Abstract
Purpose of Review
Perioperative neurocognitive disorders are common after surgery and have serious socioeconomic impacts. Despite this, these disorders remain under-recognized and underdiagnosed. To facilitate detection and direct patients toward appropriate preventative interventions, assessment of cognition during the perioperative period is of critical importance. However, there are considerable barriers to the widespread clinical implementation of cognitive assessments, including a lack of consensus regarding the optimal tool for use in specific clinical scenarios.
Recent Findings
We provide an overview of the most widely used and validated cognitive assessment tools, including those that permit telemedicine-enabled patient encounters.
Summary
No single tool is optimal for all contexts. This narrative review can help clinicians to identify the appropriate cognitive screening tool for their needs by describing the advantages and disadvantages of several available tools, thereby enabling the identification of patients at risk of cognitive decline and facilitating optimization of patient-focused perioperative care.

TAKE-HOME POINTS
→ Perioperative neurocognitive disorders (PNDs) are a common complication after surgery with serious socioeconomic consequences for patients and their families.
→ One barrier to effective perioperative cognitive screening for PND is identifying the best assessment tool from among available options.
→ No single screening tool is ideal for every clinical setting, and several factors must be considered in selecting an appropriate test.
→ Cognitive screening is a critical priority in older surgical patients beyond the perioperative setting.
→ Further research is warranted to evaluate the predictive utility and implementation of screening in routine clinical practice.
Perioperative neurocognitive disorders (PNDs) comprise several cognitive conditions, including preexisting cognitive impairment (CI), postoperative delirium, and both mild and major postoperative neurocognitive disorders (P-NCD).1 PND is associated with increased postoperative morbidity, mortality, health-care costs, risk of complications, prolonged hospital admission, lower functional capacity, and reduced quality of life.2-6 Although PND may be attenuated or prevented,7-9 it is frequently underdiagnosed, without clear consensus regarding which patients should undergo cognitive screening or how screening should be performed. Failure to identify high-risk patients and institute preventative or therapeutic strategies will increase the burden of PND as the surgical population ages. A consistent and practical strategy is urgently needed to identify perioperative cognitive deficits.
One major barrier to effectively screening perioperative cognition is the sheer breadth of available testing tools.10 Many factors should be considered when choosing appropriate cognitive tests for specific clinical scenarios. This review summarizes common cognitive screening tools that can be used perioperatively. This will assist clinicians by removing 1 key obstacle to implementation of perioperative cognitive screening and inform decision-making related to early detection and management of P-NCD. Screening for other forms of PND, such as postoperative delirium, is also important. However, an assessment of tools designed to detect delirium is beyond the scope of this review, and comprehensive reviews can be found elsewhere.11,12 An overview of our screening strategy is included in eMethods, links.lww.com/CPJ/A317.
Deciphering Postoperative Neurocognitive Disorders
How Important Is Testing?
Annually, more than 312 million surgeries are performed worldwide.13 With an aging population, clinicians are increasingly caring for older adults with varying levels of CI. P-NCD is among the most common postoperative complications, especially for older individuals.1 Both mild and major P-NCDs are characterized by persistent cognitive deficits (relative to baseline) lasting more than 30 days and diagnosed within 1 year after surgery.1 Recently, P-NCD has evolved from a research-based classification to a formal diagnosis with diagnostic criteria, nomenclature, and best practices that align with definitions for cognitive decline in the Diagnostic and Statistical Manual for Mental Disorders (5th edition).1 Specifically, mild and major P-NCDs are defined by decreases in cognitive function of 1 to <2 and ≥2 SDs, respectively, relative to a control group (i.e., normal scores of aged-matched individuals from the general population or a nonoperative, age-matched cohort).1,14 Notably, no specific testing criteria or cognitive tests have been recommended.1,14
Major P-NCD affects approximately 10% and 25% of patients 3 months after noncardiac15,16 and cardiac surgery,17 respectively. P-NCD has a complex, not yet fully elucidated etiology, despite identification of several risk factors, including advanced age, lower education level, severity of surgical insult, depth and duration of anesthesia, and preexisting CI.7,16,18
The negative effects of PND are not limited to cognition but also affect other important postoperative outcomes. Major P-NCD is associated with increased morbidity, mortality, and caregiver support and decreased quality of life and functional capacity.2,4,5 P-NCD is also associated with an increased risk of subsequent dementia.2,19,20 P-NCD negatively affects patients' mental health, with perceived postoperative cognitive decline strongly predicting depression and anxiety.21 Finally, PND has substantial economic costs with the increased costs in the first postoperative year estimated at $17,275 per patient.6
Who Should Be Screened?
Older surgical patients are predisposed to CI and have a higher prevalence of associated comorbidities (e.g., atherosclerosis and chronic inflammation).18,22-24 Among the most important risk factors associated with P-NCD is preexisting CI, which is frequently subclinical and unrecognized.25-27 A large observational study showed that preexisting CI, which affects almost 20% of surgical patients 65 years or older, was associated with a significant increase in the risk of postoperative delirium (odds ratio 2.53).28
Awareness of perioperative cognitive decline, including preexisting CI and P-NCD, remains low. Recent studies indicate that preexisting CI is undiagnosed in 88%–98% of cases.25,26 Furthermore, mild cases of CI may be missed if there is a reliance on health-care providers to recognize neurocognitive disorders (NCD). A recent study showed that health-care providers blinded to Mini-Cog screening scores identified only half of patients with preexisting CI.29 To combat this, systematic and objective cognitive assessments are needed.
The American Society of Anesthesiologists (ASA) Brain Health Initiative Summit has promoted awareness of this problem and issued calls for further research.14 A recent survey of ASA members reported that most respondents did not screen for preexisting CI and delirium (i.e., screened less than 10% of patients) and did not discuss the risks of postoperative cognitive changes.30 Increased screening alone can lead to practice changes that may benefit individuals susceptible to cognitive decline. A recent analysis of primary care providers documented significantly more interventions after reports consistent with mild NCD on a computerized screening test, even after accounting for physicians' and patients' perceptions of cognitive ability.31
When Should Testing Occur?
Routine preoperative cognitive screening should optimally occur in preoperative evaluation clinics (0–3 months before procedure). The goal is to accurately capture the individual's cognitive status at a time that is not so close to procedure dates that assessments are clouded by preoperative stress and anxiety. Postoperative screening is most important in the first 3 months after surgery, when the highest rates of cognitive decline occur; however, the residual effects of surgery and anesthesia may still confound testing results. After 3 months, these effects are expected to have subsided, allowing for an accurate assessment.32 Beyond 1 year, cognitive changes can no longer be reliably linked to surgery and anesthesia.1
Which Test to Use?
No single cognitive screening test is ideal for every clinical setting, and several factors must be considered when selecting an appropriate test. The ‟ideal tool” should have high sensitivity, specificity, and positive predictive value; be easy and quick to administer; and have high test-retest reliability. Important factors that are potential sources of error include learning, ceiling, and floor effects.33,34 The ideal test should accurately gauge cognitive changes in individuals with different levels of education and baseline capabilities. Finally, the requirement for trained personnel or testing equipment must be considered and matched to the context and available resources.
Computerized testing has several inherent advantages, including remote administration, little or no required input from assessors, elimination of data handling errors, automatic score calculation, increased standardization, and reduced assessor bias. However, computerized tests depend on device availability (e.g., tablets) and may be proprietary, and the results may be influenced by participants' computer literacy.35 In the following sections, we compare and discuss core features of several well-validated and widely available cognitive screening tools (Tables 1–5).
Table 1.
Pen and Paper Cognitive Assessment Tools—Practical Considerations
Table 2.
Computerized Cognitive Assessment Tools—Practical Considerations
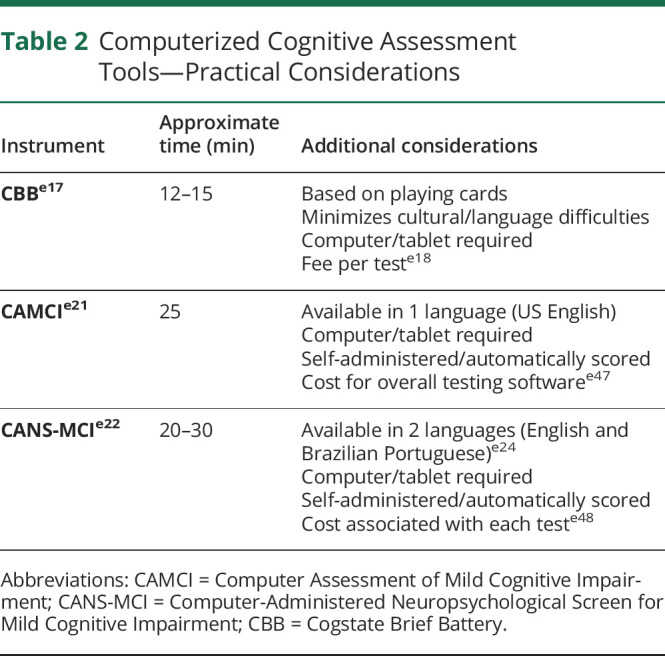
Table 3.
Cognitive Assessment Tools: Test Accuracy (Sensitivity and Specificity)
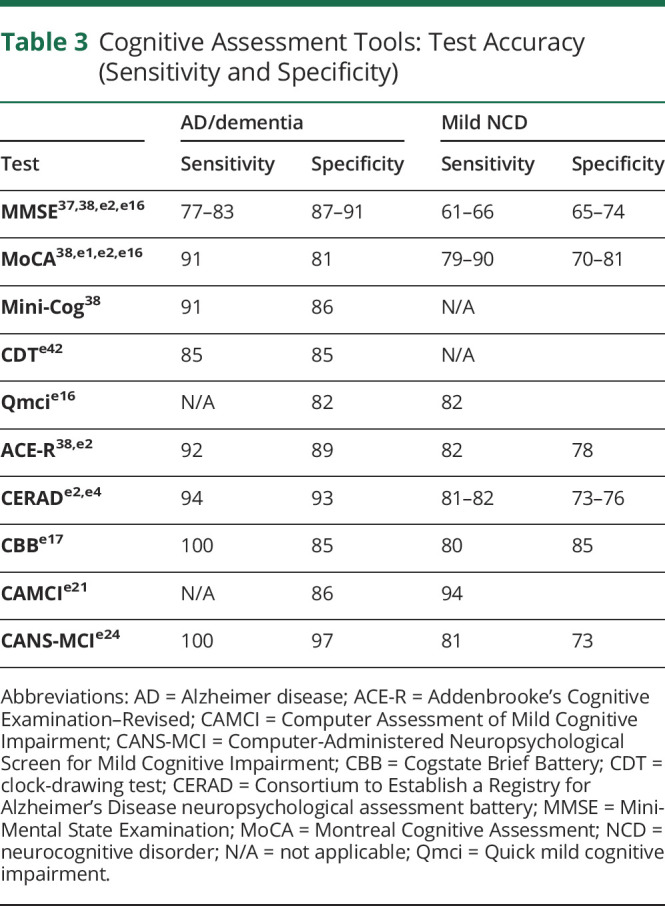
Table 4.
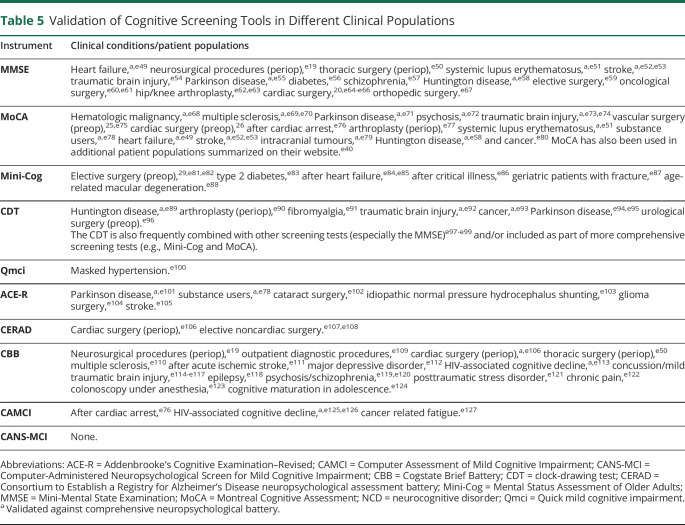
Applications of Cognitive Screening Tools
Table 5.
Validation of Cognitive Screening Tools in Different Clinical Populations
Evaluating Tools for Routine Assessment of Perioperative Cognitive Decline
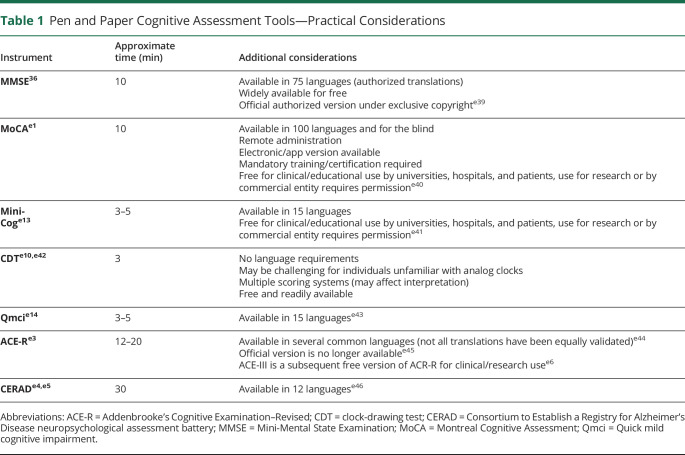
Among the most frequently used cognitive tests is the Mini-Mental State Examination (MMSE).36 A meta-analysis of 34 studies demonstrated that MMSE had moderate sensitivity and specificity for detecting dementia (sensitivity 77%–83% and specificity 87%–91%, depending on clinical context); however, its ability to identify mild NCD was limited (sensitivity 63% and specificity 65%).37 A subsequent meta-analysis reported similar sensitivity (62%) for mild NCD.38 A variation of the MMSE, the modified MMSE (mMMSE or 3 multiple sclerosis), has been developed to address a much broader range of cognitive domains.39 The mMMSE has greater sensitivity (84%) for mild NCD relative to MMSE (58%)40 but is less studied and used.
A popular alternative to MMSE is the Montreal Cognitive Assessment (MoCA), which detects mild NCD (sensitivity 90%) better than MMSE and mMMSE.e1 A recent meta-analysis demonstrated the superiority of MoCA (sensitivity 81%, specificity 74%, 24 studies, and n = 4,095) over MMSE (sensitivity 66%, specificity 74%, 46 studies, and n = 17,749) for detecting mild NCD, albeit with more modest margins.e2 For the detection of frank dementia, another meta-analysis showed that MoCA was superior (91%) to MMSE (81%) and mMMSE (86%).38
The same meta-analysis examined 2 additional comprehensive cognitive tests, Addenbrooke's Cognitive Examination–Revised (ACE-R)e3 and the Consortium to Establish a Registry MoCA for Alzheimer's Disease neuropsychological assessment battery (CERAD),e4,e5 both of which are used less frequently but have similar sensitivity (82%, both tests) and specificity (78% and 76%, respectively) to MoCA for detecting mild NCD.e2 A newly developed version of the ACE-R test, known as ACE-III,e6 has been shown in 1 patient population to have superior sensitivity and specificity (97% and 91%, respectively) for mild NCD relative to MoCA.e7 However, there is insufficient evidence to fully support its use perioperatively because it has not been validated in this patient population.
In addition to these more comprehensive tests, shorter, simpler tests have also proven useful for detecting cognitive decline. Among these is the clock-drawing test (CDT)e8-e10 (Tables 1, 3–5). The CDT lacks a memory assessment, which limits its utility for cognitive screening, especially in the context of early CI, where memory impairment is often a driving element.e11
A similar test, the Mini-Cog consists of a clock-drawing task coupled with a 3-item memory recall teste12,e13 showed superior sensitivity (91%) and specificity (86%) for detecting dementia relative to the MMSE (sensitivity 81% and specificity 89%) and comparable with MoCA (sensitivity 91% and specificity 81%) and ACE-R (sensitivity 92% and specificity 89%).38 Although well-validated for identifying dementia, the Mini-Cog has limited evidence for detecting mild NCD. By contrast, the Quick Mild CI (Qmci) test was developed specifically for detecting mild NCD.e14,e15 A systematic review reported that the Qmci was superior for differentiating mild NCD from normal controls (sensitivity 82% and specificity 82%) relative to MMSE (sensitivity 61% and specificity 67%) and MoCA (sensitivity 79% and specificity 70%), despite both of the latter tests taking longer to administer (Table 1).e16
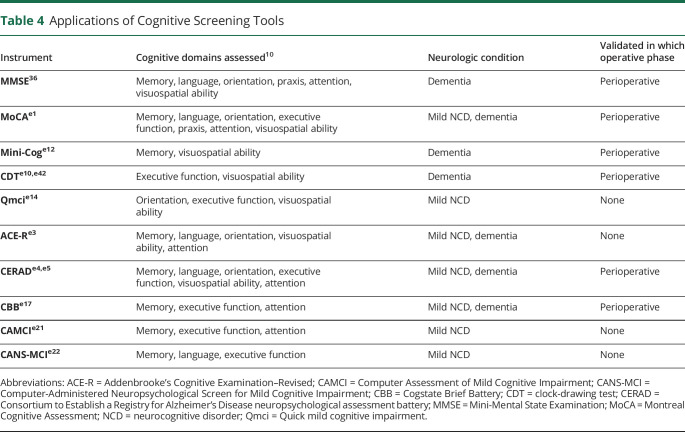
In addition to traditional “pen and paper” cognitive tests, technological advances have recently led to the development of computer-based assessments. Among the best-studied is the Cogstate Brief Battery (CBB),e17,e18 which has been validated in multiple contexts, including the postoperative setting,e19 and is suitable for detecting mild NCD in adults across a range of ages, including those with no previous computer experience.e20 Relative to MoCA, CBB has similar sensitivity (80%) and specificity (85%) for detecting mild NCD.10,e17 Drawbacks include its proprietary nature (i.e., additional cost) and potential privacy concerns, given the online nature of the assessment.
Two computer-based alternatives are the Computer Assessment of Mild CI (CAMCI)e21 and the Computer-Administered Neuropsychological Screen for Mild CI (CANS-MCI).e22 The CAMCI has been validated for detecting mild NCD in patients over 60 years of age, a population in which it performed favorably (sensitivity 86% and specificity 94%) relative to the MMSE (sensitivity 45% and specificity 80%).e21 Similarly, the CANS-MCI has good sensitivity (81%) and specificity (73%) for detecting mild NCD relative to the MoCA and ACE-R.e23,e24 Like the CBB, CAMCI is feasible to use in a variety of populations, including those without previous computer experiencee25; however, both CAMCI and CANS-MCI take longer than CBB to complete (15–30 minutes).10
Matching Tools to Disease to Improve Clinical Care and Patient Outcomes
No single cognitive screening tool is ideal for use in every clinical setting because distinct disease phenotypes and perioperative scenarios require different approaches. Clinicians must therefore be familiar with a variety of readily available tools to help with detection, interpretation, and management of perioperative cognitive conditions.
Optimal cognitive screening encompasses both preoperative and postoperative evaluations to allow patients to be compared with their own preoperative baseline (as comparisons with normative population data may be limited by personal variability). However, preoperative screening alone may still be beneficial for risk assessment (i.e., identifying preexisting CI, which can predict postoperative cognitive complications). Similarly, postoperative follow-ups may still be useful for directing impaired patients to appropriate care, even in the absence of preoperative testing. Thus, the following section highlights considerations specific to each perioperative setting. Notably, to track cognitive trajectories perioperatively, it is important to choose a single screening tool appropriate for both preoperative and postoperative screening because scores are not readily comparable between tools. Several such tests are described below.
Preoperative Cognitive Screening
When patients need to be screened in busy settings (e.g., emergency departments and preoperative clinics), rapid administration is desirable. Furthermore, because mild NCD is often subclinical and unrecognized, preoperative screening should be performed for all older patients, not only those with subjective complaints or those in whom CI is already suspected.29 Therefore, tests taking more than a few minutes to complete are suboptimal for this setting. Tests with low sensitivity for mild NCD (e.g., MMSE, CDT) are of limited utility for preoperative screening because even mild preexisting CI is a strong indicator of postoperative complications, both cognitive and otherwise.20,e26
Among paper-based screening tests, an argument could be made for the Qmci because of its ease and speed of administration, as well as its high sensitivity and specificity compared with other tests such as MoCA and MMSE (Table 3).e16 However, Qmci has not yet been used perioperatively. Alternatively, the Mini-Cog has been widely used for preoperative screening because of its rapid administration and sensitivity for dementia29,e27; however its utility is limited by an inability to effectively detect mild NCD. Thus, although it takes longer to administer, MoCA, which has been more widely validated and has similar sensitivity,e2,e16 may be a more appropriate screening tool. Regardless of the test used, individuals flagged by initial screening could then be directed toward more rigorous testing to establish a definitive diagnosis.
An alternative to screening in preoperative clinics is to have patients self-administer cognitive tests remotely at home before their appointment. Here, administration time is less important, and sensitivity for mild NCD and remote administration capability can be prioritized. CAMCI may be most useful because it has the highest sensitivity for mild NCD (86%) among computerized testse21 and has been validated for self-administration in older populations (although not specifically in the preoperative setting).e25
In-Hospital Assessment of Postoperative Cognitive Changes
During the postoperative period, speed of administration is less important than in the preoperative clinic. Here, the accuracy of identifying cognitive change is crucial, followed closely by test-retest reliability, given that repeated postoperative assessments may be performed to assess a patient's cognitive trajectory.
Regarding traditional tests, MoCA is a reasonable option because it has good test-retest reliability (r = 0.88–0.92)10 and good sensitivity and specificity for mild NCD (Table 3). It has also been validated for perioperative use.e2 Although the ACE-R and CERAD tests have similar sensitivity and specificity (Table 3), data for MoCA are based on a much larger pool in a broader range of clinical settings, increasing its generalizability.
Where electronic devices (laptops and tablets) are available, CBB and CAMCI are appropriate because both can reduce bias, errors, and learning effects relative to more traditional “pen and paper” tests. The CBB was explicitly designed to minimize practice effects by restricting assessment to memory, attention, and executive functions.e18 It has been validated in the perioperative setting, has comparable sensitivity and specificity with MoCA, and requires a similar amount of time (Tables 1–4). Although CAMCI has not been validated perioperatively and takes longer to administer than MoCA, it has reported higher sensitivity (86%) and specificity (94%) for detecting mild NCD in 1 studye21 relative to that reported for MoCA in a recent meta-analysis (sensitivity 81% and specificity 74%).e2
Assessment of Postoperative Cognitive Change After Hospital Discharge
Remote administration is highly advantageous for following patient's cognitive trajectory after hospital discharge. This minimizes return visits to hospital and increases access to cognitive testing, particularly for individuals in remote or rural communities. Here, computerized tests (e.g., CBB and CAMCI) may be superior. Additional information related to virtual and telemedicine-based cognitive screening can be found in eAppendix 1 and eTable 1, links.lww.com/CPJ/A316.
Patients for whom tests show evidence of cognitive decline could then be selected for follow-up, including more comprehensive cognitive or neuropsychological assessments with their primary physician or a specialist. Additional factors including patients' subjective reports of poor memory and interference with activities of daily living should be considered when determining a definitive diagnosis, although an objective, validated screening tool may still be useful.
Discussion
There is increasing awareness of the grave consequences of PND and the value of preoperative cognitive screening. However, formal screening programs are rare, and the risks of negative postoperative cognitive outcomes are seldom discussed with patients. To better care for older patients undergoing surgery, work is urgently needed to define and overcome the barriers to testing at the institutional level. Although even brief cognitive screening tools will take time, effort, and motivation to incorporate into routine clinical practice, several encouraging studies have demonstrated the feasibility of adopting large-scale preoperative screening.29,e28 Equivalent studies focused on postoperative cognitive follow-up outside of a research context are likewise warranted.
Ideally, every surgical patient would be assessed preoperatively to identify preexisting CI and other risk factors for PND. Unfortunately, logistic challenges may prevent this. Instead, institutions would benefit from an evidence-based, hierarchical screening strategy that prioritizes patients who are at greatest risk for perioperative CI. Further research is needed to identify which risk factors (e.g., subjective reports of cognitive decline and existence of comorbidities) best predict cognitive challenges.
A comprehensive preoperative screening strategy might also include a broader set of tests designed to measure other important factors in parallel with cognition, such as frailty, substance use, sensory impairment, and depression. Although some frameworks exist for this type of assessment (e.g., Comprehensive Geriatric Assessmente29 and Delirium Elderly At Riske30), it is unclear how these screening tools and strategies can be optimally implemented to reduce PND.
Emerging evidence suggests that biomarkers may also assist in risk stratificatione31-e33; however, the identification of reliable biomarkers that predict postoperative cognitive complications is very preliminary.e34 Similarly, the pathophysiologic basis of specific cognitive phenotypes is not well understood, and additional work is needed to characterize pathways linking cognitive changes and disease pathologies. Another challenge is the gap between research observations and translation into clinical practice. Effective, easy-to-use tools are essential to bridge this divide and dissect the different levels at which minor brain dysfunction may trigger specific brain degeneration in different forms of PND.
Nonpharmacological strategies such as orientation protocols (e.g., whiteboards with care team names in patients rooms) as well as ensuring proper perioperative sleep hygiene (e.g., noise reduction protocols), adequate nutrition, and the availability of vision/hearing aids have shown considerable success in reducing incidence of PND.7,e35,e36 Implementation of these strategies can be resource intensive and varies greatly between institutions and specialties. Although adherence to these strategies would arguably be beneficial for all surgical patients, they are especially important in those at-risk, highlighting the importance of cognitive screening for identification. Coupling cognitive screening with patient, caregiver, and physician education regarding the cognitive risks of surgery and effective preventative strategies will further improve patient outcomes.
Newly developed or repurposed pharmacologic interventions for PND are also urgently needed alongside cognitive screening. Similarly, routine cognitive screening will facilitate future clinical research into treatment and prevention. Ongoing work in this area includes 2 prospective studies by our group.27,e37,e38 The first study aims to better characterize PND in patients undergoing arthroplasty, which is among the most common surgical procedures for older people (NCT03147937).e37 The second is a randomized trial that aims to prevent P-NCD after cardiac surgery by targeting the central neuroinflammatory aspect of surgery (NCT04289142).e38 Both studies will also validate implementation of targeted perioperative cognitive screening.
Comprehensive cognitive screening is an indispensable tool for unmasking, predicting, and reducing the cognitive risks associated with surgery and directing at-risk patients to targeted therapies. Despite the growing clinical importance of evidence-based practice, there is a gap between what should be done and what is currently happening in the field of PND.30 This review creates additional clinician awareness of the requirement for objective perioperative cognitive assessment. With no gold standard and a lack of consensus about which test measures should be used, physicians can use this concise summary to guide their choice of feasible cognitive screening tools.
Appendix. Authors
Contributor Information
Andrew Fleet, Email: andrew.fleet@sunnybrook.ca.
Connor T.A. Brenna, Email: connor.brenna@mail.utoronto.ca.
Beverley A. Orser, Email: beverley.orser@utoronto.ca.
Study Funding
The authors report no targeted funding.
Disclosure
L. Kaustov, A. Fleet, and C.T.A. Brenna declare no disclosures relevant to the manuscript. B.A. Orser has a Canadian Patent No. 2852978: Methods for the prevention and/or treatment of memory impairment (Date of Patent: October 10, 2017), Filing date: October 21, 2011, a US Patent No. 9517265 B2: Methods for the prevention and/or treatment of memory impairment (Date of Patent: December 15, 2016), Filing date: November 8, 2011, and a pending US patent No. 62/268,137: Cell-permeable inhibitory agent and method of use thereof in treatment of cognitive and mood disorders, Filing date: December 16, 2015, Response to Office Action July 1, 2020, pending. S. Choi receives in-kind support for no-cost access to the CogState Brief Battery (CBB) from Cogstate Ltd for 2 ongoing studies and receives support for dedicated research time from the Sunnybrook Anesthesia Academic Partnership (SAAP). Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129(5):872-879. [DOI] [PubMed] [Google Scholar]
- 2.Evered L, Silbert B, Scott DA, Ames D, Maruff P, Blennow K. Cerebrospinal fluid biomarker for alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology. 2016;124(2):353-361. [DOI] [PubMed] [Google Scholar]
- 3.Ahlgren E, Lundqvist A, Nordlund A, Aren C, Rutberg H. Neurocognitive impairment and driving performance after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2003;23(3):334-340. [DOI] [PubMed] [Google Scholar]
- 4.Price CC, Garvan CW, Monk TG. Type and severity of cognitive decline in older adults after noncardiac surgery. Anesthesiology. 2008;108(1):8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS, the ISPOCD Group. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548-555. [DOI] [PubMed] [Google Scholar]
- 6.Boone MD, Sites B, von Recklinghausen FM, Mueller A, Taenzer AH, Shaefi S. Economic burden of postoperative neurocognitive disorders among US medicare patients. JAMA Netw Open. 2020;3(7):e208931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen TL, Alberts AR, Hooft L, Mattace-Raso F, Mosk CA, van der Laan L. Prevention of postoperative delirium in elderly patients planned for elective surgery: systematic review and meta-analysis. Clin Interv Aging. 2019;14:1095-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peden CJ, Miller TR, Deiner SG, Eckenhoff RG, Fleisher LA. Improving perioperative brain health: an expert consensus review of key actions for the perioperative care team. Br J Anaesth. 2021;126(2):423-432. [DOI] [PubMed] [Google Scholar]
- 9.Rengel KF, Pandharipande PP, Hughes CG. Special considerations for the aging brain and perioperative neurocognitive dysfunction. Anesthesiol Clin. 2019;37(3):521-536. [DOI] [PubMed] [Google Scholar]
- 10.De Roeck EE, De Deyn PP, Dierckx E, Engelborghs S. Brief cognitive screening instruments for early detection of Alzheimer's disease: a systematic review. Alzheimers Res Ther. 2019;11(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helfand BKI, D'Aquila ML, Tabloski P, et al. Detecting delirium: a systematic review of identification instruments for non-ICU settings. J Am Geriatr Soc. 2021;69(2):547-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RN, Cizginer S, Pavlech L, et al. Assessment of instruments for measurement of delirium severity: a systematic review. JAMA Intern Med. 2019;179(2):231-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiser TG, Haynes AB, Molina G, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94(3):201-209F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123(4):464-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paredes S, Cortínez L, Contreras V, Silbert B. Post-operative cognitive dysfunction at 3 months in adults after non-cardiac surgery: a qualitative systematic review. Acta Anaesthesiol Scand. 2016;60(8):1043-1058. [DOI] [PubMed] [Google Scholar]
- 16.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18-30. [DOI] [PubMed] [Google Scholar]
- 17.Greaves D, Psaltis PJ, Ross TJ, et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol. 2019;289:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avadhani R, Fowler K, Barbato C, et al. Glycemia and cognitive function in metabolic syndrome and coronary heart disease. Am J Med. 2015;128(1):46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative cognitive performance and postoperative delirium are independently associated with future dementia in older people who have undergone cardiac surgery: a longitudinal cohort study. Crit Care Med. 2017;45(8):1295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo LC, Malek MJ, Gilbertson AD, Moore JL. Perceived cognitive function and emotional distress following coronary artery bypass surgery. J Behav Med. 2005;28(5):433-442. [DOI] [PubMed] [Google Scholar]
- 22.Holmes C, Butchart J. Systemic inflammation and Alzheimer's disease. Biochem Soc Trans. 2011;39(4):898-901. [DOI] [PubMed] [Google Scholar]
- 23.Bubu OM, Pirraglia E, Andrade AG, et al. Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep. 2019;42(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma G, Parihar A, Talaiya T, Dubey K, Porwal B, Parihar MS. Cognitive impairments in type 2 diabetes, risk factors and preventive strategies. J Basic Clin Physiol Pharmacol. 2020;31(2). [DOI] [PubMed] [Google Scholar]
- 25.Partridge JS, Dhesi JK, Cross JD, et al. The prevalence and impact of undiagnosed cognitive impairment in older vascular surgical patients. J Vasc Surg. 2014;60(4):1002-1011.e3. [DOI] [PubMed] [Google Scholar]
- 26.Smith NA, Yeow YY. Use of the Montreal Cognitive Assessment test to investigate the prevalence of mild cognitive impairment in the elderly elective surgical population. Anaesth Intensive Care. 2016;44(5):581-586. [DOI] [PubMed] [Google Scholar]
- 27.Brenna CTA, Orser BA, Avramescu S, Fleet A, Kaustov L, Choi S. Cognitive decline among older adults: a hidden preexisting condition and its role in ‟brain-at-risk” surgical patients. Brain Behav. 2021;11(5):e02095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119(2):316-323. [DOI] [PubMed] [Google Scholar]
- 29.Decker J, Kaloostian CL, Gurvich T, et al. Beyond cognitive screening: establishing an interprofessional perioperative brain health initiative. J Am Geriatr Soc. 2020;68(10):2359-2364. [DOI] [PubMed] [Google Scholar]
- 30.Deiner S, Fleisher LA, Leung JM, Peden C, Miller T, Neuman MD. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA Committee on Geriatric Anesthesia-Perioperative Brain Health Initiative ASA member survey. Perioper Med (Lond). 2020;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney MC, Charles J, Naglie G, Jaakkimainen L, Moineddin R. The effects of computerized cognitive testing of older patients on primary care physicians' approaches to care: a Canadian study. Alzheimer Dis Assoc Disord. 2017;31(1):62-68. [DOI] [PubMed] [Google Scholar]
- 32.Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127(2):496-505. [DOI] [PubMed] [Google Scholar]
- 33.Rasmussen LS, Larsen K, Houx P, Skovgaard LT, Hanning CD, Moller JT. The assessment of postoperative cognitive function. Acta Anaesthesiol Scand. 2001;45(3):275-289. [DOI] [PubMed] [Google Scholar]
- 34.Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59(5):1289-1295. [DOI] [PubMed] [Google Scholar]
- 35.Werner P, Korczyn AD. Willingness to use computerized systems for the diagnosis of dementia: testing a theoretical model in an Israeli sample. Alzheimer Dis Assoc Disord. 2012;26(2):171-178. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411-431. [DOI] [PubMed] [Google Scholar]
- 38.Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458. [DOI] [PubMed] [Google Scholar]
- 39.Teng EL, Chui HC. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314-318. [PubMed] [Google Scholar]
- 40.Van Patten R, Britton K, Tremont G. Comparing the Mini-Mental State Examination and the modified Mini-Mental State Examination in the detection of mild cognitive impairment in older adults. Int Psychogeriatr. 2019;31(5):693-701. [DOI] [PubMed] [Google Scholar]
- Additional eReferences e1-e127 available at: links.lww.com/CPJ/A318.