Dear Editor,
We appreciate the positive comment, from Drs. Menno Hoekstra Ezra J. van der Wel, and Miranda Van Eck, on our recent work of PLTP deficiency-mediated atherosclerosis regression [1]. We agree that the lesion regression effect observed by us can also be attributed to the effect of PLTP deficiency specifically in macrophages, although the mechanism remains unclear.
There are certain important mechanisms are involved in atherosclerosis regression through macrophages in mouse models[2]: 1) suppression of monocyte infiltration into the atherosclerotic plaques. 2) depletion of M1 and enrichment of M2 microphages; 3) upregulation of macrophage CCR7 and increase of CCR7-dependent egress of resident macrophages from atheroma; 4) increasing cholesterol efflux from macrophages on the lesions. The results reported Hoekstra M et al. have enriched the existing mechanisms. They found that 1) treatment of hypercholesterolemia could facilitate CCR7-positive macrophage polarization and migration; and 2) new macrophages continuously infiltrate regressing atherosclerotic lesions.
In fact, PLTP deficiency can effectively reverse diet-induced or LDL receptor deficiency-mediated hypercholesterolemia [1]. Pltp knockout (KO) mice have lower circulating levels of interleukin-6 (IL-6)[3, 4] and less infiltrating macrophages in aortic tissue [5], compared with controls. All these effects could contribute to promote the regression of atherosclerosis, although PLTP activity has no effect on macrophage cholesterol efflux in mouse models [6].
Previously, it was reported that global PLTP deficiency not only greatly reduced plasma HDL-cholesterol and apoAI levels but also greatly reduced plasma sphingosine 1 phosphate (S1P) levels (60%), compared with controls [7]. Recently, we confirmed that both male and female Pltp KO mice significantly reduced S1P in the circulation (about 45%) (Fig. 1A) and we also found that inducible male and female Pltp KO mice, under a high fat and high cholesterol diet, had about 50% reduction of plasma S1P (Fig. 1B), respectively. This PLTP deficiency-mediated S1P reduction could contribute to reduction of atherosclerosis progression and induction of regression[1].
Figure 1:
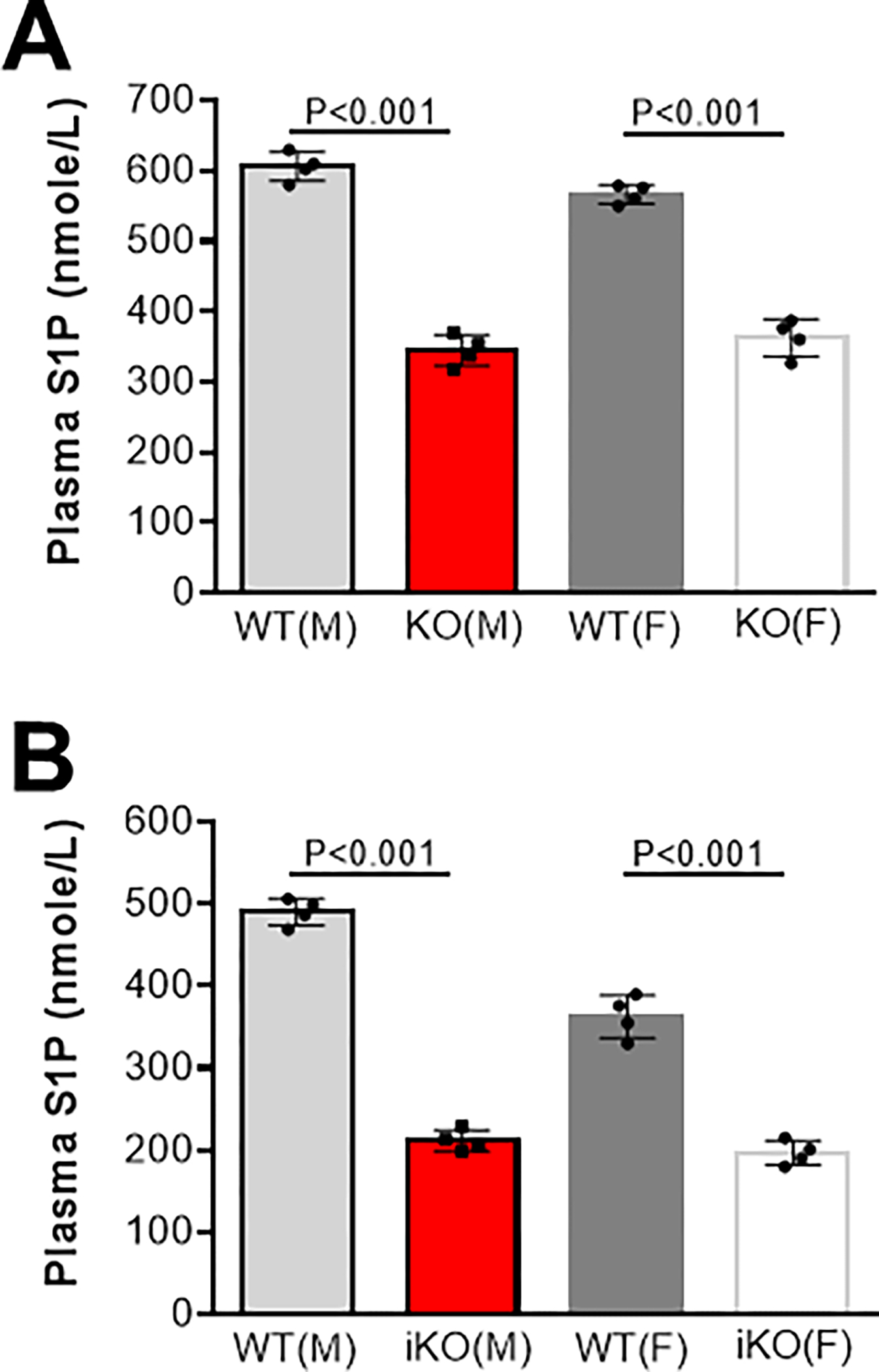
Plasma S1P levels in mice. (A), WT and Pltp KO mice on chow diet. (B), WT and inducible Pltp KO mice on a high fat high cholesterol diet (0.15% cholesterol, 20% saturated fat). M, male; F, female. Values are mean ± SD.
S1P is a potent lipid mediator composed of one long hydrophobic chain and one phosphoric acid group. S1P in blood is produced primarily by red blood cells (RBC), platelets, and endothelial cells[8], and secreted by major facilitator superfamily transporter 2b (Mfsd2b) [9] and S1P transporter spinster homolog 2 (Spns2) [10], respectively. S1P acts on macrophages to alter their functional phenotype[11]. S1P activates NF-κB [12], promotes chemotaxis, and stimulates the production of TNF-α in macrophages and/or monocytes[13]. S1P exerts potent physiological effects through five S1P receptors (S1PR1–5) located on cell membranes. The order of S1P receptor expression levels in macrophages are as follows: S1PR2 > S1PR1 >>S1PR3 and SIPR4 and there is no detectable S1PR5 [14]. The association between S1PR1 and CCR7 has been reported [15]. Activation of S1PR1 by a specific compound, KRP-203, can inhibit atherosclerosis by modulating macrophage and lymphocyte function [16]. S1PR2 signaling in macrophages is sufficient to promote atherosclerosis in the apolipoprotein (Apo) E KO mice [14]. Moreover, S1P promotes inflammatory M1 polarization of macrophage and promotes macrophages chemotaxis [17]. These studies provides support for S1P-mediated proatherogenic property.
Due to its hydrophobic nature, S1P is poorly water soluble and requires carrier proteins for efficient transport and circulation. Based on previous reports, plasma S1P is carried by HDL and albumin[18]. HDL-bound apoM as a physiologically-relevant S1P chaperone [19], which is defined as S1P carrier protein that facilitates specific receptor activation and biological response. despite the potential of the apoM-S1P axis as an endothelium-protective mechanism, the effect of apoM-S1P on atherosclerosis is still controversy [20, 21]. Moreover, global apoM deficiency causes about 45% reduction of plasma S1P [19, 21], global albumin deficiency has no significant impact on S1P in the circulation [22], and global apoM/albumin double deficient mice still have sufficient amount of plasma S1P [22]. These observations suggested that there are other S1P chaperones exist to mediate S1P functions, such as apoA4 [22]. Thus, PLTP (as a lipid carrier) depletion and PLTP deficiency-mediated HDL dramatical reduction could be two potential reasons for the dramatical reduction of S1P in the circulation. This effect of PLTP deficiency is independent from apoM, since global PLTP deficiency has no significant impact on plasma apoM (Fig. 2A) [7], and apoM deficiency has no effect on plasma PLTP activity (Fig. 2B). Unlike apoM, it is known that PLTP deficiency prevents and PLTP overexpression promotes atherosclerosis in animal models. Thus, we hypothesize that there is a PLTP-S1P axis, which is different from apoM-S1P axis, affecting atherosclerosis regression through influencing macrophages.
Figure 2:
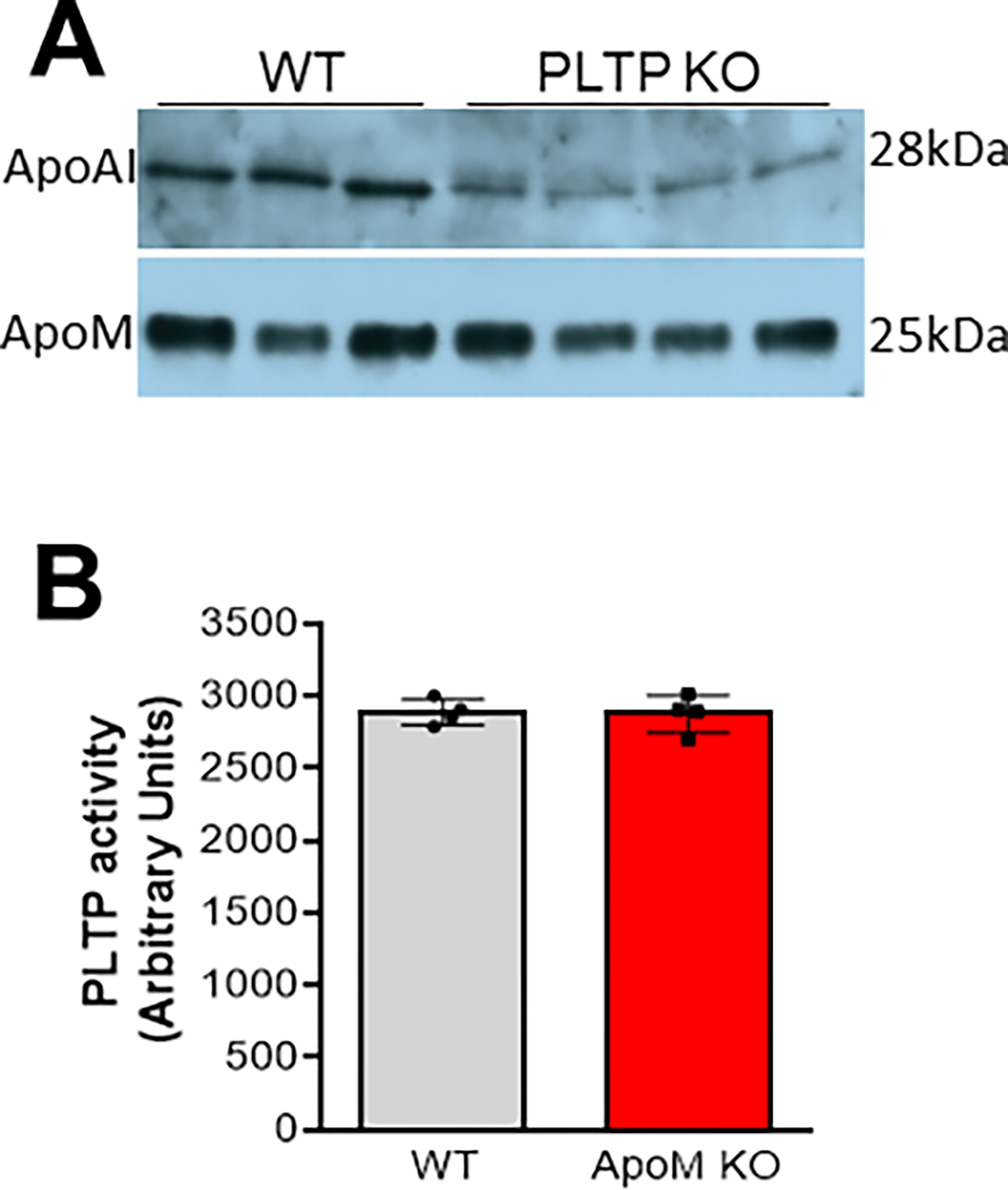
Western blot analysis for plasma apoAI and apoM in WT and Pltp KO mice (A); Plasma PLTP activity in the WT and Apom KO mice (B). Values are mean ± SD.
We agree that more detailed mechanistic studies are warranted into the possible effect of (inducible) PLTP deficiency on atherosclerosis regression. In particular, we would like to investigate the effect of PLTP deficiency-mediated S1P reduction on monocyte migration and macrophage differentiation and polarization as well as emigration of macrophages from atherosclerotic lesions.
ACKNOWLEDGEMENTS
This study was supported by VA Merit, I01BX000900 and NIH, HL139582, both to Xian-Cheng Jiang. Apom KO and control mouse plasma was a gift from Dr. Timothy Hla, Harvard Medical School. Pltp KO and control mouse plasma was a gift from Dr. Laurent Lagrost, Université Bourgogne Franche-Comté.
References
- [1].Zhang K, Zheng J, Chen Y, Dong J, Li Z, Chiang YP, He M, Huang Q, Tang H, Jiang XC, Inducible phospholipid transfer protein deficiency ameliorates atherosclerosis, Atherosclerosis, 324 (2021) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fisher EA, Regression of Atherosclerosis: The Journey From the Liver to the Plaque and Back, Arterioscler Thromb Vasc Biol, 36 (2016) 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schlitt A, Liu J, Yan D, Mondragon-Escorpizo M, Norin AJ, Jiang XC, Anti-inflammatory effects of phospholipid transfer protein (PLTP) deficiency in mice, Biochimica et biophysica acta, 1733 (2005) 187–191. [DOI] [PubMed] [Google Scholar]
- [4].Shelly L, Royer L, Sand T, Jensen H, Luo Y, Phospholipid transfer protein deficiency ameliorates diet-induced hypercholesterolemia and inflammation in mice, Journal of lipid research, 49 (2008) 773–781. [DOI] [PubMed] [Google Scholar]
- [5].Deckert V, Kretz B, Habbout A, Raghay K, Labbe J, Abello N, Desrumaux C, Gautier T, Lemaire-Ewing S, Maquart G, Le Guern N, Masson D, Steinmetz E, Lagrost L, Development of abdominal aortic aneurysm is decreased in mice with plasma phospholipid transfer protein deficiency, The American journal of pathology, 183 (2013) 975–986. [DOI] [PubMed] [Google Scholar]
- [6].Kuwano T, Bi X, Cipollari E, Yasuda T, Lagor WR, Szapary HJ, Tohyama J, Millar JS, Billheimer JT, Lyssenko NN, Rader DJ, Overexpression and deletion of phospholipid transfer protein reduce HDL mass and cholesterol efflux capacity but not macrophage reverse cholesterol transport, J Lipid Res, 58 (2017) 731–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yu Y, Guo S, Feng Y, Feng L, Cui Y, Song G, Luo T, Zhang K, Wang Y, Jiang XC, Qin S, Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability, Lipids, 49 (2014) 183–190. [DOI] [PubMed] [Google Scholar]
- [8].Obinata H, Hla T, Sphingosine 1-phosphate and inflammation, Int Immunol, 31 (2019) 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM, Torta F, Cazenave-Gassiot A, Matsumura T, Kim S, Toh SES, Suda T, Silver DL, Wenk MR, Nguyen LN, Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets, Nature, 550 (2017) 524–528. [DOI] [PubMed] [Google Scholar]
- [10].Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N, The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors, Science, 323 (2009) 524–527. [DOI] [PubMed] [Google Scholar]
- [11].Weigert A, Olesch C, Brune B, Sphingosine-1-Phosphate and Macrophage Biology-How the Sphinx Tames the Big Eater, Front Immunol, 10 (2019) 1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shatrov VA, Lehmann V, Chouaib S, Sphingosine-1-phosphate mobilizes intracellular calcium and activates transcription factor NF-kappa B in U937 cells, Biochemical and biophysical research communications, 234 (1997) 121–124. [DOI] [PubMed] [Google Scholar]
- [13].Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B, Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis, Circulation research, 108 (2011) 314–323. [DOI] [PubMed] [Google Scholar]
- [14].Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T, Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis, Arteriosclerosis, thrombosis, and vascular biology, 31 (2011) 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ishimaru N, Yamada A, Nitta T, Arakaki R, Lipp M, Takahama Y, Hayashi Y, CCR7 with S1P1 signaling through AP-1 for migration of Foxp3+ regulatory T-cells controls autoimmune exocrinopathy, Am J Pathol, 180 (2012) 199–208. [DOI] [PubMed] [Google Scholar]
- [16].Poti F, Gualtieri F, Sacchi S, Weissen-Plenz G, Varga G, Brodde M, Weber C, Simoni M, Nofer JR, KRP-203, sphingosine 1-phosphate receptor type 1 agonist, ameliorates atherosclerosis in LDL-R−/− mice, Arterioscler Thromb Vasc Biol, 33 (2013) 1505–1512. [DOI] [PubMed] [Google Scholar]
- [17].Liao CY, Song MJ, Gao Y, Mauer AS, Revzin A, Malhi H, Hepatocyte-Derived Lipotoxic Extracellular Vesicle Sphingosine 1-Phosphate Induces Macrophage Chemotaxis, Frontiers in immunology, 9 (2018) 2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F, Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions, The Biochemical journal, 352 Pt 3 (2000) 809–815. [PMC free article] [PubMed] [Google Scholar]
- [19].Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B, Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M, Proc Natl Acad Sci U S A, 108 (2011) 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Christoffersen C, Jauhiainen M, Moser M, Porse B, Ehnholm C, Boesl M, Dahlback B, Nielsen LB, Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice, J Biol Chem, 283 (2008) 1839–1847. [DOI] [PubMed] [Google Scholar]
- [21].Bosteen MH, Madsen Svarrer EM, Bisgaard LS, Martinussen T, Madsen M, Nielsen LB, Christoffersen C, Pedersen TX, Effects of apolipoprotein M in uremic atherosclerosis, Atherosclerosis, 265 (2017) 93–101. [DOI] [PubMed] [Google Scholar]
- [22].Obinata H, Kuo A, Wada Y, Swendeman S, Liu CH, Blaho VA, Nagumo R, Satoh K, Izumi T, Hla T, Identification of ApoA4 as a sphingosine 1-phosphate chaperone in ApoM- and albumin-deficient mice, J Lipid Res, 60 (2019) 1912–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]


