Abstract
The incidence of infertility has recently risen. Semen quality is an important male fertility indicator, and dietary factors can affect semen quality. We conducted this systematic review and meta-analysis to determine the effects of healthy dietary patterns on semen quality. A literature search was conducted in 3 databases (Embase, Web of Science and PubMed) on August 21, 2021. The included cross-sectional studies examined the influence of the Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and prudent diet patterns on semen quality parameters; six studies (1244 subjects) were included. By comparing high consumption with low consumption of healthy dietary patterns, the results of the meta-analysis showed significantly higher sperm concentrations (mean difference [MD] = 6.88 × 106 ml−1, 95% confidence interval [CI]: 1.26 × 106 ml−1–12.49 × 106 ml−1; P < 0.05), a significant increase in total sperm count (MD = 16.70 × 106, 95% CI: 2.37 × 106–31.03 × 106; P < 0.05), and a significant increase in progressive sperm motility (MD = 5.85%, 95% CI: 2.59%–9.12%; P < 0.01). The sperm concentration, progressive sperm motility, and total sperm count were significantly higher in men with higher versus lower consumption of healthy dietary patterns. However, the results must be interpreted with caution.
Keywords: cross-sectional study, healthy dietary pattern, meta-analysis, semen quality, systematic review
INTRODUCTION
Infertility has been identified by the World Health Organization (WHO) as a worldwide problem. A study showed that the prevalence of infertility after 12 months of trying to conceive ranges from 3.5% to 16.7% in more developed countries and from 6.9% to 9.3% in less developed countries.1 It is estimated that 186 million people worldwide are affected by infertility,2 which brings adverse social consequences such as marital instability and abuse.3 In the case of infertility, male infertility accounts for 50% of the incidence in couples.4 Abnormal semen parameters such as sperm concentration, total motility and morphology, semen volume, and motile sperm count may cause male infertility.4 Therefore, the analysis of semen quality is key in assessing male fertility. From 2001 to 2015, a study in China examined semen from 30 636 adult men. It was found that the sperm concentration, normal morphology, sperm progressive motility, and total sperm count decreased during a 15-year observation period, and the proportion of qualified semen donors dropped from 55.78% in 2001 to 17.80% in 2015.5 Therefore, the outlook regarding male semen quality is not optimistic. It is important to explore the factors that affect semen quality to improve semen quality.
Studies have found that sperm quality may be related not only to environmental pollution but also to unhealthy lifestyle factors, such as smoking and alcoholism.6,7,8 It has been reported that human sperms are very sensitive to nutrient flux, whether changes occur in sperm motility or in sperm transfer RNA (tRNA)-derived small RNA.9 Diet, as a factor that is closely related to human beings, has a major impact on our lives. In a review, the authors encouraged men to follow a dietary pattern containing seafood, poultry, nuts, whole grains, fruits, and vegetables, which may be beneficial for male fertility.10 In addition, in a systematic review, the authors concluded that a diet rich in processed meats, soy foods, potatoes, full-fat dairy products, coffee, alcohol, and sugary drinks was associated with decreased semen quality.11 However, a study concluded that the consumption of artificially sweetened beverages and processed red meat and poultry was not closely related to semen quality indicators.12 Thus, it is important to study the impact of dietary patterns on semen quality; it is also important to study whether the food groups and nutrients included in healthy dietary patterns can have a beneficial effect on improving semen quality.
The traditional Mediterranean diet includes a large intake of fruits, olive oil, grains, nuts, vegetables, and dairy products; moderate consumption of fish and poultry; low consumption of red meat; and small-to-moderate consumption of wine.13 One study suggested that this dietary pattern can reduce the risk of cardiovascular disease.14 Two randomized controlled trial (RCT) studies showed that the consumption of a Mediterranean diet improved semen concentration, sperm count, and other semen parameters;15,16 however, a cross-sectional study also found that the consumption of a Mediterranean diet only showed a positive association with total sperm count, and no significant association was found with other semen parameters.17 The Dietary Approaches to Stop Hypertension (DASH) diet, which is based on fruits and vegetables and includes moderate consumption of low-fat dairy products, low consumption of animal protein, and high consumption of plant protein in the form of legumes and nuts, significantly reduced systolic and diastolic blood pressure in people with hypertension and normal blood pressure.18 The DASH diet not only has a good effect on blood pressure, lowering systolic and diastolic blood pressure in people with hypertension and normal blood pressure, but may also have an effect on semen quality. A study showed that there was a significant positive association between the DASH diet and total motile sperm count, total sperm count, and sperm concentration.19 A prudent diet is a diet rich in vegetables, legumes, fruits, and whole grains as determined using principal component analysis based on collected Food Frequency Questionnaire (FFQ) data.20 The prudent dietary pattern is characterized by high intakes of vegetables, fruits, legumes, low-fat dairy products, whole grains, fish, and poultry.21 Studies have shown that the prudent dietary pattern is associated with reducing the risk of cardiovascular disease and diabetes.21,22 A cross-sectional study showed that adherence to the prudent dietary pattern was associated with better semen quality, such as total sperm count and sperm morphology;23 however, another study did not find a significant association between the prudent dietary pattern and semen parameters.24
Healthy dietary patterns can be defined as being rich in health-promoting foods such as vegetables, fruits, whole grains, and legumes, and limiting the intake of red meat and processed meats, which may have a role in reducing the incidence of noncommunicable diseases and overall mortality.25 The Mediterranean, prudent, and DASH diets have something in common: after factor analysis and major component analysis, they all included the consumption of more fruits, whole grains, legumes, vegetables, and low-fat dairy products, which are part of a healthy dietary pattern. Dietary patterns investigate the effects of overall diet and represent a broader consumption of foods and nutrients that may be better predictors of disease risk than foods and nutrients alone.26 Healthy dietary patterns, as a modifiable factor, have been reported to be associated with male semen parameters; however, the findings about this relationship were not consistent. Therefore, the aim of this analysis was to pool these results to explore the effect of healthy dietary patterns on male semen quality.
MATERIALS AND METHODS
We conducted this systematic review and meta-analysis based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.27 The guidelines are shown in Supplementary Table 1. The PICOS (Participants, Intervention, Comparison, Outcomes, and Study design) criteria that were used to structure the research question are shown in Supplementary Table 2.
Supplementary Table 1.
Preferred reporting items for systematic reviews and meta-analyses guidelines
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 2–5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS | 5 Table 1 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number | No |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 6–7 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 6 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 6 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6–7 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 7 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | No |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 7–8 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 8–9 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2 for each meta-analysis) | 8–9 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 8–9 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | No |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 9 Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 10–11 Table 2 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 12 Table 3 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | 12–15 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 15–16 Figure 2 and 3 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 15–16 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | No |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 17–20 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 20–21 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 21 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 22 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). PRISMA: The PRISMA Statement. PLoS Med 6 (6): e1000097. doi: 10.1371/journal.pmed1000097. PICOS: Participants, Interventions, Comparisons, Outcomes, and Study design; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Supplementary Table 2.
Participants, Interventions, Comparisons, Outcomes, and Study design criteria for inclusion of studies
| Parameter | Criterion |
|---|---|
| Participants | Male with healthy dietary pattern |
| Intervention | Studies confirming diet assessed by dietary patterns, dietary scores, dietary indices, and food patterns |
| Comparison | Lower consumption of healthy dietary pattern |
| Outcomes | Six semen parameters |
| Study design | Cross-sectional studies |
PICOS: Participants, Interventions, Comparisons, Outcomes, and Study design
Search strategy
A literature search was conducted in 3 databases (Embase, Web of Science, and PubMed) on August 21, 2021. The keywords for semen quality were “Semen Analysis”, “Semen Analyses”, “Semen Quality Analysis”, “Semen Quality Analyses”, “Semen Quality”, and “Semen Qualities”; the keywords for the healthy dietary pattern were “Diet, Mediterranean”, “Mediterranean Diet”, “Mediterranean Diets”, “Dietary Approaches to Stop Hypertension”, “DASH Diet”, “DASH Diets”, “Diet, DASH”, “Diets, DASH”, “Dietary Approaches to Stop Hypertension Diet”, “Diet, Healthy”, “Healthy Diet”, “Diets, Healthy”, “Healthy Diets”, “Healthy Eating”, “Eating, Healthy”, “Healthy Nutrition”, “Nutrition, Healthy”, “Prudent Diet”, “Diet, Prudent”, “Diets, Prudent”, “Prudent Diets”, “Healthy Eating Index”, “Healthy Eating Indices”, “Index, Healthy Eating”, and “Indices, Healthy Eating”. We searched by subject, abstract, and keywords. The retrieved documents were imported into Endnote X9 software (Clarivate Analytics, Philadelphia, PA, USA) for screening, and the documents that met the inclusion criteria were screened out.
Selection criteria
The inclusion criteria were as follows: (1) the subjects in all the selected literature were adult males; (2) the research content included the influence of healthy dietary patterns on semen qualities, and according to the consumption, the dietary pattern was scored and grouped; (3) the outcome measures included one or more of the following: sperm concentration, total sperm count, normal sperm morphology, total sperm motility, progressive sperm motility, and semen volume; (4) the results were presented as the median and interquartile or mean and 95% confidence interval (CI) values; and (5) the studies were published in English.
The exclusion criteria were as follows: (1) reviews, animal experiments, systematic reviews, and meta-analyses; (2) the research content was food groups or nutrients; (3) literature quality was assessed as low quality by the Agency for Healthcare Research and Quality (AHRQ)-recommended checklist; or (4) the information provided could not be pooled.
Data extraction
Data were extracted by three reviewers (LLC, JJC, and SJW). The title and abstract screening and full-text review were performed by 2 independent reviewers (LLC and JJC). Through discussion with a third reviewer (SJW), any discrepancies regarding suitability were resolved. An Excel spreadsheet was set up for data extraction. We extracted the following data: author, year, country, survey time, research design, sample size, study population, age, diet assessment method, dietary pattern recognition, and semen parameters.
Quality assessment
We used 11 checklists recommended by the AHRQ to assess the quality of the literature included in this systematic review and meta-analysis. The specific items were as follows: (1) define the source of the information (survey and record review); (2) list the inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications; (3) indicate the time period used for identifying patients; (4) indicate whether or not the subjects were consecutive, if the study was not population-based; (5) indicate if the evaluators of the subjective components of study were masked to other aspects of the status of the participants; (6) describe any assessments undertaken for quality assurance purposes (e.g., test/retest of the primary outcome measurements); (7) explain any patient exclusions from the analysis; (8) describe how confounding was assessed and/or controlled; (9) if applicable, explain how missing data were handled in the analysis; (10) summarize the patient response rates and the completeness of data collection; and (11) clarify what follow-up, if any, was expected and the percentage of patients, for which incomplete data or follow-up was obtained. If the answer was “no” or “unclear”, the item was scored as “0”; if the answer was “yes”, then the item was scored as “1”. The article quality was evaluated by scores as follows: low quality = 0–3; moderate quality = 4–7; and high quality = 8–11.28
Statistical analyses
We used Stata/SE version 15.1 (Stata Corp LLC, College Station, TX, USA) software to perform meta-analysis calculations. Data on the effects of healthy dietary patterns on the six outcomes (sperm concentration, total sperm count, normal sperm morphology, total sperm motility, progressive sperm motility, and semen volume) were pooled. The mean difference (MD) was used as the combined effect size, and its 95% CI was calculated. Statistical significance was set at P < 0.05, and the forest plot was mapped at the same time.
The outcome data are presented as the mean ± standard deviation (s.d.). For studies that did not provide the mean ± s.d. of the semen parameters, we extracted the data from the articles and transformed them into mean ± s.d. according to the formulae provided in the Cochrane Handbook or references. If the median and interquartile range were provided, they were converted to the mean ± s.d. according to Wan et al.29 If the means and 95% CIs were provided, we converted them to mean ± s.d. according to the Cochrane Handbook.30 Cochran's Q test and I2 statistics were used to assess the heterogeneity between studies, and the significance level was set at P < 0.10. Heterogeneity was considered acceptable when I2 < 50%. In this case, a fixed-effects model was used, and the inverse variance method was used for the pooled data. Otherwise, the heterogeneity was considered significant, and a random-effects model was used. We used Egger's regression test to test whether there was publication bias, and P < 0.05 represented a significant publication bias.
RESULTS
Study screening
A total of 490 articles were retrieved from the three databases. After the literature was imported into Endnote software, a total of 123 duplicate studies were removed, leaving 367 studies. After reading the titles and abstracts, a total of 348 articles were deleted because the research type was an animal experiment, systematic review, or review or study on the relationship between specific food and nutrients on semen quality. After full-text readings of the remaining 19 studies, we found 10 studies with no eligible data for meta-analysis; two articles studied food groups rather than dietary patterns, and dietary patterns were not grouped in 1 study. Finally, this systematic review and meta-analysis included a total of 6 articles (Figure 1).17,31,32,33,34,35
Figure 1.
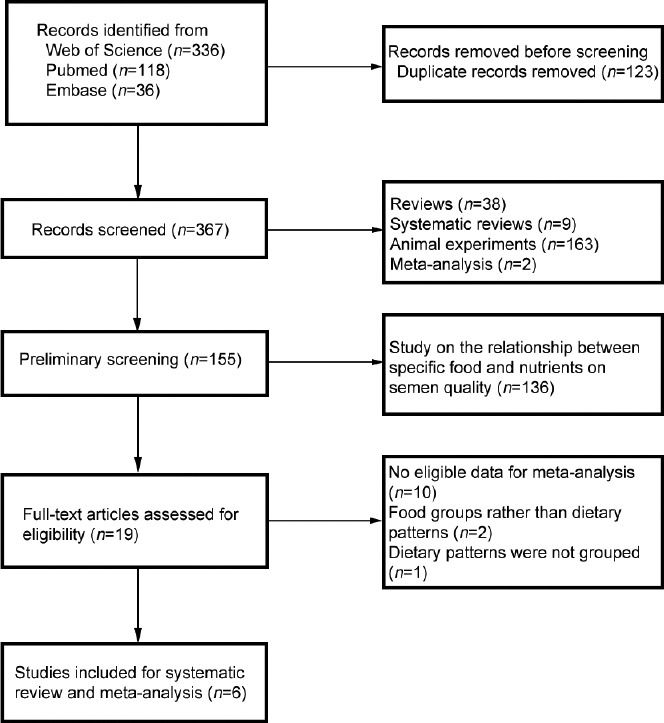
Flowchart of the progress of acquiring the qualified literature and studies included in the systematic review and meta-analysis.
Characteristics of the studies
The characteristics of the six studies are shown in Table 1. The six articles were cross-sectional studies involving 1244 subjects from 2009 to 2018. In the meta-analysis, we included 708 subjects with the lowest and highest adherence to a healthy dietary pattern. The included studies were from five countries: two were from Spain and the remaining four were from the USA, Greece, Italy, and Poland, respectively. In addition, the subjects ranged in age from 18 years to 60 years. The health status and semen analysis criteria of the study subjects are shown in Table 2. Four studies examined the effects of a Mediterranean diet on semen quality in men. They were divided into groups based on their scores for the Mediterranean diet from low to high adherence. The effect of the dietary pattern on semen quality was compared by comparing the difference in semen parameters between the high adherence group and the low adherence group. Among them, the subjects of two studies were infertile men,33,34 and the subjects of the other two studies were healthy men.17,35 The remaining two studies examined the DASH diet and the prudent diet, and the subjects were all healthy men. These studies used different FFQs. In the study of Cutillas-Tolín et al.,17 a semiquantitative FFQ of 101 foods was used to assess daily food and nutrient intake. They estimated 17 foods and food groups by grouping specific foods in the FFQ. This questionnaire had nine possible responses, ranging from “never or less than once per month” to “six or more times per day”. The study of Salas-Huetos et al.35 used a validated 143-item semiquantitative FFQ. In this questionnaire, food intake was grouped into 13 food groups. There were nine options for the frequency of food consumption, from never to more than six times per day. The two studies were conducted in Spain, so these FFQs contained foods that were popular in Spain at the time (e.g., kiwi). There were also some foods of interest for the Mediterranean diet (e.g., onion, garlic, olive oil, and wine).
Table 1.
The characteristics of 6 studies
| Study | Year | Country | Survey time | Study population | Study design | Cases (n) | Age (year), range | Diet assessment method | Dietary pattern identified | Semen parameters |
|---|---|---|---|---|---|---|---|---|---|---|
| Gaskins et al.32 | 2012 | USA | 2009–2010 | Healthy | CS | 188 | 18–22 | 131-item FFQ | Prudent pattern, western pattern | Sperm concentration, progressive motility, and morphology |
| Cutillas-Tolín et al.17 | 2015 | Spain | 2010–2011 | Healthy | CS | 209 | 18–23 | 101-item FFQ | MED, western diet | Semen volume, sperm concentration, total sperm count, morphologically normal sperm, and total motile sperm |
| Karayiannis et al.33 | 2017 | Greece | 2013–2016 | Infertility | CS | 225 | 26–55 | 75-item FFQ | MED | Semen volume, sperm concentration, total sperm count, motility, progressive motility, and morphology |
| Salas-Huetos et al.35 | 2019 | Spain | 2015–2017 | Healthy | CS | 106 | 18–35 | 143-item FFQ | MED | Semen volume, total sperm count, sperm concentration, vitality, total motility, progressive motility, and morphology |
| Ricci et al.34 | 2019 | Italy | 2014–2016 | Infertility | CS | 390 | 27–60 | 78-item FFQ | MED | Semen volume, sperm concentration, and total count |
| Danielewicz et al.31 | 2019 | Poland | 2014–2018 | Healthy | CS | 207 | 20–55 | 165-item FFQ | DASH | Semen volume, sperm concentration, sperm count, total motility, progressive motility, and morphology |
CS: cross-sectional study; FFQ: Food Frequency Questionnaire; MED: Mediterranean diet; DASH: Dietary Approaches to Stop Hypertension
Table 2.
Study population sources and semen analysis methods
| Study | Population | Sperm concentration | Total sperm count | Normal sperm morphology | Total sperm motility | Progressive sperm motility | Semen volume |
|---|---|---|---|---|---|---|---|
| Gaskins et al.32 2012 | Healthy men from a cross-sectional study recruited at a university to study the effects of environmental pollutants on semen quality | Hemocytometer | Volume × sperm concentration | The slides were Papanicolaou stained and assessed using the Tygerberg strict criteria | The WHO criteria 4th edition | The WHO criteria 4th edition | Weighing |
| Cutillas-Tolín et al.17 2015 | Healthy men from a cross-sectional study aimed at studying the influence of environmental and lifestyle factors on semen quality and reproductive hormone levels | Hemocytometer | Volume × sperm concentration | The slides were prepared, air-dried, fixed, Papanicolaou stained, and assessed using the Tygerberg strict criteria | The WHO criteria 5th edition | NA | Weighing |
| Karayiannis et al.33 2017 | Infertile men from a cross-sectional study conducted at a fertility clinic in Athens, Greece | Hemocytometer | Volume × sperm concentration | The slides were Papanicolaou stained and assessed using the Tygerberg strict criteria | The WHO criteria 5th edition | The WHO criteria 5th edition | Weighing |
| Salas-Huetos et al.35 2019 | Healthy men from a parallel randomized clinical trial aimed to assess the effects of nut supplementation on sperm quality parameters | Hemocytometer | Volume × sperm concentration | The WHO criteria 5th edition | The WHO criteria 5th edition | The WHO criteria 5th edition | A Pasteur pipette |
| Ricci et al.34 2019 | Infertile men were invited to participate in an ongoing prospective cohort study on the role of lifestyle habits and diet on assisted reproduction technologies outcome from an Italian Fertility Clinic | Hemocytometer | Volume × sperm concentration | NA | NA | NA | Weighing |
| Danielewicz et al.31 2019 | Healthy men from a cross-sectional study who attended the left of reproductive medicine in Olsztyn (Poland) or voluntarily joined the study in 2014–2018 | A four-chamber GoldCyto slide (10 μm depth) by applying 4 μl of semen | CASA | The slides were Diff-Quick stained by using WHO criteria 5th edition | A four-chamber GoldCyto slide (10 μm depth) by applying 4 μl of semen | A four-chamber GoldCyto slide (10 μm depth) by applying 4 μl of semen | The WHO criteria 5th edition |
WHO: World Health Organization; NA: not available; CASA: computer-aided semen analysis
The study of Karayiannis et al.33 used a 75-item FFQ validated for the Greek population. Twelve main food groups (i.e., dairy products, eggs, meat, fish, legumes, vegetables, fruits, sweets, fats, oils, alcohol, and stimulants) were created. This questionnaire had six possible responses, ranging from “never/rarely” to “two or more times per day”. Compared to 101/143-food-item semiquantitative FFQs, although the food groups were similar, the foods included in each group were different (e.g., missing garlic and onion). Ricci et al.34 used a previously validated 78-item FFQ; this FFQ was divided into eight sections: bread and cereals; meat and meat substitutes; vegetables; fruits; sweets, desserts, and soft drinks; milk, hot beverages, and sweeteners; alcoholic beverages; and fish. There were no questions about vitamin and mineral supplementation in the FFQ, as they are extremely rare in Italy.
Danielewicz et al.31 used the validated 165-food-item semiquantitative FFQ. Food items in this FFQ were divided into 23 food groups, and the DASH diet score comprised eight food groups. The food groups included a wide variety of foods but no item for alcohol. Gaskins et al.32 used a previously validated 131-item FFQ. The food items in this FFQ were divided into 40 food groups. This questionnaire had nine possible responses, ranging from “almost never” to “six or more times per day”. More details on the outcomes are shown in (Supplementary Table 3).
Supplementary Table 3.
The data of the included studies in semen parameters
| Study | Treat | Control | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Size | Mean | s.d. | Size | Mean | s.d. | |
| Sperm concentration (millions ml−1) | ||||||
| Gaskins et al.32 2012 | 47 | 51.20 | 68.63 | 47 | 44.30 | 58.92 |
| Cutillas-Tolín et al.17 2015 | 52 | 44.20 | 46.51 | 53 | 35.70 | 36.46 |
| Karayiannis et al.33 2017 | 66 | 37.90 | 26.43 | 76 | 30.50 | 27.14 |
| Salas-Huetos et al.35 2019 | 27 | 25.63 | 15.26 | 30 | 30.13 | 34.72 |
| Ricci et al.34 2019 | 35 | 37.67 | 36.15 | 31 | 34.30 | 44.56 |
| Danielewicz et al.31 2019 | 70 | 41.13 | 51.09 | 62 | 24.10 | 24.21 |
| Total sperm count (millions) | ||||||
| Cutillas-Tolín et al.17 2015 | 52 | 138.00 | 134.7 | 53 | 91.90 | 84.71 |
| Karayiannis et al.33 2017 | 66 | 82.10 | 70.75 | 76 | 63.80 | 72.67 |
| Salas-Huetos et al.35 2019 | 27 | 74.50 | 45.00 | 30 | 82.30 | 84.61 |
| Ricci et al.34 2019 | 35 | 85.77 | 75.62 | 31 | 81.43 | 107.29 |
| Danielewicz et al.31 2019 | 70 | 154.27 | 188.78 | 62 | 90.53 | 102.02 |
| Normal sperm morphology (%) | ||||||
| Gaskins et al.32 2012 | 47 | 7.80 | 6.30 | 47 | 8.70 | 6.30 |
| Cutillas-Tolín et al.17 2015 | 52 | 8.17 | 5.57 | 53 | 9.25 | 6.15 |
| Karayiannis et al.33 2017 | 66 | 4.60 | 2.44 | 76 | 4.30 | 2.63 |
| Salas-Huetos et al.35 2019 | 27 | 6.43 | 2.35 | 30 | 6.27 | 2.80 |
| Danielewicz et al.31 2019 | 70 | 8.33 | 6.81 | 62 | 6.33 | 3.79 |
| Total sperm motility (%) | ||||||
| Cutillas-Tolín et al.17 2015 | 52 | 56.10 | 11.31 | 53 | 56.20 | 10.70 |
| Karayiannis et al.33 2017 | 66 | 42.60 | 20.33 | 76 | 32.10 | 20.57 |
| Salas-Huetos et al.35 2019 | 27 | 73.37 | 13.70 | 30 | 55.23 | 26.15 |
| Danielewicz et al.31 2019 | 70 | 53.90 | 20.59 | 62 | 50.90 | 17.30 |
| Progressive sperm motility (%) | ||||||
| Gaskins et al.32 2012 | 47 | 63.70 | 20.26 | 47 | 57.10 | 19.58 |
| Karayiannis et al.33 2017 | 66 | 26.00 | 16.26 | 76 | 20.00 | 16.63 |
| Salas-Huetos et al.35 2019 | 27 | 52.60 | 14.87 | 30 | 40.17 | 20.50 |
| Danielewicz et al.31 2019 | 70 | 33.80 | 17.49 | 62 | 30.80 | 14.88 |
| Semen volume (ml) | ||||||
| Cutillas-Tolín et al.17 2015 | 52 | 2.97 | 1.99 | 53 | 2.50 | 1.61 |
| Karayiannis et al.33 2017 | 66 | 2.10 | 0.81 | 76 | 2.00 | 1.31 |
| Salas-Huetos et al.35 2019 | 27 | 3.27 | 1.80 | 30 | 3.07 | 2.02 |
| Ricci et al.34 2019 | 35 | 2.73 | 1.88 | 31 | 2.77 | 1.58 |
| Danielewicz et al.31 2019 | 70 | 3.77 | 1.89 | 62 | 4.17 | 1.59 |
s.d.: standard deviation
Study quality
A total of six cross-sectional studies17,31,32,33,34,35 were included in our systematic review and meta-analysis. We used 11 checklist items recommended by the AHRQ to assess the quality of the literature included in this systematic review and meta-analysis. In the studies we included, two32,35 received a score of 7, three17,33,34 received a score of 8, and one31 received a score of 9. The results of the quality assessments are shown in Table 3.
Table 3.
Quality rating of included studies using AHRQ
| Study | Item | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total score | |
| Gaskins et al.32 2012 | √ | √ | √ | √ | √ | √ | √ | 7 | ||||
| Cutillas-Tolín et al.17 2015 | √ | √ | √ | √ | √ | √ | √ | √ | 8 | |||
| Karayiannis et al.33 2017 | √ | √ | √ | √ | √ | √ | √ | √ | 8 | |||
| Salas-Huetos et al.35 2019 | √ | √ | √ | √ | √ | √ | √ | 7 | ||||
| Ricci et al.34 2019 | √ | √ | √ | √ | √ | √ | √ | √ | 8 | |||
| Danielewicz et al.31 2019 | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | ||
AHRQ: Agency for Health Care Research and Quality
Qualitative analysis of sperm concentration
In the included articles, a total of six studies17,31,32,33,34,35 involving 708 subjects reported a healthy dietary pattern relationship with sperm concentration. Four studies31,33,34,35 divided the dietary scores into three groups, and two studies17,32 divided the dietary scores into four groups. There are contradictions among the results of the six studies. Karayiannis et al.33 found that in comparison to the men with the highest scores for the Mediterranean diet, among the men with the lowest scores, there was a higher percentage with a sperm concentration that was lower than the WHO reference value; the results from Danielewicz et al.31 showed that a higher sperm concentration was associated with adherence to the DASH diet. In the other four studies,17,32,34,35 no significant correlation was found.
Qualitative analysis of total sperm count
In the included articles, a total of five studies17,31,33,34,35 involving a total of 614 subjects reported a relationship between a healthy dietary pattern and total sperm count. Four studies31,33,34,35 divided the dietary scores into three groups, and one study17 divided the dietary scores into four groups. Karayiannis et al.33 found that in comparison to the men with the highest scores for the Mediterranean diet, among the men with the lowest scores, there was a higher percentage with a total sperm count that was lower than the WHO reference value. Cutillas-Tolín et al.17 found that Mediterranean dietary patterns were positively correlated with total sperm count. Danielewicz et al.31 also showed that a higher total sperm count was associated with adherence to the DASH diet. In the other two studies,34,35 no significant correlation was found.
Qualitative analysis of normal sperm morphology
In the included articles, a total of five studies17,31,32,33,35 involving a total of 530 subjects reported a relationship between a healthy dietary pattern and normal sperm morphology. Three studies31,33,35 divided the dietary scores into three groups, and two17,32 divided them into four groups. Karayiannis et al.33 found that in comparison to the men with the highest scores for the Mediterranean diet, among the men with the lowest scores, the proportion of males with normal sperm morphology lower than WHO reference value was higher. In the other four studies,17,31,32,35 no significant correlation was found.
Qualitative analysis of total sperm motility
In the included articles, a total of four studies17,31,33,35 involving a total of 436 study subjects reported a relationship between a healthy dietary pattern and total sperm motility. Three studies31,33,35 divided the dietary scores into three groups, and one study17 divided the dietary scores into four groups. Salas-Huetos et al.35 found that total sperm motility was statistically higher in those with the highest scores than that in those with the lowest scores for the Mediterranean diet. Karayiannis et al.33 found that in comparison to the men with the highest scores for the Mediterranean diet, among the men with the lowest scores, there was a higher percentage with total sperm motility lower than the WHO reference value. In the other two studies,17,31 no significant correlation was found.
Qualitative analysis of progressive sperm motility
Four cross-sectional studies31,32,33,35 involving a total of 425 study subjects reported the relationship between healthy dietary patterns and progressive sperm motility. Three studies31,33,35 divided the dietary scores into three groups, and one study32 divided the dietary scores into four groups. Salas-Huetos et al.35 found that progressive sperm motility was statistically higher in those with the highest scores for the Mediterranean diet than that in those with the lowest scores. Karayiannis et al.33 found that in comparison to the men with the highest scores for the Mediterranean diet, among the men with the lowest scores, there was a higher percentage with progressive sperm motility lower than the WHO reference value. Gaskins et al.32 found that a prudent diet was positively correlated with progressive sperm motility in a multivariate model. In the one remaining study,31 no significant correlation was found.
Qualitative analysis of semen volume
In the included articles, a total of five studies17,31,33,34,35 involving 614 subjects reported on the relationship between a healthy dietary pattern and semen volume. Four studies31,33,34,35 divided the dietary scores into three groups, and one17 divided them into four groups. None of the five studies17,31,33,34,35 showed any association between healthy dietary patterns and semen volume.
Quantitative analysis of sperm concentration
From the data of the six cross-sectional studies17,31,32,33,34,35 that were included, it was concluded that the sperm concentration of the subjects with higher consumption of a healthy dietary pattern was significantly higher than that of the subjects with lower consumption (MD: 6.88 × 106 ml−1, 95% CI: 1.26 × 106 ml−1–12.49 × 106 ml−1; P < 0.05). Significant heterogeneity did not exist (I2 = 0.6%, P > 0.1), and there was no publication bias in the research (Egger's test, P = 0.854; Figure 2a).
Figure 2.
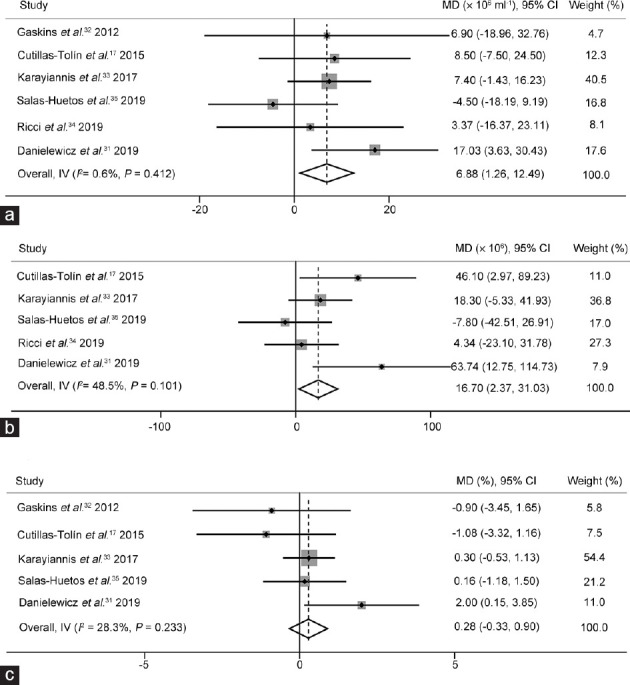
The effects of healthy dietary pattern on semen quality. The forest plots of (a) sperm concentration, (b) total sperm count, and (c) normal sperm morphology use a fixed-effects estimate method. MD: mean difference; CI: confidence interval; IV: Inverse-variance.
Quantitative analysis of total sperm count
From the data of five cross-sectional studies,17,31,33,34,35 it was concluded that the total sperm count in the subjects with higher consumption of a healthy dietary pattern was significantly higher than that of the subjects with lower consumption (MD: 16.70 × 106, 95% CI: 2.37 × 106–31.03 × 106; P < 0.05). Significant heterogeneity did not exist (I2 = 48.5%, P > 0.1), and there was no publication bias in the research (Egger's test, P = 0.279; Figure 2b).
Quantitative analysis of normal sperm morphology
Data from five cross-sectional studies17,31,32,33,35 showed that there was no significant association between a healthy dietary pattern and normal sperm morphology (MD: 0.28%, 95% CI: −0.33%–0.90%; P > 0.05). Significant heterogeneity did not exist (I2 = 28.3%, P > 0.1), and there was no publication bias in the research (Egger's test, P = 0.744; Figure 2c).
Quantitative analysis of total sperm motility
From the data of four cross-sectional studies,17,31,33,35 there was no significant correlation between healthy dietary patterns and total sperm motility (MD: 6.86%, 95% CI: −0.25%–13.96%; P > 0.05). Significant heterogeneity did not exist (I2 = 78.6%, P = 0.003), and there was no publication bias in the research (Egger's test, P = 0.074; Figure 3a).
Figure 3.
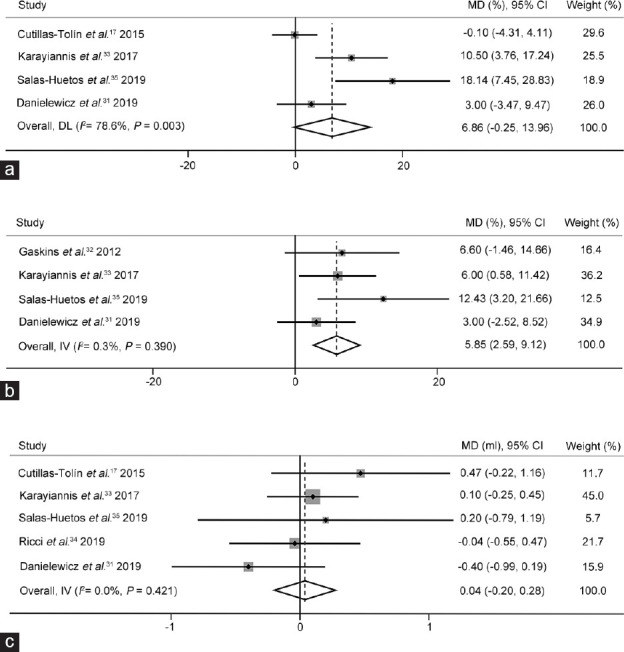
The effects of healthy dietary pattern on semen quality. (a) The forest plots of total sperm motility use a random-effects estimate method. The forest plots of (b) progressive sperm motility and (c) semen volume use a fixed-effects estimate method. MD: mean difference; CI: confidence interval; IV: Inverse-variance; DL: DerSimonian-Laird.
Quantitative analysis of progressive sperm motility
Data from four cross-sectional studies31,32,33,35 showed that the men with high consumption of a healthy dietary pattern had significantly higher progressive sperm motility than those with low consumption (MD: 5.85%, 95% CI: 2.59%–9.12%, P < 0.01). Significant heterogeneity did not exist (I2 = 0.3%, P > 0.1), and there was no publication bias in the research (Egger's test, P = 0.193; Figure 3b).
Quantitative analysis of semen volume
Five studies17,31,33,34,35 involving 614 subjects reported healthy dietary patterns and semen volumes. The results showed that there was no significant association between a healthy dietary pattern and semen volume (MD: 0.04 ml, 95% CI: −0.20 ml–0.28 ml; P > 0.05). Significant heterogeneity did not exist (I2 = 0, P > 0.1), and there was no publication bias in the research (Egger's test, P = 0.89; Figure 3c).
DISCUSSION
We reviewed and statistically analyzed six published articles to explore the influence of healthy dietary patterns on semen quality. We explored the relationship between healthy dietary patterns and the following six semen parameters: sperm concentration, total sperm count, progressive sperm motility, normal sperm morphology, total sperm motility, and semen volume. From the results, it can be seen that the sperm concentration, progressive sperm motility, and total sperm count were significantly higher in the group with high consumption of a healthy dietary pattern than those in the group with low consumption; however, no clear relationship was found between normal sperm morphology, total sperm motility, and semen volume.
Healthy dietary patterns are rich in plant-based foods, where saturated fats provide only a small percentage of the total energy intake. It has been suggested that a high-fat diet may have an effect on semen quality,36 and some animal experiments have investigated the effects of exercise and diet on semen parameters in mice. In a study of whether a high-fat diet early in life affects sperm quality in adulthood, the results of the study showed that mice fed a high-fat diet had lower sperm motility and viability even into early adulthood.37 In addition, in a systematic review and meta-analysis, strong and consistent evidence was found in animal studies that a high-fat diet is detrimental to male fertility.38 The authors found a higher proportion of sperm with DNA damage and signs of oxidative stress in mice fed a high-fat diet, which affected the metabolic state. There is a link between systemic metabolic status and sperm function, and animal studies have shown that abnormal sperm parameters caused by obesity can be reversed by a low-fat diet.39
Some antioxidants, such as antioxidant vitamins and folic acid, are more abundant in vegetables and fruits. Oxidative stress (OS) has been widely studied and has been shown to have harmful effects on sperm function. Reactive oxygen species (ROS) are oxygen-derived molecules that are formed as intermediary products of cellular metabolism.40 At high levels, ROS can influence sperm membranes and alter sperm DNA.41 Antioxidants, as scavengers of ROS, can ameliorate the adverse effects of high concentrations of active oxygen on semen. From two systematic reviews of studies, we can conclude that the use of antioxidants improves the live birth rate of couples experiencing poor sperm quality and infertility.42,43 In a study of healthy men who did not smoke, the results showed that higher antioxidant intake was related to higher total sperm count, sperm concentration, and sperm progressive motility,44 which is consistent with our findings. Antioxidants found in fruits and vegetables may influence semen quality.
Folic acid participates in lipid and protein synthesis, removing active oxidative free radicals and aiding in DNA repair. When the intake of folic acid is low, OS will increase accordingly, thereby changing the integrity of DNA and subsequently participating in the molecular processes of spermatogenesis and embryogenesis.45 Sevilla y Ruiz et al.46 showed that excessive estradiol or its metabolites may affect normal sperm production; therefore, reducing plasma estrogen levels may have a beneficial effect on sperm. The major sources of fiber are whole grains, vegetables, and fruits, which bind directly to unbound estrogen, thereby lowering plasma estrogen levels and reducing estrogen absorption.47 In our research, the food groups contained in the healthy dietary patterns included vegetables, fruits, legumes, whole grains, and low-fat dairy products. The antioxidants and other substances contained in these food groups are advantageous for semen quality. This may explain the significant increase in sperm concentration, progressive sperm motility, and total sperm count in those with high consumption of a healthy dietary pattern compared with those with low consumption.
In a study of the relationship between the intake of dairy products and semen parameters, it was found that the intake of low-fat dairy foods, especially low-fat milk, had a strong positive correlation with sperm concentration and vitality.48 Low-fat milk intake was associated with higher insulin-like growth factor-1 (IGF-1) and circulating insulin levels.49 Animal models have shown that insulin increases the total sperm count and sperm motility in rats with type 1 diabetes50 and rescues sperm production in mice with type 1 diabetes.51 Spermatogenesis requires insulin and IGF-1 to bind and activate Leydig cell insulin receptors.52 Leydig cells can be affected by insulin and IGF-1, Sertoli cells, or spermatogonia, and the increase in sperm concentration and motility associated with low-fat dairy products may represent a biological effect in humans.48
The six studies17,31,32,33,34,35 that we included covered a total of five countries, including Spain, the USA, Greece, Poland, and Italy. Because these countries are located in different geographical locations with different population types and cultural beliefs, dietary differences among the populations are relatively large, so when determining dietary patterns, an FFQ must be adapted and validated for each specific population.53 Information about the amount and frequency of consumption of food and food groups and the time of eating is not available to us, and this is a limitation of the FFQ. In our included studies, the FFQs used were validated and appropriate for the study population. Therefore, the dietary patterns obtained by the analysis are locally representative; these long-term dietary patterns are stable and may not have much impact on the analysis of the results. To some degree, different FFQ items could reflect the different dietary habits of people in the included studies. Thus, difference of FFQ items across studies might also increase the heterogeneity of the results.
Of the included studies, two studies33,34 investigated infertile men and four studies17,31,32,35 investigated healthy men. Due to the different health statuses of the included subjects, the semen quality of infertile men may be poor relative to that of healthy men, which may have an impact on our combined results. In terms of semen analysis, Gaskins et al.32 used the 4th edition of the WHO manual,54 and the other studies17,31,33,34,35 used the 5th edition of the WHO manual.55 In the sperm morphology test, the 4th edition of the WHO manual has no reference value for the percentage of morphologically normal sperm but considers that the percentage of morphologically normal sperm above 15% does not affect the fertilization rate of in vitro fertilization (IVF), while the 5th edition of the WHO manual has a reference value of 4%, which will have an impact on the results of sperm morphology assessment and thus on the reliability of our results. For semen volume measurement, Salas-Huetos et al.35 used a Pasteur pipette for measurement, while others used the direct weighing method. Measurement using the Pasteur pipette may lose some semen and therefore underestimate semen volume.55 In conclusion, due to the inconsistency of the included studies in various aspects such as study population and methodology, there may be some influence on the reliability of our results.
Limitations and strengths
To better interpret our results, we must acknowledge the limitations of our research. First, the studies we included were all cross-sectional studies, and it is impossible to determine the causal relationship between the high intake of healthy dietary patterns and semen quality. Second, after literature screening, the number of studies we included was small, and there was not enough literature to support us in drawing reliable conclusions. Third, among the outcome variables of our study, total sperm motility had the greatest heterogeneity because the number of studies was small, and no subgroup analysis was performed to analyze the heterogeneity. By reading the literature, we estimate that the source of heterogeneity may be related to the health status of the research subjects, different semen analysis criteria, different dietary patterns, the methods of determining dietary patterns, the confounding factors of adjustment, etc.
The main advantages of this study are that we studied six semen parameters, and these semen parameters can better reflect the semen quality of men. The included studies grouped the dietary patterns according to consumption and clearly compared the difference between low consumption and high consumption.
CONCLUSION
Our analysis showed that healthy dietary patterns had beneficial effects on sperm concentration, total sperm count, and progressive sperm motility in males, which affect male fertility. We recommend that dietary interventions should be considered in clinical work as part of the therapies improving male semen quality. From a public health perspective, healthy dietary patterns may promote male reproductive health and thus improve semen quality in the population.
AUTHOR CONTRIBUTIONS
LLC and PYS designed the study. LLC and JJC wrote the manuscript and performed the statistical analysis. LLC, JJC, SJW, YHL, MYY, GFW, and PYS commented in detail on the drafts. LLC, SJW, and JJC participated in the quality assessment. LLC, JJC, SJW, YHL, MYY, GFW, and PYS performed the data analysis and explanation. LLC, JJC, and PYS reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Nature Science Foundation of China (No. 82173539 and No. 81874268).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–12. doi: 10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- 2.Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21:411–26. doi: 10.1093/humupd/dmv016. [DOI] [PubMed] [Google Scholar]
- 3.Dyer SJ, Abrahams N, Hoffman M, van der Spuy ZM. 'Men leave me as I cannot have children': women's experiences with involuntary childlessness. Hum Reprod. 2002;17:1663–8. doi: 10.1093/humrep/17.6.1663. [DOI] [PubMed] [Google Scholar]
- 4.Kobori Y. Home testing for male factor infertility: a review of current options. Fertil Steril. 2019;111:864–70. doi: 10.1016/j.fertnstert.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 5.Huang C, Li B, Xu K, Liu D, Hu J, et al. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril. 2017;107:83–8.e2. doi: 10.1016/j.fertnstert.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Pizzol D, Foresta C, Garolla A, Demurtas J, Trott M, et al. Pollutants and sperm quality: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2021;28:4095–103. doi: 10.1007/s11356-020-11589-z. [DOI] [PubMed] [Google Scholar]
- 7.Ricci E, Al Beitawi S, Cipriani S, Candiani M, Chiaffarino F, et al. Semen quality and alcohol intake: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34:38–47. doi: 10.1016/j.rbmo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Harlev A, Agarwal A, Esteves SC. Cigarette smoking and semen quality: a new meta-analysis examining the effect of the 2010 World Health Organization laboratory methods for the examination of human semen. Eur Urol. 2016;70:635–45. doi: 10.1016/j.eururo.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Nätt D, Kugelberg U, Casas E, Nedstrand E, Zalavary S, et al. Human sperm displays rapid responses to diet. PLoS Biol. 2019;17:e3000559. doi: 10.1371/journal.pbio.3000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nassan FL, Chavarro JE, Tanrikut C. Diet and men's fertility: does diet affect sperm quality? Fertil Steril. 2018;110:570–7. doi: 10.1016/j.fertnstert.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23:371–89. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 12.Meldgaard M, Brix N, Gaml-Sørensen A, Ernst A, Ramlau-Hansen CH, et al. Consumption of sugar-sweetened or artificially sweetened beverages and semen quality in young men: a cross-sectional study. Int J Environ Res Public Health. 2022;19:282. doi: 10.3390/ijerph19020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402s–6s. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 14.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 15.Caruso P, Caputo M, Cirillo P, Scappaticcio L, Longo M, et al. Effects of Mediterranean diet on semen parameters in healthy young adults: a randomized controlled trial. Minerva Endocrinol. 2020;45:280–7. doi: 10.23736/S0391-1977.20.03362-3. [DOI] [PubMed] [Google Scholar]
- 16.Montano L, Ceretti E, Donato F, Bergamo P, Zani C, et al. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt randomized controlled trial. Eur Urol Focus. 2021;8:351–9. doi: 10.1016/j.euf.2021.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Cutillas-Tolín A, Mínguez-Alarcón L, Mendiola J, López-Espín JJ, Jørgensen N, et al. Mediterranean and western dietary patterns are related to markers of testicular function among healthy men. Hum Reprod. 2015;30:2945–55. doi: 10.1093/humrep/dev236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336:1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 19.Cutillas-Tolín A, Adoamnei E, Navarrete-Muñoz EM, Vioque J, Moñino-García M, et al. Adherence to diet quality indices in relation to semen quality and reproductive hormones in young men. Hum Reprod. 2019;34:1866–75. doi: 10.1093/humrep/dez157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA. 2007;298:754–64. doi: 10.1001/jama.298.7.754. [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Rimm EB, Stampfer MJ, Ascherio A, Spiegelman D, et al. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–21. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 22.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136:201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 23.Nassan FL, Jensen TK, Priskorn L, Halldorsson TI, Chavarro JE, et al. Association of dietary patterns with testicular function in young Danish men. JAMA Netw Open. 2020;3:e1921610. doi: 10.1001/jamanetworkopen.2019.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirani M, Saneei P, Nouri M, Maracy MR, Abbasi H, et al. Associations of major dietary patterns and dietary diversity score with semen parameters: a cross-sectional study in Iranian infertile men. Int J Fertil Steril. 2020;14:185–92. doi: 10.22074/ijfs.2020.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willett W, Rockström J, Loken B, Springmann M, Lang T, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–92. doi: 10.1016/S0140-6736(18)31788-4. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J, Dong Y, Chen X, Liu Y, Ma D, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. 2015;61:78–89. doi: 10.1016/j.comppsych.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JP, Li T, Deeks JJ. Chapter 6: choosing effect measures and computing estimates of effect. Cochrane Handbook for Systematic Reviews of Interventions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, et al., editors. 2nd ed. Chichester (UK): John Wiley & Sons; 2019. pp. 143–76. [Google Scholar]
- 31.Danielewicz A, Morze J, Przybyłowicz M, Przybyłowicz KE. Association of the dietary approaches to stop hypertension, physical activity, and their combination with semen quality: a cross-sectional study. Nutrients. 2019;12:39. doi: 10.3390/nu12010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, et al. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod. 2017;32:215–22. doi: 10.1093/humrep/dew288. [DOI] [PubMed] [Google Scholar]
- 34.Ricci E, Bravi F, Noli S, Ferrari S, De Cosmi V, et al. Mediterranean diet and the risk of poor semen quality: cross-sectional analysis of men referring to an Italian fertility clinic. Andrology. 2019;7:156–62. doi: 10.1111/andr.12587. [DOI] [PubMed] [Google Scholar]
- 35.Salas-Huetos A, Babio N, Carrell DT, Bulló M, Salas-Salvadó J. Adherence to the Mediterranean diet is positively associated with sperm motility: a cross-sectional analysis. Sci Rep. 2019;9:3389. doi: 10.1038/s41598-019-39826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and nutritional factors in male (in)fertility-underestimated factors. J Clin Med. 2020;9:1400. doi: 10.3390/jcm9051400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crisóstomo L, Videira RA, Jarak I, Starčević K, Mašek T, et al. Diet during early life defines testicular lipid content and sperm quality in adulthood. Am J Physiol Endocrinol Metab. 2020;319:E1061–73. doi: 10.1152/ajpendo.00235.2020. [DOI] [PubMed] [Google Scholar]
- 38.Crean AJ, Senior AM. High-fat diets reduce male reproductive success in animal models: a systematic review and meta-analysis. Obes Rev. 2019;20:921–33. doi: 10.1111/obr.12827. [DOI] [PubMed] [Google Scholar]
- 39.Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab. 2012;302:E768–80. doi: 10.1152/ajpendo.00401.2011. [DOI] [PubMed] [Google Scholar]
- 40.Combelles CM, Gupta S, Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online. 2009;18:864–80. doi: 10.1016/s1472-6483(10)60038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremellen K. Oxidative stress and male infertility – a clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 42.Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, et al. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 2010;20:711–23. doi: 10.1016/j.rbmo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Smits RM, Mackenzie-Proctor R, Yazdani A, Stankiewicz MT, Jordan V, et al. Antioxidants for male subfertility. Cochrane Database Syst Rev. 2019;3:1465–858. doi: 10.1002/14651858.CD007411.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, et al. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20:1006–12. doi: 10.1093/humrep/deh725. [DOI] [PubMed] [Google Scholar]
- 45.Hoek J, Steegers-Theunissen RP, Willemsen SP, Schoenmakers S. Paternal folate status and sperm quality, pregnancy outcomes, and epigenetics: a systematic review and meta-analysis. Mol Nutr Food Res. 2020;64:e1900696. doi: 10.1002/mnfr.201900696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sevilla y Ruiz A, Moya Gordillo C, Torres Lili G, Canales Perez ES. Serum concentrations of estradiol and testosterone in patients with oligoasthenozoospermia and asthenozoospermia. Ginecol Obstet Mex. 1991;59:313–5. Article in Spanish. [PubMed] [Google Scholar]
- 47.Goldin BR, Adlercreutz H, Gorbach SL, Warram JH, Dwyer JT, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982;307:1542–7. doi: 10.1056/NEJM198212163072502. [DOI] [PubMed] [Google Scholar]
- 48.Afeiche MC, Bridges ND, Williams PL, Gaskins AJ, Tanrikut C, et al. Dairy intake and semen quality among men attending a fertility clinic. Fertil Steril. 2014;101:1280–7. doi: 10.1016/j.fertnstert.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsilidis KK, Travis RC, Appleby PN, Allen NE, Lindström S, et al. Insulin-like growth factor pathway genes and blood concentrations, dietary protein and risk of prostate cancer in the NCI Breast and Prostate Cancer Cohort Consortium (BPC3) Int J Cancer. 2013;133:495–504. doi: 10.1002/ijc.28042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh S, Malini T, Rengarajan S, Balasubramanian K. Impact of experimental diabetes and insulin replacement on epididymal secretory products and sperm maturation in albino rats. J Cell Biochem. 2009;108:1094–101. doi: 10.1002/jcb.22337. [DOI] [PubMed] [Google Scholar]
- 51.Schoeller EL, Albanna G, Frolova AI, Moley KH. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes. 2012;61:1869–78. doi: 10.2337/db11-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holly JM, Perks CM. Insulin-like growth factor physiology: what we have learned from human studies. Endocrinol Metab Clin North Am. 2012;41:249–63. doi: 10.1016/j.ecl.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Qiu X, Zhong C, Zhang K, Xiao M, et al. Reproducibility and relative validity of a semi-quantitative food frequency questionnaire for Chinese pregnant women. Nutr J. 2015;14:56. doi: 10.1186/s12937-015-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 55.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]


