SUMMARY
Phospholipids are vital membrane constituents that determine cell functions and interactions with the environment. For bacterial pathogens, rapid adjustment of phospholipid composition to changing conditions during infection can be crucial for growth and survival. Fatty acid synthesis (FASII) regulators are central to this process. This review puts the spotlight on FabT, a MarR-family regulator of FASII characterized in streptococci, enterococci, and lactococci. Roles of FabT in virulence, as reported in mouse and nonhuman primate infection models, will be discussed. We present FabT structure, the FabT regulon, and changes in FabT regulation according to growth conditions. A unique feature of FabT concerns its modulation by an unconventional corepressor, acyl-acyl-carrier protein (ACP). Some bacteria express two ACP proteins, which are distinguished by their interactions with endogenous or exogenous fatty acid sources, one of which causes strong FabT repression. This system seems to allow preferred use of environmental fatty acids, thereby saving energy by limiting futile FASII activity. Control of fabT expression and FabT activity link various metabolic pathways to FASII. The various physiological consequences of FabT loss summarized here suggest that FabT has potential as a narrow range therapeutic target.
KEYWORDS: fatty acid synthesis, FabT, repressor, feedback regulation, acyl-ACP, binding sites
INTRODUCTION
All cells are delimited by lipid membranes, which form a selective permeable barrier that regulates the transport of nutrients and waste and receives and transmits environmental signals that adjust metabolic processes according to environmental conditions. Cell membranes commonly comprise a phospholipid bilayer composed of a polar head and an apolar body comprising two fatty acid (FA) chains per glycerol-phosphate backbone, whose features contribute to membrane composition and dynamics. FA structure and length are decisive for membrane topology and properties such as fluidity, permeability, and integrity. These features are crucial for the adaptation of bacteria to various environments (1).
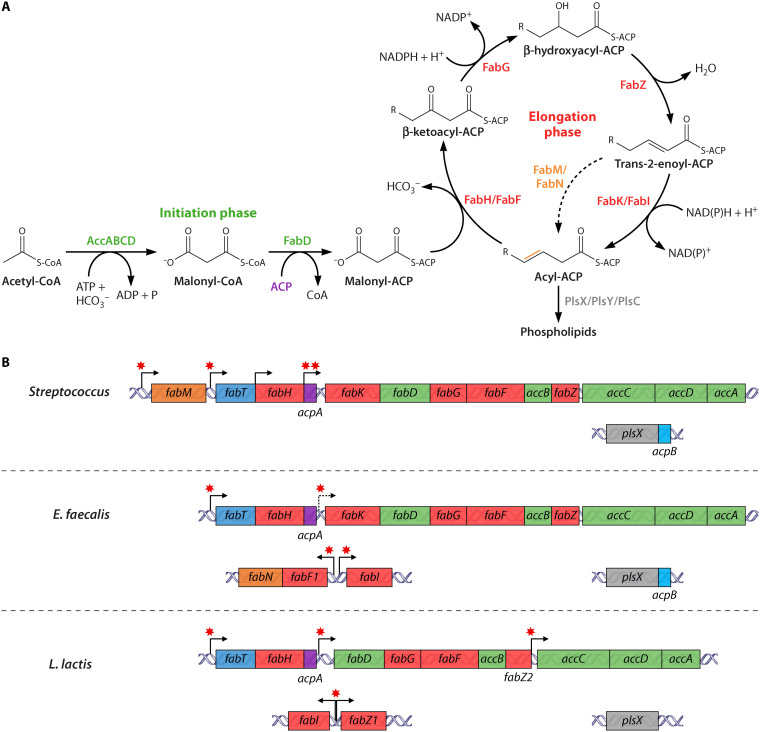
Phospholipids comprise two main FA classes, saturated (straight and branched chain FA) and unsaturated (including mono- and poly-unsaturated FA). The FA synthesis pathway (FASII) comprises an initiation phase followed by a recursive elongation cycle, which produces acyl-acyl carrier protein (acyl-ACP) (Fig. 1A). Various bacteria including streptococci, Enterococcus faecalis, and Lactococcus lactis produce monounsaturated FAs, which are synthesized via a shunt within the cycle (Fig. 1A). Many bacteria also generate cyclic FA via an enzyme that acts postsynthetically on membrane phospholipids (2). The process of cyclopropanation generates a three-membered ring at the site of an unsaturation on the carbon chain. The numerous possible FA products (different chain lengths, saturated or unsaturated, cyclized) may facilitate membrane adjustments according to shifts in environmental conditions.
FIG 1.
Fatty acid synthesis pathway (FASII) in food and pathogenic Lactobacillales. (A) FASII comprises a first initiation phase for precursor synthesis, followed by the recursive elongation cycle. The mature acyl-ACP products supply fatty acids for phospholipid synthesis. Products of FabM/FabN are unsaturated (in gold), while those of FabI/FabK are saturated. Proteins performing the same functions may vary according to species. (B) FASII genes are clustered in streptococci (S. pneumoniae, S. agalactiae, S. pyogenes), E. faecalis, and L. lactis, and share a similar genetic organization. Proteins (in panel A) or coding genes (in panel B) are in green for initiation functions, red for elongation functions, brown for FA-modifying functions, and gray for phospholipid synthesis functions. fabF1 is also named fabO. In panel A, the dashed arrow represents the shunt leading to unsaturated FA synthesis; in (B) dark blue, fabT; purple and light blue, acpA and acpB, respectively. Bent arrows, transcription start sites (12, 15); dashed bent arrow, suggested transcription reinitiation site (11); *FabT binding sites; a single star represents one or two closely localized consensus sequences.
FASII is widespread among bacteria, but genetic organization and regulation of FASII genes are not that conserved. However, these features are conserved among lactic acid bacteria (Fig. 1B). A gene within the main FASII locus encodes a member of the MarR family of regulators, named FabT (Fig. 1B) (3). FASII regulation plays a crucial role in adjusting FA production under different conditions. Both feedback and feedforward mechanisms of FASII regulation are described for different bacterial species (4, 5). In this review, we summarize the current state of knowledge concerning FabT, a FASII regulator studied in streptococci, enterococci, and lactococci. While lactococci are mainly known as food-fermenting bacteria, the genus includes a fish pathogen, and commensals of plants, intestinal tracts, and the vagina, where FabT is highly conserved; its characterization in lactococci thus contributes to understanding FabT functionality. The unique features of FabT-family regulators will be highlighted, and findings linking FabT and virulence will be discussed.
FABT INVOLVEMENT IN STREPTOCOCCUS PYOGENES VIRULENCE
The natural occurrence and potential consequences of fabT mutations during Streptococcus pyogenes in vivo infections motivated us to write this review. S. pyogenes (group A Streptococcus [GAS]) is an important human pathogen responsible for a large variety of clinical manifestations ranging from mild superficial infections to more life-threatening invasive infections including necrotizing fasciitis or streptococcal toxic shock syndrome. GAS infections are also responsible for postinfectious complications such as rheumatic arthritis and glomerulonephritis and, altogether, GAS infections are responsible for 517,000 deaths annually worldwide (6, 7). In one study, 74 clinical isolates of the same genotype, from either invasive infections (mainly streptococcal toxic shock syndrome), or noninvasive infections, were analyzed (8). fabT point mutations were only detected in noninvasive infections (2 out of 38 isolates). This suggested that spontaneous mutations in fabT are counterselected in invasive infections and infrequent in noninvasive ones. Similarly, another study identified only nine single nucleotide polymorphisms or nonsynonymous insertions/deletions in fabT when comparing the genomic sequences of more than 3,615 clinical isolates of S. pyogenes of the same genotype from invasive and noninvasive infections (9, 10). In the study of Eraso et al. (10), fabT mutant distribution between invasive and noninvasive infections was not considered. In striking contrast with the low incidence of fabT polymorphisms among invasive isolates, their occurrence rose to 12% among isolates recovered at the site of infection from nonhuman primates in necrotizing fasciitis experiments (44 amino acid replacements at 30 different FabT positions were identified). Infection was then compared using a reference strain and its isogenic fabT deletion mutant in the nonhuman primate necrotizing fasciitis model. The mutant strain multiplied less at the inoculation point and caused significantly less spread, smaller lesions, and less tissue destruction than the wild-type strain; it was also more sensitive to killing in blood and in polymorphonuclear neutrophils (10). Another study in a murine subcutaneous infection model used wild-type strains, their fabT deleted derivatives, or clinical isolates harboring spontaneous fabT point mutations and a fabT-complemented strain. Virulence was strongly attenuated for all fabT mutant strains (8), in support of the above observations.
In conclusion, fabT mutants appear spontaneously among noninvasive isolates and, when mutated, may compromise bacterial virulence. Characterization of the genes controlled by FabT and FabT repression mechanisms and how FabT influences bacterial physiology will shed light on how and why fabT mutants emerge in weakly pathogenic isolates.
IDENTIFICATION OF FABT AS THE FASII REGULATOR
Bioinformatics analysis revealed a similar genetic organization of the FASII main cluster in three Streptococcus species, and in Clostridium acetobutylicum (3, 11), L. lactis, and E. faecalis (Fig. 1B). In streptococci, FASII genes are grouped in a single locus, whereas in L. lactis and E. faecalis, they are found in two distinct loci. The main acyl carrier protein (ACP) encoded by acpA is always present on the main locus, in an operon comprising three or more genes, the first of which is the MarR family regulator FabT (Fig. 1B) (12–14). A second transcript starting with acpA may indicate the existence of a second operon, or of a stabilized part of a longer mRNA (12, 15). When present, a second ACP encoded by acpB is colocalized with plsX and will be discussed below.
FabT was shown in S. pneumoniae by the Rock laboratory to be a FASII transcriptional regulator (12, 16). Its role was further demonstrated in S. pyogenes, E. faecalis, and L. lactis and suggested by synteny and sequence similarity analyses in C. acetobutylicum (3). FabT is conserved among the streptococci; the 144 amino acid S. pneumoniae FabT protein, respectively, shares 64%, 62%, 51%, 59%, and 45% sequence identity with S. pyogenes, Streptococcus agalactiae, E. faecalis, L. lactis, and C. acetobutylicum FabT (unpublished data). FabT is distinct from FapR-type regulators as described in Bacillus, Staphylococcus, and Listeria, which involve a feed-forward-type regulation (17). In that system, FapR repression is alleviated by binding to FASII precursors malonyl-CoA or malonyl-ACP (4, 5, 18).
THE FABT REGULON
The roles of FabT and the breadth of its regulon, which includes FASII and non-FASII genes, were reported in different transcriptional studies. For this, expression was compared in wild-type and fabT deleted or overproducing mutants, using various growth conditions, including those requiring adjustments in membrane composition (10, 12, 19–21).
FASII Gene Regulation by the FabT Repressor
FASII genes are organized in three to five transcriptional units in Streptococcus and four and five in E. faecalis and L. lactis, respectively (Fig. 1B). Internal transcriptional start sites within the operon beginning with fabT were uncovered in S. pneumoniae and S. pyogenes, suggesting that expression may be finely controlled (12, 15). In E. faecalis, overlapping RT-PCR demonstrated that the 12-gene cluster is organized as a single operon that accommodates a putative transcription reinitiation site upstream from fabK (13).
In S. pneumoniae and S. pyogenes, at 37°C, fabT loss of function mutants showed 2- to 9-fold increases in FASII gene expression compared to the wild-type strain, although species differences were observed (12, 21). Those studies showed that in S. pneumoniae, FabT represses expression of all FASII genes with the exception of fabM (required for FA unsaturation) (12). In L. lactis, only three genes from the main locus, fabD, fabG1, and accB, and two genes in a separate locus, fabZ1 and fabI, displayed increased expression in a fabT deleted strain, suggesting that repression was too weak to be detected for other FASII genes in the test condition (19). Indeed, in an L. lactis strain overproducing fabT by ~135-fold, all FASII genes except fabH were repressed by a factor of 2.5 to 5.3, indicating that these genes are controlled by FabT (19). The moderate effects of FabT on FASII gene expression in these extreme conditions, evokes the possibility that FabT activity is modulated by a cofactor (see The FabT corepressor). In Streptococcus mutans, FASII gene derepression in a fabT deletion mutant was observed under both neutral and acidic pH conditions (20). In S. pyogenes, variations in FASII gene transcription were analyzed between wild-type and fabT deletion strains, at different temperatures and growth phases. The authors concluded that FabT controls all FASII pathway genes, although fabM was less tightly controlled at 35°C than the other genes (10). Weaker control of fabM is reminiscent of results obtained in S. pneumoniae, where it was not repressed by FabT (12). In conclusion, FabT represses FASII genes in a coordinated but nonidentical way. Differential regulation of fluidifying fatty acids (requiring FabM) might be important for adjustment to stress conditions.
Non-FASII Gene Regulation by the FabT Repressor
Transcriptomic studies revealed numerous non-FASII genes whose expression is affected by the FabT status. In S. pneumoniae, comparison of transcription profiles between strains grown in laboratory medium showed that fabT deletion led to mostly minor (up to 5-fold) and 4 major (12- to 50-fold) expression changes for 85 upregulated and 4 downregulated genes, involved in transport, DNA metabolism and protein synthesis, and proteins of unknown function (12). The likely indirect effects of the fabT deletion may reflect bacterial adaptation to the altered ratio of saturated to unsaturated FAs (see reference 7; FabT and in vitro bacterial physiology). In support of this finding, comparing transcriptomes of wild-type and fabT deleted strains in L. lactis revealed expression changes of only 10 non-FASII genes (19). The use of dairy strains in that study may limit the identified targets when compared to a lactococcal pathogen (Lactococcus garvieae), or L. lactis plant, vaginal, or intestinal isolates, which express identical FabT proteins. However, fabT overexpression affected 125 genes belonging to the same functional categories as those identified in S. pneumoniae. It is likely that most expression changes are indirect consequences of FabT control.
Interestingly, FabT represses the expression of two non-FASII genes involved in FA and phospholipid metabolism. i- fakB3 encodes FakB3, which is involved in the incorporation of polyunsaturated FAs, which are not synthesized by Firmicutes (22, 23); and ii- plsC encodes PlsC, which catalyzes joining of a second FA to the 1-acyl-sn-glycerol 3-phosphate (bearing a single FA) to complete phospholipid acylation. S. pneumoniae FabT thus couples regulation of FA and phospholipid synthesis, similar to FapR regulation in B. subtilis and Staphylococcus aureus, where the regulatory mechanisms are different (24, 25). A direct or indirect role of FabT in regulating fakB3 and plsC expression remains to be shown.
In S. mutans, transcription profile comparisons of wild-type and fabT deleted strains were performed after growth at pH 7 or pH 5 (20). The expression of 25% of S. mutans genes was altered at pH 7 and 7% at pH 5. They also belonged to the same functional categories as described in S. pneumoniae. Deletion of fabT may thus have more consequences at neutral pH, as normally found in the oral cavity, than at acidic pH, as produced during S. mutans growth. Alternatively, low pH already creates the membrane perturbations that are similarly generated by FabT mutation, so that the changes are already in place.
Transcriptional analyses of a fabT deletion strain were also performed in S. pyogenes, in which growth phase and temperature were varied. Stationary growth phase and to a lesser extent 40°C induced the highest number of differentially expressed genes, i.e., up to 28.5%, between wild-type and fabT strains. The majority of affected genes was less expressed in the fabT mutant (10). Significantly, expression of many genes of carbohydrate metabolism and purine and pyrimidine synthesis pathways was modified, particularly during the stationary phase. This suggests that fabT mutations may lead to metabolic dysbiosis. Temperature, growth phase, biofilm status, and the nutritional environment were documented to affect bacterial FA composition and thereby impact membrane fluidity (26–29). Thus, in bacteria using FabT as the FASII regulator, FabT would ensure the maintenance of membrane homeostasis in response to membrane stress.
Altogether, these studies indicate that FabT likely has similar roles in different streptococcaceae. They show that the consequences of fabT mutation on bacterial physiology are numerous; possibly, these pleiotropic effects are triggered by changes in membrane composition. Our previous works showed that FASII transcription is turned off in FA-rich environments (11); in light of current knowledge, this repression involves FabT. The above observations show the far-reaching consequences of FASII regulation by FabT in bacterial lifestyle.
FABT MECHANISM OF REPRESSION
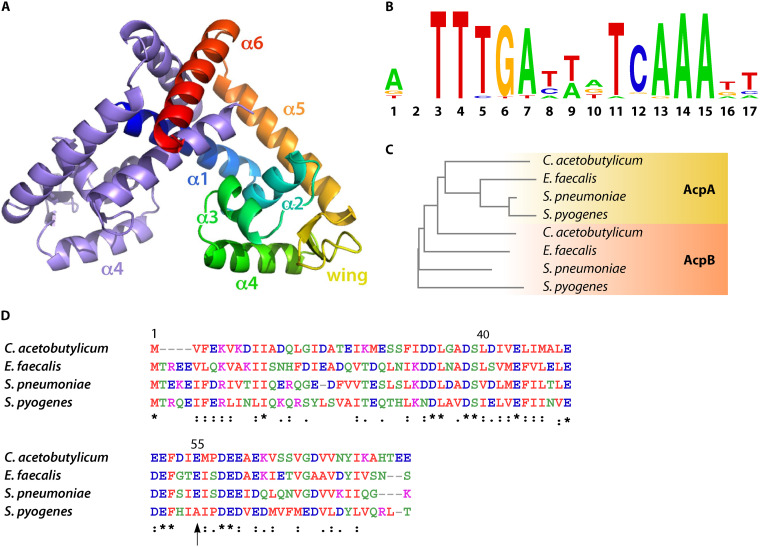
Like other MarR-family proteins, the FabT regulator comprises six alpha helices and three beta strands: α1-α2-β1-α3-α4-β2-β3-α5-α6. These regulators exist as homodimers both in their free or DNA-bound forms, and each subunit contributes a winged helix-turn-helix DNA-binding motif (Fig. 2A) (30, 31). MarR regulators have varying DNA targets and interact with either an inducer or a corepressor that modulates their affinity for their targets. Identification of these cofactors has been invaluable for understanding FabT regulatory mechanisms.
FIG 2.
Structural features of FabT, the ACP corepressor, and the FabT-DNA binding motif. (A) Overall structure of MarR family member dimer; one monomer is represented multicolored and the other in blue. Reprinted from reference 30 with permission. (B) FabT DNA binding motif; top, calculated from 56 FabT DNA binding sequences (https://regprecise.lbl.gov/regulog.jsp?regulog_id=3571). The relative size of letters corresponds to the relative frequency at which each nucleotide is present. (C) Phylogenetic neighbor-joining tree obtained from a multiple sequence alignment carried out using Clustal W (1.83). Branch lengths correspond to: C. acetobutylicum AcpA, 0.28257; E. faecalis AcpA, 0.19187; S. pneumoniae, AcpA 0.0325; S. pyogenes, AcpA 0.04858; C. acetobutylicum AcpB, 0.25594; E. faecalis AcpB, 0.29157; S. pneumonia, AcpB, 0.25143; S. pyogenes, AcpA, 0.35383. (D) AcpB alignment; multiple sequence alignment was carried out using Clustal W (1.83) (unpublished results), color code follows functional classification; *, identical in all four species: the same structural class; vertical arrow highlights amino acid residue 55, which is adjacent to the FabT-AcpB binding (32) but is not conserved in S. pyogenes.
FabT Dimerization and Target Gene Interaction
The crystal structure of the S. pneumoniae FabT-DNA complex revealed that FabT structure is similar to that of the MarR prototype protein (32). The FabT α helix 1 on one subunit intertwines with α helices 5 and 6 of the second subunit, creating a very compact and stable dimer; its α2, α3, and α4 helices, together with the hairpin comprising the β1-β2 strands, compose the DNA binding domain. However, the acyl-Acp-FabT complex binds DNA with more affinity than FabT alone. Weak FabT-DNA binding could be nonspecific, as only a single amino acid residue (Arg89)-nucleotide (T) interaction was highlighted (32). Further structural analysis will be needed to determine the biological relevance and molecular interactions involved in an effective FabT-DNA binding.
In S. pneumoniae, palindromes upstream of FASII operon genes fabM, fabT, and fabK were detected (Fig. 1B) (16). Their roles as FabT binding sites were confirmed by electrophoretic mobility shift assays (12). The positions of these binding sequences in S. pneumoniae, 10 and 30 bp from the ribosome binding sites of fabT and fabK, respectively, suggested that FabT inhibits RNA polymerase recruitment. Alternatively, the palindrome close to the fabK ribosome binding site could stop elongation of an RNA whose transcription starts upstream of acpA (12). FabT binding sites were also identified in silico in L. lactis, S. mutans, S. pyogenes, and E. faecalis, and were confirmed for L. lactis and S. mutans (10, 19–21, 33, 34). They were found upstream of various FASII genes fabM, fabT, and fabK or fabD, further supporting the existence of two or more FabT-regulated transcriptional units in the main cluster (Fig. 1B). A consensus FabT binding motif was proposed, based on 56 predicted or confirmed sequences (https://regprecise.lbl.gov/regulog.jsp?regulog_id=3571) (Fig. 2B). Most MarR proteins are sequence specific but may still show some degeneracy (31). More data are needed to determine whether FabT binds to degenerate motifs.
The presence of FabT binding sites adjacent to transcriptional start sites of specific genes is indicative of direct FabT regulation. Interestingly, FabT binding sites outside the main FASII locus are present in the intergenic regions between divergent FASII genes, such that FabT likely controls both transcriptional units. This organization was described for other MarR targets (31). Hence, while the location of the FabT binding sites appear to vary according to the species, a common feature of FabT is that it regulates all FASII genes, albeit at different levels, and depending on the growth conditions. Moreover, the low number of additional binding sites predicted in different species suggests that the FabT direct target genes are almost exclusively FASII genes.
The FabT Corepressor
The FabT ligand, acyl-ACP.
MarR family regulators generally possess a ligand acting as a corepressor or inducer. However, screening for known effectors of other MarR regulators failed to identify the FabT ligand (35). FabT was found to bind a unique ligand, acyl-ACP, the FASII end product, to modulate FASII expression. FabT-(acyl-ACP) binds DNA with high affinity. This feedback regulation strategy contrasts with the feed-forward strategy used by other Firmicutes (see Identification of FabT as the FASII Regulator). However, it is not a simple feedback regulation. AcpA is encoded in the FASII main locus and acpA expression is controlled by FabT, implying that the excess of acyl-AcpA stops acpA synthesis. This in turn leads to lower production of acyl-AcpA-FabT production and subsequent derepression of the FASII genes. This regulation favors fine tuning of the available pools of Acyl-AcpA.
It is notable that FabT-(acyl-ACP) is not a unique molecule, and its binding to DNA targets varies according to the acyl moiety (32). While FabT reportedly has the same affinity for all acyl-ACPs, the affinity of the FabT-acyl-ACP interaction with DNA increases with the length of the acyl carbon chain, with a marked increase beyond 14 carbons in S. pneumoniae (35). Thus, the length of the acyl-ACP chain is essential for consolidating FabT binding to DNA. Also, FabT-DNA binding is greater when FabT is complexed with C18:1-ACP than with C16:0-ACP. This suggested that chain length, and/or saturation status of the bound fatty acid might determine FabT-DNA binding affinity. In E. faecalis, FabT-mediated repression of FA synthesis is similarly exacerbated by C16:1 and C18:1 (cis-9), but less so in C18:1 (trans-9) and even less so in C16:0 (36). This argues in the favor of cis unsaturated FA maximizing FabT-DNA binding affinity.
The corepression mechanism mediated by S. pneumoniae FabT and acyl-ACP was characterized by combining crystallographic, biochemical, and genetic approaches (32). One acyl-ACP molecule binds to each subunit of the FabT dimer. It was suggested that acyl-ACP more readily binds FabT than does free ACP and that the acyl-ACP-FabT complex provokes a change in FabT conformation favoring the interaction with DNA (32, 35). Evidence for this interaction was obtained by replacing FabT lysine 97 and ACP aspartate 50 by cysteine residues; protein interactions and DNA binding were observed in the absence but not in the presence of DTT (32). The acyl moiety binds directly within a hydrophobic pocket of FabT, favoring ACP interaction within the FabT α5 helix, leading to formation of a stable FabT-acyl-ACP complex (32). The length of the acyl moiety increases stability of the acyl-ACP-FabT complex; this makes physiological sense, as accumulation of full-length phospholipid precursors should arrest FASII.
An increased concentration of 18:1-ACP during electrophoretic mobility shift assays performed with a fixed concentration of FabT protein led to a more intense FabT-DNA band (35). Possibly, the acyl-ACP cofactor could be a limiting factor for interactions between FabT-(acyl-ACP) and DNA in the bacterium (32). This would explain the modest difference in repression of FASII genes in L. lactis when FabT was overproduced 135-fold (19).
Why are there two ACPs in some species?
Numerous species of the order Lactobacillales, which comprise the Streptococcaceae, possess two ACPs. AcpA is encoded within the FASII locus; AcpB is encoded in the same operon as PlsX (glycerol-3-phosphate acyltransferase), required for phospholipid synthesis (Fig. 1B) (14, 32, 37). E. faecalis and S. pneumoniae encode the second ACP, AcpB, while L. lactis does not. AcpB is also found in C. acetobutylicum in the same operon as plsX (unpublished result). AcpA and AcpB share approximately 40%, 24%, 30%, and 45% sequence identity in S. pneumoniae, S. pyogenes, E. faecalis, and C. acetobutylicum, respectively (unpublished results; references 14, 32, 37). A phylogenetic tree based on multialignment comparison of AcpA and AcpB shows clustering of the four AcpA proteins but not of the four AcpBs. In C. acetobutylicum, AcpB is more closely related to the AcpA proteins than to the other AcpB proteins (Fig. 2C). Closer homology between AcpA proteins suggests that it may have more functional constraints than AcpB. Multialignment comparison of AcpB sequences highlights clusters of similarity (Fig. 2D). However, BLASTP analysis indicates that AcpB is absent or severely truncated in over half of sequenced S. agalactiae strains available in NCBI (unpublished results). These observations raise questions about the need for a second acyl carrier protein in some species.
Distinguishing features of AcpA and AcpB with respect to FASII and to interactions with FabT were investigated in E. faecalis (14). Whereas the acpA gene is essential, acpB is not. Furthermore, the acpB mutant grows without exogenous FA supplementation, unlike FASII mutants (13). AcpA and AcpB involvement in FA synthesis was assessed by studying their interactions with FabI and FabK enoyl-ACP reductases, which catalyze the last step in FASII synthesis to produce acyl-ACP (38) (Fig. 1A). FabI was reportedly more effective than FabK in reducing the acyl-AcpA precursor; conversely, FabK reduced the acyl-AcpB precursor more effectively than FabI (14). However, interactions of AcpA and AcpB were not studied in streptococci, which possess only FabK. These results suggested that FabI and AcpA are main actors in FA de novo synthesis in E. faecalis, whereas the role of AcpB in FA synthesis remains in question. To define the involvement of AcpA and AcpB in the phospholipid synthesis step, the authors then followed the activity of PlsX, which catalyzes reversible transfer of the acyl moiety between ACP and a phosphate group. Acyl transfer from AcpA proved much more efficient than from AcpB. In contrast, in the presence of exogenous FAs and PlsX, acyl transfer to AcpB (the reverse reaction) was the more efficient. This indicated that acyl-AcpA is the preferred PlsX substrate to synthesize acyl-phosphate, which then initiates phospholipid production via PlsY. In contrast, free AcpB would be a PlsX substrate, which produces Acyl-AcpB in the reverse reaction. Acyl-AcpB would then act as corepressor of FabT, signaling availability of the exogenous FA sources. Acyl-AcpB could additionally be a precursor for phospholipid synthesis that favors entry of exogenous fatty acids (14) Altogether, this suggested that AcpA is the main FASII enzyme needed for FA de novo synthesis, but may also regulate FASII repression in the presence of exogenous FA; AcpB, which is encoded in the same operon as plsX and is nonessential, may only have a role in facilitating exogenous FA utilization for phospholipid synthesis (14, 36). However, part of these conclusions were recently revised (36; see below).
Although some features of AcpB have been elucidated, our understanding of its role in fatty acid metabolism remains incomplete: a comparison of membrane FA composition and FASII gene repression in the presence of exogenous FAs indicated that acpB and fabT mutant strains had the same phenotypes, suggesting that AcpB is a corepressor of FabT (14). In keeping with this observation, acyl-AcpB, but not AcpB alone, increased FabT-(acyl-ACP) binding to DNA in S. pneumoniae (32). In silico comparison of AcpB sequences shows that amino acid residues involved in interactions with FabT are only partially conserved in S. pyogenes. AcpB Asp50 interacts with the FabT Lys97; however, the spatially adjacent AcpB Glu55 is not conserved, which may weaken the interaction (Fig. 2D) (unpublished results); reference (32). Moreover, AcpB is absent in about half the S. agalactiae strains and in L. lactis. Consistent with the above observations, a recent report clarified that the role of AcpB in controlling FabT repression was minor, compared to earlier conclusions that the acpB mutant exerted a dominant effect on FabT repression; a secondary mutation in fabT was identified, which accounted for this revised explanation (14, 36). In a reconstructed E. faecalis acpB deletion strain, addition of exogenous FA showed just partial repression of fabT promoter expression (36). Also, and in contrast to the situation in S. pneumoniae, in E. faecalis, acyl-AcpA enhances FabT binding to the fabT promoter in vitro (32, 36). This confirms that in E. faecalis, AcpA is also a FabT corepressor. Altogether, these results suggest that AcpA and AcpB as studied in E. faecalis and S. pneumoniae do not have totally distinct functions and that their properties may not be conserved in all related strains and species (14). The differential roles of the two ACP proteins merit further study.
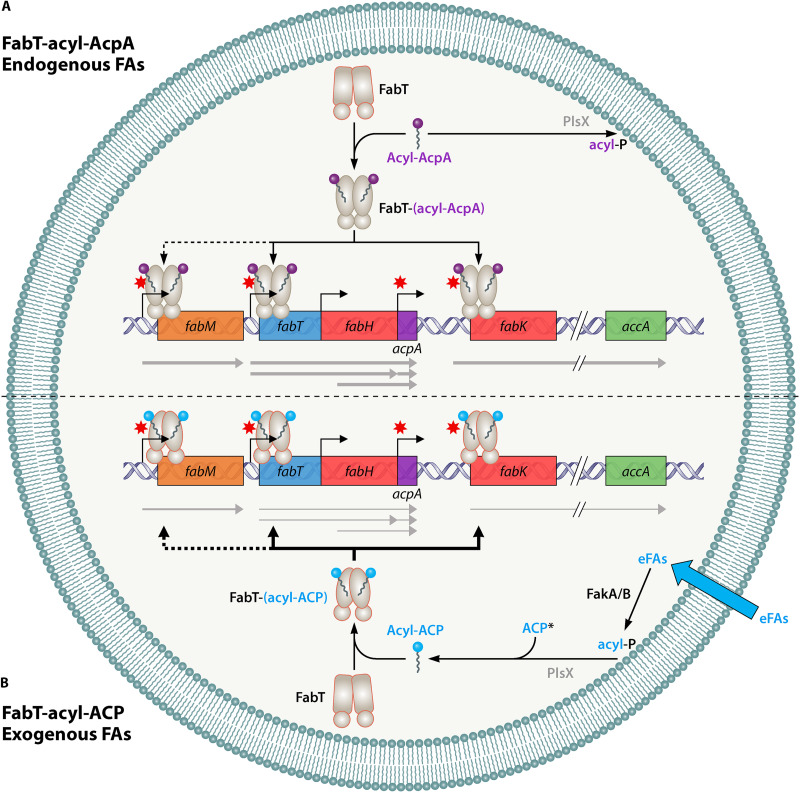
Since AcpB is constitutively synthesized in exponential growth (10, 12, 21), the concentration of acyl-AcpB and consequently its repression efficiency may depend primarily on the amounts and composition of exogenous FAs. A regulatory model was proposed based on the above considerations (Fig. 3). It is likely that the repressive effects of exogenous FA on FASII gene transcription, as we previously described (11, 13), are carried out at least in part via AcpB.
FIG 3.
Model of the FabT repression mechanism in S. pneumoniae and E. faecalis. (A) Endogenous fatty acid (FA) synthesis: an excess of FA synthesis generates acyl-AcpA, which binds to FabT; the complex binds with low affinity to the promoter regions of its target transcriptional units, leading to weak repression of FabT directly controlled genes, including acpA. Acyl-AcpA is also a substrate for the bidirectional glycerol-3-phosphate acyltransferase PlsX, leading to phospholipid synthesis (not shown). The minor apparent regulatory role of FabT when interacting with acyl-AcpA contrasts with its prominent role when exogenous FAs are present, e.g., as is common in vivo. (B) Exogenous FA utilization: entering FAs may be phosphorylated by fatty acid kinase complex FakA coupled to one of the FA-binding FakB subunits, each of which has FA preferences (22). Acyl-P is then converted to acyl-AcpB or AcpA (36) by a reverse PlsX reaction. Acyl-ACP binds to FabT, and the complex binds with high affinity to the promoter regions of its transcriptional unit targets, strongly repressing gene expression. The information contained in this figure corresponds to results shown in at least one species. Gray arrows under the genes represent the various transcriptional units described; their thickness symbolizes the level of transcription. Black arrows above or below the genes represent the binding of acyl-Acp to promoter regions; their thickness indicates repression efficiency; dotted arrows indicate that the repression level is species dependent. Note that only FabT-related functions are indicated. eFAs: exogenous FAs; acyl-P, acyl-phosphate. Note that acyl-AcpA is the FASII product; both acyl-AcpB and acyl-AcpA appear to be involved in eFA incorporation and reverse activity of PlsX.
REGULATION OF FABT TRANSCRIPTION AND FABT ACTIVITY
FabT regulates FASII synthesis and, concomitantly, exogenous FA utilization and phospholipid synthesis. To understand how this regulon responds to bacterial metabolism, we must first know how FabT itself is regulated.
In S. mutans, in-depth analysis of genes affected by deletion of fabT suggested that catabolite control protein CcpA, a master regulator of catabolic repression in Gram-positive bacteria, also controls their expression. CcpA DNA binding motifs were identified upstream of different fab genes and confirmed to be active. Importantly, fabT is regulated by CcpA (20). By controlling fabT expression, CcpA links carbon and FA metabolism. Whether CcpA exerts similar roles in fabT regulation in species related to S. mutans remains to be confirmed.
In S. pneumoniae, fabT expression is regulated by a two-component system called VicKR (also known as WalKR), which responds to parietal stress. Upon membrane stress, the VicK kinase autophosphorylates and then phosphorylates the VicR regulator, leading to repression of VicRK regulon genes within 10 min (39, 40). In the absence of parietal stress, the nonphosphorylated VicR regulator binds to the fabT promoter region, thus directly repressing its transcription (41). fabT repression in the nonstress state leads to upregulation of the fabK operon, which results in longer FA chains (39). These findings indicate that bacterial membranes would comprise a higher proportion of long-chain FAs in the absence than in the presence of parietal stress; this regulation would contribute to bacterial adaptation to each condition. This adjustment of membrane composition at the fabT transcriptional level may bypass the need for FASII regulation via (acyl-Acp)-FabT during parietal stress.
In S. pyogenes, Stk is the kinase of a two-component histidine kinase/regulator system (33). Transcriptomic analysis of the stk deleted strain suggested that Stk indirectly activates expression of FASII pathway genes. Two hypotheses were proposed. The simplest is that Stk phosphorylates FabT, preventing it from exerting its role as a transcriptional repressor. The second postulates that Stk phosphorylates FASII enzymes and that a loss of function leads to feedback activation of FASII gene expression. Kinases may have important roles in modulating FASII activity that warrant further studies.
Taken together, FabT is subject to various selective pressures and types of regulation, which seem to differ according to the Streptococcus species. Whether regulatory controls on fabT cross species boundaries remains to be tested. However, these reports underscore the importance of FabT in coordinating FASII-mediated membrane biogenesis with other metabolic processes to optimize bacterial fitness.
FABT AND BACTERIAL PHYSIOLOGY
Invasive S. pyogenes infections cause >150,000 deaths/year, thus calling for new treatment targets and strategies. We discussed above that emergence of fabT variants correlated with noninvasive infections (see Identification of FabT as the FASII Regulator). Mutations in fabT may thus be counterselected in invasive infections due to lower virulence of these mutants. Assessing the consequences of mutating fabT on bacterial physiology and on potential penalties and/or benefits during infection could give insight into the use of FabT as a therapeutic target to reduce severity of infection.
The extent to which FabT affects bacterial survival in vitro varies with the species and possibly with test conditions. In E. faecalis and L. lactis, fabT deleted strains are viable, although the E. faecalis mutant displays a growth delay (14, 19). An S. pneumoniae fabT mutant containing a stop codon early in the orf is viable, with no growth defects reported in rich medium (12). In contrast, construction of S. pyogenes fabT deletion mutants, as attempted in three separate studies, suggested that fabT has an important role: fabT mutants could not be obtained without deleting clpX (a protease ATP-binding subunit) or gdpP (encoding a c-di-AMP phosphodiesterase) (21). In a second study, a S. pyogenes fabT deleted strain displayed highly altered growth: longer latency time, slower growth and lower bacterial yield in rich medium (8). Growth was restored in medium supplemented with unsaturated FAs (cis-vaccenic acid, C18:1, or cis-eicosenoic acid, C20:1). Unlike fabT deletion mutants, fabT point mutants were obtained with no effects on in vitro growth. In a third study, a S. pyogenes fabT deletion mutant displayed no major doubling time defect but did show a longer latency period (10). All the S. pyogenes constructs respected the integrity of fabH, the gene directly downstream of fabT (Fig. 1B); hence, the discrepancies between reports can best be explained by wild-type strain differences and/or variations in test conditions. Despite these phenotypic differences, a common feature of the fabT point mutant and deleted strains was that both displayed attenuated virulence (8, 10).
Since FabT regulates the quasi-ensemble of FASII pathway genes (see The FabT Regulon), its inactivation should have direct consequences on membrane FA composition. In S. pneumoniae and L. lactis, the proportion of long-chain FAs is higher in the fabT mutant than in the wild-type strain, in keeping with derepression of FASII (12, 19). A greater proportion of saturated FAs was also reported. Interestingly, fabT inactivation leads to greater upregulation of fabK than fabM. FabM (required for unsaturated FA synthesis) and FabK compete for the same substrate (Fig. 1A); the relative increase in FabK expression compared to FabM accounts for this ratio variation. Moreover, in S. pneumoniae, FabT also controls phospholipid synthesis gene plsC (see The FabT Regulon). These observations are consistent with findings described above, that fabT repression results in longer FA chains (39) (see Regulation of FabT Transcription and FabT Activity).
Membrane permeability studies were performed to determine how FabT mutation-induced alterations of FA ratios impact membrane properties. Measurements of ethidium bromide incorporation in L. lactis indicated that permeability of the fabT membrane is greater than that of the wild-type strain (19). Membrane rigidity is known to depend in part on the ratio of saturated/unsaturated FAs. Different ratios in wild-type and fabT strains can explain the reported differences in membrane permeability.
Osmotic homeostasis is critical for all cells, and membrane FA composition is a key determinant of bacterial survival under osmotic pressure. Interestingly, fabT mutations arose when S. agalactiae was submitted to osmotic stress conditions: in that work, strains were grown in a minimal medium supplemented with 0.5 mM potassium (42). One could speculate that higher relative amounts of saturated FAs in the fabT mutant and consequent modified permeability may be responsible for this natural selection. Alternatively, but not exclusively, altered membrane protein composition may contribute to the response to potassium excess (see below).
Modifying membrane composition by environmental FAs that are not normally present may affect bacterial growth or survival. For example, ~20 μg/mL free linoleic acid (C18:2), a main FA constituent of soy bean oil, inhibits growth of lactobacilli. Increased consumption of soybeans in the USA and Europe was suggested to correlate with a decrease of L. reuteri and Lactobacillus johnsonii prevalence in the human intestinal microbiota (43). Di Rienzi et al. (43) considered a possible link between this decrease and enriched linoleic acid in the diet. Isolation of linoleic acid-resistant L. reuteri mutants identified mutations in fabT. In hindsight, the selection of fabT mutations would lead to increased proportions of membrane saturated FAs, which could equilibrate FA composition in linoleic-acid-rich environments.
During a search for a narrow-spectrum antibiotic, the alkylated dicyclohexyl carboxylic acid 2CCA-1, a polyunsaturated FA mimetic, was identified as targeting S. pneumoniae. Investigation of its bactericidal effect led to the isolation of resistant mutants (23). One mutation mapped to fakB3, encoding a FA binding protein involved in the uptake of polyunsaturated FAs. The second mapped to fabT. fakB3 is less expressed in the fabT mutant than in the wild-type strain. Consequently, in both mutant strains, 2CCA-1 incorporation would be reduced, rendering the strain resistant to the FA analog.
S. pyogenes strains were selected for resistance to polymyxin B, an antibiotic targeting anionic lipids (21). Sequencing showed that fabT point mutants displayed high polymyxin B resistance. In line with this result, fabT deleted strains are more resistant to polymyxin B (10, 19). Thus, changes in FA synthesis associated with fabT mutations may alter lipid composition, with consequences on antibiotic resistance, particularly to antibiotics targeting membrane constituents.
Deletion of fabT impacts S. pneumoniae adaptation to different known stresses (pH, reactive oxygen species [ROS], and detergents) (12). The fabT mutant proved to be more sensitive than the wild-type strain to acidic pH and detergents such as deoxycholate, suggesting that FabT may have specific roles in bacterial adaptation to these conditions. Resistance to ampicillin, vancomycin, and ROS was unchanged. Mutations in the FabT partner AcpB would expectedly give similar effects as the fabT mutant, and could further strengthen these findings.
Membrane modifications may also affect structure, function, and abundance of membrane, parietal, and secreted proteins. Indeed, some membrane protein complexes, such as the general secretion system Sec or sortases, are involved in the secretion and/or surface-anchoring of numerous proteins (44). Changes in phospholipid characteristics could affect both expression levels and activities of membrane proteins, and thereby impact bacterial adaptation to environmental changes. Studies based on exogenous FA incorporation capacities of E. faecalis confirm that disturbance of the membrane FA ratio modifies bacterial morphology, growth, and adaptation capacities (45, 46). Current studies in our laboratory will monitor the effects of S. pyogenes fabT mutations (which alter the membrane FA ratio) on protein expression and bacterial fitness. This study might help explain the emergence of mutations under some stress conditions.
Studies thus far show that FASII deregulation when FabT is mutated has direct and indirect consequences on membrane composition and modifies bacterial adaptation to its environment. Selective advantages driven by fabT mutations during noninvasive infection remain to be assessed. Accumulation of pathogen mutations during infection raises the possibility that fabT revertants might be selected because they restore bacterial invasive capacity.
CONCLUSIONS
The emergence of multiresistant bacteria and growing awareness of the need to preserve the microbiota have spurred development of therapeutic targets specific to bacterial pathogens. FASII was thought to be essential for bacterial survival, which drove the development of numerous FASII inhibitors. The understanding that Gram-positive bacteria, such as S. aureus, E. faecalis, and streptococci, incorporate exogenous FAs that allow these bacteria to bypass FASII inhibition has modified the rationale of monotherapy, as anti-FASII reaches its targets but fails to block streptococcal, staphylococcal, and enterococcal growth in FA-rich host environments (11, 13, 47–50). Thus, anti-FASII are ineffective as stand-alone in vivo antibacterial agents against these bacteria.
Here we summarized the roles of FabT as a major regulator that acts as repressor in the presence of long chain acyl-ACP. Whether acyl-ACP comprises AcpA or AcpB, which are stakeholders of a feedback or an exogenous FA-sensing mechanism, respectively, FabT-acyl-Acp-mediated repression limits futile FASII activity. FabT mutants arise spontaneously when bacteria undergo osmotic stress and seem to emerge in noninvasive infections, suggesting that fabT mutations may improve bacterial fitness in certain conditions. Since FabT controls FA length and the saturated to unsaturated FA ratio, the state of FabT is a key factor in bacterial fitness and adaptation. Development of FabT inhibitors, such as FAs leading to acyl-Acp toxic analogues, could pave the way for new narrow-spectrum therapeutic tools.
ACKNOWLEDGMENTS
We thank A. Grove for permission to reproduce a figure from her article (30).
Research of authors on the topic of this review was funded by the following French granting agencies: the regional DIM-Malinf, DIM-OneHealth Projet 00002247, the Agence Nationale de la Recherche (StaphEscape project ANR-16-CE15-0013), and the Fondation pour la Recherche Medicale (DBF20161136769), the Université de Paris (to C.L.).
Biographies

Clara Lambert is a fourth-year PhD student in Pr. Claire Poyart and Dr. Agnès Fouet’s laboratory at Institut Cochin, Paris, France. She received her bachelor’s degree in biology from Université Paris Saclay in 2016 and in 2018 her master’s in fundamental microbiology from Université Paris Cité in collaboration with Institut Pasteur. She is currently investigating the role of FabT in Streptococcus pyogenes invasive infections. She characterizes the link between the deregulation of fatty acid synthesis and the virulence defect in S. pyogenes. Using various approaches, her project provides a holistic view of the consequences of FabT mutations on bacterial environmental adaptation. She has expertise in bacterial physiology, omic techniques such as transcriptomic, proteomic, lipidomic and metabolomic, and in vivo and ex vivo analysis of bacteria-host interaction. She will defend her thesis in December 2022. After graduating, she would like to investigate the importance of the bacterial cell-wall in bacterial adaptation.

Claire Poyart, MD, PhD, studied medicine at the Universities Pierre&Marie Curie and Paris Descartes and her PhD at Institut Pasteur. In 2003, nominated Professor at Hospital Cochin, she heads the Clinical bacteriological laboratory. Since 2006, she heads the National Reference Center for Streptococci and codirects an INSERM Team at the Institut Cochin affiliated to Université Paris Cité. Her research combines clinical and fundamental approaches towards characterizing the virulence factors of group A and B Streptococcus. The main fundamental research topics are: surface proteins, environmental adaptation and expression of virulence genes, genetic variability, and virulence. She showed, together with Alexandra Gruss, that fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. As a clinical microbiologist her clinical research aims at the epidemiological characterization of GAS and GBS strains responsible for invasive infections and colonization, the development of diagnostic tools for the detection, and the identification of Gram-positive bacteria.

Alexandra Gruss obtained her PhD in Microbiology from the New York University Medical Center, where she worked on plasmid replication determinants in Staphylococcus aureus. She then established her lab at the French National Institute for Agriculture, Food and Environment (INRAE, Jouy en Josas). Her research team identified the recombination “crossover hotspot instigator” motif Chi in various Gram-positive bacteria; it also isolated thermosensitive plasmids currently used for genetic manipulation. Her subsequent research on dairy bacteria uncovered that exogenous heme activated respiratory metabolism, greatly improving lactococcal biomass and stability; this fundamental observation was successfully applied to industrial starter culture production. Fatty acid utilization has been a research focus of authors’ labs since 2006. A major finding was that environmental fatty acids, as used by essentially all Firmicutes, can overcome antibiotics targeting the fatty acid synthesis (FASII) pathway. A current focus is on combinatorial therapy approaches that block S. aureus development.

Agnes Fouet obtained her PhD at the Pasteur Institute, Paris, on the regulation of genes encoding proteins involved in sucrose transport and catabolism in Bacillus subtilis. She then moved to Tufts Medical School, Boston, as a post-doc where she studied the relationship between catabolic repression and repression of sporulation initiation in B. subtilis. In 1990, she established her group at the Pasteur Institute, where her research focused on surface components and regulation of synthesis of virulence factors in Bacillus anthracis. In 2011, she moved to the Institut Cochin, Paris, and initiated an entirely new project, investigating the mechanisms involved in initial steps of Streptococcus pyogenes infection. In this context, she uncovered a pivotal role of fatty acid metabolism in S. pyogenes-host cell interaction. She collaborates with Alexandra Gruss at the Micalis Institute, Jouy en Josas, France, on fatty acid metabolism in other Firmicutes, including Enterococcus faecalis.
Contributor Information
Alexandra Gruss, Email: alexandra.gruss@inrae.fr.
Agnes Fouet, Email: agnes.fouet@inserm.fr.
REFERENCES
- 1.Zhang YM, Rock CO. 2008. Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233. 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 2.Grogan DW, Cronan JE, Jr.. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev 61:429–441. 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osterman A, Overbeek R. 2003. Missing genes in metabolic pathways: a comparative genomics approach. Curr Opin Chem Biol 7:238–251. 10.1016/S1367-5931(03)00027-9. [DOI] [PubMed] [Google Scholar]
- 4.Cronan JE. 2020. The Escherichia coli FadR transcription factor: too much of a good thing? Mol Microbiol 15:1080–1085. 10.1111/mmi.14663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YM, Rock CO. 2009. Transcriptional regulation in bacterial membrane lipid synthesis. J Lipid Res 50 Suppl:S115–S119. 10.1194/jlr.R800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham MW. 2000. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13:470–511. 10.1128/CMR.13.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carapetis JR, Steer AC, Mulholland EK, Weber M. 2005. The global burden of group A streptococcal diseases. Lancet Infect Dis 5:685–694. 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 8.Tatsuno I, Okada R, Matsumoto M, Hata N, Matsui H, Zhang Y, Isaka M, Hasegawa T. 2016. Relevance of spontaneous fabT mutations to a streptococcal toxic shock syndrome to non-streptococcal toxic shock syndrome transition in the novel-type Streptococcus pyogenes isolates that lost a salRK. APMIS 124:414–424. 10.1111/apm.12521. [DOI] [PubMed] [Google Scholar]
- 9.Nasser W, Beres SB, Olsen RJ, Dean MA, Rice KA, Long SW, Kristinsson KG, Gottfredsson M, Vuopio J, Raisanen K, Caugant DA, Steinbakk M, Low DE, McGeer A, Darenberg J, Henriques-Normark B, Van Beneden CA, Hoffmann S, Musser JM. 2014. Evolutionary pathway to increased virulence and epidemic group A Streptococcus disease derived from 3,615 genome sequences. Proc Natl Acad Sci USA 111:E1768–76. 10.1073/pnas.1403138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraso JM, Olsen RJ, Beres SB, Kachroo P, Porter AR, Nasser W, Bernard PE, DeLeo FR, Musser JM. 2016. Genomic landscape of intrahost variation in group A Streptococcus: repeated and abundant mutational inactivation of the fabT gene encoding a regulator of fatty acid synthesis. Infect Immun 84:3268–3281. 10.1128/IAI.00608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinster S, Lamberet G, Staels B, Trieu-Cuot P, Gruss A, Poyart C. 2009. Type II fatty acid synthesis is not a suitable antibiotic target for Gram-positive pathogens. Nature 458:83–86. 10.1038/nature07772. [DOI] [PubMed] [Google Scholar]
- 12.Lu YJ, Rock CO. 2006. Transcriptional regulation of fatty acid biosynthesis in Streptococcus pneumoniae. Mol Microbiol 59:551–566. 10.1111/j.1365-2958.2005.04951.x. [DOI] [PubMed] [Google Scholar]
- 13.Hays C, Lambert C, Brinster S, Lamberet G, Du Merle L, Gloux K, Gruss A, Poyart C, Fouet A. 2021. Type II fatty acid synthesis pathway and cyclopropane ring formation are dispensable during Enterococcus faecalis systemic infection. J Bacteriol 203:e0022121. 10.1128/JB.00221-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu L, Zou Q, Cao X, Cronan JE. 2019. Enterococcus faecalis encodes an atypical auxiliary acyl carrier protein required for efficient regulation of fatty acid synthesis by exogenous fatty acids. mBio 10:e00577-19. 10.1128/mBio.00577-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosinski-Chupin I, Sauvage E, Fouet A, Poyart C, Glaser P. 2019. Conserved and specific features of Streptococcus pyogenes and Streptococcus agalactiae transcriptional landscapes. BMC Genomics 20:236. 10.1186/s12864-019-5613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marrakchi H, Choi KH, Rock CO. 2002. A new mechanism for anaerobic unsaturated fatty acid formation in Streptococcus pneumoniae. J Biol Chem 277:44809–44816. 10.1074/jbc.M208920200. [DOI] [PubMed] [Google Scholar]
- 17.Albanesi D, Reh G, Guerin ME, Schaeffer F, Debarbouille M, Buschiazzo A, Schujman GE, de Mendoza D, Alzari PM. 2013. Structural basis for feed-forward transcriptional regulation of membrane lipid homeostasis in Staphylococcus aureus. PLoS Pathog 9:e1003108. 10.1371/journal.ppat.1003108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez MA, Zaballa ME, Schaeffer F, Bellinzoni M, Albanesi D, Schujman GE, Vila AJ, Alzari PM, de Mendoza D. 2010. A novel role of malonyl-ACP in lipid homeostasis. Biochemistry 49:3161–3167. 10.1021/bi100136n. [DOI] [PubMed] [Google Scholar]
- 19.Eckhardt TH, Skotnicka D, Kok J, Kuipers OP. 2013. Transcriptional regulation of fatty acid biosynthesis in Lactococcus lactis. J Bacteriol 195:1081–1089. 10.1128/JB.02043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faustoferri RC, Hubbard CJ, Santiago B, Buckley AA, Seifert TB, Quivey RG Jr, 2015. Regulation of fatty acid biosynthesis by the global regulator CcpA and the local regulator FabT in Streptococcus mutans. Mol Oral Microbiol 30:128–146. 10.1111/omi.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Port GC, Vega LA, Nylander AB, Caparon MG. 2014. Streptococcus pyogenes polymyxin B-resistant mutants display enhanced ExPortal integrity. J Bacteriol 196:2563–2577. 10.1128/JB.01596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gullett JM, Cuypers MG, Frank MW, White SW, Rock CO. 2019. A fatty acid-binding protein of Streptococcus pneumoniae facilitates the acquisition of host polyunsaturated fatty acids. J Biol Chem 294:16416–16428. 10.1074/jbc.RA119.010659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reithuber E, Nannapaneni P, Rzhepishevska O, Lindgren AEG, Ilchenko O, Normark S, Almqvist F, Henriques-Normark B, Mellroth P. 2020. The bactericidal fatty acid mimetic 2CCA-1 selectively targets pneumococcal extracellular polyunsaturated fatty acid metabolism. mBio 11:e03027-20. 10.1128/mBio.03027-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paoletti L, Lu YJ, Schujman GE, de Mendoza D, Rock CO. 2007. Coupling of fatty acid and phospholipid synthesis in Bacillus subtilis. J Bacteriol 189:5816–5824. 10.1128/JB.00602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathania A, Anba-Mondoloni J, Gominet M, Halpern D, Dairou J, Dupont L, Lamberet G, Trieu-Cuot P, Gloux K, Gruss A. 2021. (p)ppGpp/GTP and malonyl-CoA modulate Staphylococcus aureus adaptation to FASII antibiotics and provide a basis for synergistic bi-therapy. mBio 12:e03193-20. 10.1128/mBio.03193-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois-Brissonnet F, Naitali M, Mafu AA, Briandet R. 2011. Induction of fatty acid composition modifications and tolerance to biocides in Salmonella enterica serovar Typhimurium by plant-derived terpenes. Appl Environ Microbiol 77:906–910. 10.1128/AEM.01480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juneja VK, Foglia TA, Marmer BS. 1998. Heat resistance and fatty acid composition of Listeria monocytogenes: effect of pH, acidulant, and growth temperature. J Food Prot 61:683–687. 10.4315/0362-028X-61.6.683. [DOI] [PubMed] [Google Scholar]
- 28.Dubois-Brissonnet F, Trotier E, Briandet R. 2016. The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front Microbiol 7:1673. 10.3389/fmicb.2016.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiwari KB, Gatto C, Wilkinson BJ. 2018. Interrelationships between fatty acid composition, staphyloxanthin content, fluidity, and carbon flow in the Staphylococcus aureus membrane. Molecules 23:1201. 10.3390/molecules23051201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Pande A, Sabrin A, Thapa SS, Gioe BW, Grove A. 2019. MarR Family transcription factors from Burkholderia species: hidden clues to control of virulence-associated genes. Microbiol Mol Biol Rev 83:e00039-18. 10.1128/MMBR.00039-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deochand DK, Grove A. 2017. MarR family transcription factors: dynamic variations on a common scaffold. Crit Rev Biochem Mol Biol 52:595–613. 10.1080/10409238.2017.1344612. [DOI] [PubMed] [Google Scholar]
- 32.Zuo G, Chen ZP, Jiang YL, Zhu Z, Ding C, Zhang Z, Chen Y, Zhou CZ, Li Q. 2019. Structural insights into repression of the Pneumococcal fatty acid synthesis pathway by repressor FabT and co-repressor acyl-ACP. FEBS Lett 593:2730–2741. 10.1002/1873-3468.13534. [DOI] [PubMed] [Google Scholar]
- 33.Bugrysheva J, Froehlich BJ, Freiberg JA, Scott JR. 2011. Serine/threonine protein kinase Stk is required for virulence, stress response, and penicillin tolerance in Streptococcus pyogenes. Infect Immun 79:4201–4209. 10.1128/IAI.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu YJ, White SW, Rock CO. 2005. Domain swapping between Enterococcus faecalis FabN and FabZ proteins localizes the structural determinants for isomerase activity. J Biol Chem 280:30342–30348. 10.1074/jbc.M504637200. [DOI] [PubMed] [Google Scholar]
- 35.Jerga A, Rock CO. 2009. Acyl-Acyl carrier protein regulates transcription of fatty acid biosynthetic genes via the FabT repressor in Streptococcus pneumoniae. J Biol Chem 284:15364–15368. 10.1074/jbc.C109.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou Q, Dong H, Zhu L, Cronan JE. 2022. The Enterococcus faecalis FabT transcription factor regulates fatty acid synthesis in response to exogenous fatty acids. Front Microbiol 13:877582. 10.3389/fmicb.2022.877582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longo M, De Jode M, Plainvert C, Weckel A, Hua A, Chateau A, Glaser P, Poyart C, Fouet A. 2015. Complete genome sequence of Streptococcus pyogenes emm28 clinical isolate M28PF1, responsible for a puerperal fever. Genome Announc 3:e00750-15. 10.1128/genomeA.00750-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bi H, Zhu L, Wang H, Cronan JE. 2014. Inefficient translation renders the Enterococcus faecalis fabK enoyl-acyl carrier protein reductase phenotypically cryptic. J Bacteriol 196:170–179. 10.1128/JB.01148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohedano ML, Overweg K, de la Fuente A, Reuter M, Altabe S, Mulholland F, de Mendoza D, Lopez P, Wells JM. 2005. Evidence that the essential response regulator YycF in Streptococcus pneumoniae modulates expression of fatty acid biosynthesis genes and alters membrane composition. J Bacteriol 187:2357–2367. 10.1128/JB.187.7.2357-2367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wayne KJ, Li S, Kazmierczak KM, Tsui HC, Winkler ME. 2012. Involvement of WalK (VicK) phosphatase activity in setting WalR (VicR) response regulator phosphorylation level and limiting cross-talk in Streptococcus pneumoniae D39 cells. Mol Microbiol 86:645–660. 10.1111/mmi.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohedano ML, Amblar M, de la Fuente A, Wells JM, Lopez P. 2016. The response regulator YycF inhibits expression of the fatty acid biosynthesis repressor FabT in Streptococcus pneumoniae. Front Microbiol 7:1326. 10.3389/fmicb.2016.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devaux L, Sleiman D, Mazzuoli MV, Gominet M, Lanotte P, Trieu-Cuot P, Kaminski PA, Firon A. 2018. Cyclic di-AMP regulation of osmotic homeostasis is essential in Group B Streptococcus. PLoS Genet 14:e1007342. 10.1371/journal.pgen.1007342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Rienzi SC, Jacobson J, Kennedy EA, Bell ME, Shi Q, Waters JL, Lawrence P, Brenna JT, Britton RA, Walter J, Ley RE. 2018. Resilience of small intestinal beneficial bacteria to the toxicity of soybean oil fatty acids. Elife 7:e32581. 10.7554/eLife.32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dramsi S, Bierne H. 2017. Spatial organization of cell wall-anchored proteins at the surface of gram-positive bacteria. Curr Top Microbiol Immunol 404:177–201. 10.1007/82_2016_4. [DOI] [PubMed] [Google Scholar]
- 45.Saito HE, Harp JR, Fozo EM. 2014. Incorporation of exogenous fatty acids protects Enterococcus faecalis from membrane-damaging agents. Appl Environ Microbiol 80:6527–6538. 10.1128/AEM.02044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito HE, Harp JR, Fozo EM. 2018. Enterococcus faecalis responds to individual exogenous fatty acids independently of their degree of saturation or chain length. Appl Environ Microbiol 84:e01633-17. 10.1128/AEM.01633-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morvan C, Halpern D, Kenanian G, Hays C, Anba-Mondoloni J, Brinster S, Kennedy S, Trieu-Cuot P, Poyart C, Lamberet G, Gloux K, Gruss A. 2016. Environmental fatty acids enable emergence of infectious Staphylococcus aureus resistant to FASII-targeted antimicrobials. Nat Commun 7:12944. 10.1038/ncomms12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morvan C, Halpern D, Kenanian G, Pathania A, Anba-Mondoloni J, Lamberet G, Gruss A, Gloux K. 2017. The Staphylococcus aureus FASII bypass escape route from FASII inhibitors. Biochimie 141:40–46. 10.1016/j.biochi.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Gloux K, Guillemet M, Soler C, Morvan C, Halpern D, Pourcel C, Vu Thien H, Lamberet G, Gruss A. 2017. Clinical relevance of type II fatty acid synthesis bypass in Staphylococcus aureus. Antimicrob Agents Chemother 61:e02515-16. 10.1128/AAC.02515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenanian G, Morvan C, Weckel A, Pathania A, Anba-Mondoloni J, Halpern D, Gaillard M, Solgadi A, Dupont L, Henry C, Poyart C, Fouet A, Lamberet G, Gloux K, Gruss A. 2019. Permissive fatty acid incorporation promotes staphylococcal adaptation to fasii antibiotics in host environments. Cell Rep 29:3974–3982.e4. 10.1016/j.celrep.2019.11.071. [DOI] [PubMed] [Google Scholar]