | 0.966 | 1.090 |
| Remdesivir admission, Yes |
0.417 | 1.156 | 0.816 | 1.638 |
| Tocilizumab admission, Yes |
0.015 | 1.543 | 1.089 | 2.185 |
| Amantadine admission, Yes |
0.001 | 0.242 | 0.169 | 0.348 |
| Gender, Male |
0.023 | 1.526 | 1.063 | 2.190 |
| Hypertension, Yes |
0.829 | 0.963 | 0.684 | 1.357 |
| Heart failure, Yes |
0.779 | 1.093 | 0.591 | 2.022 |
| Diabetes, Yes |
0.840 | 1.038 | 0.728 | 1.480 |
| Obesity, Yes |
0.397 | 0.860 | 0.607 | 1.220 |
Abbreviation: HR, Hazard ratio.
3.8. Characteristics of patients diagnosed with wild-type of SARS-CoV-2 depending on amantadine intake
We confirmed wild-type SARS-CoV-2 infection in 143 patients. Out of 143 patients, 99 (70%) received amantadine for treatment. The groups differed in terms of BMI (amantadine 30.56 [26.67-35.15] vs non-amantadine 27.81 [26.39-29.92], P-value = 0.039). There were no statistical differences in terms of gender, age, height, weight, percentage of lung involvement, or basic laboratory tests. Obesity and arterial hypertension were much more common in the group treated with amantadine (45 [45%] vs 10 [23%], P-value = 0.009) and (58 [59%] vs 16 [36%], P-value = 0.014), respectively. The remaining comorbidities and administration of TCZ or remdesivir did not differ statistically. Mortality rate was significantly lower in group treated that amantadine (57% vs 91%, P-value = 0.001).
We constructed multivariate Cox proportional hazards models for all statistically significant factors, administration of amantadine in line with the main goal of our study, comorbidities in line with the secondary goal of the study, and administration of remdesivir and TCZ in line with the tertiary goal. In multivariate proportional Cox hazards models, administration of TCZ was associated with higher mortality (HR: 2.200 [CI: 1.248-3.876], P-value = 0.006), while administration of amantadine associated with lower risk of death (HR: 0.212 [CI: 0.123-0.365], P-value = 0.001).
3.9. Characteristics of patients diagnosed with Alpha strain of SARS-CoV-2 depending on amantadine intake
We confirmed Alpha (B.1.1.7) strain SARS-CoV-2 infection in 98 patients. Out of 98 patients in this group, 42 (43%) received amantadine for treatment. The groups differed in terms of D-dimer level (amantadine 0.855 [0.409-2.064] vs non-amantadine 0.512 [0.264-0.9715], P-value = 0.023). There were no statistical differences in terms of gender, age, height, weight, BMI, percentage of lung involvement, or basic laboratory tests. The comorbidities and administration of TCZ or remdesivir did not differ statistically. Mortality rate was significantly lower in group treated with amantadine (66% vs 91%, P-value = 0.002).
Similarly, we constructed multivariate Cox proportional hazards models for all statistically significant factors, administration of amantadine in line with the main goal of our study, comorbidities in line with the secondary goal of the study and administration of remdesivir and TCZ in line with the tertiary goal. In multivariate proportional Cox hazards models administration of amantadine associated with lower risk of death (HR: 0.216 [CI: 0.128-0.364], P-value = 0.001). Other factors were not statistically significant.
4. Discussion
To our knowledge, this is the first study to investigate the survival effects of amantadine in ICU patients treated for severe COVID-19 respiratory failure and ARDS. Our study demonstrates the survival benefits of IV administration of amantadine sulfate in patients treated in ICU alone and in combination with immunomodulatory TCZ. The majority of admitted patients presented the laboratory features of cytokine storm, with the median IL-6 levels in the group exceeding 100 pg/ml, regardless of amantadine use, which strengthens the possibility for the immunomodulatory effect in the analyzed cases. Furthermore, survival was improved in patients who received amantadine late, after 5 days from ICU admission, and also when cases who died within 48 hours of admission were excluded, which strengthens the suggestion that the survival benefit is a relevant scientific finding. It must be emphasized that we discard the possibility of the direct antiviral but not immunomodulatory effect at this stage of the disease. Possible immunomodulatory properties of amantadine include effects on altered T cell function (Wandinger et al., 1999), production of IL-2, reduction of the production of the pro-inflammatory cytokines, specifically interferon-γ and tumor necrosis factor-α (Kubera et al., 2009).
In our study, a negative effect of TCZ on ICU survival was observed. It is most likely related to the more severe general condition of patients treated with TCZ. To avoid a sample selection bias, as TCZ has a proven therapeutic effect in the treatment of patients with COVID-19 in severe general condition and cytokine storm (Biran et al., 2020; Chober et al., 2022), we have created additional analyzes to show the effectiveness of amantadine in treating patients in the context of the use of other immunomodulatory drugs. Survival benefit associated with amantadine was retained in this group.
There are no published data on the amantadine treatment in patients with COVID-19 and ARDS requiring mechanical ventilation and ICU treated; however, amantadine has been widely used as a neurostimulant in the intensive care setting before, for reasons other than severe COVID-19 respiratory failure. The study by Leclerc et al. (2021) examined the effect of neurostimulants such as amantadine and modafinil on the treatment effects of patients hospitalized for intracerebral hemorrhage, ischemic stroke, or subarachnoid hemorrhage. A total of 87 patients were assessed over the 3.7 year study period. Of the 62 patients treated with amantadine monotherapy, 34 (55%) were considered treatment responders. Respondents were more likely to be discharged home or for emergency rehabilitation compared to non-responders. The results of our studies cannot be directly compared, while both studies showed a positive effect of the use of amantadine in patients hospitalized in the ICU. Furthermore, in the study by Rahman Abbasivash et al, the effect of oral administration of amantadine on the neurological outcomes of patients with diffuse axonal injury in the ICU was evaluated (Abbasivash et al., 2019). All patients in this study were intubated and received mechanical ventilation. They were divided into two groups: patients receiving amantadine and placebo. There were no significant differences in the duration of mechanical ventilation, hospitalization and mortality in both groups. The study found no effect of amantadine on the final results, probably due to the small group of patients studied, and the low overall mortality among the patients studied (n = 5; 7%). In an analysis by Saniova et al. (2004), patients with severe brain injuries treated with amantadine sulfate in addition to standard therapy had a lower mortality rate and higher Glasgow Coma Scale than those treated with standard therapy alone. These studies demonstrate the favorable effects of amantadine in the ICU setting, but cannot be directly translated into the COVID-19 patients.
The main advantage of our analysis is a large dataset of patients characterized by high homogeneity, which allows the impact of the drug under study to be assessed. The collection of data from one center allowed us to exclude the possibility of an error related to different standards of individual care. Being an observational study, we are aware of the high risk of information bias which could be the cause of unexpected results. Paradoxical results (regardless of their statistical significance) raise doubts about the accuracy of the data. As examples for repeated unexpected results: The resence of heart failure decreases the hazard of death by 10% (HR = 0.9), presence of diabetes mellitus lower the hazard of death by 30%. and presence of hypertension decrease the hazard of death by 13% (HR = 0.83). A further randomized clinical trial is needed to approve or disapprove our results. This can be considered as a pilot study. A randomized clinical trial is needed to confirm the utility of amantadine in treatment of COVID-19 patients.
5. Conclusion
Patients treated in ICUs due to severe respiratory failure may benefit from the initiation of amantadine sulfate treatment. The significantly lower mortality among patients who started treatment later in hospitalization suggests no effect of amantadine on the acute phase of SARS-CoV-2 infection. The exact way the medicine works is unknown and requires further studies. It seems justified to conduct further studies, including a randomized controlled trial, especially due to the low cost of possible treatment if the effectiveness is confirmed.
6. Limitations
Our research has several limitations that we are aware of. They mainly concern with its retrospective nature, lack of randomization of the treatment groups, and the lack of clear criteria for inclusion in the administration of amantadine. However, our analyzes were multidirectional and point to a potential survival signal, and therefore seem highly valuable basis for future considerations.
Also, the administration of amantadine was based on the independent decision of the clinician, which may introduce a decision bias. For this purpose, analyses excluding early mortality patients were performed.
Funding
The study was funded by the National Centre For Research and Development, Agreement No. SZPITALE-JEDNOIMIENNE/27/2020, November 20, 2020, for implementation and financing of a non-competitive project (PREVENTION AND TREATMENT: COVID-19) titled: “Development of modern laboratory technologies, IT and bioinformatics dedicated to the diagnosis and prevention of SARS-CoV-2 infections” implemented as part of the recruitment “Support for homonymous hospitals in combating the spread of SARS-CoV-2 infection and treating COVID-19”.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki, and protocol was approved by the Bioethical Committee of Pomeranian Medical University, Szczecin, Poland (approval number: KB-0012/92/2020).
Data availability statement
The original anonymous dataset is available on request from the corresponding author at Daniel.chober@pum.edu.pl.
CRediT authorship contribution statement
Daniel Chober: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Zenon Czajkowski: Conceptualization, Validation, Resources, Supervision, Project administration. Bogusz Aksak-Wąs: Resources. Katarzyna Dalewska-Kucharczyk: Resources. Katarzyna Hołubczak: Resources. Sylwia Karasińska-Milchert: Resources. Mateusz Jaremko: Resources. Miłosz Skowron: Resources. Malwina Karasińska-Cieślak: Resources. Miłosz Parczewski: Validation, Resources, Supervision, Project administration.
Declaration of competing interest
The authors have no competing interests to declare.
Acknowledgments
We would like to acknowledge all the medical personnel involved in the care of COVID-19 patients.
References
- Abbasivash R, Valizade Hasanloei MA, Kazempour A, Mahdkhah A, Shaaf Ghoreishi MM, Akhavan Masoumi G. The effect of oral administration of amantadine on neurological outcome of patients with diffuse axonal injury in ICU. J Exp Neurosci. 2019;13 doi: 10.1177/1179069518824851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biran N, Ip A, Ahn J, Go RC, Wang S, Mathura S, et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol. 2020;2:e603–e612. doi: 10.1016/S2665-9913(20)30277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Han Y, Yang L, Kim T, Nair M, Harschnitz O, Wang P, Zhu J, Koo SY, Tang X, Lacko L, Chandar V, Bram Y, Zhang T, Zhang W, He F, Caicedo J, Huang Y, Evans T, van der Valk P, Titulaer MJ, Spoor JKH, Furler RL, Canoll P, Goldman J, Przedborski S, Schwartz R, Ho D, Studer L. SARS-CoV-2 infection causes dopaminergic neuron senescence. Research Square. 21 May 2021 doi: 10.1016/j.stem.2023.12.012. https://www.researchsquare.com/article/rs-513461/v1 (accessed DD Month YYYY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chober D, Aksak-Wąs B, Bobrek-Lesiakowska K, Budny-Finster A, Hołda E, Mieżyńska-Kurtycz J, Jamro G, Parczewski M. Effectiveness of tocilizumab in patients with severe or critical lung involvement in COVID-19: a retrospective study. J Clin Med. 2022;11:2286. doi: 10.3390/jcm11092286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Yano HH, Kalpana GV, Javitch JA, Berman JW. Dopamine receptor activation increases HIV entry into primary human macrophages. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler MW, Sliwinski M, Coyle PK, Masur DM, Doscher C, Krupp LB. The effects of amantadine and pemoline on cognitive functioning in multiple sclerosis. Arch Neurol. 1996;53:185–188. doi: 10.1001/archneur.1996.00550020101021. [DOI] [PubMed] [Google Scholar]
- Ghalaenovi H, Fattahi A, Koohpayehzadeh J, Khodadost M, Fatahi N, Taheri M, et al. The effects of amantadine on traumatic brain injury outcome: a double-blind, randomized, controlled, clinical trial. Brain Inj. 2018;32:1050–1055. doi: 10.1080/02699052.2018.1476733. [DOI] [PubMed] [Google Scholar]
- Giacino JT, Whyte J, Bagiella E, Kalmar K, Childs N, Khademi A, et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med. 2012;366:819–826. doi: 10.1056/NEJMoa1102609. [DOI] [PubMed] [Google Scholar]
- Gualtieri T, Chandler M, Coons TB, Brown LT. Amantadine: a new clinical profile for traumatic brain injury. Clin Neuropharmacol. 1989;12:258–270. [PubMed] [Google Scholar]
- How long does it take for amantadine to start working? Drugs.com. https://www.drugs.com/medical-answers/long-amantadine-start-working-3546573/, 2022 (accessed 22 May 2022).
- Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology. 2012;78:1096–1099. doi: 10.1212/WNL.0b013e31824e8f0d. [DOI] [PubMed] [Google Scholar]
- Influenza antiviral drug resistance, Centers for Disease Control and Prevention. https://www.cdc.gov/flu/treatment/antiviralresistance.htm, 2021 (accessed 22 May 2022).
- Jiménez-Jiménez FJ, Alonso-Navarro H, García-Martín E, Agúndez JAG. Anti-inflammatory effects of amantadine and memantine: possible therapeutics for the treatment of Covid-19? J Pers Med. 2020;10:1–15. doi: 10.3390/jpm10040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Smith GS, Butters M, Donnell AJ, Dixon E, Yilong C, et al. Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: a study using positron emission tomography (PET) Brain Inj. 2005;19:471–479. doi: 10.1080/02699050400025059. [DOI] [PubMed] [Google Scholar]
- Kubera M, Maes M, Budziszewska B, Basta-Kaim A, Leśkiewicz M, Grygier B, et al. Inhibitory effects of amantadine on the production of pro-inflammatory cytokines by stimulated in vitro human blood. Pharmacol Rep. 2009;61:1105–1112. doi: 10.1016/s1734-1140(09)70173-2. [DOI] [PubMed] [Google Scholar]
- Leclerc AM, Riker RR, Brown CS, May T, Nocella K, Cote J, et al. Amantadine and modafinil as neurostimulants following acute stroke: a retrospective study of intensive care unit patients. Neurocrit Care. 2021;34:102–111. doi: 10.1007/s12028-020-00986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang P, Liu Y, Wu S, Yi X, Zhang S, et al. Early amantadine treatment reduces the risk of death in patients with large hemisphere infarctions:a Chinese hospital-based study. BMC Neurol. 2021;21:419. doi: 10.1186/s12883-021-02444-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto AS, Arden NH. Implications of viral resistance to amantadine in control of influenza. Clin Infect Dis. 1992;15:362–367. doi: 10.1093/clinids/15.2.362. discussion 368-9. [DOI] [PubMed] [Google Scholar]
- Peeters M, Romieu P, Maurice T, Su TP, Maloteaux JM, Hermans E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. Eur J Neurosci. 2004;19:2212–2220. doi: 10.1111/j.0953-816X.2004.03297.x. [DOI] [PubMed] [Google Scholar]
- Saniova B, Drobny M, Kneslova L, Minarik M. The outcome of patients with severe head injuries treated with amantadine sulphate. J Neural Transm (Vienna) 2004;111:511–514. doi: 10.1007/s00702-004-0112-4. [DOI] [PubMed] [Google Scholar]
- Shafiee S, Ehteshami S, Moosazadeh M, Aghapour S, Haddadi K. Placebo-controlled trial of oral amantadine and zolpidem efficacy on the outcome of patients with acute severe traumatic brain injury and diffuse axonal injury. Caspian J Intern Med. 2022;13:113–121. doi: 10.22088/cjim.13.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanjuntak Y, Liang JJ, Lee YL, Lin YL. Japanese encephalitis virus exploits dopamine D2 receptor-phospholipase C to target dopaminergic human neuronal cells. Front Microbiol. 2017;8:651. doi: 10.3389/fmicb.2017.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmaschuk S, Will MC, Meyers T. Amantadine to treat cognitive dysfunction in moderate to severe traumatic brain injury. J Trauma Nurs. 2015;22:194–203. doi: 10.1097/JTN.0000000000000138. quiz E1–2. [DOI] [PubMed] [Google Scholar]
- Stoof JC, Booij J, Drukarch B. Amantadine as N-methyl-D-aspartic acid receptor antagonist: new possibilities for therapeutic applications? Clin Neurol Neurosurg. 1992;94:S4–S6. doi: 10.1016/0303-8467(92)90006-o. [DOI] [PubMed] [Google Scholar]
- Wandinger KP, Hagenah JM, Klüter H, Rothermundt M, Peters M, Vieregge P. Effects of amantadine treatment on in vitro production of interleukin-2 in de-novo patients with idiopathic Parkinson's disease. J Neuroimmunol. 1999;98:214–220. doi: 10.1016/s0165-5728(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Zhong J, Li J, Ni C, Zuo Z. Amantadine alleviates postoperative cognitive dysfunction possibly by preserving neurotrophic factor expression and dendritic arborization in the hippocampus of old rodents. Front Aging Neurosci. 2020;12:427. doi: 10.3389/fnagi.2020.605330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original anonymous dataset is available on request from the corresponding author at Daniel.chober@pum.edu.pl.