Abstract
Purpose:
Tendon injuries are a challenging clinical problem with few treatment options. Identifying the molecular regulators of tendon is required for the development of new therapies. While the Wnt pathway is critical for the maintenance and differentiation of many tissues, the role of Wnt signaling in tendon cell biology remains largely unexplored.
Methods:
The effects of Wnt activation were tested in vitro using neonatal tendon-derived cells cultured in 2D and 3D conditions. The inducible Axin2CreERT2 was then used to label Axin2+ cells in vivo and cells were traced during neonatal tendon regeneration.
Results:
We showed that activation of Wnt signaling results in proliferation of neonatal tendon cells. While tendon marker expression was inhibited by Wnt activation under 2D conditions, Scx expression was not affected under 3D uniaxial tension, suggesting that the microenvironment contextualizes tendon cell response to Wnt signaling. Using an in vivo model of neonatal tendon regeneration, we further showed that Wnt signaling cells comprise a sub-population of tenocyte and epitenon cells that proliferate after injury and are recruited during regeneration.
Discussion:
Collectively, these studies suggest that Wnt signaling may play a role in tendon cell proliferation, differentiation, and regeneration.
Keywords: tendon, Wnt, TGFβ, 3D culture, regeneration
Introduction
Tendons are connective tissues that integrate muscle to their proper skeletal sites to enable motion. This function is mediated by highly aligned type I collagen-rich fascicles that are synthesized and maintained by resident tenocytes expressing characteristic markers such as Scleraxis (Scx), Tenomodulin (Tnmd), and Mohawk (Mkx) 1,2. Surrounding tendon and tendon fascicles are the epitenon and endotenon, respectively, which are maintained by cells expressing laminin, α-smooth muscle actin (αSMA), and platelet-derived growth factor receptor α (PDGFRα) 3–6.
Tendon function is often permanently compromised by injuries, which are common and clinically challenging. These injuries can occur due to mechanical over-use or acute trauma 7,8. While damage can range from mild inflammation to complete tear, disorganized scar formation typically accompanies adult tendon injuries, and native structure is not restored 9,10. To date, treatment to address tendon pain and damage is largely limited to corticosteroids, physical therapy, or surgical repair. However, none of these treatments restore native tendon structure or function. Biologic treatments, such as platelet-rich plasma and stem cells, remain controversial with mixed clinical efficacy 11,12. A better understanding of the cell and molecular regulators underlying basic tendon biology is therefore required for the development of effective therapies.
One cell type of interest with therapeutic relevance is tendon stem/progenitor cells (TSPCs) 13. The first characterization of these cells demonstrated expression of Scx in addition to cell surface markers associated with mesenchymal stem cells 14. Further, TSPCs undergo clonal expansion and can differentiate toward cartilage, bone, fat, and tendon fates. While TSPCs were initially proposed to reside within the tendon fascicle, recent research identified TSPC-like cells near blood vessels (CD146+) and in the epitenon (Tppp3+/PDGRα+, αSMA+), which are activated by tendon injury 3,5,15–17. That TSPCs may originate from multiple sources is supported by experiments showing that both epitenon and tendon fascicle cells can be isolated, expanded in culture, and induced toward multiple lineages 18. While these studies were carried out using juvenile (8–10 week old) or older animals, other studies showed that tendon-derived cells from neonatal postnatal day 7 (P7) rat have improved proliferative and differentiation potential compared to 8 week old rats 19. We previously showed that functionally regenerative healing in neonatal P5 mice compared to adult mice (4–6 months) is driven by proliferation and recruitment of Scx-lineage tenocytes and non-Scx-lineage cells that adopt a tenogenic fate 20,21. However, the molecular cues and signaling pathways that regulate tendon regeneration and TSPC populations have not yet been fully elucidated.
To date, the transforming growth factor beta (TGFβ) signaling family remains the best studied pathway for tendon. TGFβ signaling is critical for induction and maintenance of tendon progenitors during development as loss of signaling results in loss of tendons or progressive degeneration 22,23. During healing, TGFβ can play contextually different roles.: While TGFβ signaling is strongly associated with fibrotic scar formation in adults, in neonates, it is required for tenocyte recruitment during regeneration 21,24–26. Although a number of other pathways have been identified for tendon healing, including fibroblast growth factor (FGF), connective tissue growth factor (CTGF), and platelet-derived growth factor (PDGF), these pathways are relatively less studied 5,15,16,27–30. One intriguing pathway is the Wnt pathway, which is well-established in the maintenance of tissue-specific stem cells in many tissues including intestine, mammary glands, skin 31–34. However, the role of Wnt signaling in tendon is largely known. To address this question, we combine in vitro and in vivo models to test the hypothesis that Wnt signaling may identify TSPCs in neonatal tendon, which have regenerative potential. We find that while Wnt activation increases cell proliferation, tendon differentiation was context-specific, and labeling of Wnt signaling cells by Axin2CreERT2 identified a subset of recruited tenogenic cells in the regenerated neonatal neotendon.
Materials and Methods
Mice
The following mouse lines were used: ScxGFP 35, ScxCreERT2, Axin2CreERT2 31, and ROSA26-TdTomato Ai14 36. Labeling of Axin2-expressing, Wnt signaling cells was performed via delivery of tamoxifen in corn oil by oral gavage (1.25 mg/pup) at postnatal days 2 and 3. Proliferating cells were labeled by subcutaneous EdU injection (0.05 mg/pup) 2 hours prior to harvest. All animal procedures were approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai.
Tendon and mesenchymal stromal cell isolation and culture
Achilles tendons were dissected from postnatal day 7 (P7) mice in ice cold 1X HBSS+1% Pen/Strep. Tendons were enzymatically digested in 0.25% Trypsin without EDTA (Gibco) for 30 mins at 37°C, followed by centrifugation at 2,000 rpm for 5 minutes and resuspension in 0.5 mg/ml Collagenase type 2 (Worthington), 0.25% Trypsin without EDTA in 1X HBSS+1% Pen/Strep. The digest was incubated at 37°C for one hour with 15 minute vortex intervals. Enzymes were inactivated by adding serum-containing media (DMEM+10% FBS+1% Pen/Strep), and the tendon digest was filtered through a 70um filter to remove debris. Cells were plated on tissue culture treated dishes, cultured in DMEM+10% FBS+1% Pen/Strep in hypoxic culture (5% O2) until confluent, and used for experiments at passage 2–4.
Bone marrow-derived mesenchymal stromal cells (MSCs) were isolated from the long bones of 6–8 week old mice. Briefly, bone marrow was flushed from tibias and femurs with cold PBS+1% Pen/Strep using a 26G needle, red blood cells were lysed in ice cold RBC Lysis Buffer (eBioscience), strained through a 70 μm cell strainer and resuspended in serum containing media (DMEM+10% FBS+1% Pen/Strep). Cells were plated on a tissue culture treated dish and media was changed the following day to remove any unattached or dead cells. Cells were subsequently cultured in DMEM+10% FBS+1% Pen/Strep in hypoxic culture (5% O2) until confluent and used for experiments at passage 2–4.
Flow Cytometry
Cultured tendon cells were stained using antibodies against CD45, CD31, CD11b, Sca1, CD29,, CD90, CD105, and CD140a (Biolegend). After gating out dead cells and debris, isotype controls were used to determine CD45- CD31- CD11b- Triple Negative (TN), non-hematopoietic cells for further analysis. Experiments were run on an LSRFortessa X-20 (BD Biosciences) and Attune NxT (ThermoFisher) and data was analyzed using FCSExpress software (DeNovo) and FlowJoV10 (TreeStar).
Cell proliferation assay
To assay cell proliferation, 2,500 cells were plated in triplicate for each time point in DMEM+10% FBS+1% Pen/Strep supplemented with 10 ng/mL TGFβ2 or 5 μM CHIR. The Cell Counting Kit-8 (CCK-8, Dojindo) was used to measure cell proliferation by absorbance according to manufacturer’s directions. Cells were incubated with CCK-8 solution for 2 hours and absorbance detected using the SpectraMaxi3 plate reader at 450nm. Phenol red-containing media was used as a negative control and media absorbance was subtracted from the absorbance values of experimental samples.
Osteogenic and chondrogenic differentiation assays
For osteogenic differentiation, cells were seeded at 5.3 × 104 cells/cm2 in αMEM+10% FBS+1% Pen/Strep. After 24 hours, cells were switched over to differentiation media containing 10mM betaglycerophosphate and 50 μg/ml ascorbic acid in αMEM+10% FBS+1% Pen/Strep and cultured for 14 days. Media was refreshed twice a week. Cells were fixed in 4% paraformaldehyde and stained with Alizarin Red (1%, aqueous) (Poly Scientific R&D) for 20 minutes. Wells were then washed in deionized water, air dried, and imaged using a Leica M165FC stereoscope.
For chondrogenic differentiation, cells were cultured as micromasses (2 × 105 cells in 10 μl of DMEM+10% FBS+1% Pen/Strep. After 2 hours at 37°C to allow adherence, cell culture media was added to the micromasses. After 24 hours, cells were incubated in serum-free differentiation media containing 1% ITS, 1X sodium pyruvate, 50 μg/ml ascorbic acid, 0.1 μM dexamethasone, 40 μg/ml L-proline and 10 ng/ml TGFβ3 in high glucose DMEM+1%Pen/Strep and cultured for 14 days. Media was refreshed twice a week. Cells were fixed in 4% paraformaldehyde and stained with Alcian Blue (0.5% in 3% acetic acid, pH 1.0, Poly Scientific R&D) overnight. Wells were washed in 3% acetic acid (pH 1.0) followed by 2 washes in 3% acetic acid (pH 2.5), air dried, and imaged using a Leica M165FC stereoscope.
Fabrication of 3D engineered tendon constructs under uniaxial tension
Neonatal tendon cells were trypsinized and embedded into 3D type I collagen gels at 250,000 cells per 2 mg/mL PureCol as previously described 37. Cell-seeded gels were then cast into sterile PDMS dishes between pinned prolene sutures 37. Gels were placed in a humidified 37°C incubator for 1 hr to allow the gel to set before flooding with DMEM+10% FBS+1% Pen/Strep only or DMEM+10% FBS+1% Pen/Strep supplemented with 10 ng/mL TGFβ2, 5μM CHIR, or 10 ng/mL TGFβ2+5μM CHIR for 21 days. Media was refreshed twice per week.
RNA extraction, reverse transcription, and real time quantitative polymerase chain reaction
Cells were lysed in Trizol (Life Technologies) and total RNA was extracted using Trizol/chlorofrom. Total RNA was quantified using NanoDrop 2000 and 0.5–1 μg of RNA was reverse transcribed to generate cDNA using the Super Script VILO mastermix (Invitrogen). Gene expression was determined by qPCR using PowerUp SYBR Green Mastermix (Applied Biosystems) and validated primers (Table 1) by the Mount Sinai qPCR Core Facility. Product specificity was confirmed by melt curve analysis, and relative expression was determined using 2-ΔΔCT analysis after normalization to β actin expression.
Table 1:
List of primers used.
| Target Name | Forward Primer | Reverse Primer |
|---|---|---|
| Osterix | GATGGCGTCCTCTCTGCTTG | GCCATAGTGAGCTTCTTCCTCAA |
| Sox9 | CGTGCAGCACAAGAAAGACC | GGACCCTGAGATTGCCCAGA |
| Col2a1 | ATCTTGCCGCATCTGTGTGT | GGCCCTAATTTTCCACTGGC |
| Scleraxis | AGAAAGTTGAGCAAAGACCGTG | TCAGTGGCATCCACCTTCAC |
| Mohawk | CGTGACAACCCGTACCCTAC | TTTGACACCTGCACTAGCGT |
Achilles tendon transection surgeries
Full transection of the Achilles tendon was carried out without repair at postnatal day 5 (P5) as previously described 20. Mice were sacrificed and hindlimbs collected at 3 and 28 days post injury. All animals were included in analyses.
Histology
Hindlimbs were fixed overnight in 4% paraformaldehyde in PBS at 4°C, decalcified in 50mM EDTA in PBS for 14 days at 4°C, followed by 1 hour in 5% sucrose then overnight in 30% sucrose at 4°C. Hindlimbs were then embedded in Optimal Cutting Temperature media (OCT) (Fisher). Alternate transverse sections (12 μm) were collected on charged slides through the entire length of the Achilles tendon from the calcaneal to muscle insertion. EdU labeled cells were detected using the Click-iT EdU detection kit (Life Technologies) as per manufacturer’s instructions. Tendon constructs were fixed in 4% paraformaldehyde in PBS at room temperature for 30 minutes, embedded in OCT, and transverse sections (12 μm) collected. Immunostaining for proliferating cells was carried out using antibody against Ki67 (Biolegend). For all staining, DAPI counterstain was used to visualize cell nuclei. All fluorescent imaging was carried out using the Zeiss Axio Imager microscope with Apotome optical sectioning.
Statistical analyses
Data are reported as mean ± standard deviation and analyzed using paired or unpaired t-tests where appropriate. For experiments with multiple comparisons, one-way and two-way ANOVAs with Tukey’s posthoc analyses were used. Significance was determined at p<0.05. Sample size was chosen based on previous data. All statistics were performed using GraphPad Prism software.
Results
Neonatal tendon cells express MSC markers and demonstrate reduced differentiation potential toward chondrogenesis and osteogenesis compared to bone-marrow MSCs
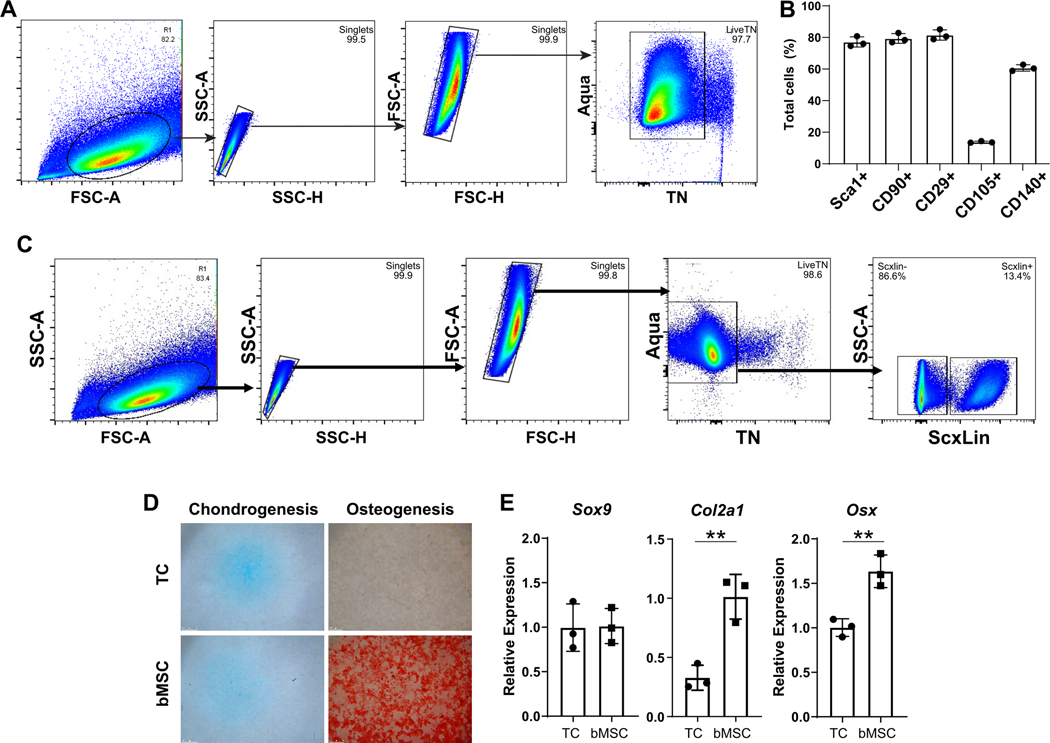
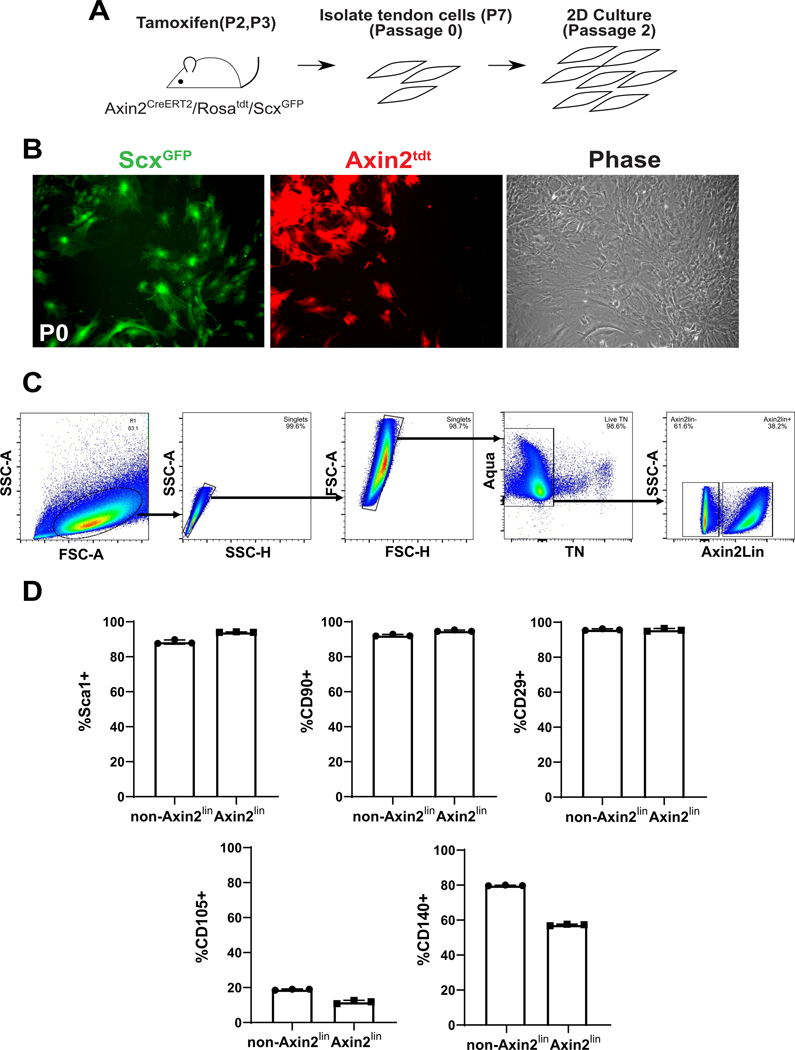
To characterize tendon cells derived from neonatal mice and cultured in 2D, we used a panel of common mesenchymal stromal cell (MSC) markers previously used to define tendon stem progenitor cell (TSPC) populations 13,14. After gating for singlets and live cells, the majority of cells (>98%) were triple negative for hematopoietic, endothelial, and myeloid markers CD45, CD31, and CD11b, respectively (Figure 1A). Consistent with previous studies from juvenile or adult murine TSPCs, a large percentage of these triple negative cells expressed MSC markers Sca1, CD90, CD29, and CD140a (~60–80%) (Figure 1B). However, few cells expressed CD105 (~10%) (Figure 1B). To determine the proportion of the cultured cells that were derived from tenocytes versus epitenon, we labeled Scx-expressing cells at days 2 and 3 after birth by tamoxifen administration of ScxCreERT2/Rosa26-TdTomato pups and isolated tendon cells at 7 days after birth. After passage 3, flow analysis of TdTomato+ cells indicated that 13% of cells were derived from tenocytes while the majority of the cells were likely of epitenon origin (Figure 1C). Analysis of ScxGFP+ cells 24 hours after initial isolation and plating showed that the initial population of plated cells were 80±6% ScxGFP (ie. intrinsic tenocytes, n=3 mice), indicating that epitenon cells likely out-proliferate tenocytes with continued 2D culture and passage (Supplemental Figure 1).
Figure 1: Characterization of neonatal tendon-derived cells.
(A) Flow cytometry gating and (B) quantification of established MSC and tendon stem/progenitor cell markers. (C) Flow cytometry gating and quantification of Scxlin and non-Scxlin cells after culture. (D) Day 21 Alcian Blue and Alizarin Red staining and (E) gene expression for neonatal tendon cell (TC, top) and juvenile bMSCs (bottom) undergoing chondrogenesis and osteogenesis, respectively. n=3 ** indicates p<0.01.
Since previous studies suggested that adult CD105-negative TSPCs give rise to ectopic cartilage and heterotopic ossification in response to injury 38, we tested the chondrogenic and osteogenic capacities of neonatal tendon cells to further characterize the cells. Compared to bone marrow-derived MSCs (bMSCs), neonatal tendon cells showed comparable differentiation potential toward chondrogenesis but minimal differentiation toward osteogenesis, as evidenced by similar Alcian Blue and poor Alizarin Red staining, respectively (Figure 1D). Gene expression confirmed lower expression of osteogenic differentiation markers Osx in neonatal TSPCs, while Sox9 expression was comparable between groups with reduced Col2a1 (Figure 1E). This data suggests that chondrogenic potential may be incomplete in neonatal tendon cells compared to bMSCs.
Effect of Wnt signaling on neonatal tendon cells is contextualized by the microenvironment.
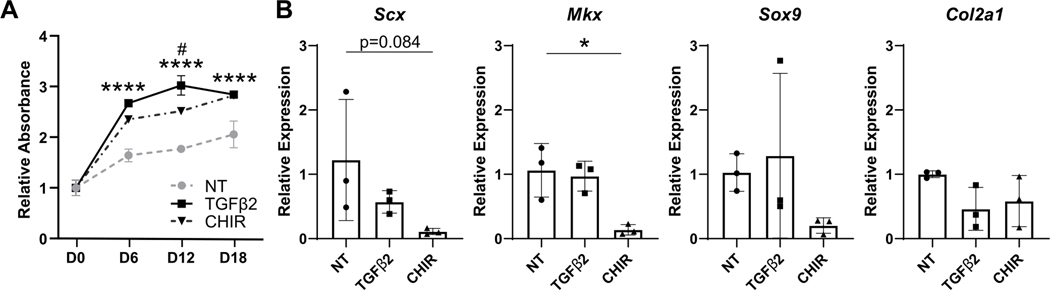
Since Wnt signaling is essential in the maintenance of multiple stem cell populations, we next asked whether Wnt signaling is required to maintain neonatal tendon cells. Using the Wnt agonist CHIR, we tested the effect of Wnt activation on neonatal tendon cells proliferation and differentiation. Cells were used after 2D expansion, similar to Figure 1 experiments above. Since TGFβ signaling is a known regulator of proliferation and tenogenic/chondrogenic differentiation, TGFβ2 was used as a positive control. In 2D culture, TGFβ2 or CHIR treatment resulted in enhanced neonatal tendon cell proliferation at all timepoints, compared to basal media conditions (no treatment, NT) (Figure 2A). Gene expression analysis showed reduced expression of tendon markers Scx and Mkx with CHIR treatment at day 15 with no difference in cartilage markers Sox9 and Col2a1 (Figure 2B). No difference in gene expression was observed with TGFβ2 treatment compared to NT.
Figure 2: Activation of Wnt signaling by CHIR treatment increases neonatal tendon cell proliferation but inhibits tendon gene expression in 2D.
(A) TGFβ2 and CHIR treatment increases proliferation compared to no treatment (NT). (B) Tendon and cartilage gene expression with TGFβ2 and CHIR treatment at D15. n=3 **** p<0.0001 TGFβ2, CHIR vs NT # p<0.05 TGFβ2 vs CHIR. *p<0.05.
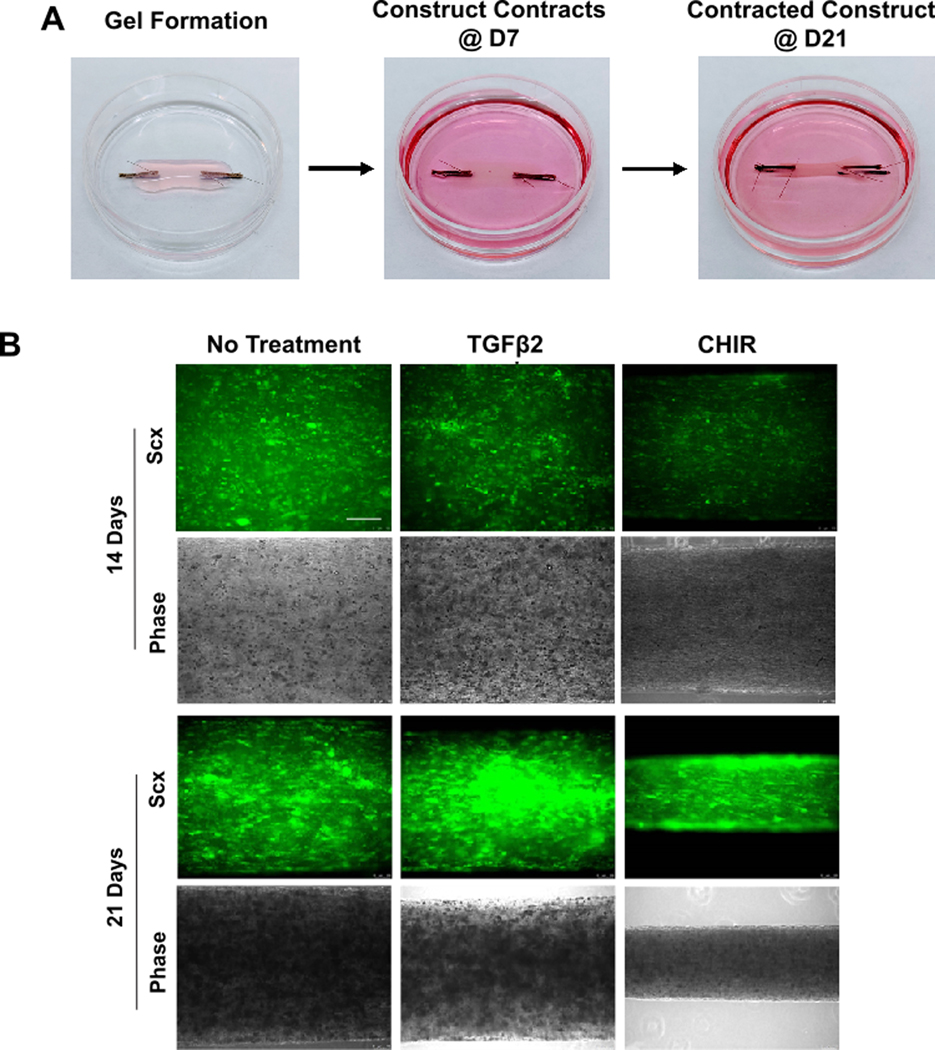
Although these results suggested that CHIR suppresses tendon differentiation of neonatal tendon cells, it is well established that proper tendon differentiation depends on culture microenvironment (previous studies have demonstrated the loss of tendon marker expression in tendon cells in 2D culture while tenogenesis is enhanced under 3D uniaxial tension 39–42. To test whether these effects of Wnt activation is maintained in a more physiologic environment, we therefore embedded neonatal tendon cells (after 2D expansion) in 3D collagen gels held under static tension (Figure 3A). Differences in cell-mediated gel contraction were detectable by 14 days of culture by whole-mount imaging of constructs. The presence of CHIR resulted in enhanced contraction relative to TGFβ2 or no treatment (Figure 3B). Qualitatively, ScxGFP expression was most intense at day 21 for all constructs, and full contraction was observed with CHIR treatment (Figure 3B).
Figure 3: ScxGFP expression is induced and maintained in neonatal tendon cells under 3D uniaxial tension.
(A) Cell-seeded collagen gels contract to form tensioned linear constructs by 21 days. (B) ScxGFP expression and phase contrast imaging of whole mount constructs after 14 and 21 days of culture in 3D. Scale bar: 200 μm.
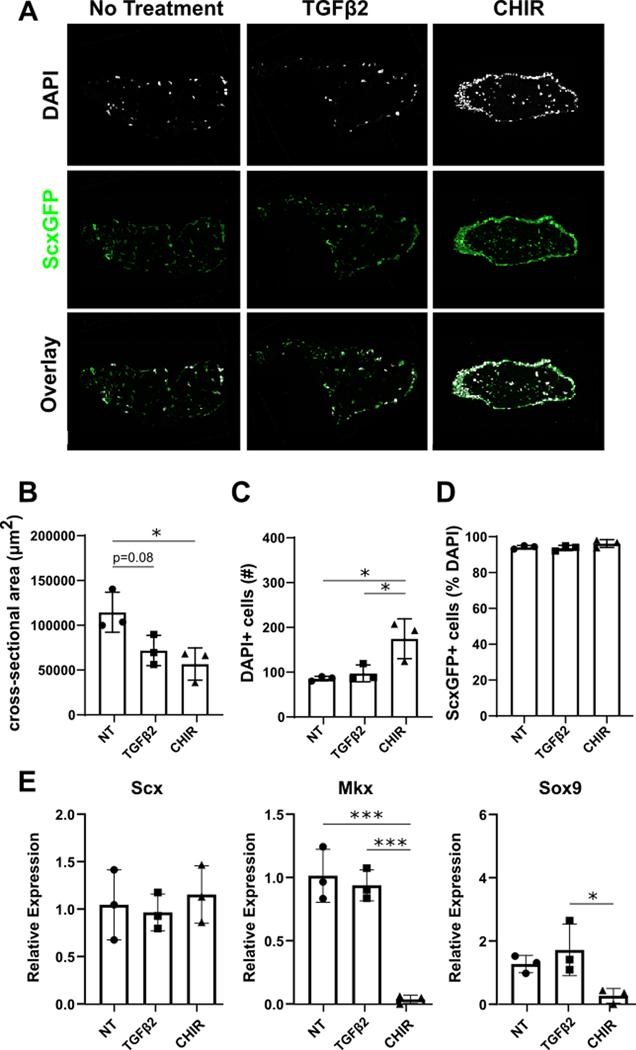
Consistent with whole mount imaging, quantitative analysis of transverse cryosections showed significantly reduced cross-sectional area between CHIR relative to NT or TGFβ2 treatment at day 21, indicative of enhanced contraction (Figure 4A, B). The number of DAPI+ cells was increased with CHIR treatment relative to NT and TGFβ2, however staining for Ki67+ proliferating cells at day 21 did not reveal any difference in proliferation between groups (Figure 4C, Supplemental Figure 2). Analysis of ScxGFP expression showed that almost all of the cells within 3D constructs expressed ScxGFP, independent of treatment condition (Figure 4D). CHIR treatment however, exhibited a distinctive dense ring of ScxGFP+ cells in the periphery, that was not observed in NT or TGFβ2. Gene expression analysis by qPCR confirmed no difference in Scx expression between groups, but Mkx and Sox9 were minimally expressed with CHIR treatment compared to NT and TGFβ2 (Figure 4E). Col2a1 expression was not detected in any samples (not shown). Collectively, these data indicate that Wnt activation improved cell number in both 2D and 3D environments. While Wnt activation suppressed tendon markers in 2D culture with no effect on cartilage markers, ScxGFP was induced and maintained under 3D tension, suggesting the importance of the biomechanical microenvironment in contextualizing the effect of Wnt signaling.
Figure 4: CHIR treatment enhances neonatal tendon cell contraction and proliferation in 3D.
(A) Transverse cryosections with 21 days of TGFβ2 and CHIR treatment. Quantification of (B) cross-sectional area, (C) DAPI+ cells, and (D) ScxGFP+ cells in transverse cryosections with treatments. (E) Gene expression analysis of tendon and cartilage markers. n=3 *p<0.05 *** p<0.001.
Neonatal tendon cells are derived from both Axin2lin and non-Axin2lin cells
To determine whether Wnt signaling cells represent a population of stem/progenitor cells in neonatal tendons, we used the tamoxifen-inducible Axin2CreERT2/Rosa26-TdTomato line to label Wnt-signaling cells at P2 and P3 after birth (Figure 5A). Since Axin2 is a Wnt target gene, all cells undergoing active Wnt signaling would be labeled at time of tamoxifen administration. At passage 0 confluency, we observed the presence of both ScxGFP+ and ScxGFP- cells within the cultured population. Similarly, both Axin2lin+/ScxGFP+ and Axin2lin+/ScxGFP- cells were identified (Figure 5B). After 2 passages, flow cytometry showed that nearly 40% of the cultured population were Axin2lin cells, indicating that non-Axin2lin cells were also capable of expansion. Analysis of MSC marker expression showed that the majority of cultured cells expressed Sca1, CD90, and CD29 with minimal expression of CD105 (Figure 5C). The only notable difference in surface marker expression was CD140a, which was expressed by ~80% of non-Axin2lin cells compared to ~60% of Axin2lin cells. CD140a is expressed by epitenon cells 43, indicating that Axin2lin cells may be derived from both the tendon fascicle as well as the epitenon. This hypothesis is supported by our Scxlin cell data, (Figure 1C), which only comprised 13% of the culture population. Overall, these data suggest few differences in stemness marker profiles of Axin2lin versus non-Axin2lin cells in culture.
Figure 5: Characterization of Axin2lin cells isolated from neonatal tendons.
(A) Schematic describing tamoxifen labeling of Axin2lin cells, cell isolation, and 2D culture. (B) Fluorescence and phase contrast imaging of cells at passage 0 (P0). (C) Flow cytometry gating for Axin2lin and non-Axin2lin tendon cells with culture. (D) Flow cytometry analysis of Axin2lin cells using established mesenchymal stem cell and tendon stem/progenitor cell markers.
Axin2lin cells proliferate and contribute to neo-tendon formation after neonatal tendon injury
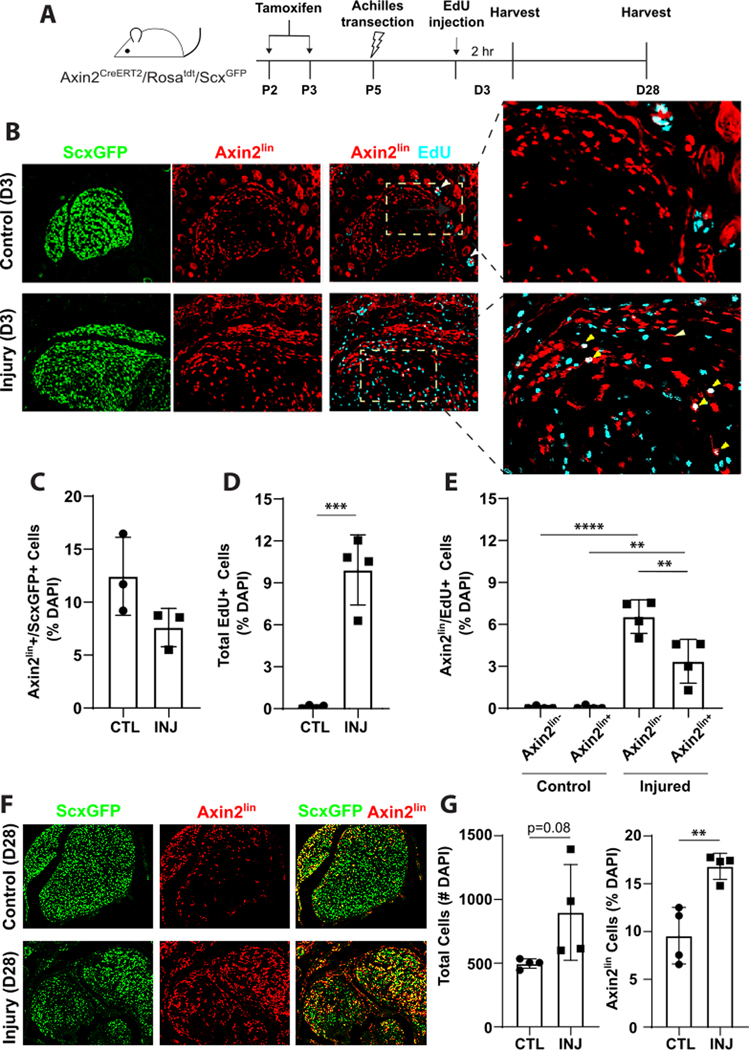
The presence of Axin2lin+/ScxGFP+ and Axin2lin+/ScxGFP- cells suggested that Axin2CreERT2 may label both neonatal tenocytes and epitenon cells in vivo. Since our previous studies showed that Scxlin and non-Scxlin cells contributed to neo-tendon formation during neonatal tendon regeneration 21, we next tested whether the recruited cells were derived from Axin2lin sub-populations (Figure 6A). Analysis of transverse sections of uninjured contralateral tendon at 3 days post-injury (DPI) confirmed Axin2lin+/ScxGFP+ tenocytes, localized mostly to the Achilles tendon periphery (Figure 6B). A few Axin2lin+/ScxGFP- epitenon cells were also detected. With injury at 3DPI, the proportion of Axin2lin+/ScxGFP+ cells was unchanged, while the number of proliferating EdU+ cells increased relative to uninjured control (Figure 6B–D). Most of the proliferating cells were Axin2lin-, but proliferating Axin2lin+ cells were also observed (Figure 6E). By 28 DPI, abundant Axin2lin+ cells were observed within the forming neo-tendon (Fig 6F, 6G). Transverse sections through the neo-tendon showed that the majority of Axin2lin+ cells were also ScxGFP+, however numerous Axin2lin-/ScxGFP+ cells were also detected (N=1). This data suggests that Axin2lin cells are one of the regenerative cell sources that proliferate and contribute to neonatal tendon repair in response to injury.
Figure 6: Axin2lin cells proliferate and are recruited during neonatal tendon regeneration.
(A) Schematic describing tamoxifen labeling of Axin2lin cells and Achilles transection injury. (B) EdU detection and (C-E) quantification of Axin2lin and ScxGFP cells at D3 post-injury in transverse cryosections of contralateral control (CTL) and injured (INJ) hindlimbs. (F) Fluorescence imaging and (G) quantification of Axin2lin cells at D28 post-injury in transverse cryosections. n=4 **p<0.01 ***p<0.001 ****p<0.0001.
Discussion
In this study, we characterized neonatal tendon-derived cells after expansion and found that expanded cells included a mixed population of Scxlin and non-Scxlin cells that expressed most MSC markers including Sca1, CD90, CD29, and CD140a. While cells were capable of chondrogenic and tenogenic differentiation, osteogenic differentiation was quite limited, suggesting that neonatal tendon cells may have restricted potential or may be more akin to progenitor cells rather than multipotent stem cells. We also found that activation of Wnt signaling enhanced neonatal tendon cell proliferation and differentiation in two in vitro models. While the proliferative effects of Wnt activation were observed in both 2D and 3D culture, regulation of tendon gene expression was contextualized by the culture environment. Under 2D conditions, we showed that Wnt signaling suppressed tendon markers, similar to previous reports 44. Under 3D uniaxial tension, however, ScxGFP and Scx expression were maintained. The tendon differentiation and maturation marker Mkx was suppressed in both 2D and 3D, however, indicating that Wnt activation may be promoting a tendon progenitor fate rather than a differentiated or mature tendon fate. The importance of the cell microenvironment in differential signal transduction is well established. For example, cells maintained in rounded morphologies adopt chondrogenic or adipogenic phenotypes, while cells maintained in elongated morphologies adopt fibrochondrogenic or osteogenic phenotypes under similar media conditions 45–47. Other microenvironmental cues, such as substrate stiffness, can also modulate cell differentiation independent of chemical cues 48. Our data support the use of 3D uniaxial culture conditions to better represent the physiologic microenvironment of tendon cells.
In 2D culture, it was previously reported that Wnt activation antagonizes TGFβ signaling in tendon stem/progenitor cells via inhibition of Smad2/3 phosphorylation 44. Future studies will test potential interactions by using Wnt and TGFβ inhibitors in 3D culture and combining Wnt and TGFβ activation. Surprisingly, treatment with TGFβ2 did not result in increased proliferation under 3D conditions, in contrast to previous findings in mouse embryonic fibroblasts or MSCs 40,49. This may be due to differences in cell type or the use of serum in the media, as serum is known to contain unidentified growth factors. Significant research in chondrogenic cultures, for example, suggests that the presence of serum introduces variability as well as impairs chondrogenic induction, compared to serum-free formulations 50. To date, a chemically-defined media formulation has not been established for tenogenic differentiation. Although increased DAPI+ cells was observed with CHIR treatment in 3D culture, analysis of cell proliferation at the final timepoint did not reveal differences between groups. This may suggest that differences in proliferation occurred at earlier timepoints or that the reduced cell numbers stemmed from apoptosis. The dynamics of proliferation and apoptosis with CHIR and TGFβ treatment will be determined in future studies.
Numerous studies in vitro and in vivo showed that Wnt signaling maintains undifferentiated stem cells in many tissues 51 including hair follicle 52, intestines 53 54, lungs 55,56, neural crest 57, eyes 58, and uterus 59. A recent study identified novel tissue-specific and common human Wnt target genes using bulk RNA sequencing on human endoderm tissue-derived organoids 60. By removing Wnt in vitro, this study also demonstrated that Wnt signaling is necessary for stem cell maintenance in a 3D culture microenvironment. However, the role of Wnt signaling in tendon is less clear. Our findings showed Wnt-mediated maintenance of ScxGFP expression in vitro and labeling of Axin2lin+/ScxGFP+ tenocytes and Axin2lin+/ScxGFP-neg cells in neonatal control tendons in vivo. Upon injury, these cell populations proliferate at 3 days post injury and contribute to tendon repair at 28 days post injury. These data indicate that, in neonatal mice, Wnt signaling may have multiple functions. Since Axin2lin+ and Axin2lin- cells were detected in roughly equal proportions with culture, future studies will determine the proportion of Axin2lin+ cells at initial isolation, to determine whether shifts may occur with 2D expansion.
While the Axin2lin+ subpopulation within the tendon fascicle and epitenon may represent a pool of progenitor cells, we also identified a population of ScxGFP+ cells in the neo-tendon at 28 days post-injury that were Axin2lin-neg. This may be due to incomplete recombination or suggest that Axin2lin cells do not represent the sole source of regenerative cells. Quantification after injury showed proliferation of both Axin2lin and non-Axin2lin cells. The proliferating non-Axin2lin population likely includes other stem/progenitor subpopulations as well as immune cells, including tissue-resident and bone marrow-derived macrophages, which we and others previously showed to be present at day 3 after injury 61. Other cells may include non-Axin2lin tenocytes or aSMA+ myofibroblasts. One limitation to these studies is we infer that Axin2+/ScxGFP- cells are epitenon cells due to their localization to the tendon periphery. However these may very well comprise an additional population of cells that are not derived from either tendon fascicle or epitenon. Emerging single cell RNA sequencing datasets will allow more precise phenotyping of the various cell populations present in tendon and their functions.
Future studies will determine whether Wnt signaling is required for functional neonatal tendon regeneration, using genetic deletion or in vivo delivery of small molecules. We will also test whether the Axin2+ TSPC subpopulation becomes more restricted with adult tendon maturation. Insights gained from these studies can be applied to adult tendon injury models with an objective of informing better therapeutic strategies to shift adult tendon repair from fibrotic to regenerative.
Supplementary Material
Supplemental Figure 1: ScxGFP cells at 24 hours after neonatal tendon isolation. Representative image of neonatal tendon-derived cells 24 hours after isolation and plating..
Supplemental Figure 2: Quantification of Ki67+ proliferating cells under 3D culture. Transverse cryosections with 21 days of TGFβ2 and CHIR treatment immunostained for Ki67 expression and DAPI. Quantification showed no difference between groups (n=3)...
Acknowledgments
We thank Ms. Gina Viavattene, the Flow Cytometry CoRE and the qPCR Core at the Icahn School of Medicine at Mount Sinai for their assistance.
Funding
This work was supported by NIH/NIAMS R01AR069537 and NYSTEM IDEA C32570GG grants to AHH.
Footnotes
Disclosures
The authors declare no conflicts of interest.
References
- 1.Benjamin M.& Ralphs JR The cell and developmental biology of tendons and ligaments. Int Rev Cytol 196, 85–130 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Huang AH, Lu HH & Schweitzer R.Molecular regulation of tendon cell fate during development. J Orthop Res, doi: 10.1002/jor.22834 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Dyment N.et al. Lineage Tracing of Resident Tendon Progenitor Cells during Growth and Natural Healing. PloS one 9, doi: 10.1371/journal.pone.0096113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor S.et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PloS one 6, doi: 10.1371/journal.pone.0016337 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey T, Flamenco S.& Fan CM A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol 21, 1490–1503, doi: 10.1038/s41556-019-0417-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staverosky J, Pryce B, Watson S.& Schweitzer R.Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Developmental dynamics : an official publication of the American Association of Anatomists 238, 685–692, doi: 10.1002/dvdy.21865 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Jozsa L.& Kannus P.Human Tendons: Anatomy, Physiology and Pathology. (Human Kinetics, 1997). [Google Scholar]
- 8.Killian ML, Cavinatto L, Galatz LM & Thomopoulos S.The role of mechanobiology in tendon healing. Journal of shoulder and elbow surgery / American Shoulder and Elbow Surgeons ... [et al. ] 21, 228–237, doi: 10.1016/j.jse.2011.11.002 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BR, Sarver JJ, Buckley MR, Voleti PB & Soslowsky LJ Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech 47, 2028–2034, doi: 10.1016/j.jbiomech.2013.10.054 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BR, Gordon JA & Soslowsky LJ The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles, ligaments and tendons journal 4 (2014). [PMC free article] [PubMed] [Google Scholar]
- 11.Saltzman BM et al. Does the Use of Platelet-Rich Plasma at the Time of Surgery Improve Clinical Outcomes in Arthroscopic Rotator Cuff Repair When Compared With Control Cohorts? A Systematic Review of Meta-analyses. Arthroscopy 32, 906–918, doi: 10.1016/j.arthro.2015.10.007 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Warth RJ, Dornan GJ, James EW, Horan MP & Millett PJ Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy 31, 306–320, doi: 10.1016/j.arthro.2014.09.007 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Walia B.& Huang AH Tendon stem progenitor cells: Understanding the biology to inform therapeutic strategies for tendon repair. J Orthop Res 37, 1270–1280, doi: 10.1002/jor.24156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bi Y.et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine 13, 1219–1227, doi: 10.1038/nm1630 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Lee CH et al. Harnessing endogenous stem/progenitor cells for tendon regeneration. J Clin Invest 125, 2690–2701, doi: 10.1172/JCI81589 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CH, Shah B, Moioli EK & Mao JJ CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 125, 3992, doi: 10.1172/JCI84508 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyment NA et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS One 8, e59944, doi: 10.1371/journal.pone.0059944 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mienaltowski MJ, Adams SM & Birk DE Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng Part A 19, 199–210, doi: 10.1089/ten.TEA.2012.0182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J.et al. Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Sci Rep 6, 22946, doi: 10.1038/srep22946 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howell K.et al. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep 7, 45238, doi: 10.1038/srep45238 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaji DA, Howell KL, Balic Z, Hubmacher D.& Huang AH TGFbeta signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife 9, doi: 10.7554/eLife.51779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pryce B.et al. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development (Cambridge, England) 136, 1351–1361, doi: 10.1242/dev.027342 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan GK et al. Tgfbeta signaling is critical for maintenance of the tendon cell fate. eLife 9, doi: 10.7554/eLife.52695 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzel E.et al. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 29, 684–693, doi: 10.1002/jor.21235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HM et al. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res 52, 87–98, doi: 10.3109/03008207.2010.483026 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Ngo M, Pham H, Longaker MT & Chang J.Differential expression of transforming growth factor-beta receptors in a rabbit zone II flexor tendon wound healing model. Plast Reconstr Surg 108, 1260–1267 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Chen CH et al. Tendon healing in vivo: gene expression and production of multiple growth factors in early tendon healing period. J Hand Surg Am 33, 1834–1842, doi:S0363-5023(08)00570-4 [pii] 10.1016/j.jhsa.2008.07.003 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Thomopoulos S.et al. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng 38, 225–234, doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomopoulos S, Harwood FL, Silva MJ, Amiel D.& Gelberman RH Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am 30, 441–447, doi:S0363-5023(05)00015-8 [pii] 10.1016/j.jhsa.2004.12.006 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Tsubone T, Moran SL, Amadio PC, Zhao C.& An KN Expression of growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg 53, 393–397, doi:00000637-200410000-00018 [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 31.van Amerongen R, Bowman A.& Nusse R.Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell stem cell 11, 387–400, doi: 10.1016/j.stem.2012.05.023 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Clevers H.Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480, doi: 10.1016/j.cell.2006.10.018 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Glass DA, 2nd et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8, 751–764, doi:S1534-5807(05)00097-3 [pii] 10.1016/j.devcel.2005.02.017 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Haegebarth A.& Clevers H.Wnt signaling, lgr5, and stem cells in the intestine and skin. The American journal of pathology 174, 715–721, doi: 10.2353/ajpath.2009.080758 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pryce B, Brent A, Murchison N, Tabin C.& Schweitzer R.Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Developmental dynamics : an official publication of the American Association of Anatomists 236, 1677–1682, doi: 10.1002/dvdy.21179 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Madisen L.et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience 13, 133–140, doi: 10.1038/nn.2467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gehwolf R.et al. 3D-Embedded Cell Cultures to Study Tendon Biology. Methods Mol Biol 2045, 155–165, doi: 10.1007/7651_2019_208 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Asai S.et al. Tendon Progenitor Cells in Injured Tendons Have Strong Chondrogenic Potential: The CD105-Negative Subpopulation Induces Chondrogenic Degeneration. Stem Cells 32, 3266–3277, doi: 10.1002/stem.1847 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo CK & Tuan RS Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue engineering. Part A 14, 1615–1627, doi: 10.1089/ten.tea.2006.0415 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Chien C, Pryce B, Tufa SF, Keene DR & Huang AH Optimizing a 3D model system for molecular manipulation of tenogenesis. Connect Tissue Res, 1–14, doi: 10.1080/03008207.2017.1383403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Breidenbach AP et al. Fibrin Gels Exhibit Improved Biological, Structural, and Mechanical Properties Compared with Collagen Gels in Cell-Based Tendon Tissue-Engineered Constructs. Tissue Eng Part A, doi: 10.1089/ten.TEA.2013.0768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaji DA, Montero AM, Patel R.& Huang AH Transcriptional profiling of mESC-derived tendon and fibrocartilage cell fate switch. Nat Commun 12, 4208, doi: 10.1038/s41467-021-24535-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harvey T, Flamenco S.& Fan CM. A Tppp3+Pdgfra+ tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol 21, 1490–1503 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kishimoto Y.et al. Wnt/β-catenin signaling suppresses expressions of Scx, Mkx, and Tnmd in tendon-derived cells. PloS one 12, doi: 10.1371/journal.pone.0182051 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McBeath R, Pirone DM, Nelson CM, Bhadriraju K.& Chen CS Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6, 483–495 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Huang AH, Stein A, Tuan RS & Mauck RL Transient exposure to TGF-beta3 improves the mechanical properties of MSC-laden cartilage constructs in a density dependent manner. Tissue Eng Part A 15, 3461–3472, doi: 10.1089/ten.TEA.2009.0198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baker BM & Mauck RL The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 28, 1967–1977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Theodossiou SK, Tokle J.& Schiele NR TGFbeta2-induced tenogenesis impacts cadherin and connexin cell-cell junction proteins in mesenchymal stem cells. Biochem Biophys Res Commun 508, 889–893, doi: 10.1016/j.bbrc.2018.12.023 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Byers BA, Mauck RL, Chiang IE & Tuan RS Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A 14, 1821–1834, doi: 10.1089/ten.tea.2007.0222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clevers H, Loh KM & Nusse R.Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012, doi: 10.1126/science.1248012 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Lim X, Tan SH, Yu KL, Lim SB & Nusse R.Axin2 marks quiescent hair follicle bulge stem cells that are maintained by autocrine Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 113, E1498–1505, doi: 10.1073/pnas.1601599113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuhnert F.et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A 101, 266–271, doi: 10.1073/pnas.2536800100 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y.et al. A growth factor-free culture system underscores the coordination between Wnt and BMP signaling in Lgr5(+) intestinal stem cell maintenance. Cell Discov 4, 49, doi: 10.1038/s41421-018-0051-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Summers ME et al. Balanced Wnt/Dickkopf-1 signaling by mesenchymal vascular progenitor cells in the microvascular niche maintains distal lung structure and function. Am J Physiol Cell Physiol 320, C119–C131, doi: 10.1152/ajpcell.00277.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Summers ME et al. Resident mesenchymal vascular progenitors modulate adaptive angiogenesis and pulmonary remodeling via regulation of canonical Wnt signaling. FASEB J 34, 10267–10285, doi: 10.1096/fj.202000629R (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleber M.et al. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol 169, 309–320, doi: 10.1083/jcb.200411095 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalez S, Oh D, Baclagon ER, Zheng JJ & Deng SX Wnt Signaling Is Required for the Maintenance of Human Limbal Stem/Progenitor Cells In Vitro. Invest Ophthalmol Vis Sci 60, 107–112, doi: 10.1167/iovs.18-25740 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seishima R.et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun 10, 5378, doi: 10.1038/s41467-019-13363-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boonekamp KE et al. Identification of novel human Wnt target genes using adult endodermal tissue-derived organoids. Dev Biol 474, 37–47, doi: 10.1016/j.ydbio.2021.01.009 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Howell KL et al. Macrophage depletion impairs neonatal tendon regeneration. FASEB J 35, e21618, doi: 10.1096/fj.202100049R (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: ScxGFP cells at 24 hours after neonatal tendon isolation. Representative image of neonatal tendon-derived cells 24 hours after isolation and plating..
Supplemental Figure 2: Quantification of Ki67+ proliferating cells under 3D culture. Transverse cryosections with 21 days of TGFβ2 and CHIR treatment immunostained for Ki67 expression and DAPI. Quantification showed no difference between groups (n=3)...