Abstract
Prostate cancer (PCa) is the second-most common cancer among men. Both active surveillance or watchful waiting (AS/WW) and focal laser ablation (FLA) can avoid the complications caused by radical treatment. How to make the choice between these options in clinical practice needs further study. Therefore, this study aims to compare and analyze their effects based on overall survival (OS) and cancer-specific survival (CSS) to obtain better long-term benefits. We included patients with low-risk PCa from the Surveillance Epidemiology and End Results database of 2010–2016. Multivariate Cox proportional hazard analyses were conducted for OS and CSS in the two groups. To eliminate bias, this study applied a series of sensitivity analyses. Moreover, Kaplan–Meier curves were plotted to obtain survival status. A total of 18 841 patients with low-risk PCa were included, with a median of 36-month follow-up. According to the multivariate Cox proportional hazard regression, the FLA group presented inferior survival benefits in OS than the AS/WW group (hazard ratio [HR]: 2.13, 95% confidence interval [CI]: 1.37–3.33, P < 0.05). After adjusting for confounders, the result persisted (HR: 1.69, 95% CI: 1.02–2.81, P < 0.05). According to the results of the sensitivity analysis, the inverse probability of the treatment weighing model indicated the same result in OS. In conclusion, AS/WW and FLA have the advantage of fewer side effects and the benefit of avoiding overtreatment compared with standard treatment. Our study suggested that AS/WW provides more survival benefits for patients with low-risk PCa. More relevant researches and data will be needed for further clarity.
Keywords: active surveillance, cancer-specific survival, focal laser ablation, low-risk prostate cancer, overall survival, watchful waiting
INTRODUCTION
Prostate cancer (PCa) is the second-most frequent cancer and causes the fifth-highest mortality for men. It was estimated that there were nearly 1.3 million new prostate cancer cases and 359 000 related deaths worldwide in 2018. It is also the most diagnosed cancer among men in more than half of the world (105 out of 185 countries).1 By the end of 2020, it is estimated that approximately 606 520 Americans will die of cancer, of which 321 160 will be male, and PCa will be ranked second in mortality. Among the estimated new cases, PCa ranks first in male patients.2 During this same period, because of the high adoption of prostate-specific antigen (PSA) screening, the global diagnosis of low-risk and intermediate PCa has increased.3 From 2004 to 2014 in the USA, 34.26% of all patients with PCa were diagnosed with low-risk PCa.4 As this represents a large proportion of affected patients, it is thus very important to ensure effective management and treatment of these cases.
Conventionally, most patients with low-risk PCa receive either radical prostatectomy (RP) or radiotherapy.5,6 However, the side effects of these treatments, such as urinary incontinence, erectile dysfunction, postoperative infection, hematuria, and pain, maybe more detrimental to the patient versus the benefit of the treatment itself.7,8,9 In addition to radiotherapy and RP, active surveillance (AS) is recommended as another standard treatment for patients with low-risk localized PCa.5,6 Several studies have consistently suggested that men with low-risk PCa should consider AS as a valid treatment option.7,10,11 AS and watchful waiting (WW) are collectively referred to conservative or deferred treatment in guidelines and in research,12,13,14,15 and the aim of both is to reduce overtreatment.
Another new treatment is local therapy, an approach centered on retaining key structures and ensuring stable urogenital function. It specifically destroys known tumor areas and maintains the survival benefits of aggressive treatments.16 Several energy therapies, including focal laser ablation (FLA), cryotherapy, and photodynamic therapy, have been developed to promote the local treatment of low- and medium-risk PCa.17 FLA has undergone significant development as a focus therapy model, and the process is often guided under magnetic resonance imaging (MRI).18,19 Based on the results of phase II trials, FLA was associated with greater beneficial tumor prognosis in the short term, and FLA-treated patients did not show significant urinary, sexual, or intestinal side effects.20 Furthermore, a small-scale research study reported that FLA as a treatment modality exhibited early tumor control and resulted in fewer complications and improved quality of life.21 A larger retrospective study showed that 83% of patients undergoing FLA had no relapse in 1 year, and no obvious changes were observed in sexual and urinary function after undergoing FLA.22 In addition, several other trials of FLA have shown encouraging short-term results, with the overall conclusion that FLA is a realistic treatment option.21,23,24,25 Despite the encouraging potential of FLA based on these findings, the present trials investigating FLA as a treatment modality for PCa do not have a double-arm design and do not collect long-term oncology results, namely the outcome of overall survival (OS) and cancer-specific survival (CSS).
Although AS/WW and FLA avoid complications caused by overtreatment, how to choose between them in clinical practice needs further study. In particular, a long-term tumor prognosis trial for patients with low-risk PCa is lacking. Furthermore, there has been no direct comparison between FLA and AS/WW. Therefore, the aim of this study was to verify the efficacy of FLA and AS/WW in patients with low-risk PCa. To evaluate the long-term benefits of these approaches, OS and CSS were analyzed and compared in our patient cohort.
PATIENTS AND METHODS
Patients selection
In 2018, the new dataset was released containing AS/WW data from the Surveillance, Epidemiology, and End Results (SEER) Program in the United States,26 from which we identified known cases of low-risk PCa from April 2010 to April 2016. Our initial study cohort consisted of 57 631 patients, who underwent further evaluation based on inclusion and exclusion criteria. Our inclusion criteria included patients with low-risk PCa, which was defined as a clinical tumor stage between T1 and T2a, Gleason score <7, and PSA <10 ng ml−1. Exclusion criteria were as follows: (1) tumors with different histology or unknown histology from adenocarcinoma; (2) absence of positive histological confirmation; (3) patients who rejected active treatment or WW as the treatment modality or patients without doctor's advice to take that treatment; or (4) patients who underwent treatment other than AS/WW or FLA. Based on these criteria, our final cohort consisted of 18 841 patients. The evaluation process for screening patients is shown in Figure 1. All information was downloaded from the SEER database.
Figure 1.
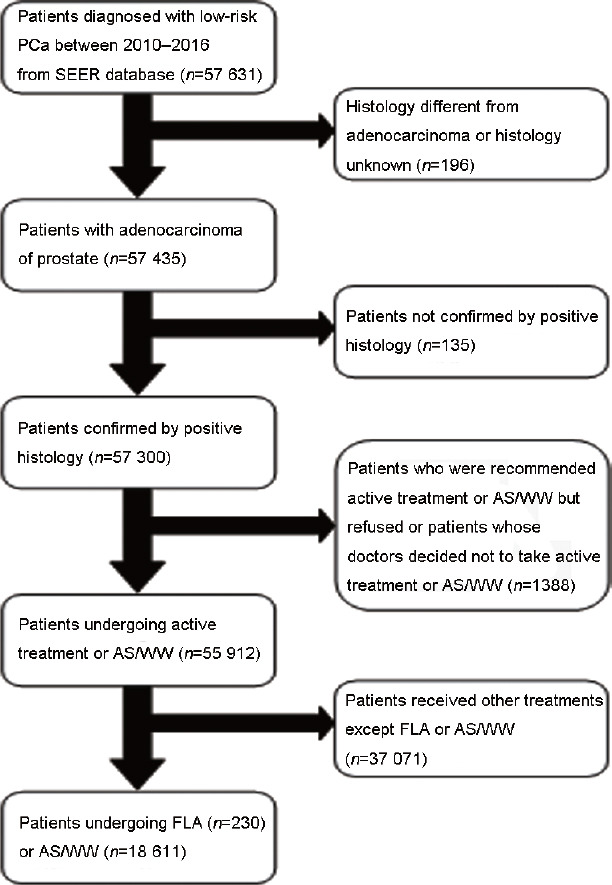
Flowchart of the patients' selection. PCa: prostate cancer; SEER: Surveillance Epidemiology and End Results; AS/WW: active surveillance or watchful waiting; FLA: focal laser ablation.
Propensity score matching (PSM)
Since baseline characteristics would influence the choice, we used PSM with a ratio of 1:4 and caliper width of 0.05 standard deviations. Our intent was to ensure that the FLA and AS/WW groups had similar baseline characteristics. In addition, we applied logistic regression to adjust for the differences between groups.27 This process was executed according to the nearest neighbor matching principle, and the matching process was considered balanced when P > 0.05.
Data analyses
Baseline indicators were contrasted before and after matching in the FLA and AS/WW groups. We applied a two-tailed samples t-test, Chi-square test, Kruskal–Wallis test, rank-sum test, and Fisher's exact test for the respective variables. Kaplan–Meier survival curves were generated to estimate the OS and CSS of patients in our two groups. In addition, we performed a multivariate Cox proportional hazard model to analyze the pre- and post-PSM cohorts of the FLA and AS/WW groups. OS and CSS are expressed by hazard ratios (HRs) with 95% confidence intervals (CIs). We also performed subgroup analyses based on race, age, tumor stage, and PSA level.
To verify the reliability of the main results, we conducted the following series of sensitivity analyses: (a) OS and CSS analyses after correcting for imbalanced covariates between the AS/WW group and FLA group; (b) CSM analysis after adjusting propensity scores; (c) PSM double-adjustment for multivariate Cox proportional hazard model; and (d) application of inverse probability of treatment weighting (IPTW) model used after PSM.
All data analyses were performed using the R packages (The R Foundation, Boston, MA, USA) and EmpowerStats (X&Y Solutions, Inc., Boston, MA, USA). P < 0.05 was considered statistically significant.
RESULTS
Our study cohort consisted of 18 841 patients with low-risk PCa between 2010 and 2016 from the SEER database. It included 18 611 patients undergoing AS/WW and 230 patients undergoing FLA. The median follow-up time of this study was 36.0 months. The baseline characteristics of our cohort are presented in Table 1. We found that patients undergoing FLA were older than those undergoing AS/WW (P < 0.001), and that patients receiving FLA had a lower PSA level (P < 0.05) and longer survival rate (P < 0.001) compared with those found in patients receiving AS/WW. In addition, these two groups had differences in the baseline characteristics of insurance status (P = 0.034) and year of diagnosis (P < 0.001; Table 1).
Table 1.
Baseline demographic and clinicopathologic characteristics by active surveillance or watchful waiting versus focal laser ablation
| Characteristic | All patients | AS/WW | FLA | P |
|---|---|---|---|---|
| Patients (n) | 18 841 | 18 611 | 230 | |
| Age at diagnosis (year), mean±s.d. | 64.09±7.59 | 64.05±7.58 | 67.77±7.96 | <0.001 |
| PSA (ng ml−1), mean±s.d. | 5.61±1.89 | 5.62±1.88 | 5.18±2.21 | 0.002 |
| Survival (month), mean±s.d. | 36.00±23.55 | 35.92±23.53 | 41.99±24.48 | <0.001 |
| Insurance status, n (%) | 0.034 | |||
| Insured | 14 303 (75.9) | 14 145 (76.0) | 158 (68.7) | |
| Insured/no specifics | 2554 (13.6) | 2516 (13.5) | 38 (16.5) | |
| Any medicaid | 570 (3.0) | 560 (3.0) | 10 (4.4) | |
| Uninsured | 218 (1.2) | 217 (1.2) | 1 (0.4) | |
| Unknown | 1196 (6.4) | 1173 (6.3) | 23 (10.0) | |
| Year of diagnosis, n (%) | <0.001 | |||
| 2010 | 1881 (10.0) | 1840 (9.9) | 41 (17.8) | |
| 2011 | 2389 (12.7) | 2352 (12.6) | 37 (16.1) | |
| 2012 | 2489 (13.2) | 2456 (13.2) | 33 (14.4) | |
| 2013 | 3048 (16.2) | 3018 (16.2) | 30 (13.0) | |
| 2014 | 2773 (14.7) | 2743 (14.7) | 30 (13.0) | |
| 2015 | 2946 (15.6) | 2914 (15.7) | 32 (13.9) | |
| 2016 | 3315 (17.6) | 3288 (17.7) | 27 (11.7) | |
| Race, n (%) | 0.754 | |||
| White | 14 788 (78.5) | 14 606 (78.5) | 182 (79.1) | |
| Black | 2545 (13.5) | 2512 (13.5) | 33 (14.4) | |
| Other | 1034 (5.5) | 1025 (5.5) | 9 (3.9) | |
| Unknown | 474 (2.5) | 468 (2.5) | 6 (2.6) | |
| T stage, n (%) | <0.001 | |||
| T1a | 51 (0.3) | 0 (0) | 51 (22.2) | |
| T1b | 8 (<0.01) | 0 (0) | 8 (3.5) | |
| T1c | 17 386 (92.3) | 17 235 (92.6) | 151 (65.7) | |
| T1NOS | 76 (0.4) | 68 (0.4) | 8 (3.5) | |
| T2a | 1320 (7.0) | 1308 (7.0) | 12 (5.2) | |
| Total Gleason score, n (%) | 0.746 | |||
| 3 | 9 (0.1) | 9 (0.1) | 0 (0) | |
| 4 | 18 (0.1) | 18 (0.1) | 0 (0) | |
| 5 | 72 (0.4) | 72 (0.4) | 0 (0) | |
| 6 | 18 742 (99.5) | 18 512 (99.5) | 230 (100.0) |
AS/WW: active surveillance or watchful waiting; FLA: focal laser ablation; PSA: prostate-specific antigen; s.d.: standard deviation; T1NOS: T1 not otherwise specified
Based on Kaplan–Meier survival curves (Figure 2), we found increased OS in patients from the AS/WW group compared with the FLA group (P = 0.009; Figure 2a), and no significant difference in CSS was found between the two groups (P = 0.32; Figure 2b). Next, using multivariate Cox proportional hazard regression analysis, we found that patients from the FLA group had worse OS than patients from the AS/WW group (HR: 2.13, 95% CI: 1.37–3.33, P < 0.001). Regarding CSS, no obvious difference between groups was observed before adjustment (HR: 1.96, 95% CI: 0.27–14.32, P = 0.509). After adjusting for age, insurance status, year of diagnosis, race, tumor stage, and PSA level, we similarly found worse OS in the FLA group than in the AS/WW group (HR: 1.69, 95% CI: 1.02–2.81, P = 0.043) and no significant difference in CSS between groups (HR: 1.97, 95% CI: 0.27–14.58, P = 0.505; Table 2). We then performed subgroup analysis and found that differences in race, age, PSA level, and tumor stage between the two groups exerted varying effects on OS and CSS. However, despite these findings, we found no evidence of an interaction on OS or CSS in our cohort (P > 0.05; Table 3).
Figure 2.
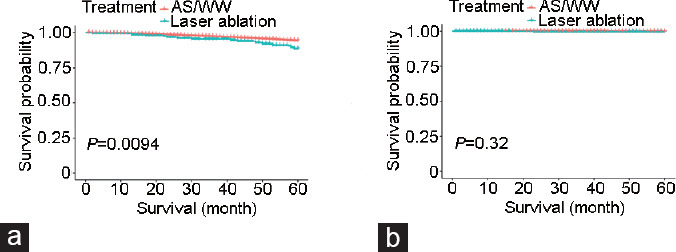
Kaplan–Meier survival curves of AS/WW versus focal laser ablation of (a) overall survival and (b) cancer-specific survival. AS/WW: active surveillance or watchful waiting.
Table 2.
Multivariate cox regression analyses for overall survival and cancer-specific survival in the total cohort
| Characteristic | Nonadjusted | Adjusted | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| CSS | ||||
| AS/WW | 1 | 1 | ||
| FLA | 1.96 (0.27–14.32) | 0.5092 | 1.97 (0.27–14.58) | 0.5050 |
| OS | ||||
| AS/WW | 1 | 1 | ||
| FLA | 2.13 (1.37–3.33) | 0.0009 | 1.69 (1.02–2.81) | 0.0430 |
The adjusted model adjusted by age, insurance status, year of diagnosis, race, T stage and PSA level. AS/WW: active surveillance or watchful waiting; FLA: focal laser ablation, CSS: cancer-specific survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; PSA: prostate-specific antigen
Table 3.
Subgroup analysis for overall survival and cancer-specific survival by active surveillance or watchful waiting versus focal laser ablation
| Subgroup | Patient (n) | CSS | OS | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P | HR (95% CI) | P | ||
| Race | |||||
| White | 14 788 | 1.33 (0.80–2.19) | 0.2709 | 1.18 (1.03–1.35) | 0.0163 |
| Black | 2545 | - | 1.03 (0.72–1.48) | 0.8557 | |
| Other | 1034 | - | - | ||
| Unknown | 474 | NA | - | ||
| P value of interaction | 0.8551 | 0.7331 | |||
| Age at diagnosis (year) | |||||
| <65 | 9402 | - | 0.93 (0.57–1.53) | 0.7812 | |
| ≥65 | 9439 | 1.29 (0.78–2.13) | 0.3265 | 1.17 (1.02–1.33) | 0.0224 |
| P value of interaction | 0.3853 | 0.3334 | |||
| PSA (ng ml−1) | |||||
| <4 | 2785 | 2.71 (1.16–6.31) | 0.0208 | 1.33 (1.03–1.73) | 0.0314 |
| ≥4 | 16 056 | - | 1.10 (0.95–1.27) | 0.2055 | |
| P value of interaction | 0.0547 | 0.2387 | |||
| T stage | |||||
| T1 | 17 521 | - | 1.16 (1.03–1.30) | 0.0112 | |
| T2 | 1320 | 2.39 (1.17–4.89) | 0.0166 | 1.12 (0.68–1.87) | 0.6551 |
| P value of interaction | 0.1123 | 0.9350 | |||
-: the number of people in the corresponding subgroup was too small to get results; CSS: cancer-specific survival; OS: overall survival; PSA: prostate-specific antigen; HR: hazard ratio; CI: confidence interval; NA: not available
We performed PSM matching 177 patients undergoing FLA with 708 patients undergoing AS/WW in a ratio of 1:4. Age, race, tumor stage, PSA level, and insurance status were chosen as covariates; the difference in PSA level was negated after PSM. All baseline variables are shown in Supplementary Table 1. Following PSM, we analyzed the matched cohorts using regression analysis and unexpectedly found that the CSS of patients receiving FLA was significantly worse than that of patients receiving AS/WW (HR: 17.76, 95% CI: 1.15–275.02, P = 0.040), whereas no obvious difference was observed in OS between the two groups (P = 0.158). As there may be baseline differences after PSM, we performed a double-adjustment with PSM. After adjusting for age, race, tumor stage and insurance status, we found that the results were consistent with those after PSM but before double adjustment (CSS, HR: 19.48, 95% CI, 1.03–369.49, P = 0.048; OS, P = 0.872; Supplementary Table 2). To further verify the robustness of our findings, we used IPTW after PSM, and found that OS was lower in the FLA group than that in the AS/WW group, a result consistent with the main finding of our study. Details regarding PSM and IPTW analyses are available in Supplementary Table 2.
Supplementary Table 1.
Baseline variables by active surveillance or watchful waiting versus focal laser ablation after propensity score matching
| Treatment group | AS/WW | FLA | SD | P |
|---|---|---|---|---|
| Number of patients | 708 | 177 | ||
| Age at diagnosis (year), mean±s.d. | 61.76±7.51 | 67.05±8.02 | 0.6814 | <0.0001 |
| PSA (ng ml−1), mean±s.d. | 5.18±2.54 | 5.52±2.00 | 0.1461 | 0.1039 |
| Survival months, mean±s.d. | 31.99±23.26 | 45.77±24.01 | 0.5832 | <0.0001 |
| Insurance status, n (%) | ||||
| Insured | 602 (85) | 115 (65) | 0.4761 | <0.0001 |
| Insured/no specifics | 69 (9.7) | 30 (16.9) | 0.213 | |
| Any medicaid | 18 (2.5) | 9 (5.1) | 0.133 | |
| Uninsured | 6 (0.8) | 1 (0.6) | 0.0337 | |
| Unknown | 13 (1.8) | 22 (12.4) | 0.4206 | |
| Year of diagnosis, n (%) | ||||
| 2010 | 52 (7.3) | 38 (21.5) | 0.4106 | <0.0001 |
| 2011 | 77 (10.9) | 34 (19.2) | 0.2347 | |
| 2012 | 83 (11.7) | 27 (15.3) | 0.1035 | |
| 2013 | 111 (15.7) | 22 (12.4) | 0.0936 | |
| 2014 | 104 (14.7) | 22 (12.4) | 0.066 | |
| 2015 | 100 (14.1) | 19 (10.7) | 0.1029 | |
| 2016 | 181 (25.6) | 15 (8.5) | 0.467 | |
| Race, n (%) | ||||
| White | 595 (84) | 135 (76.3) | 0.1957 | 0.0016 |
| Black | 68 (9.6) | 31 (17.5) | 0.2326 | |
| Other | 37 (5.2) | 5 (2.8) | 0.1224 | |
| Unknown | 8 (1.1) | 6 (3.4) | 0.1525 | |
| T stage, n (%) | ||||
| T1b | 0 | 6 (3.4) | 0.2649 | <0.0001 |
| T1c | 68 (9.6) | 151 (85.3) | 2.3246 | |
| T1NOS | 4 (0.6) | 8 (4.5) | 0.2533 | |
| T2a | 636 (89.8) | 12 (6.8) | 2.9876 | |
| Gleason total, n (%) | ||||
| 3 | 4 (0.6) | 0 | 0.1066 | 0.5686 |
| 4 | 1 (0.1) | 0 | 0.0532 | |
| 5 | 3 (0.4) | 0 | 0.0923 | |
| 6 | 700 (98.9) | 177 (100) | 0.1512 |
AS/WW: active surveillance or watchful waiting; FLA: focal laser ablation; PSA: prostate-specific antigen; s.d.: standard deviation
Supplementary Table 2.
Multivariate cox regression analyses for overall survival and cancer-specific survival in the matched cohort, double-adjustment of propensity score matching and inverse probability of treatment weighing model
| HR (95% CI), P | |
|---|---|
| In PSM | |
| CSS | |
| AS/WW | 1 |
| Laser ablation | 17.76 (1.15–275.02), 0.0396 |
| OS | |
| AS/WW | 1 |
| Laser ablation | 1.59 (0.84–3.01), 0.1581 |
| In double-adjustment of PSM | |
| CSS | |
| AS/WW | 1 |
| Laser ablation | 19.48 (1.03–369.49), 0.0480 |
| OS | |
| AS/WW | 1 |
| Laser ablation | 0.92 (0.33–2.56), 0.8722 |
| In IPTW model | |
| CSS | |
| AS/WW | 1 |
| Laser ablation | 10.32 (0.03–3552.15), 0.4336 |
| OS | |
| AS/WW | 1 |
| Laser ablation | 1.99 (1.29–3.07), 0.0018 |
The double-adjustment of propensity score matching model adjusted by age, insurance status, year of diagnosis, race, T stage, and PSA level. AS/WW: active surveillance or watchful waiting; FLA: focal laser ablation; CSS: cancer-specific survival; OS: overall survival; PSM: propensity score matching; IPTW: inverse probability of treatment weighing; HR: hazard ratio; CI: confidence interval; PSA: prostate-specific antigen
DISCUSSION
Based on our study cohort of 18 841 patients with low-risk PCa from 2010 to 2016 from the SEER database, the main finding of our study was that the OS of patients receiving AS/WW treatment was better than that of patients receiving FLA treatment. In a median follow-up of 36.0 months, we found that both OS and CSS showed similar results after model adjustments, PSM, and sensitivity analysis.
Using available data, we further examined data of the AS/WW group, which included patients receiving either AS or WW treatment. Although some guidelines and researchers have collectively grouped AS and WW,12,28,29,30 and they are treated as a single variable in SEER data, there are important differences between the two approaches. AS is a monitoring strategy for patients with low-risk PCa, allowing patients to delay active treatment without cancer progression. Its purpose is to achieve treatment of progressive diseases without losing the therapeutic window.31 In contrast, WW is a conservative treatment of patients who are considered unsuitable for treatment from the beginning. It requires observation of patients and palliative treatment according to symptoms to maintain quality of life.28 Although we applied many methods to eliminate bias between AS/WW and FLA in our groups, it is still important to consider potential intragroup differences within AS/WW.
Similarly, in our consideration of AS/WW, there are a number of considerations regarding FLA. It is a new protective therapy for PCa, in which thermal ablation using a laser fiber can lead to cell death by raising the temperature above 60°C. The intent behind FLA is to reduce complications and improve the quality of life of patients, albeit with no effect on tumor control.21,25 To date, a small number of studies with a maximum follow-up of 1 year have reported the clinical application of FLA, but they lack long-term evidence.23,25 Nevertheless, it was determined that FLA provided benefits for patients with low-risk PCa and furthermore concluded that FLA was a feasible and safe minimally invasive treatment for patients with low-risk PCa. However, van Luijtelaar et al.32 postulated that FLA should not be applied to candidates for AS and that patients receiving FLA should be closely followed. Therefore, the FLA and AS groups were comparable for clinical use, and the findings match those from our study. OS did differ between patients with low-risk PCa from the AS/WW-treated and FLA-treated groups.
Currently, precision surgery is the primary concept promoting PCa surgery with the aim of optimizing oncological outcomes and minimizing the impact on patient quality of life. Except for traditional focal therapies (i.e., FLA or cryotherapy), some new technologies have been developed. Recently, Checcucci et al.33 first introduced a new focal one high-intensity focused ultrasound platform. They prospectively included 20 patients with radiorecurrence and concluded that this therapy allows continuous monitoring and tailoring of the treatment and minimizes adverse events. Besides, Enikeev et al.34 introduced focal irreversible electroporation that could destroy tumors without thermal effects. They suggested that the margin size of ablation should be increased because of the higher rate of outfield relapse in localized PCa. In conclusion, focal irreversible electroporation might be safely performed in selected patients with adequate short-term cancer control and a low morbidity rate.34 Both technologies could play an emerging role in low-risk PCa in the future, and more prospective studies are warranted.
It is not unreasonable to consider that prognostic differences exist for different treatments. A study showed that in a series of men who received AS with selective deferred therapy, many patients who eventually received RP were found to have advanced disease.35 It was also reported that short-term oncology results of FLA are promising, in which 50% of patients have no evidence of a tumor in the postoperative biopsy and 67% of patients have no tumor in the resection area.36 Such differences may be reflected in the survival and prognosis between these two treatments. As shown in our study, although no significant differences were observed in CSS, we still found distinguishing characteristics between the AS/WW and FLA groups. Our main results showed an obvious and robust difference in OS between the two groups, a finding that suggests that the treatment approach of AS/WW is superior to that of FLA. In addition, we conducted a series of sensitivity analyses to evaluate stability and reliability and found that CSS significantly differed between our groups after PSM. Although this finding was not identical to the main result of our investigation, it is still aligned with the fact that AS/WW improved survival status significantly over FLA. Furthermore, our findings following IPTW analysis confirm this conclusion by identifying differences in OS between groups.
This investigation is a comparative study of FLA and AS/WW in PCa. Our study had a number of advantages. First, we were able to recruit a large cohort with over 18 000 patients with low-risk PCa. Second, in contrast to present studies focusing on short-term tumor control, which have focused on short-term tumor control, our cohort had a medium-term follow-up, and we also investigated long-term survival. Despite the attempt to randomize our study using statistical methods, a retrospective study cannot have the same level of evidence as a randomized controlled trial. Then, there are no individual AS and WW data in the SEER database, and the bias of the AS/WW group still remained. In addition, the baseline data of patients are not comprehensive, and therefore, there may be latent confounders. Although we used PSM to address these limitations, we cannot avoid the possibility of potential bias in the AS/WW group. Furthermore, patients with a long-life expectancy would lack long-term AS/WW data.
CONCLUSION
Compared with standard treatment, AS/WW and FLA have the advantage of fewer side effects and the benefit of avoiding overtreatment. Our study showed that treatment using AS/WW confers survival benefits to patients with low-risk PCa. Further research is required to investigate the clinical applicability of these treatment modalities to ensure that the best treatment is available for patients with low-risk PCa.
AUTHOR CONTRIBUTIONS
JKL and CCZ wrote the main manuscript text. SQ, YGB, LY and QW are responsible for the conception and design of the study. JKL, CCZ, LY, QW and SQ analyzed the data. LY, QW, BYC, QMY and XYX prepared figures. LST, DJ, XHZ and KJ prepared tables. All authors read and approved the manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors gratefully thank Dr. Chang-Zhong Chen, Chi Chen, and Xin-Lin Chen (EmpowerStats X&Y Solutions, Inc., Boston, MA, USA) for providing statistical methodology consultation.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Huland H, Graefen M. Changing trends in surgical management of prostate cancer: the end of overtreatment? Eur Urol. 2015;68:175–8. doi: 10.1016/j.eururo.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher SA, von Landenberg N, Cole AP, Gild P, Choueiri TK, et al. Contemporary national trends in prostate cancer risk profile at diagnosis. Prostate Cancer Prostatic Dis. 2020;23:81–7. doi: 10.1038/s41391-019-0157-y. [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. doi: 10.6004/jnccn.2019.0023. [DOI] [PubMed] [Google Scholar]
- 6.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90. doi: 10.1016/j.juro.2017.11.095. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou R, Croswell JM, Dana T, Bougatsos C, Blazina I, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:762–71. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 10.Briganti A, Fossati N, Catto JW, Cornford P, Montorsi F, et al. Active surveillance for low-risk prostate cancer: the European association of urology position in 2018. Eur Urol. 2018;74:357–68. doi: 10.1016/j.eururo.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, et al. Uptake of active surveillance for very-low-risk prostate cancer in Sweden. JAMA Oncol. 2017;3:1393–8. doi: 10.1001/jamaoncol.2016.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernooij RW, Lancee M, Cleves A, Dahm P, Bangma CH, et al. Radical prostatectomy versus deferred treatment for localised prostate cancer. Cochrane Database Syst Rev. 2020;6:Cd006590. doi: 10.1002/14651858.CD006590.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adolfsson J. Watchful waiting and active surveillance: the current position. BJU Int. 2008;102:10–4. doi: 10.1111/j.1464-410X.2008.07585.x. [DOI] [PubMed] [Google Scholar]
- 14.Calzada O, Switchenko JM, Maly JJ, Blum KA, Grover N, et al. Deferred treatment is a safe and viable option for selected patients with mantle cell lymphoma. Leuk Lymphoma. 2018;59:2862–70. doi: 10.1080/10428194.2018.1455973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herden J, Weissbach L. Utilization of active surveillance and watchful waiting for localized prostate cancer in the daily practice. World J Urol. 2018;36:383–91. doi: 10.1007/s00345-018-2175-0. [DOI] [PubMed] [Google Scholar]
- 16.Valerio M, Ahmed HU, Emberton M, Lawrentschuk N, Lazzeri M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014;66:732–51. doi: 10.1016/j.eururo.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: modalities, findings and future considerations. Nat Rev Urol. 2010;7:562–71. doi: 10.1038/nrurol.2010.142. [DOI] [PubMed] [Google Scholar]
- 18.Stafford RJ, Shetty A, Elliott AM, Klumpp SA, McNichols RJ, et al. Magnetic resonance guided, focal laser induced interstitial thermal therapy in a canine prostate model. J Urol. 2010;184:1514–20. doi: 10.1016/j.juro.2010.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindner U, Lawrentschuk N, Weersink RA, Davidson SR, Raz O, et al. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol. 2010;57:1111–4. doi: 10.1016/j.eururo.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Eggener SE, Yousuf A, Watson S, Wang S, Oto A. Phase II evaluation of magnetic resonance imaging guided focal laser ablation of prostate cancer. J Urol. 2016;196:1670–5. doi: 10.1016/j.juro.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 21.Lepor H, Llukani E, Sperling D, Fütterer JJ. Complications, recovery, and early functional outcomes and oncologic control following in-bore focal laser ablation of prostate cancer. Eur Urol. 2015;68:924–6. doi: 10.1016/j.eururo.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Walser E, Nance A, Ynalvez L, Yong S, Aoughsten JS, et al. Focal laser ablation of prostate cancer: results in 120 patients with low- to intermediate-risk disease. J Vasc Interv Radiol. 2019;30:401–9.e2. doi: 10.1016/j.jvir.2018.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindner U, Weersink RA, Haider MA, Gertner MR, Davidson SR, et al. Image guided photothermal focal therapy for localized prostate cancer: phase I trial. J Urol. 2009;182:1371–7. doi: 10.1016/j.juro.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Perera M, Krishnananthan N, Lindner U, Lawrentschuk N. An update on focal therapy for prostate cancer. Nat Rev Urol. 2016;13:641–53. doi: 10.1038/nrurol.2016.177. [DOI] [PubMed] [Google Scholar]
- 25.Oto A, Sethi I, Karczmar G, McNichols R, Ivancevic MK, et al. MR imaging-guided focal laser ablation for prostate cancer: phase I trial. Radiology. 2013;267:932–40. doi: 10.1148/radiol.13121652. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Prostate with Watchful Waiting Database (2010-2016) [Last accessed on 2021 Dec 10]. Available from: https://seer.cancer.gov/seerstat/databases/prostate-ww/index.html .
- 27.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 28.Mottet N, van den Bergh RC, Briers E, Van den Broeck T, Cumberbatch MG, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79:243–62. doi: 10.1016/j.eururo.2020.09.042. [DOI] [PubMed] [Google Scholar]
- 29.Kang J, Shin DW, Han K, Park SH, Lee WG, et al. Risk of dementia in prostate cancer survivors: a nationwide cohort study in Korea. Curr Probl Cancer. 2020;44:100578. doi: 10.1016/j.currproblcancer.2020.100578. [DOI] [PubMed] [Google Scholar]
- 30.Breidenbach C, Roth R, Ansmann L, Wesselmann S, Dieng S, et al. Use of psycho-oncological services by prostate cancer patients: a multilevel analysis. Cancer Med. 2020;9:3680–90. doi: 10.1002/cam4.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welty CJ, Cooperberg MR, Carroll PR. Meaningful end points and outcomes in men on active surveillance for early-stage prostate cancer. Curr Opin Urol. 2014;24:288–92. doi: 10.1097/MOU.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Luijtelaar A, Greenwood BM, Ahmed HU, Barqawi AB, Barret E, et al. Focal laser ablation as clinical treatment of prostate cancer: report from a Delphi consensus project. World J Urol. 2019;37:2147–53. doi: 10.1007/s00345-019-02636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Checcucci E, De Luca S, Piramide F, Garrou D, Mosca A, et al. The realtime intraoperative guidance of the new HIFU Focal-One®platform allows to minimize the perioperative adverse events in salvage setting. J Ultrasound. 2021 doi: 10.1007/s40477-021-00594-8. Doi: 10.1007/s40477-021-00594-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enikeev D, Taratkin M, Morozov A, Shpikina A, Singla N, et al. Focal irreversible electroporation for localized prostate cancer management: prospective assessment of efficacy and safety. Minerva Urol Nefrol. 2020;72:644–5. doi: 10.23736/S0393-2249.20.03840-0. [DOI] [PubMed] [Google Scholar]
- 35.Klotz L. Active surveillance with selective delayed intervention for favorable risk prostate cancer. Urol Oncol. 2006;24:46–50. doi: 10.1016/j.urolonc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Lindner U, Lawrentschuk N, Trachtenberg J. Focal laser ablation for localized prostate cancer. J Endourol. 2010;24:791–7. doi: 10.1089/end.2009.0440. [DOI] [PubMed] [Google Scholar]


