Abstract
Background
As enhanced recovery programs (ERPs) have continued to evolve, the length of hospitalization (LOS) following elective minimally invasive colorectal surgery has continued to decline. Further refinements in multimodal perioperative pain management strategies have resulted in reduced opioid consumption. The interest in ambulatory colectomy has dramatically accelerated during the COVID-19 pandemic. Severe restrictions in hospital capacity and fear of COVID transmission forced surgical teams to rethink strategies to further reduce length of inpatient stay.
Methods
Members of the SAGES Colorectal Surgery Committee began reviewing the emergence of SDD protocols and early publications for SDD in 2019. The authors met at regular intervals during 2020–2022 period reviewing SDD protocols, safe patient selection criteria, surrogates for postoperative monitoring, and early outcomes.
Results
Early experience with SDD protocols for elective, minimally invasive colorectal surgery suggests that SDD is feasible and safe in well-selected patients and procedures. SDD protocols are associated with reduced opioid use and prescribing. Patient perception and experience with SDD is favourable. For early adopters, SDD has been the natural evolution of well-developed ERPs. Like all ERPs, SDD begins in the office setting, identifying the correct patient and procedure, aligning goals and objectives, and the perioperative education of the patient and their supporting significant others. A thorough discussion with the patient regarding expected activity levels, oral intake, and pain control post operatively lays the foundation for a successful application of SDD programs. These observations may not apply to all patient populations, institutions, practice types, or within the scope of an existing ERP. However, if the underlying principles of SDD can be incorporated into an existing institutional ERP, it may further reduce the incidence of post operative ileus, prolonged LOS, and improve the effectiveness of oral analgesia for postoperative pain management and reduced opioid use and prescribing.
Conclusions
The SAGES Colorectal Surgery Committee has performed a comprehensive review of the early experience with SDD. This manuscript summarizes SDD early results and considerations for safe and stepwise implementation of SDD with a specific focus on ERP evolution, patient selection, remote monitoring, and other relevant considerations based on hospital settings and surgical practices.
Keywords: Same day colectomy, Ambulatory colectomy, Same day discharge, Enhanced recovery after surgery, Enhanced recovery program, Enhanced recovery protocols, Colectomy, Minimally invasive surgery, Ambulatory colectomy
Same day discharge: definitions, rationale, and perceptions
Same day discharge (SDD) is defined as a patient being discharged home the same calendar day as the date of the operation. For the purposes of this review, the operation refers to elective, minimally invasive (MIS), major abdominal colorectal surgery (CRS), with procedures including partial or subtotal colectomy, ileostomy and colostomy reversal. The pioneers first evaluating the safety and feasibility of SDD referred to their experience as “ambulatory colectomy” [1–3]. Not all SDD patients will meet discharge criteria the same date as surgery, just as not all patients in an ERP will avoid post operative ileus (POI). As SDD protocols continue to evolve, the goal of going home the same date as the operation, rather than postoperative day (POD) one or two, has become a reality.
The evolution of ERPs towards SDD following MIS CRS began in centers with highly functional and advanced multi-disciplinary ERPs [4]. As institutional ERPs continued to further evolve, they recognized that some otherwise healthy patients, who had undergone MIS CRS procedures with no adverse events, were well suited for early discharge and home recovery. Consideration for SDD, much like the transition from inpatient open appendectomy and cholecystectomy to outpatient laparoscopic procedures nearly three decades ago, has been primarily driven by patient interest. During the early phase of the COVID-19 pandemic, the push towards SDD was driven by both patients’ demand for early discharge to avoid inpatient COVID exposure, as well as hospital inpatient bed capacity shortages for elective surgery cases. Even as we emerge from the COVID-19 pandemic, there is an ongoing interest from hospital systems and providers to optimize bed capacity, and further explore ERPs that allow patients to recover at home.
Another consideration in favor of SDD is the need to standardize postoperative pain management and narcotic regimens in order to curb overuse and prescribing. The success of ERPs in reducing the incidence of POI and length of stay (LOS) is often compromised by ad hoc administration of opioids in response to pain scores during inpatient hospital stay. By implementing SDD protocols, narcotic administration can be more tightly controlled by a few health care providers supervising SDD and outpatient pain management.
However, there can be obstacles to SDD protocol development and implementation. These obstacles may include provider and hospital system inexperience with remote monitoring in the early postoperative recover period, the need for multi-disciplinary support and ongoing evolution of ERPs in several different patient care settings, language barriers for patients and educational materials, level of sufficient social support at home, and the impact of distance from the hospital. To further explore SDD implementation and these hurdles to SDD, a SAGES SDD MIS CRS questionnaire was sent out to members of the SAGES Colorectal Surgery Masters Program Collaboration Facebook Group in February 2021. Among more than 3700 members of this social media group, 263 responses were received. The majority (73%) reported practicing in a community setting. Most respondents (74%) reported additional training in one or more fellowships or specialty training: MIS/bariatric/GI surgery (44%), colorectal surgery (36%), surgical oncology (7%), acute care (2%) and trauma (2%). Seventy-two percent of respondents reported being in practice for more than 5 years, 76% performed 1–10 colorectal resections per month, and 77% of their colorectal resections were performed using MIS. Regarding implementation of ERPs, 92% of respondents endorsed having implemented ERPs for all elective MIS colon and rectal resections. Over half (58%) reported performing intra-corporeal anastomosis for right colectomies.
Only 21% reported that most of their elective MIS colon and rectal cases were discharged home on POD 1. Current utilization of SDD was rare, and only reported by 16 surgeons (6%). In this small group of SDD practicing surgeons, SDD was offered to 39% of their patient population, on average, although practices varied widely (1–99%). Exclusion criteria for SDD included patient factors (malnutrition, comorbidities, poor functional status, elderly, inflammatory bowel disease), operative factors (open surgery, operative difficulties, rectal surgery, stoma), and social factors (difficult access for postoperative monitoring, patients living alone, distance from hospital). Three obstacles achieved > 70% consensus agreement including concerns regarding feasibility of remote monitoring, patient preference for hospitalization postoperatively, and the risk of POI development and management. Notably, concerns regarding reimbursement were only reported by 26% of respondents.
All of these concerns are highly relevant. Regardless of the surgeons’ interest in SDD, addressing these issues and navigating around initial obstacles will help stimulate further improvements in ERPs and best practices. There are several key elements that need to be introduced, well established, and then mastered in an ERP prior to the implementation of SDD. These elements include, but are not limited to favorable patient and procedure selection, a well functioning and communicating multidisciplinary ERP team and program, and post-operative remote patient monitoring plan.
Transitioning from current ERP to same day discharge for elective MIS colorectal resections
When discussing the feasibility of SDD programs, one must consider how much progress has been made since the 1970s and 1980s when the average LOS following colorectal surgery was two to three weeks [5, 6]. In the intervening decades, owing to the advent of MIS techniques and ERPs, the LOS has steadily decreased to an average of 2–3 days. [7, 8] Beyond reduction in LOS, ERPs have been associated with up to 50% reduction in surgical complications, early return of gastrointestinal function, reduced deconditioning, earlier return to work and higher patient satisfaction [8]. In patients with colorectal cancer undergoing resection, ERPs have been associated with earlier initiation of adjuvant chemotherapy and higher 5-year overall survival [9, 10]. ERPs are also associated with similar or lower rates of readmission and lower hospital associated costs [11–13] (Table 1).
Table 1.
Benefits of ERPs in colorectal surgery
| Downsides of hospitalization following surgery | Benefits of implementation of basic ERPs |
|---|---|
| • Hospital acquired infections/exposure risks | • Earlier return of gi function |
| • Non-compliance of nursing staff and allied health care professionals with early and sustained ambulation and out of bed programs | • Reduced length of hospital stay |
| • Increased narcotic usage and prescribing | • Reduced deconditioning |
| • Earlier return to work | |
| • Earlier initiation of systemic chemotherapy for patients with colorectal cancer (which is associated with better cancer outcomes) | |
| • Higher patient satisfaction | |
| • Reduced health care utilization and costs | |
| • Multidisciplinary teamwork to align health care delivery in all phases of preparation, education, admission, surgery, anesthesia, multi-modal pain management, and recovery | |
| • Reduced re-admission rates | |
| • Improved perioperative patient education | |
| • Reduced variability in care | |
| • Surgical culture evolution |
Multidisciplinary collaboration and a quality-focused culture have consistently been demonstrated as key elements to ERP success [4]. Hospital LOS following elective surgery is a balancing act between many competing, yet interrelated factors such as the patient’s support system, hospital cost/capacity, early recognition of complications, and hospital readmission. On the other hand, there are many advantages to recovering from surgery in the comforts of one’s own home—improved sleep hygiene, emotional support from extending family and friends, immediate independence and access to pain management when needed, and earlier return to individual food and beverage preferences. The avoidance of hospital exposure to hospital-associated infections is another potential advantage of home recovery [14].
The French ambulatory colectomy experience
The initial experience and publications on SDD colectomy used the terminology “ambulatory colectomy” to describe outcomes of patients discharged home within 24–48 h after surgery. These pioneers in ambulatory colectomy from France should be commended for their forward thinking and dedication to the ongoing evolution of robust ERPs, collaborations amongst surgeons, anesthesiologists, and nurses in the implementation of the ambulatory ERP in a stepwise fashion. In 2015, Gignoux and colleagues first described five patients who underwent SDD after left colectomy. This group demonstrated that it was not only possible, but that patient satisfaction with this ERP was high. All patients tolerated a solid diet on the first post-operative day and no patient required readmission [1]. The multidisciplinary group built on this initial experience and demonstrated that 39 out of 40 patients who underwent left colectomy were able to be discharged the same calendar day. No patients required readmission and only two patients required a follow-up visit prior to their first scheduled visit on POD 10 [2].
In their subsequent series of 157 consecutive patients undergoing ambulatory colectomy between 2013 and 2016, 93% of patients were successfully discharged home the same calendar day. This study included right, transverse, and total colectomies, although the majority of patients (74%) underwent left-sided colectomies [3], most commonly for diverticular disease (62%). Failure to discharge on POD 0 was noted in 11 (7%) patients due to intraoperative difficulties (4), medical (4) and social reasons (3). Among the 146 patients discharged on POD 0, 21% required an unplanned visit, many prompted by elevated CRP levels collected by visiting nurses. Hospital readmission and re-operative rates were 6% and 4%, respectively. Of the 6 patients requiring re-operation, 3 of them were for anastomotic leak and required an operation on POD 2, 3, and 8, respectively. The overall morbidity in this study was 25%.
Preliminary experience with POD0-POD1 colectomy and ileostomy closure
A small pilot study in the United Kingdom (UK) investigated SDD in 15 patients undergoing loop ileostomy closure [15]. In this study, patients were contacted by telephone 24 and 72 h after discharge and were also given the telephone number of the surgical team for additional concerns. All patients were discharged on POD 0, and only 1 (6%) patient required readmission at 72 h for a urinary tract infection. Another patient required in-person assessment at 72 h for nausea, but did not require readmission. There was no other morbidity for the remaining patients.
Analysis of the ACS-NSQIP database reported that only 1.6% of 1905 cases of elective colectomy from 2012 to 2017 and 2.7% of the 24,393 cases of elective stoma closure from 2005 to 2016 were discharged within one day of surgery [16, 17]. Results from single-center series suggest that discharge within 24 h occurred in 22–35% of patients undergoing laparoscopic colorectal resection that were managed with an enhanced recovery pathway [18–20]. Readmission rates in these patients ranged from 4 to 9%. Younger patients, American Society of Anesthesiologists physical status I and II, and those undergoing right hemi-colectomy (especially with intra-corporeal anastomosis) were more likely to be discharged within 24 h.
A review of NSQIP data by Huettemann et al. evaluating the incidence of SDD for laparoscopic right colectomy between 2012 and 2017 reported that only 0.6% of patients were discharged on POD 0 (114 patients out of 19,798) [21]. They concluded that SDD appears to be safe and was not associated with increased readmission or reoperation rates, mortality, and overall complications when compared to patients discharged on POD 1–2. One significant limitation of the NSQIP administrative database is coding errors. The dataset may have included patients who underwent an extended appendectomy or partial cecectomy and were coded as a 44,205 rather than limiting it to patients undergoing an ileocecectomy or right hemi-colectomy.
How to successfully implement SDD following elective MIS CRS
A well-established, highly functional ERP includes multidisciplinary expertise and collaboration. Same Day Discharge protocols are built upon well-established ERPs that have demonstrated reduced opioid use, LOS, and complications. Early adopters of SDD report that their ERPs have undergone multiple iterative improvements since initial implementation, further demonstrating the ongoing multidisciplinary review of experiences and outcomes. The SAGES Colorectal Surgery Committee has identified three key areas that should be addressed prior to SDD implementation (Table 2).
Table 2.
The SAGES colorectal surgery committee has identified three key areas to help further evolve erps and establish prior to SDD implementation
| SDD protocol “KEY THREE” |
|---|
| (1) Assessing discharge readiness prior to return of gastrointestinal function |
| (a) I-FEED score |
| (b) Early mobilization and initiation of oral electrolyte beverages in the post operative recovery area |
| (2) Reducing postoperative pain improves efficacy of oral analgesia post-operatively |
| (a) Multi-modal, opioid sparing pain management |
| (b) Intra-operative use of opioid sparing drips such as dexmedetomidine hydrochloride, lidocaine, ketamine, propofol |
| (c) TAPP blocks, rectus sheath blocks |
| (d) Less painful specimen extraction incision locations (ex. Pfannenstiel) |
| (3) Established post-discharge remote monitoring plan |
| (a) Apps, telephone visits, video visits |
Assessing discharge readiness prior to return of gastrointestinal function
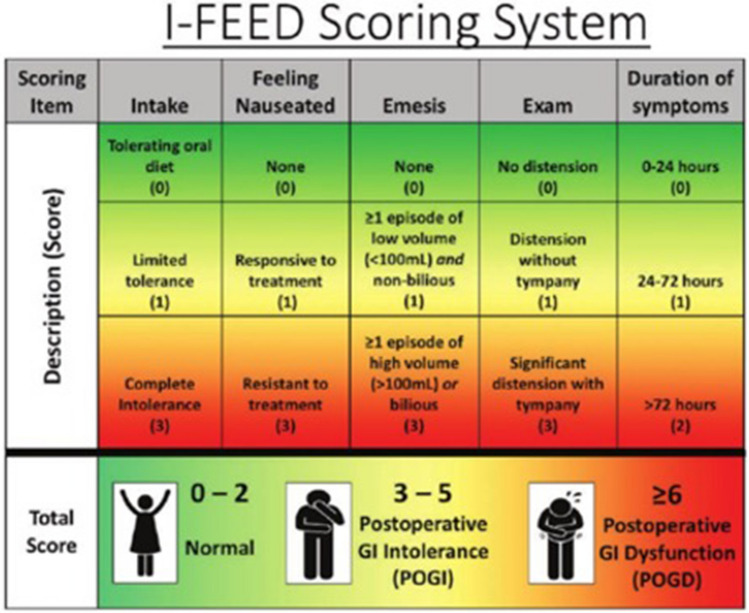
The I-FEED Scoring System is a metric for assessing and predicting sustained GI recovery [22] (Fig. 1). This new tool developed by the American Society for Enhanced Recovery and Perioperative Quality Initiative groups, as part of a joint consensus statement on assessing postoperative gastrointestinal function and dysfunction within an enhanced recovery pathway for elective colorectal surgery. The I-FEED scoring system provides a consistent and objective definition of postoperative GI function. The scoring system attributes 0–2 points for each of the five components based on clinical findings and oral tolerance. There are three categories: normal (0–2), post-operative GI intolerance (3–5), and postoperative GI dysfunction (≥ 6) [22].
Fig. 1.
“The I-FEED scoring system was created out of the need for a consistent objective definition of impaired postoperative GI function”. I intake, F feeling nauseated, E emesis, E exam, D duration of symptoms [22]
Most early colorectal ERPs require tolerance of oral intake AND recovery of lower gastrointestinal (GI) function prior to hospital discharge (flatus and/or bowel movement). Same day discharge patients will typically be discharged prior to experiencing flatus and/or bowel movement. This has been a major hurdle in the advancement of many ERPs. Frequently, a high proportion of patients remain hospitalized within their current ERPs only to confirm return of GI function (flatus and/or bowel movement) prior to hospital discharge [23]. However, the minority (15%) of patients experience delayed recovery of GI function [24, 25]. Pilot data from Lee and colleagues at McGill demonstrated that patients who tolerate a clear liquid diet on POD 0 were likely to experience an uncomplicated recovery of GI function (clear liquid diet was defined as at least 300 cc of an electrolyte clear liquid) [26].
Furthermore, Lee and colleagues investigated the trajectory of GI recovery after colorectal surgery using the I-FEED score, and have provided evidence supporting the validity of daily scoring for tracking the return of gastrointestinal function following colorectal surgery [27]. These observations support the utility of the I-FEED scoring system, in that the majority of patients with minimal initial GI symptoms (Score of 0–2) during the early post operative period will have uncomplicated GI recovery [27]. Other studies have reported similar findings [28]. These observations suggest that discharge prior to the passage of flatus and/or bowel movement is safe in otherwise clinically stable patients. In addition, these observations and associations demonstrate that patients at risk of delayed or complicated GI recovery can be identified early in the postoperative course.
Reducing postoperative pain improves efficacy of oral analgesia postoperatively
Another important discharge criteria is adequate pain control with only oral analgesia [29, 30]. This is usually defined as the ability to rest and mobilize without significant pain (sit up and walk, unless unable to do so preoperatively). The level of postoperative pain control is typically assessed by asking the patient to rate their pain level using a 0–10 numeric scale (0—no pain, 10—horrific pain). In the hospital setting, if the patient reports that their pain is controlled or rates their pain score 4 or lower, they are considered to have adequate pain control [30, 31].
Reducing postoperative pain results in improved efficacy of oral analgesia during recovery. This can be achieved by using several modifications to an existing ERP. First, an opioid-sparing approach is highly effective to further reduce the risk of POI and other postoperative complications [32]. This includes reliance on perioperative multimodal analgesia such as high dose acetaminophen (1000 mg TID), gabapentin TID, and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, intraoperative analgesia with opioid-sparing agents such as dexmedetomidine, lidocaine, ketamine, and propofol drips, have resulted in reduced postoperative pain and side effects related to high levels of intraoperative opioids and longer acting agents. Intraoperative use of routine transverse abdominus plane (TAP) and/or or rectus sheath blocks, has been shown to improve analgesia and further reduce opioid consumption [33]. In cases where there may not be access to ultrasound-guided TAP blocks, studies support that laparoscopic-guided TAP block performed by the surgeon is equivalent and may be a viable alternative [33].
Other intra-operative measures used to reduce postoperative pain and sustain adequate analgesia is to select a less painful site for specimen extraction incisions. This requires moving incisions away from the midline to an off-midline site, usually a muscle-splitting transverse or a Pfannenstiel incision. These off-midline incisions are associated with less pain and opioid consumption compared to midline incisions [34] and have lower rates of incisional hernia long term [35, 36]. However, in order to use a Pfannenstiel incision for specimen extraction, the surgeon may have to transition to intra-corporeal anastomosis creation for all ileocolic and colo-colonic anastomoses.
Post-discharge remote-monitoring options
An important concern of SDD implementation is that patients could potentially experience complications, both minor and life threatening, within the early postoperative period at home, whereas within a ‘standard ERP’ they may still be in hospital. The feasibility of remote monitoring was identified as one of the most common concerns for surgeons considering implementation of SDD in the SAGES Colorectal Surgery Masters Program Collaboration Facebook Group survey. The largest series to date on SDD laparoscopic colectomy included 157 patients with a 21% emergency room visit and 6% readmission rates from two institutions in France [3]. However, all patients had 1–2 daily home visits by a homecare nurse for the first 10 postoperative days, including blood tests on POD 1, 3, and 7. While encouraging, these results may not be widely applicable due to the impracticality of daily home visits. New technologies that enable remote follow-up such as mobile digital health (mHealth) technology [37] allow for a less resource-intensive approach to SDD colectomy by transmitting relevant medical information without relying on a physical visit. A digital remote follow-up through app-based encounters has been as effective as a face-to-face visit [37]. Additional remote monitoring options include daily telephone or video visits.
Preliminary results with SDD CRS: the US and Canadian experience
There has also been strong interest in evaluating which patient factors are associated with discharge on the same day of surgery. Askenasy reviewed his experience with SDD in 81 patients (Tables 3, 6). The following characteristics were associated with successful same day discharge following elective MIS CRS: operative duration < 180 min, estimated blood loss (EBL) < 50 mL, total intravenous fluid (IVF) < 1 L, and minimal or no narcotic use. Other factors that trended towards a higher rate of same day discharge, but did not meet statistical significance were absence of diabetes mellitus type II and male sex. Interestingly, BMI, ASA level, wound class, age, and type of surgery (right, left, anterior resection), were not significant predictors of successful same day discharge (Table 3).
Table 3.
Factors associated with discharge the same date as operation in the University of Texas Health SDD enhanced recovery program (unpublished data)
| Perioperative factors | % SDD | n (81) | p value |
|---|---|---|---|
| Patient factors | |||
| Male sex | 78.4 | 37 | 0.099 |
| Female sex | 61.4 | 44 | |
| Age 65 or younger | 75.0 | 44 | 0.213 |
| Above age 65 | 62.2 | 37 | |
| Previous abdominal surgery | 66.7 | 45 | 0.591 |
| No previous abdominal surgery | 72.2 | 36 | |
| On anticoagulation | 66.7 | 6 | 0.892 |
| No anticoagulation (excluding low dose aspirin) | 64.2 | 75 | |
| BMI < 25 | 64.3 | 14 | 0.846 |
| BMI > 25 | 65.5 | 29 | |
| BMI > 30 | 71.4 | 21 | |
| BMI > 35 | 76.5 | 17 | |
| Presence of DM | 45.5 | 11 | 0.067 |
| Absence of DM | 72.9 | 70 | |
| Case factors | |||
| Incision after 9 AM | 72.0 | 25 | 0.709 |
| Incision before 9 AM | 67.9 | 56 | |
| Finished before 12 PM | 80.5 | 41 | 0.081 |
| Finish after 12 PM | 58.3 | 24 | |
| Finished after 1 PM | 56.3 | 16 | |
| Blood loss < 50 mL | 77.1 | 61 | 0.007 |
| Blood loss > 50 mL | 45.0 | 20 | |
| Total IVF < 1 L | 86.2 | 29 | 0.013 |
| Total IVF > 1 L | 59.6 | 52 | |
| Operative duration < 180 min | 85.0 | 40 | 0.002 |
| Operative duration < 180 min | 53.7 | 41 | |
| ASA 2 | 74.2 | 31 | 0.515 |
| ASA 3 | 67.4 | 49 | |
| Wound class 2 | 70.4 | 71 | 0.755 |
| Wound class 3 | 50.0 | 2 | |
| Wound class 4 | 62.5 | 8 | |
| Right colectomy | 66.7 | 30 | 0.46 |
| Left/sigmoid/LAR colectomy | 74.5 | 47 | |
| Malignant indication | 66.7 | 36 | 0.667 |
| Benign indication | 71.1 | 45 | |
| Any narcotics (including operation) | 56.8 | 37 | 0.027 |
| No narcotics (including operation) | 79.6 | 44 | |
| Hydromorphone 0.6 mg or less | 77.2 | 57 | 0.016 |
| Greater than hydromorphone 0.6 mg | 50.0 | 24 | |
Bold indicate statistically significant p values (p < 0.05)
Table 6.
Comparison of SDD protocol outcomes between the original french study by Gignoux et al., McGill, Kaiser Permanente LAMC, MultiCare Tacoma General Hospital, and UT Health experience
| Chasseranta (n = 157) | Leeb (n = 114) | McLemorec (n = 37) | Rashidid (n = 185) | Askenasye (n = 81) | |
|---|---|---|---|---|---|
| Mean age | 59.1 (SD 11.4) | 58.7 (SD 12.8) | 55.6 (SD 14.0) | 57.0 (SD 13.7) | 61.0 (SD 13.8) |
| Male sex | 55% | 52% | 40.5% | 41.7% | 45.7% |
| BMI | 26.5 (SD 5.1) | 26.5 (SD 5.6) | 28.2 (SD 5.9) | 30 (SD 5.8) | 30.6 (SD 6.5) |
| Indication for surgery | |||||
| Neoplasm | 34% | 53% | 57% | 59% | 60% |
| Diverticular disease | 62% | 4% | 14% | 24% | 37% |
| IBD | 0% | 4% | 0% | 6% | 2% |
| Stoma | 0% | 33% | 27% | 0% | 0% |
| Other | 3% | 7% | 2% | 11% | 1% |
| MIS procedure type | |||||
| Left/sigmoid | 85% | 26% | 25% | 8% | 36% |
| Right/transverse | 14% | 25% | 27% | 24% | 38% |
| Total colectomy | 1% | 0% | 0% | 0% | 0% |
| LAR | 0% | 16% | 17% | 52% | 26% |
| Stoma closure | 0% | 33% | 31% | 0% | 0% |
| Other | 0% | 0% | 0% | 16% | 0% |
| Discharged on POD 0 | 93% | 84% | 70% | 62% | 69% |
| 30-day unscheduled evaluation | 21% | 14% | 14% | 12% | 16% |
| 30-day readmission | 6% | 11% | 13.5% | 0.8% | 9.9% |
| Anastomotic leak | 3 (1.9%) | 4 (3.5%) | 1 (2.7%) | 0 | 3 (3.7%) |
| Re-operation | 6 (3.8%) | 3 (2.6%) | 1 (2.7%) | 0 | 3 (3.7%) |
aGignoux et al.
bMcGill
cKaiser Permanente LAMC
dMultiCare Tacoma General Hospital
eUT Health
McGill, Canadian experience—SDD is feasible and safe
From a Canadian Health Care perspective, bed occupancy rates are frequently well above 85% capacity, leading to potential hospital bed shortages and cancellations of elective operations [38]. In addition to being highly distressing to patients and wasteful to the healthcare system [39, 40], these delays may lead to worse outcomes in the context of colon cancer surgery [41]. Lee and colleagues hypothesized that SDD colectomy may alleviate some of these issues by decreasing the need for hospital admission and inpatient resources, thereby optimizing use of inpatient hospital resources and minimizing case cancellations.
New technologies that enable remote follow-up such as mobile digital health (mHealth) technology [37] demonstrated the potential for a less resource-intensive approach to SDD colectomy by transmitting relevant medical information without relying on a physical visit. Based on these data, Lee and colleagues implemented an mHealth smartphone application for remote follow-up after elective colorectal resection to investigate its effect on post-discharge outcomes and its usability. In this study, use of a smartphone app for remote follow-up was associated with a significant reduction in preventable Emergency Department (ED) visits (IRR 0.34, 95%CI 0.12–0.97) and was associated with high usability and patient satisfaction [42]. This experience confirmed that patients were able to reliably convey important symptoms and concerns through the app in the post-discharge setting. Importantly, many of these issues were managed remotely through app-based communication or a phone call, without an ED visit.
Next, a SDD protocol for laparoscopic colectomy and stoma reversal with remote post-discharge follow-up with a mHealth phone app was implemented in February 2020 [43]. Strict inclusion/exclusion criteria were used (Table 4). In this study, patients were discharged on the day of surgery directly from the post-anesthesia care unit (PACU) if they tolerated clear liquids, had adequate oral analgesia, and were able to ambulate and urinate independently. Preliminary results with 48 patients undergoing laparoscopic colectomy and planned for SDD reported that 77% of patients were successfully discharged on the same day as surgery [43]. None of the patients required an ED visit within the first 72 h after discharge. The overall 30-day complication rate was 17%, similar to a previous ERP colectomy group that also used the same mHealth remote follow-up intervention (Table 5). There were no instances of morbidity related to SDD. Unplanned ED visits and hospital readmissions were also similar. Patients were prescribed acetaminophen, celecoxib, and five tablets of oxycodone 5 mg for post-discharge analgesia. This was well tolerated with no instances of patients requiring additional opioid refills, suggesting that postoperative analgesia was not a significant barrier to SDD.
Table 4.
| Patient selection factors | Case selection factors |
|---|---|
| Patient inclusion criteria | Case inclusion criteria |
| • Adult | • Elective surgery |
| • Hgb > 10 | • Minimally invasive surgery |
| • Albumin > 3.5, pre-albumin > 20 | • Off midline specimen extraction site: examples: pffanensteil extraction incision, natural orifice extraction, ostomy site extraction |
| • Ambulatory | |
| • Functionally independent | |
| • No contraindications to TAP block (incl. allergies to dexamethasone or bupivacaine) or opioid-sparing analgesia (i.e. NSAIDs or acetaminophen) | |
| • Adequate home support | |
| • Owns and is capable of using a telephone or ‘smart’ mobile device running iOS or Android (or other device needed for remote monitoring) | |
| Patient exclusion criteria | Case exclusion criteria |
| • Cognitive impairment | • Intraoperative complications |
| • Significant cardiopulmonary disease | • Prolonged operative time |
| • Anemia (Hgb < 10) | • More than one bowel anastomosis created |
| • Malnutrition (albumin < 3.5) | • Any revisions needed for the initial anastomosis |
| • Active tobacco/nicotine use | • Excessive intra-operative bleeding/transfusion |
| • Coronary artery disease | • Creation of new ostomy |
| • Cardiac arrhythmia | • Locally advanced malignancy requiring multi-visceral resection |
| • Chronic anticoagulation or coagulopathy | |
| • Liver or renal failure | |
| • Chronic opioid use | |
| • Inflammatory bowel disease (crohns disease, ulcerative colitis) | |
| • Home > 1 h travel from institution | |
| • Lack of adequate home support | |
| • Language barrier (primary language other than patient education instructions) | |
| • Unable to participate in remote monitoring: no telephone, or no ‘smart’ mobile device |
Table 5.
Comparison of outcomes of SDD vs. ERP with 3-day target length of stay; both cohorts had post-discharge remote follow-up with a mHealth phone app [43]
| Same day discharge (n = 48) | Standard ERP (n = 73) | p-value | |
|---|---|---|---|
| Mean age, years (SD) | 60.2 (10.5) | 56.5 (13.1) | 0.111 |
| Male gender | 22 (46%) | 43 (59%) | 0.158 |
| Body mass index, kg/m2 (SD) | 26.3 (5.0) | 27.7 (5.3) | 0.171 |
| ASA physical status | 0.499 | ||
| 1 | 4 (8%) | 4 (5%) | |
| 2 | 27 (55%) | 38 (52%) | |
| 3 + | 17 (35%) | 31 (43%) | |
| Indication for surgery | 0.027 | ||
| Neoplasm | 25 (52%) | 47 (64%) | |
| Inflammatory bowel disease | 3 (6%) | 12 (16%) | |
| Stoma closure | 15 (31%) | 7 (10%) | |
| Diverticular disease | 2 (4%) | 4 (5%) | |
| Other | 3 (6%) | 3 (4%) | |
| Procedure performed | 0.022 | ||
| Right colectomy | 14 (29%) | 33 (45%) | |
| Left/sigmoid colectomy | 12 (25%) | 22 (30%) | |
| Low anterior resection | 7 (15%) | 11 (15%) | |
| Stoma closure | 15 (31%) | 7 (10%) | |
| Mean procedure time, min (SD) | 116 (56) | 177 (74) | < 0.001 |
| Median estimated blood loss, mL [IQR] | 5 [5–100] | 100 [28–200] | 0.089 |
| Mean PACU time, min (SD) | 311 (242) | 260 (216) | 0.242 |
| Median length of stay, days [IQR] | 0 [0–0] | 2 [1-4] | < 0.001 |
| 30-day complications | 8 (17%) | 11 (15%) | 0.813 |
| 30-day ED visit | 5 (10%) | 6 (8%) | 0.664 |
| 30-day readmission | 3 (6%) | 3 (4%) | 0.681 |
Since the initial patient in February 2020, Lee and colleagues have recruited over 100 patients for SDD [44]. Overall, SDD accounts for 25% of their major elective colorectal resections, and approximately 50% of the patients undergoing eligible procedures are recruited to SDD. The most common reason for ineligibility for SDD was lack of smart phone mobile device (required for the mHealth post-discharge remote follow-up) or significant medical comorbidities. The proportion of patients discharged on the day of surgery has increased to 84%, compared to 77% in their initial experience. Half of the patients not discharged on POD 0 were admitted for operative concerns, and the other patients required admission for failure to meet discharge criteria in the recovery room. Almost all the patients who failed discharge criteria were discharged the next day, with a single patient remaining hospitalized until POD 2 for nausea. Amongst those discharged on POD 0, only 5 patients (5%) had an unplanned ED visit within the first 72 h after surgery, and the overall 30-day ED visit and readmissions compare favorably to standard inpatient ERP outcomes [45–47]. Table 6 summarizes the experience and comparative outcomes amongst the initial pioneers and early adopters, Chasserant, Lee, McLemore, Rashidi, and Askenasy.
Kaiser Permanente, LAMC, USA experience—SDD is associated with reduced opioid use and prescribing
In May of 2020, after validation of the I-FEED scoring system to accurately predict sustained GI recovery, McLemore and colleagues initiated a quality improvement pilot program to implement and assess the safety and feasibility of SDD in healthy patients undergoing elective colorectal surgery as an alternative to postoperative hospitalization at Kaiser Permanente, Los Angeles Medical Center (KP LAMC) [48]. The KP LAMC multi-disciplinary ERP team met bi-monthly to review upcoming SDD cases, and the outcomes following prior SDD cases. Adjustments and improvements were made on an ongoing basis as a result of these meetings. From May 2020 to October 2021, 35 patients met the highly selective eligibility criteria for “healthy patient and healthy anastomosis” (Table 4). SDD occurred in 69% of patients (24 patients). The remaining 11 patients were discharged home on POD 1. The main indication for hospitalization in these 11 patients was unresolved nausea and/or patient preference for hospitalization.
McLemore and colleagues utilized an already existing remote visit option, the telephone appointment visit (TAV) encounter. This type of patient encounter had been in place prior to the COVID-19 pandemic and patients, scheduling administrative staff, and health care providers were familiar with this form of virtual visit. TAV was performed daily for remote monitoring during the first 7 days after the operation with the following variables recorded prospectively: I-FEED score, pain score, pain management (oral analgesia frequency and amount), bowel function, dietary advancement, any complications and/or re-admissions.
Before the pilot program began, the hypothesis was that SDD patients would require more narcotics upon discharge. Patients were discharged with 40 tablets of 5 mg oxycodone at the beginning of this pilot program. As it became evident that patients were taking very few tablets at home despite an average pain score of 4.5 (SD 1.8) on POD 1, the discharge opioid dosage was reduced to 20 tablets. Mean opioid usage was 5.2 tablets of 5 mg oxycodone over the entire 7 days despite high pain scores (Figs. 2 and 3). Based on this finding, discharge opioid dosage was further reduced to 10 tablets.
Fig. 2.
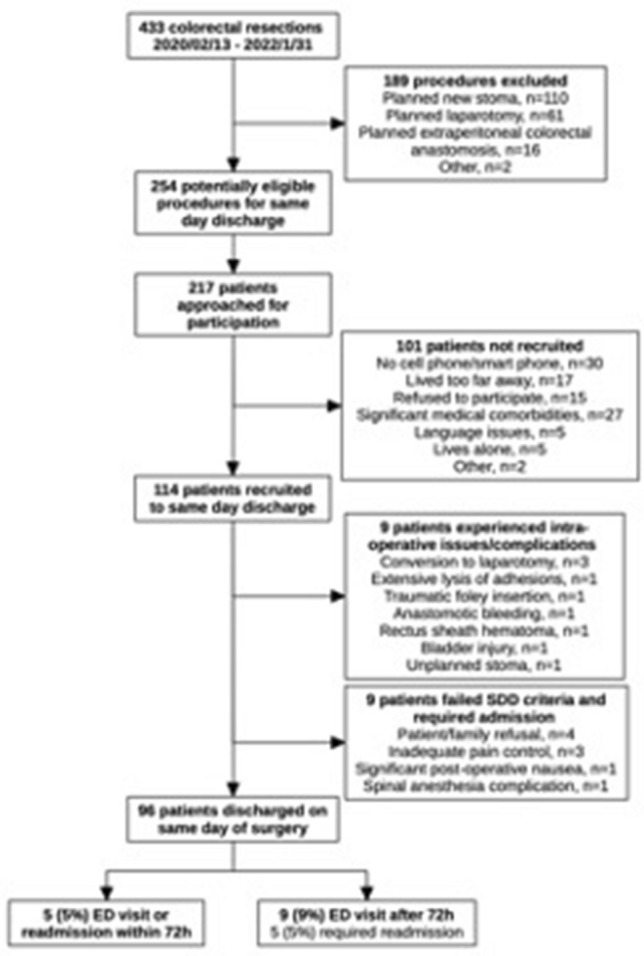
McGill SDD selection pathway, February 2020 to January 2022 [44]
Fig. 3.
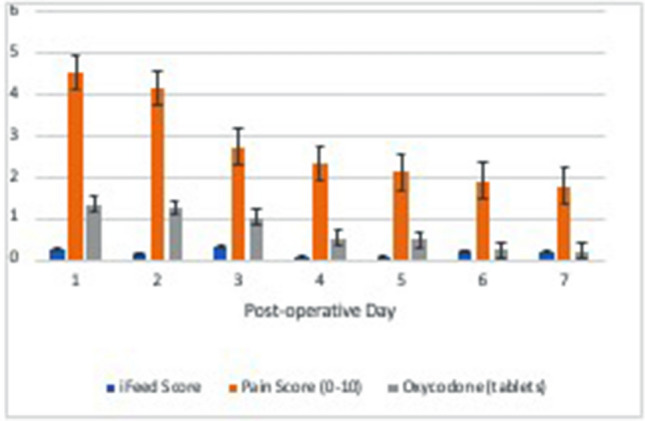
SDD home recovery trends of pain score (red), oxycodone 5 mg tablet use (green), and I-FEED score (blue) in the first 7 days after surgery. Data was obtained during the daily telephone remote visits on POD 1–7. Despite initially high pain scores on POD 1 and 2, patient opioid use was low with a mean number of five tablets used per patient during the entire postoperative recovery [48] (Color figure onlne)
As postoperative ERPs continue to evolve, opioid pain management requires a combination of realism and moderation. Postoperative pain management is an integral part of surgical recovery [49]. Opioid prescribing for postoperative pain also has the potential for over-prescribing, overuse, and abuse. ERPs have successfully demonstrated the efficacious use of multimodal analgesia with an emphasis on reducing overall opioid use [50]. SDD is associated with a significantly lower than hypothesized opioid usage, resulting in a change in clinical practice and reduction of prescribed discharge opioids.
Tacoma, WA & McGill experience—SDD is associated with high patient satisfaction
In the McGill SDD series, patient satisfaction scores were high [43]. The majority of patients did not feel like they needed to stay in hospital for their recovery and would still choose to go home on the day of surgery if they had surgery again [43]. There was also high satisfaction for the mobile phone app, with only one patient that expressed dissatisfaction in with the mobile digital health (mHealth app) remote monitoring [37]. Amongst the open-ended reports of patient experiences, only four patients expressed pain issues in the first 72 h, although none requested additional analgesics. In particular, the use of a mHealth app for remote follow-up was associated with improved patient-physician communication post-discharge, which is essential for SDD [42]. Patients felt more secure after discharge because of the ability to easily communicate with their provider. This suggests that patients’ questions and concerns post-discharge were satisfactorily managed remotely.
Similarly, Rashidi and colleagues in Tacoma, WA, created a survey composed of eleven questions to assess their SDD patient experience. The survey was performed either at postoperative follow up office visits or over the phone with an 81% participation rate. The group reported that 85% of patients who underwent SDD would do so again if given the opportunity. The majority of patients felt that they were active decision makers in their care and discharge, and they reported a heightened level of patient and family comfort with discharge. Over 95% of patients reported that they felt comfortable and safe with the preoperative education they received regarding SDD as well as with the instructions they were provided on discharge [51].
Stepwise approach to implementing SDD
Historically, ERPs have challenged surgical dogma regarding what is needed and what is best following major abdominal surgery. Mandates such as regular use of nasogastric drainage, surgical drain placement, high volume fluid use, and slow return of oral intake were once widely held notions of best practices [52]. SDD is the next step in ERP evolution (Table 7).
Table 7.
Standard ERPs vs. SDD for MIS CRS
| Standard ERPs for elective colorectal surgery | SDD for elective colorectal surgery |
|---|---|
| Surgery consultation and preparation phase | |
| Patient and family/social support education | Extensive patient and family/social support education |
| • Setting up expectations for early ambulation, multi-modal pain management, hospital length of stay | • Setting up expectations for early ambulation, multi-modal pain management, hospital length of stay |
| • Social support at home | |
| • Distance from hospital assessment | |
| • Plan ahead/worst case scenario: don’t delay, and return to same hospital/emergency department as surgery location if problems or complications arise | |
| • Set up remote monitoring plan (phone, video, or app remote monitoring) | |
| • Provide contact information for Surgical Department, Hospital, etc. for urgent questions or issues | |
| Preoperative optimization & pre-habilitation | Preoperative optimization |
| • Weight loss (Ideal BMI 30) if possible | • Weight loss (Ideal BMI 30) if possible |
| • Exercise/conditioning 20 min daily sustained activity (in ambulatory patients) | • Exercise/conditioning 20 min daily sustained activity (in ambulatory patients) |
| Preoperative nutritional assessment | Preoperative nutritional assessment |
| • Alternatives to anastomosis planning for sub-optimal nutrition levels | • If low nutritional levels, not an ideal candidate for SDD ERAS (Alb < 3.5 or Pre-albumin < 21) |
| • If NEW ostomy (temporary or permanent needed), not an ideal candidate for SDD ERAS | |
| Management of anemia | Management of anemia |
| • Alternatives to anastomosis planning for sub-optimal Hgb/Hct levels vs. pre-operative correction of anemia (IV Iron, pRBC Transfusion, etc.) |
• Anemia is a contra-indication for SDD Note: If low Hgb/Hct (< 10/ < 30), this is a contra-indication for SDD |
| Day prior to surgery preparations | |
| ± Bowel preparation | ± Bowel preparation |
| Electrolyte therapy/hydration | Electrolyte therapy/hydration |
| Decreased fasting | Decreased fasting |
| Antimicrobial prophylaxis and skin preparation | Antimicrobial prophylaxis and skin preparation |
| Dietary supplementation (Immunotherapy drinks) | Dietary supplementation (Immunotherapy drinks) |
| Day of surgery preparations and pre-op anesthesia | |
| Dietary supplementation (Immunotherapy drinks) | Dietary supplementation (Immunotherapy drinks) |
| Decreased fasting | Decreased fasting |
| Pre-operative warming | Pre-operative warming |
| Maintain normal glycemic levels | Maintain normal glycemic levels |
| Thromboprophylaxis | Thromboprophylaxis |
| ± Alvimopan | ± Alvimopan |
| Pre-operative patient/family/support re-education | |
| • Early ambulation after surgery (sitting in chair, then walking) | |
| • Multi-modal analgesia plan | |
| • Patient check In–solicit their intent to proceed with SDD vs. standard of care post operative hospitalization | |
| Intra-operative care | |
| Minimize intra-operative fluids/hemodynamic goal directed therapy | Minimize intra-operative fluids/hemodynamic goal directed therapy |
| • 500–700 mL maximal IVF goal | |
| • Approximately 3 mL/kg/h for an average 70 kg patient | |
| Surgical approach | Surgical approach |
| • Minimally invasive surgery | • Minimally invasive surgery |
| • Less painful specimen extraction site: natural orifice, pfannenstiel | |
| • Intra-corporeal anastomosis | |
| Avoid nasogastric tubes and unnecessary drains | Avoid nasogastric tubes and unnecessary drains |
| Prevent intraoperative hypothermia | Prevent intraoperative hypothermia |
| Maintain normal glycemic levels | Maintain normal glycemic levels |
| Analgesia/anesthesia | Analgesia/anesthesia |
| • Multimodal anesthesia | • Multimodal anesthesia |
| • Narcotic sparing approach | • Narcotic sparing approach |
| • ± Epidural–only recommended in open cases | • Abdominal wall blocks (TAP/rectus sheath) |
| • ± Spinal anesthesia for MIS cases | • Propofol, lidocaine, dexmedotomidine, ketamine hydrochloride infusions |
| • Abdominal wall blocks (TAP/Rectus Sheath) | • Bispectral index (BIS™) monitoring |
| • ± Posteromedial quadratus lumborum (QL) block Note: Epidural/spinal blocks not recommended for SDD programs at this time due to potential for urinary retention, vasovagal responses, and need for hospital monitoring | |
| Post-operative recovery phase | |
| Postoperative fluid and electrolyte therapy (avoid over resuscitation) | Postoperative fluid and electrolyte therapy (avoid over resuscitation) |
| Prevention of postoperative ileus | Prevention of postoperative ileus |
| • Limited opioid use/focus on short acting opioids | • Limited opioid use/focus on short acting opioids |
| • Multimodal analgesia therapy | • Multimodal analgesia therapy |
| • Avoiding routine NGT | • Avoiding routine NGT |
| • Maintaining fluid balance | • Maintaining fluid balance |
| • Alviompan (if given pre-op) | • Alvimopan (2nd and last dose; if given pre-op) |
| • ± Chewing gum, magnesium oxide | |
| • Early out of bed to chair (within 1 h of PACU arrival) | |
| Post-operative glycemic control | Post-operative glycemic control |
| Post-operative nutritional care | Post-operative nutritional care |
| • Offer clear liquids immediately (typically does not occur until Med/Surg hospital admission) | • Offer electrolyte clear liquids immediately In PACU (once sitting in chair) |
| Post-operative ambulation | Post-operative mobilization and ambulation |
| • Encourage early ambulation | • Early out of bed to chair (within 1 h of PACU arrival) |
| • Ambulation once full level of alertness achieved | |
| • ± Visit with physical therapist in PACU per hospital/PACU staffing and availability | |
| Post-operative deep breath teaching | Post-operative deep breath teaching |
| • Incentive spirometer education In PACU | |
| Urinary drainage | Urinary drainage |
| • Foley removal POD 0–1 in colon surgery |
• Avoid routine foley in colon surgery or anterior resection Note: LAR/APR patients with diverting loop ileostomy are not considered candidates for SDD ERAS (ostomy teaching/high output ileostomy management and prevention, etc.) |
| • Foley removal POD 2–3 in rectal surgery | |
| Discharge criteria | |
| Full recovery from anesthesia | Full recovery from anesthesia |
| Tolerating liquids or solids without nausea or vomiting | Tolerating liquids without nausea or vomiting |
| • I-FEED score: 0–1 | |
| • Early/immediate anesthesia emergence nausea and vomiting with resolution is acceptable, so long as I-FEED score is 0–1 prior to discharge | |
| Absence of clinical findings suspicious for infection or bleeding | n/a |
| ± Flatus/BM | n/a |
| Voiding independently | Voiding independently |
| Discharge instructions | Discharge instructions |
| Wound care, diet, after hours contact information, regular business office hours contact information, post operative visit(s) scheduled, pain management reviewed, when to call/what to be concerned about during recovery | Wound care, diet, after hours contact information, regular business office hours contact information, post operative visit(s) scheduled, pain management reviewed, when to call/what to be concerned about during recovery |
| • Review and confirm social support at home | |
| • Distance from hospital re-assessment | |
| • Worst case scenario action plans: don’t delay, and return to same hospital/emergency department as surgery location if problems or complications arise | |
| Review and confirm remote monitoring plan is in place (phone, video, or app remote monitoring) | |
SDD protocols have several small advancements throughout all phases of care resulting in patients recovering in a shorter period of time. Patient and case selection for SDD is essential (Table 3) [2, 3, 53, 54]. Informed consent follows a shared decision-making format. The patient is informed that the standard of care is to be hospitalized following surgery, at least overnight, and then be discharged on POD 1, or later, after all discharge criteria are met. An alternative is SDD with a plan to be discharged when the patient meets discharge criteria on the day of surgery. If case selection and/or discharge criteria are not met on the day of surgery, or the patient feels uncomfortable going home, then the patient proceeds with the standard hospitalization following surgery.
Perioperative nursing education and admissions education are also key factors to consider when considering SDD. Take the time to educate nursing units, perioperative anesthesia units, anesthesia, hospital administrators, admissions office staff, and office staff before getting started. This will help remove unanticipated barriers and hurdles to SDD implementation. Like all ERP advancements, re-education and SDD protocol updates will be needed over time.
Regarding key stakeholder buy-in and interest in SDD, our experience has demonstrated that it takes time to develop an ERP culture within an institution. However, once this has taken place, and MIS colorectal surgery patients begin to more routinely meet discharge criteria on POD 1 or 2, it may be observed that some patients meet discharge criteria within the first 6–12 h following the operation. Making the necessary changes to move forward with home recovery and plans for discharge on POD 0 is the next natural step in advancing an institution’s ERP forward and being able to offer SDD to select patients.
Billing and reimbursement considerations
Hospital systems use diagnosis related group (DRG) codes to categorize and bill for health care related services. These codes help classify and designate inpatient vs. observation vs. outpatient hospital encounters for billing and reimbursement. These DRG codes are further modified by patient factors such as severity of illness, treatment difficulty, mortality, comorbidities and resource intensity. The average reimbursement, in the USA, for a colectomy ranges from $30,000 to $40,000 depending on the aforementioned factors. Conversely, hospital costs associated with performing a colectomy can be challenging to determine as cost structures vary depending on hospital systems. Hospital cost information is typically not readily available as it is carefully monitored, analyzed, and guarded by hospital administrators for proprietary reasons. With that understanding, the range of hospital cost for patients undergoing SDD versus a 2-day hospital stay may be in the $15,000–$17,000 range with the vast majority of this cost incurred from the operation itself. The average daily cost to provide care for a patient in a medical-surgical unit is significantly lower ($500–$750) when compared to the costs associated with the operation itself.
There has been some concern regarding reimbursement if colectomies change from an inpatient procedure to a same day discharge or outpatient procedure. For example, joint replacements were associated with an approximately 40% decrease in reimbursement when moved to the outpatient setting. Currently, there is no DRG code for SDD colectomy and hospitals are currently submitting the same DRG code as for an inpatient procedure. As the field evolves and SDD becomes more common, the hospital’s profit margin may decrease if reimbursements are cut at a similar rate as joint replacement procedures. Forecasting has always been a field fraught with inaccuracy and similarities between joint and colon surgeries can differ significantly including variability in case complexity, potential for significant physiologic derangements, technical difficulties, and complication profile associated with colorectal resections. In addition, at this point, only a minority of patients and cases are suited for SDD (Table 4). Therefore, although there is some concern, it may be mitigated by other factors. Lastly, from a feasibility standpoint, patients who are pre-operative candidates for SDD can be admitted following the operation, and then later evaluated and discharged if criteria are met.
Conclusions
Early institutional experience with SDD protocols for MIS CRS suggests that SDD is feasible and safe in well-selected patients and procedures. These SDD protocols have also been associated with reduced opioid use and prescribing. Patient perception and experience with SDD is favourable. As worldwide interest in SDD is rising, it is important to be well-informed regarding the benefits and risks of implementing SDD programs. For early adopters, SDD has been the natural evolution of their well-developed ERPs. Like all ERPs, SDD begins in the office setting with identifying the correct patient and procedure, aligning goals and objectives, and the perioperative education of the patient and their supporting family members and/or significant others.
A thorough discussion with the patient regarding expected activity levels, oral intake, and pain control post operatively lay the foundation for a successful application of SDD programs. These observations may not be applicable to all patient populations, institutions, practice types, or within the scope of an existing ERP. However, if the underlying principles of SDD can be incorporated into an existing institutional ERP, it may further reduce POI, LOS, and improve the effectiveness of oral analgesia for postoperative pain management resulting in reduced opioid use and prescribing. Programs considering SDD should be mindful that patient and case selection is essential for success (Table 3). Prior to initiating a SDD program, institutional teams should review, prepare for, and implement the three key areas that are essential to have in place with your multi-disciplinary ERP team (Table 2). Lastly, frequent feedback and review of SDD outcomes by the multi-disciplinary ERP team will help further refine ERPs and increase their effectiveness.
Acknowledgements
The authors would like to acknowledge the following members of the SAGES Colorectal Surgery Committee who have reviewed this manuscript and contributed to the final production of the manuscript: Nawar Alkhamesi, Antonio Caycedo-Marulanda, Alan Harzman, Dana Hayden, Andreas Kaiser, and Deborah Keller
Declarations
Disclosures
Popowich is a consultant for Bard, Boehringer, GI Windows. Popowich has received financial payment for expert testimony. Lee has received grants and/or contracts from Johnson & Johnson for investigator initiated research. Askenazy is a consultant for Intuitive Surgical. Hedrick is a consultant for Ethicon/Johnson & Johnson. Rashidi is a consultant for Intuitive Surgical. McLemore and Sylla has no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gignoux B, Pasquer A, Vulliez A, Lanz T. Outpatient colectomy within an enhanced recovery program. J Visc Surg. 2015;152(1):11–15. doi: 10.1016/j.jviscsurg.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Chasserant P, Gosgnach M. Improvement of peri-operative patient management to enable outpatient colectomy. J Visc Surg. 2016;153(5):333–337. doi: 10.1016/j.jviscsurg.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Gignoux B, Gosgnach M, Lanz T, Vulliez A, Blanchet MC, Frering V, Faucheron JL, Chasserant P. Short-term outcomes of ambulatory colectomy for 157 consecutive patients. Ann Surg. 2019;270(2):317–321. doi: 10.1097/SLA.0000000000002800. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster E, Wick E. Standardized care pathways as a means to improve patient safety. Surg Clin North Am. 2021;101(1):49–56. doi: 10.1016/j.suc.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Höjer H. The effect on total antimicrobial consumption and hospitalization time after prophylactic treatment with doxycycline in colorectal surgery. Acta Chir Scand. 1978;144(3):175–179. [PubMed] [Google Scholar]
- 6.Hackford AW, Schoetz DJ, Coller JA, Veidenheimer MC. Surgical management of complicated diverticulitissssss. The Lahey Clinic experience 1967 to 1982. Dis Colon Rectum. 1985;28(5):317–321. doi: 10.1007/BF02560431. [DOI] [PubMed] [Google Scholar]
- 7.Basse L, Thorbøl JE, Løssl K, Kehlet H. Colonic surgery with accelerated rehabilitation or conventional care. Dis Colon Rectum. 2004;47(3):271–278. doi: 10.1007/s10350-003-0055-0. [DOI] [PubMed] [Google Scholar]
- 8.Thiele RH, Rea KM, Turrentine FE, Friel CM, Hassinger TE, McMurry TL, Goudreau BJ, Umapathi BA, Kron IL, Sawyer RG, Hedrick TL. Standardization of care: impact of an enhanced recovery protocol on length of stay, complications, and direct costs after colorectal surgery. J Am Coll Surg. 2015;220(4):430–443. doi: 10.1016/j.jamcollsurg.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Hassinger TE, Mehaffey JH, Martin AN, Bauer-Nilsen K, Turrentine FE, Thiele RH, Sarosiek BM, Reilley MJ, Hoang SC, Friel CM, Hedrick TL. Implementation of an enhanced recovery protocol is associated with on-time initiation of adjuvant chemotherapy in colorectal cancer. Dis Colon Rectum. 2019;62(11):1305–1315. doi: 10.1097/DCR.0000000000001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lohsiriwat V, Lertbannaphong S, Polakla B, Riansuwan W. Implementation of enhanced recovery after surgery and its increasing compliance improved 5-year overall survival in resectable stage III colorectal cancer. Updates Surg. 2021;73(6):2169–2179. doi: 10.1007/s13304-021-01004-8. [DOI] [PubMed] [Google Scholar]
- 11.Shah PM, Johnston L, Sarosiek B, Harrigan A, Friel CM, Thiele RH, Hedrick TL. Reducing readmissions while shortening length of stay: the positive impact of an enhanced recovery protocol in colorectal surgery. Dis Colon Rectum. 2017;60(2):219–227. doi: 10.1097/DCR.0000000000000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiele RH, Sarosiek BM, Modesitt SC, McMurry TL, Tiouririne M, Martin LW, Blank RS, Shilling A, Browne JA, Bogdonoff DL, Bauer TW, Hedrick TL. Development and impact of an institutional enhanced recovery program on opioid use, length of stay, and hospital costs within an academic medical center: a cohort analysis of 7774 patients. Anesth Analg. 2021;132(2):442–455. doi: 10.1213/ANE.0000000000005182. [DOI] [PubMed] [Google Scholar]
- 13.Lee L, Feldman LS. Recovery after surgery: economic impact and value. Surg Clin North Am. 2018;98(6):1137–1148. doi: 10.1016/j.suc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Lynch KT, Cramer CL, Kane WJ, Hedrick TL, Friel C, Vemuru S, Hoang SC. A history of clostridioides difficile infection portends infection recurrence and worse outcomes after stoma reversal. Surgery. 2021;170(1):55–60. doi: 10.1016/j.surg.2020.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla A, Peacock O, Tierney GM, Tou S, Hurst NG, Speake WJ, Williams JP, Lund JN. Day-case closure of ileostomy: feasible, safe and efficient. Colorectal Dis. 2015;17(9):820–823. doi: 10.1111/codi.12961. [DOI] [PubMed] [Google Scholar]
- 16.Saadat LV, Mahvi DA, Jolissaint JS, Gabriel RA, Urman R, Gold JS, Whang EE. Twenty-three-hour-stay colectomy without increased readmissions: an analysis of 1905 cases from the national surgical quality improvement program. World J Surg. 2020;44(3):947–956. doi: 10.1007/s00268-019-05257-8. [DOI] [PubMed] [Google Scholar]
- 17.Taylor JP, Stem M, Chen SY, Yu D, Fang SH, Gearhart SL, Safar B, Efron JE. The safety of outpatient stoma closure: on the verge of a paradigm shift? J Gastrointest Surg. 2019;23(10):2019–2026. doi: 10.1007/s11605-018-4001-9. [DOI] [PubMed] [Google Scholar]
- 18.de Moreira Azevedo JG, Mendes CRS, Meyline LA, de Paula S, Pessoa JC, São Julião GP, Perez RO, Vailati BR. Laparoscopic colorectal surgery and discharge within 24 h-who is at risk for readmission? Colorectal Dis. 2021;23(10):2714–2722. doi: 10.1111/codi.15791. [DOI] [PubMed] [Google Scholar]
- 19.Gash KJ, Greenslade GL, Dixon AR. Enhanced recovery after laparoscopic colorectal resection with primary anastomosis: accelerated discharge is safe and does not give rise to increased readmission rates. Colorectal Dis. 2012;14(10):1287–1290. doi: 10.1111/j.1463-1318.2012.02969.x. [DOI] [PubMed] [Google Scholar]
- 20.Studniarek A, Borsuk DJ, Kochar K, Park JJ, Marecik SJ. Feasibility assessment of outpatient colorectal resections at a tertiary referral center. Int J Colorectal Dis. 2021;36(3):501–508. doi: 10.1007/s00384-020-03782-w. [DOI] [PubMed] [Google Scholar]
- 21.Huettemann R (2020) Same day discharge is safe in selected patients following laparoscopic right colectomy in the National Surgical Quality Improvement Project. SSAT/DDW abstract. http://meetings.ssat.com/abstracts/2020-Virtual/506.cgi
- 22.Hedrick TL, McEvoy MD, Mythen MMG, Bergamaschi R, Gupta R, Holubar SD, Senagore AJ, Gan TJ, Shaw AD, Thacker JKM, Miller TE, et al. Perioperative quality initiative (POQI) 2 workgroup american society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative gastrointestinal dysfunctionwithin an enhanced recovery pathway for elective colorectal surgery. Anesth Analg. 2018;126(6):1896–1907. doi: 10.1213/ANE.0000000000002742. [DOI] [PubMed] [Google Scholar]
- 23.Munk-Madsen P, Eriksen JR, Kehlet H, Gögenur I. Why still in hospital after laparoscopic colorectal surgery within an enhanced recovery program? Colorectal Dis. 2019;21(12):1438–1444. doi: 10.1111/codi.14762. [DOI] [PubMed] [Google Scholar]
- 24.Vather R, Bissett I. Management of prolonged post-operative ileus: evidence-based recommendations. ANZ J Surg. 2013;83(5):319–324. doi: 10.1111/ans.12102. [DOI] [PubMed] [Google Scholar]
- 25.Alhashemi M, Fiore JF, Safa N, Mahroos MA, Mata J, Pecorelli N, Baldini G, Dendukuri N, Stein BL, Liberman AS, Charlebois P, Carli F, Feldman LS. Incidence and predictors of prolonged postoperative ileus after colorectal surgery in the context of an enhanced recovery pathway. Surg Endosc. 2019;33(7):2313–2322. doi: 10.1007/s00464-018-6514-4. [DOI] [PubMed] [Google Scholar]
- 26.Leung V, Baldini G, Liberman S, Charlebois P, Stein B, Feldman LS, Fr FJF, Lee L. Tolerating clear fluids diet on postoperative day 0 predicts early recovery of gastrointestinal function after laparoscopic colectomy. Surg Endosc. 2022 doi: 10.1007/s00464-022-09151-8. [DOI] [PubMed] [Google Scholar]
- 27.Leung V, Baldini G, Liberman S, Charlebois P, Stein B, Fiore JF, Feldman LS, Lee L. Trajectory of gastrointestinal function after laparoscopic colorectal surgery within an enhanced recovery pathway. Surgery. 2022;171(3):607–614. doi: 10.1016/j.surg.2021.08.062. [DOI] [PubMed] [Google Scholar]
- 28.Slim K, Reymond T, Joris J, Paul S, Pereira B, Cotte E. Intolerance to early oral feeding in enhanced recovery after colorectal surgery: an early red flag? Colorectal Dis. 2020;22(1):95–101. doi: 10.1111/codi.14785. [DOI] [PubMed] [Google Scholar]
- 29.Fiore JF, Browning L, Bialocerkowski A, Gruen RL, Faragher IG, Denehy L. Hospital discharge criteria following colorectal surgery: a systematic review. Colorectal Dis. 2012;14(3):270–281. doi: 10.1111/j.1463-1318.2010.02477.x. [DOI] [PubMed] [Google Scholar]
- 30.Fiore JF, Bialocerkowski A, Browning L, Faragher IG, Denehy L. Criteria to determine readiness for hospital discharge following colorectal surgery: an international consensus using the delphi technique. Dis Colon Rectum. 2012;55(4):416–423. doi: 10.1097/DCR.0b013e318244a8f2. [DOI] [PubMed] [Google Scholar]
- 31.Fiore JF, Faragher IG, Bialocerkowski A, Browning L, Denehy L. Time to readiness for discharge is a valid and reliable measure of short-term recovery after colorectal surgery. World J Surg. 2013;37(12):2927–2934. doi: 10.1007/s00268-013-2208-1. [DOI] [PubMed] [Google Scholar]
- 32.Fiore JF, Olleik G, El-Kefraoui C, Verdolin B, Kouyonumdijian A, Alldrit A, Figueiredo AG, Valanci S, Marquez-GdeV JA, Schulz M, Moldoveanu D, Nguyen-Powanda P, Best G, Banks A, Landry T, Pecorelli N, Baldini G, Feldman LS. Preventing opioid prescription after major surgery: a scoping review of opioid-free analgesia. Br J Anaesth. 2019;123(5):627–636. doi: 10.1016/j.bja.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Oh TK, Lee SJ, Do SH, Song IA. Transversus abdominis plane block using a short-acting local anesthetic for postoperative pain after laparoscopic colorectal surgery: a systematic review and meta-analysis. Surg Endosc. 2018;32(2):545–552. doi: 10.1007/s00464-017-5871-8. [DOI] [PubMed] [Google Scholar]
- 34.Trépanier M, ValinThorburn A, Kouyoumdjian A, Dumitra T, Alhashemi M, Kaneva P, Liberman AS, Charlebois P, Stein BS, Fried GM, Feldman LS, Lee L. Intracorporeal versus extracorporeal anastomosis for right colectomy does not affect gastrointestinal recovery within an enhanced recovery after surgery program. Surg Endosc. 2020;34(10):4601–4608. doi: 10.1007/s00464-019-07204-z. [DOI] [PubMed] [Google Scholar]
- 35.Lee L, Abou-Khalil M, Liberman S, Boutros M, Fried GM, Feldman LS. Incidence of incisional hernia in the specimen extraction site for laparoscopic colorectal surgery: systematic review and meta-analysis. Surg Endosc. 2017;31(12):5083–5093. doi: 10.1007/s00464-017-5573-2. [DOI] [PubMed] [Google Scholar]
- 36.Lee L, Mata J, Droeser RA, Kaneva P, Liberman S, Charlebois P, Stein B, Fried GM, Feldman LS. Incisional hernia after midline versus transverse specimen extraction incision: a randomized trial in patients undergoing laparoscopic colectomy. Ann Surg. 2018;268(1):41–47. doi: 10.1097/SLA.0000000000002615. [DOI] [PubMed] [Google Scholar]
- 37.Vegesna A, Tran M, Angelaccio M, Arcona S. remote patient monitoring via non-invasive digital technologies: a systematic review. Telemed J E Health. 2017;23(1):3–17. doi: 10.1089/tmj.2016.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finlayson GS, Stewart D, Tate RB, MacWilliam L, Roos N. Anticipating change: how many acute care hospital beds will manitoba regions need in 2020? Can J Aging Spring. 2005;24(Suppl 1):133–140. doi: 10.1353/cja.2005.0045. [DOI] [PubMed] [Google Scholar]
- 39.Viftrup A, Dreyer P, Nikolajsen L, Holm A. Surgery cancellation: A scoping review of patients' experiences. J Clin Nurs. 2021;30(3–4):357–371. doi: 10.1111/jocn.15582. [DOI] [PubMed] [Google Scholar]
- 40.Sommer JL, Jacobsohn E, El-Gabalawy R. Impacts of elective surgical cancellations and postponements in Canada. Can J Anaesth. 2021;68(3):315–323. doi: 10.1007/s12630-020-01824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaltenmeier C, Shen C, Medich DS, Geller DA, Bartlett DL, Tsung A, Tohme S. Time to surgery and colon cancer survival in the united states. Ann Surg. 2021;274(6):1025–1031. doi: 10.1097/SLA.0000000000003745. [DOI] [PubMed] [Google Scholar]
- 42.Eustache J, Latimer EA, Liberman S, Charlebois P, Stein B, Fiore JF, Feldman LS, Lee L. Patient-physician communication and reduces emergency department visits after colorectal surgery. Dis Colon Rectum. 2021 doi: 10.1097/DCR.0000000000002187. [DOI] [PubMed] [Google Scholar]
- 43.Lawrence Lee L, Eustache J, Baldini G, Liberman AS, Charlebois P, Fiore JF, Feldman LS. Enhanced recovery 2.0—same day discharge with mobile app follow-up after minimally invasive colorectal surgery. Ann Surg. 2021 doi: 10.1097/SLA.0000000000004962. [DOI] [PubMed] [Google Scholar]
- 44.Lee L, Eustache J, Tran-McCaslin M, Basam M, Baldini G, Rudikoff AG, Liberman S, Feldman LS, McLemore EC. North American multicenter evaluation of a same-day discharge protocol for minimally invasive colorectal surgery using mHealth or telephone remove post-discharge monitoring. Surg Endosc. 2022 doi: 10.1007/s00464-022-09208-8. [DOI] [PubMed] [Google Scholar]
- 45.Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, Tsai TC, Ko CY, Bilimoria KY. Underlying reasons associated with hospital readmission following surgery in the united states. JAMA. 2015;313(5):483–495. doi: 10.1001/jama.2014.18614. [DOI] [PubMed] [Google Scholar]
- 46.Turrentine FE, McMurry TL, Adams RB, Jones RS, Zaydfudim VM. Early patient discharge in selected patients is not associated with higher readmission after major abdominal operations. Ann Surg. 2020 doi: 10.1097/SLA.0000000000004582. [DOI] [PubMed] [Google Scholar]
- 47.Miller PE, Dao H, Paluvoi N, Bailey M, Margolin D, Shah N, Vargas HD. Comparison of 30-day postoperative outcomes after laparoscopic vs robotic colectomy. J Am Coll Surg. 2016;223(2):369–373. doi: 10.1016/j.jamcollsurg.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Tran-McCaslin M, Basam M, Rudikoff A, Thuraisingham D, McLemore EC. Reduced opioid use and prescribing in a same day discharge enhanced recovery program in healthy patients undergoing colorectal surgery during the Covid-19 pandemic. Am Surg. 2022 doi: 10.1177/00031348221109467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamdar NV, Hoftman N, Rahman S, Cannesson M. Opioid-free analgesia in the era of enhanced recovery after surgery and the surgical home: implications for postoperative outcomes and population health. Anesth Analg. 2017;125(4):1089–1091. doi: 10.1213/ANE.0000000000002122. [DOI] [PubMed] [Google Scholar]
- 50.Ni X, Jia D, Chen Y, Wang L, Suo J. Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J Gastrointest Surg. 2019;23(7):1502–1512. doi: 10.1007/s11605-019-04170-8. [DOI] [PubMed] [Google Scholar]
- 51.Curfman KR, Poola AS, Blair GE, Thilo EL, Hawkins ME, Rashidi L. Ambulatory colectomy: a pilot protocol for same day discharge in minimally invasive colorectal surgery. Am J Surg. 2022 doi: 10.1016/j.amjsurg.2022.04.039. [DOI] [PubMed] [Google Scholar]
- 52.Carmichael JC, Keller DS, Baldini G, Bordeianou L, Weiss E, Lee L, Boutros M, McClane J, Feldman LS, Steele SR. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the american society of colon and rectal surgeons and society of american gastrointestinal and endoscopic surgeons. Dis Colon Rectum. 2017;60(8):761–784. doi: 10.1097/DCR.0000000000000883. [DOI] [PubMed] [Google Scholar]
- 53.Campbell S, Fichera A, Thomas S, Papaconstantinou H, Essani R. Outpatient colectomy—a dream or reality? Proc (Bayl Univ Med Cent) 2021;35(1):24–27. doi: 10.1080/08998280.2021.1973327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bourgouin S, Monchal T, Schlienger G, Franck L, Lacroix G, Balandraud P. Eligibility criteria for ambulatory colectomy. J Visc Surg. 2022;159(1):21–30. doi: 10.1016/j.jviscsurg.2020.11.012. [DOI] [PubMed] [Google Scholar]