Abstract
Background
More deprived populations typically experience higher cancer incidence rates and smoking prevalence compared to less deprived populations. We calculated the proportion of cancer cases attributable to smoking by socio-economic deprivation in England and estimated the impact smoking has on the deprivation gap for cancer incidence.
Methods
Data for cancer incidence (2013–2017), smoking prevalence (2003–2007) and population estimates (2013–2017) were split by sex, age-group and deprivation quintile. Relative risk estimates from meta-analyses were used to estimate the population attributable fraction (PAF) for 15 cancer types associated with smoking. The deprivation gap was calculated using age-specific incidence rates by deprivation quintile.
Results
Smoking-related cancer PAFs in England are 2.2 times larger in the most deprived quintile compared to the least deprived quintile (from 9.7% to 21.1%). If everyone had the same smoking prevalence as the least deprived quintile, 20% of the deprivation gap in cancer incidence could have been prevented. If nobody smoked, 61% of the deprivation gap could have been prevented.
Conclusions
The majority of the deprivation gap in cancer incidence could have been prevented in England between 2013–2017 if nobody had smoked. Policy makers should ensure that tobacco control policies reduce overall smoking prevalence by tackling smoking inequalities.
Background
Smoking is the main cause of preventable cancer and death in the UK [1, 2]. In England, smoking accounted for 15% (around 44,000 cases) of all cancer cases in 2015 [1]. Smoking causes at least 15 different types of cancer, and the proportion of cases caused by smoking varies greatly by cancer type, ranging from 0.3% for ovarian cancer to 72% for lung cancer in England.
Cancer incidence varies by socio-economic position across the UK [3–6]. For example, cancer incidence rates for all cancers combined in England are 17% higher in the most deprived quintile compared to the least [7].
The majority of cancer types’ incidence rates are positively associated with deprivation in England, leading to an estimated 27,200 deprivation-associated cancer cases each year [3]. Many of the cancer types associated with deprivation are also associated with smoking [3, 8].
A clear socio-economic divide is observed for adult smoking prevalence in the UK [9]. Of the general population in England, smoking prevalence is around 2.5 times higher in the lowest income group compared to the highest [9, 10]. In line with this, previous studies in France and Australia have reported that more deprived populations had a higher burden of cancer incidence attributable to smoking [11, 12]. These studies also investigated the impact of the removal of smoking inequalities, which estimated that 7–13% of all cancers caused by smoking in men and 8–9% in women, could be prevented if everyone smoked like the least deprived quintile.
We aimed to estimate the proportion of cancer cases attributable to smoking by socio-economic position in England. Additionally, we estimated what proportion of the observed deprivation gap in cancer incidence in England could have been prevented if: 1) everyone had the same smoking prevalence as the least deprived group; 2) nobody smoked.
Methods
Cancer types
To calculate the proportion of cancer cases attributable to smoking we included 15 cancer types which have ‘sufficient’ evidence of a causal association with smoking based on the International Agency for Research on Cancer (IARC) Monograph [8]: oral cavity, pharynx, nasopharynx (nasal cavity and paranasal sinus), larynx, oesophagus, stomach, colorectal, liver (including bile duct), pancreas, lung, cervix uteri, kidney (including renal pelvic and ureter), bladder, ovarian (mucinous) and acute myeloid leukaemia (see S1 Table in S1 File for International Classification of Diseases version 10 codes). These cancers contribute to 44% (around 134,300 cases) of the total cancer incidence in England every year (2013–2017). We will refer to these cancer types as ‘smoking-related cancers’.
Only cancer types positively associated with deprivation—defined as having significantly higher age-standardised incidence rates in the most deprived quintile compared to the least deprived—between 2013 and 2017 in England were included for calculation of the observed deprivation gap in cancer incidence [3]: head and neck (oral cavity, salivary glands, pharynx, nasopharynx, larynx, nasal cavity and middle ear, accessory sinuses), oesophagus, stomach, colorectal, liver, pancreas, lung, cervix uteri, kidney, bladder, small intestine, anal, gallbladder, vulva, vagina, uterus, penis, Hodgkin lymphoma and cancer of unknown primary (S1 Table in S1 File for ICD-10 codes). These cancers contribute to 50% (around 154,000 cases) of the total cancer incidence in England every year (2013–2017). We will refer to these cancer types as ‘deprivation-related cancer types’.
Data sources
Cancer incidence for England between 2013 and 2017 was provided by Public Health England and population estimates between 2013 and 2017 were provided by the Office for National Statistics. Each data set was split by sex, 5-year age band and quintiles of the Income domain from the Index of Multiple Deprivation 2015 (IMD 2015). The Income domain of the IMD is a relative, objective, local-level measure of deprivation based on the proportion of the population in that area estimated to experience deprivation because of low income which includes both out-of-work and employed adults on low earnings. The indicators that comprise the income domain relate to the proportion of adults and children in receipt of certain means-tested benefits [13]. It was not possible to use individual-level self-reported income as this information is not available in the cancer data. Nor was it possible to split the smoking and cancer data by income plus other domains of inequality such as ethnic group, age, or sex, as this would have impacted reliability through reduced sample size. The cancer data was additionally split by ICD-10 3-digit code, or International Classification of Diseases for Oncology version 3 (ICD-O-3) code (e.g. mucinous ovarian, oesophageal adenocarcinoma and oesophageal squamous cell carcinoma).
Adult (16+ years) smoking prevalence between 2003–2007 and second-hand smoking prevalence was collated from Health Survey for England (HSE) datasets (2003–2007) and categorised by sex, 10-year age band and equivalised household income quintiles, accessed through the UK Data Service. A 10-year latency period between smoking exposure and subsequent cancer incidence was used in line with previous methodology [1, 14]. Smoking prevalence for 2004 had to be imputed using a simple linear model based on available years: 2003, 2005, 2006 and 2007. Question wording was reasonably consistent across survey years used (S2 Appendix in S1 File).
Relative risk (RR) estimates (S1 Table in S1 File) were obtained from meta-analyses through a literature search using previously defined search terms (see S3 Table in S1 File) [1]. The literature was reviewed between two researchers (NP and KB) to decide on the most appropriate RR estimate to use for each cancer type. An alternative collection of RRs for smoking-attributable disease has recently been published in the UK, however the estimates we collated are generally more conservative and are more closely aligned with how cancer types are grouped in this analysis [15].
Population attributable fraction formula
To calculate the proportion of cancer cases attributable to smoking by deprivation quintile, the standard population attributable fraction (PAF) formula was used [14]:
Where p1 is the proportion of ‘current cigarette smokers’ in England, p2 is the proportion of ‘ex-regular cigarette smokers’, ERR1 is the excess relative risk (relative risk– 1) for current smokers and ERR2 is the excess relative risk (relative risk– 1) for ex-smokers. Lung cancer is the only cancer type to be causally associated with second-hand smoke according to IARC classifications [8], therefore we implemented a specific adjustment to the calculation which included an extension of the formula above to account for second-hand smoke exposure prevalence (S4 Appendix in S1 File).
Smoking-attributable cases were calculated for each cancer type and then summed to obtain figures for all smoking-related cancer types combined. Overall PAF estimates used the smoking-attributable cancer cases as the numerator and all cancers combined excluding non-melanoma skin cancer (C00-C97 excl. C44) as the denominator, by sex and deprivation quintile. PAF estimates are presented for all ages combined (0–99+ years) and broken down by two broad age groups (24–64 years and 65+ years). Confidence intervals were not calculated, all comparisons are based on point estimates.
Observed deprivation gap in cancer incidence and smoking
To further investigate the contribution of smoking to cancer incidence by socio-economic position in England, we grouped smoking-related cancer types together to form a combined age-standardised incidence rate (ASR) by deprivation quintile (2013–2017). We then modelled ASR’s for two hypothetical smoking scenarios based on smoking-related cancer types where: scenario 1) everyone had the same smoking prevalence as the least deprived quintile; scenario 2) nobody smoked. Rates were age-standardised to the 2013 European Standard Population [16].
To calculate the proportion of avoidable cases under each smoking scenario, we used deprivation-associated cases for deprivation-related cancer types (representing the observed deprivation gap in cancer incidence) as the denominator, and the number of deprivation-associated cases in scenario 1 and scenario 2 as the numerator.
Deprivation-associated cases were calculated using age-specific incidence rates, as has been previously described [17]. Briefly, ‘expected’ cases were estimated by applying the age-specific incidence rate from the least deprived quintile to each population of the remaining 4 quintiles. The ‘expected’ cases were then subtracted from their corresponding observed cases to produce excess deprivation-associated cases. For the remainder of this article, ‘deprivation-associated cases’ will be used to refer to excess cases due to higher incidence rates in more deprived populations compared to the least deprived.
Confidence intervals were calculated for ASRs, but not for deprivation-associated case estimates. See S5 and S6 Appendices in S1 File for more detailed information on these calculations.
Sensitivity analysis
Due to lack of data, the measure of deprivation used for smoking prevalence (equivalised household income) and cancer incidence (income domain of IMD) was not a direct match. To assess the robustness of the main results to differences in deprivation measurement, PAFs were also calculated with smoking prevalence by ‘all domains’ IMD (7 domains: income, employment, health and disability, education, barriers to housing and services, crime and living environment) from HSE datasets (2003–2007), accessed through the UK Data Service.
Results
Population Attributable Fraction (PAF) of cancer related to smoking by deprivation quintile
A strong deprivation gradient was observed for the proportion of cancer cases attributable to smoking in England (Table 1). For all ages combined, the smoking PAF was 2.2 times larger in the most deprived quintile compared to the least deprived quintile. The smoking PAF increased from 9.7% in the least deprived quintile to 21.1% in the most deprived quintile. Similar relative increases in PAFs were observed for both sexes, but the PAFs were generally larger for males compared to females.
Table 1. Average number and proportion of smoking-attributable cancer cases per year by sex, age and deprivation quintile, England, 2013–2017.
| Deprivation quintile | 25–64 years | 65+ years | All ages (0–99+ years) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Observed cases | PAFa | Smoking attributable cases | Observed cases | PAFa | Smoking attributable cases | Observed cases | PAFa | Smoking attributable cases | |
| Females | |||||||||
| 1 (least) | 11,675 | 4.6% | 542 | 18,654 | 9.7% | 1,817 | 30,626 | 7.7% | 2,359 |
| 2 | 11,919 | 6.3% | 751 | 19,969 | 10.9% | 2,184 | 32,176 | 9.1% | 2,935 |
| 3 | 11,404 | 8.2% | 930 | 18,934 | 13.1% | 2,482 | 30,654 | 11.1% | 3,413 |
| 4 | 11,202 | 10.3% | 1,153 | 17,042 | 16.9% | 2,872 | 28,569 | 14.1% | 4,026 |
| 5 (most) | 10,990 | 14.3% | 1,566 | 15,009 | 19.9% | 2,991 | 26,398 | 17.3% | 4,558 |
| Males | |||||||||
| 1 (least) | 8,832 | 9.2% | 810 | 24,049 | 12.6% | 3,031 | 33,203 | 11.6% | 3,841 |
| 2 | 9,067 | 12.1% | 1,099 | 25,223 | 15.1% | 3,812 | 34,616 | 14.2% | 4,911 |
| 3 | 8,954 | 14.9% | 1,332 | 22,743 | 17.5% | 3,979 | 32,011 | 16.6% | 5,311 |
| 4 | 8,945 | 18.1% | 1,619 | 19,768 | 21.4% | 4,227 | 29,059 | 20.1% | 5,846 |
| 5 (most) | 9,228 | 23.4% | 2,161 | 17,198 | 26.3% | 4,529 | 26,829 | 24.9% | 6,690 |
| Persons | |||||||||
| 1 (least) | 20,507 | 6.6% | 1,352 | 42,703 | 11.4% | 4,848 | 63,828 | 9.7% | 6,200 |
| 2 | 20,986 | 8.8% | 1,850 | 45,192 | 13.3% | 5,996 | 66,792 | 11.7% | 7,846 |
| 3 | 20,358 | 11.1% | 2,262 | 41,677 | 15.5% | 6,461 | 62,665 | 13.9% | 8,724 |
| 4 | 20,147 | 13.8% | 2,772 | 36,810 | 19.3% | 7,099 | 57,628 | 17.1% | 9,871 |
| 5 (most) | 20,217 | 18.4% | 3,727 | 32,206 | 23.3% | 7,520 | 53,227 | 21.1% | 11,247 |
a PAF: Population attributable fraction out of all cancers (excl. non-melanoma skin cancer)
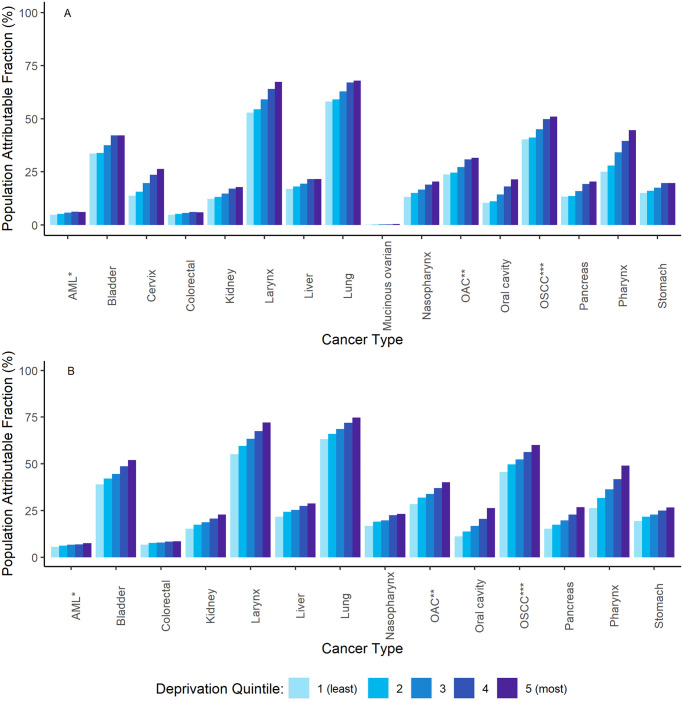
A similar deprivation gradient was found for each broad age group. However, the smoking PAFs were generally smaller in the younger age group compared to the older age group. There was variation in PAFs by cancer type, with both lung cancer and laryngeal cancer having the largest PAFs, as well as strong deprivation gradients (Fig 1A and 1B).
Fig 1. A (females) and B (males).
Population Attributable Fraction (PAF) for smoking, by cancer type and deprivation quintile, England, 2013–2017. *Acute myeloid leukaemia; **Oesophageal adenocarcinoma; ***Oesophageal squamous cell carcinoma.
Cancer incidence by deprivation quintile
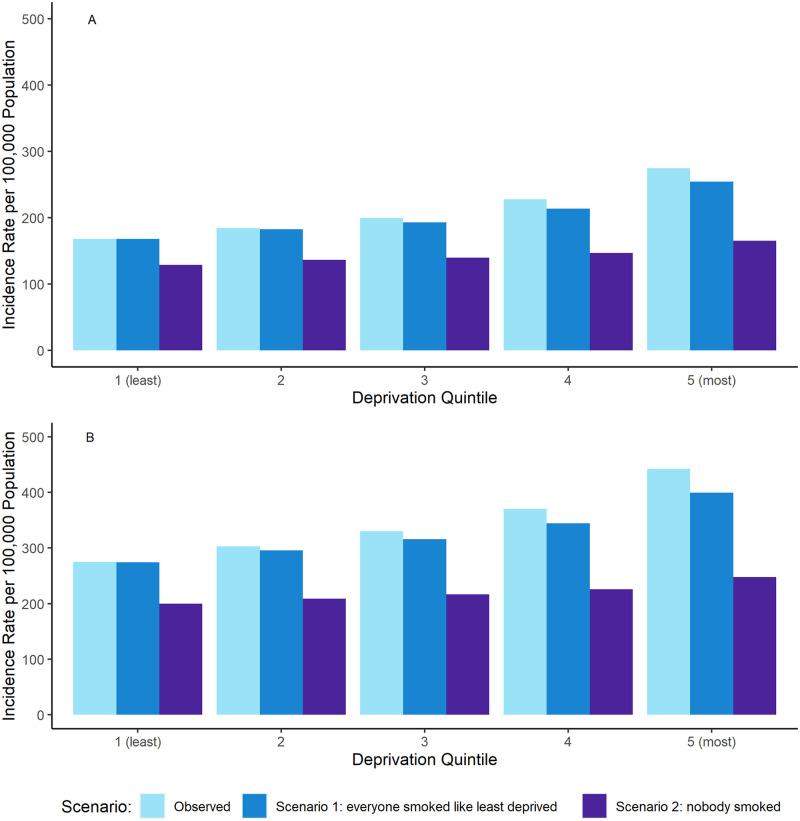
Age-standardised incidence rates by deprivation quintile and sex are displayed in Fig 2A and 2B. A clear deprivation gradient is observed for smoking-related cancer types for both sexes, with a 63% and a 60% relative increase in ASR between the least and most deprived quintiles for females and males, respectively.
Fig 2. A (females) and B (males).
Combined European Age-Standardised incidence rates (ASR) per 100,000 population for smoking-related cancer types* by deprivation quintile and sex, for observed cancer incidence (the current situation), scenario 1 and scenario 2, England, 2013–2017. *oral cavity, pharynx, nasopharynx, larynx, oesophagus, stomach, colorectal, liver, pancreas, lung, cervix uteri, kidney, bladder, ovarian (mucinous) and leukaemia (acute myeloid).
The deprivation gap for incidence rates between the least and most deprived quintiles is partly reduced in scenario 1 to a 51% and 45% relative increase in ASR between the least and most deprived quintile for females and males, respectively. For scenario 2 where nobody smoked, there is a marked reduction in both the cancer incidence rate and the deprivation gradient, which shows a 28% and 24% relative increase in ASR between the least and most deprived quintile for females and males, respectively.
Deprivation gap in cancer incidence and smoking
A summary of deprivation-associated cases and the proportion of the observed deprivation gap in cancer incidence that could have been prevented in scenarios 1 and 2 is presented in Table 2. For deprivation-related cancer types, it is estimated that there were an average of 27,156 cases (11,851 in females and 15,305 in males) associated with deprivation every year in England between 2013 and 2017.
Table 2. Estimated average number of deprivation-associated cases per year for deprivation-related cancer types* and smoking-related cancer types**, scenario 1 and scenario 2; and the estimated number of deprivation-associated cases and proportion of the observed deprivation gap in cancer incidence that could have been prevented, in England, in 2013–2017.
| 25–64 years | 65+ years | All ages (0–99+ years) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Persons | Female | Male | Persons | Female | Male | Persons | ||
| Deprivation-associated cases | Deprivation -related cancer types | 4,380 | 5,242 | 9,622 | 7,467 | 10,043 | 17,510 | 11,851 | 15,305 | 27,156 |
| Smoking-related cancer types | 3,562 | 4,782 | 8,344 | 6,413 | 9,248 | 15,661 | 10,009 | 14,057 | 24,066 | |
| Scenario 11 | 2,605 | 3,481 | 6,086 | 5,403 | 7,012 | 12,415 | 8,043 | 10,519 | 18,562 | |
| Scenario 22 | 1,154 | 1,474 | 2,628 | 2,281 | 2,552 | 4,833 | 3,470 | 4,052 | 7,522 | |
| Preventable deprivation-associated cases (Preventable proportion of the observed deprivation gap in cancer incidence) a | Scenario 11 | 957 (21.9%) | 1,301 (24.8%) | 2,258 (23.5%) | 1,010 (13.5%) | 2,236 (22.3%) | 3246 (18.5%) | 1,966 (16.6%) | 3,538 (23.1%) | 5,504 (20.3%) |
| Scenario 22 | 2,408 (55.0%) | 3,308 (63.1%) | 5,716 (59.4%) | 4.132 (55.3%) | 6,696 (66.7%) | 10,828 (61.8%) | 6,539 (55.2%) | 10,005 (65.4%) | 16,544 (60.9%) | |
1Scenario where everyone has the same smoking prevalence as the least deprived quintile;
2Scenario where nobody smoked
aCalculation: 957 = 3562–2605; 21.9% = 957/4380
*head and neck (oral cavity, salivary glands, pharynx, nasopharynx, larynx, nasal cavity and middle ear, accessory sinuses), oesophagus, stomach, colorectal, liver, pancreas, lung, cervix uteri, kidney, bladder, small intestine, anal, gallbladder, vulva, vagina, corpus uteri, penis, Hodgkin Lymphoma and cancer of unknown primary
**oral cavity, pharynx, nasopharynx, larynx, oesophagus, stomach, colorectal, liver, pancreas, lung, cervix uteri, kidney, bladder, ovarian (mucinous) and leukaemia (acute myeloid)
If everyone had the same smoking prevalence as the least deprived quintile 20.3% (5,504 cases every year) of deprivation-associated cases could have been prevented. If nobody smoked, 60.9% (16,544 cases every year) of deprivation-associated cases could have been prevented.
Sensitivity analysis
The PAFs estimated from smoking prevalence by IMD all domains were similar to the PAFs estimated from smoking prevalence by equivalised household income. For females, the PAFs increased from 7.9% in the least deprived quintile to 18.4% in the most deprived. For males, the PAFs increased from 12.1% in the least deprived to 24.3% in the most deprived (see S7 Table in S1 File).
Discussion
Interpretation of main findings
We observed a strong deprivation gradient for the proportion of cancer cases attributable to smoking in England, which reflects the clear and longstanding socio-economic inequality observed for smoking prevalence in England [10, 18].
Smoking is a key driver of socio-economic inequality in cancer incidence in England. If everyone had the same smoking prevalence as the least deprived quintile 20% (5,504 cases every year) of deprivation-associated cancer cases between 2013 and 2017 could have been prevented. If no one in England had smoked, 61% (16,544 cases every year) of deprivation-associated cases could have been prevented, indicating that smoking explained the majority of the observed deprivation gap in cancer incidence in England between 2013 and 2017.
Though the majority of the observed deprivation gap in cancer incidence can be explained by smoking for both sexes, other risk factors are probably contributing to the remainder of the gap. Obesity (body mass index [BMI] 30+) is positively associated with deprivation for adults in England [10], as well as being related to 8 cancer types that are also related to deprivation [3, 8]. Routine and manual workers may have higher risk of exposure to occupational risk factors (e.g. asbestos, silica, aromatic amines) that are related to cancers of the lung, head and neck and bladder [19–22]. Prevalence of the human papillomavirus (HPV) infection and helicobacter pylori infection are positively associated with deprivation in the UK, and are linked to numerous cancers that are more common in deprived areas [8, 23, 24].
Other research has addressed the hypothetical removal of socio-economic inequality in risk factor exposure on subsequent cancer incidence or mortality, however direct comparisons are precluded by methodological differences (e.g. measure of deprivation, RRs, outcome measures). A French study estimated that 43.4% and 27.5% of deprivation-associated cancer cases for smoking-related cancer types could have been prevented if everyone smoked like the least deprived, in females and males respectively [11]. In Australia it was estimated that 4% of all cancer cases could have been prevented if smoking, overweight and obesity and physical activity prevalence matched the least deprived across all deprivation quintiles [12]. Smoking accounted for the vast majority of these deprivation-associated cases. A UK team showed that 30% of lung and laryngeal cancer deaths in men, and 23% of those in women, could be prevented if everyone smoked like those with tertiary education [25].
Policy implications
The UK government’s prevention green paper recently set the aim of England becoming smoke free by 2030, defined as smoking prevalence below 5% [26]. Successful UK public health initiatives have contributed to overall smoking prevalence declining over time [27], but smoking inequalities have widened [9, 17]. The Marmot review of 2010 argued that action is needed across the social gradient ‘with a scale and intensity that is proportionate to the level of disadvantage’ [28]. To incorporate this, action is needed at both a national and local level.
Fiscal measures provide a national cost-effective approach to help target and reduce smoking prevalence, particularly for future generations, whilst also increasing government revenues [29]. And fiscal measures may also be effective for more deprived smokers where price is more of a potential barrier to consumption [30, 31]. A study modelling the impact of a 10% increase per annum in the price of cigarettes in England and Wales projected a 74% and 86% reduction in the socio-economic gap in lung cancer incidence by 2050, in females and males, respectively [32].
Local level support can aid smoking cessation for current smokers, particularly for those from the most deprived communities. Smokers from deprived backgrounds are subject to barriers (e.g. lack of social support, high nicotine dependence) that makes it difficult for them to quit [33–35]. Local Stop Smoking Services provide multi-faceted smoking cessation support within communities that can engage with smokers from deprived communities [32, 36]. However, these services are increasingly threatened due to central funding cuts, making it difficult for them provide support locally across the country.
A UK parliamentary group has set out a comprehensive set of recommendations that argue for targeted investment along with behavioural change campaigns to reduce inequalities in smoking-related ill-health. Among these suggestions, they argue for regional mass media campaigns in regions of the country where smoking rates are higher—and generally where the population is more deprived—as a cost-effective means to tackle smoking inequalities. But investment for such media campaigns has dropped by 90% in the last decade [37].
Sustained funding into smoking cessation initiatives would likely help tackle smoking inequalities and prove cost-effective, by reducing smoking-related ill-health that negatively impacts on the National Health Service and productivity [38, 39].
Strengths and limitations
We provide a unique quantification of the relationship between socio-economic deprivation, smoking and subsequent cancer incidence in England. Modelling like this may help inform and reinforce policy to prevent smoking-related cancer and improve health more generally in deprived populations. The analysis used high quality cancer incidence and smoking prevalence data, which was averaged over 5 years to reduce the risk of spurious results as a consequence of any year-on-year fluctuation.
This analysis is not without limitations. The same RRs for current and ex-smoking prevalence were applied across all deprivation quintiles. This may reduce the accuracy of the point estimates if the risk associated with those broad definitions varies by deprivation quintile. For example, more deprived smokers may smoke more heavily and start smoking younger [40, 41]. They are also more likely to have multiple cancer risk factors [42], including those which combine synergistically with smoking to raise cancer risk, such as alcohol [43, 44], obesity [45, 46] and occupational exposures [47, 48]. However, the net effect of this is likely to be underestimation of the deprivation gap in smoking PAFs.
We used significant difference in ASRs between the least and most deprived to distinguish a cancer type’s association with deprivation. This means some cancer types with a less strong association with deprivation could have been missed, resulting in a potential over estimation of the percentage of deprivation-associated cancer cases that could have been avoided. However, the analysis is based on five years of data to provide sufficient power to detect true differences in incidence by deprivation. In addition, the number of cancer cases with no significant association with deprivation either positive or negative constitutes a small proportion (~13%) of the England total cancer cases. Therefore, the margin of error around the absolute numbers and proportions presented here remains relatively low.
Restricting the cancer types included in the analysis to those with IARC-classified ‘sufficient’ evidence may have resulted in an underestimation in the total number of smoking-attributable cancer cases. However, currently only breast cancer is classified as having ‘limited evidence’ [8]. Including this cancer type would markedly complicate interpretation of results because it is inversely associated with deprivation due primarily to screening uptake and reproductive behaviour [49, 50].
These calculations can only be considered estimates because of the PAF methodology used, which is an indirect and relatively simple method that is subject to some uncertainty around point estimates. We used a 10-year latency period, in line with Parkin et al.’s methodology [14], and to correspond with the average follow-up period of the most recent relative risk sources. Choice of latency period is a contentious issue with no agreed solution. It may be inappropriate to use older relative risks with a longer follow-up to correspond with a longer latency period if smoking patterns and products have changed over time. But using a shorter latency period to match with more recent relative risks may under-represent the true lag time between smoking exposure and subsequent cancer incidence. Additionally, this analysis required smoking prevalence by deprivation quintile, and increasing the latency period would have led to issues around reliable data availability. A 10-year latency period also assumes people will remain in the same deprivation group from exposure through to recording of cancer incidence. The cancer incidence data uses highly granular area-level rather than individual-level deprivation, meaning these findings may be subject to ecological fallacy.
Conclusion
Smoking is an important driver of cancer incidence inequalities in England. Efforts to reduce smoking prevalence should focus on minimising smoking inequalities. Future research should assess the projected impact of no intervention on smoking prevalence on the deprivation gap in cancer incidence compared to varying levels of smoking cessation interventions. More research is required to better understand and overcome the complex barriers that smokers from deprived populations face in order to enhance smoking cessation interventions.
Supporting information
(DOCX)
Acknowledgments
We acknowledge the work of the England cancer registry, as without their work there would be no incidence data. This work uses data provided by patients and collected by the NHS as part of their care and support. We also acknowledge the work of NHS Digital of the Health Survey for England team at the Health and Social Survey Research group at the Department of Epidemiology at University College London with NatCen Social Research for their smoking prevalence data. Their data provides detailed and reliable breakdowns across a range of different metrics and over many years, that helped enable this research to take place.
Data Availability
The cancer incidence data analysed during the current study are available from the National Cancer Registration and Analysis Service (part of Public Health England), on request through the Office for Data Release but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Population estimates are available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Smoking prevalence data was taken from the Health Survey for England, accessed through the UK Data Archive: https://www.data-archive.ac.uk/.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Brown KF., Rumgay H., Dunlop C, Ryan M, Quartly F, Cox A, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland and the United Kingdom in 2015. Br J Cancer. 2018; 118: 1130–1141. doi: 10.1038/s41416-018-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Health Data Exchange. Global Burden of Disease (GBD) Results Tool. https://ghdx.healthdata.org/gbd-2019
- 3.Cancer Research UK (2020). Incidence of common cancers by deprivation. https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Five.
- 4.Information Services Division Scotland. Cancer Statistics. https://www.isdscotland.org/Health-Topics/Cancer/Cancer-Statistics/All-Types-of-Cancer/.
- 5.Welsh Cancer Intelligence and Surveillance Unit. Cancer incidence in Wales 2002–2018. https://phw.nhs.wales/services-and-teams/welsh-cancer-intelligence-and-surveillance-unit-wcisu/cancer-incidence-in-wales-2002-2018/.
- 6.Northern Ireland Cancer Registry. Cancer information. https://www.qub.ac.uk/research-centres/nicr/CancerInformation/official-statistics/
- 7.Cancer Research UK. Cancer in the UK 2020: Socio-economic deprivation. https://www.cancerresearchuk.org/sites/default/files/cancer_inequalities_in_the_uk.pdf
- 8.International Agency for Research on Cancer (2021). Agents classified by the IARC Monographs, Volumes 1–129. https://monographs.iarc.who.int/agents-classified-by-the-iarc/
- 9.Office for National Statistics. Adult smoking habits in the UK: 2019. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare
- 10.NHS Digital. Health Survey for England 2019. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england
- 11.Menvielle G, Kulhánová I, Bryere J, Launoy G, Eilstein D, Delpierre C, et al. Tobacco-attributable burden of cancer according to socioeconomic position in France. Int J Cancer. 2018; 143(3): 478–485. doi: 10.1002/ijc.31328 [DOI] [PubMed] [Google Scholar]
- 12.Wilson LF, Green AC, Jordan SJ, Neale RE, Webb PM, Whiteman DC. The proportion of cancers attributable to social deprivation: A population-based analysis of Australian health data. Cancer Epi. 2020; 67: 101742. doi: 10.1016/j.canep.2020.101742 [DOI] [PubMed] [Google Scholar]
- 13.Ministry of Housing, Communities & local Government. Indices of Deprivation 2019: income and employment domains combined for England and Wales—guidance note. https://www.gov.uk/government/statistics/indices-of-deprivation
- 14.Parkin DM. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011; 105(2), S77–S81. doi: 10.1038/bjc.2011.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health England. Response to consultation on proposed changes to the calculation of smoking attributable mortality and hospital admissions. https://assets.publishing.service.gov.uk/government/Consultation_response_on_proposed_changes_to_smoking_relative_risks.pdf
- 16.Eurostat. Revision of the European Standard Population. https://ec.europa.eu/eurostat/documents
- 17.Cancer Research UK and National Cancer Intelligence Network. Cancer by deprivation in England: Incidence, 1996–2010, Mortality, 1997–2011. 2014. London: NCIN. http://www.ncin.org.uk/about_ncin/cancer_by_deprivation_in_england
- 18.Cancer Intelligence Team, Cancer Research UK (2019). Smoking prevalence trends by occupation group in Health Survey for England. https://www.cancerresearchuk.org/sites/default/files/smoking_prevalence_trends_occupation_final_2020.pdf
- 19.McCormack V, Peto J, Byrnes G, Straif K, Boffetta P. Estimate the asbestos-related lung cancer burden from mesothelioma mortality. Br J Cancer. 2012; 106(3): 575–84. doi: 10.1038/bjc.2011.563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poinen-Rughooputh S, Rughooputh M, Guo Y, Rong Y, Chen W. Occupational exposure to silica dust and risk of lung cancer: an updated meta-analysis of epidemiological studies. BMC Public Health. 2016; 16(1): 1–17. doi: 10.1186/s12889-016-3791-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown T, Darnton A, Fortunato L, Rushton L. Occupational cancer in Britain. Respiratory cancer sites: larynx, lung and mesothelioma. Br J Cancer. 2012; 107: S56–70. doi: 10.1038/bjc.2012.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown T, Slack R, Rushton L. British Occupational Cancer Burden Study Group. Occupational cancer in Britain. Urinary tract cancers: bladder and kidney. Br J Cancer. 2012; 19: S76–84. doi: 10.1038/bjc.2012.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanton C, Soldan K, Beddows S, Mercer CH, Waller J, Field N, et al. High-risk human papillomavirus (HPV) infection and cervical cancer prevention in Britain: Evidence of differential uptake of interventions from a probability survey. Cancer Epi & Prev Bio. 2015; 24(5): 842–53. doi: 10.1158/1055-9965.EPI-14-1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalifa MM, Sharaf RR, Aziz RK. Helicobacter pylori: a poor man’s gut pathogen? Gut Path. 2012; 2(1): 1–2. doi: 10.1186/1757-4749-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulik MC, Hoffmann R, Judge K, Looman C, Menvielle G, Kulhanova I, et al. Smoking and the potential for reduction of inequalities in mortality in Europe. Eur J Epi. 2013; 28(12): 959–71. doi: 10.1007/s10654-013-9860-5 [DOI] [PubMed] [Google Scholar]
- 26.Department of Health and Social Care (2019). Advancing our health: prevention in the 2020s –consultation document. https://www.gov.uk/government/consultations/advancing-our-health-prevention-in-the-2020s
- 27.Office for National Statistics. Adult smoking habits in Great Britain. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/drugusealcoholandsmoking/datasets/adultsmokinghabitsingreatbritain
- 28.Marmot M, Goldblatt P, Allen J. et al. Fair society, healthy lives. Pub Health. 2012; 126: S4–10. doi: 10.1016/j.puhe.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 29.Chaloupka FJ, Straif K, Leon ME. Effectiveness of tax and price policies in tobacco control. Tob Control. 2011; 20(3): 235–238. doi: 10.1136/tc.2010.039982 [DOI] [PubMed] [Google Scholar]
- 30.Townsend JL, Roderick P, Cooper J. Cigarette smoking by socioeconomic group, sex, and age: effects of price, income, and health publicity. Br Med J. 1994; 309: 923–926. doi: 10.1136/bmj.309.6959.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrelly MC, Bray JW, Pechacek T, Woollery T. The response by adults to increases in cigarette prices by sociodemographic characteristics. S Econ J. 2001; 68(1): 156–165. doi: 10.2307/1061518 [DOI] [Google Scholar]
- 32.Soerjomataram I, Barendregt JJ, Gartner C, Kunst A, Moller H. Reducing inequalities in lung cancer incidence through smoking policies. Lung Cancer. 2011; 73(3): 286–273. doi: 10.1016/j.lungcan.2011.01.009 [DOI] [PubMed] [Google Scholar]
- 33.Brose LS, McEwen A. Neighbourhood deprivation and outcomes of stop smoking support—an observational study. PLoS One. 2016; 11(1): e0148194. doi: 10.1371/journal.pone.0148194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiscok R, Dobbie F, Bauld L. Smoking cessation and socioeconomic status: an update of existing evidence from a national evaluation of English Stop Smoking Services. BioMed Research International. 2015; (2015). doi: 10.1155/2015/274056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Centre for Smoking Cessation and Training (2013). Stop Smoking Services and Health Inequalities. https://www.ncsct.co.uk/usr/pub/NCSCT_briefing_effect_of_SSS_on_health_inequalities.pdf
- 36.Smith CE, Hill SE, Amos A. Impact of specialist and primary care stop smoking support on socio-economic inequalities in cessation in the United Kingdom: a systematic review and national equity analysis. Addiction. 2020; 115(1): 34–46, doi: 10.1111/add.14760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.All Party Parliamentary Group on Smoking and Health. Delivering a Smokefree 2030: The All Party Parliamentary Group on smoking and health recommendations for the tobacco Control Plan 2021. https://ash.org.uk/wp-content/uploads/2021/06/APPGTCP2021.pdf
- 38.Allender S, Balakrishnan R, Scarborough P, Rayner M. The burden of smoking-related ill health in the UK. Tobacco Control. 2009; 18(4): 262–267. doi: 10.1136/tc.2008.026294 [DOI] [PubMed] [Google Scholar]
- 39.Baker CL, Flores NM, Zou KH, Bruno M, Harrison VJ. Benefits of quitting smoking on work productivity and activity impairment in the United States, the European Union and China. International journal of clinical practice. 2017; 71(1): e12900. doi: 10.1111/ijcp.12900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarvis MJ, Wardle J, Waller J, Owen L. Prevalence of hardcore smoking in England, and associated attitudes and beliefs: cross sectional study. BMJ. 2003; 326(7398): 1061. doi: 10.1136/bmj.326.7398.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belvin C, Britton J, Holmes J, Langley T. Parental smoking and child poverty in the UK: an analysis of national survey data. BMC Public Health. 2015; 15(1): 1–8. doi: 10.1186/s12889-015-1797-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.NHS digital. Health Survey for England 2017. Multiple risk factors. http://healthsurvey.hscic.gov.uk/media/78655/HSE17-MRF-rep.pdf.
- 43.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiology Prevention Biomarkers. 2009; 18(2): 541–550. doi: 10.1158/1055-9965.EPI-08-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of oesophageal squamous cell carcinoma: a meta-analysis. Official journal of the American College of Gastroenterology; 2014; 109(6): 822–827. doi: 10.1038/ajg.2014.71 [DOI] [PubMed] [Google Scholar]
- 45.Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok AS. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. Journal of Hepatology. 2005; 42(2): 218–224. doi: 10.1016/j.jhep.2004.10.005 [DOI] [PubMed] [Google Scholar]
- 46.Roos ET, Lallukka T, Lahelma E, Rahkonen O. Joint associations between smoking and obesity as determinants of premature mortality among midlife employees. The European Journal of Public Health. 2017; 27(1): 135–139. doi: 10.1093/eurpub/ckw111 [DOI] [PubMed] [Google Scholar]
- 47.Lee PN. Relation between exposure to asbestos and smoking jointly and the risk of lung cancer. Occupational and Environmental Medicine. 2001; 58(3): 145–153. doi: 10.1136/oem.58.3.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hertz-Picciotto I, Smith AH, Holtzman D, Lipsett M, Alexeeff G. Synergism between occupational arsenic exposure and smoking in the induction of lung cancer. Epidemiology. 1992; 3(1): 23–31. doi: 10.1097/00001648-199201000-00006 [DOI] [PubMed] [Google Scholar]
- 49.Lundqvist A, Andersson E, Ahlberg I, Nilbert M, Gerdtham U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—a systematic review and meta-analysis. Eur J Public Health. 2016; 26(5): 804–813. doi: 10.1093/eurpub/ckw070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith D, Thomson K, Bambra C, Todd A. The Breast Cancer Paradox: A Systematic Review of the Association Between Area-Level Deprivation and Breast Cancer Screening Uptake in Europe. Cancer Epidemiology. 2019; 60: 77–85. doi: 10.1016/j.canep.2019.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The cancer incidence data analysed during the current study are available from the National Cancer Registration and Analysis Service (part of Public Health England), on request through the Office for Data Release but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Population estimates are available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates. Smoking prevalence data was taken from the Health Survey for England, accessed through the UK Data Archive: https://www.data-archive.ac.uk/.