Abstract
The incidence of Parkinson’s disease (PD) is expected to increase as our population ages and will likely strain the projected capacity of our health care system. Despite being the most common movement disorder, there have been few noninvasive therapeutic advances for people with PD since the first levodopa clinical trial in 1961. The study of PD pathogenesis, combined with an appreciation for the biochemical mechanisms by which physical activity and exercise may impact physiology, has resulted in emerging hypotheses for new modifiable risk factors for PD. Physical activity and exercise as a means of preventing PD, or maintaining the functionality of people with PD, are a promising area of investigation. Conversely, physical inactivity is implicated in many disease states, some of which are also correlated with the development of PD, such as metabolic syndrome. The primary relationship between these diseases is likely rooted in heightened inflammation and oxidative stress at the cellular level. Physical activity and exercise as a means of attenuating inflammation have led to increased interest in related potential therapeutic targets for PD. Ultimately, these findings may translate into low-cost, universally available therapies for PD disease modification or prevention.
Keywords: Parkinson’s disease, exercise, metabolic syndrome, physical activity, disease modification
Better to hunt in fields for health unbought
Than fee the doctor for a nauseous draught.
The wise for cure on exercise depend;
God never made his work for man to mend.
John Dryden, Epistle to John Dryden of Chesterton, 1700
Parkinson’s disease (PD) is expected to increase significantly in future decades. Identifying measures for preventing the consequent increased burden on people with PD and on society is imperative. Drugs that target the underlying causes of PD would be expected to reduce disease burden, but the etiology of PD is likely multifactorial, including genetic and nongenetic factors, and an effective disease-modifying therapy remains elusive.1–9 A complementary approach would be to identify modifiable risk factors for PD. Physical activity,10 defined as any bodily movement caused by skeletal muscles resulting in energy expenditure, and exercise,10 defined as structured, repetitive physical activity with a goal of physical fitness, have long been known to reduce mortality and lower the risk of developing many diseases, including cardiovascular disease, stroke, diabetes, and, more recently, dementia.11–21 Conversely, physical inactivity is a contributing factor in many diseases, including metabolic syndrome (MetS), which is a constellation of symptoms including abdominal adiposity, insulin resistance, dyslipidemia, and hypertension.22–24 Inflammation and oxidative stress, the central mechanisms underlying MetS, are also putative pathogenetic mechanisms in PD.25,26 Given the integral role of oxidative stress and inflammation in the pathogenesis of both PD and MetS, it is not surprising that MetS may increase the risk of developing PD.27,28 In this review, we will consider the benefits of physical activity, including exercise, and the adverse metabolic effects of physical inactivity as they relate to Parkinson’s disease risk and progression.
Physical Activity, Exercise, and Reduction of Parkinson’s Disease Risk — Epidemiologic Studies
A beneficial relationship between physical activity and PD was first suggested in 1992, when Sasco and colleagues reported that the future risk of PD was reduced in men who played sports in college and adult life and that PD risk was increasingly lower as activity levels increased.29 This finding has been replicated in nearly all subsequent epidemiologic studies30,31 (Table 1). For example, among the more than 200,000 participants in the NIH-AARP Diet and Health Study cohort, those who participated in consistent and frequent moderate to vigorous activities had a 40% lower risk of developing PD compared with sedentary participants. Similarly, risk of PD was reduced in participants in the Cancer Prevention Study II Nutrition Cohort who engaged in vigorous but not light physical activity.32 In the latter 2 large prospective cohort studies, greater intensity of physical activity was associated with greater reduction in PD risk.32,33
TABLE 1.
Prospective cohort studies investigating the impact of physical activity on Parkinson’s disease
| Lead author, year | Duration of follow-up | Population | Independent variable | Dependent variable | Results |
|---|---|---|---|---|---|
| Sasco, 199229 | Harvard: 1916–1950 to 1978; U Penn: 1931–1940 to 1976 | 50,002 university-educated men | Participation in college varsity teams or moderate or heavy sports in adulthood | PD diagnosis | Lower, but nonsignificant, risk of PD for sports teams participants (any duration: OR, 0.78; 95% CI, 0.34–1.8). Similarly, lower but nonsignificant risk of PD for adult exercise (any exercise: OR, 0.74; 95% CI, 0.31–1.7). |
| Chen, 200536 | Men: 1986–2000; women: 1976–1998 | 48,574 men from the Health Professionals Follow-Up Study and 77,254 women from the Nurses’ Health Study | Average time spent on specified activities (eg, walking, playing tennis) per week, number of stairs climbed per day), resulting in calculating METs | PD diagnosis | Higher levels of physical activity reduced the risk of PD for men (RR, 0.5; 95% CI, 0.3–0.09; P = 0.007) but in women only strenuous physical activity aged 18–22 trended toward significance (RR, 1.2 for 3 months vs 0.5; 95% CI, 0.2–1.4 for 10–12 months of strenuous activity; test for trend, P = 0.06). |
| Logroscino, 2006115 | 1988–1993, plus death certificates to 1997 | 10,714 men from the Harvard Alumni Health Study (subset of Sasco, 1992, excluding PD prevalent in 1988) | Physical activity for 1988: daily number of blocks walked and stairs climbed, participation in sports and recreational activities in the past week, resulting in estimated energy expenditure categories | PD diagnosis after 1988 | Nonsignificant trend to reduction of PD risk with physical activity in 1988; RR, 1000–1999 kcal/week activity: 1.15 (95% CI, 0.71–1.88), RR ≦ 3000 kcal/week: 0.63 (95% CI, 0.36–1.12); P for trend = 0.12. |
| Thacker, 200832 | 1992–2001 | 143,325 participants in the Cancer Prevention Study II Nutrition Cohort | Light compared with moderate- to vigorous-intensity recreational activity | PD diagnosis | Reduced risk of developing PD most significant for moderate to vigorous activity (RR, 0.6; 95% CI, 0.4–1.0; P = 0.02). No gender difference. |
| Xu, 201033 | 1996–2006 | 213,701 participants of the NIH-AARP Diet and Health Study cohort | Physical activities over 4 periods (aged 15–18, 19–29, and 35–39 and in the past 10 years) | PD diagnosis | Those with moderate to vigorous physical activity aged 35–39 (OR, 0.62; 95% CI, 0.48–0.81; P = 0.005) or in past 10 years (OR, 0.65; 95% CI, 0.51–0.83; P = 0.0001) had significantly lower risk of PD. |
| Saaksjarvi, 201467 | 1973–1999 | 6,715 men and women aged 50–79 years from the Finnish Mobile Clinic Health Examination Survey | Leisure-time physical activity | PD Diagnosis | Heavy leisure-time physical activity associated with lower PD risk compared with those with no activity (RR, 0.27; 95% CI, 0.08–0.90). |
| Yang, 201534 | 1997–2010 | 43,368 individuals from the Swedish National March Cohort | Household, commuting, and occupational activities, leisure-time exercise, total daily physical activity; exercise during different age periods; estimated MET hours per day | PD diagnosis | For men there was a reduction in PD for medium vs low physical activity (HR, 0.55; 95% CI, 0.35–0.87), high vs low household, commuting, and leisure-time exercise (HR, 0.53; 95% CI, 0.33, 0.85) and for a longer duration of household and commuting physical activity (HR, 0.50; 95% CI, 0.31, 0.81). No risk reduction for women. |
CI, confidence interval; HR, hazard ratio; OR, odds ratio; PD. Parkinson’s disease; RR, relative risk; MET, metabolic equivalent.
Participants in the Swedish National March reported on a broad spectrum of physical activities, including leisure, occupational, household, and commuting activities, at study enrollment.34 Over approximately 13 years of follow-up, 286 of 43,368 participants developed PD. People with greater than 6 hours per week of activities when enrolled had a 43% lower risk of PD. This study is particularly important, as it demonstrates that all physical activity, not just exercise, can reduce the risk of PD. Incremental lifestyle changes, such as walking to work or taking the stairs, may be more realistically achievable for many. Although not studied directly, similar incremental changes may benefit people with PD, given the many barriers that people with PD face in order to exercise.35
Two studies suggested that physical activity may influence PD risk differently for men and women. In the Health Professionals Follow-Up and Nurses’ Health studies, higher levels of exercise reduced the risk of PD for men but not for women, although more strenuous exercise showed a nonsignificant reduced risk in women.36 Similarly, in the Swedish National March study, physical activity was associated with a decreased PD risk for men, but the benefit was less clear for women.34 Men and women may have different biological responses to physical activity. For instance, the long-term metabolic consequences of exercise appear to be of greater benefit in men, whereas women exhibit increased stabilization of resting metabolic rate, post-prandial triglyceride concentrations, blood glucose concentrations, fuel selection, and fasting lipolytic rates, with a consequent reduction in the overall impact on metabolism after exercise for women compared with men.37 Although differential effects in men and women are plausible, other large studies found physical activity to similarly benefit both men and women, were limited to men, or did not evaluate sexes separately.29,31–33 Determining whether physical activity has different effects in men and women will be important in developing appropriate interventions.
Taken together, these studies provide compelling evidence for an inverse association between physical activity or exercise and risk of PD. Understanding the mechanisms underlying this beneficial effect may lead to key understandings for the prevention of PD.
Molecular Mechanisms Underlying the Impact of Physical Activity and Exercise on Parkinson’s Disease Physiology
Exercise has been shown to upregulate the production of growth factors and receptors, attenuate dopaminergic neuron damage, and reduce cellular inflammation and oxidative stress (Fig. 1).20,38–41 Rodent models of PD using the neurotoxins 6-hydroxydopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) enabled researchers to study the impact of exercise on the striatum in greater detail.40 In rodent models, brain-derived neurotrophic factor (BDNF) protects dopaminergic cells from 6-OHDA- and MPTP-induced damage, as well as widely promotes synaptic transmission.42 In healthy humans, exercise promotes neuroprotective growth factors such as BDNF and glial-derived neurotrophic factor.43 Furthermore, serum levels of BDNF are significantly reduced in people with early PD compared with healthy controls.44 Exercise may also increase the production of dopamine receptors in the striatum. For example, in a small study of people with PD, those randomized to undergo intensive treadmill training exhibited an increase in dopamine receptor DA-D2R expression using positron emission tomography imaging.45 Dopaminergic signaling neuroplasticity may be integral to affecting PD pathogenesis.
FIG. 1.
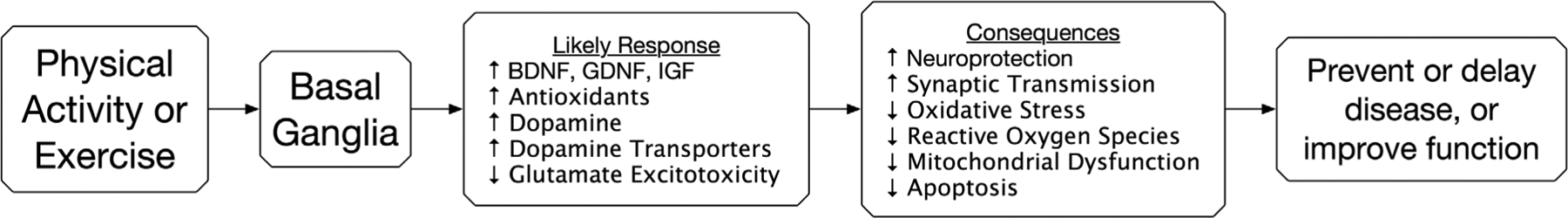
Proposed action of physical activity and exercise on the basal ganglia at the cellular level.
Abbreviations: BDNF, Brain-Derived Neurotrophic Factor; GDNF, Glial-Derived Neurotrophic Factor; IGF, Insulin-like Growth Factor
Exercise also attenuates damage to dopaminergic neurons within the motor circuits. Dopamine depletion, as seen in PD, may result in glutamatergic-mediated hyperexcitability that damages the medium spiny neurons critical to the functionality of the basal ganglia. However, rodent models have shown that exercise can attenuate this cellular injury. For example, exercise may induce postsynaptic alterations of glutamatergic neurotransmission because of changes in the expression of the alpha-amino-3-hydroxy-5-methyl-4-isoazoleproprionic acid receptors within the medium spiny neurons, resulting in tempering of this pathologic hyperexcitability.46 Rodent models of PD exposed to 6-OHDA or MPTP have shown preserved striatal dopamine levels with treadmill running postexposure and increased loss of dopamine neurons with forced nonuse of a forelimb from casting.47–50 In addition, in 1 series of animal experiments, rodent limb disuse from casting resulted in decreased levels of dopamine markers in the striatal dopamine system, including the dopamine transporter, vesicular monoamine transporter-2, and tyro-sine hydroxylase, negatively impacting the homeostasis of this system.51 Importantly, the beneficial effects of exercise may use different mechanisms in normal than in diseased basal ganglia.52 The decreased motor function that results from loss of striatal dopaminergic neurons and overall dopamine depletion may be both a symptom of and a contributor to further destruction of this integral circuit.51
Another important factor is the impact of exercise on oxidative stress and mitochondrial dysfunction. For example, exercise is associated with protection against proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6.25 This is particularly important in the substantia nigra, which is especially susceptible to mitochondrial dysfunction and neuroinflammatory processes as a result of chronic oxidative damage.53,54 Rodent models of PD demonstrated both elevations of p53 gene expression and increased release of cytochrome c from the mitochondria, resulting in cell death and ultimately further neurodegeneration within this network.40 Such mitochondrial disturbances may be attenuated by exercise.40 Although exercise acutely increases the production of reactive oxygen species, rodent models demonstrated that activity chronically suppresses systemic oxidative stress through an adaptive process that increases antioxidants (eg, superoxide dismutase and glutathione) and oxidative damage repair enzymes.55,56 Consistent exercise can even increase serum levels of glutathione in humans.57 This is especially important because in human studies, people with PD have decreased baseline levels of antioxidants, such as glutathione and urate, compared with people without PD, providing support for recent clinical trials.58–61
Physical activity and exercise disrupt the molecular mechanisms underlying PD pathogenesis in numerous ways. Reduction of cellular oxidative stress, mitochondrial dysfunction, and inflammation paired with upregulation of beneficial growth factors and antioxidants results in a compelling explanatory model for why physical activity and exercise should have an integral role in discussions of PD therapy.
Physical Inactivity: Parkinson’s Disease and Metabolic Syndrome
Physical inactivity is associated with the development of many devastating chronic diseases, including type 2 diabetes and obesity.24 Some studies suggest a relationship between insulin resistance, diabetes, obesity, dyslipidemia, or diet and the development of PD (Table 2).27,62–71 Together, these traits define an endocrine disease of epidemic proportion rooted in systemic inflammation and oxidative stress: metabolic syndrome (MetS).26 MetS is a cluster of abnormalities that contribute to an increased risk of cardiovascular disease, type 2 diabetes mellitus, kidney disease, and overall mortality.22,23 Although diabetes is known to cause nerve damage such as retinopathy and peripheral neuropathy, little is understood about the relationship between MetS and the development of PD.27,72
TABLE 2.
Selected studies investigating the relationship between components of metabolic syndrome and Parkinson’s disease
| Lead author, year | Study type | Population | Independent variable | Dependent variable | Results |
|---|---|---|---|---|---|
| Abbott, 200263 | Cohort | 7990 men in the Honolulu Heart program with adiposity measures in 1965–1968, followed for 30 years | BMI, subscapular skin-fold thickness (SSF), and triceps skin-fold thickness (TSF) | PD diagnosis | 3-Fold increased PD incidence in highest quartile of TSF vs lowest (3.7/100,000 to 11.1/100,000 person-years); similar but weaker pattern for SSF, BMI. |
| Hu, 200666 | Cohort | 22,367 Finnish men and 23,439 women aged 25–59, with a follow-up period of 18.8 years | BMI category | PD diagnosis | Multivariate-adjusted direct association between BMI and the risk of PD (for subjects aged 25–49 and 50–59 years, never smokers and smokers, participants diagnosed with PD before and after 65 years of age). |
| Hu, 200769 | Cohort | 51,552 Finnish men and women aged 25–74 years old, with a follow-up period of 18 years. | Type 2 diabetes diagnosis | PD diagnosis | Type 2 diabetes was associated with an increased risk of PD (male HR, 1.80; 95% CI, 1.03–3.15; female HR, 1.93; 95% CI, 1.05–3.53; combined HR, 1.85: 95% CI, 1.23–2.80). |
| Driver, 200879 | Cohort | 21,841 US male physicians in the Physicians’ Health Study cohort over 23 years | Type 2 diabetes | PD diagnosis | Increased risk of PD in participants with diabetes (RR, 1.34; 95% CI, 1.01–1.77). |
| Palacios, 201174 | Cohort | 147,096 participants in the Cancer Prevention Study II Nutrition Cohort from 1992 to 2005 | BMI, waist circumference, history of diabetes | PD diagnosis | Neither diabetes nor BMI was associated with PD risk (P > 0.05). |
| Xu, 201178 | Cohort | 288,662 participants with self-reported diabetes in 1995–1996 in the National Institutes of Health-AARP Diet and Health Study | Diabetes diagnosis | PD diagnosis | Significantly higher PD risk among diabetic patients, especially for individuals who had diabetes for more than 10 years (OR, 1.75; 95% CI, 1.36–2.25). |
| Cereda, 201280 | Case-Control | 89 patients with a diagnosis of diabetes prior to PD onset matched to control group by BMI and PD duration, from 2007 to 2010 | Diabetes diagnosis | UPDRS motor score | Diabetes was associated with higher UPDRS motor scores, activities of daily living scores, more severe Hoehn & Yahr staging, and higher levodopa treatment doses (P < 0.05). |
| Saaksjarvi, 201467 | Cohort | 6,715 men and women from the Finnish Mobile Clinic Health Examination Survey | BMI category | PD diagnosis | Elevated risk of developing PD for higher BMI levels (after excluding the first 15 years of follow-up, P = 0.02). |
| Chen, 201465 | Meta-analysis | PubMed, EMBASE, and the Chinese National Knowledge Infrastructure (CNKI) databases to identify studies with key words overweight, obesity, and PD, retrieved until September 30, 2013. | BMI category | PD diagnosis | Being overweight may increase risk of PD; however, there was only a statistically significant difference between 25 ≤ BMI < 30 and BMI < 25 (RR, 1.17; 95% CI, 1.03–1.32; P = 0.03). |
| Wang, 201568 | Meta-analysis | 10 prospective studies from a PubMed search | BMI category | PD diagnosis | Higher BMI was not associated with PD risk (summary RR, 1.00; 95% CI, 0.89–1.12). |
BMI, body mass index; CI, confidence interval; HR, hazard ratio; OR, odds ratio; PD, Parkinson’s disease; RR, relative risk; UPDRS, Unified Parkinson’s Disease Rating Scale.
Most studies have evaluated the risk of PD from a single component of MetS, rather than the sum of its parts. For example, increased body mass index and adiposity are associated with the risk of PD in a dose-dependent fashion in some62,64,65 but not all studies.67,73 Rodent studies suggest a possible mechanism for this, as obesity has been shown to produce a-synucleinopathy through changes in Akt phosphorylation states, which are important for insulin and neurotropic factor signaling.74 Furthermore, adipocytes secrete the cytokines TNF-α and IL-6, which precipitate a proinflammatory intracellular cascade, subsequently inducing oxidative stress and an inflammatory state25,75; however, IL-6 is even increased in subjects with low levels of physical activity independent of obesity.76
Several prospective studies have implicated diabetes as an independent risk factor for PD.68,77,78 Furthermore, diabetes that precedes PD has been associated with more severe PD symptomology, as determined by higher UPDRS motor scores and larger doses of levodopa.79,80
The proposed impact of diabetes on PD risk has a plausible molecular basis. The substantia nigra contains numerous dopaminergic neurons and insulin receptors.81 Selective loss of the dopaminergic cells is integral to the development of PD, and immunologic studies suggest dysfunction of the insulin/insulin receptor system in PD as well.82,83 For example, Moroo and colleagues used immunohistochemistry to stain brain tissue of people diagnosed with PD using anti-insulin receptor antibodies.83 They found that neurons in multiple regions, including the substantia nigra pars compacta, did not stain, suggesting defects in the insulin/insulin receptor system in these people.83 Levels of insulin receptor mRNA and tyrosine hydroxylase mRNA — essential for the rate-limiting step of dopa-mine biosynthesis — were also found to be depressed in people with PD compared with healthy controls.84,85 In addition to impaired insulin signaling, PD and diabetes share similar pathways of molecular dys-regulation, including mitochondrial dysfunction, oxidative stress, and augmented inflammation.86,87
In a related field, the composition of gut microbiota has been correlated with the development of obesity, type 2 diabetes mellitus, and MetS and is now suggested to also be associated with PD.88 Keshavarzian and colleagues obtained sigmoid mucosal biopsies and fecal samples from people with and without PD. The subjects with PD were found to have a significantly higher quantity of “proinflammatory” gut bacteria in their colonic mucosa.88 In a related study, urinary concentrations of indican, a metric directly associated with dysbiosis and small intestinal bacterial overgrowth, were measured in people with PD and healthy subjects.89 Indican urinary concentrations were significantly higher in people with PD compared with controls even when adjusted for factors such as body mass index, constipation, or the consumption of dairy products, suggesting greater intestinal dysbiosis in these people.89
Understanding the existence of common molecular and pathophysiologic pathways between PD and MetS may point toward novel therapies for PD. Just as physical inactivity is correlated with the development of MetS,24 physical activity may be one solution for this disease.90–92 Current targeted treatments for MetS may also be appropriated for people with PD symptoms. The potential relationship between MetS and PD necessitates attention given the high prevalence of MetS and the expected increase in the incidence of both diseases.22
Potential Interventions for Parkinson’s Disease and Future Therapeutic Directions
Certain forms of physical activity such as physical therapy have long been recognized to provide short-term benefits for motor impairment in people with Parkinson’s disease.93 Recently, randomized trials investigating many types of physical activity of varying degrees of intensity, including treadmill training, dance, and tai chi, have similarly demonstrated improvement in certain PD motor symptoms.94–96 There have been few systematic comparisons among types of physical activity, and to date no one approach appears to provide superior benefit.97 Although follow-up was relatively short in most studies, a few trials have investigated the effects of physical activity continued over several years (Table 3).96,98 In the PRET-PD trial, Corcos et al conducted a randomized, controlled trial comparing the effects of progressive resistance exercise (PRE) versus a combination of stretching, balance, and strengthening exercises (modified fitness count [mFC]) on the off-medication UPDRS-III scores of people with mild to moderate PD after 24 months.99 Although the PRE and mFC groups showed similar improvements in UPDRS-III at 6 months, participants undergoing PRE exhibited a significant decrease in UPDRS-III scores at 12, 18, and 24 months compared with the mFC cohort.99 In addition, improvements in cognition were observed in both the PRE and mFC groups at 24 months, suggesting a more general benefit of physical activity on brain health in PD.100 In a second study with 2-year follow-up, Frazzitta and colleagues evaluated the effects of monthlong multidisciplinary intensive rehabilitation treatments (MIRTs), including aerobic treadmill, strength, stretching, and balance training, at baseline and 12 months compared with no treatment in rasagaline-treated people with early PD.101 After 2 years, motor function in the MIRT group was improved compared with baseline, and antiparkinsonian therapy dose (measured in levodopa equivalents) was lower when compared with the group not receiving MIRT, suggesting a lasting motor benefit that could reflect modification of disease progression. In further support of a disease-modifying effect of this intervention, after the initial 28-day treatment with MIRT or no therapy, BDNF levels were increased in the aerobic exercise group compared with the control group.102 Collectively, these trials reinforce the importance of structured exercise programs for people with PD. Further study is warranted to better understand if a particular type of exercise is most beneficial or if less vigorous physical activity — as described observationally by Yang et al34 and Li et al96 — would yield sufficiently similar gains. The sustained improvement in motor symptoms for 12 months, increase in BDNF, and improvement in cognition as well as movement also suggests a possible change in disease trajectory for these people with PD.99,103
TABLE 3.
Selected studies investigating emerging Parkinson’s disease interventions
| Lead author, year | Study type | Population | Independent variable | Dependent variable | Results |
|---|---|---|---|---|---|
| Fisher, 200899 | Randomized, controlled trial | 30 people with PD, within 3 years or more of diagnosis, with Hoehn and Yahr stages 1–2. | High-intensity exercise (treadmill; 24 sessions over 8 weeks), low-intensity exercise (physical therapy; 24 sessions over 8 weeks), or zero-intensity education group (6 classes over 8 weeks). | UPDRS, biomechanical analysis of self-selected fast walking and sit-to-stand tests, corticomotor excitability using transcranial magnetic stimulation (TMS). | The high-intensity group subjects showed most consistent improvements in fast gait and sit-to-stand tests, as well as normalization of corticomotor excitability, compared with low- or zero-intensity groups. |
| Duncan and Earhart, 2012104 | Randomized, controlled trial | 62 people with PD with Hoehn and Yahr stages 1–4. | Twice-weekly community-based Argentine tango program for 12 months or no intervention. | Primary: MDS-UPDRS-3. Secondary: MDS-UPDRS-1 and -2, Mini-BESTest balance test, Freezing of Gait Questionnaire (FOG_Q), 6-Minute Walk Test, gait velocity, Nine-Hole Peg Test. | Significant reduction in MDS-UPDRS-3 off medication for tango group compared with control (28.7%, or 12.8 points), as well as improvements in balance, gait, and upper-extremity function. |
| Li, 201297 | Randomized, controlled trial | 195 people with PD with Hoehn and Yahr stages 1–4, qualified from May 2008 to November 2010; total of 176 persons completed the intervention. | Tai chi, resistance training, or stretching for 60 minutes twice weekly for 24 weeks. | Limits-of-stability test; UPDRS scores, measures of gait and strength, scores on functional-reach and timed up-and-go tests, number of falls. | The tai chi group performed better than the resistance training (5.55 percentage points; 95% CI, 1.12–9.97 percentage points) or stretching groups (11.98 percentage points; 95% CI, 7.21–6.74 percentage points) on maximum excursion; performed better on all secondary outcomes compared with the stretching group; performed better in stride length and functional reach compared with resistance group. Gains maintained at 3 months. |
| Corcos, 2013100; David, 2015101 | Randomized, controlled trial | 51 people with PD from September 2007 to July 2001: 20 in progressive resistance exercise (PRE) and 18 in modified fitness counts (mFC) completed the trial. | Presence of either PRE or mFC. | Off-medication UPDRS-III score; measures of cognition (digit span, Stroop, brief test of attention [BTA]). | The PRE group exhibited a significant reduction in UPDRS-III scores compared with the mFC group (mean difference, −7.3 points; 95% CI, −11.3 to −3.6; P < 0.001). mFC improved on digit span and Stroop, PRET improved on digit span, Stroop, and BTA. |
| Frazzitta, 2014,103 2015102 | Randomized, controlled trial with blinded rater | 40 newly diagnosed, rasagaline-treated people with PD. | Randomized to 28-day interventions at baseline and 12 months of multidisciplinary intensive rehabilitation treatments (MIRT; including aerobic exercise) or to no therapy. | BDNF levels (after first month); UPDRS, Berg Balance Scale, 6-minute walking test, l-dopa equivalents at 24 months. | The treatment group had a significant increase in BDNF levels; all measures of motor function were improved at 24 months in MIRT group, but worsened in control. Levodopa-equivalent dose was lower in MIRT group than in controls. |
BDNF, brain-derived neurotrophic factor; CI, confidence interval; HR, hazard ratio; OR, odds ratio; PD, Parkinson’s disease; RR, relative risk; UPDRS, Unified Parkinson’s Disease Rating Scale.
One understandable concern is the potential cost of certain types of physical activity for people with PD. Structured, intensive programs such as those implemented by Frazzitta et al102 may be expensive, whereas community-based dance programs103 would be less so, and individual lifestyle adaptations, such as increased walking during daily activities, would entail little or no extra expense. For many, instruction and support in establishing and sustaining an exercise program may be important. Despite the initial cost of an exercise program, such an investment will likely be cost-effective given the potential decreased risk of falls and associated reduction in injuries, hospital stay days, and other comorbidities.104–106
Medications important in the battle against MetS and diabetes, such as drugs affecting the glucagon-like peptide-1 (GLP-1) receptor, which is found throughout the brain, show putative neuroprotective effects in animal models and may provide therapeutic targets for PD.86,107,108 In an open-label study of the antidiabetic drug exenatide, a centrally-active synthetic analogue of the GLP-1 agonist exendin-4, people with moderate PD treated for 12 months showed improved MDS-UPDRS scores compared with the control group.109 Motor benefit was maintained for 2 months posttreatment, suggesting an effect on the underlying disease process.110
Understanding the beneficial metabolic effects of physical activity, including anti-inflammatory and antioxidant actions, may also support new directions for disease-modifying therapeutic interventions. For example, treatments currently under investigation as disease-modifying agents for PD60,61,111 directly or indirectly result in antioxidant benefits analogous to those associated with physical activity. Other elements directly or indirectly associated with MetS, such as statin use and dietary modifications affecting the intake of dairy and certain vitamins, are also under active study.70,112,113 Ultimately, regimens combining physical activity and pharmacologic interventions may prove most useful in modifying progression in people with PD.101
Considerations
Although current studies provide evidence for the benefits of both physical activity and exercise with regard to improvement in PD symptoms, additional, larger studies are needed. Current studies use inconsistent terminology, and terms with different meanings may be used synonymously, for example, using “physical activity,” “exercise,” and/or “physical fitness” interchangeably.29,36,114 This lack of standardized language, protocols, interventions, and outcome measures limits aggregation of these data or comparisons. Whether reduced activity is a risk for PD may be confounded by the possibility that it is an early, prodromal disease feature, although studies demonstrating an effect of exercise many decades before the onset of PD argue against that possibility.33 Ideally, future studies will take into account total activity, including exercise, leisure sports, occupational activities (which may vary widely in level of exertion), daily walking routines, and other activities such as stair climbing. Utilization of standard measures such as metabolic equivalent of task for all activities will provide a more comprehensive measure and facilitate comparisons among studies.
Conclusions
The collective evidence supports encouraging physical activity or exercise for PD prevention. Physical activity can be implemented in any population at a relatively low cost,104,105,115 with few adverse effects and many health benefits.15 Physical activity is equally important for maintenance of function in people already afflicted with PD. This is especially important because people with PD are more likely to become physically inactive compared with people without PD.116 Physical activity is well tolerated98 and should be encouraged in all patients. Physical therapy programs should be used to maintain conditioning in people with PD.93 Current evidence suggests that higher-intensity exercise may be more beneficial for improvement of PD symptoms, although even leisurely physical activity will likely provide some benefit when compared with inactivity.26,90,96,98,99,102–106,117 Further understanding of the relationship between physical activity, inactivity, MetS, and the pathogenesis of PD may lead to disease-modifying treatments. Appropriation of pharmaceuticals already in use to address components of MetS has resulted in multiple novel therapies currently under investigation.61,109,118
Physical activity and exercise provide a low-cost and universally available adjunct to current therapies during a time of soaring medical expenditures. Given the projected increase in the number of people with PD over the coming decades, further clarification of the role of physical activity and exercise is imperative. ■
Relevant conflicts of interest/financial disclosures:
Dr. LaHue has no disclosures. Dr. Comella serves on the editorial board of Clinical Neuropharmacology, Sleep Medicine, and Continuum. She receives research support from the NIH R01NS074343, U54NS065701, Dystonia Medical Research Foundation, Allergan Inc., Ipsen Biopharmaceuticals, Inc, and Merz Pharmaceutical. She receives compensation/honoraria for services as a consultant or an advisory committee member from Allergan, Inc, Impax Pharmaceuticals, Ipsen Biopharmaceuticals, Inc, Medtronic Inc., Merz Pharmaceuticals, US World Meds, Acadia Pharmaceuticals, and Teva Neurosciences. She receives royalties from Cambridge, Humana Press, and Wolters Kluwer. She receives research support from the Parkinson’s Disease Foundation. Dr. Tanner, serves on the editorial boards of the Annals of Neurology, Journal of Parkinson’s Disease, Parkinsonism and Related Disorders, and NPJ Parkinson’s Disease. She serves on the Scientific Advisory Boards of the Michael J. Fox Foundation and the National Spasmodic Dysphonia Association as a voluntary consultant and has provided paid consulting services to Ultragenyx Pharmaceuticals, Neurocrine Biosciences, Cynapsus, and Adamas. She has received compensation for serving on Data Monitoring Committees from Biotie Therapeutics, Voyager Therapeutics, and Intec Pharma. She receives grant support from the Michael J. Fox Foundation, the Parkinson’s Disease Foundation, the Department of Defense, and the National Institutes of Health.
References
- 1.Checkoway H, Nelson LM. Epidemiologic approaches to the study of Parkinson’s disease etiology. Epidemiology 1999;10:327–336. [PubMed] [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol 2006;5:525–535. [DOI] [PubMed] [Google Scholar]
- 3.Tanner CM, Goldman SM. Epidemiology of Parkinson’s disease. Neurol Clin 1996;14:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA 2014;311:1670–1683. [DOI] [PubMed] [Google Scholar]
- 5.Gardner RC, Burke JF, Nettiksimmons J, Goldman S, Tanner CM, Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann Neurol 2015;77:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol 2002;52:276–284. [DOI] [PubMed] [Google Scholar]
- 7.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol 2001;50:56–63. [DOI] [PubMed] [Google Scholar]
- 8.Tanner CM, Goldman SM, Ross GW, Grate SJ. The disease intersection of susceptibility and exposure: chemical exposures and neurodegenerative disease risk. Alzheimers Dement 2014;10:S213–225. [DOI] [PubMed] [Google Scholar]
- 9.Tanner CM. The role of environmental toxins in the etiology of Parkinson’s disease. Trends Neurosci 1989;12:49–54. [DOI] [PubMed] [Google Scholar]
- 10.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 11.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694–2703. [DOI] [PubMed] [Google Scholar]
- 12.Wessel TR, Arant CB, Olson MB, et al. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA 2004;292:1179–1187. [DOI] [PubMed] [Google Scholar]
- 13.Gorelick PB, Sacco RL, Smith DB, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA 1999; 281:1112–1120. [DOI] [PubMed] [Google Scholar]
- 14.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ 2013;347:f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006;16(Suppl 1): 3–63. [DOI] [PubMed] [Google Scholar]
- 16.Lakka TA, Venalainen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med 1994;330:1549–1554. [DOI] [PubMed] [Google Scholar]
- 17.Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986;314:605–613. [DOI] [PubMed] [Google Scholar]
- 18.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med 2001;161:1703–1708. [DOI] [PubMed] [Google Scholar]
- 19.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol 2015;11:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 2006;144:73–81. [DOI] [PubMed] [Google Scholar]
- 22.Cornier MA, Dabelea D, Hernandez TL, et al. The metabolic syndrome. Endocr Rev 2008;29:777–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 24.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2012;2:1143–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 2016;167:257–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golbidi S, Mesdaghinia A, Laher I. Exercise in the metabolic syndrome. Oxid Med Cell Longev 2012;2012:349710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Tian B. Metabolic syndrome: an important risk factor for Parkinson’s disease. Oxid Med Cell Longev 2014;2014: 729194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang O Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 2013;22:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasco AJ, Paffenbarger RS, Jr., Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson’s disease. Arch Neurol 1992;49:360–365. [DOI] [PubMed] [Google Scholar]
- 30.Frigerio R, Elbaz A, Sanft KR, et al. Education and occupations preceding Parkinson disease: a population-based case-control study. Neurology 2005;65:1575–1583. [DOI] [PubMed] [Google Scholar]
- 31.Shih IF, Liew Z, Krause N, Ritz B. Lifetime occupational and leisure time physical activity and risk of Parkinson’s disease. Parkinsonism Relat Disord 2016;28:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson’s disease. Mov Disord 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 2010;75:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang F, Trolle Lagerros Y, Bellocco R, et al. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain 2015;138:269–275. [DOI] [PubMed] [Google Scholar]
- 35.Ellis T, Boudreau JK, DeAngelis TR, et al. Barriers to exercise in people with Parkinson disease. Phys Ther 2013;93:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 37.Henderson GC. Sexual dimorphism in the effects of exercise on metabolism of lipids to support resting metabolism. Front Endocrinol (Lausanne) 2014;5:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galassetti PR, Nemet D, Pescatello A, Rose-Gottron C, Larson J, Cooper DM. Exercise, caloric restriction, and systemic oxidative stress. J Investig Med 2006;54:67–75. [DOI] [PubMed] [Google Scholar]
- 39.Zoladz JA, Majerczak J, Zeligowska E, et al. Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol 2014;65:441–448. [PubMed] [Google Scholar]
- 40.Patki G, Lau YS. Impact of exercise on mitochondrial transcription factor expression and damage in the striatum of a chronic mouse model of Parkinson’s disease. Neurosci Lett 2011;505:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsou YH, Shih CT, Ching CH, et al. Treadmill exercise activates Nrf2 antioxidant system to protect the nigrostriatal dopaminergic neurons from MPP 1 toxicity. Exp Neurol 2015;263:50–62. [DOI] [PubMed] [Google Scholar]
- 42.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 2011;77:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol 2008;59(Suppl 7):119–132. [PubMed] [Google Scholar]
- 44.Scalzo P, Kummer A, Bretas TL, Cardoso F, Teixeira AL. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J Neurol 2010;257:540–545. [DOI] [PubMed] [Google Scholar]
- 45.Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 2013;24:509–514. [DOI] [PubMed] [Google Scholar]
- 46.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res 2010;88:650–668. [DOI] [PubMed] [Google Scholar]
- 47.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 2003;119: 899–911. [DOI] [PubMed] [Google Scholar]
- 48.Poulton NP, Muir GD. Treadmill training ameliorates dopamine loss but not behavioral deficits in hemi-parkinsonian rats. Exp Neurol 2005;193:181–197. [DOI] [PubMed] [Google Scholar]
- 49.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci 2002;22:6790–6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neuro-chemical effects of 6-hydroxydopamine. J Neurosci 2001;21: 4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caudle WM, Tillerson JL, Reveron ME, Miller GW. Use-dependent behavioral and neurochemical asymmetry in MPTP mice. Neurosci Lett 2007;418:213–216. [DOI] [PubMed] [Google Scholar]
- 52.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci 2007;27:5291–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dias V, Junn E, Mouradian MM. The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 2013;3:461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson’s disease. Biochim Biophys Acta 2010;1802:29–44. [DOI] [PubMed] [Google Scholar]
- 55.Monteiro-Junior RS, Cevada T, Oliveira BR, et al. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson’s disease. Med Hypotheses 2015;85(5): 537–541. [DOI] [PubMed] [Google Scholar]
- 56.Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 2008; 44:153–159. [DOI] [PubMed] [Google Scholar]
- 57.DiFrancisco-Donoghue J, Lamberg EM, Rabin E, Elokda A, Fazzini E, Werner WG. Effects of exercise and B vitamins on homocysteine and glutathione in Parkinson’s disease: a randomized trial. Neurodegener Dis 2012;10:127–134. [DOI] [PubMed] [Google Scholar]
- 58.Maher P The effects of stress and aging on glutathione metabolism. Ageing Res Rev 2005;4:288–314. [DOI] [PubMed] [Google Scholar]
- 59.Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol 2007;166:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katz M, Won SJ, Park Y, et al. Cerebrospinal fluid concentrations of N-acetylcysteine after oral administration in Parkinson’s disease. Parkinsonism Relat Disord 2015;21:500–503. [DOI] [PubMed] [Google Scholar]
- 61.Parkinson Study Group S-PDI, Schwarzschild MA, Ascherio A, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 2014;71:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson’s disease. Neurology 2002;59:1051–1057. [DOI] [PubMed] [Google Scholar]
- 63.Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuro-muscular and neurodegenerative diseases. Biomed Res Int 2014; 2014:474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen J, Guan Z, Wang L, Song G, Ma B, Wang Y. Meta-analysis: overweight, obesity, and Parkinson’s disease. Int J Endocrinol 2014;2014:203930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu G, Jousilahti P, Nissinen A, Antikainen R, Kivipelto M, Tuomilehto J. Body mass index and the risk of Parkinson disease. Neurology 2006;67:1955–1959. [DOI] [PubMed] [Google Scholar]
- 66.Saaksjarvi K, Knekt P, Mannisto S, et al. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur J Epidemiol 2014;29:285–292. [DOI] [PubMed] [Google Scholar]
- 67.Wang YL, Wang YT, Li JF, Zhang YZ, Yin HL, Han B. Body mass index and risk of Parkinson’s disease: a dose-response meta-analysis of prospective studies. PLoS One 2015;10:e0131778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2007; 30:842–847. [DOI] [PubMed] [Google Scholar]
- 69.Sandyk R, Awerbuch GI. The association of diabetes mellitus with dementia in Parkinson’s disease. Int J Neurosci 1992;64: 209–212. [DOI] [PubMed] [Google Scholar]
- 70.Agim ZS, Cannon JR. Dietary factors in the etiology of Parkinson’s disease. Biomed Res Int 2015;2015:672838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okubo H, Miyake Y, Sasaki S, et al. Dietary patterns and risk of Parkinson’s disease: a case-control study in Japan. Eur J Neurol 2012;19:681–688. [DOI] [PubMed] [Google Scholar]
- 72.Tian T, Li Z, Lu H. Common pathophysiology affecting diabetic retinopathy and Parkinson’s disease. Med Hypotheses 2015;85(4): 397–398. [DOI] [PubMed] [Google Scholar]
- 73.Palacios N, Gao X, McCullough ML, et al. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord 2011;26:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rotermund C, Truckenmuller FM, Schell H, Kahle PJ. Diet-induced obesity accelerates the onset of terminal phenotypes in alpha-synuclein transgenic mice. J Neurochem 2014;131:848–858. [DOI] [PubMed] [Google Scholar]
- 75.Golbidi S, Laher I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res 2014;2014:726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fischer CP, Berntsen A, Perstrup LB, Eskildsen P, Pedersen BK. Plasma levels of interleukin-6 and C-reactive protein are associated with physical inactivity independent of obesity. Scand J Med Sci Sports 2007;17:580–587. [DOI] [PubMed] [Google Scholar]
- 77.Xu Q, Park Y, Huang X, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care 2011;34:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2008;31:2003–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cereda E, Barichella M, Cassani E, Caccialanza R, Pezzoli G. Clinical features of Parkinson disease when onset of diabetes came first: A case-control study. Neurology 2012;78:1507–1511. [DOI] [PubMed] [Google Scholar]
- 80.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson’s disease. Mov Disord 2013;28:257. [DOI] [PubMed] [Google Scholar]
- 81.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 1991;36:343–362. [DOI] [PubMed] [Google Scholar]
- 82.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med 1998;339:1044–1053. [DOI] [PubMed] [Google Scholar]
- 83.Moroo I, Yamada T, Makino H, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol 1994;87:343–348. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi M, Yamada T, Tooyama I, et al. Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett 1996;204:201–204. [DOI] [PubMed] [Google Scholar]
- 85.Haavik J, Toska K. Tyrosine hydroxylase and Parkinson’s disease. Mol Neurobiol 1998;16:285–309. [DOI] [PubMed] [Google Scholar]
- 86.Santiago JA, Potashkin JA. System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol Dis 2014;72 Pt A:84–91. [DOI] [PubMed] [Google Scholar]
- 87.Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res 2012; 2012:941868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord 2015;30:1351–1360. [DOI] [PubMed] [Google Scholar]
- 89.Cassani E, Barichella M, Cancello R, et al. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord 2015;21:389–393. [DOI] [PubMed] [Google Scholar]
- 90.Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med 2013;43:121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yamaoka K, Tango T. Effects of lifestyle modification on metabolic syndrome: a systematic review and meta-analysis. BMC Med 2012;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gordon B, Chen S, Durstine JL. The effects of exercise training on the traditional lipid profile and beyond. Curr Sports Med Rep 2014;13:253–259. [DOI] [PubMed] [Google Scholar]
- 93.Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst Rev 2013;9:CD002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mehrholz J, Kugler J, Storch A, Pohl M, Hirsch K, Elsner B. Treadmill training for patients with Parkinson’s disease. Cochrane Database Syst Rev 2015;9:CD007830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharp K, Hewitt J. Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev 2014;47:445–456. [DOI] [PubMed] [Google Scholar]
- 96.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med 2012;366: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bloem BR, de Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 2015;30:1504–1520. [DOI] [PubMed] [Google Scholar]
- 98.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil 2008;89:1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 2013;28:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.David FJ, Robichaud JA, Leurgans SE, et al. Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov Disord 2015;30:1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson’s disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair 2015;29: 123–131. [DOI] [PubMed] [Google Scholar]
- 102.Frazzitta G, Maestri R, Ghilardi MF, et al. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabil Neural Repair 2014;28:163–168. [DOI] [PubMed] [Google Scholar]
- 103.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair 2012;26:132–143. [DOI] [PubMed] [Google Scholar]
- 104.Fletcher E, Goodwin VA, Richards SH, Campbell JL, Taylor RS. An exercise intervention to prevent falls in Parkinson’s: an economic evaluation. BMC Health Serv Res 2012;12:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li F, Harmer P. Economic evaluation of a tai ji quan intervention to reduce falls in people with Parkinson disease, Oregon, 2008–2011. Prev Chronic Dis 2015;12:E120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pressley JC, Louis ED, Tang MX, et al. The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 2003;60:87–93. [DOI] [PubMed] [Google Scholar]
- 107.Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 2002;302: 881–888. [DOI] [PubMed] [Google Scholar]
- 108.Liu W, Jalewa J, Sharma M, Li G, Li L, Holscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 2015;303:42–50. [DOI] [PubMed] [Google Scholar]
- 109.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 2013;123:2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Foltynie T, Aviles-Olmos I. Exenatide as a potential treatment for patients with Parkinson’s disease: first steps into the clinic. Alzheimers Dement 2014;10:S38–S46. [DOI] [PubMed] [Google Scholar]
- 111.Parkinson Study G Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD). Mov Disord 2013;28: 1823–1831. [DOI] [PubMed] [Google Scholar]
- 112.Gao X, Simon KC, Schwarzschild MA, Ascherio A. Age, statin use, and the risk for incident Parkinson disease-reply. Arch Neurol 2012;69:1381. [DOI] [PubMed] [Google Scholar]
- 113.Park M, Ross GW, Petrovitch H, et al. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 2005;64:1047–1051. [DOI] [PubMed] [Google Scholar]
- 114.Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM. Physical activity and risk of Parkinson’s disease: a prospective cohort study. J Neurol Neurosurg Psychiatry 2006;77:1318–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Farag I, Sherrington C, Hayes A, et al. Economic evaluation of a falls prevention exercise program among people With Parkinson’s disease. Mov Disord 2016;31:53–61. [DOI] [PubMed] [Google Scholar]
- 116.van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, et al. Physical inactivity in Parkinson’s disease. J Neurol 2011;258: 2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen H, O’Reilly EJ, Schwarzschild MA, Ascherio A. Peripheral inflammatory biomarkers and risk of Parkinson’s disease. Am J Epidemiol 2008;167:90–95. [DOI] [PubMed] [Google Scholar]
- 118.Weinreb O, Mandel S, Youdim MB, Amit T. Targeting dysregulation of brain iron homeostasis in Parkinson’s disease by iron chelators. Free Radic Biol Med 2013;62:52–64. [DOI] [PubMed] [Google Scholar]


