Abstract
Background and Objectives
COVID-19 outcomes in patients with neurodegenerative disorders (NDs) are not well understood, and we hypothesize that there may be increased morbidity and mortality in this group.
Methods
This was a retrospective cohort study performed at 3 hospitals in the Chicagoland area. All patients hospitalized with COVID-19 infection with ND during a 3-month period (March 15, 2020–June 15, 2020) were included and compared with age-matched controls (CL) at 1:1 ratio. Primary outcomes were death, intensive care unit (ICU) admission, and invasive ventilation. Secondary outcomes included presenting COVID-19 symptoms, development of encephalopathy, supplementary oxygen use, discharge disposition, and risk factors for mortality.
Results
The study included 132 patients with neurodegenerative disorders and 132 age-matched CL. Ninety-day mortality (ND 19.7% vs CL 23.5%, p = 0.45) and ICU admission (ND 31.5% vs CL 35.9%, p = 0.43) rates were not significantly different between the 2 groups. Patients with ND had a lower rate of invasive ventilation (ND 11.4% vs CL 23.2%, p = 0.0075) and supplementary oxygen use (ND 83.2% vs CL 95.1%, p = 0.0012). Patients with ND were also more likely to have altered mental status or confusion as their presenting COVID-19 symptom and less likely to present with respiratory symptoms. Patients with ND were discharged to nursing home or hospice at higher rates compared with CL.
Discussion
We found that there was no difference in short-term mortality of patients with ND hospitalized for COVID-19 compared with CL, but they may have higher rates of neurologic complications and disability. Future studies should address long-term outcomes.

As of December 2020, the COVID-19 pandemic has resulted in over 1.5 million deaths worldwide,1 and although there are several promising vaccines in the pipeline, COVID-19 will likely remain a considerable threat for the near future. Several risk factors are associated with mortality due to COVID-19 infection including age, obesity, hypertension, cardiovascular disease, diabetes, chronic kidney, male sex, and more.2 However, it is not well understood whether underlying neurodegenerative disease affects COVID-19 outcome. Cohorts published early during the COVID-19 pandemic were limited due to absent or inconsistent reporting of neurologic comorbidities.3 The mortality rate for patients with Parkinson disease (PD) with acute COVID-19 infection is cited as 5.7%–44%,4-8 although most of these studies lack control (CL) comparison,4-6 and most reports are from outside the United States. Dementia may be associated with increased mortality from COVID-19 infection, as suggested by cohort studies from Europe,9,10 Asia,11 and New York.12,13
Given the geographic variability in COVID-19 epidemiology,14 we sought to assess outcomes within our population in the Chicagoland area. The objective of this study was to compare short-term morbidity and mortality in hospitalized patients with COVID-19 with preexisting neurodegenerative disorders (NDs) vs age-matched CL. We hypothesize that patients with neurodegenerative disorders would have increased risk of mortality and complications, particularly encephalopathy, given their baseline neurologic dysfunction and disability.
Methods
Study Design, Setting, and Patients
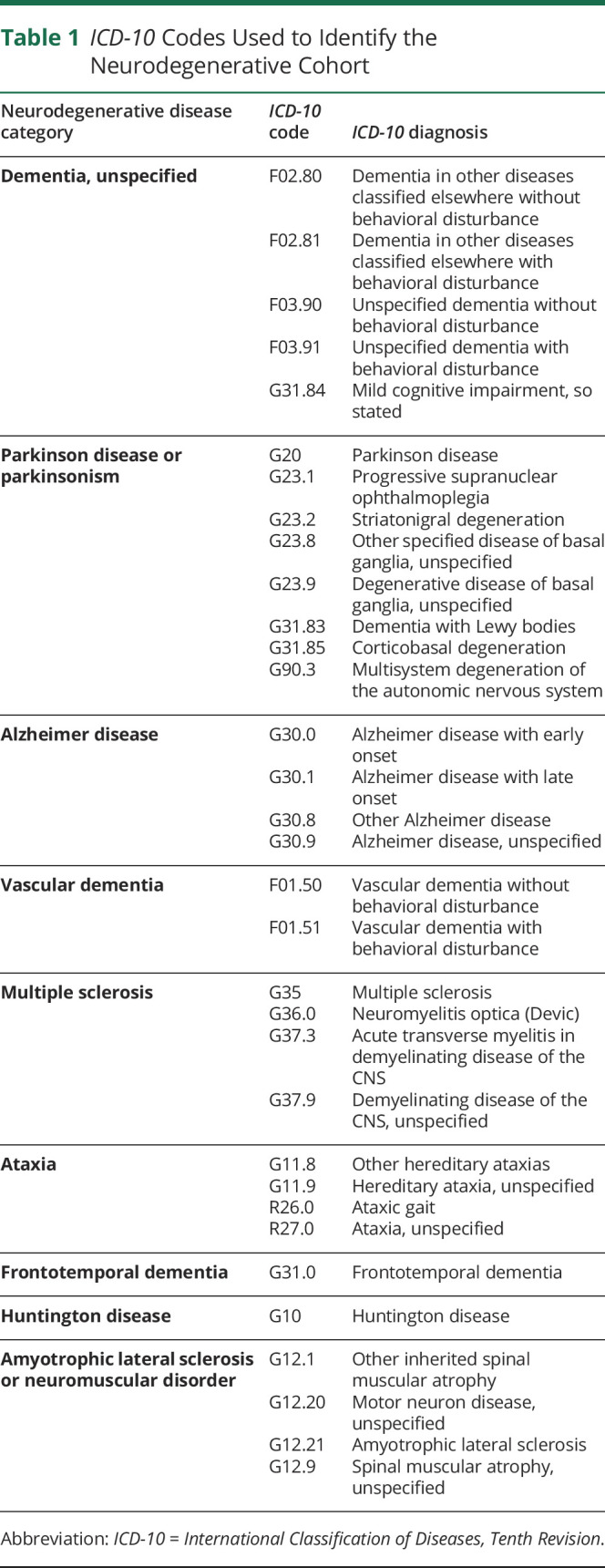
This was a retrospective cohort study. Patients were identified from the Rush COVID-19 Registry, a data repository of all patients tested for SARS-CoV2 within Rush hospital system established by the Rush Bioinformatics and Biostatistics Core. Registry data were sourced from 3 hospitals including Rush University Medical Center, an urban academic center in Chicago, and Rush Oak Park and Rush Copley Hospitals, which are community suburban hospitals in the Chicagoland area. All patients included in the study were adults (age ≥18 years) with confirmed positive SARS-CoV2 testing by either molecular or antigen testing who were hospitalized between March 15 and June 15, 2020. Two cohorts were created: ND cohort were patients with a preexisting neurodegenerative disease identified using International Classification of Diseases, Tenth Revision (ICD-10) code (Table 1), and CL cohort were patients without preexisting neurodegenerative disease and were age matched with patients with ND at 1:1 ratio.15
Table 1.
ICD-10 Codes Used to Identify the Neurodegenerative Cohort
Procedures
All data were collected by extraction from electronic medical record database or manual chart review. Baseline characteristics included age, sex, body mass index (BMI), medical comorbidities based on ICD-10 codes (Asthma or chronic lung disease J40-J47; Hypertension or Cardiovascular disease I10-I15, I20-I25, and I30-I52; Diabetes E10-E11; Chronic kidney disease N18; and Chronic liver disease K74), race, ethnicity, estimated median household income, living situation before hospitalization, and number of hospitalizations for COVID-19 (i.e., some patients had multiple admissions). Estimated median household income was determined by zip code based on Census Bureau American Community Survey, 2018 inflation-adjusted dollars, 5-year estimates.16 ND patient neurodegenerative diagnosis was identified using ICD-10 codes (Table 1) and verified by manual chart review. Primary outcomes were death (defined as all-cause death within 90 days of hospital admission), intubation or mechanical ventilation, and intensive care unit (ICU) admission. Secondary outcomes were presenting COVID-19 symptoms, hospital length of stay, oxygen therapy, development of encephalopathy, neuroleptic therapy (haloperidol, quetiapine, risperidone, and olanzapine were included), code status on admission (full code vs do not resuscitate or do not intubate [DNR/DNI]), discharge disposition, and COVID-19 medication therapy. COVID-19 medications hydroxychloroquine, azithromycin, remdesivir, and tocilizumab were included because these were the agents being used during the time period of March 15 to June 15, 2020; therefore, they may not reflect current standard for COVID-19 treatment, which has evolved since these early months. Complication of encephalopathy was identified using ICD-10 codes (G92, G93.4, F05, and R41) and verified by manual chart review.
Statistical Analysis
No sample size calculation was performed because this was a convenience sample. ND and CL cohorts were compared using the independent t-test for continuous variables and the χ2 or Fisher exact test for categorical variables. The Cochran-Mantel-Haenszel test was used to identify covariate for intubation outcome. Binary logistic regression was used to identify risk factors for 90-day mortality (with dependent variable as death vs no death). Only univariate analysis was performed to assess potential risk factors of mortality for each cohort (multivariable analysis was not performed), and ND and CL cohorts were analyzed separately. p Value ≤0.05 was considered statistically significant. In domains where multiple comparisons were made between ND and CL cohorts and null hypothesis was rejected, Holm-Bonferroni correction17 was performed to protect against type 1 error. All analyses were performed using SAS software (SAS Studio 3.8, SAS Institute Inc, Cary, NC).
Standard Protocol Approvals, Registrations, and Patient Consents
This study protocol was approved by ethical standards committee: Institutional Review Board (IRB) at Rush University Medical Center. It was determined by the IRB that written informed consent by participants was not needed due to the retrospective nature of this study and lack of identifiable personal health information being used.
Data Availability
All study data, including raw data and analyzed data, are available from the corresponding author on reasonable request.
Results
Demographics
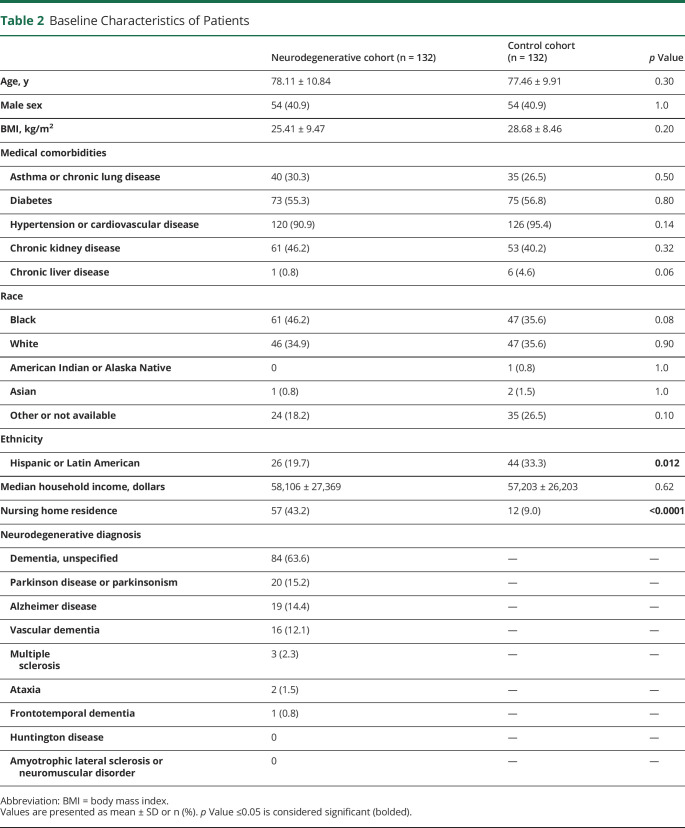
Two hundred sixty-two patients were included in the study, with 132 in the ND group and 132 in the CL group. Data were complete for all assessed variables and outcomes. Baseline characteristics were similar between the 2 groups including age, sex, BMI, medical comorbidities, race, and median household income based on zip code (Table 2). There were more patients with Hispanic or Latin American ethnicity in the CL group compared with the ND group (33.3% vs 19.7%, respectively, p = 0.012). In addition, a higher portion of patients with ND resided in nursing home (NH) before admission compared with CL (43.2% vs 9.0%, respectively, p < 0.0001). Baseline NDs are listed for the ND group, with the most common being unspecified dementia, followed by PD or parkinsonism, Alzheimer disease, vascular dementia, multiple sclerosis, ataxia, and frontotemporal dementia. None of the patients had Huntington disease or amyotrophic lateralizing sclerosis. Some patients had multiple diagnoses (e.g., vascular dementia and PD).
Table 2.
Baseline Characteristics of Patients
Primary Outcomes
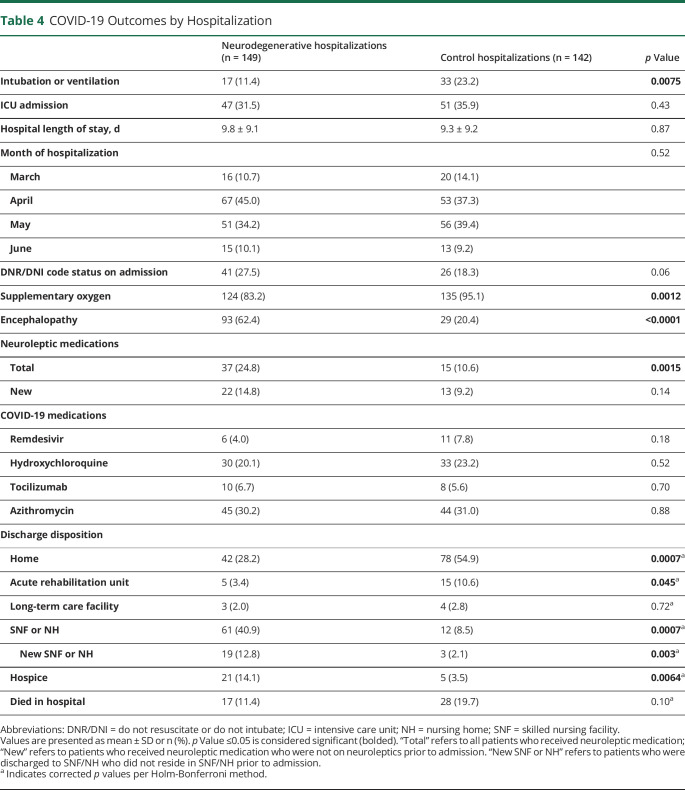
Death was similar between the 2 groups (Table 3; ND 19.7% vs CL 23.5%, p = 0.45), as was the ICU admission rate (Table 4; ND 31.5% vs CL 35.9%, p = 0.87). Intubation or mechanical ventilation was more frequent in the CL group (Table 4; CL 23.2% vs ND 11.4%, p = 0.0075). After adjusting for code status, there was no longer a difference in ventilation or intubation rate between the 2 groups. Relative risk (RR) of intubation or ventilation in the ND group without controlling for DNR/DNI code status was 0.88, 95% confidence interval (CI) 0.79–0.98, and RR after controlling for DNR/DNI code status was 1.04, 95% CI 0.92–1.16.
Table 3.
COVID-19 Outcomes by Patient
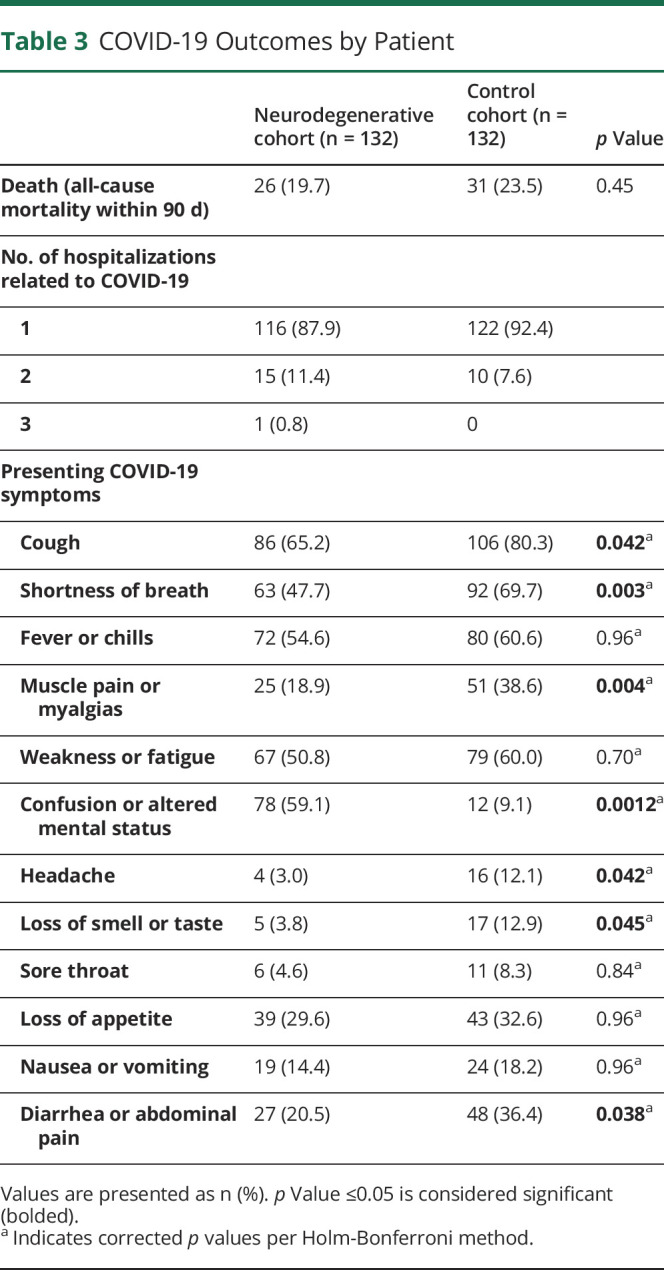
Table 4.
COVID-19 Outcomes by Hospitalization
Secondary Outcomes
Most patients in both groups had 1 hospitalization for COVID-19, and a minority of patients in each group had 2 or 3 hospitalizations (Table 3). With regard to presenting COVID-19 symptoms, fever or chills, weakness or fatigue, loss of appetite, nausea or vomiting, and sore throat were similar between the 2 groups. Cough, shortness of breath, muscle pain or myalgias, diarrhea or abdominal pain, headache, and loss of smell or taste were reported more frequently in the CL cohort, whereas altered mental status or confusion was more common in the ND cohort (Table 3).
During the hospitalization, CL had a higher rate of supplementary oxygen use (Table 4; CL 95.1% vs ND 83.2%, p = 0.0012), whereas patients with ND had a higher rate of encephalopathy (Table 4; ND 62.4% vs CL 20.4%, p < 0.0001). Both groups had similar length of stay and similar rates of DNR/DNI code status on admission and COVID-19 medication prescription. Patients with ND were prescribed neuroleptic medications more frequently (ND 24.8% vs CL 10.6%), but there was no difference in new neuroleptic prescription, i.e., when we removed patients who were prescribed neuroleptic medication before hospitalization. Discharge disposition was different between the 2 groups, with CL being discharged to home and acute rehabilitation unit more frequently and patients with ND discharged to NH or skilled nursing facility (SNF) or hospice more frequently (Table 4). The difference in discharge to NH of SNF between the 2 groups was maintained even when we removed patients who lived in NH before hospitalization (Table 4; new SNF or NH, ND 12.8% vs CL 2.1%, p = 0.003).
Risk Factors for Mortality
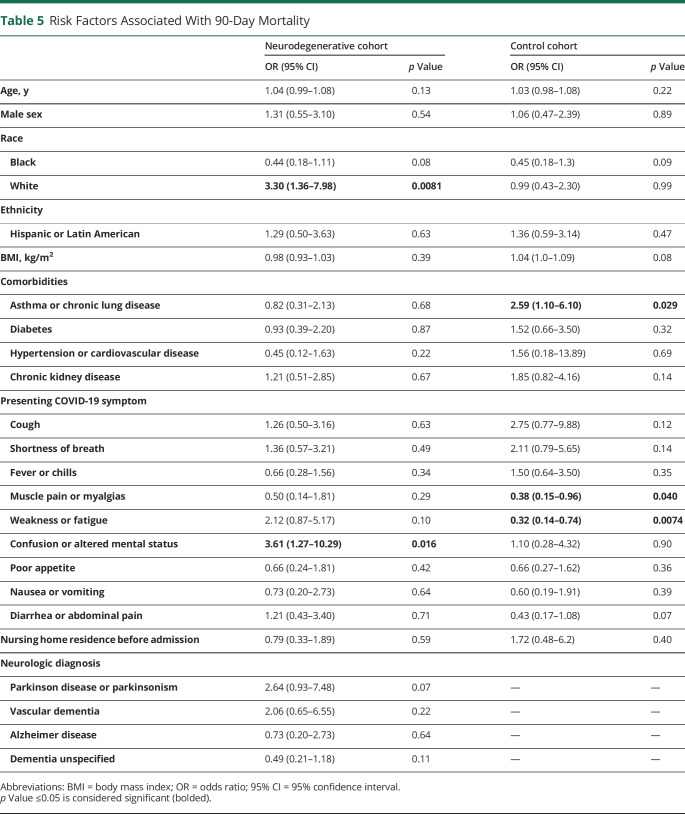
Risk factors for 90-day mortality after COVID-19 admission based on baseline characteristics are depicted in Table 5. In the ND cohort, risk factors for mortality included White race (OR 3.3, 95% CI 1.36–7.98, p = 0.0081) and presenting COVID-19 symptom of confusion or altered mental status (OR 3.61, 95% CI 1.27–10.29, p = 0.016). Whereas in the CL cohort, asthma or chronic lung disease comorbidity (OR 2.59, 95% CI 1.10–6.10, p = 0.29) was associated with increased risk for mortality, and presenting COVID-19 symptoms of muscle pain or myalgias (OR 0.38, 95% CI 0.15–0.96, p = 0.040) and weakness or fatigue (OR 0.32, 95% CI 0.14–0.74, p = 0.0074) were associated with reduced risk of mortality.
Table 5.
Risk Factors Associated With 90-Day Mortality
Discussion
In this retrospective cohort study from 3 hospitals in the Chicagoland area, we found that among patients hospitalized for COVID-19 infection, those with preexisting neurodegenerative disease had similar mortality and ICU admission rates compared with age-matched CL, and they had lower rates of intubation or mechanical ventilation. Of note, our ND cohort comprised 89% dementia (combining patients with unspecified dementia, Alzheimer disease, vascular dementia, and frontotemporal dementia), 15% PD or parkinsonism, and 2% multiple sclerosis.
The mortality rate of 19.7% among hospitalized patients with ND with COVID-19 infection is comparable to reports in the literature. Among patients with dementia in the literature, mortality from COVID-19 varies from 8% to 62%,10,12,18-22 with differences in study design, geography, and demographics likely accounting for the wide range of outcomes. In studies that specifically assessed hospitalized patients with COVID-19 with dementia, there was 62% mortality rate in a cohort from Northern Italy,10 although their cohort was older compared with ours (mean age 82.6 years) and diagnosis of dementia was based on more rigorous criteria (combination of history and Clinical Dementia Rating Scale), so their subjects may have had more severe cognitive dysfunction. In hospitalized patients with Alzheimer disease (AD) in the Wuhan region of China, mortality from COVID-19 was 11%, lower than our study; and there was no difference when compared with non-AD age-matched CL, which is similar to our findings. In a large study using UK Biobank data, over 65,000 patients with age ≥65 years hospitalized with COVID-19 were assessed, of which 28% died, and dementia was an independent risk factor for mortality (OR 7.3, 95% CI 3.28–16.21) in models adjusted for comorbidities, age, sex ethnicity, and education, which differed from our study in which dementia was not found to be a risk factor for mortality. This difference may be due to the sample size, with our study possibly being underpowered, and the heterogeneity of our ND cohort may have also contributed. In PD, mortality from COVID-19 is reported between 5.7% and 44%,4-8 with dementia and longer PD duration associated with increased risk of mortality6 and atypical parkinsonism associated with increased risk of hospitalization.8 Multiple sclerosis has lower COVID-19 mortality reported in the literature of 2–8%,23-25 and this condition comprised a minority of the patients with ND in our study.
We found that patients with ND were more likely to present for COVID-19 admission with confusion or altered mental status and less likely to report respiratory symptoms such as cough or shortness of breath. Similarly, studies have reported hypoactive delirium as the most frequent presenting symptom among patients with dementia hospitalized with COVID-19,10 and hospitalized patients with dementia who died of COVID-19 presented with cough less frequently.26 However, our finding differs from a report of similar clinical presentations—with regard to fever and respiratory symptoms—between AD and non-AD age-matched CL hospitalized with COVID-19, although they did not specifically assess for altered mental status symptom.20 We found that presentation with altered mental status or confusion was an independent risk factor for mortality in the ND cohort (OR 3.61, 95% CI 1.27–10.29, p = 0.016). A similar finding is reported in COVID-19–positive patients in a dementia facility in Northern Italy (delirium associated with death, OR 17.0, 95% CI 2.8–102.7, p = 0.002)27 and older adults presenting to US emergency departments for COVID-19 with delirium at presentation (delirium associated with death, adjusted RR 1.24, 95% CI 1.0–1.55).28
We expected that neuroleptic use would be higher in the ND cohort given their high rate of encephalopathy (62%) during hospitalization, and although overall neuroleptic prescription was higher in patients with ND compared with CL, the difference was not significant when we removed patients who were on neuroleptics before hospitalization. This may be because COVID-19 is associated with hypoactive delirium more than hyperactive or agitated delirium10,27,28; thus, it may be less likely to require sedating medication.
In our cohort, patients with ND had a similar ICU admission rate compared with CL; however, they had lower rates of intubation and mechanical ventilation. This may be due to difference in admission code status. It may also be related to difference in respiratory involvement of COVID-19 infection, with CL being more likely to present with respiratory symptoms (i.e., cough and shortness of breath) and having higher rates of supplementary oxygen use compared with ND. This differed from a study that cited similar rates of oxygen therapy and invasive ventilation between AD and non-AD age-matched CL who were hospitalized with COVID-19;20 and another study reported a lower rate of ICU admission among patients with dementia who died of COVID-19 compared with age-matched CL.26 These conflicting reports suggest that the decision to pursue advanced care for COVID-19 in patients with neurodegenerative disorders is complex, and many factors may contribute including code status, presence of respiratory symptoms or hypoxia, and palliative and goals of care discussions between providers and patients or health care proxies.29
In addition to presentation with altered mental status or confusion, White race was also found to be an independent risk factor for mortality in our ND cohort. This was surprising given that large epidemiologic studies have described higher COVID-19 burden among racial and ethnic minorities, although reports vary and some studies suggest that mortality from COVID-19 among hospitalized patients may not differ between White and Black patients, in particular.30,31 In addition, it is possible that White patients in our ND cohort had more advanced neurocognitive disorder; studies in dementia and PD cohorts suggest that increased severity of cognitive impairment6,10 and longer disease duration6 were associated with COVID-19 mortality risk; and we did not account for these factors in our study. In contrast to other studies, we did not find that baseline NH residence,21 sex,27 or age10 was associated with mortality in our ND cohort, which may have been due to our study being underpowered and having an older cohort to begin with.
Patients with ND were more likely to be discharged to SNF/NH, whereas CL were more likely to be discharged to home or acute rehabilitation facility. This suggests that patients with ND may have increased disability after COVID-19 infection, thus requiring increased level of care after hospital discharge. More studies are needed to characterize long-term recovery and rehabilitation process of patients with ND after COVID-19 infection.
Strengths of our study include large sample size, comparison of the ND group with age-matched CL (to reduce confounding potential of age), inclusion of data from 3 different hospitals in the Chicagoland area serving both urban and suburban areas, and racially and ethnically diverse sample. One limitation is the process used to identify patients with ND—using ICD-10 codes and verification by chart review—which is not as rigorous as other methods of disease classification, and did not allow assessment of neurodegenerative disease duration or severity. In addition, our ND cohort was heterogeneous and included numerous diseases, which may have led to reduced effect size. Although multiple comparisons were made between the 2 groups (ND vs CL), we tried to protect against type 1 error by applying Holm-Bonferroni correction to domains in which multiple comparisons were made and null hypothesis was rejected (i.e., presenting COVID-19 symptom, Table 3; discharge disposition, Table 4). Last, because this was a chart review retrospective study, our data were based on information available in the electronic medical records that may have been incomplete.
Our study describes COVID-19 outcomes in hospitalized patients with neurodegenerative disorders in the Chicagoland area. Acknowledging geographic and demographic factors that can account for variations in outcomes, we think that results of our study are generalizable. In particular, health care providers should be aware that patients with neurodegenerative disorders may be more likely to have atypical presentation of COVID-19 infection with mental status changes, as opposed to respiratory symptoms. In addition, although patients with neurodegenerative disorders had a similar short-term mortality rate compared with CL, they had higher rates of encephalopathy and NH or hospice discharge, suggesting that they may have more disability and require longer time recovery. Future studies should investigate long-term outcomes of patients with neurodegenerative disorders who survive COVID-19 infection and targeted intervention strategies to protect this vulnerable population.
TAKE-HOME POINTS
→ Patients with neurodegenerative disorders hospitalized for COVID-19 had similar 90-day mortality and ICU admission rates compared with age-matched controls, and they were intubated less frequently.
→ Altered mental status or confusion was a common presenting symptom for patients with neurodegenerative disorders hospitalized with COVID-19, and this presentation was an independent risk factor for mortality.
→ Patients with neurodegenerative disorders hospitalized with COVID-19 developed encephalopathy at higher rates than controls, and they were more likely to be discharged to nursing home or hospice.
Appendix. Authors
Footnotes
Contributor Information
Glenn T. Stebbins, Email: glenn_stebbins@rush.edu.
Ekta B. Kishen, Email: ekta_b_kishen@rush.edu.
Brandon Barton, Email: brandon_r_barton@rush.edu.
Study Funding
No targeted funding reported.
Disclosure
The authors have no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.COVID-19 Map–Johns Hopkins Coronavirus Resource Center. Accessed December 10, 2020. coronavirus.jhu.edu/map.htm.
- 2.Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID-19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology. 2020;95(2):77-84. [DOI] [PubMed] [Google Scholar]
- 4.Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson's Disease patients affected by COVID‐19. Mov Disord. 2020;35(6):905-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Prete E, Francesconi A, Palermo G, et al. Prevalence and impact of COVID-19 in Parkinson's disease: evidence from a multi-center survey in Tuscany region. J Neurol. 2021;268(4):1179-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A, Elia AE, Dallocchio C, et al. Predictors of COVID-19 outcome in Parkinson's disease. Parkinsonism Relat Disord. 2020;78:134-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasano A, Cereda E, Barichella M, et al. COVID-19 in Parkinson's disease patients living in Lombardy, Italy. Mov Disord. 2020;35(7):1089-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vignatelli L, Zenesini C, Belotti LMB, et al. Risk of hospitalization and death for COVID‐19 in people with Parkinson's disease or parkinsonism. Mov Disord. 2021;36(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchetti A, Rozzini R, Guerini F, et al. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24(6):560-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esme M, Koca M, Dikmeer A, et al. Older adults with coronavirus disease 2019: a nationwide study in Turkey. Journals Gerontol Ser A. 2021;76(3):e68-e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyashita S, Yamada T, Mikami T, Miyashita H, Chopra N, Rizk D. Impact of dementia on clinical outcomes in elderly patients with coronavirus 2019 (COVID-19): an experience in New York. Geriatr Gerontol Int. 2020;20(7):732-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gerwen M, Alsen M, Little C, et al. Risk factors and outcomes of COVID-19 in New York City; a retrospective cohort study. J Med Virol. 2021;93(2):907-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialek S, Bowen V, Chow N, et al. Geographic differences in COVID-19 cases, deaths, and incidence: United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortensen LQ, Andresen K, Burcharth J, Pommergaard H-C, Rosenberg J. Matching cases and controls using SAS software. Front Big Data. 2019;2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Income in Past 12 Months (in 2018 Inflation-Adjusted Dollars). US Census Bureau. data.census.gov/cedsci/table?q=median income 2018&tid=ACSST1Y2018.S1901&hidePreview=true (accessed 2 November 2020). [Google Scholar]
- 17.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Bueno JA, Mena-Vázquez N, Ojea-Ortega T, et al. Case fatality of COVID-19 in patients with neurodegenerative dementia. Neurologia. 2020;35(9):639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caratozzolo S, Zucchelli A, Turla M, et al. The impact of COVID-19 on health status of home-dwelling elderly patients with dementia in East Lombardy, Italy: results from COVIDEM network. Aging Clin Exp Res. 2020;32(10):2133-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Long X, Huang H, et al. Resilience of Alzheimer's disease to COVID-19. J Alzheimers Dis. 2020;77(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matias-Guiu JA, Pytel V, Matias-Guiu J. Death rate due to COVID-19 in Alzheimer's disease and frontotemporal dementia. J Alzheimers Dis. 2020;78(2):537-541. [DOI] [PubMed] [Google Scholar]
- 22.Giorgi Rossi P, Marino M, Formisano D, Venturelli F, Vicentini M, Grilli R. Characteristics and outcomes of a cohort of COVID-19 patients in the province of Reggio Emilia, Italy. Forloni G, ed. PLoS One. 15(8);2020:e0238281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrotta E, Kister I, Charvet L, et al. COVID-19 outcomes in MS: observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sormani MP. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canevelli M, Palmieri L, Raparelli V, et al. Prevalence and clinical correlates of dementia among COVID‐19‐related deaths in Italy. Alzheimers Dement (Amst). 2020;12(1):e12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poloni TE, Carlos AF, Cairati M, et al. Prevalence and prognostic value of Delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. EClinicalMedicine. 2020;26:100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy M, Helfand BKI, Gou RY, et al. Delirium in older patients with COVID-19 presenting to the Emergency Department. JAMA Netw Open. 2020;3(11):e2029540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domenico BG, Claudia G, Monika O, Ralf J. CoviD-19: decision making and palliative care. Swiss Med Wkly. 2020;150(13-14):w20233. [DOI] [PubMed] [Google Scholar]
- 30.Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA Netw Open. 2020;3(8):e2018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PLoS Med. 2020;17(9):e1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data, including raw data and analyzed data, are available from the corresponding author on reasonable request.