Abstract
During the coronavirus disease (COVID-19) epidemic, there have been concerns about the impact of vaccines on people's fertility, including the fertility of those who are currently preparing for pregnancy and those who might become pregnant in future. However, there is still a lack of research on the effect of the COVID-19 vaccine on male fertility, and it is not surprising that couples and donors have concerns regarding vaccination. In this study, a retrospective cohort study was conducted to examine semen quality before and after receipt of the inactivated COVID-19 vaccine. There were no statistically significant changes in semen parameters (volume, sperm concentration, progressive motility, and total progressive motile count) after two doses of vaccine (all P > 0.05). In summary, our study updates the most recent studies on the effects of the COVID-19 vaccine on male fertility, and the information from this study could be used to guide fertility recommendations for assisted reproductive technology (ART) patients and donors.
Keywords: assisted reproductive technology, coronavirus disease vaccine, male fertility, semen parameters, sperm donation
INTRODUCTION
Coronavirus disease (COVID-19) is highly infectious and continues to spread worldwide, posing a serious threat to global public health. Until the end of March 2022, there have been over 472 000 000 confirmed global COVID-19 infection cases and over 6 093 000 global deaths since 2019.1 Effective prevention and control measures are urgently needed to respond to the virus. Vaccination is an important method to prevent the spread of infectious diseases and to effectively protect the body from infection. As the epidemic promoted the development of vaccines,2 by the end of 2020, a number of vaccine products successively finished third clinical trials and were authorized for emergency use, including the inactivated vaccine in China and mRNA vaccines produced by Pfizer and Moderna.3,4,5 Existing immunization programs which based on first edition guidelines of vaccination for COVID-19 in China target people aged 18 years and above, including people preparing for pregnancy or receiving assisted reproductive technology (ART) treatment.6
While everyone is hoping to be protected by the vaccine, some people are troubled by it, and there have been concerns about the impact of the vaccine: the quality of a man's sperm is a common concern among many couples who are clinically treated with ART, as well as among volunteers who donate to sperm banks, and this concern has led many men to delay their plans to have children or refrain from visiting sperm banks to donate.7,8 Specifically, people trying to become pregnant are concerned about the side effects of vaccines as well as not being protected by vaccines during the COVID-19 epidemic. Studies have shown that COVID-19 infection or its side effects such as fever significantly decreased semen parameters such as semen concentration, total sperm count, and total motile count.9,10,11 There is another study showing that spermatogenic function of the testicle was impaired by COVID-19 infection.12 Given these studies, while there is still a lack of research on the effect of the COVID-19 vaccine on male fertility, it is not surprising that couples and donors are found to have concerns about their decisions.
In this context, the importance of determining the impact of the COVID-19 vaccine on males' semen quality is not just for medical research purposes but also for decision-making by couples and donors. Therefore, it is worthwhile to study the effect of inactivated COVID-19 vaccines on male sperm quality. Although a recent mRNA COVID-19 vaccine study highlighted that there were no significant decreases in any semen parameters before and after two doses of vaccine,13 little is known about the impact of inactivated COVID-19 vaccine on semen quality in Chinese reproductive-age males. Our study focuses on evaluating semen quality, including volume, sperm concentration, progressive motility (PR), and total progressive motile count (TPMC), before and after receiving inactivated COVID-19 vaccine. This study could be used to improve decision-making for ART patients or donors and to guide future fertility recommendations.
PARTICIPANTS AND METHODS
Study design and participants
A retrospective cohort study was conducted at the Human Sperm Bank of Fudan University (Shanghai, China) between December 2020 and August 2021. This research was approved by the Ethics Committee in Human Sperm Bank of Fudan University (approval No. EC2018050702). All participants provided and signed informed consent prior to the study. All participants met the following criteria: (1) had decided to voluntarily receive a COVID-19 vaccine (had chosen to receive 2 doses of inactivated vaccine) prior to participating in the study; (2) had never been infected with COVID-19; (3) were over 21 years of age; (4) met the initial screening criteria for China's donation requirements;14 (5) followed the donation requirements during the donation period: each donation followed the recommended 3–5 days of sexual abstinence, had no physical discomfort, and tried to live their usual lifestyle; and (6) their data were valid for the study, specifically that the donation time met the screening conditions.
A total of 894 donors were initially screened in September 2021 in Human Sperm Bank of Fudan University databases who finished vaccination. Of these 894 donors, 60 donors were eligible for inclusion, of whom 43 (71.7%) ultimately participated. The inclusion procedure is summarized in the inclusion flowchart (Figure 1).
Figure 1.
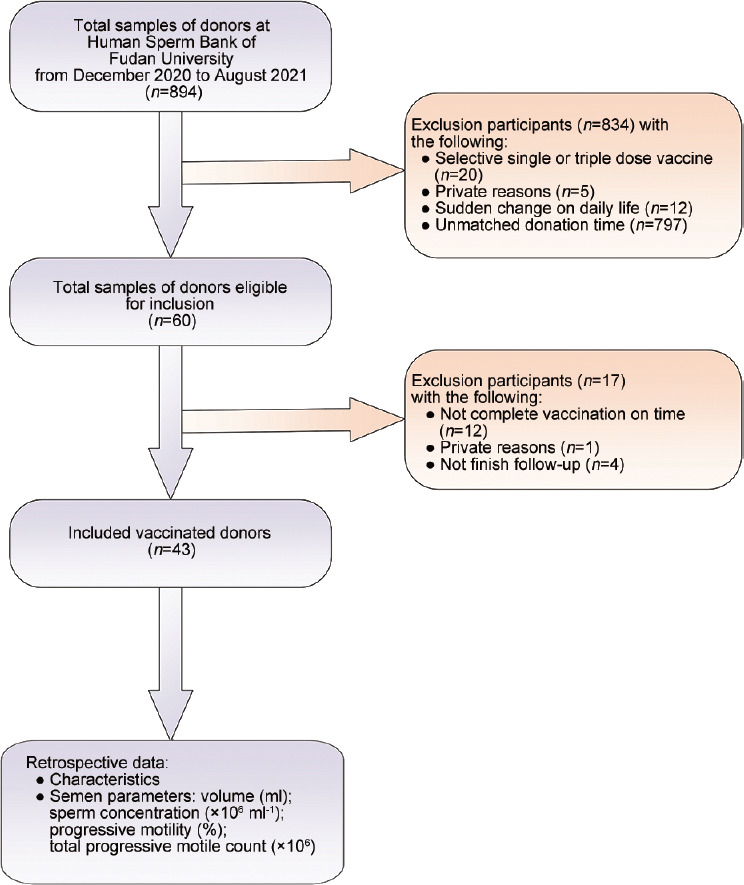
Study inclusion flowchart.
Study procedures
All donors had the intention of COVID-19 vaccination before participating in the study and were voluntarily vaccinated. The inactivated COVID-19 vaccine products available to donors had successfully finished third clinical trials and were authorized for emergency use in China. The immunization procedure required two doses of vaccine with an interval of ≥3 weeks. Donors who opted for other immunization procedures (single or triple dose) or other types of vaccines (e.g., mRNA vaccine and adenovirus vaccine) were excluded from the study. Participants agreed to the sperm bank collecting semen parameters from the database. Qualified participants (data) were defined as meeting the following requirements: (1) donation (T0) within one month before the first dose of the COVID-19 vaccine; (2) donation (T1) within 21 days of receiving the first dose of the COVID-19 vaccine; and (3) donation (T2) within 60 days of receiving the second dose of the COVID-19 vaccine. Donation data, including vaccination date, participant characteristics (age, height, and weight), semen parameters (volume, sperm concentration, PR, and TPMC), and andrology medical history, were collected from the Human Sperm Bank of Fudan University database.
Statistical analyses
SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) software was used for statistical analysis. The measurement data were expressed as the mean ± standard deviation (s.d.). A paired t test was used to compare and analyze the data before and after vaccination. When the P < 0.05, the difference was considered statistically significant.
RESULTS
Basic information
A total of 43 donors were involved in this study. The age (mean ± s.d.) was 28.6 ± 5.9 years, the height (mean ± s.d.) was 1.8±0.1 m, the weight (mean ± s.d.) was 69.3 ± 7.3 kg, and the body mass index (BMI; mean ± s.d.) was 22.7 ± 2.1 kg m–2. Andrological physical examination showed that the testicular volume (mean ± s.d.) was estimated as 14.3 ± 1.5 ml and 14.5 ± 1.4 ml for the left testicle and right testicle, respectively, using the testicular palpation. The time range (mean ± s.d.) of the three semen collections (T0 to T2) was 61.8 ± 36.2 days. The second semen collection (T1) was completed within 9.1 ± 8.1 days (mean ± s.d.) after the first vaccine injection, and the third semen collection (T2) was completed within 30.1 ± 23.3 days (mean ± s.d.) after the second vaccine injection. The third semen collection (T2) was completed within 59.5 ± 24.1 days (mean ± s.d.) after the first vaccine injection (Figure 2).
Figure 2.
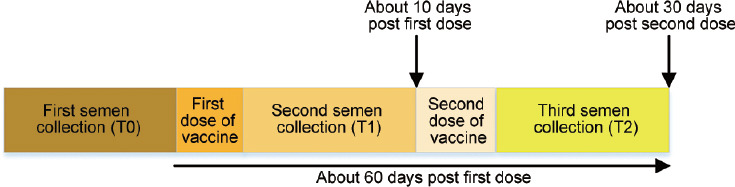
Semen collection and immunization procedure timeline.
Semen parameters
Comparing semen parameters before and after vaccination, we found that the volume (mean ± s.d.) decreased from 3.1 ± 1.3 ml to 2.8 ± 1.2 ml after the first COVID-19 vaccine injection and recovered to 3.3 ± 1.7 ml after the second injection. The sperm concentration (mean ± s.d.) increased from 55.6 × 106 ± 21.7 × 106 ml–1 to 61.9 × 106 ± 23.3 × 106 ml–1 after the first injection and decreased to 55.9 × 106 ± 18.5 × 106 ml–1 after the second injection. The PR (mean ± s.d.) decreased from 49.9% ± 10.7% to 48.7% ± 10.5% after the first injection and recovered to 49.8% ± 10.7% after the second injection. The TPMC (mean ± s.d.) decreased from 88.5 × 106 ± 56.4 × 106 to 84.2 × 106 ± 50.6 × 106 after the first injection and recovered to 87.9 × 106 ± 49.6 × 106 after the second injection. No statistically significant changes in these semen parameters (volume, sperm concentration, PR, and TPMC) were observed after two doses of vaccine (all P > 0.05; Table 1).
Table 1.
Comparison of semen parameters before and after vaccination
| Semen parameter | Before first dose (T0) | Within 21 days of receiving the first dose (T1) | Within 60 days of receiving the second dose (T2) | P |
|---|---|---|---|---|
| Semen volume (ml), mean±s.d. | 3.1±1.3 | 2.8±1.2 | 3.3±1.7 | >0.05 |
| Sperm concentration (×106 ml−1), mean±s.d. | 55.6±21.7 | 61.9±23.3 | 55.9±18.5 | >0.05 |
| PR (%), mean±s.d. | 49.9±10.7 | 48.7±10.5 | 49.8±10.7 | >0.05 |
| TPMC (×106), mean±s.d. | 88.5±56.4 | 84.2±50.6 | 87.9±49.6 | >0.05 |
PR: progressive motility; TPMC: total progressive motile count; s.d.: standard deviation
Before vaccination, the semen parameters of all the volunteers were consistent with the normal reference values of the 5th edition of the WHO guidelines.15 However, there were 3 special cases, as two volunteers' PR was lower than normal on Day 1 and Day 7 after the first dose of vaccine (30.0% and 31.0%, respectively) but recovered to normal on Day 1 and Day 17 after the second dose (60.0% and 60.0%, respectively). In another case, the PR was lower than normal 14 days after the second injection (30.0%) and returned to normal 41 days after vaccination (35.0%).
Adverse reactions
Information related to the types of adverse reactions of the donors to the inactivated vaccines was collected after receiving the first and second doses of vaccine. We found that nearly 86.1% and 93.0% of donors reported no adverse reactions after the first and second injections, respectively. The most common symptom was a sore arm for both the first and second injections (nearly 13.9% and 4.7%, respectively). Only one (2.3%) donor reported feeling tiredness after the second dose. Of these 43 donors, no one reported fever, body aches, headaches, or other symptoms (Table 2).
Table 2.
Adverse reactions experienced by the donors after receiving the first and second doses of vaccine (n=43)
| Type of adverse reaction | First dose, n (%) | Second dose, n (%) |
|---|---|---|
| Soreness (arm) | 6 (14.0) | 2 (4.7) |
| Fever | 0 (0) | 0 (0) |
| Body aches | 0 (0) | 0 (0) |
| Tiredness | 0 (0) | 1 (2.3) |
| Headaches | 0 (0) | 0 (0) |
| None | 37 (86.0) | 40 (93.0) |
| Other | 0 (0) | 0 (0) |
DISCUSSION
This study indicates that the inactivated COVID-19 vaccine does not cause infertility in men and does not affect male sperm quality. Our findings suggest that there were no significant changes in any semen parameters in healthy males before and after two injections (doses) of inactivated COVID-19 vaccine.
There have been concerns about the vaccine causing damage to male fertility. Most of the concerns stem from reports of the effect of coronavirus on the reproductive system.16,17 The coronavirus invades host target cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is highly expressed in male renal tubular cells, the interstitial cells of the testicle and the seminiferous tubule, and the testicle is highly susceptible to coronavirus infection.18 Whereas the principle of the inactivated vaccine is to use African green monkey kidney (Vero) cells for virus culture amplification, β-propiolactone inactivated virus retains antigen components to induce an immune response and adds aluminum hydroxide adjuvant to improve immunogenicity. Inactivated vaccines, such as inactivated influenza vaccines, are generally considered safe during preparation for pregnancy and during pregnancy.19
There are concerns that rare adverse reactions to the inactivated vaccine may affect fertility, especially fever, consistent with previous studies.9,10,11 It is well known that febrile episodes are associated with high risk of damage to semen parameters and DNA integrity,20 so the results of adverse reaction symptoms are particularly important to couples. There are few reports on the side effects of and adverse reactions to inactivated COVID-19 vaccines, and our study presents the adverse reactions experienced by donors after receiving first and second doses of vaccine, though with a limited study group. Notably, 86.1% and 93.0% of donors reported no adverse reactions after the first and second injections, respectively, and a small group of donors reported arm soreness, with no one reporting fever. There is room for additional study of the adverse reactions of inactivated COVID-19 vaccines to semen parameters, particularly fever.
In addition, considering that the timing of spermatogenesis is approximately 60 days to 70 days, we analyzed the timing in our study. The third semen collection (T2) was completed about 60 days after the first vaccine injection, which approximated the spermatogenesis cycle. Since there were no statistically significant changes between the first semen collection and third semen collection, the first dose had no negative effects on semen parameters. Based on the above understanding of vaccine safety and the results of our study, it can be inferred that inactivated vaccines do not affect the reproductive ability of pregnant couples.
Based on our research, there are small fluctuations in semen parameters (volume, sperm concentration, PR, and TPMC) after the first dose of inactivated COVID-19 vaccine, and the parameters return to the prevaccination level after the second dose. The Society for Male Reproduction and Urology (SMRU) and the Society for the Study of Male Reproduction (SSRM) emphasized that COVID-19 vaccines should not be withheld from men desiring fertility.21 Similarly, the American Society for Reproductive Medicine (ASRM) encouraged patients who underwent ART to be vaccinated.22 Although the limited data and studies provide some assurance that inactivated COVID-19 vaccines are safe with regard to sperm quality in men, due to the lack of long-term data, couples who are preparing for pregnancy and receiving ART in future can be vaccinated as per the general recommendations.
Recommendations from the international society on COVID-19 vaccination for people preparing for pregnancy and receiving ART were based on different vaccines. The European Society for Human Reproduction and Embryology (ESHRE)23 recommended a delay of at least 2 months before starting ART including sperm extraction, ovulation induction, and embryo transfer. The Joint Committee on Vaccination and Immunisation (JCVI) and the Centers for Disease Control and Prevention (CDC) recommended that people trying to become pregnant now, or who might become pregnant in future, do not need to delay pregnancy after receiving the mRNA vaccine.24 The Beijing Human Assisted Reproductive Technology Quality Control and Improvement Center experts advised that for couples who need to receive ART, it is recommended to start treatment after one month of vaccination to stable immune response.5
Unlike the people preparing for pregnancy and receiving ART, there appears to be concerns from sperm banks regarding donors postponing their donation plan and the safety associated with further use of the samples. A major challenge is donors delaying donation plans because they assume that the COVID-19 vaccine affects semen quality. According to the technical guidelines for vaccination of COVID-19 (first edition) of the three inactivated coronavirus vaccine products approved by the National Health Commission of the People's Republic of China, two doses should be administered.6 The interval between the two doses is recommended to be ≥3 weeks, and the second dose should be completed as soon as possible within 8 weeks.6 Therefore, based on the above information, sperm banks should do more to educate donors on the optimal sperm donation schedules. In addition, to avoid further studies on the negative effects of vaccines on semen quality, sperm banks can independently label samples donated after the donor has received COVID-19 vaccines during the COVID-19 pandemic for further processing.25
This study significantly updates the most recent studies on the effects of the COVID-19 vaccine on male fertility. Since vaccines remain the most effective form of protection and research on male fertility has rarely been conducted, the information from this study could be used to guide future recommendations for ART patients and donors. Our study has several limitations. The sample size was limited, and all the participants' data were collected in a single center, which limited the diversity. The follow-up period needs to be expanded to long-term research to further understand the effect of inactivated COVID-19 vaccines on male fertility.
CONCLUSIONS
According to our study, there were no significant changes in any semen parameters in healthy males before and after two injections (doses) of inactivated COVID-19 vaccine. The concerns regarding the impact of the inactivated vaccine on the semen quality can be addressed by improving professional recommendations.
AUTHOR CONTRIBUTIONS
HZ and XW contributed to conception and design of the study. BLF and FJ contributed to management and coordination responsibility for the research. FZ, YZ, and MRD contributed to drifting and reviewing the manuscript and acquisition of the financial support for the funding. ZWT, GQL, and JR contributed to the data collection and processing. CS, HTM, and YDL contributed to the laboratory analysis of semen samples. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This project was supported by the Shanghai Municipal Science and Technology Major Project (grant No. 2017SHZDZX01).
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. Global confirmed of COVID-19 from Coronavirus Resource Center. [Last accessed on 2022 Mar 22]. Available from: https://coronavirus.jhu.edu .
- 2.Graham BS. Rapid COVID-19 vaccine development. Science. 2020;368:945–6. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. DRAFT Landscape of COVID-19 Candidate Vaccines. [Last accessed on 2021 Dec 27]. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines .
- 4.Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–4. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Group for Beijing Human Assisted Reproductive Technology Center For Quality Control and Improvement. COVID-19 vaccination strategy for planning pregnancy and assisted reproductive technology treatment: expert recommendations. Chin J Reprod Contracep. 2021;41:296–9. [Google Scholar]
- 6.Bureau of Disease Control and Prevention; National Health Commission of the People's Republic of China. [Guidelines of vaccination for COVID-19 vaccines in China (first edition)] Chin J Clin Infect Dis. 2021;14:89–90. Article in Chinese. [Google Scholar]
- 7.Lo SP, Hsieh TC, Pastuszak AW, Hotaling JM, Patel DP. Effects of SARS CoV-2, COVID-19, and its vaccines on male sexual health and reproduction: where do we stand? Int J Impot Res. 2021;27:1–7. doi: 10.1038/s41443-021-00483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson AK, McQueen DB, Swanson AC, Confino R, Feinberg EC, et al. Psychological distress and postponed fertility care during the COVID-19 pandemic. J Assist Reprod Genet. 2021;38:333–41. doi: 10.1007/s10815-020-02023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, et al. Assessment of SARS-CoV-2 in human semen – a cohort study. Fertil Steril. 2020;114:233–8. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo TH, Sang MY, Bai S, Ma H, Wan YY, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23:479–83. doi: 10.4103/aja.aja_31_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53:e13912. doi: 10.1111/and.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YF, Wang J, Ren JL, Zhao YB, Chen J, et al. Effect of COVID-19 on male reproductive system – a systematic review. Front Endocrinol (Lausanne) 2021;12:677701. doi: 10.3389/fendo.2021.677701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez DC, Nassau DE, Khodamoradi K, Ibrahim E, Blachman-Braunet R, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA. 2021;326:273–4. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ping P, Zhu WB, Zhang XZ, Li YS, Wang QX, et al. Sperm donation and its application in China: a 7-year multicenter retrospective study. Asian J Androl. 2011;13:644–8. doi: 10.1038/aja.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. pp. 26–44. [Google Scholar]
- 16.Yan RH, Zhang YY, Li YN, Xia L, Guo YY, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–8. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groner MF, de Carvalho RC, Camillo J, Abrão Ferreira PR, Fraietta R. Effects of COVID-19 on male reproductive system. Int Braz J Urol. 2021;47:185–90. doi: 10.1590/S1677-5538.IBJU.2021.99.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang ZP, Xu XJ. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maertens K, Paulien Orije MR, Van Damme P, Leuridan E. Vaccination during pregnancy: current and possible future recommendations. Eur J Pediatr. 2020;179:235–42. doi: 10.1007/s00431-019-03563-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88:970.e1–7. doi: 10.1016/j.fertnstert.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Society for the Study of Male Reproduction. Joint Statement Regarding COVID-19 Vaccine in Men Desiring Fertility from the Society for Male Reproduction and Urology (SMRU) and the Society for the Study of Male Reproduction (SSMR) [Last accessed on 2021 Dec 06]. Available from: https://ssmr.org/news/statement-from-ssmr-about-covid-19-vaccine.aspx .
- 22.American Society for Reproductive Medicine. New Study Reveals COVID Vaccine Does Not Cause Female Sterility. [Last accessed on 2021 Jan 24]. Available from: https://www.asrm.org/news-andpublications/news-and-research/press-releases-and-bulletins/new-study-revealscovid-vaccine-does-not-cause-female-sterility .
- 23.Veiga A, Gianaroli L, Ory S, Horton M, Feinberg E, et al. Assisted reproduction and COVID-19: a joint statement of ASRM, ESHRE and IFFS. Fertil Steril. 2020;114:484–5. doi: 10.1016/j.fertnstert.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The Joint Committee on Vaccination and Immunisation. JCVI Statement Regarding a COVID-19 Booster Vaccine Programme for Winter 2021 to 2022. [Last accessed on 2021 Sep 14]. Available from: https://www.gov.uk/government/publications/jcvi-statement-september-2021-covid-19-boostervaccine-programme-for-winter-2021-to-2022/jcvi-statement-regarding-a-covid-19-booster-vaccine-programme-for-winter-2021-to-2022 .
- 25.Huang C, Zhou SF, Gao LD, Li SK, Cheng Y, et al. Risks associated with cryopreserved semen in a human sperm bank during and after the COVID-19 pandemic. Reprod Biomed Online. 2021;42:589–94. doi: 10.1016/j.rbmo.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


