Abstract
Reductions of the auditory N100 are present in schizophrenia, even at the first episode (FESz). Because most studies examine auditory N100 on active target detection oddball tasks, it is unclear if the abnormality in FESz results from sensory deficits or impaired enhancement of N100 by selective attention or both. N100 was recorded from 21 FESz and 22 matched healthy controls (HC) on a single-tone task and a two-tone oddball task. Overall, N100 was smaller in FESz (p=.036). Attention enhanced N100 amplitude (p<.001), but this differed between groups, with FESz impaired in N100 modulation (group x attention, p=.012). The oddball task showed greater N100 enhancement than the single-tone task (p<.001) in both groups. Group differences in N100 enhancement in the oddball task were large (Cohen’s d=0.85). Exploratory correlations showed that better N100 enhancement on the oddball task in FESz was associated with better MATRICS Overall Composite scores (cognitive tasks highly sensitive to psychosis), lower PANNS Negative factor and SANS scores, and better interpersonal (social) and role functioning in the last year. N100 during ignore conditions showed no significant difference between groups, albeit smaller in FESz, with small to medium effect sizes. Although sensory deficits in N100 are likely present, they are compounded by a failure to enhance N100 with attention. The failure of N100 enhancement by attentional gain control in FESz suggests functional dysconnection between cognitive control areas and sensory cortex. N100 amplitude on active attention tasks may be a useful outcome biomarker for targeted enhancement of the cognitive control system.
Keywords: N100, attention, gain, enhancement, schizophrenia, first episode psychosis
Graphical Abstract
Introduction
Schizophrenia (Sz) results in severe social, behavioral, and cognitive impairment. Earlier clinical intervention may improve outcome (Penttilä et al., 2014; Yada, Yoshimura & Kishi, 2015: Rosenheck et al., 2016). However, the field still lacks a mechanistic understanding of the biological systems involved in the emergence of psychosis. Early interventions would benefit from precision medicine approaches that target specific neuro-biological deficits present at, or prior to, the emergence of psychosis (Kane et al., 2016; Rosenheck et al., 2016; Correll et al., 2018). For example, cognitive strategies coupled with targeted non-invasive brain stimulation (NIBS) might help ameliorate some of the early deficits associated with psychosis and thereby improve prognosis. However, candidate systems for targeted intervention need to be identified.
Individuals early in the course of emerging psychosis experience problems in selective attention, the ability to focus on one item among others in the context of distracting stimuli. Selective attention has long been understood as a fundamental cognitive deficit in psychosis across the course of the disorder (Bleuler ,1911/1950; Everett, Laplante, & Thomas, 1989). Its presence in the prodromal pre-psychosis phase of Sz makes it a promising candidate neural process for targeted intervention (Cornblatt & Erlenmeyer-Kimling, 1985; Mohamed et al., 1999). To that end, the broad concept of selective attention needs to be reduced to an objective biological process and the associated neural system identified and isolated.
Auditory processing deficits are also reflected in many of the symptoms of long-term Sz, such as verbal hallucinations and impaired auditory perception (e.g., Turetsky et al., 2009), including tone-matching and emotion identification (Leitman et al., 2010, Kantrowitz et al., 2013), and sarcasm perception (Kantrowitz et al., 2014). A study of the interaction of executive control in service of selective attention and sensory processing with first-episode psychosis may shed light on basic mechanisms of pathology in Sz, thereby isolating a distributed neural system for targeted intervention. Examination of the modulation of sensory auditory evoked potentials (AEPs) by attention, specifically amplitude enhancement of the N100 AEP, can operationalize this interplay to an objective quantifiable measure.
The N100 is a negative-going scalp-recorded brainwave that occurs approximately 100 ms after the onset of an auditory stimulus. On passive tasks, N100 is reduced in long-term Sz (Connolly et al., 1985; Iwanami, Suga, & Kanamori, 1994; O’Donnell et al., 1994; Shelley, Silipo, & Javitt, 1999), indicating impaired bottom-up cortical auditory sensory-perceptual deficits in the disorder. Further, Leavitt et al. (2007) showed that the N1 deficit on passive tasks in long-term illness was preceded by deficits in AEPs as early as the medial geniculate nucleus auditory relay of the thalamus, suggesting quite extensive early sensory deficits.
Although N100 occurs obligatorily in the lack of attentional focus, its amplitude is enhanced when a subject attends a task-relevant stimulus, such as during the oddball task where participants actively detect rare deviant stimuli among standard stimuli (Hillyard et al., 1973). Individuals with long-term Sz lack the attentional modulation (enhancement) of the N100 response (O’Donnell et al., 1994; Ogura et al., 1991; Foxe et al., 2011; Force et al., 2008; see Rosburg, Boutros, & Ford, 2008 for review of passive and active N100 in Sz), indicating a deficit in the long-range executive modulation of sensory processing. Importantly, because a lack of attentional enhancement is additive with any sensory deficit, observed N100 deficits in attend tasks tend to be larger than in passive tasks.
There has been interest in N100 as a possible endophenotype for Sz. The relatively small number of studies suggest N100 may show trait effects. Ahveninen et al. (2006) showed reduced passive N100 in Sz probands and in their discordant twins. The degree of N100 deficit was greater in monozygotic twins than in dizygotic twins. Foxe et al. (2011) showed reduced N100 on an active target detection oddball (thus including N100 enhancement in the measure) in unaffected relatives of Sz probands. Force et al. (2008) showed reduction of N100 in unaffected relatives of Sz probands, but normal N100 enhancement, suggesting a relatively pure sensory deficit in relatives, but normal top-down executive modulation of sensory processing.
The picture in first-episode schizophrenia (FESz) is less clear. Salisbury, Collins, and McCarley (2010) and Foxe et al. (2011) showed reduced N100 recorded from standard stimuli in FESz on an active oddball attention task but were unable to determine if the deficit was primarily sensory or executive in this early stage of disease. Thus, N100 deficits in FESz could be related to sensory processing deficits, attentional modulation deficits, or (more likely) both. The current study is the first experiment to determine to what extent early sensory and later cognitive deficits contribute to N100 reduction in FESz. N100 reduction in FESz during passive listening would indicate sensory abnormalities, and support the notion of sensory N100 deficits as an endophenotype. A reduction only in N100 enhancement with attention would indicate selective cognitive-level impairment, and suggest that sensory deficits in N100 observed in long-term Sz and unaffected relatives might develop with late maturational processes during the 20’s and 30’s. Furthermore, the number of to-be attended stimuli was manipulated across two tasks to see if stimulus complexity differentially affected N100 enhancement overall or affected the ability of FESz to enhance N100. Previous literature shows that Sz individuals show greater than normal deficits in task performance with increasing task demand, and our own work (Salisbury et al., 1994) showed that target detection in an oddball task was disproportionately affected in Sz as stimulus discriminability decreased. Thus, we examined if presentation of two tones instead of one tone led to greater N100 enhancement in healthy individuals (more resources deployed) and/or less N100 enhancement in FESz (task complexity causing system failure). Finally, and more practically, if N100 enhancement deficits were present in FESz, this brainwave abnormality could serve as an objective measure of system-level selective attention circuit pathophysiology and would be a viable outcome measure for targeted interventions to improve top-down executive function in the disorder.
Materials and Methods
Participants
Twenty-one FESz recruited from consecutive admissions to Western Psychiatric Hospital (WPH) inpatient and outpatient services were compared with 22 matched healthy controls (HC). FESz were recruited first. HC were recruited from the general population via advertisements and selected to approximately match FESz demographics on a one-to-one basis. Subsequently, samples were matched at group-level on age, sex, premorbid estimates of intelligence based on the Wechsler Abbreviated Scale of Intelligence (WASI) IQ, and parental socioeconomic status (Table 1). The 4-factor Hollingshead Scale was used to measure socioeconomic status (SES) in participants and in their parents. Diagnosis utilized the Structured Clinical Interview for DSM-IV (SCID-P). HC also underwent structured interviews for Axis II disorders (SCID II or SCID5PD). Within the FESz group, 13 were diagnosed with schizophrenia (paranoid =10, undifferentiated =3), 2 with schizoaffective disorder (depressed type =1, bipolar type =1), 4 with psychotic disorder not otherwise specified (NOS), and 2 with schizophreniform disorder. Symptom ratings utilized the Positive and Negative Symptom Scale (PANSS), Scale for Assessment of Positive Symptoms (SAPS), and Scale for Assessment of Negative Symptoms (SANS). FESz had less than two months of lifetime antipsychotic medication exposure (medicated =19, unmedicated =2) and their average length of illness since their first psychiatric service contact was 4.24 months (7.34 SD, one individual was excluded due to unconfirmable date). As mentioned, premorbid intellect was estimated from the WASI. The MATRICS Cognitive Consensus Battery estimated current intellectual functioning. The Global Assessment Scale, the brief UCSD Performance-based Skills Assessment (UPSA-B), Global Functioning: Social and Role scales, and the Social Functioning Scales were utilized to assess social functioning.
Table 1.
Subject Demographics and Measure of Cognitive Function.
| FESz | HC | Statistics | |
|---|---|---|---|
| Number | 21 | 22 | |
| Gender | 16M/5F | 15M/7F | X2 =0.74, p =0.40 |
| Age | 21.81 ± 4.39 | 23.64 ± 5.97 | t(41) =−1.14, p =0.26 |
| SES | 26.00 ± 11.01 | 35.55 ± 15.17 | t(41) =−2.35, p =0.02 |
| PSES | 42.97 ± 14.18 | 49.82 ± 13.05 | t(39) =−1.61, p =0.12 |
| WASI IQ | 105.38 ± 14.20 | 109.64 ± 8.79 | t(41) =−1.18, p =0.25 |
| MATRICS | 36.19 ± 15.55 | 49.73 ± 6.80 | t(41) =−3.54, p =0.001 |
Note: Bold indicates significant group differences. SES =Socioeconomic status. PSES = parental SES. WASI IQ = Wechsler Abbreviated Scales of Intelligence Intelligence Quotient. MATRICS = MATRICS Cognitive Consensus Battery composite score.
All subjects had normal hearing as assessed by audiometry (within 30dB nHL and less than 15dB difference between ears from 500–4000 Hz), at least nine years of schooling, and an estimated IQ over 75. None of the subjects had a history of concussion or head injury with sequelae, history of alcohol or drug dependence, detox in the last five years, or neurological comorbidity. All participants provided written informed consent and were paid for participation. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human subjects/patients were approved by University of Pittsburgh IRB.
Procedure
EEG was collected while participants partook in two different auditory tasks containing passive and active attention conditions. Binaural auditory stimuli were generated with Tone Generator (NCH Software) and presented at 80 dB using Presentation (Neurobehavioral Systems, Inc.) over Etymotic 3A insert earphones. Intensity was confirmed with a sound meter. During both attend and ignore conditions, participants were presented a silent nature video.
Stimuli
Single-tone Task.
Stimuli consisted of repeated tones (1kHz, 50 ms duration, 5 ms rise/fall) presented with a stimulus onset asynchrony of 1050–1550 ms. A total of 420 tones were presented. A 500 ms duration 40Hz click train was inserted 250 ms, 300 ms, or 350 ms after each tone in the inter-tone interval, but the results of this gamma oscillation investigation will not be further discussed in this paper. In one block, participants were asked to ignore the tones and focus on a silent nature video. In another, participants were asked to pay attention to the tones and press a button to every seventh tone. Due to a programming error, button-press responses were not recorded. Blocks were counterbalanced. Half of the participants were asked to focus on the tones first, and the other half were asked to ignore the tones first.
Oddball Task.
Stimuli consisted of a standard tone (1kHz, 50 ms duration, 5 ms rise/fall) and a deviant tone (1200 Hz, 50 ms duration, 5 ms rise/fall) presented with a stimulus onset asynchrony of 1050–1550 ms. A total of 420 tones were presented, including 357 standard tones (85%) and 63 deviant tones (15%). As for the single-tone task, a 500 ms duration 40Hz click train was inserted in the inter-tone interval, but the results of the gamma-band oscillation will not be further discussed. In one block participants were asked to ignore the tones and focus on a silent nature video. In another, participants were asked to pay attention to the tones and press a button to every deviant tone. Due to a programming error, button-press responses were not recorded. Blocks were counterbalanced. Half of the participants were asked to focus on the tones first, and the other half were asked to ignore the tones first. Only responses to standard tones (not deviant oddballs) were analyzed.
EEG Recording
EEG was recorded using a low impedance 60-electrode EEG array based on the 10–10 system using an Elekta Neuromag system with a sampling rate of 1000 Hz and an online bandpass filter of 0.1 – 330 Hz. Impedance was below 15 kΩ. EEG recordings were referenced to the left mastoid. The right mastoid was used as ground. Additional bipolar leads placed above and below the left eye (VEOG), and lateral to the outer canthi of both eyes (HEOG), were used to monitor blinks and horizontal eye movements. Bipolar EKG leads were placed just below the left and right clavicles.
EEG Signal Preprocessing
Pre-processing was done off-line with the MATLAB-based EEGLAB Toolbox (Delorme & Makeig 2004). EEG was filtered at 0.5 Hz to remove DC drifts and skin potentials. Data were visually examined, bad channels removed, and any intervals with prolonged (> 1 sec) movement marked as bad. Adaptive Mixture ICA (AMICA) was performed to remove one eye-blink/vertical eye-movement, one horizontal eye-movement, and 1–2 ECG components (QRS complex and/or pulsation) in the EEG data. After AMICA, initially removed bad channels were interpolated.
EEG Analysis
Following pre-processing in EEGLAB, event-related potentials were processed using BrainVision Analyzer2 (Brain Products GMBH). EEG data were re-referenced to the averaged mastoids and low-pass filtered at 20 Hz to remove muscle and other high-frequency artifacts. EEG data were then segmented into epochs starting 100ms prior to the stimulus and ending 1000ms after. Epochs were baseline-corrected using the 100ms pre-stimulus baseline, and epochs with voltage exceeding ±50 μV were rejected. Averages were constructed for the identical tones in the single-tone task and for standard tones in the oddball task. Overall, FESz had fewer trials rejected than HC (See Table 2 for accepted trials per task and condition). The N100 response was measured as the average amplitude between 105 and 115 ms at Fz (peak ±5 ms). In addition, N100 enhancement on each task was calculated by subtracting N100 amplitude during the ignore condition from N100 amplitude during the attend condition.
Table 2.
Trials included for each task and condition.
| TASK | Group | t-test |
|---|---|---|
| Ignore AEP | FESz 359.4 (72.3) | t =0.74, p = .46 |
| HC 343.6 (67.9) | ||
| Attend AEP | FESz 361.9 (60.7) | t =2.53, p = .016 |
| HC 304.1 (86.3) | ||
| Ignore Oddball | FESz 300.4 (68.4) | t =1.66, p = .105 |
| HC 265.5 (69.9) | ||
| Attend Oddball | FESz 301.9 (52.7) | t =2.05, p = .047 |
| HC 258.5 (82.0) |
Note: Values are Mean (SD). Bold indicates significant group differences. AEP = Auditory Evoked Potential, single-tone task. Oddball = Two-tone task. Bold indicates significant group differences. For each attend waveform, FESz had more trials than HC.
Analyses
Group demographics and neuropsychological scores were compared using t-tests and chi-squared tests where appropriate. N100 analyses utilized repeated-measures ANOVA with group (FE, HC) as the between-subject factor and task (AEP or oddball) and attention condition (attend or ignore) as within-subject factors. Follow-up analyses utilized repeated-measures ANOVA or t-tests where appropriate. Exploratory (uncorrected) analyses of correlations between N100 values and N100 enhancement, and symptoms, cognition, and social functioning were performed using Spearman’s rho. Values are reported as Mean ± SD. Significance was attained at p<0.05. Partial eta squared estimates of effect size are reported for ANOVAs. Cohen’s d effect sizes were calculated for group differences in ignore N100, attend N100, and N100 enhancement separately for each task.
Results and Statistical Analyses
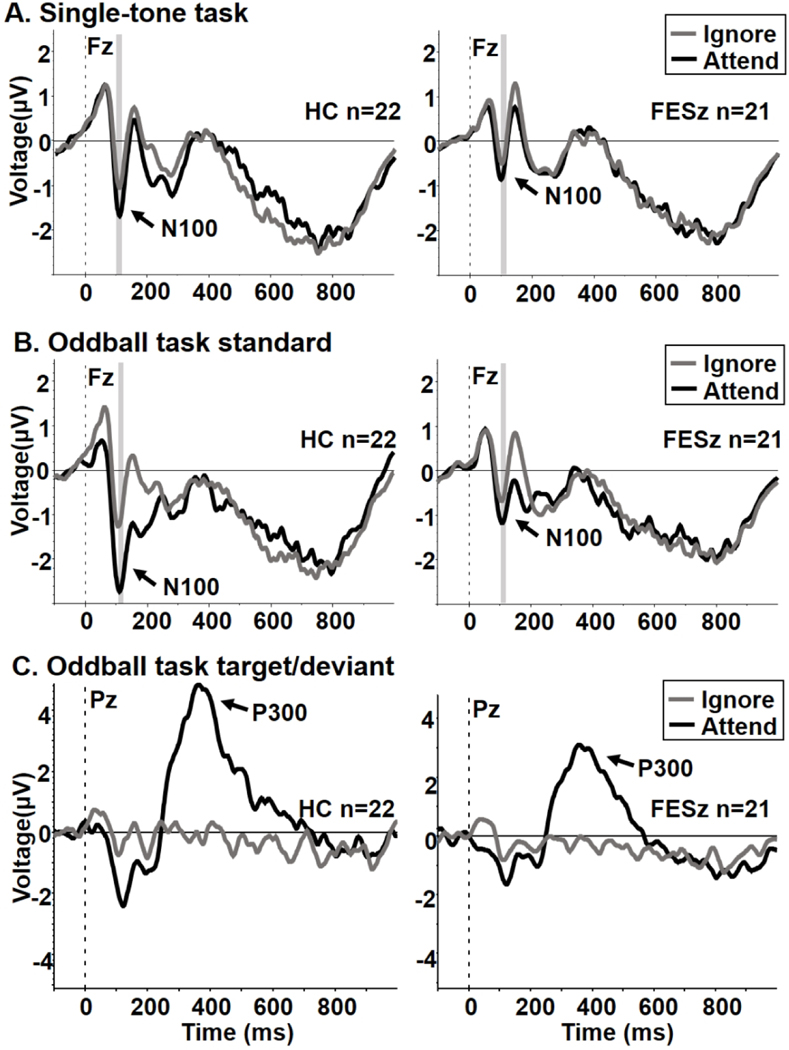
Single-tone (AEP) task waveforms for each group are presented in Figure 1, Panel A, and two-tone (oddball) task waveforms are presented in Figure 1, Panel B. Despite the lack of overt behavioral data, inspection of Figure 1, Panel C shows that oddball targets elicited a P300 in the attend but not ignore condition in both groups, prima facie evidence of the successful manipulation of attention. Measured N100 for each task and condition, along with uncorrected t-test statistics between and within groups, are graphically presented in Figure 2, and mean (SD) N100 amplitudes for each task and condition, as well as N100 enhancement on each task, are presented in Table 3.
Figure 1. N100 Averages.
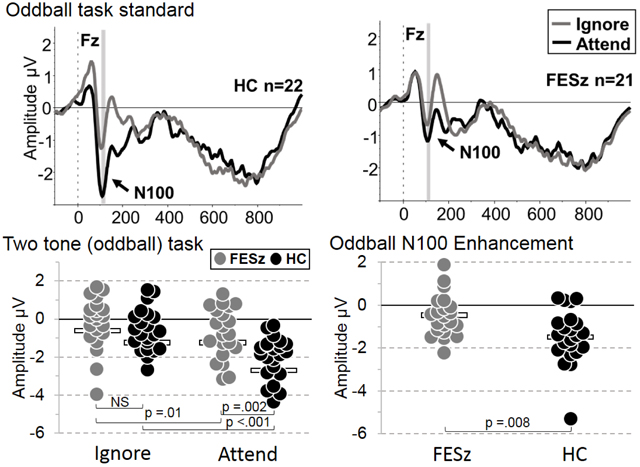
A. Attend and ignore single-tone task (AEP) waveforms for HC (left panel) and FESz (right panel). B. Attend and ignore oddball task standard tone waveforms for HC (left panel) and FESz (right panel). Note the generally larger increase in N100 with attention in both groups, albeit larger in HC. C. Attend and ignore oddball task target/deviant tone ERP waveforms for HC (left panel) and FESz (right panel). The presence of P300 in attend but not ignore conditions indicates the controlled deployment of selective attention. HC = Healthy comparison participants. FESz = First-episode schizophrenia participants. Shaded area indicates peak N100 ± 5msec.
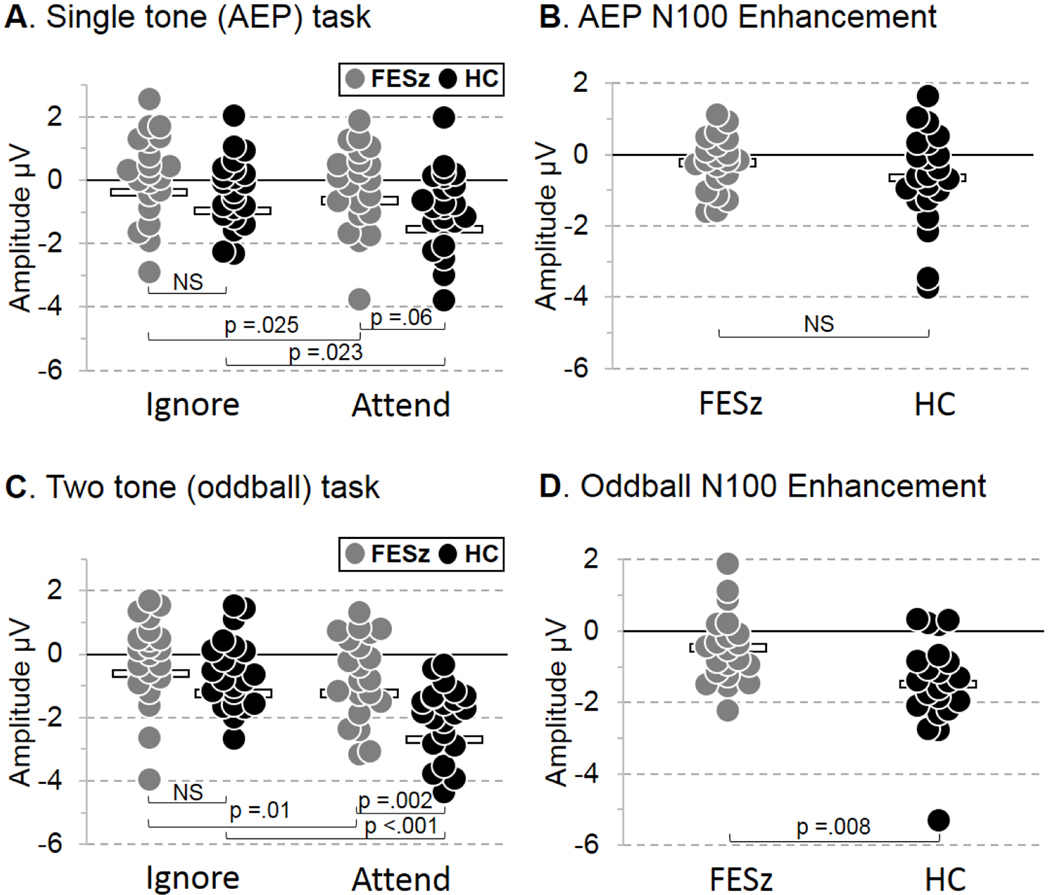
Figure 2. N100 peak ±10 msec amplitudes and N100 enhancement.
A. Single-tone (AEP) task N100 distributions at Fz for ignore and attend conditions in each group. B. N100 enhancement by attention on the AEP task for each group. C. Two-tone (oddball) task N100 distributions at Fz for ignore and attend conditions in each group. D. N100 enhancement by attention on the oddball task for each group. Note: Bars in the scattergrams represent the group mean. Statistics are for uncorrected t-tests.
Table 3.
N100 Amplitudes, N100 Enhancement, and Effect Sizes.
| TASK | FESz | HC | t-test | Effect Size |
|---|---|---|---|---|
| Ignore AEP | −0.42 (1.65) | −0.98 (1.21) | t =1.27, p = .21 | Cohen’s d =0.39 |
| Attend AEP | −0.68 (1.78) | −1.63 (1.46) | t =1.92, p = .06 | Cohen’s d =0.58 |
| Ignore Oddball | −0.66 (1.69) | −1.23 (1.26) | t =1.26, p = .22 | Cohen’s d =0.38 |
| Attend Oddball | −1.17 (1.67) | −2.71 (1.38) | t = 3.30, p = .002 | Cohen’s d =1.01 |
| AEP Enhancement | −0.26 (0.77) | −0.65 (1.32) | t =1.18, p = .24 | Cohen’s d =0.36 |
| Oddball Enhancement | −0.51 (0.98) | −1.47 (1.27) | t =2.79, p = .008 | Cohen’s d =0.85 |
Note: Values are Mean (SD). Bold indicates significant group differences. AEP = Auditory Evoked Potential, single-tone task. Oddball = Two-tone task. Bold indicates significant group differences.
Omnibus ANOVA revealed overall significant reductions of N100 in FESz (F(1,41) =4.69, p =.036, partial eta squared =0.103). The single-tone task N100 responses were significantly smaller than those of the oddball task (F(1,41) =22.14, p <.001, partial eta squared =0.351), and this effect did not differ between groups. N100 was larger across tasks in the attend condition compared to the ignore condition (F(1,41) =31.61, p <.001, partial eta squared =0.435), but this N100 enhancement differed between groups (F(1,41) =6.99, p =.012, partial eta squared =0.146), with FESz impaired in their degree of enhancement relative to HC. The oddball task showed greater enhancement than the single-tone task (F(1,41) =5.80 p =.021, partial eta squared =0.124), and this effect did not differ between groups.
Due to the question of sensory versus attentional enhancement contributions to the N100 amplitude deficits in FESz, each condition was compared between and within groups (Figure 2, Table 3). N100 during the ignore condition was not significantly different between groups on either task in these small samples, but showed small to medium effect sizes for group differences that would attain significance with >60 participants per group (Table 3). N100 to attended tones showed trend-level reductions on the single-tone task (p =.06, d =0.58, medium effect size) and significant reduction on the oddball task (p =.008, d =0.85, large effect size, Table 3), indicating a general reduction in executive enhancement of N100 in FESz.
Correlations
In FESz, there were no significant correlations between single-tone (AEP) ignore N100, single-tone (AEP) attend N100, single-tone (AEP) N100 enhancement, or Oddball ignore N100 with WASI IQ, MATRICS Composite Score, PANSS Total, Positive, or Negative factors, SANS or SAPS global scores, or GF:S or GF:R current functioning, or high or low functioning in the last year. N100 on the attend oddball task was significantly inversely correlated with MATRICS composite scores (rho =−.51, p =.017), indicating higher scores on tests sensitive to the effect of psychosis were associated with larger attend oddball N100 amplitudes, and with the highest occupational role functioning (GF:R) in the last year (rho =−.51, p =.019), indicating better occupational functioning was associated with larger attend oddball N100 amplitudes. N100 enhancement on the oddball task in FESz was significantly inversely correlated with MATRICS composite scores (rho =−.55, p =.009) and with the highest role (GF:R) functioning in the last year (rho =−.49, p =.024) and highest social (GF:S) functioning in the last year (rho =−.44, p =.049), and positively associated with the PANSS negative factor (rho =−.55, p =.016) and SANS global scores (rho =−.57, p =.007), indicating greater negative symptoms were associated with less N100 enhancement. In HC, the only significant correlation was a positive association between WASI IQ and oddball task enhancement (rho =.58, p =.005), indicating higher intellect in this group was associated with less enhancement.
Discussion
The current study utilized both single-tone and oddball tasks during attend and ignore conditions to disentangle sensory and cognitive contributions, on the one hand, and stimulus complexity, on the other, to N100 deficits in FESz. Overall N100 amplitudes were reduced in FESz, but this deficit was particularly compounded by deficits in attentional enhancement (gain) of N100. Although the comparisons of ignore conditions on each task between FESz and HC did not attain significance, both comparisons would have attained significance with larger Ns, and several previous studies have demonstrated reduced N100s in FESz (see Introduction). Perhaps more remarkable then are the sizeable group differences detected during attend conditions, suggesting that lack of attentional gain contributes substantially to N100 deficits observed in FESz.
We also detected an increase in N100 and N100 enhancement in the oddball task relative to the single-tone task. We speculate that this reflects the increased resources needed for the two-tone task. In the single-tone task, participants simply counted the occurrence of the same sound, indicating every 7th tone. On the oddball task, participants were required to discriminate between two-tones, putatively more resource consuming and necessitating greater cognitive control. Alternatively, it is possible that the video was somehow more distracting in the single-tone task, as the lower resources needed may make participants more susceptible to distraction on that task, although we can neither support nor reject this alternative. Still both groups showed increases from the single-tone (AEP) task to the two-tone (oddball) task, albeit the N100 enhancement in FESz was impaired relative to HC. To the extent that both groups showed N100 enhancement across tasks but the deficit in FESz was relatively constant on each task, we suspect the overall N100 differences were due to stimulus complexity rather than differential distractibility.
Although we focused on the peak N100 difference, the exact morphology of N100 enhancement is not clear. Several studies report attention increased N100 and P200 (the subsequent positive AEP) directly (Woldorff et al., 1993; O’Donnell et al., 1994; Boutros et al., 2004). This N100 enhancement is reduced in Sz (O’Donnell et al., 1994). This morphology is consistent with our single-tone (AEP) data, where enhancement for N100 peak (gain) is clear. On the other hand, other groups reported a broad longer-lasting negativity that increases N100 and reduces P200, the negative difference (Nd; Hansen & Hillyard, 1980) or processing negativity (PN; Näätänen, 1982). This broader Nd morphology is consistent with our two-tone (oddball) data. This broader Nd component contains at least two phases (Hansen & Hillyard, 1980), and the early phase occurs during the N100 period, and appears to have similar generators as N100 (Woldorff et al., 1993). Thus, it likely reflect direct gain modulation of N100 processes by attention. Still, regardless of whether the attentional enhancement leads to a discrete or broad negativity, N100 enhancement (even if reflecting early Nd) is an objective measure of selective attention and executive modulation of sensory processing, and can be used to assess the integrity of frontal-temporal systems-level functioning.
Diminution of N100 amplitude enhancement in FESz indicates a deficit of top-down control during auditory processing. It is well-known that the frontal cortex is highly involved in top-down attentional control (Hopfinger, Buonocore, & Mangun, 2000; Tobyne et al., 2017). Our results reflect a long-range functional disconnection between cognitive control cortical areas (i.e. frontal cortex) and the auditory temporal lobes early in the disease course. This system may be preferentially affected early in the disease course and serve as a systems-level neurobiological target for precision medicine interventions. The hypothesis that N100 enhancement is sensitive to frontal-temporal pathology is strengthened by the correlation of N100 enhancement on the more complex oddball task with cognition, negative symptoms, and best social functioning (inter-personal and role) in the last year, all functions known to have substantial contributions from frontal lobe function. For example, Asami et al. (2012) found FESz showed persistent negative symptoms associated with widespread, progressive gray matter reductions in frontal cortex, and longitudinal gray matter volume loss were linked to persistent and worsening negative symptoms. Still, further work is necessary, as the sample sizes were small and the correlations were exploratory, without correction for multiple comparisons. Studies examining the neurophysiological, gray matter, and white matter concomitants of attentional control between the prefrontal cortex and auditory cortex in FESz are currently underway in new, larger samples. In HC, higher intellect was associated, perhaps counter-intuitively, with less oddball enhancement (within the significant group increase of enhancement on the task). We speculate that the task complexity may have been more trivial for these individuals, necessitating little increase of sensory gain, but, because of the small sample size and exploratory nature of the correlations, future work examining the association between intellect, N100 enhancement, and task difficulty is warranted.
Given that neuropsychological deficits predate overt psychosis (Woodberry, Giuliano, & Seidman 2008) this finding suggests the reduction of N100 enhancement on the oddball task may be a candidate biomarker for imminent psychosis in the prodromal state. Although reductions of N100 have been reported in the prodrome (Brockhaus-Dumke et al. 2008; del Re et al. 2015), to our knowledge specific assessment of attend and ignore conditions have not been made. (Recall that the N100 enhancement was associate with negative symptoms and role and interpersonal functioning more robustly than the raw attend oddball N100.) Future work should assess whether N100 enhancement may be a more sensitive biomarker to emerging psychosis in the prodromal state.
We propose that the failure of N100 enhancement with attention on the oddball task is due to impaired frontal cortex top-down modulation of the auditory processing system in FESz. N100 enhancement during the oddball task may thus serve as an objective measure of selective attention circuit pathophysiology, and therefore an objective outcome measure for targeted interventions. A future direction of this research program is to use concurrently recorded EEG and magnetoencephalography (MEG) data to determine cortical sources using realistic head models from individual structural MRI images to create a more complete picture of the underlying brain circuitry associated with auditory N100 enhancement, and pathophysiology of this circuit in FESz. Such an approach would allow investigation of cortical dynamics and help localize the cortical areas most amenable to targeted intervention (e.g. NIBS).
Several caveats of the current experiment must be discussed. The insertion of click trains between tones led to a broad negativity between 300 and 800 ms as shown in Figure 1. It is unclear to what extent this affected the N100 enhancement. Currently we are testing participants without the click trains inserted. Another potential limitation is that the majority of patients were on antipsychotic medication at the time of participation. However, no participant had a long course of treatment. We did not observe an effect of medication on the ability to modulate N100 response with attention (p’s >.05). We are ethically obligated to treat patients for their symptoms, and it is increasingly rare that individuals are not treated with antipsychotics in the United States before first contact with psychiatric services. Additionally, the sample size in the current study is relatively small. We suspect the lack of group differences is due to the relatively small sample size, given the extant literature demonstrating such deficits early in disease course. The small sample sizes also necessitate that the exploratory (uncorrected) correlations be interpreted with caution. Within the FESz group the gender ratio (3 male to 1 female) was unbalanced. Current work is testing a replication sample, and increasing the number of women included. Finally, due to a programming error, key-press responses and corresponding reaction times were not recorded, so we were unable to report behavioral performance measures. However, our technicians carefully monitored task performance to make sure participants paid attention and were making target button presses, and, as indicated in Figure 1C, P300 was elicited only in the attend condition but not ignore condition as expected with and without attention.
In summary, individuals in the first-episode schizophrenia spectrum exhibited impaired attentional modulation of N100 that compounded overall N100 deficits. This finding indicates that abnormal attentional processing can be objectively measured from neurophysiological sensory/perceptual responses at the start of psychosis. Because such deficits can be associated with a distinct executive control system, the N100 enhancement deficit helps indicate a neurobiological system for targeted intervention. Further, deficits in N100 enhancement may provide clues to neurobiological abnormalities in the psychosis prodrome. Reductions in the ability to enhance N100 in the service of auditory selective attention are present in the first episode schizophrenia spectrum and provide a sensitive index of cognitive impairments due to psychosis and negative symptoms.
Acknowledgements
This study was supported by the Brain and Behavior Foundation (NARSAD Independent Investigator Award) and the National Institute of Mental Health (R01 MH108568) to DFS, and a Conte Center for Translational Mental Health Research Undergraduate Fellowship to SNF. We thank the faculty and staff of the WPIC Psychosis Recruitment and Assessment Core, the Conte Center for Translational Mental Health Research (P50 MH103204, David Lewis, MD, Director), and the University of Pittsburgh Clinical Translational Science Institute (UL1 RR024153, Steven E. Reis, MD) for their assistance in recruitment, diagnostic and psychopathological assessments, and neuropsychological evaluations.
Abbreviations
- AEPs
Auditory Evoked Potentials
- EEG
Electroencephalogram
- FESz
First Episode Schizophrenia
- GF:R
Global Functioning Role Scale
- GF:S
Global Functioning Social Scale
- HC
Healthy Controls
- MEG
Magnetoencephalography
- NIBS
Non-invasive Brain Stimulation
- NOS
Not Otherwise Specified
- PANSS
Positive and Negative Symptom Scale
- SANS
Scale for Assessment of Negative Symptoms
- SAPS
Scale for Assessment of Positive Symptoms
- SCID
Structured Clinical Interview for DSM-IV
- SES
Socioeconomic Status
- Sz
Schizophrenia
- UPSA-B
UCSD Performance-based Skills Assessment
- WPH
Western Psychiatric Hospital
- WASI IQ
Wechsler Abbreviated Scale of Intelligence Intelligence Quotient
Footnotes
Competing Interests
None.
Attention increases the gain (amplitude) of auditory signals, assisting in focusing on task-relevant sensory information in the auditory landscape. Individuals at their first episode of psychosis show impairments in this cognitive control process that requires effective connectivity between frontal control areas and temporal lobe auditory cortex. This frontal-temporal dysconnectivity, evident at psychosis onset, provides a potentially tractable neural system for targeted remediation.
Data Availability
The data is available upon request to Prof. Salisbury.
References
- Asami T, Bouix S, Whitford TJ, Shenton ME, Salisbury DF and McCarley RW, 2012. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage, 59(2), pp.986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahveninen J, Jääskeläinen IP, Osipova D, Huttunen MO, Ilmoniemi RJ, Kaprio J, Lönnqvist J, Manninen M, Pakarinen S, Therman S and Näätänen R, 2006. Inherited auditory-cortical dysfunction in twin pairs discordant for schizophrenia. Biological psychiatry, 60(6), pp.612–620. [DOI] [PubMed] [Google Scholar]
- Bleuler E, 1950. Dementia praecox or the group of schizophrenias. [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A & Bell M Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res. 126, 203–215 (2004). [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J and Ruhrmann S, 2008. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biological psychiatry, 64(5), pp.376–384. [DOI] [PubMed] [Google Scholar]
- Connolly JF, Manchanda R, Gruzelier JH and Hirsch SR, 1985. Pathway and hemispheric differences in the event-related potential (ERP) to monaural stimulation: a comparison of schizophrenic patients with normal controls. Biological Psychiatry, 20(3), pp.293–303. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA and Erlenmeyer-Kimling L, 1985. Global attentional deviance as a marker of risk for schizophrenia: specificity and predictive validity. Journal of Abnormal Psychology, 94(4), p.470. [DOI] [PubMed] [Google Scholar]
- Correll CU, Galling B, Pawar A, Krivko A, Bonetto C, Ruggeri M, Craig TJ, Nordentoft M, Srihari VH, Guloksuz S and Hui CL, 2018. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA psychiatry, 75(6), pp.555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re EC, Spencer KM, Oribe N, Mesholam-Gately RI, Goldstein J, Shenton ME, Petryshen T, Seidman LJ, McCarley RW and Niznikiewicz MA, 2015. Clinical high risk and first episode schizophrenia: auditory event-related potentials. Psychiatry Research: Neuroimaging, 231(2), pp.126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A and Makeig S, 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods, 134(1), pp.9–21. [DOI] [PubMed] [Google Scholar]
- Everett J, Laplante L and Thomas J, 1989. The selective attention deficit in schizophrenia: limited resources or cognitive fatigue?. Journal of Nervous and Mental Disease. [DOI] [PubMed] [Google Scholar]
- Force RB, Venables NC and Sponheim SR, 2008. An auditory processing abnormality specific to liability for schizophrenia. Schizophrenia research, 103(1–3), pp.298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Yeap S, Snyder AC, Kelly SP, Thakore JH and Molholm S, 2011. The N1 auditory evoked potential component as an endophenotype for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives, first-episode, and chronic schizophrenia patients. European archives of psychiatry and clinical neuroscience, 261(5), pp.331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JC and Hillyard SA, 1980. Endogeneous brain potentials associated with selective auditory attention. Electroencephalography and clinical neurophysiology, 49(3–4), pp.277–290. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL and Picton TW, 1973. Electrical signs of selective attention in the human brain. Science, 182(4108), pp.177–180. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH and Mangun GR, 2000. The neural mechanisms of top-down attentional control. Nature neuroscience, 3(3), pp.284–291. [DOI] [PubMed] [Google Scholar]
- Iwanami A, Suga I and Kanamori R, 1994. N1 component derived from the temporal region during an auditory passive event-related potential paradigm in schizophrenics. Clinical Electroencephalography, 25(2), pp.50–53. [DOI] [PubMed] [Google Scholar]
- Kane JM, Robinson DG, Schooler NR, Mueser KT, Penn DL, Rosenheck RA, Addington J, Brunette MF, Correll CU, Estroff SE and Marcy P, 2016. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE early treatment program. American Journal of Psychiatry, 173(4), pp.362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Jakubovitz A, Scaramello N, Laukka P, Silipo G and Javitt DC, 2013. Are Schizophrenia Patients Amusical?: The Role of Pitch and Rhythm in Auditory Emotion Recognition Impairments in Schizophrenia. In Biological Psychiatry (Vol. 73, No. 9, pp. 18S–18S). [Google Scholar]
- Kantrowitz JT, Hoptman MJ, Leitman DI, Silipo G and Javitt DC, 2014. The 5% difference: Early sensory processing predicts sarcasm perception in schizophrenia and schizoaffective disorder. Psychological medicine, 44(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt VM, Molholm S, Ritter W, Shpaner M and Foxe JJ, 2007. Auditory processing in schizophrenia during the middle latency period (10–50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits. Journal of psychiatry & neuroscience. [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G and Javitt DC, 2010. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. American Journal of Psychiatry, 167(7), pp.818–827. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’Leary D, Arndt S and Andreasen N, 1999. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Archives of general psychiatry, 56(8), pp.749–754. [DOI] [PubMed] [Google Scholar]
- Näätänen R, 1982. Processing negativity: An evoked-potential reflection. Psychological bulletin, 92(3), p.605. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Hokama H, McCarley RW, Smith RS, Salisbury DF, Mondrow E, Nestor PG and Shenton ME, 1994. Auditory ERPs to non-target stimuli in schizophrenia: relationship to probability, task-demands, and target ERPs. International Journal of Psychophysiology, 17(3), pp.219–231. [DOI] [PubMed] [Google Scholar]
- Ogura C, Nageishi Y, Matsubayashi M, Omura F, Kishimoto A and Shimokochi M, 1991. Abnormalities in event‐related potentials, N100, P200, P300 and slow wave in schizophrenia. Psychiatry and Clinical Neurosciences, 45(1), pp.57–65. [DOI] [PubMed] [Google Scholar]
- Penttilä M, Jääskeläinen E, Hirvonen N, Isohanni M and Miettunen J, 2014. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. The British Journal of Psychiatry, 205(2), pp.88–94. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN and Ford JM, 2008. Reduced auditory evoked potential component N100 in schizophrenia—a critical review. Psychiatry research, 161(3), pp.259–274. [DOI] [PubMed] [Google Scholar]
- Rosenheck R, Leslie D, Sint K, Lin H, Robinson DG, Schooler NR, Mueser KT, Penn DL, Addington J, Brunette MF and Correll CU, 2016. Cost-effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE Early Treatment Program. Schizophrenia bulletin, 42(4), pp.896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC and McCarley RW, 2010. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophrenia bulletin, 36(5), pp.991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, O’DONNELL BF, McCARLEY RW, Nestor PG, Faux SF and Smith RS, 1994. Parametric manipulations of auditory stimuli differentially affect P3 amplitude in schizophrenics and controls. Psychophysiology, 31(1), pp.29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley AM, Silipo G and Javitt DC, 1999. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophrenia research, 37(1), pp.65–79. [DOI] [PubMed] [Google Scholar]
- Tobyne SM, Osher DE, Michalka SW and Somers DC, 2017. Sensory-biased attention networks in human lateral frontal cortex revealed by intrinsic functional connectivity. Neuroimage, 162, pp.362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG and Gur RE, 2009. Profile of auditory information-processing deficits in schizophrenia. Psychiatry research, 165(1–2), pp.27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D and Bloom FE, 1993. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of the National Academy of Sciences, 90(18), pp.8722–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ and Seidman LJ, 2008. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry, 165(5), pp.579–587. [DOI] [PubMed] [Google Scholar]
- Yada Y, Yoshimura B and Kishi Y, 2015. Correlation between delay in initiating clozapine and symptomatic improvement. Schizophrenia research, 168(1–2), p.585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available upon request to Prof. Salisbury.