Abstract
Background and Objectives
To describe the prevalence of high adverse childhood experiences (ACEs) among neurology outpatients and determine their association with health care utilization rates and comorbid medical and psychiatric disease.
Methods
This was a cross-sectional study of adults seen for outpatient neurology follow-up at the University of Pennsylvania. Participants completed the ACE questionnaire and depression/anxiety screenings. Health care utilization metrics (emergency department [ED] visits, hospitalizations, and outpatient calls) were obtained for all participants. High ACE scores were defined as a score of ≥4. The prevalence of high ACE scores in our cohort was compared with US historical controls. Statistical associations were adjusted for age, sex, and race/ethnicity.
Results
One hundred ninety-eight patients were enrolled in the study. Neurology patients were more likely to have elevated ACE scores compared with US population estimates (23.7% vs 12.6%, p < 0.01). High ACE scores were associated with increased ED utilization (odds ratio [OR] = 21, 95% CI [5.8–76.0], p < 0.01), hospitalizations (OR = 5.2, 95% CI [1.7–15.0], p < 0.01), and telephone encounters (OR 3, 95% CI [1.1–8.2], p < 0.05). High ACEs were also associated with medical and psychiatric comorbidities (OR 5.8, 95% CI [2.0–17.0], p < 0.01 and OR 4.5, 95% CI [2.1–9.6], p < 0.01) and high depression and anxiety scores (OR = 6.9, 95% CI [2.8–17.0], p < 0.01, and OR = 4.3, [95% CI 1.7–11.0], p < 0.01).
Discussion
Patients with neurologic conditions are more likely to have high ACEs than the US population, which was associated with higher rates of health care utilization, increased number of medical and psychiatric comorbidities, and higher anxiety and depression scores. Addressing ACEs may be a way to improve the health outcomes of patients with neurologic conditions.

Adverse childhood experiences (ACEs) are traumatic events experienced before age 18 years and are related to higher risk of disease and worse health outcomes. Prior studies have shown that the greater number of ACEs leads to a higher risk of cardiovascular disease, diabetes, obesity, polysubstance use, mental health illness, and, notably, a decrease in life expectancy.1,2 Furthermore, ACEs are associated with negative health behaviors in adulthood (e.g., smoking and polysubstance use), and biomarkers of disease severity and progression, including elevated inflammatory markers, and epigenetic changes leading to differences in endocrine, immune, and neurotransmitter genes.3-5 Childhood adversity has also been associated with limited medical adherence, greater health care utilization, and prolonged hospitalizations, all of which are major drivers of health care costs.6-8
Despite the growing body of evidence on the important role childhood adversity plays in increasing the risk, severity, and adverse outcomes of numerous medical conditions, little is known about its effect on neurologic disease. To date, studies have reported an association between ACEs and a greater incidence of headaches, worse symptom severity in patients with functional neurologic disorder, and earlier onset of multiple sclerosis.9-11 Prior studies of the relationship between high ACEs and stroke have either not observed an association or seen that it was confounded by other variables such as adult socioeconomic status and smoking.1,12 Unfortunately, even less is known about the relationship between common neurologic conditions such as epilepsy and ACEs. Furthermore, the implications on health outcomes, such as rates of health care utilization for neurologic is not known.
To address this knowledge gap, our study examined the prevalence of ACEs in an outpatient neurology practice in Philadelphia and health care utilization patterns in this patient cohort. We also describe the prevalence of comorbid medical and psychiatric conditions in neurology patients with high ACEs to generate hypotheses on the potential pathways in which ACEs contribute to neurologic disease and adverse outcomes.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of the Perelman School of Medicine at the University of Pennsylvania. Written informed consent was obtained from all participants in the study.
Subject Recruitment
In this cross-sectional study, we surveyed follow-up patients aged 18 years and older who were evaluated at the outpatient neurology practice at the Perelman Center for Advanced Medicine at the Hospital of the University of Pennsylvania between July 2019 and March 2020. We excluded patients without an established neurologic diagnosis, cognitive impairment (Montreal Cognitive Assessment <26), and any patient unable to provide consent or fill out the survey in English.
Data Collection
Participants were asked to complete a 6-page survey (eAppendix 1, links.lww.com/CPJ/A319). The survey included the 10-item ACE questionnaire used in the original 1998 study by the Centers for Disease Control and Kaiser Permanente.1 The questionnaire was created using the literature on trauma, abuse, and conflict scales and then tested and validated.13 Since then, the questionnaire has been used by different public health agencies at state and national levels throughout the last 20 years with similar results rates of ACEs found when compared with the original 1998 study.14-16 Currently, 48 states collect and publicly report ACE data.17 The World Health Organization has also adopted versions of the initial questionnaire worldwide, with other countries corroborating validity of the questionnaire as a measure of childhood trauma and have shown associations with chronic health conditions in adulthood.18,19
Each item in the ACE questionnaire asks whether an individual experienced some form of abuse, neglect, or childhood dysfunction before age 18 years. A yes response in the ACE questionnaire is given 1 point, for a total possible score of 10. This is what is known as the ACE score. A score of 10 indicates the highest possible number of ACEs. Here is an example of one of the items:
Before your 18th birthday, did a parent or other adult in the household often or very often…swear at you, insult you, put you down, or humiliate you? or act in a way that made you afraid that you might be physically hurt?
The survey also included brief depression and anxiety screens (Patient Health Questionnaire-2 [PHQ2] and Generalized Anxiety Disorder-2 [GAD2]). A score of 3 or higher in the PHQ2 or GAD2 was considered elevated and potentially indicative of major depression or generalized anxiety.20,21 Participants who scored high on these 2 screening assessments were asked whether they were interested in talking to a social worker in addition to and independent of their study participation. On completion of the survey, all participants were provided with educational resources in regards ACEs, as well as local behavioral health resources.
We also collected demographics and clinical and health care utilization measures from the medical record. We defined telephone encounters of 15 or higher, 3 or more overnight inpatient hospitalizations, and 4 or more emergency department (ED) visits over the last year as markers of high health care utilization.24-27
Data Analysis
Study data were collected and managed using Research Electronic Data Capture electronic data capture tools hosted at the University of Pennsylvania.22,23
Chart Review
The chart review process included verification of each participants' neurologic diagnosis as charted by their neurologist during their most recent office visit. Other data collected included number of telephone encounters or messages in the past 12 months, participants' home zip code, and insurance payor. In hopes to minimize errors during data entry, the chart review data were linked to each participant's questionnaire using REDCap software. Telephone encounters were filtered in the electronic medical record to only populate neurology-specific telephone encounters.
Data Analysis
We describe the proportion of our sample with high ACEs using a cutoff score of 4 as per prior studies. Our primary outcome was identifying the proportion of elevated ACEs across individual neurologic conditions. We then compared demographic, clinical characteristics, rates of psychiatric comorbidities, and health care utilization between individuals with high and low ACE scores. Per previous studies and current reporting conventions, 3 or more overnight inpatient hospitalizations and 4 or more ED visits over the last year were considered markers of high health care utilization.24-27 Telephone encounters of 15 or higher in the previous year were also considered a measure of high utilization. Logistic regression models were built in a stepwise fashion to determine the independent relationship between covariates and high ACE scores. Notably, participants were able to identify as more than 1 race or ethnicity. Statistical testing was 2 sided, and the significance level was set at α = 0.05. All statistical analyses were performed using R statistical software, version 3.6.1.
In secondary analysis, we compared the prevalence of high ACEs in our participant cohort to publicly available, general population estimates (i.e., the Kaiser-insured original ACE study and additional studies with similar estimates).28 We performed a 2-sided proportion test to assess for differences in the proportion of elevated ACEs stratified by sex in both the total cohort, and between specific neurologic conditions, compared with the general population.
Data Availability
Anonymized for this study and is available on request to the authors.
Results
Patient Characteristics
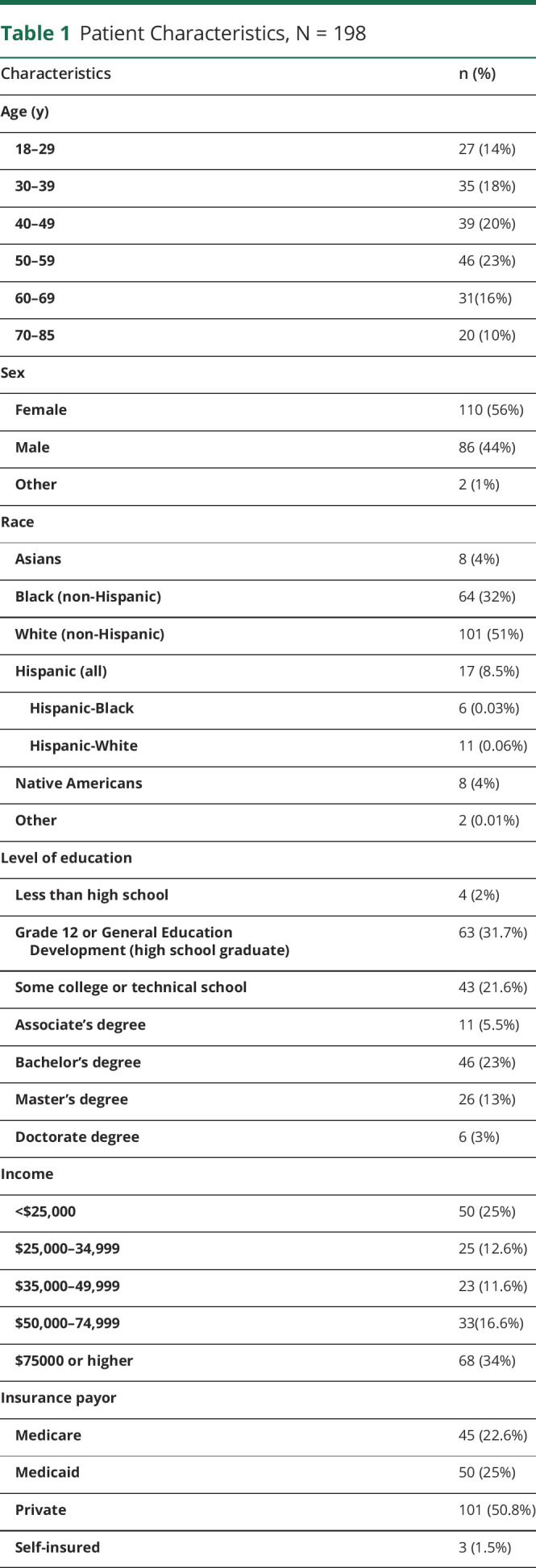
Of the 248 surveys distributed, 198 were eligible for analysis. Patient demographics including age, sex, race, and socioeconomic characteristics are available in Table 1. Most participants were aged between 50 and 59 years (23%), with women representing a little more than half of the cohort (56%). There was no relationship between the age of patients and the ACE score (eTable 1, links.lww.com/CPJ/A321). Approximately half of the participants were White/non-Hispanic (51%); however, some patients were multiracial and thus accounted for in more than 1 race category. Native Americans were more likely to have an elevated ACE score compared with other races (odds ratio [OR] 5.9, 95% CI [1.3, 26.0], p = 0.018). Black and Hispanic populations also had higher ACE scores; however, this was not statistically significant (OR 1.9, 95% CI [0.97, 3.7], p = 0.06, and OR 1.4, 95% CI [0.46, 4.1], p = 0.57, respectively). Medicaid beneficiaries were more likely to have an elevated ACE score (OR 3.4, 95% CI [1.4, 8.2], p = 0.001) compared with the reference population of patients with private insurance. There was no difference between income or level of education and elevated ACE scores when treated as categorical values. However, when education was treated as a continuous discrete variable, having a high ACE score was associated with lower levels of education after adjusting for age, sex, and race/ethnicity (p = 0.02, slope estimate −0.6, [95% CI −1.11 to −0.11]). We did not see this association when income was treated as a continuous discrete variable. eFigure 1 (links.lww.com/CPJ/A320) depicts the distribution of elevated ACE scores across different zip codes in the Philadelphia area, where most study participants reside.
Table 1.
Patient Characteristics, N = 198
Health Care Utilization
Health care utilization in patients with neurologic conditions and elevated ACE scores was measured by the number of hospitalizations, ED visits, and telephone encounters while adjusting for age, sex, and race/ethnicity (Table 2). Having a neurologic condition and elevated ACE score was associated with an increased likelihood of having 3 or more hospitalizations or any hospitalization compared with those without elevated ACEs (OR 5.2, 95% CI [1.7, 15.0], p = 0.003, and OR 2.8, 95% CI [1.3, 5.8], p = 0.007, respectively). In addition, these individuals were more likely to have 4 or more ED visits and any ED visit (OR 21, 95% CI [5.8, 76.0], p ≤ 0.001, and OR 6.2, 95% CI [2.5, 15.0], p ≤ 0.001, respectively). Finally, they were also more likely to have 15 or more telephone encounters in a 1-year period (OR 3, 95% CI [1.1, 8.2] p = 0.03).
Table 2.
Health Care Utilization in Patients With Neurologic Conditions and Elevated ACE Scores

Medical Comorbidities
In patients with a neurologic condition, there was a strong association between an elevated ACE score and having multiple medical and psychiatric comorbidities (Table 3). For those with 2 medical comorbidities, each additional comorbidity was associated with a higher adjusted odds ratio and a greater statistical significance (Table 3). Elevated ACE scores were associated with having any medical comorbidity (OR 5.8, 95% CI [2.0, 17.0] p = 0.001). However, having a neurologic disease and elevated ACE score was not associated with having an additional comorbid neurologic disease (OR 1.2, 95% CI [0.45, 3.1] p = 0.74).
Table 3.
Risk of Multiple Medical Comorbidities in Patients With Neurologic Conditions and Elevated ACEs

Psychiatric Comorbidities
Patients with neurologic conditions and elevated ACE score had an increased likelihood of comorbid psychiatric conditions (OR 4.5, 95% CI [2.1–9.6], p < 0.001) (Table 3). Furthermore, patients with elevated ACE scores were more likely to score 3 or more on the GAD2 questionnaire and PHQ2 questionnaire (OR 4.3, 95% CI [1.7, 11.0], p = 0.002, and OR 6.9, 95% CI [2.8, 17.0], p < 0.001, respectively) (Table 4).
Table 4.
Anxiety and Depression Scales and ACE Scores

Secondary Analysis
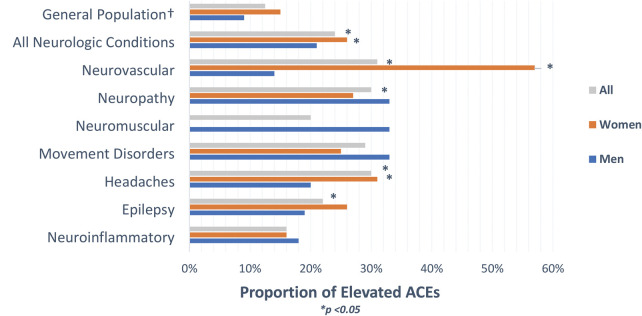
Although we did not find any statistically significant differences between elevated ACE scores and individual neurologic conditions, we performed proportional analysis testing to assess whether there was a difference between the prevalence of high ACEs in patients with neurologic conditions compared with patients with high ACEs in the general population (Figure). Taken together, 24% of all participants had elevated ACEs, which is higher than the general population (p < 0.001, 95% CI [18–31%]). In our cohort, 21% of men and 26% of women reported an elevated ACE score. Compared with the general population, both men and women with neurologic conditions were more likely to have elevated ACE scores (p < 0.001, 95% CI [13–31%] and p = 0.002, 95% CI [19–36%]). Within specific neurologic conditions, 57% of women (p < 0.001, 95% CI [7–61%] and 31% of all participants (p < 0.001, 95% CI [17–48%]) seen for neurovascular follow-up had elevated ACE scores. Women and all patients seen for headache follow-up also had higher ACEs (31%, p = 0.02, 95% CI [17–49%] and 30%, p = 0.01, 95% CI [17–47%]. A greater proportion of high ACE scores was found in patients seen for epilepsy and neuropathy follow-up (22%, p = 0.03, 95% CI [12–36%]; 30%, p = 0.04, 95% CI [13–54%].
Figure . Proportion of Elevated ACEs Compared With the General Population.
Discussion
ACEs have been linked to a variety of medical conditions but have not been studied in detail in patients with neurologic disease. In this cross-sectional study, we found that patients with neurologic diseases are more likely to have elevated ACEs than the general population. Patients with neurologic conditions and high ACEs also had high rates of health care utilization, multiple medical comorbidities, psychiatric comorbidities, and high scores in anxiety and depression screens. Although associations between high ACEs and health care utilization, medical comorbidities, and psychiatric disease have been documented in the literature, this is one of few studies evaluating these relationships among patients with neurologic conditions.
The high prevalence of elevated ACEs in patients with neurologic conditions in comparison to the general population is an important finding, although a causal relationship is difficult to elucidate. For example, it is possible that certain behaviors linked to elevated ACEs such as smoking, obesity, and substance use may in part explain why there was a high proportion of elevated ACEs among patients seen in neurovascular and neuropathy follow-up. However, elevated ACEs have also been associated with proinflammatory markers and epigenetic changes, which may be directly linked to neurologic diseases, independent of adverse health behaviors.29 In a study on ACEs and migraines in women, elevated ACEs were associated with a higher incidence of vascular and inflammatory biomarkers, all which have been associated with an increased stroke risk.9 We also know that a history of anxiety and depression is associated with an increased risk of developing epilepsy,30-32 and it is possible that this relationship may be mediated by a common neurobiological pathway.31 In keeping with the existent literature, our study showed that high ACEs are associated with a history of psychiatric disease and high scores in anxiety and depression screening. It is possible that this may be a causal pathway between high ACEs and epilepsy, with the development of psychiatric disease as an intermediate step. Further studies are necessary to better understand the extent to which neurobiological changes or individuals' behaviors drive the relationship between high ACEs and neurologic disease.
A second finding in our study is the association between high ACEs and high health care utilization. Although the number of ED visits and hospitalizations in the previous year was not neurology specific, the number of telephone encounters was filtered to only capture calls to our neurology clinic. This may suggest greater symptom severity, disability, or greater medical needs in neurology patients with high ACEs. This level of health care use also highlights the importance of early recognition of ACEs, appropriate referral to social work and behavioral health resources, and the overall importance of multidisciplinary neurology clinics. An initial role of social work referral is to provide an opportunity to mitigate ongoing detrimental effects of childhood and intergenerational trauma. This type of referral may also aid in identifying ways of mitigating not only the individual effects of childhood trauma but also identifying how childhood and adult socioeconomic status may also play a role in a patient's neurologic outcomes. Other examples include identifying barriers to health care access (both geographic, social, or cost-related barriers), referral to community resources specific to patients with high ACEs, and mental health referral for resilience training, cognitive behavioral therapies among others. Many of these interventions among patients with high ACEs have been proven to reduce the rates of mental health illness and substance use disorders, as well as improving overall health outcomes.33 It has been proposed that patients who have sustained significant childhood distress are also less likely to maintain long-term relationships with their primary providers, thus resulting in increased ED visits.34 In an outpatient neurology clinic, all of these interventions have the promise to identify ACEs as a potential contributor to disease severity, comorbid psychiatric disease directly affecting neurologic outcomes, and high volume of telephone encounters/calls to neurology practices. Early identification and referral to appropriate services has the potential to improve rapport between patients and neurologists, improve treatment adherence, and hopefully reduce ED visits and hospitalizations. Even in clinics, which may not have readily accessible social work referrals, many public health initiatives such as ACEs Aware in the State of California have provided online resources for training in assessment and referrals for treatment of high ACEs.35 A multidisciplinary approach addressing ACEs (with aid of social work, mental health, and or pastoral care) may improve neurologic and overall health care outcomes in patients seen in outpatient neurology clinics and overall may reduce costs to our health care system. Some of the subgroup analyses were limited due to small sample sizes, and as such, certain results should be interpreted with caution. Unfortunately, due to the COVID-19 pandemic, recruitment for the study was stopped earlier than anticipated secondary to clinic closures. Because the survey was only administered in English, we did not have an appropriate representation of how ACEs may affect patients from a non–English-speaking country of origin. There is also a limited representation of numerous neurologic subspecialties such as Movement Disorders and Traumatic Brain Injury, which was largely due to these clinics being located at a different location. As with any questionnaire, the study is also subject to recall bias given that patients need to self-report their ACEs and number of ED and hospital visits. Particularly as it pertains to childhood trauma, it is unclear to what extent these experiences may affect the social, emotional, and cognitive development of these individuals and to what extent this may affect subject recall, responses, and interpretations of ACE questions. Patient selection bias may also be a factor because the study was conducted at a single center, with a large proportion of patients seen in resident clinic, which serves a large portion of Medicare and Medicaid patients. Finally, the possibility of human error arises during the chart review process. In keeping with the commonly cited ACE literature, we looked at the aggregate of yes responses and categorize our independent variable as a high ACE score. Nonetheless, this approach may not capture the contribution of an individual childhood experience to different outcomes of interests. Although the current ACE questionnaire captures different forms of childhood trauma, other traumatic experiences such as famine, war violence, and others are worth exploring, particularly when studying childhood trauma and health care outcomes in countries outside of the United States. Although socioeconomic status in adulthood was not associated with high ACEs, childhood socioeconomic status was an area we did not explore, yet important to include in future ACE studies. Despite these limitations, our study was able to systematically address ACEs across different neurologic conditions and can serve as an initial model for subsequent, larger, longitudinal studies in different settings.
TAKE-HOME POINTS
→ ACEs are seen at higher rates in patients with neurologic conditions and are directly associated with a higher number of medical and psychiatric comorbidities and high scores in generalized anxiety disorder and major depressive disorder screens.
→ High ACEs in patients with neurologic disease are associated with increased health care utilization rates.
→ Early recognition of high ACEs and appropriate referral to social work and behavioral health resources may provide patient support and connection to social and mental health resources, all of which could reduce health care costs and ultimately improve neurologic outcomes.
→ The impact of ACEs is relatively understudied within neurology and merits further studies of potential biological pathways in neurologic disease and long-term neurologic outcomes such as increased morbidity and mortality.
Acknowledgment
The authors acknowledge Gloria Young, BSN, at the Hospital of the University of Pennsylvania, who helped with data collection for the study and Kathleen Reeves, Senior Associated Dean of Health Equity, Diversity, and Inclusion, at Lewis Katz School of Medicine at Temple University, who gave them the idea to study ACEs in adults with neurologic disease.
Appendix. Authors

Contributor Information
Cody L. Nathan, Email: cody.nathan@nm.org.
Pouya Khankhanian, Email: philly.pouya@gmail.com.
Marissa Anto, Email: antom@chop.edu.
Cynthia Clyburn, Email: cynthia.clyburn@pennmedicine.upenn.edu.
Alexandra Acaba-Berrocal, Email: acabaalex@gmail.com.
Louise Breen, Email: louise.n.breen@gmail.com.
Nabila Dahodwala, Email: nabila.dahodwala@uphs.upenn.edu.
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to this manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14(4):245-258. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert LK, Breiding MJ, Merrick MT, et al. Childhood adversity and adult chronic disease. Am J Prev Med. 2015;48(3):345-349. [DOI] [PubMed] [Google Scholar]
- 3.Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, Miller GE. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: findings from the MIDUS study. Dev Psychol. 2015;51(11):1630-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brockie TN, Heinzelmann M, Gill J. A framework to examine the role of epigenetics in health disparities among native Americans. Nurs Res Pract. 2013;2013:410395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su S, Jimenez MP, Roberts CTF, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17(10):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korhonen MJ, Halonen JI, Brookhart MA, et al. Childhood adversity as a predictor of non-adherence to statin therapy in adulthood. PLoS One. 2015;10(5):e0127638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellis M, Hughes K, Hardcastle K, et al. The impact of adverse childhood experiences on health service use across the life course using a retrospective cohort study. J Health Serv Res Pol. 2017;22(3):168-177. [Google Scholar]
- 8.Chartier MJ, Walker JR, Naimark B. Separate and cumulative effects of adverse childhood experiences in predicting adult health and health care utilization. Child Abuse Negl. 2010;34(6):454-464. [DOI] [PubMed] [Google Scholar]
- 9.Tietjen GE, Khubchandani J, Herial NA, Shah K. Adverse childhood experiences are associated with migraine and vascular biomarkers. Headache J Head Face Pain. 2012;52(6):920-929. [DOI] [PubMed] [Google Scholar]
- 10.Anda R, Tietjen G, Schulman E, Felitti V, Croft J. Adverse childhood experiences and frequent headaches in adults. Headache J Head Face Pain. 2010;50(9):1473-1481. [DOI] [PubMed] [Google Scholar]
- 11.Shaw MT, Pawlak NO, Frontario A, Sherman K, Krupp LB, Charvet LE. Adverse childhood experiences are linked to age of onset and reading recognition in multiple sclerosis. Front Neurol. 2017;8(June):8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nandi A, Glymour MM, Kawachi I, Vanderweele TJ. Using marginal structural models to estimate the direct effect of adverse childhood social conditions on onset of heart disease, diabetes, and stroke. Source Epidemiol. 2012;23(2):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethell C, Carle A, Hudziak J, et al. Technical appendix methods to assess adverse childhood experiences of children and families: towards resilience and well-being based approaches in policy and practice. Accessed March 13, 2021. who.int [DOI] [PMC free article] [PubMed]
- 14.Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr. 2018;172(11):1038-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bethell CD, Carle A, Hudziak J, et al. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 2017;17(7S):S51-S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wade R, Becker BD, Bevans KB, Ford DC, Forrest CB. Development and evaluation of a short adverse childhood experiences measure. Am J Prev Med. 2017;52(2):163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behavioral risk factor surveillance system ACE data |Violence Prevention|Injury Center|CDC. Accessed March 13, 2021. cdc.gov/violenceprevention/aces/ace-brfss.html
- 18.Kazeem OT. A Validation of the Adverse Childhood Experiences Scale in Nigeria, Vol 5; 2015. Accessed March 13, 2021. iiste.org [Google Scholar]
- 19.Almuneef M, Qayad M, Aleissa M, Albuhairan F. Adverse childhood experiences, chronic diseases, and risky health behaviors in Saudi Arabian adults: a pilot study. Child Abus Negl. 2014;38(11):1787-1793. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The patient health questionnaire-2. Med Care. 2003;41(11):1284-1292. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317-325. [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Center for Health Statistics N. Table P-10. Number of Overnight Hospital Stays during the Past 12 Months, by Selected Characteristics: United States; 2018. Accessed April 14, 2020. cdc.gov/nchs/nhis/SHS/tables.htm [Google Scholar]
- 25.Mcdermott KW, Elixhauser A, Sun R. Trends in Hospital Inpatient Stays in the United States, 2005-2014; 2005. Accessed April 14, 2020 hcup-us.ahrq.gov/faststats/landing.jsp [Google Scholar]
- 26.Vinton DT, Capp R, Rooks SP, Abbott JT, Ginde AA. Frequent users of US emergency departments: characteristics and opportunities for intervention. Emerg Med J. 2014;31(7):526-532. [DOI] [PubMed] [Google Scholar]
- 27.Locker TE, Baston S, Mason SM, Nicholl J. Defining frequent use of an urban emergency department. Emerg Med J. 2007;24(6):398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Got your ACE score? ACEs too high. Accessed July 25, 2020. acestoohigh.com/got-your-ace-score/.
- 29.McEwen BS. Neurobiological and systemic effects of chronic stress. Chronic Stress. 2017;1:247054701769232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47(2):246-249. [PubMed] [Google Scholar]
- 31.Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2006;59(1):35-41. [DOI] [PubMed] [Google Scholar]
- 32.Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia. 2014;55(7):1120-1127. [DOI] [PubMed] [Google Scholar]
- 33.Larkin H, Felitti VJ, Anda RF. Social work and adverse childhood experiences research: implications for practice and health policy. Soc Work Public Health. 2014;29(1):1-16. [DOI] [PubMed] [Google Scholar]
- 34.Hargreaves MK, Mouton CP, Liu J, Zhou YE, Blot WJ. Adverse childhood experiences and health care utilization in a low-income population. J Health Care Poor Underserved. 2019;30(2):749-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Home | ACEs Aware – Take Action. Save Lives. Accessed February 15, 2021. acesaware.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized for this study and is available on request to the authors.