SUMMARY
Convalescent plasma (CP) recurs as a frontline treatment in epidemics because it is available as soon as there are survivors. The COVID-19 pandemic represented the first large-scale opportunity to shed light on the mechanisms of action, safety, and efficacy of CP using modern evidence-based medicine approaches. Studies ranging from observational case series to randomized controlled trials (RCTs) have reported highly variable efficacy results for COVID-19 CP (CCP), resulting in uncertainty. We analyzed variables associated with efficacy, such as clinical settings, disease severity, CCP SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) antibody levels and function, dose, timing of administration (variously defined as time from onset of symptoms, molecular diagnosis, diagnosis of pneumonia, or hospitalization, or by serostatus), outcomes (defined as hospitalization, requirement for ventilation, clinical improvement, or mortality), CCP provenance and time for collection, and criteria for efficacy. The conflicting trial results, along with both recent WHO guidelines discouraging CCP usage and the recent expansion of the FDA emergency use authorization (EUA) to include outpatient use of CCP, create confusion for both clinicians and patients about the appropriate use of CCP. A review of 30 available RCTs demonstrated that signals of efficacy (including reductions in mortality) were more likely if the CCP neutralizing titer was >160 and the time to randomization was less than 9 days. The emergence of the Omicron variant also reminds us of the benefits of polyclonal antibody therapies, especially as a bridge to the development and availability of more specific therapies.
KEYWORDS: COVID-19, convalescent plasma, randomized clinical trial, propensity score-matched, neutralizing antibodies, viral neutralization tests
INTRODUCTION
In the first 21 years of the 21st century, humanity has experienced six major multinational epidemics. The agents involved were severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), influenza A(H1N1) virus, Ebola virus, Zika virus, and SARS-CoV-2. For the five most lethal of these outbreaks, the response included the use of convalescent plasma (CP) (reviewed in references 1 and 2), and it was considered for the less lethal sixth (Zika virus). The attraction of CP is that it is readily available as soon as there are convalescing survivors, that unlike drugs or monoclonal antibodies it needs no development, and it is polyclonal, affordable, and deployable even in resource-poor countries. Despite suffering from some logistical hurdles (dedicated collection, testing, and handling procedures, heterogeneity, standardization of the therapeutic dose, blood type matching, and intravenous delivery), CP has been proposed as a first-line response to new pandemics (3) and was deployed during the COVID-19 pandemic in March 2020 in countries that experienced the early waves of disease, such as China (4, 5) and Italy (6). The relatively low COVID-19 case-fatality rate (compared to the other epidemic agents noted above) allowed for testing of CP across a wider spectrum of disease severity.
While in early 2020 most clinical use was reported in case series or small phase II clinical trials (7), beginning in late March 2020, the U.S. expanded access program (EAP) generated a large and robust treatment data set, with insights into safety and optimal use. This database provided the first clear evidence that CP is safe, which was important given that early in the pandemic there were significant concerns about antibody-dependent enhancement (8). Later, an analysis of the first 3,082 patients within the EAP database provided evidence that associated early administration of high-titer COVID-19 CP (CCP) to nonventilated hospitalized patients with reduced mortality (9). Before the FDA granted emergency use authorization (EUA), the U.S. EAP provided CCP to as many as 94,287 patients.
During the past year, many studies employing either randomized control (RCTs) or propensity score-matched (PSM) controls have been published: as of 28 January 2022, PubMed, which is also indexing studies on the medRxiv prepublication server, had reported 30 RCTs and 13 PSM studies on CCP, and the ClinicalTrials.gov database had reported 24 more RCTs that were completed, active, or recruiting across several continents. RCTs and PSM studies reported so far have had largely opposite outcomes, with most but not all RCTs finding little overall effect on mortality and the PSM studies and many smaller trials reporting mortality benefits. Several RCTs did not have mortality as a primary endpoint or it was part of a composite endpoint (5, 10–12). These disparate results have led to confusion for both the public and clinicians, leading to reduced enthusiasm for the use of CP, in part because RCT data are more influential in affecting the opinion of many physicians, specialty societies, and government regulators.
As with any other medical treatment, several key factors should be taken into account when evaluating a trial, including the indication (which can be estimated by timing or clinical severity), the therapeutic dose, and the intended outcomes. The choices made by the trial designers determine whether the trial will demonstrate clinical benefit. While much attention is appropriately focused on the performance features of clinical trials (sample size, fidelity to randomization, appropriate analysis), the biological rationale for the hypothesis being tested is critically important but not always taken into account.
THE INDICATION
While it would be desirable to have a single drug that works at any disease stage, it was not reasonable to expect neutralizing antibody (NAb)-based treatments such as CCP to have a significant effect in later stages of disease. COVID-19 is now well defined as a disease with two stages, an initial viral phase characterized by flu-like and upper and lower respiratory symptoms, followed, in severe cases, by an inflammatory phase that is characterized by inflammation-driven damage to multiple organ systems, including the lungs, which can impair gas exchange and cause life-threatening hypoxia and damage to multiple organs, including the brain and blood vessels (13). Accordingly, lack of endogenous NAb at baseline has been associated with a higher risk of viremia, but Marconato et al. showed that CCP recipients benefit from high-titer CCP even after adjustment for their endogenous NAb (14). Specific intact antibodies in CCP are expected to neutralize SARS-CoV-2 in the intravascular system and, in some patients, prevent progression from early to severe and life-threatening disease (as seen in animal models [15–17]). However, this antiviral therapy cannot be expected to reverse the inflammatory phase of the disease nor neutralize infectious viruses invading the extravascular system. Thus, the impact of CCP in COVID-19 is similar to that seen in influenza, a disease in which antivirals are effective early in disease but have no effect in later stages when the symptomatology stems largely from the inflammatory response. The rationale for administering CCP as early as possible in the course of COVID-19 stems from the neutralization stoichiometry itself: the larger the number of actively replicating virions in the body, the higher the NAb dose needed for neutralization (18). Some uncontrolled studies have reported a lack of association between early intervention and outcomes (19, 20), but in these studies, the level of NAb or the overall anti-Spike antibody level in the infused CCP was unknown, leaving room for alternative explanations.
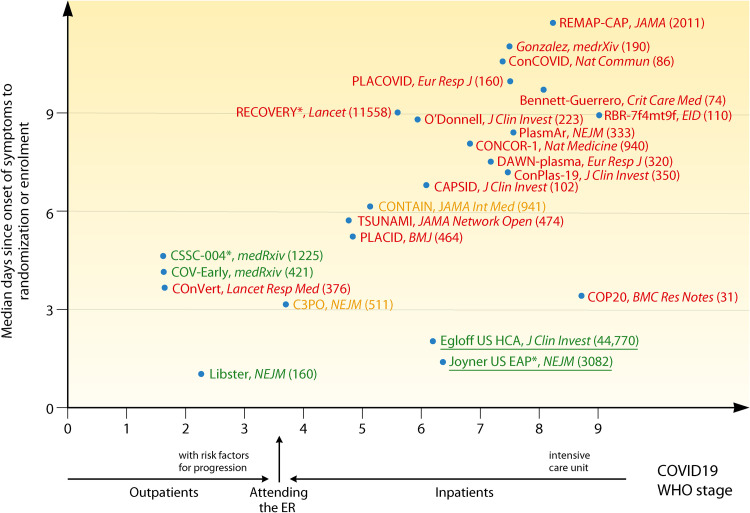
At the beginning of the pandemic, some investigators and opinion leaders, riding the wave of CCP successes in anecdotal reports in the media and small case series, introduced CCP to the general public as a panacea for any patient with COVID-19, including life-threatening cases, leading to confusing messaging: after reports of failure in severely ill patients emerged, opinions became polarized and the debate became anything but scientific (21). We use the word “failure” with care and considerable nuance, since negative trials can be very important in teaching us about populations that do not benefit from CCP or variables that affect its efficacy. In clinical trials, the indication (i.e., the baseline clinical setting) has been variously defined by patient status (outpatient versus presenting to the emergency room versus hospitalized versus intensive care unit [ICU] admitted), disease severity (using the 5-category COVID-19 Outpatient Ordinal Outcome Scale [22], a 6-category ordinal scale [12], a 7-category COVID-19 severity scale [23], the WHO 8-category [24]or 11-category [25]ordinal scale, or pneumological scores such as sequential organ failure assessment [SOFA]), the time elapsed before recruitment (also variably defined as from molecular diagnosis, from onset of hospitalization, from diagnosis of pneumonia, or from onset of symptoms), or by serological status (presence of antibodies or the ability to neutralize SARS-CoV-2). This variability in inclusion criteria for studies has resulted in marked heterogeneity in recruited patients. As shown by the CONTAIN trial (where those with shorter symptom durations did worse), symptom duration can be a poor indicator of “early” disease but may actually serve to indicate of severe, rapidly progressing disease (26). Disease severity marked by WHO score as opposed to symptom duration may be a more accurate tool for capturing “early” disease, as supported by the positive results with CCP in patients with low WHO scores, as summarized in Fig. 1.
FIG 1.
Simplified graphical representation of CCP RCTs and large uncontrolled trials reported to date, plotted according to earliness of intervention and disease severity, stratified according to the WHO 11-category ordinal scale (25) (0, uninfected; no viral RNA detected; 1, asymptomatic, viral RNA detected; 2, symptomatic, independent; 3, symptomatic, assistance needed; 4, hospitalized, no oxygen therapy; 5, hospitalized, oxygen by mask or nasal prongs; 6, hospitalized, oxygen by noninvasive ventilation (NIV) or high flow; 7, intubation and mechanical ventilation, partial pressure of oxygen/fraction of inspired oxygen (pO2/FiO2) ≥ 150 or oxygen saturation measured by pulse oximetry/fraction of inspired oxygen (SpO2/FiO2) ≥ 200; 8, mechanical ventilation, pO2/FiO2 < 150 [SpO2/FiO2 < 200] or vasopressors; 9, mechanical ventilation, pO2/FiO2 < 150, and vasopressors, dialysis, or extracorporeal membrane oxygenation [ECMO]). Green text indicates trials which met the primary endpoint with statistical significance; orange text indicates trials which failed to meet the primary endpoint but showed statistically nonsignificant trends in favor of CCP; red text indicates trials which failed to show and benefit from CCP in the primary endpoint. Sources cited in the figure are references 9–12, 26–30, 34–36, 41, 46, 48, 50, 52, 57, 73, 76, 77, 113, 144, and 145. Numbers in parentheses represent the number of recruited patients.
The vast majority of SARS-CoV-2 infections are mildly symptomatic, so when dealing with outpatients (WHO categories 1 to 3; the strata which are most likely to benefit from NAb-based therapies), the number needed to treat (NNTT) in order to prevent a single hospitalization or death can be very large and even larger if vaccinees are recruited. In order to be economically and logistically sustainable, it seems wise to focus on those outpatients having risk factors for disease progression, as was done with COVID19 monoclonal antibodies (MAbs). This approach was pursued by C3PO (27) and NCT04479163 but not other outpatient RCTs (CoV-Early [28], COnV-ert [29], CSSC-004 [30]). A special category is represented by outpatients recruited at the time of emergency room (ER) attendance (e.g., in C3PO [27]): they should be considered as at the border between outpatients and inpatients and hence not aggregated in outpatient meta-analyses. Among outpatients not recruited at the ER, clinical benefit has been shown up to 6 days since onset of symptoms (e.g., in the CSSC-004 RCT [30]). No benefit of CCP over fresh frozen plasma (FFP) has been proven in the single RCT of postexposure prophylaxis (CSSC-001) at preventing infection or symptomatic disease, but the study was not powered to detect a reduction in hospitalization (31).
For inpatients (WHO categories 4 to 9), clinical benefit from CCP is more likely for those in categories 4 and 5, since from category 6 on, the patients require high-flow oxygen and hence have significant respiratory failure.
THE THERAPEUTIC DOSE
Determining the effective dose of CCP is difficult in a pandemic because the antibody assays and other tests needed to assess the potency of any antibody product take time to be developed. In practice, the effective dose is the product of multiple factors, none of which is fully standardized.
The first factor is the concentration of the NAbs as measured by a viral neutralization test (VNT). At the beginning of the pandemic, only a few laboratories equipped for biosafety level 3 (BSL3) or higher studies could run a VNT using authentic live SARS-CoV-2 virus: the procedure was time-consuming (3 to 5 days), and the reports were operator dependent. Nowadays, the availability of Spike-pseudotyped viruses which can be managed under the more widely available BSL2 laboratories or cell-free ACE-2 competition assays, combined with automated (e.g., luminescence-based) readings, have standardized outcomes and shortened turnaround times (32): however, harmonization between different assays is still a work in progress (33). The VNT differs according to the type of replication-competent cell line, the viral isolate used for the challenge (which is critically important when the virus is mutating rapidly, as has been the case with emergence of variants of concern [VOCs]), the multiplicity of infection (i.e., the ratio between the viral inoculum—assessed with different measuring units—and the number of replication-competent cells within each well), the detection system (optic microscopy for cytopathic effect, immunostaining, quantitative PCR, or luminometer for engineered pseudoviruses), and finally, the threshold of neutralization (50% or 90%). The DAWN-plasma (34), C3PO (27), and REMAP-CAP (35) RCTs provide clear examples of such heterogeneity, with up to 4 different VNTs used at different participating laboratories/countries within the same study (see Table 1). It was not until August 2020, when many trials were already under way, that the FDA EUA 26382 defined high-titer CCP on the basis of correlation with a reference standard, the Broad Institute live-virus, 5-dilution VNT, as a 50% inhibitory dilution (ID50) of 1:250 or more (https://www.fda.gov/media/141481/download), and exclusive use of high-titer CCP was formally recommended by the FDA only on 9 March 2021. Table 1 summarizes the key variables in VNTs employed to date in CCP RCTs. Published trials have varied greatly in their approaches to antibody quantification, whether in measured transfused CCP units or in recipients. Several RCTs performed NAb titration but with highly heterogenous methods, which makes comparability of doses across studies difficult. Table 1 attempts to reconcile doses across those trials, suggesting that they actually differed more than is apparent by inspection of raw titers.
TABLE 1.
Details of VNTs employed in CCP RCTsa
| Virus | RCT (acronym/first author) | Cell line | No. of cells seeded/well | Virus lineage | Virus/well | MOI | Length of incubation | Assay readout | Threshold | Protocol reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Authentic live SARS-CoV-2 | NeuCoV-NET; NCT04393727 (TSUNAMI) | Vero E6 | 12,000 | SARS-CoV-2/human/ITA/PAVIA10734/2020 (D614G) | 100 TCID50 | 0.01 | Until the CPE became evident | CPE | Last serum dilution that inhibited SARS-CoV-2 CPE by 90% | 126 |
| NCT04433910 (cAPSID) | Vero E6 | NA | NA | 100 PFU | NA | 3 days | CPE | PRNT50 | 127 | |
| Broad Institute on a high-throughput platform (BROAD PRNT); part of NCT04355767 (C3PO) | Vero E6-TMPRSS2 | 10,000 | SARS-CoV-2 live virus (D614) | NA | NA | 48 h | N-protein ELISA | Samples whose curves lay above 0.5 for all the data points were considered nonneutralizing, with an ID50 of 20, while samples whose curves fell below 0.5 were considered highly neutralizing and assigned an ID50 of 10,240 | 128 | |
| NCT04359810 (O’Donnell) | Vero E6 | 10,000 | 2019-nCoV/USA-WA1-2020 | 100 TCID50 | 0.01 | 48 h | Triplex CII-SARS-CoV-2 rRT-PCR test, EUA200510 | The highest CCP dilution that prevented virus growth (CT) was rated as a neutralization titer | 11 | |
| NCT04348656 (cONCOR-1) | Vero E6 | NA | Canada/ON_ON-VIDO-01-2/2020; EPI_ISL_42517 | 50 PFU | NA | 72 h | CPE | PRNT50 | 123 | |
| NCT04342182 (ConCOVID) | Vero E6 | NA | German isolate (GISAID ID EPI_ISL 406862) | 400 PFU | NA | 8 h | CTL ImmunoSpot image analyzer | Reciprocal of the highest dilution resulting in a reduction of >50% of infected cells (PRNT50) | 36 | |
| NCT04429854 (DAWN-plasma) | Vero E6 | NA | BetaCov/Belgium/Sart-Tilman/2020/1 | 100 TCID50 | NA | 5 days | CPE | PRNT50 | 129 | |
| 18,000 | 2019-nCoV-Italy-INMI1 | 3 TCID50 | NA | 5 days | CPE | PRNT50 | 130 | |||
| NA | Belgium/GHB‐03021/2020 | 400 PFU | NA | 4 days | CPE | PRNT50 | 131 | |||
| 20,000 | Belgium/S1871/2020 | 100 TCID50 | NA | 2 days | Anti-N staining | NT50 | 132, 133 | |||
| Australian part of NCT02735707 (REMAP-CAP) | Vero E6 | 20,000 | hCoV/Australia/VIC01/202011 and hCoV/Australia/VIC2089/2020 | 200 TCID50 | 0.01 | 3 days | CPE | NA | 134 | |
| Canadian part of NCT02735707 (REMAP-CAP) | Vero E6 | NA | Canada/ON_ON-VIDO-01-2/2020 | 50 PFU | NA | 3 days | CPE | NA | 135 | |
| RBR-7f4mt9f | Vero (CCL-81) | 50,000 | NA | NA | NA | 3 days | CPE | NA | ||
| Spike pseudotyped viruses | Vitalant Research Institute (VRI) pseudovirus neutralization; part of NCT04355767 (C3PO) | ACE2- and TMPRSS2-expressing HEK293T cells | NA | VSV pseudotyped with Wuhan-Hu-1 Spike (D614G mutation and without 21 C-terminal aa) | NA | NA | 18–24 h | Chemiluminescence reader | NTs were calculated as a percentage of no-serum control, and the NT50 was estimated from the dilution curve | 128 |
| NCT04383535 (PlasmAr) | Vero-CCL81 | 20,000 | VSV pseudotyped with Spike (CoV2pp) and carrying Renilla luciferase gene in place of its G glycoprotein (VSVΔG-rLuc) | NA | NA | 18–22 h | Luminometer | IC50 is calculated as the midway point between the upper and lower plateaus of the curve. absIC80 appeared to be a more stringent measure of NAb activity, as some sera that have respectable MN absIC50 titers never achieve an absIC80; this is due in part to the difference in the dynamic ranges between a luciferase-based assay (≥3 logs RLUs) and a MN assay (∼1.5-log OD values corresponding to the amount of viral protein detected) | 136 | |
| CTRI/2020/04/024775 (PLACID) | Vero CCL-81; 293 T/ACE2 cells | 10,000 | SARS-CoV-2 strain NIV2020770 | NA | NA | 36 h | Luminometer | NA | 137 | |
| NCT04345523 (ConPlas-19) | Vero E6 | 5,000 | Lentivirus pseudotyped with Spike and luciferase | Titrated at 10 ng p24 Gag | NA | 48 h | Luminometer | ID50 expressed as the highest dilution of plasma (reciprocal dilution) which resulted in a 50% reduction in luciferase activity compared to control without serum. Sigmoid curves were generated, and ID50 neutralization titers (NT50) were calculated by nonlinear regression | 23 | |
| NCT04375098 (Elvira Balcells) | HEK293T/hACE2 | 10,000b | HIV-1–SΔ19 pseudotyped with Spike (GenBank accession no. QHU36824.1) and luciferase | NA | NA | 48 hb | Luminometer | Samples with a neutralizing activity of at least 50% at a 1:160 dilution were considered positive and used to perform titration curves and ID50 NT calculations | 138 | |
| NCT04344535 (Bennett-Guerrero) | NA | NA | PRNT and pseudovirus | NA | NA | NA | NA | NA | 57 | |
| NCT04600440 (COP20) | Vero E6 | 10,000 | Pseudovirus | 200 PFU | NA | 8 h | Indirect immunofluorescence measured by Trophos Plate Runner HD | No. of infected cells were quantified and normalized to that of virus incubated without plasma. IC50 values were calculated using normalized data and a nonlinear fit with variable slope | 111 | |
| NCT04621123 (COnV-ert) | HEK293T/hACE2 | 10,000 | HIV reported pseudovirus | 200 TCID50 | 0.02 | 48 h | Luminometer | ID50 was calculated by plotting and fitting the log of plasma dilution vs normalized response | 29 | |
| NCT04364737 (cONTAIN) | Vero | NA | VSV pseudovirus | NA | NA | 7 h | Automated enumeration of GFP-positive cells | IC50 | 139 |
Information was retrieved from the original article (including the supplementary appendix). NA, not available (details could not be retrieved); IC, inhibitory concentration; NT, neutralization titer; PRNT, plaque reduction neutralization test; VNT, virus neutralization test; MOI, multiplicity of infection; VSV, vesicular stomatitis virus; aa, amino acids; TCID50, 50% tissue culture infective dose; CPE, cytopathic effect; PRNT50, plaque reduction neutralization test of 50%; IC50, 50% inhibitory concentration; ID50, 50% infective dose; CT, threshold cycle; absIC80, absolute 80% inhibitory concentration; MN absIC50, absolute 50% inhibitory concentration; RLU, relative light units; OD, optical density; rRT-PCR, real-time reverse-transcriptase polymerase chain reaction.
These data not reported in the reference, so the corresponding author was contacted.
Despite these uncertainties, we can make estimates of likely effective doses based on the available clinical experience thus far. The lack of utility from low-titer (1:40) CCP in moderate COVID-19 was confirmed by the PLACID trial (10). As long as a clear therapeutic dose is not identified, given that a 250-mL unit of transfused CCP is diluted into the ∼2.5 L of plasma in the recipient, it seems prudent to transfuse units containing NAb titers at least 10-fold higher than the NAb titer measured before transfusion in recipient serum. Similarly, the ConCOVID RCT showed that CCP units having NAb titers similar to those of the recipients (1:160) did not confer a clinical benefit (36). CCP units with an adequate NAb titer (nowadays estimated at >1:160) are more easily found among older males who recovered from a previous symptomatic COVID-19 requiring hospitalization (37, 38): unfortunately, such donors were poorly represented in the first donation waves, which tended to obtain CCP from younger donors with mild disease and, presumably, lower NAb titers (10).
Many trials have relied on high-throughput semiquantitative or qualitative assays with a poor to moderate relationship with NAb titers. Harmonization of such high-throughput assays using the WHO International Standard of binding arbitrary units (BAU) is now possible (39). Although most trials performed a correlation analysis between VNTs and high-throughput serological assays, in many cases, the CCP units were tested only with the latter without validation, as was the case with 66% of the patients in the PlasmAr trial (12). This procedure risks an incorrect evaluation of the neutralizing CCP activity. Another cause for discrepancies in outcomes could be that although IgM, IgG, and IgA are all capable of mediating neutralization, VNT titers correlate better with binding levels of IgM and IgA1 than they do with IgG (40). In contrast, routinely used high-throughput serological assays quantify only IgG, including nonneutralizing IgGs, whose potency against SARS-CoV-2 has not been established. Trials should preferentially use VNTs to assess the serostatus of transfused units and not rely on high-throughput serology. As for any other medicinal product, CCP exhibits a dose-response relationship, which is also evident when high-throughput assays are used. In the subgroup analysis of the EAP, a gradient of mortality was seen in relation to IgG antibody levels in the transfused CCP. In the subgroup of patients who were not receiving mechanical ventilation, death within 30 days after CCP transfusion occurred in 81 of 365 patients (22.2%; 95% confidence interval [CI], 18.2 to 26.7%) in the low-titer group, 251 of 1,297 patients (19.4%; 95% CI, 17.3 to 21.6%) in the medium-titer group, and 50 of 352 patients (14.2%; 95% CI, 10.9 to 18.2%) in the high-titer group. Depending on the statistical model, the risk ratio (RR) for 30-day mortality in high-titer CCP recipients compared to low-titer CCP recipients ranged from 0.64 to 0.67, with an upper 95% confidence bound of 0.91 (8). Similarly, the large retrospective PSM study from Hospital Corporation of America (HCA) Healthcare-affiliated hospitals reported a 0.2% decreased risk of mortality for every 1 unit of signal-to-cutoff ratio (S/CO) serology level (41).
An additional limit is testing NAbs only at the time of first donation (e.g., in ConCOVID [36]) while leaving donors opportunities to repeat their donations for months: given the expected decline of NAb levels over time in convalescents, this could have overestimated the actual dose received in units collected later.
The NAb titer (or total IgG levels as measured by surrogate assays) describes only one factor involved in defining the real therapeutic dose in that it represents the concentration of just one (likely the main) active ingredient. But CCP contains additional antibodies that mediate antibody-dependent cellular cytotoxicity (ADCC), complement activation, and phagocytosis of viral particles, functions that can each contribute to its antiviral effects (42). At this time, the relative importance of NAbs versus the other antibody activities is not understood, but hopefully, retrospective analyses that correlate CCP efficacy with these activities will reveal additional variables that need to be considered in choosing optimal CCP units. This has relevance when assessing interfering factors; e.g., the impact of pathogen reduction technologies (PRT) on NAbs has experimentally been shown to be apparently minimal (43, 44), but the impact on Fc-dependent functions (such as ADCC) is still to be investigated and could be relevant for some PRTs (45) (used, e.g., in TSUNAMI [46], NCT04356534 [47], ConPlas-19 [48], and COnV-ert [29]). In this regard, we note that an early study reported that methylene blue reduced the protective function of antibodies to pneumococcus by interfering with glycosylated domains, raising concerns that it could affect Fc function (45). Of relevance, the various pathogen-reduced plasma units went through clinical evaluation for assessment of their hemostatic activity linked to coagulation factors or, at best, Fc binding to receptors (49), not their immunological activity; furthermore, none of the photochemical processes used for CCP is used in the field of plasma fractionation, and therefore we cannot make inferences from pharmaceutical-grade immunoglobulins.
The therapeutic dose of NAb is a product of its concentration in the infused CP multiplied by the overall infused CP volume, adjusted to the recipient body weight to take account of dilution into the blood volume and tissues. RCTs have varied in the provision of volume per unit (200 to 300 mL) and, most importantly, in cumulative volume per patient (1 to 4 units) and in extent of exposure to diverse antibodies from various CCP donors, and no published trials have adjusted levels of NAbs by recipient body weight (or, when attempts have been performed, they referred to the historical 10- to 15-mL/kg dose derived from the treatment of hemorrhagic coagulopathies [50]). A failure of CCP to improve outcomes when 200 mL of CCP with a 1:160 NAb titer is provided to a patient who weighs 120 kg represents quite a different scenario from failure of a 600-mL transfusion of CCP with a 1:640 NAb titer to produce improvement in a 60-kg patient. But these central issues in dosage have not been considered in the RCTs published so far.
Finally, antibodies other than NAbs can play a prominent role in viral clearance. Bahnan et al. have shown that CCP and anti-Spike MAbs induce phagocytosis but with diminishing returns when the antibody concentrations become high; activation and inhibition of phagocytosis are independent of neutralization potential, and humanized ACE2 mice are protected from intranasal challenge by nonneutralizing antibodies (51).
RELEVANCE OF CCP TO THE VIRAL VARIANT
Albeit not formally demonstrated, CCP manufactured by pooling ABO-matched units from many different donors (e.g., in PlasmAr [12]) theoretically should have greater polyclonality of NAbs than repeated CCP doses from a single donor (e.g., in CAPSID [52]) and should provide higher efficacy against viral variants. Nevertheless, pooling typically occurs among donors attending the same blood bank, making donor exposure to different viral variants less likely.
An analysis of potential variables associated with CCP efficacy associated near-sourcing with reduced mortality; the efficacy of CCP in reducing mortality fell sharply when the CCP source was more than 150 miles from where it was used (53). This finding suggests that SARS-CoV-2 variants at some geographic locations create antibody responses in CCP that are not effective against other variants at different locations (54, 55). Even though CCP is often standardized for the NAb titer to the Spike protein, the VNT could use a nonrelevant viral strain or miss major functional differences for the antibody response (42). This finding has implication for RCTs that use nationally sourced (centralized) CCP, since the attempt to standardize the therapeutic units centrally could inadvertently reduce CCP efficacy if hospitals use CCP obtained from distant loci. For example, in the C3PO RCT, which was conducted in 21 U.S. states, 95% of the donor CCP was collected in either Chicago or Denver; since only 4 of the 48 centers were in Illinois or Colorado, most CCP usage had to be from remote sources (27). The same concern applies to large multinational RCTs such as REMAP-CAP (35). In contrast, the NCT04359810 RCT in New York and Brazil, which found a beneficial effect of CCP on mortality, used CCP locally sourced in New York, and its efficacy against P.1 was tested to ensure efficacy at the other recruiting center in Brazil (11).
Although also not formally demonstrated during clinical trials, it is reasonable to assume that CCP collected during early pandemic waves could be less effective against currently circulating variants of concern (56). RCTs whose recruitment was protracted across multiple pandemic waves (e.g., ConPlas-19 [48] and TSUNAMI [46]) and which relied on CCP collected and banked months earlier could have inadvertently used CCP with reduced activity against the SARS-CoV-2 strains circulating in the community when the therapy was administered. No CCP study to date has ever annotated or retrospectively performed SARS-CoV-2 sequencing on CCP donors or on patients to identify the underlying variant; matching of the convalescent donor and the recipient for viral variant has never been formally achieved. Hence, both geography and time of collection of the CCP are important variables when considering the efficacy of the treatment.
THE INTENDED OUTCOMES
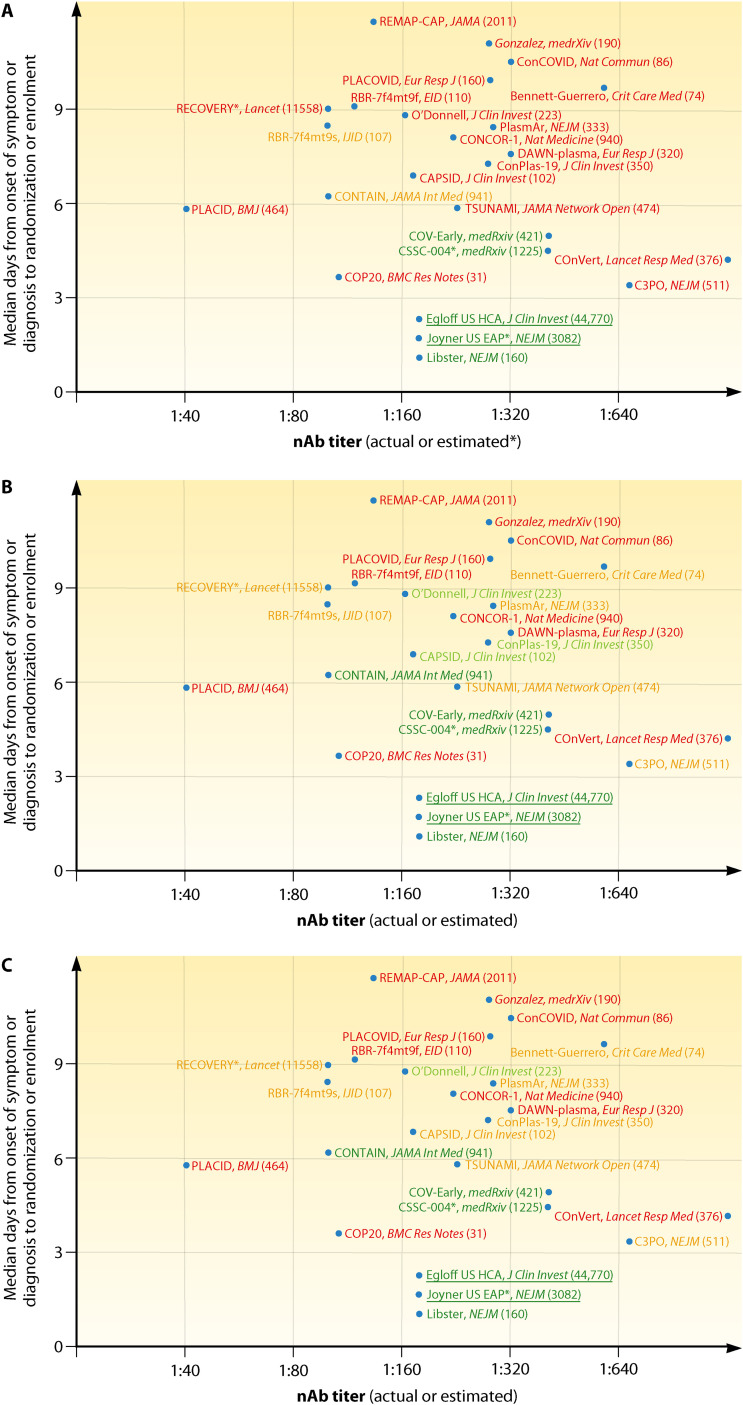
Most trials (CONTAIN [26] and PassItOn being exceptions) have used composite endpoints or specialty scores (e.g., SOFA) rather than progression in the simple WHO ordinal scale or mortality, and many were stopped because of apparent futility at a time when they may have been underpowered to detect significant benefit. As represented in Fig. 2, several studies have reported overall negative results (Fig. 2A) despite the presence of positive signals of efficacy just barely missing statistical significance (Fig. 2B and C). The significance level (i.e., P = 0.05) is largely a socially constructed convention for rejecting the null hypotheses, but it has often been misinterpreted as a measure of reality by many individuals not familiar with the nuances of statistics. For example, some CCP studies have concluded that a difference that did not achieve a P value of <0.05 indicated an absence of difference, even when mortality in the CCP arm was ∼20 to 40% lower than in controls (11, 48, 52, 57). This reasoning has played a central role in the polarized views of CCP efficacy and has impeded subsequent studies from drilling down on positive effects that were observed. The dogged pursuit of statistical significance, viewed as a measure of reality, instead of the actual reality demonstrated by the data during a public health emergency dealt a serious blow to studies of CCP and created significant confusion for clinicians. It is also important to understand that RCTs are powered to be less tolerant of type I errors than type II errors, which are conventionally set at 0.05 and 0.20, meaning that a type II error is expected four times as often as a type 1 error. This statistical convention can contribute to the absence of significance in studies that were set up early in the pandemic, when there was little information on expected effects for the various patient populations studied, when power estimates were only guesses, and when the enrolled patients were so heterogenous that only subgroups were likely to have responded to CCP treatment. Many studies were originally designed to enroll inpatients at any disease stage, and it should be no surprise that subgroup analyses on the groups that were later demonstrated to be more likely to benefit from CCP (e.g., early treated, seronegative patients, those receiving high NAb titer) were underpowered to reach statistical significance, as shown by orange color predominance in Fig. 2C. Nevertheless, favorable trends are a shared feature across such trials (5, 11, 12, 27, 48, 50, 52, 57, 58).
FIG 2.
Simplified graphical representation of CCP RCTs and large uncontrolled trials reported to date, plotted according to earliness of intervention and NAb titers in CCP. (A) Green text indicates trials which met the primary endpoint with statistical significance; orange text indicates trials which failed to meet the primary endpoint but showed statistically nonsignificant trends in favor of CCP; red text indicates trials which failed to show and benefit from CCP in the primary endpoint. (B) Green text indicates trials which showed overall mortality benefit from CCP; orange text indicates trials which showed mortality benefit from CCP in the subgroup of early arrivals or higher NAb titers; red text indicates trials which failed to show any mortality benefit from CCP. (C) Green text indicates trials which showed statistically significant mortality benefit from CCP (overall or in the subgroup of early arrivals or higher NAb titers); orange text indicates trials which showed statistical trends toward mortality benefit from CCP (overall or in the subgroup of early arrivals); red text indicates trials which failed to show any mortality benefit trend from CCP in any subgroup. Underlined text indicates large trials which were not RCT and for which NAb levels were inferred from high-throughput serology but are nevertheless reported as reference studies. Numbers in parentheses indicate the cumulative number of patients enrolled. Sources cited in the figure are references 9–12, 26–30, 34–36, 41, 46, 48, 50, 52, 57, 73, 76, 77, 113, 144, and 145.
Rigid adherence to primary outcomes that were often fixed in the early days of the pandemic, when information about disease stage and quality of CCP associated with efficacy were not understood, has contributed further to the confusion. When these outcomes were not met, trials were considered failures, even though there were often signals of efficacy in the data that were not considered as valuable since these had not been prespecified, even when they made biological sense. For example, in the New York-Brazil RCT cited above, CCP did not lower the primary endpoint of clinical status on an ordinal scale, but the statistically significant halving of mortality was acknowledged in the abstract. Would it have made sense to ignore the strong effect of CCP on mortality in this trial just because mortality was not selected as a primary outcome? RCTs of MAbs (59) and dexamethasone (60) were designed to achieve a tiny 6% and 4% reduction in mortality, respectively, while several CCP RCTs failed because their initial assumption in the magnitude of reduced mortality was more optimistic (e.g., TSUNAMI [46]).
Another misunderstood endpoint is viral clearance, defined as the conversion of nasopharyngeal swabs (NPS) from positive to negative for PCR evidence of SARS-CoV-2 in CCP-treated patients. While there was early and robust evidence for this effect from CCP (4, 10), the sampling of NPS too late after CCP treatment, when the endogenous immune response had also mounted in the control arm, could miss differences.
ANALYZING CONFLICTING OUTCOMES IN INDIVIDUAL RCTs
Keeping the factors discussed above in mind, we have analyzed individual RCTs in detail. At the very beginning, many historically or internally controlled observational studies showed clinical benefit from CCP (4–6), and this led the FDA to issue an EAP in March 2020 that was converted into an emergency use authorization (EUA) on 23 August 2020. The largest observational study is the U.S. open-label EAP (NCT04338360) led by Joyner et al., which enrolled 105,717 hospitalized patients with severe or life-threatening COVID-19 from 3 April to 23 August 2020 (61). In an analysis of the effect of antibody in CCP performed independently of the results cited above (8) and using a NAb titer in an overlapping but nonidentical group of EAP patients, the FDA showed that the 7-day mortality in nonintubated patients who were younger than 80 years of age and were treated within 72 h after diagnosis was 6.3% in those receiving high-titer CCP and 11.3% in those receiving low-titer CCP (https://www.fda.gov/media/142386/download).
In a later analysis of a larger (n = 35,322) subset of EAP patients (including 52.3% in the ICU and 27.5% receiving mechanical ventilation), the 7-day mortality rate was 8.7% in patients transfused within 3 days of diagnosis but 11.9% in patients transfused ≥4 days after diagnosis; similar findings were observed in 30-day mortality (21.6% versus 26.7%) (62). A similar halving of 28-day mortality was reported in inpatients treated within 3 days of hospitalization in large cohort studies from Argentina (18.1% mortality in 3,113 patients transfused within 3 days compared to 30.4% in 1,380 transfused at 3 to 7 days and 38.9% in 226 transfused after 7 days) (63) and Italy (14% mortality in 1,571 CCP-treated patients versus 25% in a synchronous CCP-untreated local cohort) (64). The major criticism of these results is that controls were neither randomized nor PSM; hence, a difference in treatment outcome between treated and untreated groups may be caused by a factor that predicts treatment rather than by the treatment itself. However, importantly, NAb titer analysis was retrospectively done, both patients and physicians were unaware of the NAb content in the CCP units used, the results are what would have been expected from the experience with antibody therapy, and multivariate models were used to adjust for potential confounders (1). Additionally, given the outline of an optimal use case with these data and the earlier underpowered RCT by Li et al. (5), it is unfortunate that due to (i) lack of awareness and (ii) logistical burden associated with protocol adjustments, involving repowering and new patients’ recruitment criteria, later-treatment RCTs either continued or were initiated without modifications based on newly available evidence.
The highest level of scientific evidence in primary clinical research stems from prospective PSM studies and RCTs. PSM studies (Table 2) balance treatment and control groups on a large number of covariates without losing a large number of observations. Unfortunately, no PSM study to date has investigated NAb titers by VNT or outpatients. Nevertheless, in two retrospective PSM studies from two different hospitals in New York, trends for improved outcomes in nonintubated patients and those treated within 7 days of hospitalization (hazard ratio [HR], 0.33) were observed (65, 66). These findings were later confirmed in a prospective PSM study from Houston (67, 68). Of interest, a retrospective PSM study from Providence did not show any benefit, but patients were treated at a median of 7 days after onset of symptoms (69). Another PSM study from Yale associated CCP with a 35% reduction in mortality (70). That study is notable in that it included patients on mechanical ventilation who would not normally be expected to benefit from CCP and the percentage of individuals receiving corticosteroids was very low since the study was conducted in the early days of the pandemic in the United States. Another PSM from the Washington, DC, area found a reduction in mortality with CCP use at both days 14 and 28, which reached statistical significance at the earlier date (71). Finally, a very large study from 176 community hospitals affiliated with HCA Healthcare confirmed substantial mortality reduction in hospitalized patients receiving CCP within 3 days of admission (72).
TABLE 2.
PSM CCP studies reported to datea
| Study design | Location | No. of patients + controls | Median no. of days CCP given posthospitalization | Baseline recipient WHO score (24)b | Transfused CCP vol (mL) | Statistically significant outcomes | Reason(s) for failure | Reference |
|---|---|---|---|---|---|---|---|---|
| Retrospective | Mount Sinai, NY, USA | 39 + 156 | 4 | 5 (87%), 6 (10%) | 250 + 250 | On day 14, oxygen requirements worsened in 17.9% of plasma recipients vs 28.2% of controls (aOR, 0.86). Survival improved in plasma recipients (aHR, 0.34). | No failure | 66 |
| Providence, RI, USA | 64 + 177 | >2 (<10 from onset of symptoms, median, 7) | 4 (70%), 5 (30%) | NA (2 units) | No significant differences in incidence of in-hospital mortality (12.5% and 15.8%; aHR, 0.93) or overall rate of hospital discharge (RR, 1.28; although increased among patients of >65 yrs). | Late usage | 69 | |
| Montefiore Medical Center, NY, USA | 90 + 258 | <3 (3–7 days from onset of symptoms) | 5–6 (<24 h of mechanical ventilation) | 200 | Anti-S-IgG titer ≥ 1:2,430 (median, 1:47,385); recipients <65 yrs had 4-fold-lower mortality and 4-fold-lower deterioration in oxygenation or mortality at day 28. | No failure | 65 | |
| Washington, USA | 263 + 263 | <14 | NA | 245 (median) | Reduced 7-day (9.1 vs 19.8%) and 14-day (14.8 vs 23.6%) mortality but not 28-day mortality (P = 0.06), and longer hospital stay. | Late usage; control cohort was treated, on average, 29 days prior to the CCP cohort | 71 | |
| USA (176 HCA healthcare-affiliated community hospitals) | 3,774 + 10,687 | <3 vs 4–7 | NA | NA | Lower mortality (aHR, 0.71) and faster recovery. CCP within 3 days after admission, but not 4–7 days, was associated with a significant reduction in mortality risk (aHR, 0.53). CCP serology level was inversely associated with mortality when controlling for interaction with days to transfusion (HR, 0.998) but was not significant in a univariable analysis. | No failure | 72 | |
| China | 163 + 163 | 23 | NA | 300 | Hospital stay in CCP group was significantly longer than that of matched control group (P < 0.0001). | Very late usage; more advanced disease in the CCP group (23 vs 15 days since hospital admission). | 140 | |
| Greece | 59 + 59 | 7 | ≥4 | 200–233 (days 1, 3, and 5) | Significantly reduced risk of death (HR, 0.04; 3.4% vs 13.6%), significantly better overall survival by Kaplan-Meier analysis, and increased probability of extubation (OR, 30.3). Higher levels of antibodies (as measured with Euroimmun or pseudoVNT) in CCP recipients were independently associated with significantly reduced risk of death. | No failure | 141 | |
| New Haven, CT, USA | 132 + 2,551 | <6 vs >6 days | Moderate to severe | Early CCP recipients, of whom 31 (40%) were on mechanical ventilation, had lower 14-day (15% vs 23%) and 30-day (38% vs 49%) mortality than a matched unexposed cohort, with nearly 50% lower likelihood of in-hospital mortality (HR, 0.52). Early plasma recipients had more days alive and ventilator-free at 30 days (+3.3 days) and improved WHO scores at 7 days (−0.8) and hospital discharge (−0.9) than the matched unexposed cohort. | No failure | 70 | ||
| USA | 143 + 823 (hematological cancer) | NA | NA | NA | Improved 30-day mortality (HR, 0.52; 95% CI, 0.29–0.92) among the 338 patients admitted to the ICU, mortality was significantly lower in CCP recipients than in nonrecipients (HR, 0.40). Among the 227 patients who required mechanical ventilatory support, mortality was significantly lower in CCP recipients than in nonrecipients (HR, 0.32). | No failure | 109 | |
| Prospective | Houston, TX, USA | 136 + 251 | NA | 3 (9%), 4 (63%), 5 (18%), 6 (10%), 7 (1%)b | 300 (1–2 units) | Reduction in mortality within 28 days, specifically in patients transfused <72 h of admission with CCP with an anti-RBD titer of ≥1:1,350 (i.e., ∼80% probability of a live virus in vitro neutralization titer of ≥1:160 [142]). | No failure | 67 |
| 341 + 594 | NA | 300 (1–2 units) | Reduced 28-day (aHR, 2.09 for controls) and 60-day (5.7% vs 10.7%; aHR, 1.82 for controls) mortality in those transfused with anti-RBD titer of ≥1:1,350 within 72 h posthospitalization. Optimal window of 44 h to maximize benefit in 60-day mortality (4% vs 12.3%). 91% received CCP with an anti-RBD titer of ≥1:1,350. Median S/CO ratio of 24 using Ortho Vitros. | No failure | 68 | |||
| Poland | 102 + 102 | NA | NA | NA | Lower mortality rate (13.7% vs 34.3%; OR, 0.25) related to time of first administration (12.2% at day 5, 21.5% at day 10); no significant differences in ICU stay, ventilator time, and hospitalization time. Earlier administration resulted in a ventilator being needed for a shorter length of time (r = 0.41). | No failure | 143 | |
| Brazil | 58 + 116 (kidney transplant recipients) | 6 from onset of symptoms | Mild and moderate | 200 | No differences in need for supplementary oxygen or mechanical ventilation at day 30. | Only 48% of CCP units were high titer; compared to nonsurvivors, a trend towards a higher proportion of survivors receiving higher-titer CCP | 144 | |
| Colorado (16 hospitals) | 188 + 188 | NA | NA | 1 unit if <90 kg; 2 units if >90 kg | Increased length of hospital stay in CCP-treated patients and no change in inpatient mortality compared to controls. In subgroup analysis of CCP-treated patients within 3 or 7 days of admission, there was no difference in length of hospitalization and inpatient mortality. | Covariate matching not achieved for subgroup receiving CCP < 3 days | 145 |
None of these studies determined titers of NAbs in either donors or recipients using the VNT. PSM, propensity score matched; DPH, days posthospitalization. HR, hazard ratio; aHR, adjusted HR; OR, odds ratio; aOR, adjusted OR; RBD, receptor binding domain; S-IgG, secretory IgG.
Since PSM accounts only for observed (and observable) covariates, and not latent characteristics, RCT remains the gold standard for highest-level evidence (Table 3). In the PlasmAr RCT, the small number of early arrivals (less than 72 h since symptom onset) showed superior primary and secondary outcomes in the CCP arm (n = 28) compared to the placebo arm (n = 11), but the minimal contribution of this group to the overall cohort (228 CCP and 105 placebo recipients) made the advantage disappear in the final outcomes at day 30 (12). In another Argentinean RCT of 160 outpatients older than 65 years of age with mild COVID-19 who were treated with CCP within 72 h, progression to severe COVID-19 was halved at day 30 (73). Similar findings were reported in outpatients without risk factors in CSSC-004 (30). An RCT from India reported that patients younger than 67 years treated at a median of 4 days after hospital admission manifested superior mitigation of hypoxia and survival in the CCP arm (74). Another RCT in Spain enrolling patients at less than 7 days of hospitalization showed four deaths in the control arm and none in the CCP arm (48). Similarly, the ConCOVID RCT showed reduced mortality in the CCP arm (36).
TABLE 3.
RCTs of CCP reported to date, listed according to date of (pre)publicationa
| RCT identifier (acronym/first author) | No, of participants recruited (out of expected) (randomization strategy) | Control arm (in addition to BSC) | Median days before randomization | Baseline recipient 10-point WHO scoreb (24, 25) | Transfused CCP vol (mL) (pathogen inactivaction) | Median NAb titer in CCP units | Median pretransfusion NAb titer in recipient | Main outcomes reported in abstract or conclusions | Likely reason(s) for failure | Signals of efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04479163 (Libster) | 160 (out of 210) (1:1) | BSC + normal saline | 39.6 h (from symptoms; and > 65 yrs) | 2 | 250 | NA | NA | Progression to severe COVID-19 halved at day 30 | No failure | Main outcome | 73 |
| BKH-CT-012 | 49 (1:1) | BSC | <3 (from ICU admission) | 5 | 400 | NA | NA | Duration of infection reduced by 4 days; mortality 1/21 in CCP arm vs 8/28 | No failure | Main outcome | 146 |
| CTRI/2020/05/025209 (Raj) | 80 (1:1) | BSC | 4.2 (from hospital admission) | 5 | 200 + 200 | NA | NA | Immediate mitigation of hypoxia and reduction in hospital stay, as well as survival benefit were recorded in severe COVID-19 patients with ARDS aged less than 67 yrs | No failure | Main outcome | 74 |
| ChiCTR2000029757 (Li) | 103 (out of 200) (1:1) | BSC | 30 (from symptoms) | 5–6 | 200 | ≥1:40 (inferred from correlation) | NA | No significant difference in 28-day mortality (15.7% vs 24.0%) or time from randomization to day-28 discharge (51.0% vs 36.0%) | Moderately late usage | Reduced mortality at day 28 only in patients with WHO score of 5 (HR, 2.5); negative conversion rate of viral PCR at 72 h in 87.2% of the CCP group vs 37.5% of the control group (OR, 11.39) | 5 |
| NCT04342182 (ConCOVID) | 86 (out of 426) (1:1) | BSC | 10 from symptoms; 2 from hospitalization | 5–6 | 300 | 1:320 (PRNT50) | 1:160 in 79% of recipients (11% seronegative) | No benefit at day 15 | Very late usage, high rate of seropositives | Mortality in CCP group of 14% (6 out of 43) vs 26% in control group (11 out of 43) (OR, 0.47) | 36 |
| CTRI/2020/04/024775 (PLACID) | 464 (1:1) | BSC | 6 (from symptoms) | 4–5 | 200 + 200 | 1:40 | 1:90 (17% seronegative) | No benefit at day 28 | Moderately late usage; high rate of seropositives; extremely low NAb titer in CCP | None | 10 |
| NCT04345523 (ConPlas-19) | 350 (1:1) | BSC | 8 (from symptoms) | 3 (25%); 4 (75%) | 250–300 (methylene blue, 46.3%; riboflavin, 24%; psoralen, 19.6%; unknown, 10.0%) | 1:292 | NA | No significant differences in primary endpoint (proportion of patients in category 5, 6, or 7 [death] at 14 days) | Underpowered for mortality; primary endpoint set at just 15 days | Primary endpoint significant at day +28; trends for reduced overall mortality (P = 0.087) at day +28, especially in those aged >75 yrs; primary and secondary endpoints improved in methyelen blue-treated CCP subgroup compared to controls (personal communication) | 48 |
| NCT04375098 (Elvira-Balcells) | 58 (1:1) | Late CCP | 6 (from symptoms) | 3–4 | 200 + 200 | ≥1:160 | <1:160 in 59% (16% of patients enrolled before day 5 had titer of ≥1:160 vs 60% of those enrolled after day 6) | No benefit at day 30 in death, mechanical ventilation, or prolonged hospitalization compared to CCP administration only in cases of clinical worsening or >7 days after enrollment | Underpowered, moderately late usage | None | 147 |
| NCT04383535 (PlasmAr) | 333 (2:1) | BSC + normal saline | 8 (from symptoms) | 5 | 500 (from a pool of up to 5 donors) | 1:300 IC80 | NA | No benefit at day 30 (16.2% vs 31.2%) | Moderately late usage | Early arrivals (less than 72 h) showed superior primary and secondary outcomes in the CCP arm (n = 28) compared to the placebo arm (n = 11), but the minimal contribution of this group to the overall cohort (228 CCP recipients and 105 placebo recipients) made the advantage disappear in the final outcomes at day 30 (12) | 12 |
| NCT04356534 (AlQahtani) | 40 (1:1) | BSC | NA | 4 (95%); 5 (5%) | 200 + 200 methylene blue inactivated | NA | NA | No difference in requirement for ventilation, white blood cell count, LDH, CRP, troponin, ferritin, d-dimer, procalcitonin, mortality rate at 28 days | Underpowered; the CP group was a higher-risk group with higher ferritin levels | Primary outcome measure, ventilation, was required in 6 controls and 4 patients on CCP (RR = 0.67; 95% CI, 0.22 to 2.0, P = 0.72); mean time on ventilation was 10.5 days in the control vs 8.2 days in patients on CCP (P = 0.81) | 47 |
| NCT04346446 (Bajpai) | 29 (1:1) | BSC + FFP | <3 (from symptoms) | 4–5 | 250 + 250 | NA | NA | No significant reduction in mortality or hospitalization | NAb measured with surrogate competitive assay (GenScript); beneficial factors in FFP used in control arm (92) | Better median improvement in PaO2/FiO2 at 48 h (42 vs 231) and at day 7 | 58 |
| NCT04381936 (RECOVERY) | 11,558 (1:1) | BSC | 9 from symptoms; 2 from hospitalization | 4–7 | Median, 275 ± 75 (81% got 2 units from different donors; 12% got 1 unit) | NA | 35% seronegative | No significant difference in 28-day mortality or progression to invasive mechanical ventilation; closed for futility | Late usage | Risk ratio for patients randomized within 7 days of symptom onset was 0.92 in favor of CCP versus 1.06 in patients randomized later and 0.95 for those randomized within 3 days (personal communication); a reanalysis of seronegative patients (having 10% lower mortality) with a vague prior found that the likelihood of any or modest benefit was 86 to 68% (80) | 50 |
| NCT04348656 (CONCOR-1) | 940 (out of 1,200) (2:1) | BSC | 8 from symptoms | 4–6 | 1–2 units, each 250 mL | 1:250 | NA | Closed for futility (even in the subgroup transfused within 3 days from diagnosis) in intubation or death by day 30 | Late usage (hypoxemic), sicker CCP arm (more abnormal CXR, more in ICU), varying standard of care across 72 centers in 3 countries | Each standard log increase in neutralization or ADCC independently reduced the potential harmful effect of CCP (OR = 0.74), while anti-Spike IgG increased it (OR = 1.53) (148) | 123 |
| NCT02735707 (REMAP-CAP) | 2,011 (95% from UK) | BSC | ≤3 from ICU hospitalization | 5 (25%); 6 (75%) | 2 units (550 ± 150 mL) within 48 h | ≥1:80 in Australia; ≥1:160 in Canada; NA in UK and USA | Performed but undisclosed in Australia; NA in Canada, UK, and USA | No significant difference in median organ support-free days, in-hospital mortality, or median number of days alive and free of organ support at day 21; closed for futility | Very late usage | In the small no. of participants (n = 126) with immunodeficiency at baseline, CP demonstrated potential benefit (posterior probability of superiority of 89.8%). | |
| NCT04355767 (C3PO) | 511 (out of 900) with at least 1 risk factor associated with severe COVID-19 | BSC | 4 from symptoms, presented to the emergency department | 2–3 | 1 250-mL unit | 1:641 ID50 | NA | Nonsignificant difference in risk difference (1.9%); outcomes regarding worst illness severity and hospital-free days were similar in the two groups | “All cause” outcome instead of COVID-19-related outcome; centralized CCP supply to distant sites likely affected by different SARS-CoV-2 variants (53) (since only 4 of the 48 centers were in Illinois or Colorado, most CCP usage had to be from remote sources); immunosuppressed individuals were nearly twice as common in the treatment group (12.8% vs 6.7%); designed to detect an absolute risk difference of 10% in disease progression (149) | 9.4% reduction in primary event endpoint in CCP group, which rises to 24% after exclusion of patients admitted on the index visit (P = 0.07); dyspnea emerged in 6.7% of controls but in 2.3% of those treated with CCP (P < 0.05); hospitalizations (including deaths) after the day of treatment were found in 12.8% of the CCP treated but in 19.6% of controls (P = 0.035); symptom worsening after the first day occurred in 34.9% of CCP recipients but in 41.9% of controls (P < 0.05) | 27, 150 |
| NCT04359810 (O’Donnell) | 223 (2:1) | BSC + FFP | 9 from symptoms | 5–7 | One 200- to 250-mL unit | 1:160 | NA | At 28 days, no significant improvement in clinical status | Very late usage; beneficial factors in FFP used in control arm (92) | Lower mortality (12.6% vs 24.6%) than with nonconvalescent plasma | 11 |
| NCT04381858 (Gonzalez) | 190 (2:1) | BSC + IV Ig 1.5 mg/kg | 12 from symptoms | 6-7 (85%) | Two 200-mL units 24 h apart | NA (29.5% received at least 1 unit of CCP with antibodies) | NA | No difference in mortality at day 28 | Very late usage; beneficial factors of IVIg used in control arm | None | 124 |
| NCT04344535 (Bennett-Guerrero) | 74 (out of 500) (4:1) | BSC + FFP | 9 from symptoms, 4 from hospitalization | NA | Two 200-mL units | 1:526 | NA | No difference in ventilator-free days or mortality (27% vs 33%) at day 28; terminated after FDA issued EUA | Very late usage; beneficial factors in FFP used in control arm (92) | All-cause mortality through 90 days was numerically lower in the CCP group than standard plasma group (27% vs 33%; P = 0.63) | 57 |
| NCT04433910 (CAPSID) | 105 (1:1) | BSC | 7 from symptoms | 4–7 | 3 units from same donor over 5 days (850 mL) | 1:160 (PRNT50) | 1:160 (PRNT50) | No significant difference in primary outcome (dichotomous composite outcome of survival and no longer fulfilling criteria for severe COVID-19) and secondary outcomes | Moderately late usage | Median time to clinical improvement was 26 days in the CCP group and 66 days in the control group (P = 0.27); median time to discharge from hospital was 31 days (IQR, 16–n.r.) in the CCP group and 51 days (IQR, 20–n.r.) in the control group (P = 0.24); in the subgroup that received a higher cumulative amount of Nabs, the primary outcome occurred in 56.0% (vs 32.1%), with a shorter interval to clinical improvement, shorter time to hospital discharge, and better survival than that of the control group | 52 |
| NCT04547660 (PLACOVID) | 160 (1:1) | BSC | 10 from symptoms | 5–6 (37%); 7 (66%) | Two 300- mL aliquots 2 days apart | NA | >1:80 in 83% | No difference in 28-day mortality, days alive, days free of respiratory support, duration of invasive ventilatory support, or inflammatory and other laboratorial marker values on days 3, 7, and 14 | Very late usage | None | 125 |
| NCT04429854 (DAWN-plasma) | 320 (2:1) | BSC | 7 from symptoms | 3–5 | Two 200- to 250-mL aliquots within 12 h, followed by 2 units within 36 h | NA | ≥1:320 | No significant improvement in proportion of patients that require mechanical ventilation or have died at day 15 or 30 | Late usage | None | 34, 151 |
| NCT04621123. (COnV-ert) | 376 (out of 474) (1:1) | Placebo | 4.4 from onset of symptoms (outpatients) | 2 | One 200- to 300-mL unit (methylene blue inactivated) | ID50, 1:1,379 | 85% seronegative | No reduced progression to severe illness requiring hospitalization; CP did not reduce viral load change at day 7 | Vague definition of seropositivity; NAb reduction post-methylene blue inactivation; no assessment of damage to Fc-mediated functions | None | 28, 29 |
| NCT04589949 (CoV-Early; first 20% recruitment aggregated with the first 20% of ConV-ert RCT [29]) | 421 (out of 690) (1:1) | FFP | ≤7 from symptoms (median, 5?), ≥50 yrs, and ≥1 additional factor (outpatients) | 2 | One 300-mL unit | 1:386 | 93% seronegative (21 vaccinated with 2 doses, 14 with 1 dose?) | No difference in hospital admission, death, or time to resolution (OR = 0.93), regardless of NAb titer | Moderately late usage, low titer, low volume; low hospitalization rate (9.3%), hence not powered to exclude a small treatment effect | Effect of CP on hospital admission or death was largest in patients with ≤5 days of symptoms (OR, 0.658) | 28 |
| NCT04393727 (TSUNAMI) | 417 (1:1) | BSC | >7 from radiological diagnosis | 4–5 | Two 200-mL aliquots (>90% amotosalen; <10% riboflavin inactivated) | 1:226 (each unit >1:160) | NA | No statistically significant improvement in progression to ventilatory support or death | Powered to detect an exaggerated 40% reduction in primary endpoint; underpowered for subgroup analysis; late usage; unexplained findings at site 02 (which recruited 40% of patients) (152) | Trends favoring CCP in basally seronegatives were a P/F of >300 mm Hg (P = 0.059) and recipients of units with higher NAb titers | 46 |
| NCT04600440 (COP20) | 31 (out of 100) (1:1) | BSC | <4 from positive NPS | 5 | Three 200- to 250-mL aliquots | 1:116 (each unit, >1:40) | NA | No significant difference in no. of days of oxygen treatment to keep SaO2 > 93% or mortality at 28 days; closed for futility | Focusing on patients with worse clinical course (requiring oxygen at day 4 since NPS); largely underpowered; more days passed since onset of symptoms to transfusion | None | 111 |
| NCT04397757 (PennCCP2) | 80 (1:1) | BSC | 6 since onset of symptoms, 1 since hospitalization | 3–5 | 2 units on day 1 from 2 different donors | NA | NA (60% seronegative) | Significant benefit by clinical severity score and 28-day mortality (26% vs 5%) in patients with a median of 3 comorbidities (including immunodeficiency) | No failure | Trends for WHO score better than 8 at days 14 and 28, any use of mechanical ventilation or ECMO, duration of mechanical ventilation or ECMO use, and duration of supplemental oxygen use | 88 |
| NCT04373460 (CSSC-004) | 1,225 (out of 1,344) (1:1) | FFP | 6 since onset of symptoms (outpatients) | 1–3 | One 250-mL unit | NA (1:14,580 Euroimmun) | NA | Hospitalization in 6.3% of FFP vs 2.9% of CCP (RR = 0.46) | No failure | None | 30 |
| NCT04364737 (CONTAIN) | 941 (1:1) | BSC + placebo | 7 since onset of symptoms | 5 | One 250-mL unit | 1:93 (70% < 1:160) (1:175 between April and June 2020) | NA (67% seropositive) | No improvement in WHO ordinal scale at day 14 or 28 | Low-titer (except Q2 and Q5) and low volume; centrally distributed CCP to states with different viral variants after June 2020; primary endpoint too early at day 14 | At day 28, cORs were 0.72 for participants enrolled in Q2 (April-June 2020) and 0.65 for those not receiving remdesivir and not receiving corticosteroids at randomization; at day 28, mortality was lower in 486 seropositive than 242 seronegative participants irrespective of treatment arm, and in seronegative CCP (14.4%) than placebo (17.9%) recipients | 26 |
| NCT04323800 (CSSC-001) | 180 (out of 500 because of wide vaccine availability) (1:1) | BSC + FFP | Exposed within 96 h of enrollment and 120 h of receipt of CCP (median, 2 days) | 0 | 1 unit | >1:320 | 100% seronegative | No reduction in infection or symptomatic disease rate | Not powered to show reduction in hospitalization | No COVID-19-related hospitalizations in CCP recipients and 2 in control recipients | |
| RBR-7f4mt9f | 110 (out of 120 because of interpandemic pause) (1:2) | BSC | 9 since onset of symptoms | 6–9 | 3 units of 600 mL each | 1:120 | 100% seropositive | No statistically significant reduction in mortality, requirement for invasive ventilation, and duration of hospital stay | Very late usage | At day 30, death rates were 22% for CCP group and 25% for control group; at day 60, rates were 31% for CCP and 35% for control | 112 |
NAb: neutralizing antibodies; BSC, best supportive care; FFP, fresh frozen (nonconvalescent) plasma; NA, not assessed (i.e., antivirus antibodies were assessed only using high-throughput serology); IQR, interquartile range; OR, odds ratio; ARDS, acute respiratory distress syndrome; LDH, lactate dehydrogenase; CRP, C-reactive protein; PaO2, partial pression of oxygen; FiO2, inhaled fraction of oxygen; CXR, chest X-ray; IVIg, intravenous Ig; P/F, PaO2/FiO2; cORs, cumulative adjusted odds ratio ; n.r., not reached. Moderately late usage is defined as 4 to 6 days since onset of symptoms, late usage as 7 to 10 days, and very late usage as >10 days.
Ten-point WHO scores: 0, uninfected, no viral RNA detected; 1, asymptomatic, viral RNA detected; 2, symptomatic, independent; 3, symptomatic, assistance needed; 4, hospitalized, no oxygen therapy; 5, hospitalized, oxygen by mask or nasal prongs, 6, hospitalized, oxygen by NIV or high flow; 7, intubation and mechanical ventilation, pO2/FiO2 ≥ 150 or SpO2/FiO2 ≥ 200; 8, mechanical ventilation, pO2/FiO2 < 150 (SpO2/FiO2 < 200) or vasopressors; 9, mechanical ventilation, pO2/FiO2 < 150, and vasopressors, dialysis, or ECMO; 10, dead.
An additional complexity in recruitment to CCP trials is time to treatment. Clinical trials involve administrative requirements and consent procedures, and recruitment to an RCT further requires randomization, which may produce delays in treatment. CCP therapy requires cross-matching of blood types, ordering of the CCP, which may or may not be available on site, and setting up the transfusion. This inherent delay from randomization to infusion means that RCTs may build in a disadvantage for the CCP study arm in the few RCTs using control treatments (e.g., saline or fresh frozen plasma [FFP]), where controls may have received treatment earlier in the disease course; for example, FFP was used in controls only in NCT04346446 (58), NCT04359810 (11), NCT04344535 (57), COV-Early (28), and CSSC-004 (30) RCTs. ABO-compatible CCP units may be not readily available at the local blood bank, and recruited patients may have to wait for a compatible unit of CCP. These almost inevitable delays from randomization mean that CCP may be provided later in the illness than is ideal, and even if the trial intends to treat early, in practice it may not be possible unless the RCT is designed to deliver plasma immediately after randomization.
During a pandemic, moreover, delays in treatment are magnified. The accrual of severely ill patients in emergency departments and the overwhelmed or even collapsed health care systems can create long delays from arrival in the emergency room to treatment. In the absence of quick (antigenic or molecular) tests for SARS-CoV-2, the turnaround time for final confirmation of diagnosis with PCR, which must often be run in batches, can take several hours. All these factors are likely to impact the efficacy of CCP treatment. To shorten such time, fully screened CCP collected from eligible donors (75) could be safely administered within emergency departments shortly after admission and even before the patient reaches the ward.
Figure 2 graphically places the outcomes of RCTs and PSM studies on a Cartesian plot having timeliness and NAb dose as variables (if values are disclosed in the reports). This makes immediately clear that the studies suggesting benefit cluster in the lower right quadrant (high NAb dose and early intervention), while the studies showing no benefit are scattered elsewhere (Fig. 2A), reflecting lower antibody levels infused or late treatment, or both, with the latter being the more common problem. Nevertheless, when we focus on mortality irrespective of statistical significance (Fig. 2B) or focus on statistical significance (Fig. 2C), additional RCTs showed clear benefits.
THE INADEQUACY OF META-ANALYSES
With all the heterogeneity in key drivers discussed in the previous paragraphs, it becomes clear that secondary research (ranging from umbrella reviews to meta-analyses to systematic reviews), whereby each study is considered at the same level, invariably ends up with biased and divergent conclusions. This adds confusion to the already complex field of individual trial outcomes. Amazingly, as of 21 December 2021, PubMed has indexed 26 meta-analyses on CCP efficacy, more than the RCTs reported at the same date. Until the beginning of 2021, meta-analyses (variably including observational studies) were generally in favor of CCP (76) but began to be biased toward failure after publication of the large RECOVERY trial (50), which, by enrolling as many as 11,448 patients, diluted all the signals from positive RCTs. Clear examples of this phenomenon come from a widely cited meta-analysis from Janiaud et al. in JAMA (77), which included press release data from RECOVERY, and from the living systematic review by the Cochrane Group (78). The JAMA paper was surely unprecedented in the tradition of meta-analysis, not only because it included a study based only on a news release (which proved to differ in some important respects from the published paper), but because it allowed these data from a news release to dominate the entire analysis. In November 2021, in a follow-up meta-analysis from the same group on 16,477 patients from 33 trials, RECOVERY still accounted for 69.8% of the data (79). Several groups have attempted to dissect the RECOVERY trial and others by running subgroup analyses in their systematic reviews (80–82), but these reviews were unable to restore the confidence in CCP efficacy in the clinical community that had been lost because of the publication of the overall negative findings of RECOVERY and PlasmAr (83). Basing its recommendations entirely on RCT meta-analyses, on 8 December, the WHO revised its living guidelines on drugs for COVID-19, discouraging usage of CCP (84).
Meta-analyses embedding more recent studies and performing subgroup analysis were more positive for the beneficial effects of CCP. A meta-analysis of 22,591 patients (enrolled in 10 RCTs and 15 observational studies) showed that early CCP significantly reduced mortality (RR, 0.72; P < 0.00001) but only in patients who were not suffering severe or critical disease (85). Another meta-analysis of 18 peer-reviewed clinical trials, 3 preprints, and 26 observational studies found that CCP use was associated with reduced risk of all-cause mortality in severe or critical COVID-19 patients (86). A recent umbrella review of 29 meta-analyses and systematic reviews found evidences for improvement in the CCP arms for some outcomes (overall mortality, viral clearance at day 3) but not for others (clinical improvement, length of hospital stay) (87).
Rather than pooling published RCTs, the Continuous Monitoring of Pooled International Trials of Convalescent Plasma for COVID-19 Hospitalized Patients (COMPILE) study pooled individual patient data from ongoing RCTs at 2-week intervals. Unfortunately, with the single exception of CONTAIN (26), participating RCTs largely treated patients in advanced disease stages (DAWN-plasma [34], PLACID [10], ConCOVID [36], ConPlas-19 [48], NCT04421404, PennCCP2 [88], and the Brasília Covid-19 Convalescent Plasma [BCCP]) (89). Not surprisingly, the COMPILE metanalysis failed to show any benefit from CCP in the aggregate analysis (90). Nevertheless, the investigators were able to derive a therapeutic benefit index (TBI) showing that CCP mostly benefits patients with preexisting conditions (diabetes and cardiovascular and pulmonary diseases), with blood type A or AB, and, most importantly, at early COVID-19 stage (91).
CURRENT CLINICAL UTILITY OF CCP
While CCP contains a plethora of biologically active molecules (92), we now have very strong evidence that appropriately vetted CCP from eligible convalescent donors is safe for patients (93, 94), with no evidence of increased risks of transfusion-transmitted acute lung injury or antibody-mediated enhancement concerns feared in the early days of the pandemic (95), nor is there evidence that CCP induces accelerated SARS-CoV-2 evolution (11). Polyclonal antibodies such as are present in CCP are likely to offer better protection against onset of variants than monoclonal antibodies (96–98). Importantly, Pommeret et al. showed that CCP can rescue immune escape variants that emerged during treatments with a bamlanivimab/etesevimab cocktail (99). Outcomes in immunocompromised patients treated with CCP have been successful in the long term, with minimal evidence of immune escape (100).
We have also learned that CCP is less likely to benefit patients requiring oxygen (i.e., from level 4 and up on the WHO 11-point ordinal scale), and hence, ideally, the focus should be on outpatients and in identifying that subset of patients who seek hospital care and are still sufficiently early in the course of disease such that they can benefit from CCP. This finding parallels the finding with hyperimmune serum and anti-Spike monoclonal antibodies, which at first failed in hospitalized patients (101, 102) but later succeeded for ambulatory patients with mild to moderate COVID-19 (103) and were approved for emergency use.
CCP usage per admission peaked after issuance of the EUA, with more than 40% of inpatients estimated to have received CCP between late September and early November 2020. Oladunjoye et al. showed that mortality in the second wave in the United States, when utilization of corticosteroids, remdesivir, and convalescent plasma was higher, was lower than in the first wave (104). However, following reports of RCTs that failed to show clear benefit from CCP, usage per admission declined steadily to a nadir of less than 10% in March 2021. A strong inverse correlation (Pearson correlation coefficient of −0.5176 with a P of 0.00242) was found between CCP usage and deaths occurring 2 weeks after admission, and this finding was robust for examination of deaths taking place 1, 2, or 3 weeks after admission. Changes in number of hospital admissions, prevalence of variants, and age of patients could not explain these findings. The authors estimated that the retreat from CCP usage, a phenomenon they termed “plasma hesitancy,” might have resulted in 29,000 to 36,000 excess deaths in the period from mid-November 2020 to February 2021 (105). The same analysis estimated that the United States had avoided 96,000 excess deaths from August 2020 to March 2021 by its liberal deployment of CCP.
Several lines of evidence, ranging from the EAP to clinical trials employing RCT or PSM controls, are now indicating how CCP should be used early in immunocompetent patients (106). Nevertheless, chronically immunosuppressed patients benefit from CCP even at later stages (100, 107, 108): the best evidence for this scenario comes from a prospective PSM study showing a halving of mortality in ICU-admitted oncohematological COVID-19 patients who received CCP (109). We note that while there have been concerns that use in the immunocompromised can promote the emergence of antibody-resistant variants, such variants have emerged from massive replication in susceptible populations and not from treated patients, who in any case are isolated in hospitals, where mitigation efforts to reduce transmission are employed, and are thus very unlikely to transmit their viruses further (110). Such simple concepts have been poorly communicated to the general public and the clinical community, who should be better informed on the settings where CCP has shown efficacy and the ones where it has not.
CONCLUSIONS
The absence of benefit in trials of CCP largely occurs for four reasons:
Trials that transfused insufficient therapeutic doses of CCP due to either low total IgG levels or low NAb levels (e.g., PLACID [10]).
Trials that transfused appropriate doses of CCP but too late, but which nevertheless reported signals of efficacy (e.g., RECOVERY [50], CAPSID [52], NCT04359810 [11], and TSUNAMI [46]).
Trials that were stopped too early to observe benefit or with inherent design flaws and/or were underpowered such that the likelihood of success was reduced (e.g., C3PO [27]).
Trials in which CCP was used to treat a condition not amenable to antibody intervention, such as hypoxia that is caused by pulmonary inflammation (e.g., COP20 [111], RBR-7f4mt9f [112], or REMAP-CAP [35]).
In contrast, trials showing a benefit were characterized by the initiation of CCP treatment as early as 44 to 72 h within onset of symptoms (which largely pertains to outpatients) and using CCP with a NAb titer of >1:160. Benefit within 1 week from onset of symptoms (including in hospitalized patients) is less well understood, although a benefit from higher therapeutic doses cannot be ruled out at this stage. Clinical benefit seems absent when administered after 1 week from onset of symptoms or in patients requiring ventilation or in those who receive CCP with a low NAb titer.
RECOMMENDATIONS
Stopping trials for futility is an occurrence that deserves special attention, because it represents wasted resources during a pandemic. Eight RCTs so far have been halted for futility, namely, RECOVERY (50), REMAP-CAP (35), CONCOR-1 (113), C3PO (27), NCT04361253 (ESCAPE), CoV-Early (28), COnV-ert (29), and COP20 (111), with the first one being viewed as the strongest evidence for futility (50), with its massive recruitment affecting the outcomes of systematic reviews (77). Instead of stopping trials for futility based on preset endpoints, we urge that data and safety monitoring boards (DSMBs) facing a high likelihood of lack of statistical significance provide advice on trial modifications that are likely to amplify the significance of signals of efficacy evident in these studies. This would seem a more responsible action than trial cessation, given the paucity of therapeutic alternatives in the pandemic emergency.
We also suggest that more flexibility is needed when dealing with a new virus, since estimates of efficacy and the required power can change tremendously as the data accumulate. For example, some RCTs were designed to evaluate efficacy at day 15, but subsequently, we learned that this time was too early since mortality often occurs later (26). Indeed, a Bayesian reanalysis of RECOVERY data with a wide variety of priors (vague, optimistic, skeptical, and pessimistic) calculated the posterior probability for both any benefit or a modest benefit (number needed to treat, 100). Across all patients, when analyzed with a vague prior, the likelihood of any benefit or a modest benefit was estimated to be 64% and 18%, respectively. In contrast, in the seronegative subgroup, the likelihood of any benefit or a modest benefit was estimated to be 90% and 74%, respectively (80). This finding of benefit accruing to specific subgroups, who were not determined post hoc but because they were likely to benefit based on an understanding of the principles of CP treatment, is found in nearly every trial whose overall finding is negative. This effect is more reflective of a problem with RCT design and execution than a limitation on the efficacy of CCP. Although we agree that subgroup analysis carries the risk of “cherry picking” data, such analyses are often important for hypothesis generation and are critically important during the emergency of a pandemic where neither viral pathogenesis nor therapeutic variables are well understood. When subgroup analyses are based on firm biological principles, such as focusing on those treated early in disease or lacking their own serological response, the exercise may be warranted. Christopher Columbus missed the prespecified primary endpoint of his mission, reaching India, but no one considers his discovery of the New World to be a failure! Turning to the clinical arena, most trials of anticoagulants in myocardial infarction found reductions in mortality of about 20 to 25%, which was generally not significant in these underpowered trials that declared the findings to be null, even though such a mortality reduction would clearly be of value (113). Given that conventional peer review slows down during a pandemic, prepublishing RCT results by the preprint mechanism should be encouraged to accelerate sharing of potentially life-saving therapeutic approaches and to provide prepublication review that could improve the quality of the final published study.
THE FUTURE OF CCP
CCP remains a relatively inexpensive therapy that is available throughout the world, even in resource-poor areas that cannot afford expensive antiviral drugs or monoclonal antibody therapies. Much has been learned about the variables that affect CCP efficacy, even though, as recounted here, the clinical efficacy data are mixed. Table 4 lists the RCTs whose outcomes have still to be reported after completion or which are still recruiting patients. Unfortunately, few new findings can be expected, given that most of these RCTs were designed to enroll patients having symptoms for more than 7 days. Given the heterogeneity of the product and the complex variables that contribute to efficacy, it is remarkable that many studies have reported reductions in mortality. This suggests a likely therapeutic effect that allow signals of efficacy to break through all the noise imposed by variability in the product and its clinical use. The positive evidence for CCP efficacy cannot be dismissed, while in many cases negative results can be explained. In the absence of good therapeutic options for COVID-19, CCP is likely to find a niche in the early treatment of disease. Instead of looking for unlikely superiority outcomes, noninferiority RCTs comparing MAbs with CCP in early arrivals should be initiated. Such an RCT is very unlikely to be sponsored by vendor companies, so public institutions should be sensitized to funding it.
TABLE 4.
Summary of completed but not yet reported or ongoing RCTs of CCP, as registered on ClinicalTrials.gov as of 26 August 2021a
| Status | NCT registration no. | Patient subtype | Control arm | Study design | No. planned to enroll | Study start date | Location |
|---|---|---|---|---|---|---|---|
| Completed | NCT04332835 | Severe (SOFA < 6) | BSC | Single masking (outcome assessor) | 92 | 8 August 2020 | Colombia |
| NCT04349410 | Any | 10 different arms | Single masking (investigator) | 1,800 | 11 April 2020 | USA | |
| NCT04421404 (cAPRI) | Within 3 days from hospitalization or 14 from symptoms | Placebo | Triple masking (participant, care provider, investigator) | 34 | 9 June 2020 | USA | |
| NCT04374526 | Pneumonia, age > 65 yrs, and PaO2/FiO2 ≥ 300 mm Hg and comorbidities | BSC | Open label | 29 | 27 May 2020 | Italy | |
| NCT04358783 | Hospitalized requiring supplemental oxygen | BSC | Quadruple masking (participant, care provider, investigator, outcome assessor) | 30 | 27 April 2020 | Mexico | |
| NCT04405310 | Moderate to severe requiring supplemental oxygen | Albumin | Double (participant, care provider) | 80 | 20 May 2020 | Mexico | |
| NCT04425915 | On ventilator within 3 days from onset of symptoms | BSC | Open label | 400 | 14 June 2020 | India | |
| Active, not recruiting | NCT04539275 | Ventilated and within 3 days from hospitalization | Masked saline placebo | Triple masking (participant, care provider, investigator) | 702 | 16 November 2020 | USA |
| NCT04374487 | Hospitalized and severe | BSC | Open label | 100 | 9 May 2020 | India | |
| NCT04425837 | High-risk | BSC | Single masking (outcome assessor) | 236 | July 2020 | Colombia | |
| NCT04395170 | Hospitalized | 2 arms (BSC; anti-COVID-19 IVIg) | Open label | 75 | September 2020 | Colombia | |
| NCT04391101 | Severe | BSC | Open label | 231 | June 2020 | Colombia | |
| Recruiting | NCT04516811 | Moderate to severe | Placebo | Triple masking (participant, care provider, investigator) | 600 | 21 September 2020 | South Africa |
| NCT04388410 | Hospitalized, severe disease or risk for severe diseases | BSC | Quadruple masking (participant, care provider, investigator, outcome assessor) | 410 | 25 August 2020 | Mexico | |
| NCT04385043 | Severe infection | BSC | Open label | 400 | 1 May 2020 | Italy | |
| NCT04380935 | Acute respiratory distress syndrome | BSC | Open label | 60 | 18 May 2020 | Indonesia | |
| NCT04362176 (PassItOn) | Hospitalized adults | Placebo | Triple masking (participant, care provider, outcome assessor) | 1,000 | 24 April 2020 | USA | |
| NCT04390503 | Exposed within 7 days or mild symptoms within 5 days | Albumin | Double masking (participant, outcome assessor) | 150 | 12 March 2021 | USA | |
| NCT04376034 | Severe or life-threatening | BSC | Open label | 240 | 30 March 2021 | USA | |
| NCT04366245 | Hospitalized, ventilated | BSC | Open label | 72 | 23 April 2020 | Spain | |
| NCT04333251 | Hospitalized within 7 days from symptoms | BSC | Open label | 115 | 1 April 2020 | USA | |
| NCT04345991 | Mild and within 8 days from symptoms | BSC | Open label | 120 | 15 April 2020 | France | |
| NCT04372979 | Hospitalized within 10 days from symptoms | FFP | Triple masking (participant, care provider, outcome assessor) | 80 | 14 September 2020 | France | |
| NCT04345289 | Adults with pneumonia | Placebo | Quadruple masking (participant, care provider, investigator, outcome assessor) | 1,100 | 1 May 2020 | Denmark |
BSC, best supportive care; FFP, fresh frozen plasma. Several studies were withdrawn (NCT04377568).
There is evidence that vaccinated convalescents may have even higher NAb titers than unvaccinated convalescents (114, 115) and that these NAbs are more effective against VOCs than those from unvaccinated convalescents (116, 117), offering the promise of expanded success in using CCP. The higher frequency of high-titer donors would make donor screening more cost-effective. With these donations having being collected from individuals far from hospital discharge, and eventually from periodic blood donors, it is unlikely that these units would benefit from further infectious risk minimization with PRTs, reducing the final cost and avoiding the previously discussed potential problem of the inactivation method interfering with antibody function. Future CCP trials that will start after mass vaccination campaigns should preferentially rely on CCP from vaccinated donors. That said, inclusion of vaccinated individuals (e.g., CSSC-004 [30]) in future RCTs is also a potentially confounder. Indeed, those individuals are at lower risk, thus increasing the outpatient NNTT to prevent a single disease progression and also complicating the identification of seronegative individuals who are more likely to benefit from NAbs.
Low- to middle-income countries (LMIC) are likely to benefit the most from CCP, given that they cannot afford massive deployment of MAbs or small-chemical antivirals. Research is ongoing to spare some of the cold chain requirements for CCP reliance over freeze-dried plasma (FDP), which has been shown to preserve NAb functions for months at ambient temperature (118).
On 8 November 2021, the sudden emergence of the SARS-CoV-2 Omicron VOC with its 32 Spike mutations was an alarming development because it threatened to prolong the pandemic and undermine the immunity gains made with vaccination campaigns. Computer modeling and in vitro VNTs soon confirmed full escape from clinically used MAb cocktails (including bamlanivimab plus etesevimab, REGN-CoV-2, and AZD7442) (119, 120). While it was already known that MAb cocktails were less likely to experience immune escape than single MAbs, most of the scientific community underestimated the risk from major shifts in the three-dimensional Spike structure. Combined with the lengthy manufacturing and approval process for MAb therapies, such sudden shifts largely hinder primarily MAb-based therapies, since polyclonal approaches such as CCP are less vulnerable to losing activity. While even CCP is vulnerable to losing some efficacy with VOCs that manifest partial immune escape, the probability and extent of reduction are far lower (121). In view of this, on 27 December 2021, the FDA updated the EUA expanding authorization to immunosuppressed outpatients (122).
Given the experience accumulated with COVID-19, it is almost certain that CCP will again be considered to deal with the surges yet to come in the current epidemic as well as for the next epidemic. One of the good legacies of the COVID-19 pandemic is that an enormous international research effort had produced a large body of data that teaches how to best use CCP. The lessons learned reinforce the experience of the past, namely that best results are obtained with high-titer CCP administered early in the course of disease. We are hopeful that lessons learned in this pandemic are heeded such that use and trials focus on the very early use of high-titer CCP.
ACKNOWLEDGEMENTS
This article was written in memory of Giuseppe De Donno, Director of the Pneumology Division at the Mantua Hospital, a pioneer in the field of CCP.
Author contributions: D.F. designed the paper, analyzed the data, and wrote the first draft. M.F., M.J.J., T.B., L.P., N.P., and A.C. revised the manuscript. All authors approved the final version.
We declare no conflict of interest.
Biographies

Daniele Focosi is a hematologist employed since 2009 as a resident transfusion physician at the Pisa University Hospital. He was formerly a transplant immunologist and immunogeneticist and a quality assurance manager at the largest blood bank in Italy. He has received awards from the European Federation of Immunogenetics, the European Society of Organ Transplantation, and the Italian Society of Hematology. He has a Ph.D. degree in clinical and fundamental virology and a master’s degree in clinical trials. He has authored 169 articles on topics ranging from emerging viral infections to new markers of immune competence (including more than 50 articles and a SpringerNature book on SARS-CoV-2 evolution), for an h-index of 30.

Massimo Franchini specialized in hematology (1995) at the University of Verona (Italy). He is currently Director of the Department of Hematology and Transfusion Medicine of the Hospital of Mantua (Italy). He is Associate Editor of Seminars in Thrombosis and Hemostasis and Member of the Committee of the Italian Association of Hemophilia Centers (AICE) for the revision of the Italian Guidelines on the Management of Hemophilia. He is a member of the Regional Hematology Network (REL, Lombardy Region), Subcommittee of Hemostasis, and served as a consultant to the Italian Ministry of Health–National Blood Center (2016 to 2020). His field of scientific research is predominantly dedicated to hemostasis and thrombosis. He is currently studying the COVID-19 convalescent plasma, from biological validation to its clinical use. He has authored more than 650 publications for a h-index of 83.

Liise-anne Pirofski is a physician-scientist. Her research programs focus on immunity to encapsulated pathogens and antibody-mediated immunity to infectious diseases. She is chief of the Division of Infectious Diseases and the Jacques and Selma Mitrani Chair in Biomedical Research at Albert Einstein College of Medicine and Montefiore Medical Center. She is a member of the American Association of Physicians and a fellow of the American Academy of Microbiology, the American College of Physicians, the Infectious Diseases Society of America, and the American Association for the Advancement of Science. She is deeply devoted to biomedical education and mentoring, for which she has received numerous accolades, including the American Society for Microbiology William A. Hinton Award, the Albert Einstein College of Medicine Faculty Mentoring Award, the Harry Eagle Award for Outstanding Basic Science Teaching, and the Lifetime Achievement Award from the Albert Einstein College of Medicine Alumni Association.

Thierry Burnouf is a protein biochemist who has authored more than 280 publications in the field of therapeutic blood products for an h-index of 53. He has received the 2019 Award from the International Plasma Fractionation Association. His current research interest focuses on platelet growth factors for regenerative medicine and cell therapy, plasma fractionation, viral inactivation technologies, and blood biotechnology. He is the Secretary of the Working Party on Global Blood Safety and the Treasurer of the Working Party on Cellular Therapies of the International Society of Blood Transfusion.

Nigel Paneth is a pediatrician and perinatal and child health epidemiologist with a particular interest in the causes and prevention of childhood neurodevelopmental handicaps, especially cerebral palsy. He was among the promotors of the U.S. National COVID19 Convalescent Plasma Project (CCPP10). He is Emeritus University Distinguished Professor of Epidemiology and Biostatistics and Pediatrics at Michigan State University and has authored more than 320 publications for an h-index of 86.

Michael J. Joyner has authored more than 535 publications for an h-index of 93. His laboratory is interested in how humans respond to various forms of physical and mental stress during activities such as exercise, hypoxia, standing up, and blood loss. Dr. Joyner is leading a national program sponsored by the U.S. Government to coordinate the collection and distribution of COVID-19 convalescent plasma for the treatment of individuals with severe or life-threatening disease.

Arturo Casadevall is the Chair of the Molecular Microbiology & Immunology Department, Bloomberg Distinguished Professor, and Alfred & Jill Sommer Professor and Chair Professor at the Johns Hopkins School of Public Health. He has authored more than 900 PubMed-indexed articles for an h-index of 133. His research focuses on host defense mechanisms, how fungi cause disease, and the development of antibody-based therapies for infectious diseases.
REFERENCES
- 1.Casadevall A, Pirofski LA, Joyner MJ. 2021. The principles of antibody therapy for infectious diseases with relevance for COVID-19. mBio 12:e03372-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Pirofski L-a. 2020. The convalescent sera option for containing COVID-19. J Clin Invest 130:1545–1548. 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. 2010. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med 38:e66–e73. 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 4.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. 2020. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA 117:9490–9496. 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, Ren L, Wei Q, Mei H, Hu C, Tao C, Yang R, Wang J, Yu Y, Guo Y, Wu X, Xu Z, Zeng L, Xiong N, Chen L, Wang J, Man N, Liu Y, Xu H, Deng E, Zhang X, Li C, Wang C, Su S, Zhang L, Wang J, Wu Y, Liu Z. 2020. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 324:460–470. 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perotti C, Baldanti F, Bruno R, Delfante C, Seminari E, Casari S, Percivalle E, Glingani C, Musella V, Belliato M, Garuti M, Meloni F, Frigato M, Di Sabatino A, Klersy C, De Donno G, Franchini M. 2020. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter interventional trial. Haematologica 105:2834–2840. 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Focosi D, Anderson AO, Tang JW, Tuccori M. 2020. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev 33:e00072-20. 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, Carter RE, Klompas AM, Wiggins CC, Shepherd JR, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Johnson PW, Lesser ER, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Hodge DO, Kunze KL, Buras MR, Vogt MN, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, van Buskirk CM, Winters JL, Stubbs JR, Paneth NS, Verdun NC, Marks P, Casadevall A. 2020. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest 130:4791–4797. 10.1172/jci140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, Verdun NC, Marks P, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, Casadevall A. 2021. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med 384:1015–1027. 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P, Latha B, Bundas S, Kumar V, Dosi R, Khambholja JK, de Souza R, Mesipogu RR, Srivastava S, Dube S, Chaudhary K, Mattuvar SS, Sa K, Rajendran V, Sundararajaperumal A, Balamanikandan P, Maheswari RSU, Jayanthi R, Ragunanthanan S, Bhandari S, Singh A, Pal A, Handa A, Rankawat G, Kargirwar K, Regi J, Rathod D, Pathrose E, Bhutaka N, Patel MH, Verma RJ, Malukani K, Patel S, Thakur A, Joshi S, Kulkarni R, Suthar NN, Shah NM, Purohit HM, Shah CK, Patel MN, Shah S, Shah SH, Memon T, Beriwala VR, et al. 2020. Convalescent plasma in the management of moderate COVID-19 in India: open-label parallel-arm phase II multicentre randomized controlled trial (PLACID Trial). BMJ 371:m3939. 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Donnell MR, Grinsztejn B, Cummings MJ, Justman J, Lamb MR, Eckhardt CM, Philip NM, Cheung YK, Gupta V, João E, Pilotto JH, Diniz MP, Cardoso SW, Abrams D, Rajagopalan K, Borden S, Wolf A, Sidi LC, Vizzoni A, Veloso VG, Bitan ZC, Scotto DE, Meyer BJ, Jacobson SD, Kantor A, Mishra N, Chauhan LV, Stone E, Dei Zotti F, La Carpia F, Hudson KE, Ferrera SA, Schwartz J, Stotler B, Lin W-H, Wontakal S, Shaz B, Briese T, Hod EA, Spitalnik SL, Eisenberger A, Lipkin WI. 2021. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest 131:e150646. 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, Savoy N, Giunta DH, Pérez LG, Sánchez ML, Gamarnik AV, Ojeda DS, Santoro DM, Camino PJ, Antelo S, Rainero K, Vidiella GP, Miyazaki EA, Cornistein W, Trabadelo OA, Ross FM, Spotti M, Funtowicz G, Scordo WE, Losso MH, Ferniot I, Pardo PE, Rodriguez E, Rucci P, Pasquali J, Fuentes NA, Esperatti M, Speroni GA, Nannini EC, Matteaccio A, Michelangelo HG, Follmann D, Lane HC, Belloso WH, PlasmAr Study Group . 2021. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med 384:619–629. 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirofski LA, Casadevall A. 2020. Pathogenesis of COVID-19 from the perspective of the damage-response framework. mBio 11:e01175-20. 10.1128/mBio.01175-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marconato M, Abela IA, Hauser A, Schwarzmueller M, Katzensteiner R, Braun DL, Epp S, Audige A, Weber J, Rusert P, Schindler E, Pasin C, West E, Boeni J, Kufner V, Huber M, Zaheri M, Schmutz S, Frey BM, Kouyos RD, Gunthard HF, Manz MG, Trkola A. 2021. Contribution of endogenous and exogenous antibodies to clearance of SARS-CoV-2 during convalescent plasma therapy. medRxiv. 10.1101/2021.12.09.21267513. [DOI] [Google Scholar]
- 15.Van Rompay KKA, Olstad KJ, Sammak RL, Dutra J, Watanabe JK, Usachenko JL, Immareddy R, Roh JW, Verma A, Lakshmanappa YS, Schmidt BA, Germanio CD, Rizvi N, Stone M, Simmons G, Dumont LJ, Allen AM, Lockwood S, Pollard RE, de Assis RR, Yee JL, Nham PB, Ardeshir A, Deere JD, Patterson J, Jain A, Felgner PL, Iyer SS, Hartigan-O’Connor DJ, Busch MP, Reader JR. 2021. Early post-infection treatment of SARS-CoV-2 infected macaques with human convalescent plasma with high neutralizing activity reduces lung inflammation. bioRxiv. 10.1101/2021.09.01.458520. [DOI] [PMC free article] [PubMed]
- 16.Takamatsu Y, Imai M, Maeda K, Nakajima N, Higashi-Kuwata N, Iwatsuki-Horimoto K, Ito M, Kiso M, Maemura T, Takeda Y, Omata K, Suzuki T, Kawaoka Y, Mitsuya H. 24 November 2021. Highly neutralizing COVID-19 convalescent plasmas potently block SARS-CoV-2 replication and pneumonia in Syrian hamsters. J Virol. 10.1128/JVI.01551-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deere JD, Carroll TD, Dutra J, Fritts L, Sammak RL, Yee JL, Olstad KJ, Reader JR, Kistler A, Kamm J, Di Germanio C, Shaan Lakshmanappa Y, Elizaldi SR, Roh JW, Simmons G, Watanabe J, Pollard RE, Usachenko J, Immareddy R, Schmidt BA, O’Connor SL, DeRisi J, Busch MP, Iyer SS, Van Rompay KKA, Hartigan-O’Connor DJ, Miller CJ. 2021. SARS-CoV-2 infection of rhesus macaques treated early with human COVID-19 convalescent plasma. Microbiol Spectr 9:e0139721. 10.1128/Spectrum.01397-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Focosi D, Maggi F, Franchini M, Aguzzi A, Lanza M, Mazzoni A, Menichetti F. 2021. Patient-blood management for COVID19 convalescent plasma therapy: relevance of affinity and donor-recipient differences in concentration of neutralizing antibodies. Clin Microbiol Infect 27:987–992. 10.1016/j.cmi.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alamgir J, Abid R, Garibaldi BT, Munir N, Setoguchi S, Hong SS, Chen X, Kocis PT, Yajima M, Alexander GC, Mehta HB, Madhira V, Ergas R, O'Brien TR, Bozzette S. 2021. Lack of association between convalescent plasma administration and length of hospital stay: a hospital-day stratified multi-center retrospective cohort study. medRxiv. 10.1101/2021.05.04.21256627. [DOI] [Google Scholar]
- 20.Kocayiğit H, Demir G, Karacan A, Süner K, Tomak Y, Yaylacı S, Dheir H, Kalpakci Y, Erdem AF. 2021. Effects on mortality of early vs late administration of convalescent plasma in the treatment of Covid-19. Transfus Apher Sci 60:103148. 10.1016/j.transci.2021.103148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Focosi D, Navarro D, Maggi F, Roilides E, Antonelli G. 2021. COVID-19 infodemics: the role of mainstream and social media. Clin Microbiol Infect 27:1568–1569. 10.1016/j.cmi.2021.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrell F, Lindsell C. 2021. Statistical design and analysis plan for sequential parallel-group RCT for COVID-19. Vanderbilt University School of Medicine, Nashville, TN. http://hbiostat.org/proj/covid19/bayesplan.html. [Google Scholar]
- 23.Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, Campins M, Portolés A, González-Pérez M, García Morales MT, Arana-Arri E, Aldea M, Díez-Fuertes F, Fuentes I, Ascaso A, Lora D, Imaz-Ayo N, Barón-Mira LE, Agustí A, Pérez-Ingidua C, Gómez de la Cámara A, Arribas JR, Ochando J, Alcamí J, Belda-Iniesta C, Frías J, Martínez de Soto L, Rodríguez Mariblanca A, Díaz García L, Ramírez García E, Seco Meseguer E, Stewart Balbás SM, Marín Candón A, García García I, Urroz Elizalde M, Monserrat Villatoro J, de la Rosa P, Sanz García M, López Crespo C, Mauleón Martínez V, de Madariaga Castell R, Vitón Vara L, García Rodríguez J, Buño A, López Granados E, Cámara C, Rey Cuevas E, Ayllon García P, Jiménez González M, Hernández Rubio V, et al. 2021. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 398:121–130. 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. 2020. WHO R & D blueprint: novel coronavirus: COVID19 therapeutic trial synopsis. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf?ua=1. Accessed October 1.
- 25.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. 2020. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 20:e192–e197. 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ortigoza MB, Yoon H, Goldfeld KS, Troxel AB, Daily JP, Wu Y, Li Y, Wu D, Cobb GF, Baptiste G, O'Keeffe M, Corpuz MO, Ostrosky-Zeichner L, Amin A, Zacharioudakis IM, Jayaweera DT, Wu Y, Philley JV, Devine MS, Desruisseaux MS, Santin AD, Anjan S, Mathew R, Patel B, Nigo M, Upadhyay R, Kupferman T, Dentino AN, Nanchal R, Merlo CA, Hager DN, Chandran K, Lai JR, Rivera J, Bikash CR, Lasso G, Hilbert TP, Paroder M, Asencio AA, Liu M, Petkova E, Bragat A, Shaker R, McPherson DD, Sacco RL, Keller MJ, Grudzen CR, Hochman JS, Pirofski LA, Parameswaran L. 2021. Efficacy and safety of COVID-19 convalescent plasma in hospitalized patients: a randomized clinical trial. JAMA Intern Med 182:115–126. 10.1001/jamainternmed.2021.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, El Kassar N, Foster LD, Hah JM, Jaiswal S, Kaplan A, Lowell E, McDyer JF, Quinn J, Triulzi DJ, Van Huysen C, Stevenson VLW, Yadav K, Jones CW, Kea B, Burnett A, Reynolds JC, Greineder CF, Haas NL, Beiser DG, Silbergleit R, Barsan W, Callaway CW. 2021. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med 385:1951–1960. 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millat-Martinez P, Gharbharan A, Alemany A, Rokx C, GeurtsvanKessel C, Papageourgiou G, van Geloven N, Jordans C, Groeneveld G, Swaneveld F, van der Schoot E, Corbacho-Monne M, Ouchi D, Ferreira FP, Malchair P, Videla S, Garcia VG, Ruiz-Comellas A, Ramirez-Morros A, Codina JR, Simon RA, Grifols J-R, Blanco J, Blanco I, Ara J, Bassat Q, Clotet B, Baro B, Troxel A, Zwaginga JJ, Mitja O, Rijnders B. 2021. Convalescent plasma for outpatients with early COVID-19. medRxiv. 10.1101/2021.11.30.21266810. [DOI] [Google Scholar]
- 29.Alemany A, Millat-Martinez P, Corbacho-Monné M, Malchair P, Ouchi D, Ruiz-Comellas A, Ramírez-Morros A, Rodríguez CJ, Amado SR, Videla S, Costes G, Capdevila-Jáuregui M, Torrano-Soler P, San José A, Bonet Papell G, Puig J, Otero A, Ruibal Suarez J, Zarauza Pellejero A, Llopis Roca F, Rodriguez Cortez O, Garcia Garcia V, Vidal-Alaball J, Millan A, Contreras E, Grifols J, Piccolo Ferreira F, Bonet M, Cantoni J, Prat N, Ara J, Forcada Arcarons A, Farré M, Pradenas E, Blanco J, Rodriguez-Arias M, Fernández Rivas G, Marks M, Bassat Q, Blanco I, Baro B, Clotet B, Mitjà O, GROUP C-e . 2022. High-titre methylene-blue treated convalescent plasma as an early treatment for COVID-19 outpatients: a randomized clinical trial. Lancet Respir Med 9:S2213-2600(21)00545-2. 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan D, Gebo K, Shoham S, Bloch E, Lau B, Shenoy A, Mosnaim G, Gniadek T, Fukuta Y, Patel B, Heath S, Levine A, Meisenberg B, Spivak E, Anjan S, Huaman M, Blair J, Currier J, Paxton J, Gerber J, Petrini J, Broderick P, Rausch W, Cordisco MJH, Greenblatt B, Cluzet V, Cruser D, Oei K, Abinante M, Hammitt L, Sutcliffe C, Forthal D, Zand M, Cachay E, Raval J, Kassaye S, Foster E, Roth M, Marshall C, Yarava A, Lane K, McBee N, Gawad A, Karlen N, Singh A, Ford D, Jabs D, Appel L, Shade D, et al. 2021. Randomized controlled trial of early outpatient COVID-19 treatment with high-titer convalescent plasma. medRxiv. 10.1101/2021.12.10.21267485. [DOI] [Google Scholar]
- 31.Shoham S, Bloch EM, Casadevall A, Hanley D, Lau B, Gebo K, Cachay E, Kassaye SG, Paxton JH, Gerber J, Levine AC, Currier J, Patel B, Allen ES, Anjan S, Appel L, Baksh S, Blair PW, Bowen A, Broderick P, Caputo CA, Cluzet V, Cordisco ME, Cruser D, Ehrhardt S, Forthal D, Fukuta Y, Gawad A, Gniadek T, Hammel J, Huaman MA, Jabs DA, Jedlicka A, Karlen N, Klein S, Laeyendecker O, Lane K, McBee N, Meisenberg B, Merlo C, Mosnaim G, Park H-S, Pekosz A, Petrini J, Rausch W, Shade DM, Shapiro J, Singleton JR, Sutcliffe C, Thomas DL, et al. 2021. Randomized controlled trial transfusing convalescent plasma as post-exposure prophylaxis against SARS-CoV-2 infection. medRxiv. 10.1101/2021.12.13.21267611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Focosi D, Mazzetti P, Pistello M, Maggi F. 2021. Viral infection neutralization tests: a focus on SARS-CoV-2 with implications for convalescent plasma therapy. Rev Med Virol 31:e2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen D, Simmonds P, Steenhuis M, Wouters E, Desmecht D, Garigliany M, Romano M, Barbezange C, Maes P, Van Holm B, Mendoza J, Oyonarte S, Fomsgaard A, Lassaunière R, Zusinaite E, Resman Rus K, Avšič-Županc T, Reimerink JH, Brouwer F, Hoogerwerf M, Reusken CB, Grodeland G, Le Cam S, Gallian P, Amroun A, Brisbarre N, Martinaud C, Leparc Goffart I, Schrezenmeier H, Feys HB, van der Schoot CE, Harvala H. 2021. SARS-CoV-2 neutralising antibody testing in Europe: towards harmonisation of neutralising antibody titres for better use of convalescent plasma and comparability of trial data. Euro Surveill 26:2100568. 10.2807/1560-7917.ES.2021.26.27.2100568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devos T, Van Thillo Q, Compernolle V, Najdovski T, Romano M, Dauby N, Jadot L, Leys M, Maillart E, Loof S, Seyler L, Moonen M, Moutschen M, Van Regenmortel N, Ariën KK, Barbezange C, Betrains A, Garigliany M, Engelen MM, Gyselinck I, Maes P, Schauwvlieghe A, Liesenborghs L, Belmans A, Verhamme P, Meyfroidt G. 2021. Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur Respir J 59:2101724. 10.1183/13993003.01724-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estcourt LJ, Turgeon AF, McQuilten ZK, McVerry BJ, Al-Beidh F, Annane D, Arabi YM, Arnold DM, Beane A, Bégin P, van Bentum-Puijk W, Berry LR, Bhimani Z, Birchall JE, Bonten MJM, Bradbury CA, Brunkhorst FM, Buxton M, Callum JL, Chassé M, Cheng AC, Cove ME, Daly J, Derde L, Detry MA, De Jong M, Evans A, Fergusson DA, Fish M, Fitzgerald M, Foley C, Goossens H, Gordon AC, Gosbell IB, Green C, Haniffa R, Harvala H, Higgins AM, Hills TE, Hoad VC, Horvat C, Huang DT, Hudson CL, Ichihara N, Laing E, Lamikanra AA, Lamontagne F, Lawler PR, Linstrum K, Litton E, et al. 2021. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial. JAMA 326:1690–1702. 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gharbharan A, Jordans CCE, GeurtsvanKessel C, den Hollander JG, Karim F, Mollema FPN, Stalenhoef JE, Dofferhoff A, Ludwig I, Koster A, Hassing R-J, Bos JC, van Pottelberge GR, Vlasveld IN, Ammerlaan HSM, Segarceanu E, Miedema J, van der Eerden M, Papageorgiou G, Te Broekhorst P, Swaneveld FH, Katsikis PD, Mueller Y, Okba NMA, Koopmans MPG, Haagmans BL, Rokx C, Rijnders B. 2021. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat Comm 12:3189. 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Fante C, Franchini M, Baldanti F, Percivalle E, Glingani C, Marano G, Mengoli C, Mortellaro C, Viarengo G, Perotti C, Liumbruno GM. 2021. A retrospective study assessing the characteristics of COVID-19 convalescent plasma donors and donations. Transfusion 61:830–838. 10.1111/trf.16208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehew J, Johnson R, Roberts D, Harvala H. 2020. Convalescent plasma for COVID-19: male gender, older age and hospitalisation associated with high neutralising antibody levels, England, 22 April to 12 May 2020. Euro Surveill 25:2001754. 10.2807/1560-7917.ES.2020.25.45.2001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiuzzo P, Bentley E, Hassal M, Routley S, Richardson S, Bernasconi V, Kristianse P, Harvala H, Roberts D, Semple M, Turtle L, Openshaw P, Baillie K. 2020. WHO/BS/2020.2403: Establishment of the WHO International Standard and Reference Panel for Anti-SARS-CoV-2 Antibody. WHO Expert Committee on Biological Standardization, Geneva, Switzerland. [Google Scholar]
- 40.Klingler J, Weiss S, Itri V, Liu X, Oguntuyo KY, Stevens C, Ikegame S, Hung C-T, Enyindah-Asonye G, Amanat F, Baine I, Arinsburg S, Bandres JC, Kojic EM, Stoever J, Jurczyszak D, Bermudez-Gonzalez M, Simon V, Nádas A, Liu S, Lee B, Krammer F, Zolla-Pazner S, Hioe CE. 2021. Role of IgM and IgA antibodies in the neutralization of SARS-CoV-2. J Infect Dis 223:957–970. 10.1093/infdis/jiaa784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egloff SAA, Junglen A, Restivo JSA, Wongskhaluang M, Martin C, Doshi P, Schlauch D, Fromell G, Sears LE, Correll M, Burris HA, LeMaistre CF. 2021. Convalescent plasma associates with reduced mortality and improved clinical trajectory in patients hospitalized with COVID-19. J Clin Invest 131:e151788. 10.1172/JCI151788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Natarajan H, Crowley AR, Butler SE, Xu S, Weiner JA, Bloch EM, Littlefield K, Wieland-Alter W, Connor RI, Wright PF, Benner SE, Bonny TS, Laeyendecker O, Sullivan D, Shoham S, Quinn TC, Larman HB, Casadevall A, Pekosz A, Redd AD, Tobian AAR, Ackerman ME. 2021. Markers of polyfunctional SARS-CoV-2 antibodies in convalescent plasma. mBio 12:e00765-21. 10.1128/mBio.00765-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Focosi D, Franchini M. 2021. Impact of pathogen-reduction technologies on COVID-19 convalescent plasma potency. Transfus Clin Biol 28:132–134. 10.1016/j.tracli.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bojadzic D, Alcazar O, Buchwald P. 2021. Methylene blue inhibits the SARS-CoV-2 Spike–ACE2 protein-protein interaction—a mechanism that can contribute to its antiviral activity against COVID-19. Front Pharmacol 11:600372. 10.3389/fphar.2020.600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross V. 1938. Photodynamic action of methylene blue on antipneumococcal serum. J Immunol 35:351–369. [Google Scholar]
- 46.Menichetti F, Popoli P, Puopolo M, Spila Alegiani S, Tiseo G, Bartoloni A, De Socio G, Luchi S, Blanc P, Puoti M, De Donno G, Toschi E, Massari M, Palmisano L, Marano G, Chiamenti M, Martinelli L, Franchi S, Pallotto C, Suardi L, Luciani Pasqua B, Merli M, Fabiani P, Bertolucci L, Borchi B, Modica S, Moneta S, Marchetti G, d'Arminio Monforte A, Stoppini L, Ferracchiato N, Piconi S, Fabbri C, Beccastrini E, Saccardi R, Giacometti A, Esperti S, Pierotti P, Bernini l, Bianco C, Benedetti S, Lanzi A, Bonfanti P, Massari M, Sani S, Saracino A, Castagna A, Trabace l, Lanza M, Focosi D, et al. 2021. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open 4:e2136246. 10.1001/jamanetworkopen.2021.36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, Conroy RM, Wasif P, Atkin SL, Otoom S, Abduljalil M. 2021. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci Rep 11:9927. 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Malo de Molina R, Torres F, Fernandez-Cruz A, Callejas-Diaz A, Calderon J, Payares-Herrera C, Salcedo I, Romera I, Lora-Tamayo J, Mancheno-Losa M, Paciello ML, Villegas C, Estrada V, Saez-Serrano I, Porras-Leal ML, Jarilla-Fernandez MC, Pano-Pardo JR, Moreno-Chulilla JA, Arrieta-Aldea I, Bosch A, Belhassen-Garcia M, Lopez-Villar O, Ramos-Garrido A, Blanco L, Madrigal ME, Contreras E, Muniz-Diaz E, Domingo-Morera JM, Casas-Flecha I, Perez-Olmeda M, Garcia-Perez J, Alcami J, Bueno JL, Duarte RF. 2021. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J Clin Invest 131:e152740. 10.1172/JCI152740:152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raster J, Zimmermann K, Wesche J, Aurich K, Greinacher A, Selleng K. 2021. Effect of methylene blue pathogen inactivation on the integrity of immunoglobulin M and G. Transfus Med Hemother 48:148–153. 10.1159/000514485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, Spata E, Pessoa-Amorim G, Campbell M, Roddick A, Brunskill NE, George T, Zehnder D, Tiberi S, Aung NN, Uriel A, Widdrington J, Koshy G, Brown T, Scott S, Baillie JK, Buch MH, Chappell LC, Day JN, Faust SN, Jaki T, Jeffery K, Juszczak E, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Mafham M, Roberts D, Haynes R, Landray MJ. 2021. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 397:2049–2059. 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bahnan W, Wrighton S, Sundwall M, Bläckberg A, Larsson O, Höglund U, Khakzad H, Godzwon M, Walle M, Elder E, Happonen L, André O, Kumra Ahnlide J, Hellmark T, Wendel-Hansen V, Wallin RP, Malmström J, Malmström L, Ohlin M, Rasmussen M, Nordenfelt P. 14 October 2021. Opsonization by non-neutralizing antibodies can confer protection to SARS-CoV-2 despite Spike-dependent modulation of phagocytosis. bioRxiv. 10.1101/2021.10.14.464464. [DOI] [PMC free article] [PubMed]
- 52.Koerper S, Weiss M, Zickler D, Wiesmann T, Zacharowski K, Corman VM, Gruener B, Ernst L, Spieth P, Lepper PM, Bentz M, Zinn S, Paul G, Kalbhenn J, Dollinger M, Rosenberger P, Kirschning T, Thiele T, Appl T, Mayer B, Schmidt M, Drosten C, Wulf H, Kruse JM, Jungwirth B, Seifried E, Schrezenmeier H, CAPSID Clinical Trial Group . 2021. Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients. J Clin Invest 131:e1552264. 10.1172/JCI152264:2021.05.10.21256192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kunze KL, Johnson PW, van Helmond N, Senefeld JW, Petersen MM, Klassen SA, Wiggins CC, Klompas AM, Bruno KA, Mills JR, Theel ES, Buras MR, Golafshar MA, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, Verdun NC, Marks P, Paneth NS, Fairweather D, Wright RS, van Buskirk CM, Winters JL, Stubbs JR, Senese KA, Pletsch MC, Buchholtz ZA, Rea RF, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Lesser ER, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Casadevall A, Carter RE, Joyner MJ. 2021. Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors. Nat Commun 12:4864. 10.1038/s41467-021-25113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rochman ND, Wolf YI, Faure G, Mutz P, Zhang F, Koonin EV. 2021. Ongoing global and regional adaptive evolution of SARS-CoV-2. Proc Natl Acad Sci USA 118:e02104241118. 10.1073/pnas.2104241118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Focosi D, Tuccori M, Baj A, Maggi F. 2021. SARS-CoV-2 variants: a synopsis of in vitro efficacy data of convalescent plasma, currently marketed vaccines, and monoclonal antibodies. Viruses 13:1211. 10.3390/v13071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Junker D, Dulovic A, Becker M, Wagner TR, Kaiser PD, Traenkle B, Rothbauer U, Kienzle K, Bunk S, Strümper C, Häberle H, Schmauder K, Malek N, Althaus K, Koeppen M, Bitzer M, Göpel S, Schneiderhan-Marra N. 2021. Reduced serum neutralization capacity against SARS-CoV-2 variants in a multiplex ACE2 RBD competition assay. medRxiv. 10.1101/2021.08.20.21262328. [DOI] [Google Scholar]
- 57.Bennett-Guerrero E, Romeiser JL, Talbot LR, Ahmed T, Mamone LJ, Singh SM, Hearing JC, Salman H, Holiprosad DD, Freedenberg AT, Carter JA, Browne NJ, Cosgrove ME, Shevik ME, Generale LM, Andrew MA, Nachman S, Fries BC. 2021. Severe acute respiratory syndrome coronavirus 2 convalescent plasma versus standard plasma in coronavirus disease 2019 infected hospitalized patients in New York: a double-blind randomized trial. Crit Care Med 49:1015–1025. 10.1097/ccm.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajpai M, Kumar S, Maheshwari A, Chhabra K, Kale P, Gupta A, Narayanan A, Gupta E, Trehanpati N, Bihari C, Agarwal R, Gupta K, Gupta U, Bhardwaj A, Kumar G, Islam M, Singh R, Yadav P, Maiwall R, Sarin SK. 2020. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. medRxiv. 10.1101/2020.10.25.20219337. [DOI] [Google Scholar]
- 59.Recovery Collaborative Group, Horby PW, Mafham M, Peto L, Campbell M, Pessoa-Amorim G, Spata E, Staplin N, Emberson JR, Prudon B, Hine P, Brown T, Green CA, Sarkar R, Desai P, Yates B, Bewick T, Tiberi S, Felton T, Baillie JK, Buch MH, Chappell LC, Day JN, Faust SN, Jaki T, Jeffery K, Juszczak E, Lim WS, Montgomery A, Mumford A, Rowan K, Thwaites G, Weinreich DM, Haynes R, Landray MJ. 2021. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 10.1101/2021.06.15.21258542. [DOI] [Google Scholar]
- 60.RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. 2021. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384:693–704. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senefeld JW, Johnson PW, Kunze KL, Bloch EM, van Helmond N, Golafshar MA, Klassen SA, Klompas AM, Sexton MA, Diaz Soto JC, Grossman BJ, Tobian AAR, Goel R, Wiggins CC, Bruno KA, van Buskirk CM, Stubbs JR, Winters JL, Casadevall A, Paneth NS, Shaz BH, Petersen MM, Sachais BS, Buras MR, Wieczorek MA, Russoniello B, Dumont LJ, Baker SE, Vassallo RR, Shepherd JRA, Young PP, Verdun NC, Marks P, Haley NR, Rea RF, Katz L, Herasevich V, Waxman DA, Whelan ER, Bergman A, Clayburn AJ, Grabowski MK, Larson KF, Ripoll JG, Andersen KJ, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, et al. 2021. Access to and safety of COVID-19 convalescent plasma in the United States Expanded Access Program: a national registry study. PLoS Med 18:e1003872. 10.1371/journal.pmed.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, Wiggins CC, Bruno KA, Klompas AM, Lesser ER, Kunze KL, Sexton MA, Diaz Soto JC, Baker SE, Shepherd JRA, van Helmond N, van Buskirk CM, Winters JL, Stubbs JR, Rea RF, Hodge DO, Herasevich V, Whelan ER, Clayburn AJ, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MNP, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, Paneth NS, Fairweather D, Wright RS, Carter RE, Casadevall A. 2020. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. medRxiv. 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 63.Gonzalez SE, Regairaz L, Salazar M, Ferrando N, Gonzalez V, Carrera Ramos P, Pesci S, Vidal JM, Kreplak N, Estenssoro E. 2022. Timing of convalescent plasma administration and 28-day mortality for COVID-19 pneumonia. J Investig Med 8:jim-2021-002158. 10.1136/jim-2021-002158. [DOI] [PubMed] [Google Scholar]
- 64.De Silvestro G, Marson P, La Raja M, Cattelan A, Guarnieri G, Monticelli J, Tiberio I, Vianello A, Gandini G, Gessoni G, Fiorin F, Sardella C, Astolfi L, Saia M. 2021. Outcome of SARS CoV-2 inpatients treated with convalescent plasma: one-year of data from the Veneto region (Italy) Registry. Eur J Int Med. 10.1016/j.ejim.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoon HA, Bartash R, Gendlina I, Rivera J, Nakouzi A, Bortz RH, Wirchnianski AS, Paroder M, Fehn K, Serrano-Rahman L, Babb R, Sarwar UN, Haslwanter D, Laudermilch E, Florez C, Dieterle ME, Jangra RK, Fels JM, Tong K, Mariano MC, Vergnolle O, Georgiev GI, Herrera NG, Malonis RJ, Quiroz JA, Morano NC, Krause GJ, Sweeney JM, Cowman K, Allen S, Annam J, Applebaum A, Barboto D, Khokhar A, Lally BJ, Lee A, Lee M, Malaviya A, Sample R, Yang XA, Li Y, Ruiz R, Thota R, Barnhill J, Goldstein DY, Uehlinger J, Garforth SJ, Almo SC, Lai JR, Gil MR, et al. 2021. Treatment of severe COVID-19 with convalescent plasma in the Bronx, NYC. JCI Insight 6:e142270. 10.1172/jci.insight.142270:142270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, Rodriguez D, Tandon P, Bassily-Marcus A, Bander J, Sanky C, Dupper A, Zheng A, Nguyen FT, Amanat F, Stadlbauer D, Altman DR, Chen BK, Krammer F, Mendu DR, Firpo-Betancourt A, Levin MA, Bagiella E, Casadevall A, Cordon-Cardo C, Jhang JS, Arinsburg SA, Reich DL, Aberg JA, Bouvier NM. 2020. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med 26:1708–1713. 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 67.Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, Lopez BV, Eagar TN, Yi X, Zhao P, Rogers J, Shehabeldin A, Joseph D, Leveque C, Olsen RJ, Bernard DW, Gollihar J, Musser JM. 2020. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol 190:2290–2303. 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, Lopez BV, Eagar TN, Yi X, Zhao P, Rogers J, Shehabeldin A, Joseph D, Masud F, Leveque C, Olsen RJ, Bernard DW, Gollihar J, Musser JM. 2021. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG. Am J Pathol 191:90–107. 10.1016/j.ajpath.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers R, Shehadeh F, Mylona EK, Rich J, Neill M, Touzard-Romo F, Geffert S, Larkin J, Bailey JA, Lu S, Sweeney J, Mylonakis E. 2021. Convalescent plasma for patients with severe COVID-19: a matched cohort study. Clin Infect Dis 73:e208–e214. 10.1093/cid/ciaa1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Briggs N, Gormally MV, Li F, Browning SL, Treggiari MM, Morrison A, Laurent-Rolle M, Deng Y, Hendrickson JE, Tormey CA, Desruisseaux MS. 2021. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS One 16:e0254453. 10.1371/journal.pone.0254453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shenoy AG, Hettinger AZ, Fernandez SJ, Blumenthal J, Baez V. 2021. Early mortality benefit with COVID-19 convalescent plasma: a matched control study. Br J Haematol 192:706–713. 10.1111/bjh.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnold Egloff SA, Junglen A, Restivo JSA, Wongskhauluang M, Martin C, Doshi P, Schlauch D, Fromell G, Sears LE, Correll M, Burris HA, LeMaistre CF. 2021. Association of convalescent plasma treatment with reduced mortality and improved clinical trajectory in patients hospitalized with COVID-19 in the community setting. medRxiv. 10.1101/2021.06.02.21258190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, Esteban I, Caballero MT, Wood C, Berrueta M, Rondan A, Lescano G, Cruz P, Ritou Y, Fernández Viña V, Álvarez Paggi D, Esperante S, Ferreti A, Ofman G, Ciganda Á, Rodriguez R, Lantos J, Valentini R, Itcovici N, Hintze A, Oyarvide ML, Etchegaray C, Neira A, Name I, Alfonso J, López Castelo R, Caruso G, Rapelius S, Alvez F, Etchenique F, Dimase F, Alvarez D, Aranda SS, Sánchez Yanotti C, De Luca J, Jares Baglivo S, Laudanno S, Nowogrodzki F, Larrea R, Silveyra M, Leberzstein G, Debonis A, Molinos J, González M, Perez E, Fundación INFANT–COVID-19 Group, et al. 2021. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 384:610–618. 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ray Y, Paul SR, Bandopadhyay P, D'Rozario R, Sarif J, Raychaudhuri D, Bhowmik D, Lahiri A, Vasudevan JS, Maurya R, Kanakan A, Sharma S, Kumar M, Singh P, Roy R, Chaudhury K, Maiti R, Bagchi S, Maiti A, Perwez MM, Mondal A, Tewari A, Mandal S, Roy A, Saha M, Biswas D, Maiti C, Bhaduri R, Chakraborty S, Sarkar BS, Haldar A, Saha B, Sengupta S, Pandey R, Chatterjee S, Bhattacharya P, Paul S, Ganguly D. 2022. A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19. Nat Commun 13:383. 10.1038/s41467-022-28064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Epstein J, Burnouf T. 2020. Points to consider in the preparation and transfusion of COVID-19 convalescent plasma. Vox Sang 115:485–487. 10.1111/vox.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, Grossman BJ, Henderson JP, Musser J, Salazar E, Hartman WR, Bouvier NM, Liu STH, Pirofski LA, Baker SE, van Helmond N, Wright RS, Fairweather D, Bruno KA, Wang Z, Paneth NS, Casadevall A, Joyner MJ. 2021. The effect of convalescent plasma therapy on mortality among patients with COVID-19: systematic review and meta-analysis. Mayo Clin Proc 96:1262–1275. 10.1016/j.mayocp.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, Hepprich M, Smith ER, Haber NA, Khanna N, Moher D, Goodman SN, Ioannidis JPA, Hemkens LG. 2021. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA 325:1185–1195. 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, Monsef I, Wood EM, Lamikanra AA, Roberts DJ, McQuilten Z, So-Osman C, Estcourt LJ, Skoetz N. 2021. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev 5:Cd013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Axfors C, Janiaud P, Schmitt AM, Van't Hooft J, Smith ER, Haber NA, Abayomi A, Abduljalil M, Abdulrahman A, Acosta-Ampudia Y, Aguilar-Guisado M, Al-Beidh F, Alejandria MM, Alfonso RN, Ali M, AlQahtani M, AlZamrooni A, Anaya J-M, Ang MAC, Aomar IF, Argumanis LE, Averyanov A, Baklaushev VP, Balionis O, Benfield T, Berry S, Birocco N, Bonifacio LB, Bowen AC, Bown A, Cabello-Gutierrez C, Camacho B, Camacho-Ortiz A, Campbell-Lee S, Cao DH, Cardesa A, Carnate JM, Castillo GJJ, Cavallo R, Chowdhury FR, Chowdhury FUH, Ciccone G, Cingolani A, Climacosa FMM, Compernolle V, Cortez CFN, Costa Neto A, D'Antico S, Daly J, Danielle F, et al. 2021. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis 21:1170. 10.1186/s12879-021-06829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamilton FW, Lee TC, Arnold DT, Lilford RJ, Hemming K. 2021. Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomised controlled trial. Int J Infect Dis 109:114–117. 10.1016/j.ijid.2021.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cruciani M, Bongiovanni G, Franchini M. 2021. High-titer convalescent plasma therapy for coronavirus disease 2019 and mortality. Transfusion 61:1988–1990. 10.1111/trf.16434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klassen SA, Senefeld JW, Senese KA, Johnson PW, Wiggins CC, Baker SE, van Helmond N, Bruno KA, Pirofski LA, Shoham S, Grossman BJ, Henderson JP, Wright RS, Fairweather D, Paneth NS, Carter RE, Casadevall A, Joyner MJ. 2021. Convalescent plasma therapy for COVID-19: a graphical mosaic of the worldwide evidence. Front Med (Lausanne) 8:684151. 10.3389/fmed.2021.684151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casadevall A, Grossman BJ, Henderson JP, Joyner MJ, Paneth NS, Pirofski LA, Shoham S, 2021. Please reevaluate the data on convalescent plasma for COVID-19. https://www.medpagetoday.com/opinion/second-opinions/94256?xid=nl_mpt_DHE_2021-08-30&eun=g1783322d0r&utm_source=Sailthru&utm_medium=email&utm_campaign=Daily%20Headlines%20Top%20Cat%20HeC%20%202021-08-30&utm_term=NL_Daily_DHE_dual-gmail-definition. Accessed 31 August 2021.
- 84.Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Leo Y-S, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kim Y-J, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Preller J, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, et al. 2020. A living WHO guideline on drugs for covid-19. BMJ 370:m3379. [DOI] [PubMed] [Google Scholar]
- 85.de Candia P, Prattichizzo F, Garavelli S, La Grotta R, De Rosa A, Pontarelli A, Parrella R, Ceriello A, Matarese G. 2021. Effect of time and titer in convalescent plasma therapy for COVID-19. iScience 24:102898. 10.1016/j.isci.2021.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kloypan C, Saesong M, Sangsuemoon J, Chantharit P, Mongkhon P. 2021. Convalescent plasma for COVID-19: a meta-analysis of clinical trials and real-world evidence. Eur J Clin Invest 51:e13663. 10.1111/eci.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franchini M, Focosi D, Corsini F, Cruciani M. 2021. Safety and efficacy of convalescent plasma in COVID-19: an overview of systematic reviews. Diagnostics 11:1663. 10.3390/diagnostics11091663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bar K, Shaw P, Choi G, Aqui N, Fesnak A, Yang J, Soto-Calderon H, Grajales L, Starr J, Andronov M, Mastellone M, Amonu C, Feret G, DeMarshall M, Buchanan M, Caturla M, Gordon J, Wanicur A, Monroy M, Mampe F, Lindemuth E, Gouma S, Mullin A, Barilla H, Pronina A, Irwin L, Thomas R, Eichinger R, Demuth F, Prak E, Pascual J, Short W, Elovitz M, Baron J, Meyer N, Degnan K, Frank I, Hensley S, Siegel D, Tebas P. 2021. A randomized, controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J Clin Invest 131:e155114. 10.1172/JCI155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anonymous. 2021. Continuous monitoring of pooled international trials of convalescent plasma for COVID-19 hospitalized patients. https://med.nyu.edu/departments-institutes/population-health/divisions-sections-centers/biostatistics/research/continuous-monitoring-pooled-international-trials-convalescent-plasma-covid19-hospitalized-patients. Accessed 31 August 2021.
- 90.Troxel AB, Petkova E, Goldfeld K, Liu M, Tarpey T, Wu Y, Wu D, Agarwal A, Avendaño-Solá C, Bainbridge E, Bar KJ, Devos T, Duarte RF, Gharbharan A, Hsue PY, Kumar G, Luetkemeyer AF, Meyfroidt G, Nicola AM, Mukherjee A, Ortigoza MB, Pirofski LA, Rijnders BJA, Rokx C, Sancho-Lopez A, Shaw P, Tebas P, Yoon HA, Grudzen C, Hochman J, Antman EM. 2022. Association of convalescent plasma treatment with clinical status in patients hospitalized with COVID-19: a meta-analysis. JAMA Netw Open 5:e2147331. 10.1001/jamanetworkopen.2021.47331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park H, Tarpey T, Liu M, Goldfeld K, Wu Y, Wu D, Li Y, Zhang J, Ganguly D, Ray Y, Paul SR, Bhattacharya P, Belov A, Huang Y, Villa C, Forshee R, Verdun NC, Yoon HA, Agarwal A, Simonovich VA, Scibona P, Burgos Pratx L, Belloso W, Avendaño-Solá C, Bar KJ, Duarte RF, Hsue PY, Luetkemeyer AF, Meyfroidt G, Nicola AM, Mukherjee A, Ortigoza MB, Pirofski LA, Rijnders BJA, Troxel A, Antman EM, Petkova E. 2022. Development and validation of a treatment benefit index to identify hospitalized patients with COVID-19 who may benefit from convalescent plasma. JAMA Netw Open 5:e2147375. 10.1001/jamanetworkopen.2021.47375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Focosi D, Franchini M, Pirofski L-a, Burnouf T, Fairweather D, Joyner MJ, Casadevall A. 2021. COVID-19 convalescent plasma is more than neutralizing antibodies: a narrative review of potential beneficial and detrimental co-factors. Viruses 13:1594. 10.3390/v13081594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Franchini M, Cruciani M. 2021. How safe is COVID-19 convalescent plasma? Mayo Clin Proc 96:2279–2281. 10.1016/j.mayocp.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, Wiggins CC, Senefeld JW, Klompas AM, Hodge DO, Shepherd JR, Rea RF, Whelan ER, Clayburn AJ, Spiegel MR, Baker SE, Larson KF, Ripoll JG, Andersen KJ, Buras MR, Vogt MN, Herasevich V, Dennis JJ, Regimbal RJ, Bauer PR, Blair JE, van Buskirk CM, Winters JL, Stubbs JR, van Helmond N, Butterfield BP, Sexton M, Diaz Soto J, Paneth NS, Verdun NC, Marks P, Casadevall A, Fairweather D, Carter RE, Wright RS. 2020. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clinic Proc 95:1888–1895. 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. 2020. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol 5:1185–1191. 10.1038/s41564-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Focosi D, Novazzi F, Genoni A, Dentali F, Dalla Gasperina D, Baj A, Maggi F. 2021. Emergence of SARS-CoV-2 spike escape mutation Q493R after treatment for COVID-19. Emerg Infect Dis 27:2728–2731. 10.3201/eid2710.211538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guigon A, Faure E, Lemaire C, Chopin M, Tinez C, Assaf A, Lazrek M, Hober D, Bocket L, Engelmann I, Kazali Alidjinou E. 2021. Emergence of Q493R mutation in SARS-CoV-2 spike protein during bamlanivimab/etesevimab treatment and resistance to viral clearance. J Infect 84:248–288. 10.1016/j.jinf.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jensen B, Luebke N, Feldt T, Keitel V, Brandenburger T, Kindgen-Milles D, Lutterbeck M, Freise NF, Schoeler D, Haas R, Dilthey A, Adams O, Walker A, Timm J, Luedde T. 2021. Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany. Lancet Reg Health Eur 8:100164. 10.1016/j.lanepe.2021.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pommeret F, Colomba J, Bigenwald C, Laparra A, Bockel S, Bayle A, Michot JM, Hueso T, Albiges L, Tiberghien P, Marot S, Jary A, Lacombe K, Barlesi F, Griscelli F, Colomba E. 2021. Bamlanivimab + etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann Oncol 32:1445–1447. 10.1016/j.annonc.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Focosi D, Franchini M. 2021. Potential use of convalescent plasma for SARS-CoV-2 prophylaxis and treatment in immunocompromised and vulnerable populations. Expert Rev Vaccines 2021 May 27:1–8. 10.1080/14760584.2021.1932475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anonymous. 2021. CoVIg-19 Plasma Alliance announces topline results from NIH-sponsored clinical trial of investigational COVID-19 hyperimmune globulin medicine. https://www.takeda.com/newsroom/newsreleases/2021/covig-19-plasma-alliance-announces-topline-results-from-nih-sponsored-clinical-trial-of-investigational-covid-19-hyperimmune-globulin-medicine/. Accessed 2 April 2021.
- 102.ACTIV-3/TICO LY-CoV555 Study Group, Lundgren JD, Grund B, Barkauskas CE, Holland TL, Gottlieb RL, Sandkovsky U, Brown SM, Knowlton KU, Self WH, Files DC, Jain MK, Benfield T, Bowdish ME, Leshnower BG, Baker JV, Jensen JU, Gardner EM, Ginde AA, Harris ES, Johansen IS, Markowitz N, Matthay MA, Østergaard L, Chang CC, Davey VJ, Goodman A, Higgs ES, Murray DD, Murray TA, Paredes R, Parmar MKB, Phillips AN, Reilly C, Sharma S, Dewar RL, Teitelbaum M, Wentworth D, Cao H, Klekotka P, Babiker AG, Gelijns AC, Kan VL, Polizzotto MN, Thompson BT, Lane HC, Neaton JD. 2020. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med 384:905–914. 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dougan M, Nirula A, Azizad M, Mocherla B, Gottlieb RL, Chen P, Hebert C, Perry R, Boscia J, Heller B, Morris J, Crystal C, Igbinadolor A, Huhn G, Cardona J, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Dabora MC, Klekotka P, Shen L, Skovronsky DM. 2021. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 385:1382–1392. 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oladunjoye O, Gallagher M, Wasser T, Oladunjoye A, Paladugu S, Donato A. 2021. Mortality due to COVID-19 infection: a comparison of first and second waves. J Community Hosp Intern Med Perspect 11:747–752. 10.1080/20009666.2021.1978154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Casadevall A, Dragotakes Q, Johnson PW, Senefeld JW, Klassen SS, Wright SR, Joyner MJ, Paneth N, Carter R. 2021. Convalescent plasma use in the United States was inversely correlated with COVID-19 mortality: did convalescent plasma hesitancy cost lives? Elife 4:e69866. 10.7554/eLife.69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Focosi D, Franchini M. 2021. COVID-19 convalescent plasma therapy: hit fast, hit hard! Vox Sang 116:935–942. 10.1111/vox.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Focosi D, Franchini M. 2021. COVID-19 neutralizing antibody-based therapies in humoral immune deficiencies: a narrative review. Transfus Apher Sci 60:103071. 10.1016/j.transci.2021.103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Senefeld JW, Klassen SA, Ford SK, Senese KA, Wiggins CC, Bostrom BC, Thompson MA, Baker SE, Nicholson WT, Johnson PW, Carter RE, Henderson JP, Hartman WR, Pirofski LA, Wright RS, Fairweather L, Bruno KA, Paneth NS, Casadevall A, Joyner MJ. 2021. Use of convalescent plasma in COVID-19 patients with immunosuppression. Transfusion 61:2503–2511. 10.1111/trf.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thompson MA, Henderson JP, Shah PK, Rubinstein SM, Joyner MJ, Choueiri TK, Flora DB, Griffiths EA, Gulati AP, Hwang C, Koshkin VS, Papadopoulos EB, Robilotti EV, Su CT, Wulff-Burchfield EM, Xie Z, Yu PP, Mishra S, Senefeld JW, Shah DP, Warner JL, Halmos B, Verma A, Gartrell BA, Goel S, Ohri N, Sica RA, Thakkar A, Stockerl-Goldstein KE, Butt O, Campian JL, Fiala MA, Monahan R, Zhou AY, Bohachek P, Mundt D, Streckfuss M, Tadesse E, Lammers PE, Revankar SG, Panagiotou OA, Egan PC, Farmakiotis D, Khan H, Olszewski AJ, Loaiza-Bonilla A, Del Prete SA, Angevine AH, Bar MH, Lo KS, COVID-19 and Cancer Consortium, et al. 2021. Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19. JAMA Oncol 7:1167. 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Casadevall A, Henderson J, Joyner M, Pirofski L-a. 2021. SARS-Cov2 variants and convalescent plasma: reality, fallacies, and opportunities. J Clin Invest 131:e148832. 10.1172/JCI148832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holm K, Lundgren M, Kjedlsen-Kragh J, Ljungquist O, Bottiger B, Wiken C, Oberg J, Fernstrom N, Rosendal E, Overby A, Bystrom J, Forsell M, Landin-Olsson M, Rasmussen M. 2021. Convalescence plasma treatment of COVID-19: results from a prematurely terminated randomized controlled open-label study in Southern Sweden. BMC Res Notes 14:440. 10.1186/s13104-021-05847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Santis GC, Oliveira LC, Garibaldi PMM, Almado CEL, Croda J, Arcanjo GGA, Oliveira É, Tonacio AC, Langhi DM, Jr, Bordin JO, Gilio RN, Palma LC, Santos EV, Haddad SK, Prado BPA, Jr, Pontelli MC, Gomes R, Miranda CH, Martins MA, Covas DT, Arruda E, Fonseca BAL, Calado RT. 2022. High-Dose Convalescent Plasma for Treatment of Severe COVID-19. Emerg Infect Dis 28:10.3201/eid2803.212299. 10.3201/eid2803.212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chalmers TC, Matta RJ, Smith H, Jr, Kunzler AM. 1977. Evidence favoring the use of anticoagulants in the hospital phase of acute myocardial infarction. N Engl J Med 297:1091–1096. 10.1056/NEJM197711172972004. [DOI] [PubMed] [Google Scholar]
- 114.Vickers MA, Sariol A, Leon J, Ehlers A, Locher AV, Dubay KA, Collins L, Voss D, Odle AE, Holida M, Merrill AE, Perlman S, Knudson CM. 2021. Exponential increase in neutralizing and spike specific antibodies following vaccination of COVID-19 convalescent plasma donors. Transfusion 61:2099–2106. 10.1111/trf.16401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gallian P, Le Cam S, Brisbarre N, Pastorino B, Amroun A, Malard L, de Lamballerie X, Bliem C, Richard P, Morel P, Tiberghien P. 12 December 2021. COVID-19 convalescent plasma: evolving strategies for serological screening in France. Vox Sang. 10.1111/vox.13228. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt F, Weisblum Y, Rutkowska M, Poston D, Da Silva J, Zhang F, Bednarski E, Cho A, Schaefer-Babajew DJ, Gaebler C, Caskey M, Nussenzweig MC, Hatziioannou T, Bieniasz PD. 2021. High genetic barrier to SARS-CoV-2 polyclonal neutralizing antibody escape. Nature 600:512–516. 10.1038/s41586-021-04005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Germanio CD, Simmons G, Thorbrogger C, Martinelli R, Stone M, Gniadek T, Busch MP. 30 October 2021. Vaccination of COVID-19 convalescent plasma donors increases binding and neutralizing antibodies against SARS-CoV-2 variants. medRxiv 10.1101/2021.10.28.21265622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheffield WP, Bhakta V, Howell A, Jenkins C, Serrano K, Johnson N, Lin Y-CJ, Colwill K, Rathod B, Greenberg B, Gingras A-C, Evans DH, Flaumenhaft E, Beckett A, Drews SJ, Devine DV. 2022. Retention of hemostatic and immunological properties of frozen plasma and COVID-19 convalescent apheresis fresh-frozen plasma produced and freeze-dried in Canada. Transfusion 62:418–428. 10.1111/trf.16772. [DOI] [PubMed] [Google Scholar]
- 119.Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, Metzler M, Kohmer N, Hoehl S, Helfritz FA, Wolf T, Goetsch U, Ciesek S. 2021. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv. 10.1101/2021.12.07.21267432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao YR, Wang J, Jian F, Xiao T, Song W, Yisimayi A, Huang W, Li Q, Wang P, An R, Wang J, Wang Y, Niu X, Yang S, Liang H, Sun H, Li T, Yu Y, Cui Q, Liu S, Yang X, Du S, Zhang Z, Hao X, Shao F, Jin R, Wang X, Xiao J, Wang Y, Xie XS. 2021. B.1.1.529 escapes the majority of SARS-CoV-2 neutralizing antibodies of diverse epitopes. bioRxiv. 10.1101/2021.12.07.470392. [DOI] [PMC free article] [PubMed]
- 121.Focosi D, Maggi F, Franchini M, McConnell S, Casadevall A. 11 November 2021. Analysis of immune escape variants from antibody-based therapeutics against covid-19. medRxiv. 10.1101/2021.11.11.21266207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anonymous. 27 December 2021. U.S. Food and Drug Administration (FDA) clinical memorandum. Re: EUA 26382. Product: COVID-19 convalescent plasma. Available at https://www.fda.gov/media/141477/download.
- 123.Bégin P, Callum J, Jamula E, Cook R, Heddle NM, Tinmouth A, Zeller MP, Beaudoin-Bussières G, Amorim L, Bazin R, Loftsgard KC, Carl R, Chassé M, Cushing MM, Daneman N, Devine DV, Dumaresq J, Fergusson DA, Gabe C, Glesby MJ, Li N, Liu Y, McGeer A, Robitaille N, Sachais BS, Scales DC, Schwartz L, Shehata N, Turgeon AF, Wood H, Zarychanski R, Finzi A, Marceau D, Huang A, Carr H, Lin Y, Lall R, Graham C, Arsenault C, Sales V, Sidhu D, Semret M, Hamm C, Arhanchiague E, Solh Z, Srour N, Soliman K, Yee C, Laroche V, Nahirniak S, CONCOR-1 Study Group, et al. 2021. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med 27:2012–2024. 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beltran Gonzalez JL, Gonzalez Gamez M, Mendoza Enciso EA, Esparza Maldonado RJ, Hernandez PD, Duenas CS, Ovalle RI, Macias GM, Garcia DA, Gutierrez PC, Reza EA, Tiscareno GM, Galvan GE, Dorantes Morales MR, Martinez ML, Monroy CV, Arreola GJ. 2021. Efficacy and safety of convalescent plasma and intravenous immunoglobulin in critically ill COVID-19 patients. A controlled clinical trial. medRxiv. 10.1101/2021.03.28.21254507. [DOI] [Google Scholar]
- 125.Sekine L, Arns B, Fabro BR, Cipolatt MM, Machado RRG, Durigon EL, Parolo E, Pellegrini JAS, Viana MV, Schwarz P, Lisboa TC, Dora JMS, Balsan AM, Schirmer FD, Franz JPM, da-Silveira LM, Breunig RC, Petersen V, Sosnoski M, Mesquita NF, Volpato FCZ, Sganzerla D, Falavigna M, Rosa RG, Zavascki AP. 2022. Convalescent plasma for COVID-19 in hospitalised patients: an open-label, randomised clinical trial. Eur Respir J 59:2101471. 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moscato G, Mazzetti P, Lucenteforte E, Rosellini A, Cara A, Quaranta P, Mainardi V, Villa P, Focosi D, Lanza M, Bianco I, Mazzoni A, Falcone M, Menichetti F, Maggi F, Lai M, Freer G, Pistello M. 2021. Assessment of automated high-throughput serological assays for prediction of high-titer SARS-CoV-2 neutralizing antibody. J Clin Virol Plus 1:100016. 10.1016/j.jcvp.2021.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jahrsdörfer B, Kroschel J, Ludwig C, Corman VM, Schwarz T, Körper S, Rojewski M, Lotfi R, Weinstock C, Drosten C, Seifried E, Stamminger T, Groß HJ, Schrezenmeier H. 2021. Independent side-by-side validation and comparison of 4 serological platforms for SARS-CoV-2 antibody testing. J Infect Dis 223:796–801. 10.1093/infdis/jiaa656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Germanio C, Simmons G, Kelly K, Martinelli R, Darst O, Azimpouran M, Stone M, Hazegh K, Grebe E, Zhang S, Ma P, Orzechowski M, Gomez JE, Livny J, Hung DT, Vassallo R, Busch MP, Dumont LJ. 2021. SARS-CoV-2 antibody persistence in COVID-19 convalescent plasma donors: dependency on assay format and applicability to serosurveillance. Transfusion 61:2677–2687. 10.1111/trf.16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Misset B, Hoste E, Donneau A-F, Grimaldi D, Meyfroidt G, Moutschen M, Compernolle V, Gothot A, Desmecht D, Garigliany M, Najdovski T, Laterre P-F. 2020. A multicenter randomized trial to assess the efficacy of CONvalescent plasma therapy in patients with invasive COVID-19 and acute respiratory failure treated with mechanical ventilation: the CONFIDENT trial protocol. BMC Pulm Med 20:317. 10.1186/s12890-020-01361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mariën J, Ceulemans A, Michiels J, Heyndrickx L, Kerkhof K, Foque N, Widdowson MA, Mortgat L, Duysburgh E, Desombere I, Jansens H, Van Esbroeck M, Ariën KK. 2021. Evaluating SARS-CoV-2 spike and nucleocapsid proteins as targets for antibody detection in severe and mild COVID-19 cases using a Luminex bead-based assay. J Virol Methods 288:114025. 10.1016/j.jviromet.2020.114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Betrains A, Godinas L, Woei AJF, Rosseels W, Van Herck Y, Lorent N, Dierickx D, Compernolle V, Meyfroidt G, Vanderbeke L, Vergote V, Lagrou K, Verhamme P, Wauters J, Vermeersch P, Devos T, Maes P, Vanderschueren S. 2021. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol 192:1100–1105. 10.1111/bjh.17266. [DOI] [PubMed] [Google Scholar]
- 132.Okba NMA, Müller MA, Li W, Wang C, GeurtsvanKessel CH, Corman VM, Lamers MM, Sikkema RS, de Bruin E, Chandler FD, Yazdanpanah Y, Le Hingrat Q, Descamps D, Houhou-Fidouh N, Reusken C, Bosch BJ, Drosten C, Koopmans MPG, Haagmans BL. 2020. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis 26:1478–1488. 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Amanat F, White KM, Miorin L, Strohmeier S, McMahon M, Meade P, Liu W-C, Albrecht RA, Simon V, Martinez-Sobrido L, Moran T, García-Sastre A, Krammer F. 2020. An in vitro microneutralization assay for SARS-CoV-2 serology and drug screening. Curr Protoc Microbiol 58:e108. 10.1002/cpmc.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walker GJ, Naing Z, Ospina Stella A, Yeang M, Caguicla J, Ramachandran V, Isaacs SR, Agapiou D, Bull RA, Stelzer-Braid S, Daly J, Gosbell IB, Hoad VC, Irving DO, Pink JM, Turville S, Kelleher AD, Rawlinson WD. 2021. SARS coronavirus-2 microneutralisation and commercial serological assays correlated closely for some but not all enzyme immunoassays. Viruses 13:247. 10.3390/v13020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abe KT, Li Z, Samson R, Samavarchi-Tehrani P, Valcourt EJ, Wood H, Budylowski P, Dupuis AP, II, Girardin RC, Rathod B, Wang JH, Barrios-Rodiles M, Colwill K, McGeer AJ, Mubareka S, Gommerman JL, Durocher Y, Ostrowski M, McDonough KA, Drebot MA, Drews SJ, Rini JM, Gingras A-C. 2020. A simple protein-based surrogate neutralization assay for SARS-CoV-2. JCI Insight 5:e142362. 10.1172/jci.insight.142362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oguntuyo KY, Stevens CS, Hung C-T, Ikegame S, Acklin JA, Kowdle SS, Carmichael JC, Chiu H-p, Azarm KD, Haas GD, Amanat F, Klingler J, Baine I, Arinsburg S, Bandres JC, Siddiquey MN, Schilke RM, Woolard MD, Zhang H, Duty AJ, Kraus TA, Moran TM, Tortorella D, Lim JK, Gamarnik AV, Hioe CE, Zolla-Pazner S, Ivanov SS, Kamil JP, Krammer F, Lee B. 2021. Quantifying absolute neutralization titers against SARS-CoV-2 by a standardized virus neutralization assay allows for cross-cohort comparisons of COVID-19 sera. mBio 12:e02492-20. 10.1128/mBio.02492-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu F, and Wang A, and Liu Mei and Wang Q, and Chen J, and Xia Shuai and Ling Y, and Zhang Y, and Xun J, and Lu Lu and Jiang Shibo and Lu Hongzhou and Wen Y, and Huang J. 9 April 2020. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. https://ssrn.com/abstract=3566211.
- 138.Beltrán-Pavez C, Riquelme-Barrios S, Oyarzún-Arrau A, Gaete-Argel A, González-Stegmaier R, Cereceda-Solis K, Aguirre A, Travisany D, Palma-Vejares R, Barriga GP, Gaggero A, Martínez-Valdebenito C, Corre NL, Ferrés M, Balcells ME, Fernandez J, Ramírez E, Villarroel F, Valiente-Echeverría F, Soto-Rifo R. 2021. Insights into neutralizing antibody responses in individuals exposed to SARS-CoV-2 in Chile. Sci Adv 7:eabe6855. 10.1126/sciadv.abe6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dieterle ME, Haslwanter D, Bortz RH, 3rd, Wirchnianski AS, Lasso G, Vergnolle O, Abbasi SA, Fels JM, Laudermilch E, Florez C, Mengotto A, Kimmel D, Malonis RJ, Georgiev G, Quiroz J, Barnhill J, Pirofski LA, Daily JP, Dye JM, Lai JR, Herbert AS, Chandran K, Jangra RK. 2020. A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition. Cell Host Microbe 28:486–496.e6. 10.1016/j.chom.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang W, Li W, Xiong L, Wu Q, Wu J, He B, Shen J, Pang R, Luo T, Guo Y, Yang Y, Han Y, Dai W, Zhu P, Xia X. 2020. Clinical efficacy of convalescent plasma therapy on treating COVID-19 patients: evidence from matched study and a meta-analysis. Clin Transl Med 10:e259. 10.1002/ctm2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pappa V, Bouchla A, Terpos E, Thomopoulos TP, Rosati M, Stellas D, Antoniadou A, Mentis A, Papageorgiou SG, Politou M, Kotanidou A, Kalomenidis I, Poulakou G, Jahaj E, Korompoki E, Grigoropoulou S, Hu X, Bear J, Karaliota S, Burns R, Pagoni M, Trontzas I, Grouzi E, Labropoulou S, Stamoulis K, Bamias A, Tsiodras S, Felber BK, Pavlakis GN, Dimopoulos MA. 2021. A phase II study on the use of convalescent plasma for the treatment of severe COVID-19—a propensity score-matched control analysis. Microorganisms 9:806. 10.3390/microorganisms9040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Salazar E, Kuchipudi SV, Christensen PA, Eagar T, Yi X, Zhao P, Jin Z, Long SW, Olsen RJ, Chen J, Castillo B, Leveque C, Towers D, Lavinder J, Gollihar J, Cardona J, Ippolito G, Nissly R, Bird I, Greenawalt D, Rossi RM, Gontu A, Srinivasan S, Poojary I, Cattadori IM, Hudson PJ, Josleyn NM, Prugar L, Huie K, Herbert A, Bernard DW, Dye JM, Kapur V, Musser JM. 2020. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor-binding domain IgG correlate with virus neutralization. J Clin Invest 130:6728–6738. 10.1172/JCI141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tworek A, Jaroń K, Uszyńska-Kałuża B, Rydzewski A, Gil R, Deptała A, Franek E, Wójtowicz R, Życińska K, Walecka I, Cicha M, Wierzba W, Zaczyński A, Król ZJ, Rydzewska G. 2021. Convalescent plasma treatment is associated with lower mortality and better outcomes in high risk COVID-19 patients—propensity score matched case-control study. Int J Infect Dis 105:209–215. 10.1016/j.ijid.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cristelli MP, Junior DML, Viana LA, de Andrade LGM, Martins SBS, Dreige YC, Bordim JO, Tedesco-Silva H, Medina-Pestana J. 2021. Efficacy of convalescent plasma to treat mild to moderate COVID-19 in kidney transplant patients: a propensity score matching analysis. Transplantation 10.1097/tp.0000000000003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chauhan L, Pattee J, Ford J, Thomas C, Lesteberg K, Richards E, Loi M, Dumont LJ, Annen K, Berg M, Zirbes M, Miller A, Jenkins TC, Bennett TD, Monkowski D, Boxer RS, Beckham JD. 2021. A multi-center, prospective, observational-cohort controlled study of clinical outcomes following COVID-19 convalescent plasma therapy in hospitalized COVID-19 patients. Clin Infect Dis. 10.1093/cid/ciab834:ciab834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rasheed AM, Fatak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, Abdulamir AS. 2020. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 28:357–366. [PubMed] [Google Scholar]
- 147.Balcells ME, Rojas L, Le Corre N, Martínez-Valdebenito C, Ceballos ME, Ferrés M, Chang M, Vizcaya C, Mondaca S, Huete Á, Castro R, Sarmiento M, Villarroel L, Pizarro A, Ross P, Santander J, Lara B, Ferrada M, Vargas-Salas S, Beltrán-Pavez C, Soto-Rifo R, Valiente-Echeverría F, Caglevic C, Mahave M, Selman C, Gazitúa R, Briones JL, Villarroel-Espindola F, Balmaceda C, Espinoza MA, Pereira J, Nervi B. 2021. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med 18:e1003415. 10.1371/journal.pmed.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Joyner MJ, Paneth NS, Senefeld JW, Fairweather D, Bruno KA, Wright RS, Carter RE, Casadevall A. 2022. Concerns about estimating relative risk of death associated with convalescent plasma for COVID-19. Nat Med 28:51–52. 10.1038/s41591-021-01638-6. [DOI] [PubMed] [Google Scholar]
- 149.Anonymous. 2021. National COVID19 Convalescent Plasma Project. Commentary from the COVID-19 Convalescent Plasma Project (CCPP19) Leadership Group on “Early convalescent plasma for high-risk outpatients with Covid-19”. https://ccpp19.org/news/review%20of%20NEJM%20US%20outpatient%20CP%20trial%208-23.docx. Accessed 25 August 2021.
- 150.Anonymous. 2 March 2021. NIH halts trial of COVID-19 convalescent plasma in emergency department patients with mild symptoms. https://www.nih.gov/news-events/news-releases/nih-halts-trial-covid-19-convalescent-plasma-emergency-department-patients-mild-symptoms.
- 151.Devos T, Geukens T, Schauwvlieghe A, Ariën KK, Barbezange C, Cleeren M, Compernolle V, Dauby N, Desmecht D, Grimaldi D, Lambrecht BN, Luyten A, Maes P, Moutschen M, Romano M, Seyler L, Nevessignsky MT, Vandenberghe K, van Griensven J, Verbeke G, Vlieghe E, Yombi JC, Liesenborghs L, Verhamme P, Meyfroidt G. 2020. A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial. Trials 21:981. 10.1186/s13063-020-04876-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Franchini M, Focosi D, Casadevall A, Joyner MJ, Perotti C. 2022. Convalescent plasma for COVID-19. TSUNAMI is not the final word. Eur J Intern Med 10.1016/j.ejim.2022.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]