Abstract
Leishmaniasis is a complex protozoan infectious disease and, associated with malnutrition, poor health services and unavailability of prophylactic control measures, neglected populations are particularly affected. Current drug regimens are outdated and associated with some drawbacks, such as cytotoxicity and resistance, and the development of novel, efficacious and less toxic drug regimens is urgently required. In addition, leishmanial pathogenesis is not well established or understood, and a prophylactic vaccine is an unfulfilled goal. Human kinetoplastid protozoan infections, including leishmaniasis, have been neglected for many years, and in an attempt to overcome this situation, some new drug targets were recently identified, enabling the development of new drugs and vaccines. Compounds from new drug classes have also shown excellent antileishmanial activities, some of the most promising ones included in clinical trials, and could be a hope to control the disease burden of this endemic disease in the near future. In this review, we discuss the limitations of current control methods, explore the wide range of compounds that are being screened and identified as antileishmanial drug prototypes, summarize the advances in identifying new drug targets aiming at innovative treatments and explore the state-of-art vaccine development field, including immunomodulation strategies.
This review explores the wide range of compounds that are being identified as antileishmanial drug prototypes, summarize the advances in identifying innovative treatments and explore the state-of-art of vaccines and immunomodulation strategies.
Introduction
Leishmaniasis is a devastating infectious disease caused by protozoan parasites from the Leishmania species, widespread across the globe and mainly present in parts of the tropics, subtropics, and southern Europe. This disease, transmitted to humans by female sand flies of the genera Phlebotomine (old world) and Lutzomyia (new world), is mainly found in three clinical forms: visceral, cutaneous, and mucocutaneous, that differ in their immunopathologies and degree of mortality. This disease is considered the third most important vector-borne parasitic disease, after lymphatic filariasis and malaria.1
Associated with malnutrition, poor domestic sanitary conditions, a weak immune system, and lack of financial resources, neglected populations are particularly affected, with little interest from public health authorities to implement activities to research, prevent or control the disease. According to the World Health Organization, more than 1 billion people live in areas endemic for leishmaniasis and are at risk of infection. An estimated one million new cases of cutaneous, and 30 000 new cases of visceral leishmaniasis occur annually, affecting mainly some of the poorest people on earth.1,2
Visceral leishmaniasis (VL), also known as kala-azar, is fatal if untreated in over 95% of cases. It is characterized by weight loss, irregular bouts of fever, anemia, and enlargement of the spleen and liver. Most cases occur in East Africa, Brazil, India, China, Iraq, and Nepal. An estimated 50 000 to 90 000 new cases of VL occur worldwide annually and are one of the top parasitic diseases with mortality potential.1
VL is caused by Leishmania infantum in Europe, North Africa, and Latin America, and Leishmania donovani in East Africa and the Indian subcontinent.1,3
Additionally, a considerable proportion of successfully cured VL patients develop macular, papular or nodular rashes usually on the face, upper arms, and other parts of the body after a few months or years. This condition is nominated as post-kala-azar dermal leishmaniasis, and these individuals, which are difficult to treat with current drugs, are a potential threat for disease transmission to non-endemic areas.1,4
Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis and manifests as a variety of cutaneous symptoms ranging from simple skin lesions and ulcers to disfiguring lesions on exposed parts of the body, leaving life-long scars.5 This form of the disease is not life threatening, unlike VL, but infected people are subjected to serious disability or stigma, leading to psychological damage and impaired access to employment, marriage, and education.6,7
Most of the estimated 600 000 to one million new cases worldwide annually occur in the Mediterranean basin, the Middle East and Central Asia, and the Americas (Fig. 1). Approximately 20 species of Leishmania are able to infect macrophages in the dermis, being the main species in the old world Leishmania major in dry desertic regions and Leishmania tropica in urban areas, and in the new world, mainly Latin America, Leishmania mexicana, Leishmania amazonensis, Leishmania chagasi, Leishmania panamensis, and Leishmania braziliensis are the most observed species.7
Fig. 1. Status of endemicity of visceral leishmaniasis, 2019. Source: Control of Neglected Tropical Diseases (NTD), World Health Organization.1.
Mucocutaneous leishmaniasis (ML) causes partial or complete destruction of mucous membranes of the nose, mouth, and throat, and it is considered the most disabling form of the disease. Over 90% of ML occur in Brazil, Bolivia, Ethiopia, and Peru, and Leishmania braziliensis is responsible for most cases.1,3
Leishmania–HIV co-infection
Most people who become infected with the parasite do not become sick during their lifetime. Therefore, the term leishmaniasis refers to individuals who develop symptoms of a Leishmania infection, not being only infected with the parasite. It is important to mention that leishmaniasis is often observed in neglected populations because a weak immune system increases the risk of progression from asymptomatic to symptomatic visceral or cutaneous forms. Similarly, in a state of compromised immunity, such as that observed in HIV co-infected patients, leishmaniasis is difficult to treat and is often associated with high rates of relapse and mortality.4
Limited information is available on drug interactions of antileishmanial and antiretroviral drugs. Treatment of VL-HIV co-infection is challenging, and case fatality rates are high, especially in settings still relying on antimonials, reaching up to 25%.8 High Leishmania–HIV co-infection rates are reported in Brazil, Ethiopia, and India.1,9
Pathogenesis of leishmaniasis
This parasitic infection has two life cycles: a promastigote flagellar form found in the female phlebotomine sandfly vector and the intracellular amastigote form, which develops in the mammalian host. Transmission to the human host occurs by inoculation of promastigotes of the insect vector into the skin. Parasites are internalized by macrophages and transform into amastigote forms, losing their flagella and being able to replicate in mammalian hosts. This form causes illness to humans, since Leishmania is able to survive and multiply in the acidic environment of the lysosome, causing dissemination through the lymphatic and vascular systems.3 At this moment, the parasite interacts with its host immune system in different ways, modifying the expression and secretion of cytokines, in order to modulate immune cell flux to the infection site and unfavoring an effective host response to the infection. This complex parasitic–host relationship depends on several parameters, such as host genetic and health factors and the diversity of parasite strains.10
Treatment
The pentavalent antimonial salts sodium stibogluconate and meglumine antimonate have been the main compounds used for decades to treat visceral, cutaneous, and mucocutaneous leishmaniasis. Being the only specific antileishmanial compounds used to date in the current drug regimen, and due to the emergence of parasites resistant to these compounds, other drugs have been repurposed, such as pentamidine and paromomycin (antimicrobials), amphotericin B, fluconazole and ketoconazole (antifungals) and miltefosine (antitumor) (Fig. 2). These are the only alternative medicines currently used, and are associated with serious shortcomings, such as toxicity and the requirement of prolonged administration. Additionally, it has been reported that treatment with amphotericin B does not reach a sterile cure and is also associated with increasing rates of kala azar dermal leishmaniasis and the development of resistant parasites in clinical settings.4,11–13
Fig. 2. Chemical structures of currently used drugs.
In this way, the therapeutic use of available drugs has declined due to low efficacy, high toxicity, and the advent of drug resistance. Unfortunately, we do not yet have a vaccine, either prophylactic or preventive, for leishmaniasis available for clinical use, as the leishmanial pathogenesis has not yet been fully elucidated.
In recent years, few new drugs have been approved against leishmaniasis worldwide. The greatest efforts are observed in the screening of new combinations of known drugs replacing monotherapy, as a way to reduce the emergence of resistant strains. This is a worrying fact, considering that the available drugs have some limitations in addition to the gradual increase in resistance, such as safety, toxicity, side effects, price, feasibility, and efficacy.
Clearly, the development of a vaccine is not a reality in the near future. Repositioning strategies have shown potential to enable the discovery of new medicines for this disease, but the identification of new drug targets and the development of new leishmanicidal agents should be prioritized.12,14,15
Pentavalent antimonials
Sodium stibogluconate (pentosam) and branded meglumine antimoniate have been used for several decades (they were first introduced in 1945) and remain the drugs of choice where resistance is not observed, but the growing incidence of resistance limits their use in endemic areas. These drugs are limited by the need of daily parenteral administration for all treatments, VL, ML, and CL, requiring hospitalization in most cases.16
The pentavalent antimoniate, identified as a pro-drug, is reduced to trivalent antimoniate, the active form of the drug, either in the parasite or in the macrophages, although the exact mechanisms of action remain to be identified. Some of the main studies carried out identified that after reduction to SbIII in host cells, apoptosis is induced by oxidative stress promotion and intracellular Ca2+ generation. Other studies also indicate an indirect effect of Sb on the host's immune responses.17
Recent in vitro studies on Leishmania-resistant species reveal the diminished capacity to reduce the pentavalent to trivalent form, and this loss of drug activation is closely associated with drug resistance, along with the inappropriate or incorrect use of this medication over the years in endemic areas.18,19
Amphotericin B
Amphotericin B is a widespread polyene antifungal medication that is also used to treat life-threatening protozoan infections, such as all forms of leishmaniasis and primary amoebic meningoencephalitis.20 Although there is a consensus on the effectiveness of amphotericin B formulations, some factors limit its use in clinical practice, such as the low bioavailability and the need for intravenous administration, and especially the indispensable hospitalization of patients for treatment, due to the possible serious side effects that may arise. Its use is commonly associated with severe and potentially fatal side effects, including high fever, hypotension, nausea, headache, renal and liver failure, and allergic symptoms such as anaphylaxis may also occur.15
This drug shows high affinity for ergosterol, which is found in the cell membranes of fungi and protozoa, and since these species are not able to survive without ergosterol, the enzymes that synthesize it have become important drug targets for drug discovery in recent years. Ergosterol is also a provitamin form of vitamin D2, an important nutrient for human health.18
Paromomycin
Paromomycin is a broad-spectrum antibiotic produced by bacterial spp. Streptomyces rimosus, and originally developed for the treatment of intestinal protozoans in the 1960s. Its use was later expanded to treat a number of parasitic infections, such as amoebiasis, cryptosporidiosis, and giardiasis, and was also considered an effective oral treatment for human VL in combination with miltefosine in 2002. Paromomycin belongs to the aminoglycoside drug family and is toxic to the kidneys and ears. Its pharmacological activity is based on protein synthesis inhibition in nonresistant cells, acting on ribosomal proteins and mitochondrial membrane depolarization in the parasite, but the exact mechanism of action is largely unclear.21
Although it has a good cost–benefit ratio, with the cost of treatment per patient of only 10 dollars, this medicine is poorly absorbed orally, being rapidly eliminated and excreted by the kidneys unchanged, therefore intramuscular or intravenous administration is needed. Even with parenteral administration, the need of frequent dosing can lead to serious side effects, including nephrotoxicity, ototoxicity and hepatotoxicity. Paromomycin is able to treat both VL and CL, but it has limited use in endemic regions.15,17,22
Miltefosine
Developed originally as an anticancerous agent, it is the first oral drug used for the treatment of leishmaniasis and is used to treat all forms of this disease.16 Its mode of action, in this case very similar against both Leishmania parasites and human cancer cells, involves an induction of an apoptosis-like mechanism of cell death, but only a few of the targets involved in this process have already been identified. This drug is also able to cause a disturbance of lipid-dependent cell signaling pathways and to modulate the host's immune responses to parasites, inducing the production of the proinflammatory cytokine interferon-γ.13
The most advantageous characteristic of miltefosine is related to its pharmacokinetic properties, especially its high bioavailability and slow absorption process, allowing oral administration and restricting the need for hospitalization.15 In addition to its activity against Leishmania protozoan parasites and cancer cells, this drug is also active against other kinetoplastid parasites, various pathogenic bacteria and fungi.23 Moreover, recent studies in Brazil, one of the most affected countries to leishmaniasis burden, demonstrated the absence of decreased susceptibility to miltefosine in L. infantum for the 73 strains tested.24
Although the use of miltefosine has simplified the treatment, severe gastrointestinal side effects can be observed and, after the identification of some cases in endemic areas,25 recently resistance to this drug was first reported in clinical studies. Some patients relapsed after 9–12 months after successful treatment with this drug and, additionally, its use limitations include teratogenicity and abortifacient nature, which prevents its use in pregnancy.22,26
Pentamidine
Pentamidine is an aromatic diamine, and its isothionate and methanesulfonate salts are mainly used for the treatment of VL. The exact mode of action is still unknown, but it has been reported that the drug passes through the surface protein membrane of Leishmania donovani promastigotes through arginine and polyamine transporters. Drug resistance is observed after an alteration in the polyamine carrier that might be responsible for the alteration to the surface protein nature and content leading to decreased influx of the drug.18
This drug is highly toxic, limiting its use in treatment against leishmaniasis: it causes severe hypoglycemia, diabetes mellitus, nephrotoxicity, hypotension and myocarditis. Due these to serious side effects pentamidine is rarely used in clinical practice. Additionally, it is known that Leishmania parasites inhibit hepatic drug metabolism, and this possibly decreases systemic levels of pentamidine in patients, since it is metabolized by P450 enzymes in human liver microsomes. However, these theories were never fully elucidated by a robust pharmacokinetic study of pentamidine in VL patients.17,27
Azoles
Oral antifungal azoles can be tested as an alternative for treating CL, especially fluconazole and ketoconazole, in combination with other drugs. Evaluation of this class is underway against leishmaniasis, and so far has resulted in conflicting outcomes on different continents.28,29 This administration must be individualized, since azoles, as well as all other therapies/regimens to treat CL, appear highly effective only for some strains in certain specific regions of the world. Additionally, potential benefits must be compared to the risks of hepatotoxicity and, in the case of fluconazole, the risk of agranulocytosis.16
Another antileishmanial agent considered promising as an oral treatment option was sitamaquine, an 8-aminoquinoline derivative formerly synthesized as an antimalarial prototype in the same study that identified primaquine. This compound reached phase 2-b randomized clinical trials, but severe side effects, including vomiting, cyanosis, and nephritic syndrome dyspepsia led to interruption of its development in 2017.30,31
Local therapies
These approaches are often applied to treat clinically simple CL lesions, and parasite strains not associated with an increased risk for ML insurgence. Thermotherapy and cryotherapy, intralesional injections of pentavalent antimonials, topical creams with standard available drugs such as paromomycin, photodynamics or CO2 laser treatment can be used. It is the choice treatment for such conditions, alone or in combination with systemic drug administrations, but in some cases painful for the patient, as bedsores overlying ulcers need to be previously debrided to maximize the obtained results.16
Among all, the most widespread procedure is thermotherapy, which causes local injury in skin lesions by promoting direct heat in specific infected areas and is employed with moderate success. This has the same objective of cryotherapy and CO2 laser administration, the latter being considered an improvement in the thermotherapy field, able to reduce healing time and reach higher cure rates when compared to intralesional antimonial injections.32 Other studies are on course with promising results, such as local electric field stimulation,33 and diverse topical treatment strategies are currently in clinical and preclinical trials, such as formulations containing paromomycin, imiquimod, miltefosine, morphine, and buparvaquone components.22,34
A summary of the treatment options currently available for leishmaniasis is shown in Fig. 3.
Fig. 3. Current leishmaniasis therapy: treatment options.
New drug prototypes – progress and pitfalls
Parasitic diseases such as leishmaniasis are still a threat to humanity, due to the limited effectiveness of the existing chemotherapy and the lack of incentive to develop new therapeutic agents. The progress in the development of new leishmanicidal drugs has been limited by some key factors, the most important being the perception that the high cost of investment is insufficient to guarantee a return on it because, like most cases occur in developing countries, pharmaceutical companies fear there is no market, as few people could pay high prices for new drugs. Another factor, especially for cutaneous leishmaniasis, is the belief that there is no great need for new drugs on the market because skin diseases are rarely fatal.5,7 The discovery of a new drug is a long and expensive process, estimated to cost approximately 1.8 billion dollars and last 10–17 years.35
In relation to the development of novel treatments for leishmaniasis, it is important to emphasize that the two main manifestations of the disease, VL and CL, although belonging to the same genus of parasite, differ substantially in the requirement of different pharmacokinetic profiles and compound formulations.5 Additionally, novel cutaneous antileishmanial drugs need to overcome a special feature of this disease: it is indispensable to pass through deeper vascularized dermis, other than most antibacterial and antifungal skin medicines that target avascular superficial layers of the skin.5
Despite the factors that limit the interest of large pharmaceutical companies in the development of therapeutic agents, new antileishmanial drugs have been persistently required to reduce adverse effects and to overcome the evidence of increasing resistance to the available medicines. New chemical classes are being explored through library screenings, resulting in partnerships of biotech companies and pharmaceutical industries, but a low number of validated targets or the lack of connection between molecular phenotypes and the screening tests are limitations that must be overcome.35 Repurposing strategies in the search for new treatments for leishmaniasis are also becoming useful, since information about their pharmacokinetic and safety profiles is already available.12,36 Herbal extracts have also been studied in VL endemic countries with good results.37
Given that it is considered the most serious and potentially fatal form of the disease, VL is actually the main target of most developing compounds, and new chemical entities are showing promising activities in experimental models, progressing into clinical phase 1 trials. Included in this context are the new antileishmanial classes nitroimidazoles and benzoxaboroles, with DNDI-0690 and DNDI-6148 as lead compounds, respectively, the pyrazolopyrimidine scaffold recognized as CRK-12 kinase inhibitors, developed in a partnership with GSK (GSK899/DDD853651 and GSK 245/DDD1305143), and the proteasome inhibitors, with the triazolopyrimidine LXE-408 as lead compound.38 Being under development against CL, CpG-D35 compounds, synthetic oligonucleotides that act as enhancers of the effector immune response, are under phase 1 evaluation in the treatment of post-kala-azar dermal leishmaniasis and complicated cutaneous forms. Additionally, DNDI 6174, another drug candidate with the benzoxaborole nucleus, is under evaluation in the preclinical phase, according to the portfolio of DNDi (Table 1).
DNDi leishmaniasis portfolio in June, 2021. Development of new oral combination treatments with new chemical entities (NCEs)40.
| Discovery | Translation | Development | |||
|---|---|---|---|---|---|
| Lead optimization | Preclinical | Phase 1 | Phase 2a/proof of concept | Phase 2b/3 | Registration |
| S07 series | DNDi-6174 | DNDi-6148 | None | New CL combination | New VL treatments (Latin America) |
| CF series | DNDi-0690 | ||||
| GSK899 | New treatments for PKDL | ||||
| Novartis LXE408 | New treatments for HIV/VL | ||||
| CpG-D35 for CL | |||||
| GSK245 | Miltefosine + paromomycin combination (África) | ||||
DNDI-5561, an aminopyrazole derivative developed in a screening programme with Pfizer, was the most advanced compound of this series in clinical trials up to 2019 but, due to unfavorable safety results, was withdrawn from studies in phase 1.39
Nitroimidazole class (DNDI-0690)
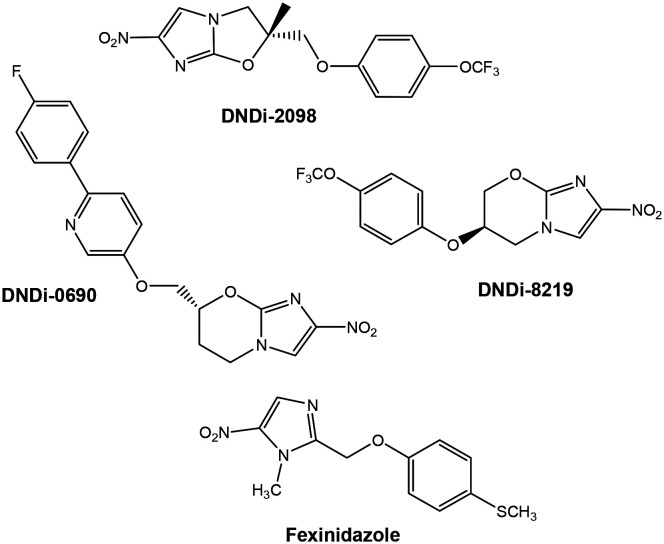
The nitroimidazole class of drugs, including nitroimidazo-oxazole and nitroimidazo-oxazine derivatives, is well known to have a broad spectrum of antiparasitic properties.41,42 The 7-substituted nitroimidazo-oxazine compound, identified as DNDi-0690 and entering phase 1 clinical development, was effective against intramacrophage forms of L. infantum and L. donovani (EC50 = 93 and 60 nM, respectively) and practically nontoxic (SI ≥ 1000) to mammalian cells (Fig. 4). Additionally, in preliminary studies, this compound presented no mutagenic potential and high oral bioavailability.15,43 This compound, a clinical drug candidate for visceral leishmaniasis, seems to be also promising against cutaneous leishmaniasis, with pharmacokinetics studies in mouse models presenting rapid accumulation and potent activity in the Leishmania-infected dermis when administered orally.44
Fig. 4. Chemical structure of nitroimidazole compounds.
Before DNDi-0690, other compounds elaborated in partnerships with DNDi had their development interrupted due to unsatisfactory results in preclinical trials against leishmaniasis, such as the lead compounds VL-2098 (ref. 42 and 45) (Fig. 4) and fexinidazole.46 The development of VL-2098 was interrupted due to adverse effects observed during toxicological studies, and fexinidazole, a DNA synthesis inhibitor, proved to be more effective against other neglected diseases and is currently used for the oral treatment of human African trypanosomiasis (HAT; also known as sleeping sickness) and Chagas' disease.15
In addition to the compounds found in clinical tests, other studies with these nuclei are underway.47,48 In one of them, a 900-compound pretomanid analog library was screened, and other 2-nitroimidazooxazine hits displaying excellent activities against Leishmania infantum and suitable solubility were identified.49 Additional studies identified the compound DNDi-8219, a trifluoromethoxy-3-phenoxy the most promising compound, mainly due to its high oral bioavailability, as a promising candidate to enter clinical trials.15 (Fig. 4)
It is believed that the nitroreductase enzyme (NTR2) is responsible for activating bicyclic nitro compounds, such as DNDI-0690, VL-2098 and DNDi-8219, to form toxic intermediates to Leishmania parasites.43,50,51
Pyrazolopyrimidine class (GSK899)
From a target-based high-throughput in vitro screening against Trypanosoma brucei GSK-3 kinase, the early compounds, a diaminothiazole carbaldehyde series, also showed little activity against L. donovani axenic amastigotes. This discovery led researchers at the University of Dundee in collaboration with Glaxo Smith Kline (GSK) to modify the core structure, maintaining the functions of the hydrogen bond acceptor and donor, and after compound optimization, developed pyrazolopyrimidine lead compound GSK3186899/DDD853651 (GSK 899) (Fig. 5).15,43,52–54
Fig. 5. Chemical structure of GSK899.
This derivative showed an EC50 value of 1.4 μM against the L. donovani intramacrophage assay, comparable to the antileishmanial drugs miltefosine (EC50 = 0.9 μM) and paromomycin (EC50 = 6.6 μM), and good selectivity against mammalian THP-1 host cells (EC 50 value > 50 μM).54 Further in vivo studies indicate good bioavailability, no undesirable toxicological effects, and a reduction of 99% in the parasite load in a mouse model of visceral leishmaniasis when dosed orally for 10 days at 25 mg kg−1.52
Proteomic studies to determine the mode of action of representative pyrazolopyrimidine analogs revealed that the cell division cycle-2-related kinase 12 (CRK12) receptor is undoubtedly the principal target of this compound series, but this scaffold also interacts with other Leishmania protein kinases, in particular CRK6 and CRK3, albeit with considerably lower affinities than CRK12.36 The identification of CRK12 and other protein kinases as potential drug targets led to further studies to identify other prototypes with better antileishmanial profiles, since protein kinases play an essential role, orchestrating the cellular signaling required for Leishmania survival throughout the life cycle.55–58
These results supported preclinical studies initiated in 2017 in collaboration with DNDi, with this compound included in phase 1 clinical trials in 2019.59
Imidazopyrimidine class (GSK245)
The compound named GSK3494245/DDD1305143 (GSK245), also identified from the in vitro screening tests that led to the discovery of GSK899, displayed remarkable activity in an intramacrophage in vitro assay, with an EC50 of 1.6 μM, also demonstrating equivalent efficacy against a diverse range of L. donovani and L. infantum clinical strains, including antimony-resistant isolates.60 After these promising results, further studies indicated good physicochemical properties, such as oral bioavailability, desirable safe profiles, and an in vivo reduction of the parasite load of over 95%, comparable to the current oral antileishmanial miltefosine in a similar mouse model.61
After a detailed mode of action study for this compound, entered into phase 1 clinical trials in 2019, it was found to act through selective inhibition of the β5 subunit of the L. donovani proteasome, specifically at the “chymotrypsin” site, and in this way was formerly classified as a proteasome inhibitor.61,62
Further studies of the relationship between the antiparasitic SAR and the identified target are also discussed in detail (Fig. 6).60 It was reported that a hydrogen bond donor (HBD) at positions 8 (N) and 1 (N or O) was essential for proteasome inhibition, while an HBD at the 7-position was harmful to activity. Heteroatoms were tolerated but not necessary in other positions of the ring, and a 6-substituent seems to be necessary for activity and metabolic stability. In relation to the central phenyl ring, 1-urea and 4-fluoro substituents were able to reach better levels of potency, metabolic stability, and solubility than their counterparts.
Fig. 6. SAR around the core scaffold of GSK245.
Triazolopyrimidine class, proteasome inhibitors (LXE-408)
After genetic and chemical validation, published by a team led by Novartis, of the parasite proteasome as a promising therapeutic target for the treatment of leishmaniasis and other kinetoplastid infections,63 further studies of lead optimization from triazolopyrimidine series yielded LXE408 (Fig. 7), a derivative that showed sustained and selective inhibition of the parasite proteasome when dosed orally in mouse disease models, with an appropriate safety profile. When applied to a murine model of CL, administration for 10 days at a dose of 20 mg kg−1 b.i.d. presented an efficacy comparable to that of amphotericin B, the most potent antileishmanial drug available today.64
Fig. 7. Chemical structure of LXE-408.
To further understand the binding mode of LXE-408 to the parasite proteasome, studies indicated that this compound selectively inhibits the chymotrypsin-like activity of the β5 proteasome subunit (PSMB5) in a noncompetitive manner.60,64 Currently, phase I studies are ongoing to establish safety and tolerability.
Oxaborole class (DNDI-6148 and DNDI 6174)
In recent years, great evolution in the development of boron-based compounds as potential therapeutic agents has been observed. It is mainly due to their ability to form covalent bonds with nucleophiles and also based on their carbon mimicry, which is especially purposeful in the inhibition of hydrolytic enzymes, which can form a covalent complex with the core structure of designed compounds.65
The most advanced compound in clinical trials is DNDi 6148, which initiated preclinical development in 2016 and, after completion of pharmacology and toxicology studies, upgraded to clinical trial application in 2019 (Fig. 8). This candidate entered clinical trials after promising in vitro results, as high levels of parasite burden reduction in liver and spleen in a visceral leishmaniasis murine model after 5 to 10 days of treatment.43,66,67
Fig. 8. Chemical structure of oxaborole compounds.

DNDi-6148 was discovered after a DNDi's screening of oxaborole compounds in collaboration with Anacor, and analogs effective against leishmaniasis and other kinetoplastid diseases, such as sleeping sickness and Chagas, were found. These compounds show activity even in strains resistant to other drugs used against leishmaniasis, and thus, it is believed that they have a different mechanism of action, although this has not yet been elucidated. Recent studies suggest that resistance to oxaboroles may not develop quickly upon clinical use, since after prolonged exposure of DNDI-6148, the emergence of compound-resistant parasites was not observed.68
Other potential derivatives with efficacious profiles were identified and were able to proceed to preclinical evaluation in the case of DNDi-6148 not succeeding in development, such as DNDi-5421 and DNDi-5610.69
Another oxaborole drug candidate, DNDi-6174, has also emerged as a promising VL and CL compound (Fig. 8), progressing to preclinical development in 2019 after being chosen from two other lead compounds from this series, DNDi-6588 and DNDi-6749.70
Oligonucleotides (CpG-D35)
CpG-D35 is under evaluation in a combination therapy for the treatment of cutaneous leishmaniasis and post-kala-azar dermal leishmaniasis (PKDL) in partnership with GeneDesign. Here, the strategy is to modulate the immune response to the parasite with CpG oligonucleotides, such as D35, to magnify the effectiveness of the treatment, reduce scarring and prevent recurrences of Leishmania infection. The goal is to develop a safe and affordable compound to CL or PKDL, which there are no satisfactory treatments, able to accelerate healing and reduce suffering of affected populations, in combination with proven chemotherapy.
Previous in vivo studies showed that macaques treated for 10 days with D35 plus 5 mg kg−1 sodium stibogluconate had smaller lesions and reduced time to re-epithelization after infection with Leishmania major, without evidenced toxicities even when using doses which were 10 times higher than usual.71,72 No adverse effects were observed, which supports the expectation of a good safety profile, and systemic activation of genes associated with innate immune responses in skin occurred, with an increased number of T cells producing IFNγ in response to Leishmania antigen.73,74
Since synthetic CpG-oligodeoxynucleotides are analogs of naturally occurring viral and bacterial DNA, it was observed that treatment with D35 results in systemic activation of T cells and dendritic cells, enabling the immune system to produce high levels of IL-12 (interleukin-12), IFNα (interferon alpha), and IFNγ (interferon gamma) and low levels of IL-10 (interleukin-10), inducing parasite clearance and lesion healing. Additionally, cytokines have been proposed as biomarkers of local innate and adaptive immune activation.75
Phase 1 clinical toxicology studies were initiated in 2020 after achieving a promising preclinical profile.76
Aminopyrazole class (DNDI-5561, discontinued)
A novel series of amino-pyrazole ureas has been identified from an HTS campaign with approximately 95 000 compounds, with excellent in vitro antileishmanial activity in preclinical models of VL.77
After this discovery, further studies of lead optimization yielded the derivatives DNDi-1044, DNDi-8012, and DNDi-1047.15,66,78 All three compounds, especially DNDi-1047, showed excellent activity across a range of Leishmania species that are known to cause cutaneous leishmaniasis, resulting in both a significant lesion size and parasite load reduction. These compounds offer novel potential drugs for the treatment of CL.66
DNDI-5561, an aminopyrazole derivative developed after a screening programme with Pfizer, and a hit-to-lead project conducted by DNDi in a partnership with Takeda Pharmaceutical and GHIT Fund was the most advanced compound of this series in clinical trials up to 2019, but due to unfavorable safety results, this compound was withdrawn from studies in phase 1. Although DNDi-5561 belongs to the substituted aminopyrazole class, its specific structure has not yet been disclosed.
Recent studies indicate that aminopyrazole activity does not suggest the rapid development of resistance, suggesting that aminopyrazole is a highly promising antileishmanial chemical series.77
In addition to the compounds found in clinical trials and the recent efforts to obtain new chemical entities able to overcome the issues related to the current treatment, such as toxicity, adverse effects, and advent of resistance, many synthesized agents, natural drugs or semisynthetic derivatives have been reported with remarkable antileishmanial activities, offering great potential for the future development of new antileishmanial chemotherapeutic drugs.79–89
As most of the above cited studies indicate, the main strategy used to obtain currently available drugs is known as the phenotypic approach, when a new drug prototype is obtained without any knowledge about its target or specific function against the disease.78 The screening of large libraries of drug candidates to obtain a hit compound and then elaborate their mechanisms of action has been successful in drug discovery but delays the progress to find the best possible prototype for that specific target. On the other hand, the target-based approach initially involves the identification of viable molecular targets, followed by designing compounds that consistently differ from those existing in the host environment and is detailed below.
Antileishmanial potential targets
The main issues related to the available antileishmanial treatment, the emergence of resistant strains and significant side effects, are also the main guide for the development of new drug strategies, such as the utilization of known validated targets for drug screening and optimization, goaling a new prototype able to fix only at the chosen target and minimize most undesirable adverse symptoms.90 This approach identifies enzymes structurally and functionally distinct from their mammalian counterparts for selective inhibition and to overcome cytotoxic effects that play essential roles in the parasite life cycle.
This strategy was greatly favored by the studies that performed the complete genome sequence of Leishmania strains, such as L. infantum, L. major, and L. brasiliensis, which enabled technological advances such as proteome analysis and bioinformatic approaches and allowed a better understanding of these microorganisms, such as the identification of unique genes of the parasite and physiological and biochemical differences between host and pathogen.91,92
Some of the main enzymes related to the parasite life cycle most recently explored in target-based drug discovery studies are the protein kinases, considered one of the best candidates as drug targets for leishmaniasis since it is able to modulate the ability of these proteins to interact with the host cell and damage its innate immune response,90 and enzymes involved in the sterol biosynthesis pathway (mainly ergosterol and stigmasterol, which differs from its mammalian counterpart cholesterol), essential components of the parasite cell membrane, vital to cellular integrity, growth and viability of various cellular functions.18 Additionally, three antioxidant enzymes that act in trypanothione metabolism, trypanothione reductase (TryR), tryparedoxin peroxidase (TxnP) and thioredoxin, which are able to minimize oxidative stress inside host phagocytes to control parasite redox homeostasis, are considered drug targets with great potential due to the high sensitivity of Leishmania species to oxidative stress.4,93
Other enzymes also reported in recent years include proteases, such as cysteine, serine, threonine, and aspartic peptidases, acting in many biochemical processes and being essential for the virulence of the parasite and its ability to enter the host cell, in addition to playing an important role in the autophagy process; glycolytic enzymes, involved in the glycolysis process as the only source of ATP generation of Leishmania species and other African trypanosomes since the parasite lacks a functional Krebs cycle;91 enzymes involved in folate biosynthesis, an essential cofactor for amino acid metabolism and synthesis of nucleic acids, essential to parasite growth and survival;94 and enzymes involved in the purine salvage pathway made in mammalian host cells, such as phosphoribosyl transferases and nucleoside diphosphate kinases, since parasitic Leishmania lacks enzymes necessary for de novo synthesis of purine nucleotides.95
Finally, the most recent studies in this area also include heat shock proteins (HSPs), a family of proteins that provides the correct three-dimensional shape to newly synthesized polypeptides, avoids misfolding and prevents aggregation of proteins, as new druggable targets with great potential, since they play key roles in cell differentiation, infectivity and overall survival of the parasitic protozoan Leishmania in the host cell. Additionally, an intrinsic correlation between the expression of HSPs and drug resistance in Leishmania was demonstrated, and it was presumed that inhibition of HSPs may reserve resistance to their respective drugs.96
In addition to metabolic enzymes, nucleoside transporters are also considered strategic drug targets. Although it remains difficult to target them with selective inhibitors, since they are able to transport nucleosides as purines and pyrimidines across the cellular membrane, it is suggested that they can also conduct toxic nucleoside analogs to the parasite, inhibiting cell growth.91
Immunotherapy
In recent years, immunotherapy has become an attractive approach since it causes fewer side effects than traditional treatments, is cost-effective and is potentially less susceptible to parasite resistance. It has been highlighted as a promising alternative treatment, particularly for patients who do not adapt or have a condition such as heart disease or nephropathy that prevents them from following conventional treatment.6
Among numerous immunotherapeutic strategies, which include therapy with antibodies and vaccines, cytokine-based immunotherapy has emerged as a promising option for the future treatment of leishmaniasis and other infectious diseases.97,98
Recent studies in the immunopathogenesis area of cutaneous and mucocutaneous leishmaniasis demonstrated that after contact with the parasite, an exacerbated inflammatory immune response caused by cytokines such as TNF and IFN-γ has an important role in tissue damage and the therapeutic complexity of this disease. It is important to mention that these inflammatory cytokines are vital for defense mechanisms of the host against parasitic infection, but their exaggerated production leads to a deleterious impact on the patient's condition. Therefore, cytokines have emerged as potential drug targets, and the immunomodulators obtained from this strategy aim to decrease the intensity of this inflammatory immune response and favor the elimination of the Leishmania protozoan parasite by the host and associated anti-Leishmania drugs. Modulation of the immune response may enable an increased production of IL-10, a cytokine able to reduce the local production of IFNγ and TNF.99
Additionally, it is known that host innate immunity, including macrophages, neutrophils, natural killer cells and dendritic cells, can contribute, after Leishmania infection, to host protection or disease progression, but it is clear that T-cell mediated immune responses, with cytokines being released from different immune cells, play a more vital role in immunopathology processes.100,101
Among the studies carried out in this area, satisfactory results have been obtained for the use of IFNγ in combination with the usual treatment, although so far no constant or reproducible results have been observed, and the use of imiquimod, an immune response modifier able to induce alpha-interferon and cytokine synthesis, has shown no or marginally superior results against CL over reference treatments.98,102,103
Imiquimod and oligodeoxynucleotides (ODNs) containing CpG motifs are some small and large compounds with immunomodulatory properties that have also attracted much interest in the drug development field of infectious diseases, autoimmune disorders and cancers, including combination therapies. As an example, antimicrobial peptides (AMPs) can induce specific cells to modulate gene expression and production of cytokines and chemoquines, with remarkable activity against different strains of Leishmania in animal models.104
Progress in this field requires a better understanding of the complex immunopathology of humans, which is not accurately reproduced in mouse models, since it involves the host immune machinery and its intricate regulations. Other factors, in addition to the modulating role of cytokines on the immune response, such as the levels of cytokine receptors, host genetics, and the diversity of Leishmania strains, can interfere with therapy outcomes.100
Vaccination
The obtainment of a vaccine against leishmaniasis is the object of study by several techniques, but it seems to be a complex goal, and so far there is no effective vaccine available for clinical practice. Approaches that have been studied include live vectors, classified as first-generation vaccines,105 second-generation recombinant proteins (produced through genetically engineered cells) and third-generation DNA motifs, in combination with immunomodulatory adjuvants,106 CpG oligonucleotides,107 and conventional adjuvants.108,109
The leishmanization approach, which includes inoculation of live virulent L. major promastigotes, was investigated in the past in different countries with some positive outcomes, but after undesirable symptoms observed in some individuals and safety issues, this practice was abandoned.110
Most efforts at the moment are concentrated on the development of a recombinant protein vaccine, and several antigens have been tested in animal models, with few of them advanced to clinical trials, such as LEISH-F1, formerly called Leish-111f. This artificial protein, encoded by three genes, a thiol-specific antioxidant of L. major (TSA) homolog, L. major stress-inducible protein-1 (LmSTI1), and L. braziliensis elongation and initiation factor (LeIF), has been shown to be immunogenic, safe and well tolerated in preliminary studies and was redesigned to overcome possible regulatory issues and to facilitate the manufacturing process, generating LEISH-F2. This vaccine was discontinued in phase 2 clinical trials after partial unsatisfactory results regarding efficacy.111,112
LEISH-F3 is another multicomponent vaccine consisting of nucleoside hydrolase (NH36) from L. donovani and sterol 24-c-methyltransferase (SMT) of L. infantum with adjuvant GLA-SE (glucopyranosyl lipid A), and has shown a robust immune response against visceral leishmaniasis. This vaccine candidate was considered safe in preliminary studies, showing increased secretion of cytokines IFN-γ, TNF-α, IL-2, IL-5 and IL-10. It was conducted in phase 1 clinical trials, together with LEISH-F3+, a homologous prototype with cysteine protease B added.113
Other studies exploring DNA vaccines (classified as third generation), which induce T cell-based immunity against leishmaniasis, are at earlier stages of development. In a very recent study, an adenovirus-vectored vaccine (ChAd63-KH) induced potent innate and cell-mediated immune responses in preliminary trials with human volunteers. This vaccine candidate employs a simian adenovirus backbone (ChAd63) and encodes two Leishmania antigens, kinetoplastid membrane protein-11 (KMP-11) and hydrophilic acylated surface protein B (HASPB), both of which have proven efficacy in preclinical animal models.114,115
One of the strategies to increase the efficiency of DNA vaccines is the employment of an effective multiantigen formulation to induce cross-protective immunity against different strains of Leishmania parasites. This strategy is used, for example, in the development of the DNA vaccine candidate HisAK70, which encodes seven Leishmania genes (H2A, H2B, H3, H4, A2, KMP11 and HSP70) and showed a specific cell-mediated immune response against L. Amazonensis in murine models.116
Adjuvants are recognized as molecules able to boost a specific humoral and cellular immune response against inoculated antigens in infected individuals in an attempt to render vaccines more effective, causing long-lasting efficacy and less toxicity.111
Recent studies have also used in silico immune-bioinformatics tools to develop recombinant chimeric protein vaccines, being observed promising results in murine models.117,118
The development of new vaccines must consider the balance between the two types of immune responses of T helper lymphocytes, Th1 and Th2. Th1 cells are responsible for releasing proinflammatory cytokines associated with the host response to parasitic infection, while Th2 cells mediate the production of anti-inflammatory cytokines, able to neutralize the deleterious action of proinflammatory cytokines but depending on their abundance, enabling disease progression. In this way, the Th1/Th2 continuous balance plays a vital role in host immune protection. Vaccines that involve both Th1 and Th2 cells are supposed to produce long-lasting immunity, and although this is a goal to be achieved, promoting reproducible modifications in this system is a complex task, since different combinations of cytokines may result in a divergent immune response.97,108
Conclusions
Despite recent advances in antileishmanial drug discovery and development, there is a lack of availability of new treatments for this disease (miltefosine was, in 2014, the only approved medicine in the last 30 years), and the advent of resistance to current agents is a constant threat. It seems urgent to develop more efficient drugs and strategies to overcome this outcome and slow down the potential emergence of resistant kinetoplastid Leishmania parasites. Some initiatives and investments must be elaborated as a way to encourage the pharmaceutical industry to invest in the development of new drugs or vaccines in this area, which it considers of great risk and little financial return. As a result of this consideration, leishmaniasis can be classified as a neglected disease.
Author contributions
Both authors, ACP and MVNS, contributed equally to the conceptualization and writing of this review article.
Conflicts of interest
There is no conflict of interest to declare.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge FAPERJ Foundation for financial support.
Biographies
Biography
Alessandra Campbell Pinheiro.

Alessandra Campbell completed her doctorate in medicinal chemistry at Federal University of Rio de Janeiro (BR) with a doctoral internship at CNRS (Paris, FR), supervised by professor Danièle Bonnet-Delpon and professor Benoit Crousse. She is a researcher at FIOCRUZ (Rio de Janeiro, BR) in the area of synthesis of pharmaceuticals and bioactive compounds. Her research interests centre on small drugs specially aimed at the treatment of neglected diseases, such as tuberculosis, malaria and leishmaniasis.
Biography
Marcus Vinícius Nora de Souza.

Marcus Nora is a researcher at FIOCRUZ (Rio de Janeiro, Brazil) with particular interest in the synthesis of substances against neglected tropical diseases, with more than 30 years of experience in this field. He obtained a PhD in organic chemistry from Université Paris XI (Paris-Sud) – France, with 3 post-doctoral degrees in organic synthesis and medicinal chemistry (University of Florida (USA), Federal University of Juiz de Fora (BR) and Genzyme Industry – Boston (USA)).
References
- World Health Organization, https://www.who.int/health-topics/leishmaniasis#tab=tab_1, (Accessed July 01, 2022)
- Torres-Guerrero E. Quintanilla-Cedillo M. R. Ruiz-Esmenjaud J. Arenas R. F1000Research. 2017;6:750. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis F. Sundar S. Hailu A. Ghalib H. Rijal S. Peeling R. W. Alvar J. Boelaert M. Nat. Rev. Microbiol. 2007;5:873. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Tiwari N. Gedda M. R. Tiwari V. K. Singh S. P. Singh R. K. Mini-Rev. Med. Chem. 2018;18:26. doi: 10.2174/1389557517666170425105129. [DOI] [PubMed] [Google Scholar]
- Bocxlaer K. V. Croft S. L. RSC Med. Chem. 2021;12:472. doi: 10.1039/D0MD00343C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caridha D. Vesely B. Van Bocxlaer K. Arana B. Mowbray C. E. Rafati S. Uliana S. Reguera R. Kreishman-Deitrick M. Sciotti R. Buffet P. Croft S. L. Int. J. Parasitol.: Drugs Drug Resist. 2019;11:106. doi: 10.1016/j.ijpddr.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle W. H. Makhoui K. Am. Fam. Physician. 2004;69:1455. [PubMed] [Google Scholar]
- Van Griensven J. Zijlstra E. E. Hailu A. PLoS Neglected Trop. Dis. 2014;8:e3023. doi: 10.1371/journal.pntd.0003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Int. J. Infect. Dis. 2014;29:103. doi: 10.1016/j.ijid.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Séguin O. Descoteaux A. Cell. Immunol. 2016;309:1. doi: 10.1016/j.cellimm.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Sahu A. Kumar D. Agrawal R. K. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2017;16:3. doi: 10.2174/1871523016666170502120210. [DOI] [PubMed] [Google Scholar]
- Charlton R. L. Rossi-Bergmann B. Denny P. W. Steel P. G. Parasitology. 2018;145:219. doi: 10.1017/S0031182017000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga S. S. Eur. J. Med. Chem. 2019;183:111660. doi: 10.1016/j.ejmech.2019.111660. [DOI] [PubMed] [Google Scholar]
- Rashidi S. Fernández-Rubio C. Manzano-Román R. Mansouri R. Shafiei R. Ali-Hassanzadeh M. Barazesh A. Karimazar M. Hatam G. Nguewa P. Parasitology. 2021;148:655. doi: 10.1017/S0031182021000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera R. M. Pérez-Pertejo Y. Gutiérrez-Corbo C. Domínguez-Asenjo B. Ordóñez C. García-Estrada C. Martínez-Valladares M. Balaña-Fouce R. Pure Appl. Chem. 2019;91:1385. doi: 10.1515/pac-2018-1102. [DOI] [Google Scholar]
- Aronson N. Herwaldt B. L. Libman M. Pearson R. Lopez-Velez R. Weina P. Carvalho E. Ephros M. Jeronimo S. Magill A. Am. J. Trop. Med. Hyg. 2017;96:24. doi: 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kip A. E. Schellens J. H. M. Beijnen J. H. Dorlo T. P. C. Clin. Pharmacokinet. 2018;57:151. doi: 10.1007/s40262-017-0570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. Kumar M. Singh R. K. Asian Pac. J. Trop. Med. 2012;5:485. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- Rath S. Trivelin L. A. Imbrunito T. R. Tomazela D. M. De Jesús M. N. Marzal P. C. Quim. Nova. 2003;26:550. doi: 10.1590/S0100-40422003000400018. [DOI] [Google Scholar]
- Grace E. Asbill S. Virga K. Antimicrob. Agents Chemother. 2015;59:6677. doi: 10.1128/AAC.01293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Pandey S. C. Samant M. Open Parasitol. J. 2018;4:e4. doi: 10.1017/pao.2018.1. [DOI] [Google Scholar]
- Roatt B. M. Cardoso J. M. O. De Brito R. C. F. Coura-Vital W. Aguiar-Soares R. D. O. Reis A. B. Appl. Microbiol. Biotechnol. 2020;104:8965. doi: 10.1007/s00253-020-10856-w. [DOI] [PubMed] [Google Scholar]
- Dorlo T. P. C. Balasegaram M. Beijnen J. H. de Vries P. J. J. Antimicrob. Chemother. 2012;67:2576. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- Espada C. R. Levatti E. V. C. Boité M. C. Lamounier D. Alvar J. Cupolillo E. Costa C. H. N. Rode J. Uliana S. R. B. Microorganisms. 2021;9:1228. doi: 10.3390/microorganisms9061228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. Mishra J. Gupta A. K. Singh A. Shankar P. Singh S. Parasites Vectors. 2017;10:49. doi: 10.1186/s13071-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G. A. S. Costa D. L. Costa C. H. N. Almeida R. P. Melo E. V. Carvalho S. F. G. Rabello A. Carvalho A. L. Souza A. Q. Leite R. D. Lima S. S. Amaral T. A. Alves F. P. Rode J. PLoS Neglected Trop. Dis. 2017;11:e0005706. doi: 10.1371/journal.pntd.0005706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta T. B. Das N. Das M. Marik R. Biochem. Biophys. Res. Commun. 2003;312:75. doi: 10.1016/j.bbrc.2003.09.227. [DOI] [PubMed] [Google Scholar]
- Galvão E. L. Rabello A. Cota G. F. PLoS One. 2017;12:e0186117. doi: 10.1371/journal.pone.0186117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prates F. V. O. Dourado M. E. F. Silva S. C. Schriefer A. Guimarães L. H. Brito M. G. O. Almeida J. Carvalho E. M. Machado P. R. L. Clin. Infect. Dis. 2017;64:67. doi: 10.1093/cid/ciw662. [DOI] [PubMed] [Google Scholar]
- Loiseau P. M. Cojean S. Schrével J. Parasite. 2011;18:115. doi: 10.1051/parasite/2011182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, USA. Clinical trials.gov website, https://clinicaltrials.gov/ct2/show/NCT00381394, Accessed July 01, 2022
- Meymandi S. S. Zandi S. Aghaie H. Heshmatkhah A. J. Eur. Acad. Dermatol. Venereol. 2011;25:587. doi: 10.1111/j.1468-3083.2010.03781.x. [DOI] [PubMed] [Google Scholar]
- Dorsey B. M. Cass C. L. Cedeño D. L. Vallejo R. Jones M. A. Pathogens. 2018;7:77. doi: 10.3390/pathogens7040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim M. Khan S. A. Ullah S. Ullah S. Anjum S. I. PLoS Neglected Trop. Dis. 2021;15:e0009099. doi: 10.1371/journal.pntd.0009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaña-Fouce R. Pertejo M. Y. P. Domínguez-Asenjo B. Gutiérrez-Corbo C. Reguera R. M. Drug Discovery Today. 2019;24:1209. doi: 10.1016/j.drudis.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Bustamante C. Ochoa R. Asela C. Muskus C. J. Comput.-Aided Mol. Des. 2019;33:845. doi: 10.1007/s10822-019-00230-y. [DOI] [PubMed] [Google Scholar]
- Sharma U. Velpandian T. Sharma P. Singh S. Parasitol. Res. 2009;105:1287. doi: 10.1007/s00436-009-1554-2. [DOI] [PubMed] [Google Scholar]
- Nagle A. Biggart A. Be C. Srinivas H. Hein A. Caridha D. Sciotti R. J. Pybus B. Kreishman-Deitrick M. Bursulaya B. Lai Y. H. Gao M.-Y. Liang F. Mathison C. J. N. Liu X. Yeh V. Smith J. Lerario I. Xie Y. Chianelli D. Gibney M. Berman A. Chen Y.-L. Jiricek J. Davis L. C. Liu X. Ballard J. Khare S. Eggimann F. K. Luneau A. Groessl T. Shapiro M. Richmond W. Johnson K. Rudewicz P. J. Rao S. P. S. Thompson C. Tuntland T. Spraggon G. Glynne R. J. Supek F. Wiesmann C. Molteni V. J. Med. Chem. 2020;63:10773. doi: 10.1021/acs.jmedchem.0c00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. DNDI-5561, https://dndi.org/research-development/portfolio/dndi-5561/ Accessed July 01, 2022
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. Research & development Portfolio, https://dndi.org/research-development/portfolio/, Accessed July 01, 2022
- Thompson A. M. O'Connor P. D. Marshall A. J. Yardley V. Maes L. Gupta S. Launay D. Braillard S. Chatelain E. Franzblau S. G. Wan B. Wang Y. Ma Z. Cooper C. B. Denny W. A. J. Med. Chem. 2017;60:4212. doi: 10.1021/acs.jmedchem.7b00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Blaser A. Yardley V. Maes L. Gupta S. Launay D. Martin D. Franzblau S. G. Wan B. Wang Y. Ma Z. Denny W. A. J. Med. Chem. 2016;59:2530. doi: 10.1021/acs.jmedchem.5b01699. [DOI] [PubMed] [Google Scholar]
- Alves F. Bilbe G. Blesson S. Goyal V. Monnerat S. Mowbray C. Ouattara G. M. Pécoul B. Rijal S. Rode J. Solomos A. Strub-Wourgaft N. Wasunna M. Wells S. Zijlstra E. E. Arana B. Alvar J. Clin. Microbiol. Rev. 2018;31:e00048. doi: 10.1128/CMR.00048-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.-J. Croft S. L. De la Flor R. Alavijeh M. Yardley V. Braillard S. Mowbray C. Bocxlaer K. V. Antimicrob. Agents Chemother. 2019;63:e00829. doi: 10.1128/AAC.00829-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. Yardley V. Vishwakarma P. Shivahare R. Sharma B. Launay D. Martin D. Puri S. K. J. Antimicrob. Chemother. 2015;70:518. doi: 10.1093/jac/dku422. [DOI] [PubMed] [Google Scholar]
- Deeks E. D. Drugs. 2019;79:215. doi: 10.1007/s40265-019-1051-6. [DOI] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Yardley V. Maes L. Launay D. Braillard S. Chatelain E. Wan B. Franzblau S. G. Ma Z. Cooper C. B. Denny W. A. ACS Med. Chem. Lett. 2021;12:275. doi: 10.1021/acsmedchemlett.0c00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Marshall A. J. Yardley V. Maes L. Gupta S. Launay D. Braillard S. Chatelain E. Wan B. Franzblau S. G. Ma Z. Cooper C. B. Denny W. A. Eur. J. Med. Chem. 2021;209:112914. doi: 10.1016/j.ejmech.2020.112914. [DOI] [PubMed] [Google Scholar]
- Thompson A. M. O'Connor P. D. Marshall A. J. Blaser A. Yardley V. Maes L. Gupta S. Launay D. Braillard S. Chatelain E. Wan B. Franzblau S. G. Ma Z. Coper C. B. Denny W. A. J. Med. Chem. 2018;61:2329. doi: 10.1021/acs.jmedchem.7b01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylle S. Roberts A. J. Norval S. Patterson S. Foth B. J. Berriman M. Read K. D. Fairlamb A. H. PLoS Pathog. 2016;12:e1005971. doi: 10.1371/journal.ppat.1005971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Patterson S. Fairlamb A. H. Antimicrob. Agents Chemother. 2013;57:901. doi: 10.1128/AAC.01788-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Thomas M. Patterson S. Crouch S. De Rycker M. Lowe R. Gresham S. Urbaniak M. D. Otto T. D. Stojanovski L. Simeons F. R. C. Manthri S. MacLean L. M. Zuccotto F. Homeyer N. Pflaumer H. Boesche M. Sastry L. Connolly P. Albrecht S. Berriman M. Drewes G. Gray D. W. Ghidelli-Disse S. Dixon S. Fiandor J. M. Wyatt P. G. Ferguson M. A. J. Fairlamb A. H. Miles T. J. Read K. D. Gilbert I. H. Nature. 2018;560:192. doi: 10.1038/s41586-018-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. G. De Rycker M. Ajakane M. Albrecht S. Alvarez-Pedraglio A. I. Boesche M. Brand S. Campbell L. Cantizani-Perez J. Cleghorn L. A. T. Copley R. C. B. Crouch S. D. Daugan A. Drewes G. Ferrer S. Ghidelli-Disse S. Gonzalez S. Gresham S. L. Hill A. P. Hindley S. J. Lowe R. M. MacKenzie C. J. MacLean L. Manthri S. Martin F. Miguel-Siles J. Nguyen V. L. Norval S. Osuna-Cabello M. Woodland A. Patterson S. Pena I. Quesada-Campos M. T. Reid I. H. Revill C. Riley J. Ruiz-Gomez J. R. Shishikura Y. Simeons F. R. C. Smith A. Smith V. C. Spinks D. Stojanovski L. Thomas J. Thompson S. Underwood T. Gray D. W. Fiandor J. M. Gilbert I. H. Wyatt P. G. Read K. D. Miles T. J. J. Med. Chem. 2019;62:1180. doi: 10.1021/acs.jmedchem.8b01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycker M. Hallyburton I. Thomas J. Campbell L. Wyllie S. Joshi D. Cameron S. Gilbert I. H. Wyatt P. G. Frearson J. A. Fairlamb A. H. Gray D. W. Antimicrob. Agents Chemother. 2013;57:2913. doi: 10.1128/AAC.02398-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broni E. Kwofie S. K. Asiedu S. O. Miller III W. A. Wilson M. D. Biomolecules. 2021;11:458. doi: 10.3390/biom11030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou A. Smirlis D. Microorganisms. 2021;9:691. doi: 10.3390/microorganisms9040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. Catta-Preta C. M. C. Neish R. Sadlova J. Powell B. Alves-Ferreira E. V. C. Geoghegan V. Carnielli J. B. T. Newling K. Hughes C. Vojtkova B. Anand J. Mihut A. Walrad P. B. Wilson L. G. Pitchford J. W. Volf P. Mottram J. C. Nat. Commun. 2021;12:1244. doi: 10.1038/s41467-021-21360-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker D. L. Marr J. J. Berens R. L. J. Biol. Chem. 1986;261:9412. doi: 10.1016/S0021-9258(18)67670-7. [DOI] [PubMed] [Google Scholar]
- Miles T. J. and Thomas M. G., PCT Int Appl., WO2016116563A1, 2016
- Thomas M. Brand S. De Rycker M. Zuccotto F. Lukac I. Dodd P. G. Ko E.-J. Manthri S. McGonagle K. Osuna-Cabello M. Riley J. Pont C. Simeons F. Stojanovski L. Thomas J. Thompson S. Viayna E. Fiandor J. M. Martin J. Wyatt P. G. Miles T. J. Read K. D. Marco M. Gilbert I. H. J. Med. Chem. 2021;64:5905. doi: 10.1021/acs.jmedchem.1c00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie S. Brand S. Thomas M. De Rycker M. Chung C. Pena I. Bingham R. P. Bueren-Calabuig J. A. Cantizani J. Cebrian D. Craggs P. D. Ferguson L. Goswami P. Hobrath J. Howe J. Jeacock L. Ko E.-J. Korczynska J. MacLean L. Manthri S. Martinez M. S. Mata-Cantero L. Moniz S. Nühs A. Osuna-Cabello M. Pinto E. Riley J. Robinson S. Rowland P. Simeons F. R. C. Shishikura Y. Spinks D. Stojanovski L. Thomas J. Thompson S. Gaza E. V. Wall R. J. Zuccotto F. Horn D. Ferguson M. A. J. Fairlamb A. H. Fiandor J. M. Martin J. Gray D. W. Miles T. J. Gilbert I. H. Read K. D. Marco M. Wyatt P. G. Proc. Natl. Acad. Sci. U. S. A. 2019;116:9318. doi: 10.1073/pnas.1820175116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. GSK3186899/DDD853651 & GSK3494245/DDD1305143, https://dndi.org/research-development/portfolio/gsk3186899-ddd853651-gsk3494245-ddd1305143/, Accessed July 01, 2022
- Khare S. Nagle A. S. Biggart A. Lai Y. H. Liang F. Davis L. C. Barnes S. W. Mathison C. J. N. Myburgh E. Gao M.-Y. Gillespie J. R. Liu X. Tan J. L. Stinson M. Rivera I. C. Bailard J. Yeh V. Groessi T. Federe G. Koh H. X. Y. Venable J. D. Bursulaya B. Shapiro M. Mishra P. K. Spraggon G. Brock A. Mottram J. C. Buckner F. S. Rao S. P. S. Wen B. G. Walker J. R. Tuntland T. Molteni V. Glynne R. J. Supek F. Nature. 2016;537:229. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle A. Biggart A. Be C. Srinivas H. Hein A. Caridha D. Sciotti R. J. Pybus B. Kreishman-Deitrick M. Bursulaya B. Lai Y. H. Gao M.-Y. Liang F. Mathison C. J. N. Liu X. Yeh V. Smith J. Lerario I. Xie Y. Chianelli D. Gibney M. Berman A. Chen Y.-L. Jiricek J. Davis L. C. Liu X. Ballard J. Khare S. Eggimann F. K. Luneau A. Groessl T. Shapiro M. Richmond W. Johnson K. Rudewicz P. J. Rao S. P. S. Thompson C. Tuntland T. Spraggon G. Glynne R. J. Supek F. Wiesmann C. Molteni V. J. Med. Chem. 2020;63:10773. doi: 10.1021/acs.jmedchem.0c00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thareja S. Zhu M. Ji X. Wang B. Heterocycl. Commun. 2017;23:137. doi: 10.1515/hc-2017-0086. [DOI] [Google Scholar]
- Bocxlaer K. V. Caridha D. Black C. Vesely B. Leed S. Sciotti R. J. Wijnant G.-J. Yardley V. Braillard S. Mowbray C. E. Ioset J.-R. Croft S. L. Int. J. Parasitol.: Drugs Drug Resist. 2019;11:129. doi: 10.1016/j.ijpddr.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. DNDi-6148, https://dndi.org/research-development/portfolio/dndi-6148/, Accessed July 01, 2022
- Kerkhof M. V. Leprohon P. Mabille D. Hendrickx S. Tulloch L. B. Wall R. J. Wyllie S. Chatelain E. Mowbray C. E. Braillard S. Ouellette M. Maes L. Caljon G. Microorganisms. 2021;9:1408. doi: 10.3390/microorganisms9071408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. DNDi-5421 & DNDI-5610, https://dndi.org/research-development/portfolio/oxaleish/, Accessed July 01, 2022
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. Leishmaniasis L205 series, https://dndi.org/research-development/portfolio/leish-l205-series/ (Accessed July 01, 2022)
- Thacker S. G. McWilliams I. L. Bonnet B. Halle L. Beaucage S. Rachuri S. Dey R. Duncan R. Modabber F. Robinson S. Bilbe G. Arana B. Verthelyl D. PLoS Neglected Trop. Dis. 2020;14:e0008050. doi: 10.1371/journal.pntd.0008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M. Oryan A. Hatam G. Immunol. Lett. 2021;233:80. doi: 10.1016/j.imlet.2021.03.011. [DOI] [PubMed] [Google Scholar]
- Verthelyi D. Gursel M. Kenney R. T. Lifson J. D. Liu S. Mican J. Klinman D. M. J. Immunol. 2003;170:4717. doi: 10.4049/jimmunol.170.9.4717. [DOI] [PubMed] [Google Scholar]
- Puig M. Grajkowski A. Boczkowska M. Ausín C. Beaucage S. L. Verthelyi D. Nucleic Acids Res. 2006;34:6488. doi: 10.1093/nar/gkl867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi D. Ishii K. J. Gursel M. Takeshita F. Klinman D. M. J. Immunol. 2001;166:2372. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- DNDi, Drugs for Neglected Diseases initiative, Geneva, Switzerland. CpG-D35 for cutaneous leishmaniasis, https://dndi.org/research-development/portfolio/cpg-d35/
- Kerkhof M. V. Mabille D. Hendrickx S. Leprohon P. Mowbray C. E. Braillard S. Ouellette M. Maes L. Caljon G. Antimicrob. Agents Chemother. 2020;64(9):e00152. doi: 10.1128/AAC.00152-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof M. V. Mabille D. Chatelain E. Mowbray C. E. Braillard S. Hendrickx S. Maes L. Caljon G. Int. J. Parasitol.: Drugs Drug Resist. 2018;8:81. doi: 10.1016/j.ijpddr.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangshetti J. N. Khan F. A. K. Kulkarni A. A. Arote R. Patil R. H. RSC Adv. 2015;5:32376. doi: 10.1039/C5RA02669E. [DOI] [Google Scholar]
- Coimbra E. S. Antinarelli L. M. R. Crispi M. A. Nogueira T. C. M. Pinheiro A. C. de Souza M. V. N. ChemMedChem. 2018;13:1387. doi: 10.1002/cmdc.201800328. [DOI] [PubMed] [Google Scholar]
- Razzaghi-Asl N. Sepehri S. Ebadi A. Karami P. Nejatkhah N. Johari-Ahar M. Mol. Diversity. 2020;24:525. doi: 10.1007/s11030-019-09953-4. [DOI] [PubMed] [Google Scholar]
- Gonçalves G. A. Spillere A. R. Das Neves G. M. Kagami L. P. Von Poser G. L. Canto R. F. S. Eifler-Lima V. L. Eur. J. Med. Chem. 2020;203:112514. doi: 10.1016/j.ejmech.2020.112514. [DOI] [PubMed] [Google Scholar]
- Rosa L. B. Aires R. L. Oliveira L. S. Fontes J. V. Miguel D. C. Abbehausen C. ChemMedChem. 2021;16:1682–1696. doi: 10.1002/cmdc.202100022. [DOI] [PubMed] [Google Scholar]
- Al-Tamimi A.-M. S. Etxebeste-Mitxeltorena M. Sanmartín C. Jiménez-Ruiz A. Syrjänen L. Parkkila S. Selleri S. Carta F. Angeli A. Supuran C. T. Bioorg. Chem. 2019;86:339. doi: 10.1016/j.bioorg.2019.01.069. [DOI] [PubMed] [Google Scholar]
- Coimbra E. S. de Souza M. V. N. Terror M. S. Pinheiro A. C. Granato J. T. Eur. J. Med. Chem. 2019;184:111742. doi: 10.1016/j.ejmech.2019.111742. [DOI] [PubMed] [Google Scholar]
- Rebello K. M. Andrade-Neto V. V. Gomes C. R. B. de Souza M. V. N. Branquinha M. H. Santos A. L. S. Torres-Santos E. C. D'Avila-Levy C. M. Front. Cell. Infect. Microbiol. 2019;9:1. doi: 10.3389/fcimb.2019.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akao Y. Canan S. Cao Y. Condroski K. Engkvist O. Itono S. Kaki R. Kimura C. Kogej T. Nagaoka K. Naito A. Nakai H. Pairaudeau G. Radu C. Roberts I. Shimada M. Shum D. Watanabe N. Xie H. Yonezawa S. Yoshida O. Yoshida R. Mowbray C. Perry B. RSC Med. Chem. 2021;12:384. doi: 10.1039/D0MD00353K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira R. A. A. Resende Jr. C. O. Martinez P. D. G. Koovits P. J. Soares B. M. Ferreira L. L. G. Michelan-Duarte S. Chelucci R. C. Andricopoulo A. D. Galuppo M. K. Uliasna S. R. B. Matheeussen A. Caijon G. Maes L. Campbell S. Kratz J. M. Mowbray C. E. Dias L. C. PLoS Neglected Trop. Dis. 2021;15:e0009196. doi: 10.1371/journal.pntd.0009196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koovits P. J. Dessoy M. A. Matheeussen A. Maes L. Caljon G. Ferreira L. L. G. Chelucci R. C. Michelan-Duarte S. Andricopoulo A. D. Campbell S. Kratz J. M. Mowbray C. E. Dias L. C. RSC Med. Chem. 2020;11:1267. doi: 10.1039/D0MD00165A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durleu E. Prina E. Leclercq O. Oumata N. Gaborlaud-Kolar N. Vougoglannopoulou K. Aulner N. Defontaine A. No J. H. Ruchaud S. Skaltsounis A.-L. Galons H. Späth G. F. Meijer L. Rachidi N. Antimicrob. Agents Chemother. 2016;60:2822. doi: 10.1128/AAC.00021-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla B. Madhubala R. J. Parasit. Dis. 2010;34:1. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura F. Rajesh R. Catta-Preta C. M. C. Moretti N. S. Cestari I. Drug Dev. Res. 2020:1. doi: 10.1002/ddr.21664. [DOI] [PubMed] [Google Scholar]
- Battista T. Colotti G. Ilari A. Fiorillo A. Molecules. 2020;25:1924. doi: 10.3390/molecules25081924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhit A. A. El-Agroudy E. Helmy A. Ibrahim T. M. Shavandi A. Bekhit A. E. A. Eur. J. Med. Chem. 2018;160:229. doi: 10.1016/j.ejmech.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Brindha J. Balamurali M. M. Kaushik C. Front. Chem. 2021;9:622286. doi: 10.3389/fchem.2021.622286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna P. Upadhyay A. Infect. Immun. 2021;89(2):e00559. doi: 10.1128/IAI.00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayakar A. Chandrasekaran S. Kuchipudi S. V. Kalangi S. K. Front. Immunol. 2019;10:670. doi: 10.3389/fimmu.2019.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taslimi Y. Zahedifard F. Rafati S. Parasitology. 2018;145:497. doi: 10.1017/S003118201600216X. [DOI] [PubMed] [Google Scholar]
- Oliveira W. N. Ribeiro L. E. Schrieffer A. Machado P. Carvalho E. M. Bacellar O. Cytokine+ 2014;66:127–132. doi: 10.1016/j.cyto.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei A. Maleki M. Masoumi E. Maspi N. Cytokine+ 2021;145:155297. doi: 10.1016/j.cyto.2020.155297. [DOI] [PubMed] [Google Scholar]
- Jawed J. J. Dutta S. Majumdar S. Biomed. Pharmacother. 2019;117:109098. doi: 10.1016/j.biopha.2019.109098. [DOI] [PubMed] [Google Scholar]
- Firooz A. Khamesipour A. Ghoorchi M. H. Nassiri-Kashani M. Eskandari S. E. Khatami A. Hooshmand B. Gorouhi F. Rashighi-Firoozabadi M. Dowlati Y. Arch. Dematol. 2006;142:1575. doi: 10.1001/archderm.142.12.1575. [DOI] [PubMed] [Google Scholar]
- Dalton J. E. Kaye P. M. Expert Rev. Anti-infect. Ther. 2010;8:739. doi: 10.1586/eri.10.64. [DOI] [PubMed] [Google Scholar]
- Abdossamadi Z. Seyed N. Rafati S. Cell. Immunol. 2016;309:23. doi: 10.1016/j.cellimm.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Mendonça S. C. F. Parasites Vectors. 2016;9:492. doi: 10.1186/s13071-016-1777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseda Y. Munakata L. Meng J. Suzuki R. Aoshi T. PLoS One. 2020;15:e0227891. doi: 10.1371/journal.pone.0227891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann J. Klinman D. M. Vaccine. 2014;32:6377. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A. S. A. Tian W. Zhang Y. Peng P. Wang F. Li T. Carbohydr. Polym. 2020;237:116120. doi: 10.1016/j.carbpol.2020.116120. [DOI] [PubMed] [Google Scholar]
- Sundar S. Singh B. Expert Rev. Vaccines. 2014;13:489. doi: 10.1586/14760584.2014.894467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebali M. Nadim A. Khamesipour A. Acta Trop. 2019;200:105173. doi: 10.1016/j.actatropica.2019.105173. [DOI] [PubMed] [Google Scholar]
- Ratnapriya S. Keerti Sahasrabuddhe A. A. Dube A. Vaccine. 2019;37:3505. doi: 10.1016/j.vaccine.2019.04.092. [DOI] [PubMed] [Google Scholar]
- Moafi M. Rezvan H. Sherkat R. Taleban R. Int. J. Prev. Med. 2019;10:95. doi: 10.4103/ijpvm.IJPVM_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coler R. N. Duthie M. S. Hofmeyer K. A. Guderian J. Jayashankar L. Vergara J. Rolf T. Misquith A. Laurence J. D. Raman V. S. Bailor H. R. Cauwelaert N. D. Reed S. J. Vallur A. Favila M. Orr M. T. Ashman J. Ghosh P. Mondal D. Reed S. G. Clin. Transl. Immunol. 2015;4:e35. doi: 10.1038/cti.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis B. M. Osman M. Khalil E. A. G. Santoro F. Furini S. Wiggins R. Keding A. Carraro M. Musa A. E. A. Abdarahaman M. A. A. Mandefield L. Bland M. Aebisher T. Gabe R. Layton A. M. Lacey C. J. N. Kaye P. M. Musa A. M. Mol. Ther. 2021;29:2366. doi: 10.1016/j.ymthe.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S. Freier A. Boussoffara T. Das S. Oswald D. Losch F. O. Selka M. Sacerdoti-Sierra N. Schönian G. Wiesmüller K.-H. Seifert K. Schroff M. Juhls C. Jaffe C. L. Roy S. Das P. Louzir H. Croft S. L. Modabber F. Walde P. Sci. Transl. Med. 2014;6:234ra56. doi: 10.1126/scitranslmed.3008222. [DOI] [PubMed] [Google Scholar]
- Martínez-Rodrigo A. Dias D. S. Ribeiro P. A. F. Roatt B. M. Mas A. Carrión J. Coelho E. A. F. Domínguez-Bernal G. Vaccines. 2019;7:183. doi: 10.3390/vaccines7040183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lage D. P. Ribeiro P. A. F. Dias D. S. Mendonça D. V. C. Ramos F. F. Carvalho L. M. Oliveira D. Steiner B. T. Martins V. T. Perin L. Machado A. S. Santos T. T. O. Tavares G. S. V. Silva J. A. O. Oliveira J. S. Roatt B. M. Ávila R. A. M. Teixeira A. L. Humbert M. V. Coelho E. A. F. Christodoulides M. npj Vaccines. 2020;5:75. doi: 10.1038/s41541-020-00224-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito R. C. F. Ruiz J. C. Cardoso J. M. O. Ostolin T. L. V. P. Reis L. E. S. Mathias F. A. S. Soares R. D. O. A. Roatt B. M. Oliveira R. C. Resende D. M. Reis A. B. Vaccines. 2020;8:252. doi: 10.3390/vaccines8020252. [DOI] [PMC free article] [PubMed] [Google Scholar]