Abstract
Background
We conducted a systematic review and meta-analysis of the cardiovascular, kidney, and safety outcomes of sodium-glucose cotransporter 2 inhibitors (SGLT2i) among patients with diabetic kidney disease (DKD).
Methods
We searched electronic databases for major randomized placebo-controlled clinical trials published up to September 30, 2021 and reporting on cardiovascular and kidney outcomes of SGLT2i in patients with DKD. DKD was defined as chronic kidney disease in individuals with type 2 diabetes. Random-effects meta-analysis models were used to estimate pooled hazard ratios (HR) and 95% confidence intervals (CI) for clinical outcomes including major adverse cardiovascular events (MACE: myocardial infarction [MI], stroke, and cardiovascular death), kidney composite outcomes (a combination of worsening kidney function, end-stage kidney disease, or death from renal or cardiovascular causes), hospitalizations for heart failure (HHF), deaths and safety events (mycotic infections, diabetic ketoacidosis [DKA], volume depletion, amputations, fractures, urinary tract infections [UTI], acute kidney injury [AKI], and hyperkalemia).
Results
A total of 26,106 participants with DKD from 8 large-scale trials were included (median age: 65.2 years, 29.7–41.8% women, 53.2–93.2% White, median follow-up: 2.5 years). SGLT2i were associated with reduced risks of MACE (HR 0.83, 95% CI 0.75–0.93), kidney composite outcomes (HR 0.66, 95% CI 0.58–0.75), HHF (HR 0.62, 95% CI 0.55–0.71), cardiovascular death (HR 0.84, 95% CI 0.74–0.96), MI (HR 0.78, 95% CI 0.67–0.92), stroke (HR 0.76, 95% CI 0.59–0.97), and all-cause death (HR 0.86, 95% CI 0.77–0.96), with no significant heterogeneity detected. Similar results were observed among participants with reduced estimated glomerular filtration rate (eGFR: < 60 mL/min/1.73m2). The relative risks (95% CI) for adverse events were 3.89 (1.42–10.62) and 2.50 (1.32–4.72) for mycotic infections in men and women respectively, 3.54 (0.82–15.39) for DKA, and 1.29 (1.13–1.48) for volume depletion.
Conclusions
Among adults with DKD, SGLT2i were associated with reduced risks of MACE, kidney outcomes, HHF, and death. With a few exceptions of more clear safety signals, we found overall limited data on the associations between SGLT2i and safety outcomes. More research is needed on the safety profile of SGLT2i in this population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01476-x.
Keywords: SGLT2 inhibitors, Diabetic kidney disease, Cardiovascular outcomes, Kidney outcomes, Safety, Meta-analysis
Background
Type 2 diabetes (T2D) remains highly common in the United States [1]. Nearly 32 million Americans currently live with T2D and its prevalence is expected to rise [2, 3]. Diabetes-related microvascular complications such as diabetic kidney disease (DKD) constitute a significant public health problem. Indeed, nearly 40% of patients with T2D develop DKD [4]. DKD is a leading cause of chronic kidney disease (CKD) and end-stage kidney disease (ESKD) [4]. Furthermore, most of the excess mortality risk observed in patients with diabetes may be related to the presence of DKD [5].
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have recently emerged as a new class of oral glucose-lowering agents with pleiotropic effects including reduction in cardiovascular and kidney outcomes among patients with T2D [6–11]. SGLT2i prescriptions have steadily increased among patients with DKD [12]. However, some uncertainty remains on the effects of SGLT2i along the entire spectrum of DKD. For instance, a relatively small number of participants reached ESKD in early clinical trials of SGLT2i [7, 13, 14]; studies of the effects of SGLT2i on clinical outcomes such as stroke among individuals with DKD have yielded inconsistent results [15]. Moreover, the cardiovascular outcome trials focusing on SGLT2i have usually been underpowered to evaluate most adverse events in people with DKD [6–10, 13, 14, 16]. Therefore, we performed a systematic review and meta-analysis of the major cardiovascular and kidney outcomes trials to summarize and update the currently available evidence on the effects of SGLT2i on cardiovascular, kidney and safety outcomes among patients with DKD.
Methods
This systematic review and meta-analysis was registered with PROSPERO (CRD42021282869)[17] and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [18].
Search strategy
We conducted a comprehensive search of PubMed and Embase for major randomized, placebo-controlled clinical trials of SGLT2i published up to September 30, 2021 and reporting on cardiovascular and kidney outcomes as well as adverse events among adults with DKD. The SGLT2i included empagliflozin, dapagliflozin, canagliflozin, ertugliflozin, and sotagliflozin. Sotagliflozin is an SGLT2i that also inhibits gastrointestinal sodium glucose cotransporter 1 receptors in the gastrointestinal tract. DKD was defined as the presence of CKD in individuals with T2D, defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and/or urine albumin-to-creatinine ratio (UACR) ≥ 30 mg/g. Two reviewers (ADK and MZ) independently identified articles and sequentially screened them for inclusion, starting with titles and abstracts, then full-text review. Additionally, reference lists of identified studies were manually scanned, and citing references screened through the ISI Web of Knowledge database, for possible additional eligible studies.
Eligibility criteria, data extraction and assessment of study quality
Studies were considered eligible if they met all of the following criteria: (1) the study design was a randomized controlled trial (RCT) of cardiovascular or kidney outcomes of SGLT2i; (2) the study reported on cardiovascular, kidney, or adverse events of SGLT2i among individuals with DKD; (3) the hazard ratio (HR) or relative risk (RR) and its corresponding 95% confidence interval (CI) or enough data to calculate them were reported. We excluded studies in people without diabetes or CKD. Two investigators (ADK and MZ) independently abstracted data from eligible studies and conducted quality assessment. Data were extracted from the primary trials’ reports as well as from secondary data analyses where available [15, 19–25]. We assessed the quality of studies using the Cochrane Risk of Bias tool [26].
Outcomes
The efficacy outcomes assessed in this meta-analysis include: (1) major adverse cardiovascular events (MACE) defined as a composite of nonfatal myocardial infarction (MI), nonfatal stroke, or cardiovascular death; (2) hospitalization for HF (HHF); (3) cardiovascular death; (4) fatal and nonfatal MI; (5) fatal and nonfatal stroke; (6) all‐cause mortality; and (7) kidney composite outcomes. The kidney composite outcome definitions varied across trials (Additional file 1: Table S1) but generally included a combination of worsening eGFR or serum creatinine, ESKD with or without renal replacement therapy or transplant, or death from renal or cardiovascular causes.
We evaluated the safety outcomes that have been reported across the clinical trials including mycotic infections, diabetic ketoacidosis (DKA), volume depletion, amputations, fractures, urinary tract infections (UTI), acute kidney injury (AKI), and hyperkalemia.
Statistical analysis
For this meta-analysis, we used published data; and not individual participant-level data. For each outcome, we sought to identify HRs and 95%CIs of the effects of SGLT2i in participants with DKD from individual studies. As HRs were not always available for adverse events, we used in order of preference HRs, then rate ratios or risk ratios to maximize the use of trial‐level data. The pooled effect estimates and associated 95% CI were computed using random-effects meta-analysis models. The random-effects model is the most conservative approach as it makes allowances for within and between-study heterogeneity [27]. For each outcome, the HR and its 95% CI limits were logarithmically transformed before the meta-analysis. The meta-analysis was implemented on the natural logarithmic scale, with results exponentiated and reported on the original HR scale. The z statistic was used to test the null hypothesis (that the SGLT2i is not associated with the outcome). We assessed the heterogeneity between studies using Cochran’s Q statistic, and I2 statistics [28, 29]. Publication bias was assessed by the Egger’s test [30]. Furthermore, we performed subgroup analyses stratified by eGFR range and albuminuria levels. Albuminuria levels were classified as moderate albuminuria (UACR 30 – 300 mg/g) or severe albuminuria (UACR ≥ 300 mg/g).
A two-sided P-value was deemed statistically significant. All analyses were performed using Stata software (Stata Corp V.14, Texas, USA).
Results
Characteristics of studies
The study selection process is summarized in Additional file 1: Fig. S1. A total of 8 placebo-controlled trials evaluating 5 SGLT2i were included (Additional file 1: Table S2). These included the EMPA‐REG OUTCOME (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients) [6], CANVAS (Canagliflozin Cardiovascular Assessment Study) Program [11], DECLARE‐TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58) [7], CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) [8], DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) [9], VERTIS CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial) [10], SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) [16], and SOLOIST-WHF (Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure) [31]. All the studies had a low risk of bias (Additional file 1: Table S3) [26].
We included data from 26,106 participants, with a median follow-up of 2.5 years (interquartile range: 1.9–3.1). Across the 8 trials, the mean age of participants ranged from 61.8 to 69 years, the proportion of women from 29.7 to 41.8%; White individuals made up the majority of participants (53.2–93.2%). The mean HbA1C ranged from 7.1 to 8.3%. The proportion of patients with a history of established CVD at baseline varied from 37.4 to 100%.
Association of SGLT2i with cardiovascular and kidney outcomes among patients with diabetic kidney disease
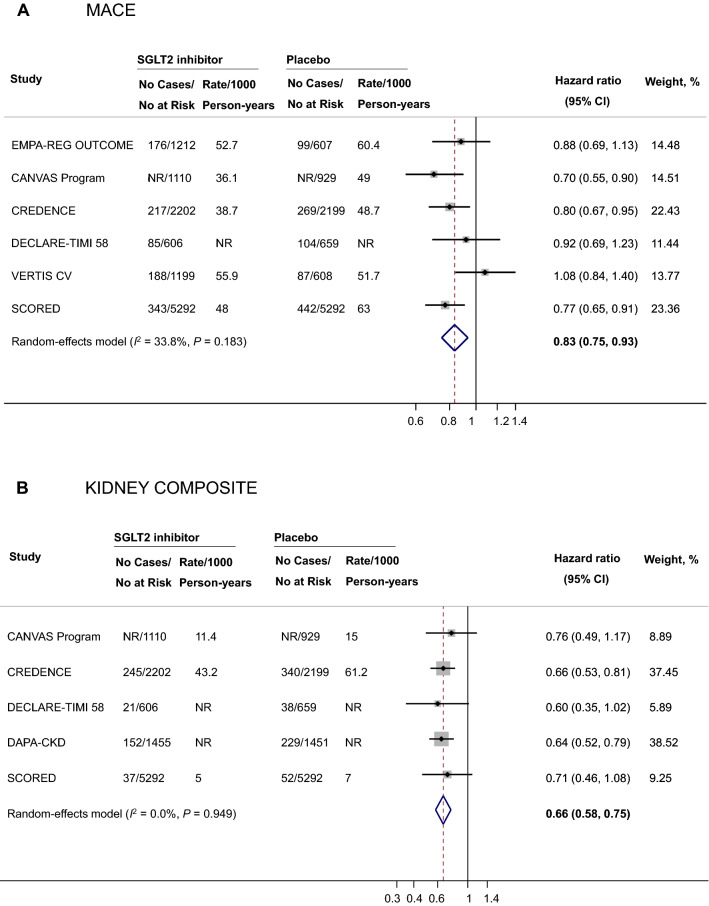
Meta-analyses for the effect of SGLT2i on clinical outcomes are summarized in Table 1. Overall, 2,271 participants experienced a MACE. The use of SGLT2i was associated with a 17% reduction in the risk of MACE (HR 0.83, 95% CI 0.75–0.93, I2 = 33.8%, P for heterogeneity = 0.183, n = 21,913, Fig. 1). Additionally, SGLT2i were associated with reductions in the risks of HHF (HR 0.62, 95% CI 0.55–0.71, I2 = 0.0%, P for heterogeneity = 0.844, n = 22,346), cardiovascular death (HR 0.84, 95% CI 0.74–0.96, I2 = 0.0%, P for heterogeneity = 0.639, n = 20,539), fatal and nonfatal MI (HR 0.78, 95% CI 0.67–0.92, I2 = 7.7%, P for heterogeneity = 0.363, n = 20,108), fatal and nonfatal stroke (HR 0.76, 95% CI 0.59–0.97, I2 = 41.3%, P for heterogeneity = 0.146, n = 20,108, Additional file 1: Fig. S2), as well as all-cause mortality (HR 0.86, 95% CI 0.77–0.96, I2 = 14.5%, P for heterogeneity = 0.322, n = 21,406).
Table 1.
Effect of SGLT2 inhibitors on clinical outcomes in adults with diabetic kidney disease
| Outcome | No. studies | No. events | Sample size | HR (95% CI) | I2, % | PHeterogeneity | PEgger test |
|---|---|---|---|---|---|---|---|
| MACE | 6 | 2271 | 21,913 | 0.83 (0.75–0.93) | 33.8 | 0.183 | 0.287 |
| Kidney composite | 5 | 1197 | 21,195 | 0.66 (0.58–0.75) | 0.0 | 0.949 | 0.513 |
| HHF | 6 | 1219 | 22,346 | 0.62 (0.55–0.71) | 0.0 | 0.844 | 0.267 |
| Cardiovascular death | 5 | 953 | 20,539 | 0.84 (0.74–0.96) | 0.0 | 0.639 | 0.996 |
| Fatal and nonfatal MI | 5 | 498* | 20,108 | 0.78 (0.67–0.92) | 7.7 | 0.363 | 0.671 |
| Fatal and nonfatal stroke | 5 | 332* | 20,108 | 0.76 (0.59–0.97) | 41.3 | 0.146 | 0.564 |
| All-cause mortality | 5 | 1451 | 21,406 | 0.86 (0.77–0.96) | 14.5 | 0.322 | 0.268 |
*The number of MI events and stroke cases from the SCORED trial were not reported in the primary trials and are not included in the table. CI indicates confidence interval; HHF hospitalization for heart failure, HR hazard ratio, I2, I-squared, MACE Major Adverse Cardiovascular Events, MI myocardial infarction, SCORED Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk, SGLT2 sodium-glucose cotransporter 2; SGLT2, sodium-glucose cotransporter 2, SOLOIST-WHF Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure, VERTIS CV Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial
Fig. 1.
Effects of SGLT2 inhibitors on major adverse cardiovascular events (A) and kidney composite outcomes (B) among individuals with diabetic kidney disease. CANVAS indicates Canagliflozin Cardiovascular Assessment Study, CI confidence interval, CREDENCE Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation, DECLARE‐TIMI 58 Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58, EMPA‐REG OUTCOME Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients, I2 I-squared, MACE major adverse cardiovascular event, NR not reported, SCORED Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk, SGLT2 sodium-glucose cotransporter 2, VERTIS CV Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial
A total of 1,197 participants experienced a kidney composite outcome. SGLT2i were associated with a 34% reduction in the risk of a kidney composite outcome (HR 0.66, 95% CI 0.58–0.75, I2 = 0.0%, P for heterogeneity = 0.949, n = 21,195, Fig. 1).
Association of SGLT2i with cardiovascular and kidney outcomes among participants with reduced eGFR
The meta-analyses of the effects of SGLT2i on clinical outcomes by baseline eGFR are displayed in Table 2. When analyses were restricted to participants with reduced eGFR (eGFR < 60 mL/min/1.73 m2) regardless of albuminuria status (n = 20,106), SGLT2i remained associated with lower risks of MACE (HR 0.82, 95% CI 0.74–0.91, I2 = 8.6%, P for heterogeneity = 0.363, n = 20,106), HHF (HR 0.61, 95% CI 0.54–0.70, I2 = 0.0%, P for heterogeneity = 0.740, n = 20,106), cardiovascular death (HR 0.86, 95% CI 0.75–0.98, I2 = 0.0%, P for heterogeneity = 0.912, n = 18,299), nonfatal and fatal MI (HR 0.75, 95% CI 0.63–0.90, I2 = 0.0%, P for heterogeneity = 0.480, n = 15,707), nonfatal and fatal stroke (HR 0.75, 95% CI 0.55–1.01, I2 = 38.3%, P for heterogeneity = 0.151, n = 15,707), and kidney composite outcomes (HR 0.65, 95% CI 0.55–0.78, I2 = 0.0%, P for heterogeneity = 0.645, n = 16,480). There was a trend towards a reduction in all-cause mortality, though confidence intervals included the null hypothesis value (HR 0.93, 95% CI 0.81–1.07, I2 = 0.0%, P for heterogeneity = 0.302, n = 13,668 participants across 3 studies).
Table 2.
Effect of SGLT2 inhibitors on clinical outcomes among participants with reduced eGFR
| Outcome | No. studies | No. events | Sample size | HR (95% CI) | I2, % | PHeterogeneity | PEgger test |
|---|---|---|---|---|---|---|---|
| Overall (eGFR < 60 mL/min/1.73m2) | |||||||
| MACE | 6 | 2102 | 20,106 | 0.82 (0.74–0.91) | 8.6 | 0.363 | 0.810 |
| Kidney composite | 4 | 530 | 16,480 | 0.65 (0.55–0.78) | 0.0 | 0.645 | 0.771 |
| HHF | 6 | 1125 | 20,106 | 0.61 (0.54–0.70) | 0.0 | 0.740 | 0.099 |
| Cardiovascular death | 5 | 834 | 18,299 | 0.86 (0.75–0.98) | 0.0 | 0.912 | 0.363 |
| Fatal and nonfatal MI | 4 | 320* | 15,707 | 0.75 (0.63–0.90) | 0.0 | 0.480 | 0.879 |
| Fatal and nonfatal stroke | 4 | 190* | 15,707 | 0.75 (0.55–1.01) | 38.3 | 0.151 | 0.855 |
| All-cause mortality | 3 | 837 | 13,668 | 0.93 (0.81–1.07) | 0.0 | 0.638 | 0.147 |
| eGFR < 45 mL/min/1.73m2** | |||||||
| MACE | 3 | 347 | 2437 | 0.75 (0.60–0.93) | 0.0 | 0.797 | 0.921 |
| Kidney composite | 2 | 225 | 1867 | 0.70 (0.54–0.92) | 0.0 | 0.841 | NA |
| HHF | 3 | 166 | 2437 | 0.60 (0.44–0.82) | 0.0 | 0.522 | 0.193 |
| Cardiovascular death | 3 | 191 | 2437 | 0.83 (0.62–1.11) | 0.0 | 0.699 | 0.925 |
| Fatal and nonfatal MI | 2 | 71 | 1124 | 0.70 (0.39–1.26) | 28.3 | 0.238 | NA |
| Fatal and nonfatal stroke | 2 | 38 | 1124 | 0.52 (0.23–1.17) | 30.2 | 0.231 | NA |
| All-cause mortality | 1 | 74 | 570 | 0.86 (0.54–1.38) | NA | NA | NA |
*The number of MI and stroke events were not reported in SCORED and are therefore not included in the table. Likewise, the number of HHF/cardiovascular death events were not reported in SOLOIST-WHF and are not included in the table. CI indicates confidence interval, eGFR estimated glomerular filtration rate, HHF hospitalization for heart failure, MACE major adverse cardiovascular events, HR hazard ratio, MI myocardial infarction, NA not applicable, NR not reported, SCORED Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk, SGLT2 sodium-glucose cotransporter 2, SOLOIST-WHF Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure
**The lower ends of eGFR ranged from 25 to 30 mL/min/1.73 m2
Association of SGLT2i with cardiovascular and kidney outcomes among participants with moderate or severe albuminuria
The meta-analyses of the effects of SGLT2i on clinical outcomes by baseline albuminuria levels are summarized in Table 3. Among participants with severely increased albuminuria, SGLT2i were associated with reduced risks of MACE (HR 0.73, 95% CI 0.65–0.83, I2 = 0.0%, P for heterogeneity = 0.444, n = 9,216), HHF (HR 0.62, 95% CI 0.51–0.75, I2 = 0.0%, P for heterogeneity = 0.915, n = 6,685), cardiovascular death (HR 0.72, 95% CI 0.59–0.87, I2 = 0.0%, P for heterogeneity = 0.408, n = 5,930), all-cause mortality (HR 0.77, 95% CI 0.65–0.90, I2 = 0.0%, P for heterogeneity = 0.415, n = 5,930), and kidney composite outcomes (HR 0.63, 95% CI 0.53–0.76, I2 = 0.8%, P for heterogeneity = 0.365, n = 8,447). There was a trend towards a reduction in MI (HR 0.81, 95% CI 0.64–1.03, I2 = 0.0%, P for heterogeneity = 0.654, n = 5,930) and stroke (HR 0.85, 95% CI 0.65–1.11, I2 = 0.0%, P for heterogeneity = 0.410, n = 5,930), although confidence intervals included the null value. Among participants with moderately increased albuminuria, the HRs were 0.92 (95% CI 0.79–1.07) for MACE, 0.60 (95% CI 0.47–0.77) for HHF, 0.70 (95% CI 0.35–1.38) for cardiovascular death, 1.01 (95% CI 0.77–1.32) for MI, 1.06 (95% CI 0.76–1.48) for stroke, 0.78 (95% CI 0.47–1.28) for all-cause mortality, and 0.98 (95% CI 0.62–1.57) for kidney composite outcomes (Table 3).
Table 3.
Effect of SGLT2 inhibitors on clinical outcomes among participants with moderate or severe albuminuria
| Outcome | No. studies | No. events | Sample size | HR (95% CI) | I2, % | PHeterogeneity | PEgger test |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| MACE | 4 | 1284* | 17,084 | 0.80 (0.71–0.90) | 25.6 | 0.234 | 0.683 |
| Kidney composite | 4 | 1124* | 17,208 | 0.66 (0.58–0.75) | 1.4 | 0.407 | 0.530 |
| HHF | 4 | 670 | 13,456 | 0.61 (0.52–0.71) | 0.0 | 0.916 | 0.791 |
| Cardiovascular death | 3 | 642 | 10,209 | 0.70 (0.55–0.88) | 50.9 | 0.086 | 0.378 |
| Fatal and nonfatal MI | 3 | 503 | 10,209 | 0.89 (0.75–1.07) | 0.0 | 0.679 | 0.355 |
| Fatal and nonfatal stroke | 3 | 382 | 10,209 | 0.92 (0.75–1.14) | 0.0 | 0.457 | 0.279 |
| All-cause mortality | 4 | 817* | 13,115 | 0.76 (0.66–0.88) | 28.8 | 0.219 | 0.285 |
| Moderate albuminuria | |||||||
| MACE | 3 | 520* | 7868 | 0.92 (0.79–1.07) | 0.0 | 0.795 | 0.342 |
| Kidney composite | 2 | 70* | 5855 | 0.98 (0.62–1.57) | 0.0 | 0.962 | NA |
| HHF | 3 | 261 | 6771 | 0.60 (0.47–0.77) | 0.0 | 0.473 | 0.283 |
| Cardiovascular death | 2 | 229 | 4279 | 0.70 (0.35–1.38) | 84.2 | 0.012 | NA |
| Fatal and nonfatal MI | 2 | 233 | 4279 | 1.01 (0.77–1.32) | 0.0 | 0.774 | NA |
| Fatal and nonfatal stroke | 2 | 153 | 4279 | 1.06 (0.76–1.48) | 0.0 | 0.363 | NA |
| All-cause mortality | 2 | 147* | 4279 | 0.78 (0.47–1.28) | 80.4 | 0.024 | NA |
| Severe Albuminuria | |||||||
| MACE | 4 | 764* | 9216 | 0.73 (0.65–0.83) | 0.0 | 0.444 | 0.428 |
| Kidney composite | 3 | 673* | 8447 | 0.63 (0.53–0.76) | 0.8 | 0.365 | 0.729 |
| HHF | 4 | 409 | 6685 | 0.62 (0.51–0.75) | 0.0 | 0.915 | 0.815 |
| Cardiovascular death | 3 | 413 | 5930 | 0.72 (0.59–0.87) | 0.0 | 0.408 | 0.348 |
| Fatal and nonfatal MI | 3 | 270 | 5930 | 0.81 (0.64–1.03) | 0.0 | 0.654 | 0.389 |
| Fatal and nonfatal stroke | 3 | 229 | 5930 | 0.85 (0.65–1.11) | 0.0 | 0.410 | NA |
| All-cause mortality | 3 | 473* | 5930 | 0.77 (0.65–0.90) | 0.0 | 0.415 | 0.234 |
*The number of MACE and kidney composite outcomes were not reported in SCORED and are therefore not included in the table. Likewise, the number of all-cause deaths by albuminuria were not reported in the CANVAS program and are not included in the table. CANVAS indicates Canagliflozin Cardiovascular Assessment Study, CI confidence interval; HHF hospitalization for heart failure, MACE major adverse cardiovascular events, HR hazard ratio, MI myocardial infarction, NA not applicable, NR not reported, SCORED Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk, SGLT2 sodium-glucose cotransporter 2
Association of SGLT2i with safety outcomes among participants with diabetic kidney disease
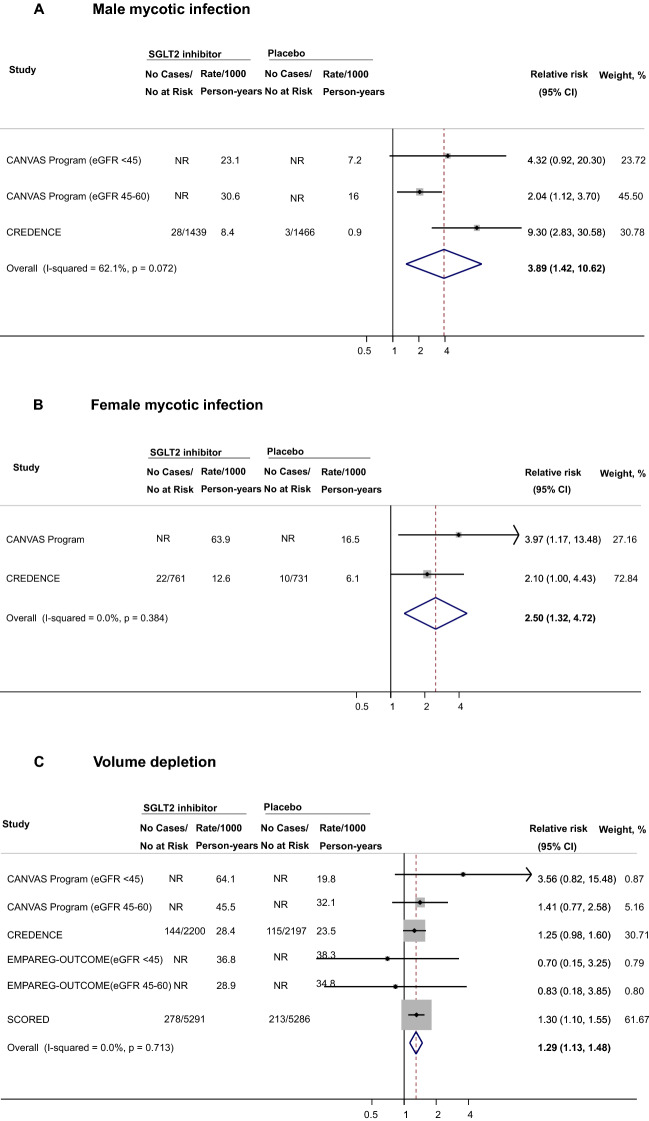
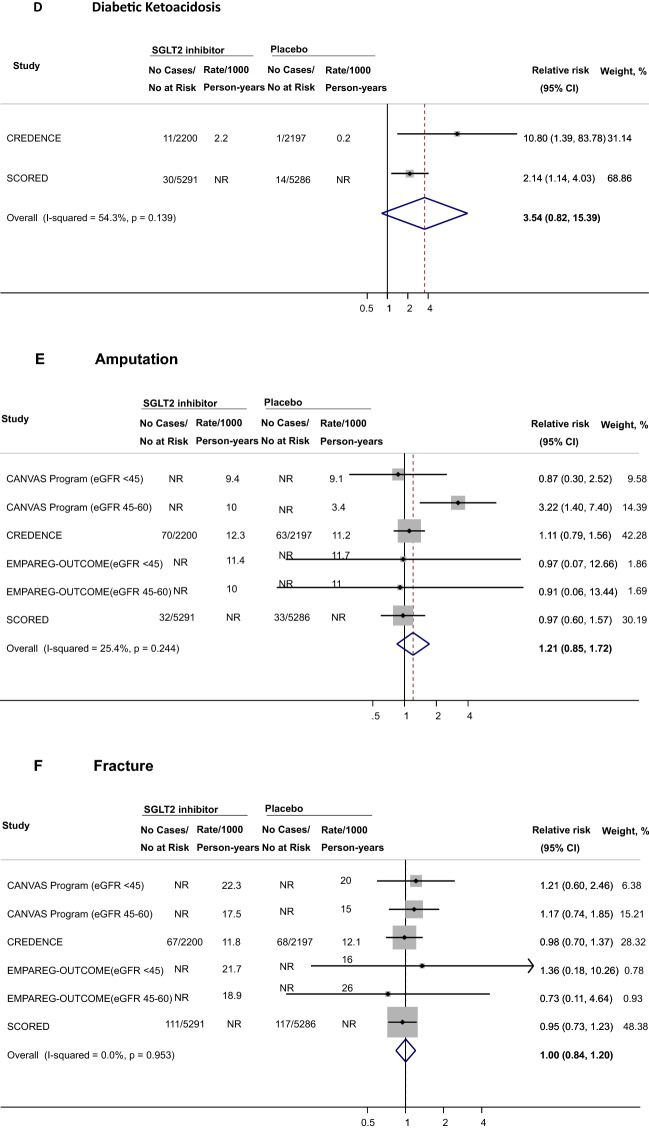
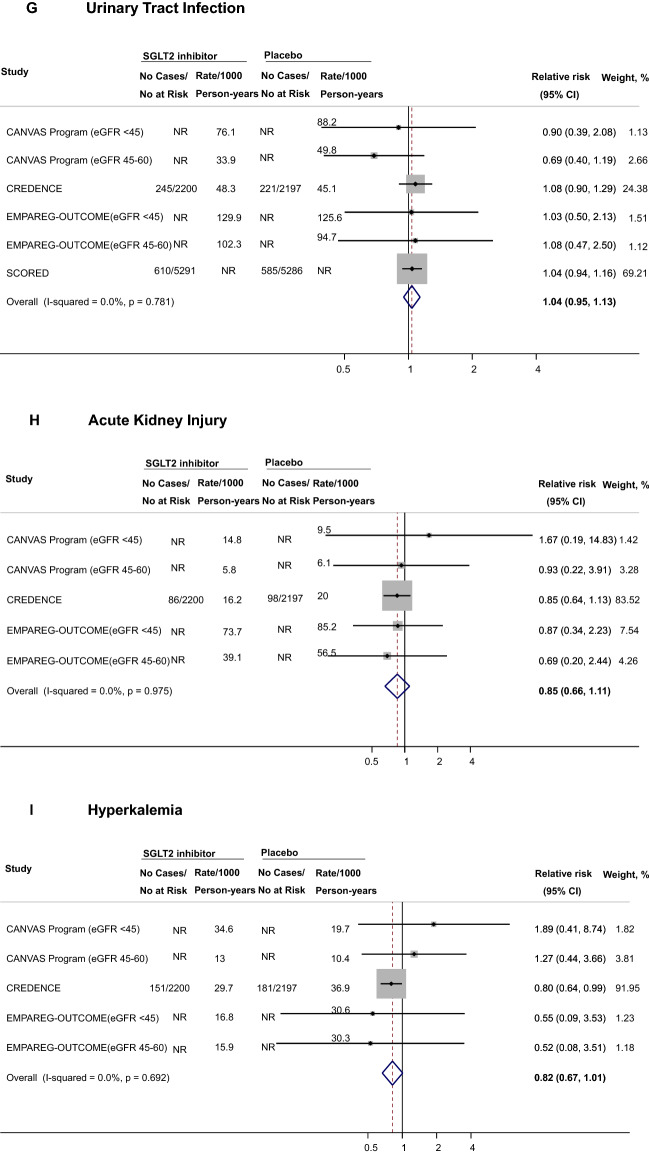
A total of 4 trials reported data on safety outcomes among participants with DKD. SGLT2i were associated with higher risks of male mycotic infections (RR 3.89, 95% CI 1.42–10.62, n = 4,091 participants across 2 studies, Fig. 2A), female mycotic infections (RR 2.50, 95% CI 1.32–4.72, n = 2,100 participants across 2 studies, Fig. 2B), and volume depletion (RR 1.29, 95% CI 1.13–1.48, n = 18,832 participants across 4 studies, Fig. 2C). With respect to DKA, the effect estimate was increased but its CI was wide (RR 3.54, 95% CI 0.82–15.39, n = 14,974 participants across 2 studies, Fig. 2D). No association was observed for amputations (RR 1.21, 95% CI 0.85–1.72, n = 18,832 participants across 4 studies, Fig. 2E), bone fractures (RR 1.00, 95% CI 0.84–1.20, n = 18,832 participants across 4 studies, Fig. 2F), and UTI (RR 1.04, 95% CI 0.95–1.14, n = 18,832 participants across 4 studies, Fig. 2G). The RRs were 0.85 for AKI (95% CI 0.66–1.11, n = 8255 participants across 3 studies, Fig. 2H) and 0.82 for hyperkalemia (95% CI 0.67–1.01, n = 8255 participants across 3 studies, Fig. 2I). Overall, confidence intervals were wide for safety outcomes, limiting the precision of point estimates (Table 4).
Fig. 2.
Effects of SGLT2 inhibitors on safety outcomes among individuals with diabetic kidney disease. CANVAS indicates Canagliflozin Cardiovascular Assessment Study, CI confidence interval, CREDENCE Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation, eGFR estimated glomerular filtration rate, EMPA‐REG OUTCOME Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients, NR not reported; SCORED Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk; SGLT2, sodium-glucose cotransporter 2
Table 4.
Effect of SGLT2 Inhibitors on safety events among patients with diabetic kidney disease
| Outcome | No studies | No events | Sample size | RR (95% CI) | I2, % | PHeterogeneity | PEgger test |
|---|---|---|---|---|---|---|---|
| Male genital mycotic infections | 2 | 98 | 4091 | 3.89 (1.42–10.62) | 62.1 | 0.072 | 0.392 |
| Female genital mycotic infections | 2 | 53 | 2100 | 2.50 (1.32–4.72) | 0.0 | 0.384 | NA |
| Diabetic ketoacidosis | 2 | 56 | 14,974 | 3.54 (0.82–15.39) | 54.3 | 0.139 | NA |
| Volume depletion | 4 | 1016* | 18,832 | 1.29 (1.13–1.48) | 0.0 | 0.713 | 0.936 |
| Amputations | 4 | 248* | 18,832 | 1.21 (0.85–1.72) | 25.4 | 0.244 | 0.767 |
| Bone fractures | 4 | 475* | 18,832 | 1.00 (0.84–1.20) | 0.0 | 0.953 | 0.447 |
| Urinary tract infections | 4 | 1739* | 18,832 | 1.04 (0.95–1.14) | 0.0 | 0.781 | 0.339 |
| Acute kidney injury | 3 | 197* | 8255 | 0.85 (0.66–1.11) | 0.0 | 0.975 | 0.535 |
| Hyperkalemia | 3 | 359* | 8255 | 0.82 (0.67–1.01) | 0.0 | 0.692 | 0.601 |
Bold values indicate statistically significant estimates
*The number of events from the EMPA-REG OUTCOME trial were not reported and therefore not included in the table for the following outcomes: volume depletion, amputations, fractures, urinary tract infection, acute kidney injury, and hyperkalemia. CI indicates confidence interval, EMPA‐REG OUTCOME Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients; I2, I-squared, RR relative risk, SGLT2 sodium-glucose cotransporter 2
Discussion
We conducted a meta-analysis of several clinical outcomes of SGLT2i focused entirely on individuals with DKD. In a large sample of adults with T2D and DKD, we found that SGLT2i reduced the risks of MACE, kidney composite outcomes, HHF, cardiovascular death, nonfatal and fatal MI, nonfatal and fatal stroke, as well as all-cause mortality. Similar trends were observed when the sample was restricted to participants with reduced eGFR and those with moderate or severe albuminuria. The risks of mycotic infections, as well as volume depletion were greater among participants receiving SGLT2i compared to those on placebo.
Our findings add to previously published meta-analyses on SGLT2i by focusing exclusively on patients with DKD, and by including the most recent trials. Furthermore, our review is unique in its evaluation of clinical outcomes by eGFR and albuminuria levels and the assessment of the effects of SGLT2i on adverse safety events in participants with DKD. Additionally, we chose a random-effects meta-analysis approach as opposed to fixed-effects models used in prior meta-analyses [32, 33], due to the heterogeneity of patient populations included across the clinical trials of SGLT2i.
Our study confirms that the beneficial effects of SGLT2i on the three-point composite of MACE (nonfatal MI, nonfatal stroke, and cardiovascular death) extends to people with DKD. These findings corroborate results from a prior meta-analysis of four SGLT2i trials which found that SGLT2i reduced the MACE composite irrespective of baseline atherosclerotic CVD, HF, or kidney function status [33]. Likewise, a recent meta-analysis of five trials observed a beneficial effect of SGLT2i against MACE in patients with T2D [34]. In our meta-analysis, the protective association of SGLT2i with MACE persisted in subgroup analyses by baseline eGFR; a similar trend was noted among participants with severely increased albuminuria. Among participants with moderately increased albuminuria, the effect of SGLT2i on MACE remained protective although with less precise estimates, likely owing to the small number of studies in this subgroup analysis.
In our meta-analysis, SGLT2i provided the highest risk reduction for kidney composite outcomes and HHF compared to placebo. We found a 34% reduction in the risk of kidney composite outcomes. This finding was consistent across kidney function levels and in patients with moderate or severe albuminuria. A possible mechanism for this association relates to the role of SGLT2i in reducing the risk of sustained eGFR decline or ESKD [35]. Our finding is consistent with current guidelines recommending the use of SGLT2i among patients with T2D at moderate kidney failure risk [35].
Our observation of a beneficial effect of SGLT2i on HHF in patients with DKD is consistent with prior published meta-analyses of individuals with T2D [33, 34]. The benefits of SGLT2i on HHF risk applied widely across the drug class and were consistent across various eGFR and albuminuria levels. These findings support current guidelines recommending the use of SGLT2i in patients with or at high risk of HF, independent of glycemic considerations. HF occurs at higher rates among patients with CKD [36] and diabetes [37]. Albuminuria and reduced eGFR compound the risks of HHF and death [36]. The prevention of HHF may reduce the risk of ESKD and death in patients with CKD [38].
We found a protective effect of SGLT2i on the risk of stroke in patients with DKD. This finding confirms that from two recent meta-analyses [32, 33], although the two prior studies included only four studies in their stroke meta-analysis and implemented fixed-effect meta-analysis models, an analytical approach that assumes the presence of one true effect size underlying all the studies in the meta-analysis. We chose to conduct random-effects meta-analyses as this approach is more conservative and allows for between-study heterogeneity [27]. A recent meta-analysis of the effect of SGLT2i on the risk of stroke in patients with DKD observed a null association using the random-effects models, likely due to low statistical power [15]. The exact mechanisms for the beneficial effect of SGLT2i on stroke risk observed in our study is unclear, but may be mediated by prevention of atrial fibrillation (AF) and atrial flutter (AFL) [39]. Indeed, dapagliflozin was found to lower the risks of first AF/AFL as well as total AF/AFL events in a large sample of adults with T2D [40]. Two recent meta-analyses also found that SGLT2i were associated with a lower risk of AF [15, 41]. SGLT2i promote natriuresis, diuresis and thus may reduce the atrial diameter. Additionally, SGLT2i reduce cardiac remodeling, left ventricular mass, blood pressure, body weight, inflammation, oxidative stress and the sympathetic drive, all of which may promote AF/AFL, and thus increase the risk of stroke [42, 43].
With respect to safety outcomes, our review identified a knowledge gap as most trials were not powered to assess adverse effects of SGLT2i in the subgroup of patients with DKD, neither did they report safety information in this population. Additionally, data from the safety outcomes in this review were derived from a total of 2 to 4 studies. Consistent with prior reports, we found that the most commonly reported side effects of SGLT2i also extend to patients with DKD [6, 7, 11]. Indeed, in our meta-analysis, SGLT2i were associated with higher risks of genital mycotic infections, as well as volume depletion. Participants on SGLT2i were also at higher risk of DKA, although estimates were imprecise, likely owing to insufficient statistical power. It is unclear if participants who developed DKA in this review were on insulin. Although SGLT2i was linked to a higher risk of volume depletion, SGLT2i did not result in a higher risk of AKI in this meta-analysis. In fact, the risk of AKI appeared lower among those on SGLT2i. Prior evidence from observational studies has suggested that SGLT2i may be linked to a decreased risk of AKI [44–47]. While the exact mechanisms underpinning this association are unclear, possible hypotheses include the attenuation of ischemic-reperfusion injury to the kidney [48], as well as a reduction in tubular injury [49].
The public health and research implications of our findings are manifold. Our findings confirm the relevance of SGLT2i in the therapeutic scheme of patients with DKD. As SGLT2i prescription rates for patients with DKD are increasing [12], our findings highlight the importance of risk mitigation strategies in this population. These strategies include the proper hygiene of genitalia to prevent fungal infections, the proactive dose reduction of diuretics in euvolemic patients to prevent volume depletion, patient education on early recognition of DKA and implementation of STOP DKA protocol (stop SGLT2i, test for ketones, maintain intake of fluid and carbohydrates, and use maintenance and supplemental insulin) as well as foot examination to reduce the risk of amputations [35]. Further research is needed to determine the effect of SGLT2i among patients with advanced DKD. As most clinical trials focus on efficacy outcomes and are typically not powered to assess rare adverse safety effects of medications [50], additional studies using real-world data are needed to better evaluate the safety profile of SGLT2i in patients with DKD.
The limitations of this study should be acknowledged. First, most of the trials included in this meta-analysis excluded participants with eGFR less than 30 mL/min/1.73 m2; therefore, our findings may not be generalizable to individuals with advanced DKD. Second, the included SGLT2i trials did not always report the effect estimates for all the relevant outcomes specifically among participants with DKD (for example, the trials did not always report on individual kidney outcomes such as ESKD that would have been defined more consistently across trials); and we did not have access to individual participant data that could have been used to calculate the effect estimates for the unreported outcomes or perform meta-regression analyses. Likewise, the individual trials did not report data specifically in the subset of participants with albuminuria and eGFR ≥ 60 mL/min/1.73 m2; thus, we could not evaluate the effects of SGLT2i on outcomes in this subgroup. Third, the limited number of available studies limited our ability to perform meaningful subgroup analyses such as analyses by age, gender, or race/ethnicity, for example. Fourth, we defined DKD as the presence of CKD in individuals with T2D and the individual studies did not exclude causes of CKD other than T2D among the participants. Finally, the definition of the kidney composite outcomes was not consistent across the trials. These limitations notwithstanding, our review has several strengths. First, this is a comprehensive review of all information available to date on the efficacy and safety outcomes of SGLT2i in patients with DKD. Second, we used random-effects meta-analysis models, a more conservative approach which makes less assumptions about the effect of SGLT2i across included trials. Third, only placebo-controlled RCTs with low risk of bias were included. Fourth, we appraised the quality of studies using a standard quality assessment tool. Finally, our review is quantitative and highlights the magnitude of the associations between SGLT2i and various clinical outcomes.
Conclusions
In conclusion, our findings confirm the beneficial effects of the SGLT2i class on cardiovascular and kidney outcomes, as well as mortality in patients with T2D and DKD. Our data support the current recommendations to prioritize the prescriptions of SGLT2i in patients with DKD, independent of glycemic control [35]. Further research is warranted to explore the effects of SGLT2i in patients with advanced DKD and to better characterize the safety profile of SGLT2i in patients with DKD.
Supplementary Information
Additional file 1: Fig S1. Selection of studies for inclusion in the meta-analysis. Fig S2. Effects of SGLT2 Inhibitors on fatal and nonfatal stroke among individuals with Diabetic Kidney disease. Table S1. Definition of kidney composite outcomes across included trials. Table S2. Characteristics of Clinical Trials Included in the Meta-analysis. Table S3. Risk of Bias Assessment.
Acknowledgements
Not applicable.
Abbreviations
- AF
Atrial fibrillation
- AFL
Atrial flutter
- AKI
Acute kidney injury
- CANVAS
Canagliflozin Cardiovascular Assessment Study
- CI
Confidence interval
- CKD
Chronic kidney disease
- CREDENCE
Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation
- CVD
Cardiovascular disease
- DAPA-CKD
Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease
- DECLARE‐TIMI 58
Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58
- DKA
Diabetic ketoacidosis
- DKD
Diabetic kidney disease
- eGFR
Estimated glomerular filtration rate
- EMPA‐REG OUTCOME
Empagliflozin Cardiovascular Outcome Event Trial in Type 2 diabetes Mellitus Patients
- ESKD
End-stage kidney disease
- HHF
Hospitalizations for heart failure
- HR
Hazard ratio
- MACE
Major adverse cardiovascular event
- MI
Myocardial infarction
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- RR
Relative risk
- SCORED
Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk
- SGLT2i
Sodium-glucose cotransporter 2 inhibitor
- SOLOIST-WHF
Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure
- T2D
Type 2 diabetes
- UACR
Urine albumin-to-creatinine ratio
- UTI
Urinary tract infection
- VERTIS CV
Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial
Authors' contributions
ADK and JMP conceived and designed the study. ADK and MZ collected data in electronic databases. ADK performed the statistical analyses. ADK, MZ, SCK, EP, and JMP contributed to the interpretation of data. ADK and JMP drafted the manuscript. ADK, MZ, SCK, EP, and JMP revised the manuscript critically for essential intellectual content. All authors read and approved the final manuscript.
Funding
This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. EP was supported by a career development grant (K08AG055670) from the National Institute on Aging. MZ was supported by an institutional research grant (DK007199) from the National Institute of Health. None of these funding sources had any role in the design or conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review or approval of the manuscript, or decision to submit the manuscript for publication.
Availability of data and materials
The data that support the findings of this study are publicly available and included in this article and in its additional file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No potential conflicts of interest relevant to this article were reported. EP is principal investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of the submitted work. SK has received research grants to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche and Bristol-Myers Squibb for studies not related to the topic of the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, Department of Health and Human Services. National Diabetes statistics report, 2020 estimates of diabetes and its burden in the United States [Internet]. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 7 Oct 2021.
- 3.Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the US. Diabetes Care. 2001;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- 4.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med . 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med . 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med . 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 9.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med . 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med . 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, De Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 12.Harris ST, Patorno E, Zhuo M, Kim SC, Paik JM. Prescribing trends of antidiabetes medications in patients with type 2 diabetes and diabetic kidney disease, a cohort study. Diabetes Care. 2021 doi: 10.2337/dc21-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 14.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med . 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Jardine MJ, Li Q, Neuen BL, Cannon CP, de Zeeuw D, et al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: results from the CREDENCE trial and meta-analysis. Stroke. 2021;52:1545–1556. doi: 10.1161/STROKEAHA.120.031623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med . 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 17.Kaze AD, Zhuo M, Paik JM. Association of SGLT2 inhibitors with clinical outcomes in patients with type 2 diabetes and diabetic kidney disease: a systematic review and meta-analysis. PROSPERO 2021; CRD42021282869.https://www.crd.york.ac.uk/prospero/#recordDetails. Accessed 14 Nov 2021.
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–129. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 20.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation. 2018;138:1537–1550. doi: 10.1161/CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol. 2019;30:2229–2242. doi: 10.1681/ASN.2019010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140:739–750. doi: 10.1161/CIRCULATIONAHA.119.042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31:1128–1139. doi: 10.1681/ASN.2019111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021;42:1216–1227. doi: 10.1093/eurheartj/ehab094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142:2205–2215. doi: 10.1161/CIRCULATIONAHA.120.050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berlin JA, Laird NM, Sacks HS, Chalmers TC. A comparison of statistical methods for combining event rates from clinical trials. Stat Med. 1989;8:141–151. doi: 10.1002/sim.4780080202. [DOI] [PubMed] [Google Scholar]
- 28.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 29.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med . 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med . 2021;384:117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L-M, Huang J-N, Qiu M, Ding L-L, Zhan Z-L, Ning J. Gliflozins for the prevention of stroke in diabetes and cardiorenal diseases: a meta-analysis of cardiovascular outcome trials. Medicine (Baltimore) 2021;100:e27362. doi: 10.1097/MD.0000000000027362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e014908. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Albajrami O, Zhuo M, Hawley CE, Paik JM. Decision algorithm for prescribing SGLT2 inhibitors and GLP-1 receptor agonists for diabetic kidney disease. Clin J Am Soc Nephrol. 2020;15:1678–1688. doi: 10.2215/CJN.02690320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, et al. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol. 2019;73:2691–2700. doi: 10.1016/j.jacc.2019.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia. 2019;62:1550–1560. doi: 10.1007/s00125-019-4926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sud M, Tangri N, Pintilie M, Levey AS, Naimark DMJ. ESRD and death after heart failure in CKD. J Am Soc Nephrol. 2015;26:715–722. doi: 10.1681/ASN.2014030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yurista SR, Silljé HHW, Rienstra M, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition as a mitochondrial therapy for atrial fibrillation in patients with diabetes? Cardiovasc Diabetol. 2020 doi: 10.1186/s12933-019-0984-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
- 41.Li H-L, Lip GYH, Feng Q, Fei Y, Tse Y-K, Wu M-Z, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. 2021;20:100. doi: 10.1186/s12933-021-01293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: The EMPA-HEART cardiolink-6 randomized clinical trial. Circulation. 2019;140:1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375. [DOI] [PubMed] [Google Scholar]
- 43.Shao S-C, Chang K-C, Lin S-J, Chien R-N, Hung M-J, Chan Y-Y, et al. Favorable pleiotropic effects of sodium glucose cotransporter 2 inhibitors: head-to-head comparisons with dipeptidyl peptidase-4 inhibitors in type 2 diabetes patients. Cardiovasc Diabetol. 2020;19:17. doi: 10.1186/s12933-020-0990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuo M, Paik JM, Wexler DJ, Bonventre JV, Kim SC, Patorno E. SGLT2 inhibitors and the risk of acute kidney injury in older adults with type 2 diabetes. Am J kidney Dis Off J Natl Kidney Found. 2021 doi: 10.1053/j.ajkd.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadkarni GN, Ferrandino R, Chang A, Surapaneni A, Chauhan K, Poojary P, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care. 2017;40:1479–1485. doi: 10.2337/dc17-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao M, Sun S, Huang Z, Wang T, Tang H. Network meta-analysis of novel glucose-lowering drugs on risk of acute kidney injury. Clin J Am Soc Nephrol. 2020;16:70–78. doi: 10.2215/CJN.11220720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rampersad C, Kraut E, Whitlock RH, Komenda P, Woo V, Rigatto C, et al. Acute kidney injury events in patients with type 2 diabetes using SGLT2 inhibitors versus other glucose-lowering drugs: a retrospective cohort study. Am J kidney Dis Off J Natl Kidney Found. 2020;76:471–479.e1. doi: 10.1053/j.ajkd.2020.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Chang Y-K, Choi H, Jeong JY, Na K-R, Lee KW, Lim BJ, et al. Correction: Dapagliflozin, SGLT2 Inhibitor, Attenuates Renal Ischemia-Reperfusion Injury. PLoS ONE. 2016 doi: 10.1371/journal.pone.0160478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dekkers CCJ, Petrykiv S, Laverman GD, Cherney DZ, Gansevoort RT, Heerspink HJL. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20:1988–1993. doi: 10.1111/dom.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferner RE. Newly licensed drugs. BMJ. 1996 doi: 10.1136/bmj.313.7066.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig S1. Selection of studies for inclusion in the meta-analysis. Fig S2. Effects of SGLT2 Inhibitors on fatal and nonfatal stroke among individuals with Diabetic Kidney disease. Table S1. Definition of kidney composite outcomes across included trials. Table S2. Characteristics of Clinical Trials Included in the Meta-analysis. Table S3. Risk of Bias Assessment.
Data Availability Statement
The data that support the findings of this study are publicly available and included in this article and in its additional file.