Abstract
The lambdoid phage Gifsy-2 contributes significantly to Salmonella enterica serovar Typhimurium virulence. The phage carries the periplasmic superoxide dismutase gene, sodCI, and other unidentified virulence factors. We have characterized the gene grvA, a single open reading frame inserted in the opposite orientation in the tail operon of the Gifsy-2 phage. Contrary to what is observed with classic virulence genes, grvA null mutants were more virulent than wild type as measured by intraperitoneal competition assays in mice. We have termed this effect antivirulence. Wild-type grvA in single copy complemented this phenotype. However, grvA+ on a multicopy plasmid also conferred the antivirulence phenotype. Neither a grvA null mutation nor the grvA+ plasmid conferred a growth advantage or disadvantage in laboratory media. The antivirulence phenotype conferred by the grvA null mutation and the grvA+ plasmid required wild-type sodCI but was independent of other virulence factors encoded on Gifsy-2. These results suggest that in a wild-type situation, GrvA decreases the pathogenicity of serovar Typhimurium in the host, most likely by affecting resistance to toxic oxygen species. These virulence phenotypes were independent of functional Gifsy-2 phage production. Our data suggest that the contribution of Gifsy-2 is a complicated sum of both positive virulence factors such as sodCI and antivirulence factors such as grvA.
Salmonella enterica serovars cause diseases ranging from a mild, self-limiting gastroenteritis to a potentially life-threatening enteric fever in a variety of hosts (38). Salmonella enterica serovar Typhimurium causes a typhoid fever-like disease in mice, which serves as a model for human typhoid fever (38). A number of virulence genes are involved in each stage of serovar Typhimurium infection (47), and many of these virulence genes are found on horizontally acquired segments of DNA such as pathogenicity islands and islets (23, 35). It is becoming apparent that bacteriophages also play a large role in the movement of virulence factors among bacteria (36, 42, 55, 56).
Two inducible prophages have recently been shown to contribute to serovar Typhimurium virulence. These phages, Gifsy-1 and Gifsy-2, are partially homologous to each other but are found in different regions of the chromosome: 57 and 23.8 centisomes, respectively (22). They are members of the lambdoid family of bacteriophages, having the same relative gene order as the prototype phage λ. Sequence homology of the putative Gifsy proteins to λ proteins ranges from unrecognizable to highly conserved, as it does in other lambdoid phages (25). Although these phages are heteroimmune, the presence of lysogenized Gifsy-1 decreases the efficiency of Gifsy-2 induction (22). Both the Gifsy-1 and the Gifsy-2 phages are induced by oxidative stress and other traditional means of phage induction and produce viable phage particles (21). It has been shown that serovar Typhimurium strains cured of Gifsy-2 are significantly attenuated in the mouse (21). The attenuation of Gifsy-2-cured strains is seen in the presence or absence of Gifsy-1 (22).
The Gifsy-2 phage has been shown to carry at least two virulence genes: sodCI and an as yet unidentified factor(s) (21). The gene sodCI encodes one of two periplasmic Cu/Zn superoxide dismutases of Salmonella (12, 20). Serovar Typhimurium strains mutant in sodCI are attenuated in macrophages as well as in mice (12). SodCI catalyzes the conversion of superoxide radical to hydrogen peroxide in the periplasm. Superoxide and nitric oxide production by macrophages is an important component of host defense against Salmonella (7, 13). Superoxide can react with nitric oxide to form toxic peroxynitrite (59). It has been proposed that SodCI protects Salmonella by depleting the superoxide pool and thereby preventing or reducing the level of peroxynitrite formation (12, 19). More recently, two groups have shown that srfH (60), or sseI (37), carried on Gifsy-2, is under the control of SsrAB, a two-component regulatory system encoded on Salmonella pathogenicity island 2 (SPI2), and is secreted by the SPI2 type III secretion system. Although srfH/sseI potentially participates in Gifsy-2 virulence, this has not been explicitly tested.
In this study, we have characterized a gene that contributes to the complex virulence of serovar Typhimurium. We show that the gene grvA (for “Gifsy-2-related virulence”) is located on the prophage Gifsy-2. A grvA null mutant showed an increase in virulence of serovar Typhimurium in mice as measured by intraperitoneal competition assays. Overexpression of grvA+ also increased virulence. The increased virulence phenotype is dependent on SodCI but independent of other virulence factors on Gifsy-2. These results suggest that in a wild-type situation, GrvA acts to decrease serovar Typhimurium virulence in mice. We have termed grvA an antivirulence gene.
MATERIALS AND METHODS
Bacterial strains, media, and genetic techniques.
The bacterial strains used are described in Table 1. All strains generated for this study are isogenic derivatives of the serovar Typhimurium strain 14028. Non-Typhimurium Salmonella strains were obtained predominately from the SARB collection (6). Strains were grown in or on Luria-Bertani (LB), NCE–0.2% glucose (33), or M63–0.2% glucose (46) medium. Ampicillin (100 μg/ml in LB, 10 μg/ml in minimal medium), tetracycline (25 μg/ml), kanamycin (50 μg/ml), and chloramphenicol (20 μg/ml) were used as selective antibiotics. In the process of optimizing Gifsy phage plaque formation, we developed G agar (10 g of tryptone, 5 g of NaCl, 5 g of K2HPO4, 2.5 g of glucose, 12.5 mg of Evans blue, 25 mg of uranine, and 15 g of agar per liter) and G top agar (G agar but 7 g of agar per liter). P22 transductions were performed according to previously described protocols using P22 HT 105/int-201 (33). DNA from a collection of MudP22 strains was obtained using a previously described protocol (61).
TABLE 1.
S. enterica serovar Typhimurium strains
| Strain | Genotype | Source or referencea |
|---|---|---|
| 14028 | Wild type | ATCCb |
| JS107 | 14028 zjg-8101::km | 34 |
| JS130 | 14028 zjg-8103::pir+ | 24 |
| JS150 | 14028 grvA1::MudCm | |
| JS151 | 14028 pTH1104 zjg-8101::km | |
| JS152 | 14028 pTH42 | |
| JS153 | 14028 pTH42 grvA1::MudCm | |
| JS154 | 14028 pTH1104 sodCI::aph | |
| JS155 | 14028 grvA1::MudCm zjg-8101::km | |
| JS156 | 14028 zcd-8109::MudCm zjg-8101::km | |
| JS157 | 14028 zcd-8110::MudCm zjg-8101::km | |
| JS158 | 14028 gftG1::MudCm zjg-8101::km | |
| JS159 | 14028 Δ(G-2 B) | |
| JS160 | 14028 zii-8104::Tn10dTc Gifsy-2[−] | |
| JS161 | 14028 Δ(G-2 B) grvA1::MudCm | |
| JS162 | 14028 pTH98 Δ(G-2 B) zjg-8103::pir+ | |
| JS163 | 14028 pTH98 zjg-8103::pir+ Gifsy-1[−] | |
| JS164 | 14028 pTH98 zjg-8103::pir+ Gifsy-1 [−] grvA1::MudCm | |
| JS178 | 14028 galE496 bio-104::Tn10 Gifsy-1[−] Gifsy-2[−] | |
| JS179 | 14028 grvA1::MudCm Gifsy-1[−] | |
| JS180 | 14028 gftG1::MudCm Gifsy-1[−] | |
| JS181 | 14028 zcd-8109::MudCm Gifsy-1[−] | |
| JS182 | 14028 zcd-8110::MudCm Gifsy-1[−] | |
| JS183 | 14028 sodCI::aph Gifsy-1[−] | |
| JS184 | 14028 attλ::pRA102::pTH1104 Gifsy-1[−] bio-104::Tn10 | |
| JS185 | 14028 attλ::pRA102::pTH42 Gifsy-1[−] bio-104::Tn10 | |
| JS186 | 14028 attλ::pRA102::pTH1104 | |
| JS187 | 14028 attλ::pRA102::pTH1104 pepN88::Tn10 Gifsy-2[−] | |
| JS188 | 14028 attλ::pRA102::pTH42 | |
| JS189 | 14028 attλ::pRA102::pTH42 pepN88::Tn10 Gifsy-2[−] | |
| JS190 | 14028 pTH98 grvA1::MudCm | |
| JS192 | 14028 sodCI::aph | |
| JS193 | 14028 sodCI::aph grvA1::MudCm | |
| JS194 | 14028 pTH98 zjg-8103::pir+ | |
| JS195 | 14028 pTH102 zjg-8103::pir+ | |
| JS197 | 14028 pTH98 zjg-8103::pir+ sodCI::aph | |
| MA5973 | 14028 Gifsy-1[−] | 22 |
| MA6275 | 14028 Gifsy-2[−] | 22 |
| TE3461 | LT2 hisD9953::MudCm hisA9944::Mud1 | 15 |
This study unless otherwise noted.
ATCC, American Type Culture Collection.
Gifsy phage titers.
Gifsy-1 or Gifsy-2 lysogens were grown in LB at 37°C overnight. One milliliter of culture was transferred to a microcentrifuge tube and vortexed briefly in the presence of approximately 250 μl of chloroform. After sitting at room temperature for 10 min, the cultures were centrifuged for 5 min at 12,000 × g. The supernatants were then transferred to clean microcentrifuge tubes and stored at 4°C in the dark for no longer than 3 days. These supernatants were considered Gifsy phage lysates. The recipient strain used for Gifsy plaque assays, JS178, was grown overnight in LB and subcultured 1:100 into the same medium and grown for 3 to 3.5 h at 37°C. Recipient subcultures (0.1 ml) were added to 3 ml of molten G top agar, poured on G agar plates, and allowed to solidify for 5 to 10 min. Gifsy lysates or dilutions of lysates (25 μl) were spotted and allowed to dry on the top agar. Plates were incubated at 25 to 30°C for 36 to 72 h before observation.
DNA sequence analysis and PCR.
DNA was sequenced at the W. M. Keck Center for Comparative and Functional Genomics, University of Illinois, Urbana-Champaign. DNA sequences were analyzed using National Center for Biotechnology Information (NCBI) BLAST searches and the Wisconsin Package (Genetics Computer Group, Inc.). PCRs were performed according to the Taq polymerase manufacturer's instructions (Life Technologies, Inc.).
Primers for DNA sequencing and PCR were synthesized at the W. M. Keck Center for Comparative and Functional Genomics. To amplify the grvA region, primers grvA P1 (5′-ACC TGC GGA TCC GGT ACA GGT CGG-3′), grvA P2 (5′-AAC TCC TAG GTT CTC CTC CAG AGT C-3′), and grvA P3 (5′-TGT TCT AGA TGA CGC CAG TCA GT-3′) were synthesized. The grvA P1 and P2 primers amplify a 550-bp fragment. The grvA P2 and P3 oligomers amplify a 930-bp product (Fig. 1A). For amplifying the region near grvA, we used primers of gftG (for “Gifsy-two gene G”), designated gftG P1 (5′-GGC CGG AGA TCT CGA ACA ATG-3′) and gftG P2 (5′-GAT GCT CAG AAT TCC GGT C-3′), which amplify a 1,020-bp product, and sodCI primers sodCI P1 (5′-GTA AAG CGG ATC CCT TAC CAC TGA C-3′) and sodCI P2 (5′-CGC CGG ATC CTC TTA ACC GTC TTT CAT TC-3′), which generate a 679-bp PCR fragment.
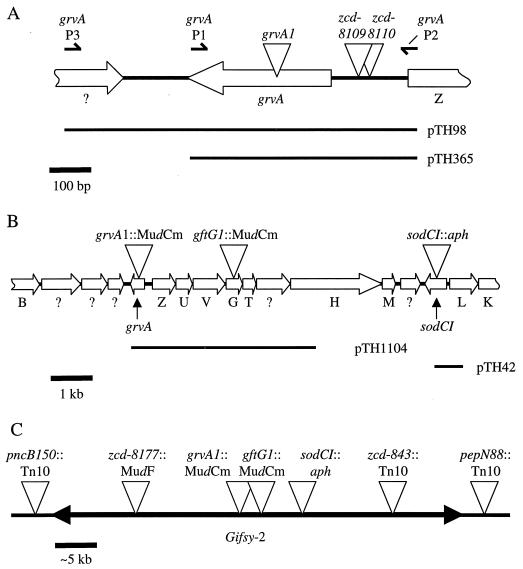
FIG. 1.
Genetic and physical maps of Gifsy-2. (A) Primers and MudCm insertions in the grvA region. The grvA P1, grvA P2, and grvA P3 primers are indicated. grvA1::MudCm is located 133 bp downstream of the putative start of the grvA open reading frame. The zcd-8109::MudCm marker is 66 bp upstream from the putative ATG initiation codon. Marker zcd-8110 is 95 bp upstream of the open reading frame and 29 bp from the zcd-8109 marker. (B) grvA-sodCI region of Gifsy-2. The relative distances and locations of grvA, sodCI, and the lambdoid tail genes are indicated. Open reading frames with single-letter designations are the gft equivalents of the lambda tail genes. Most of the open reading frames indicated with a question mark have weak homology to phage genes in the NCBI database. Marker insertions in grvA, gftG, and sodCI are also indicated. (C) Gifsy-2 region. The distances between the markers found on and near Gifsy-2 are based on genetic data from two and three factor crosses. The size of the Gifsy-2 genome is estimated to be 45 to 50 kb based on genetic data. Markers pncB150::Tn10 and pepN88::Tn10 are not located on the Gifsy-2 prophage. The breakpoints between chromosomal and phage DNA have not been precisely determined. In E. coli, pncB and pepN are separated by 260 bp.
For a control in PCRs, we designed two malate dehydrogenase primers, mdh P1 (5′-TGT ACG ACA TCG CTC CAG TGA C-3′) and mdh P2 (5′-ATG ATA TCC AGC GTG GTA ACG CC-3′), which generate a 370-bp product. These primers were designed to amplify a PCR product if chromosomal template is present and will amplify the mdh product from Escherichia coli and all Salmonella strains that we have tested.
Plasmid construction.
The plasmids used in this work require the Pi protein encoded by the pir gene in order to replicate autonomously (39). The parent plasmid of pTH1104 was the Pi-dependent pIVET1 plasmid (32, 49). The fusion plasmid pTH1104 was obtained from a random chromosomal library of partial Sau3AI-digested fragments (51). In pTH1104, the promoter of grvA drives transcription of the lacZ reporter. The sodCI P1-P2 PCR product was cloned into the BglII site of pIVET1 to create pTH42, in which the promoter of sodCI drives expression of the lacZ reporter. Plasmid pTH365 was constructed by inserting the BamHI-digested grvA P1-P2 PCR product into the BglII site of the Pi-dependent plasmid pGP704 (39). The pTH98 plasmid contained the grvA P2-P3 PCR product (BamHI and XbaI digested) cloned into the BglII-to-XbaI fragment of pGP704 (Fig. 1A). Plasmid pTH102 was generated by recombining the grvA1::MudCm allele onto plasmid pTH98 by P22 transduction (34).
Isolation of MudCm insertions in and near grvA.
MudCm was transposed onto the Pi-dependent plasmids pTH365 and pTH1104 as previously described using TE3461 as a MudCm donor (15). About 5,000 to 10,000 plasmid-bearing colonies containing random MudCm transpositions were pooled. Plasmids from these strains were isolated and used to transform a Pi+ recipient (JS130), selecting for chloramphenicol resistance to identify plasmids containing MudCm insertions. P22 lysates grown on the pooled chloramphenicol-resistant transformants were used to transduce the Pi− wild-type strain (14028) selecting for chloramphenicol resistance (34). The resulting transductants were screened for loss of the integrated plasmid (Aps). Only MudCm insertions in the chromosomal insert of the original plasmid were able to lose the Apr plasmid. This procedure allowed us to rapidly identify and isolate MudCm insertions in the desired region of DNA. MudCm insertions of interest were recombined back onto the respective plasmid and the exact location of the MudCm insertion with respect to grvA was determined by DNA sequence analysis.
Construction of a Gifsy-2 B deletion-insertion.
Deletion of the Gifsy-2 B region and concomitant insertion of a kanamycin cassette were carried out using the technique developed by Datsenko and Wanner (11). A 60-bp primer, B2–5 (5′-CTT CAC AAA CTG ACC GGA TTT CCG TCT CTT TCG TTT AAC ATG TAG GCT GGA GCT GCT-3′), and a 44-bp primer, G2pepNP2 (5′-CAG GCA CAA ATG GCC TAT TTG CTA CAT ATG AAT ATC CTC CTT AG-3′), were synthesized. These primers were used to PCR amplify from the plasmid pKD4 (11) a kanamycin resistance cassette fragment flanked by approximately 30-bp sequences corresponding to just downstream of the phage late operon gene J (B2–5) and the putative attR site of Gifsy-2 (G2pepNP2). This product was transformed into a Gifsy-1[−] strain, MA5973, containing the pKD46 plasmid, which is temperature sensitive and carries the lambda red, gam, and bet genes under arabinose control. The transformants resulting from this procedure were moved into a clean wild-type background (14028) by P22 transduction. The location of these kanamycin resistance markers was checked by P22 linkage to known markers in and surrounding Gifsy-2.
Virulence assays.
All strains used in virulence assays were derivatives of 14028, JS107, or JS130. Neither the Kmr insertion in JS107 nor the pir+ gene in JS130 has any effect on virulence (data not shown). Strains were grown overnight in LB broth at 37°C with aeration. For intraperitoneal (i.p.) assays, bacteria were washed and diluted in saline. For orally inoculated assays, overnight cultures were washed and diluted in 0.2 M H2NaPO4 (pH 8.0) buffer. For both oral and i.p. assays, BALB/c mice or C3H/HeN mice (Harlan Sprague Dawley, Inc.) were inoculated with equal mixtures of mutant and wild-type strains at 2 to 3 log units above the 50% lethal dose (LD50). This corresponds to 200 to 500 bacteria routinely inoculated in i.p. assays. Inocula were plated on LB plates and then replica plated onto appropriate selective media to determine the total number and percentage of mutant bacteria inoculated.
Mice inoculated i.p. were sacrificed after 4 to 5 days, and their spleens were removed. Orally inoculated mice were sacrificed after 6 to 8 days, and the spleens and the small intestines were removed. All organs were homogenized, diluted, and plated on LB plates. We routinely recover 107 to 108 bacteria per spleen. Replica plating on selective media allowed us to determine the percentage of mutant bacteria. The competitive index (CI) was calculated as (% mutant recovered/% wild type recovered)/(% mutant inoculated/% wild type inoculated). The competitive index of each set of assays was analyzed statistically using Student's t test. Each mutant strain was reconstructed at least once to ensure that the virulence phenotype is the result of the designated mutation. For each mutant or plasmid-bearing strain, between 7 and 25 mice were used.
In vitro growth assays.
Equal volumes of overnight cultures of mutant and wild-type bacteria were mixed and inoculated into LB medium (200 to 1,000 bacteria/2 ml of medium) or M63–0.2% glucose medium (4,000 to 10,000 bacteria/2 ml of medium). Each inoculum was plated on lab medium to precisely determine the number of bacteria and the percentage of mutants in the mixture. After 16 h (LB medium) or 48 h (minimal medium) of growth at 37°C with aeration, cultures were diluted in saline and plated on lab media. The percentages of mutant bacteria recovered were analyzed as in the in vivo competition assays. Between 5 and 12 competition assays were performed for each mutant or plasmid-bearing strain.
To determine growth curves, each culture was grown overnight in LB or NCE–0.2% glucose medium. Overnight cultures were subcultured 1:100 into rich or minimal medium and incubated at 37°C with constant aeration for 24 h in an ELx808 plate reader (Biotek Instruments, Inc.).
Integration of grvA-lacZ and sodCI-lacZ fusions at the λ attachment site.
The Pi-dependent pTH1104 and pTH42 plasmids were moved into a Gifsy-2[−] strain containing the pRA102 plasmid (9) integrated at the attλ of serovar Typhimurium. Plasmid pRA102 and the lacZ fusion plasmids pTH1104 and pTH42 share approximately 700 bp of homology. Therefore, the fusion plasmids integrate into pRA102 by homologous recombination. This was verified by cotransduction of the pRA102 and the fusion plasmids. Once verified, the attλ::pRA102::pTH1104 or attλ::pRA102::pTH42 constructs were P22 transduced into the Gifsy-1[−] Gifsy-2[−], Gifsy-1[−] Gifsy-2[+], Gifsy-1[+] Gifsy-2[−], and Gifsy-1[+] Gifsy-2[+] backgrounds.
β-Galactosidase assays.
The β-galactosidase activities of the grvA-lacZ and sodCI-lacZ fusions were determined using a microtiter plate assay as described elsewhere (50). Strains containing the fusion plasmids integrated into the chromosome were grown overnight in LB or NCE–0.2% glucose medium with ampicillin. The bacteria were then subcultured 1:100 into the tested condition in the absence of antibiotic for 3 to 4 h. The fusion strain was tested for induction in the presence and absence of magnesium or iron, as well as at various pHs and at different levels of oxygen (unaerated overnight culture), H2O2 (10 μM, 0.1 mM, and 1 mM), and nalidixic acid (0.2, 1, and 2 μg/ml).
Nucleotide sequence accession number.
The grvA sequence has been entered into GenBank under accession number AF266469.
RESULTS
Identification and sequence analysis of grvA.
The grvA (“Gifsy-2-related virulence”) gene was initially identified in a region of serovar Typhimurium chromosome with no orthologs in E. coli (5). Since many regions of the serovar Typhimurium chromosome that are not found in E. coli have been shown to harbor virulence genes (35, 36), we investigated whether grvA has any role in pathogenesis. The original grvA-purA/lacZ fusion plasmid (pTH1104), which was used to identify and study grvA expression and virulence, was isolated from a chromosomal library (51) in pIVET1 (32, 49). Based on DNA sequence analyses of this plasmid and other clones of this region, we determined that grvA encodes a single open reading frame of 351 bp. The grvA sequence had no significant homology to any gene or protein in the NCBI database.
Located 180 bp upstream of grvA are putative lambdoid phage tail genes (Fig. 1B). These genes are organized in an operon similar to the late operon of phage Lambda. Inserted in this operon is the gene grvA, which is transcribed in an orientation opposite to that of the tail genes. Subsequent analysis showed that the sodCI gene is located on a separate insertion in this tail gene operon 6.7 kb 5′ from grvA and is also transcribed in a direction opposite to that of the tail genes (Fig. 1B). The presence of phage tail genes suggested that grvA was carried on a phage and could have been horizontally transmitted, much like other known virulence factors (35, 36).
Isolation of MudCm insertions in and near grvA.
To determine if grvA played a role in virulence, we first isolated insertion mutations in and near grvA by transposing MudCm onto two Pi-dependent plasmids containing grvA (see Materials and Methods). The plasmid pTH365 contains 210 bp upstream of grvA and 340 bp of grvA itself (Fig. 1A). The pTH1104 plasmid carries the region of DNA from the middle of gftH (for “Gifsy-two tail gene H”) through 340 bp of grvA (Fig. 1B). The exact locations of MudCm insertions in the cloned fragments were determined by DNA sequence analysis. Of the three insertions isolated using pTH365, one was located in grvA and two were just upstream of the open reading frame (Fig. 1A). An insertion isolated on pTH1104 was located in the tail gene gftG (Fig. 1B). These MudCm insertions were recombined onto the chromosome of an otherwise wild-type strain via P22 transduction (34).
The grvA gene is carried on the Gifsy-2 phage
To determine the location of grvA in the chromosome, primers grvA P1 and P2 (Fig. 1A) were used in PCRs with template DNA isolated from a set of MudP22 phage lysates. MudP22 lysogens, which contain prophage integrated at various locations in the chromosome (61), were induced and template DNA was isolated from the resulting phage particles. In these reactions, the mdh (malate dehydrogenase) P1 and P2 primers were used as controls to measure the level of contaminating chromosomal DNA. We found the strongest grvA P1-P2 PCR products with DNA isolated from MudP22 phage located at 23 to 25 centisomes (data not shown). It had been shown that the Gifsy-2 phage was located at 23.8 centisomes by pulsed-field gel electrophoresis of BlnI-restricted chromosomal DNA (22). These results suggested that grvA was in the region carrying Gifsy-2.
Two and three factor crosses were performed with the insertions sodCI::aph, gftG1::MudCm, grvA1::MudCm, gftO8177::MudF, pncB150::Tn10, pepN88::Tn10, and pepN104::MudJ. It had previously been shown that the sodCI and gftO genes are located on Gifsy-2 and that pepN and pncB are located just outside Gifsy-2 (21). Our results indicated the order of and relative distances between the genes in this region as shown in Fig. 1C. According to these data, grvA is between pncB and pepN, the markers known to flank Gifsy-2 (21).
The presence of grvA on the Gifsy-2 prophage was confirmed by PCR analysis. The grvA P2 and P3, gftG P1 and P2, and sodCI P1 and P2 primers were used in individual PCRs with the mdh primers as positive controls in all reactions to amplify products using chromosomal DNA templates from the wild-type strain (14028, Gifsy-2[+]) and the Gifsy-2 cured strain (MA6275, Gifsy-2[−]). The grvA, gftG, and sodCI primers did not amplify products from the Gifsy-2[−] strain. In contrast, all primers amplified the appropriately sized products when the wild-type 14028 was used as template. The internal control mdh primers gave the appropriate product in all reactions (data not shown). The lack of grvA, gftG, and sodCI PCR products in the Gifsy-2[−] strain confirmed that these regions of DNA are all located on the Gifsy-2 phage. Thus, the results from genetic, PCR, and DNA sequence analyses established that grvA is located on Gifsy-2 near sodCI.
Loss of grvA function increases virulence.
The virulence phenotypes of the insertion mutants were determined in i.p. and oral competition assays using BALB/c mice. Each MudCm insertion mutant was competed against an isogenic wild-type strain (JS107). In i.p. studies using BALB/c mice, both the grvA null mutant (JS155) and the strain containing the zcd-8109 insertion located 66 bp upstream of the grvA open reading frame (JS156) outcompeted the wild type (Table 2). While statistical analysis showed that both of these strains were significantly more virulent than the wild type, there was no statistical difference between the two insertion strains. This suggested that mutants of grvA were more virulent and that the insertion of MudCm 66 bp upstream of grvA had an effect similar to that of a deletion of grvA.
TABLE 2.
Results of competition assays with MudCm insertion mutants
| Relevant genotypea | Median CIb | No. of micec | Pd |
|---|---|---|---|
| grvA1::MudCm | 3.8 | 25 | <0.0005 |
| grvA1::MudCm | 3.5 | 12 (C3H/HeN) | 0.063 |
| grvA1::MudCm/pgrvA+ single copy | 0.63 | 7 | NS |
| zcd-8109::MudCm | 13 | 8 | 0.047 |
| zcd-8110::MudCm | 1.1 | 17 | NS |
| gftG1::MudCm | 0.99 | 10 | NS |
Strains JS155, JS190, JS156, JS157, and JS158 were competed against the isogenic wild-type strain 14028 or JS107 (zjg-8101::km).
CI = (mutant/wild-type output)/(mutant/wild-type inoculum).
All assays were performed i.p. using BALB/c mice except where noted.
Student's t test was used to compare output versus inoculum. NS, not significant.
Other MudCm insertions farther upstream of grvA showed no such increase in virulence. The strain JS158, containing an insertion in gftG, located 2 kb from grvA, competed evenly with the wild-type strain. This demonstrated that MudCm insertions in Gifsy-2 per se would not cause the antivirulence phenotype shown by the grvA null mutants (Table 2). The mutant containing the zcd-8110 insertion located 95 bp upstream of the grvA open reading frame (JS157) also competed evenly with the wild type. Thus, although the zcd-8110::MudCm insertion was only 29 bp upstream of the zcd-8109 insertion, zcd-8110 did not show the grvA null antivirulence phenotype. Both the zcd-8109 and the zcd-8110 markers have the MudCm insertion oriented in the same direction with respect to the grvA promoter. A possible explanation for this result is that part of the grvA promoter region lies between 66 and 95 bp upstream of the grvA open reading frame. Therefore, while the zcd-8110 insertion had no effect on virulence, the zcd-8109 insertion resulted in a grvA null virulence phenotype.
In order to prove that the increased virulence phenotype of the grvA1::MudCm insertion is due to loss of grvA function, we performed a complementation test. The Pi-dependent plasmid pTH98 contains only the grvA+ open reading frame with approximately 300 bp on either side of the gene, including the putative promoter region of grvA (Fig. 1A). Plasmid pTH98 was integrated into the chromosome of the grvA1::MudCm strain to create JS190. In i.p. competition assays, the strain containing the grvA1::MudCm insertion and the single-copy pgrvA+ plasmid (JS190) competed evenly with the isogenic wild-type strain (14028; Table 2). Colonies recovered from the animal were tested to ensure that both the plasmid and the grvA1::MudCm allele were present. Thus, the single copy of grvA+ was able to restore the wild-type virulence phenotype, suggesting that the grvA1::MudCm phenotype was due to a loss of function of wild-type grvA.
The Nramp1 defect of BALB/c mice is a major factor in the susceptibility of these mice to Salmonella infection (28, 54). Although BALB/c (Nramp1−) and C3H/HeN (Nramp1+) mice vary in a number of genetic loci, a major difference in their susceptibility to Salmonella infection is in the Nramp1 gene (43). To determine whether the grvA virulence phenotype would be altered in Nramp1+ mice, we competed the grvA null mutant (JS155) against wild type in i.p. assays using C3H/HeN mice (Table 2). The results of the competition showed that the grvA null mutant outcompeted the wild type in the C3H/HeN mice. However, these results were not as statistically significant as the results of competition assays with BALB/c mice (Table 2). Thus, the Nramp1 defect does not appear to play a major role in the grvA phenotype.
The results of oral competition assays using the MudCm mutants and plasmid-bearing strains showed virulence phenotypes similar to those obtained in the i.p. competition assays. However, due to greater variability in oral assays, the results were not as statistically reliable as those of the i.p. competition assays (data not shown).
Overexpression of GrvA increases virulence.
To determine the effect of GrvA overexpression on virulence, the Pi-dependent grvA+ plasmid (pTH98) was moved into a strain producing Pi protein (JS130) to generate a strain containing a multicopy grvA+ plasmid (JS194). Competition assays showed that this strain (JS194) outcompeted the wild-type strain sixfold (Table 3). Thus, overexpression of GrvA increased virulence. This phenotype is analogous to the phenotype conferred by the grvA1::MudCm insertion in the chromosome. As a control for this experiment, we recombined the grvA1::MudCm insertion onto the plasmid. A grvA+ strain containing the grvA1::MudCm plasmid pTH102 in multiple copies (JS195) competed evenly with the wild type (Table 3). This result showed that the overproduction phenotype is dependent on grvA and provided further evidence that the grvA1::MudCm insertion is recessive.
TABLE 3.
Results of competition assays with plasmid-bearing strains
| Plasmid genotype | Chromosomea | Median CIb | No. of micec | Pd |
|---|---|---|---|---|
| grvA+ (pTH98) | Wild type | 6.3 | 16 | 0.0086 |
| grvA1::MudCm (pTH102) | Wild type | 1.1 | 8 | NS |
| grvA+ (pTH98) | sodCI::aph | 0.05 | 8 | 0.0099 |
All plasmid-bearing strains used in these competitions contain the pir+ gene (zjg-8103::pir+) inserted into the chromosome. Strains JS194, JS195, and JS197 were competed against the wild-type strain 14028. Previous studies have shown that the zjg-8103::pir+ allele has no effect on virulence.
CI = (mutant/wild-type output)/(mutant/wild-type inoculum).
All assays were performed i.p. using BALB/c mice.
Student's t test was used to compare output versus inoculum. NS, not significant.
GrvA does not affect growth in vitro.
In order to determine whether the virulence phenotypes seen in vivo were due to simple growth effects, we tested the strains used in the competition assays for in vitro growth defects or advantages. None of the insertion mutants or plasmid-bearing strains showed any growth advantage or defect compared to the wild-type strain when grown in LB or minimal-glucose M63 or NCE medium (median CI, 0.64 to 1.53 in all cases). To further show that the MudCm insertions conferred no growth phenotype, we determined growth curves for each insertion strain. For all MudCm insertion mutants, growth curves in LB or glucose-NCE medium were indistinguishable from those of the wild type. These data suggested that the MudCm insertions did not confer a simple growth advantage or defect that could explain the in vivo virulence phenotype.
The grvA phenotype is dependent on SodCI.
The other known virulence gene on Gifsy-2 is sodCI. Because of the proximity of the genes, we investigated whether grvA and sodCI interact with one another. If they act independently, we would expect that a grvA sodCI double mutant would have an intermediate phenotype because the decrease in virulence due to sodCI would be partially compensated for by the increase in virulence conferred by a grvA mutation. In contrast, if GrvA and SodCI are involved in the same function, then either the sodCI or the grvA mutation should be epistatic to the other.
We competed grvA1::MudCm and sodCI::aph single and double mutants against the wild type and each other to determine the effects of these mutations on virulence. The sodCI::aph mutant (JS192) was attenuated six- to sevenfold compared to the wild type (Table 4). A strain (JS193) containing both the grvA1::MudCm and the sodCI::aph mutations was attenuated to the same extent as the wild type (Table 4). Thus, the virulence phenotype of the double mutant was the same as that of the sodCI single mutant. When the double mutant (JS193) was assayed against the sodCI mutant (JS192), these strains were shown to compete evenly with one another. Thus, sodCI is epistatic to grvA. This suggested that GrvA and SodCI do not act independently but that GrvA requires SodCI function to elicit a phenotype.
TABLE 4.
Results of competition assays with Gifsy-2 mutants
| Strain Aa | Strain B | Median CIb | No. of micec | Pd |
|---|---|---|---|---|
| sodCI | Wild type | 0.15 | 9 | <0.0005 |
| grvA sodCI | Wild type | 0.20 | 25 | <0.0005 |
| grvA sodCI | grvA | 0.12 | 9 | <0.0005 |
| grvA sodCI | sodCI | 0.76 | 15 | NS |
| Gifsy-2[−] | Wild type | 0.0030 | 8 | <0.0005 |
| Δ(G-2 B) | Wild type | 0.11 | 7 | <0.0005 |
| grvA Δ(G-2 B) | Δ(G-2 B) | 6.7 | 3 | 0.023 |
| pTH98 Δ(G-2 B) | Δ(G-2 B) | 17 | 3 | 0.059 |
The strains used were 14028, JS192, JS193, JS150, JS160, JS159, JS161, and JS162. The Gifsy-2[−] strain (JS160) contains the zii-8014::Tn10dTc marker, which has no effect on virulence. The pTH98 Δ(G-2 B) strain contains the zjg-8013::pir+ allele, which has no effect on virulence.
CI = (strain A/strain B output)/(strain A/strain B inoculum).
All assays were performed i.p. using BALB/c mice.
Student's t test was used to compare output versus inoculum. NS, not significant.
To confirm the above hypothesis, we tested the virulence phenotype of a sodCI strain that overexpressed wild-type GrvA. The Pi-dependent grvA+ plasmid was moved into a pir+ sodCI strain. In i.p. competition assays, the resulting strain (JS197) was attenuated with respect to the wild-type strain (Table 3). Thus, although overexpression of grvA+ in an otherwise wild-type background increased virulence (Table 3), overexpression of grvA+ in the sodCI strain could not rescue the decreased virulence phenotype of the sodCI mutation. This further suggested that GrvA requires SodCI to exert its effect on virulence.
Additional virulence factors on Gifsy-2 are independent of GrvA.
It had previously been shown that an additional virulence factor(s) was located on the Gifsy-2 phage (21). To explicitly test this, we competed a strain (JS160) cured of Gifsy-2 against the isogenic wild-type strain in i.p. assays. The Gifsy-2[−] strain was attenuated >100-fold relative to the wild-type strain (Table 4). This is much greater than the sixfold attenuation conferred by the sodCI insertion. This suggests that Gifsy-2 must contribute another virulence factor(s) in addition to SodCI.
In phage lambda, genes located in the region between the phage gene J and attR, termed the B region, are not necessary for phage production (8). To determine the location of additional virulence genes on the Gifsy-2 phage, we deleted the analogous B region of the Gifsy-2 phage and inserted a kanamycin resistance cassette, designating the mutation Δ(G-2 B). Note that this deletion does not affect grvA or sodCI. Isogenic Δ(G-2 B) grvA+ (JS159), grvA null (JS161), and grvA+-overexpressing (JS162) strains were constructed and tested for virulence defects in competition assays. When the Δ(G-2 B) mutant was competed against its isogenic wild-type parent, we found that the mutant was attenuated ninefold (Table 4). This suggested that the Gifsy-2 B region contains a virulence gene(s) that contributes to the attenuation of a strain cured of the Gifsy-2 phage. Moreover, this deletion conferred approximately the same level of attenuation as a sodCI mutation.
To determine if the virulence gene(s) in the Gifsy-2 B region was affected by grvA, we competed the Δ(G-2 B) mutant against the isogenic Δ(G-2 B) mutants with altered grvA levels. In competition assays, the Δ(G-2 B) mutant was outcompeted six- to sevenfold by the Δ(G-2 B) grvA double mutant. Likewise, the grvA+-overexpressing Δ(G-2 B) strain outcompeted the Δ(G-2 B) mutant 16- to 17-fold. These results showed that the grvA antivirulence phenotype is not affected by the deletion of the virulence gene(s) in the Gifsy-2 B region. By the arguments outlined above, these results suggest that GrvA acts independently of the virulence factors encoded in the B region of the phage.
In vitro expression of grvA and sodCI.
Our competition assays suggest that GrvA depends on SodCI function to confer a phenotype. We asked if this dependence takes place at the transcriptional level by monitoring the expression of grvA and sodCI using transcriptional lac fusions (50). We integrated grvA-lacZ or sodCI-lacZ fusion plasmids (pTH1104 or pTH42, respectively) at the Salmonella attλ site in Gifsy-2[+] and Gifsy-2[−] strains or in Gifsy-1[+] and Gifsy-1[−] strains (see Materials and Methods). All of the grvA-lacZ fusion strains produced 9 to 10 U of β-galactosidase activity. Similarly, all of the sodCI-lacZ fusion strains produced 5 to 6 U of β-galactosidase activity. The data suggest that both grvA and sodCI are expressed at low but detectable levels and that the transcription of neither grvA nor sodCI was affected by any element carried on the Gifsy phages under these in vitro conditions.
To explicitly determine the effects of grvA on sodCI expression, the sodCI-lacZ fusion plasmid pTH42 was integrated into the chromosome of the grvA null mutant (JS150) to make JS153. β-Galactosidase assays were performed for JS153 and the isogenic wild-type fusion strain (JS152). These assays showed that sodCI expression was the same in both grvA and grvA+ backgrounds (5 to 6 U). In addition, the integrated grvA-lacZ fusion plasmid (pTH1104) was moved into a strain containing the sodCI::aph mutation (19) to create JS154. β-Galactosidase assay results indicated that the expression of the grvA-lacZ fusion did not change in a sodCI null background compared to the isogenic sodCI+ strain. Thus, grvA and sodCI apparently do not interact at the transcriptional level.
Many virulence genes are regulated in response to environmental conditions (31). We examined the expression of grvA under different growth conditions by measuring lacZ activity produced from the integrated grvA-lacZ fusion strain, JS151. The grvA-lacZ fusion was neither repressed nor induced in excessive or limiting iron or magnesium. Expression of grvA was also not affected when the fusion strain was grown under semianaerobic conditions or subcultured into media at pH 3, 5, or 7.
The expression of grvA and sodCI was also monitored after exposure to H2O2 and nalidixic acid, reagents that have been shown to induce production of Gifsy phage (21). We assayed the grvA-lacZ and sodCI-lacZ fusions integrated at the attλ site in Gifsy-2[+] and Gifsy-2[−] strains (JS186 and JS187 for grvA and JS188 and JS189 for sodCI). These strains were exposed to different levels of H2O2 and nalidixic acid concentrations below the levels necessary to induce production of Gifsy phage. The expression of grvA and sodCI was the same in the presence and absence of H2O2 and nalidixic acid. Therefore, neither H2O2 nor nalidixic acid apparently affects grvA or sodCI expression in the presence or absence of Gifsy-2.
Effects of insertion mutations on Gifsy-2 phage production.
It is possible that the phenotype conferred by the grvA insertion is due to some effect on Gifsy-2 phage biology. To address this question, we determined the titer of Gifsy-2 phage produced by strains containing the insertion mutations or plasmids. The presence of Gifsy-1 greatly decreases the production of Gifsy-2 (21). Therefore, it was necessary to move each insertion and plasmid clone into a Gifsy-1[−] Gifsy-2[+] background (MA5973) to determine the effect on Gifsy-2 production. For each strain, the titers of between 5 and 16 independent overnight cultures were determined for Gifsy-2 phage. The recipient strain used to plaque phage was a galE Gifsy-1[−] Gifsy-2[−] strain (JS178).
All MudCm insertions significantly decreased Gifsy-2 phage production. The grvA1::MudCm Gifsy-1[−] strain (JS179) showed a >200-fold decrease in phage titer from that of the control Gifsy-1[−] Gifsy-2[+] strain (MA5973; Table 5). Likewise, the Gifsy-1[−] strains containing the marker gftG1::MudCm (JS180), zcd-8109::MudCm (JS181), or zcd-8110::MudCm (JS182) all showed a >200-fold decrease in phage production from that of the wild-type Gifsy-1[−] Gifsy-2[+] strain (MA5973; Table 5). The simplest explanation for loss of Gifsy-2 production in the MudCm insertion mutants is that the addition of 3.4 kb of MudCm DNA prevents packaging of the complete phage genome. The fact that all of the MudCm insertions blocked phage production, yet only a subset conferred a virulence phenotype, suggests that effects on Gifsy-2 production per se are not responsible for the grvA phenotype.
TABLE 5.
Gifsy-2 phage production
| Donor | Relevant genotypea | Avg PFU/mlb |
|---|---|---|
| MA5973 | Gifsy-2[+] | 5.8 × 103 |
| JS179 | grvA1::MudCm | <40 |
| JS180 | gftG1::MudCm | <40 |
| JS181 | zcd-8109::MudCm | <40 |
| JS182 | zcd-8110::MudCm | <40 |
| JS183 | sodCI::aph | 3.9 × 103 |
| JS163 | pgrvA+/Gifsy-2[+] | 6.4 × 103 |
| JS164 | pgrvA+/grvA1::MudCm | <40 |
All strains were Gifsy-1[−].
Donor lysates were prepared as described in Materials and Methods. All experiments were performed on 5 to 15 independent lysates. Deviations from the average were insignificant.
In contrast, the Gifsy-1[−] Gifsy-2[+] strain containing the sodCI::aph insertion-deletion (JS183) produced a phage titer comparable to that of the wild-type control (MA5973; Table 5). These results show that sodCI does not affect Gifsy-2 phage production. Again, virulence defects and phage production are genetically separable. This result also suggests that the 1-kb insertion associated with the sodCI::aph mutation does not disrupt phage production.
To further show that GrvA did not affect Gifsy-2 phage production, we tested the effects of overexpression of GrvA. The pir+ Gifsy-1[−] strain bearing the grvA+ multicopy plasmid pTH98 (JS163) showed the same titer of Gifsy-2 phage production as that shown by the wild-type control (MA5973; Table 5). The grvA1::MudCm pir+ Gifsy-1[−] strain bearing the grvA+ multicopy plasmid (JS164) did not produce detectable Gifsy-2 phage (Table 5). We concluded that overexpression of GrvA had no effect on Gifsy-2 phage production. In addition, the grvA+ plasmid could not rescue the effect of grvA1::MudCm on phage production. This further suggested that it was the insertion of MudCm itself and not the loss of grvA that decreased Gifsy-2 phage production in the grvA1::MudCm strain.
Presence of grvA and sodCI in S. enterica serovars.
We were interested in determining the evolutionary distribution of grvA and sodCI in other S. enterica serovars. To investigate this, the PCR primers grvA P1 and P2 and sodCI P1 and P2 were used to screen the prevalence of grvA and sodCI in various S. enterica serovars. Primers mdh P1 and P2 were used as a positive control for the PCRs. The grvA fragment was found in serovar Typhimurium 14028 and LT2 and serovars Heidelberg (SARB23), Newport (C259), and Duisberg (SARB15). It was not found in serovars Typhi, Enteriditis, Saintpaul4 (SARB56), Derby1 and -13 (SARB9 and -10), Agona (SARB1), Salinatis, Brandenburg (SARB3), Stanley (SARB60), Wein (SARB71), Schwartzegrund (SARB57), and Muenchen (SARB32). The sodCI fragment was present and absent in the same strains as grvA with the exception of serovar Haifa (SARB22), which did yield a sodCI product but failed to amplify a grvA product.
DISCUSSION
Genetic analysis suggests that the Gifsy-2 bacteriophage contributes significantly to Salmonella pathogenesis. S. enterica serovar Typhimurium strains cured of Gifsy-2 are over 100-fold reduced in their virulence. This defect is in part due to loss of the periplasmic superoxide dismutase, SodCI, which is encoded on Gifsy-2. In addition, Gifsy-2 encodes additional virulence factors, at least some of which are encoded in the B region of the phage. We have shown that null mutations in a Gifsy-2 gene, which we have termed grvA, increased the virulence properties of serovar Typhimurium as measured in i.p. competition assays. While this null phenotype could be complemented in single copy, overexpression of wild-type GrvA also resulted in an increase in virulence. Neither the null mutant nor the strain overexpressing GrvA had any growth defect or advantage compared to the wild-type when grown in rich or minimal lab medium. The increased virulence in both cases was dependent on the presence of wild-type sodCI but independent of other virulence factors encoded by the phage.
These lines of evidence suggest that, in a wild-type situation, the grvA gene function decreases the virulence of serovar Typhimurium. Hence, we have termed this gene an antivirulence factor. Our data suggest that GrvA functions in the same pathway as SodCI. This is based on the fact that sodCI is epistatic to the grvA null mutation or grvA overexpression. Although the precise targets protected by SodCI are not known, it is believed that SodCI increases virulence of serovar Typhimurium by reducing the antimicrobial effects of the oxidative burst produced by host macrophages (12). One could argue that the defect conferred by the sodCI mutation is so severe that the grvA phenotype would never be observed, regardless of the mechanism of action. However, altering grvA levels increased virulence in the Gifsy-2 B-region deletion strain to the same extent as in the wild-type background, even though the Δ(G-2 B) strain is attenuated to approximately the same degree as the sodCI mutant (nine- versus sevenfold, respectively), and both defects are the result of mutations in Gifsy-2. Thus, the inability of grvA to exert a phenotype in the sodCI background is apparently specific. We conclude that GrvA function normally requires SodCI.
We suggest three models for GrvA function. The first model states that GrvA acts in a complex or network that either directly affects SodCI or affects the same pathway as SodCI. This complex may also require other gene products. Loss of GrvA or increased amounts of GrvA would then disrupt the complex or network. Although we believe that this general model best explains our data, GrvA could be acting in a number of ways. For example, the simplest variation would have GrvA affecting the enzymatic activity of SodCI, either directly or indirectly. An alternative is that GrvA somehow affects the target of superoxide or facilitates superoxide-related damage.
A second model suggests that GrvA is a regulator of virulence, affecting the expression of sodCI or other genes involved in sodCI function. A search of the NCBI database shows that grvA is not homologous to any known sequences at either the protein or the DNA level. Thus, if grvA is a regulator of gene expression, it does not easily fall into a previously identified class. We provide direct evidence that grvA is not transcriptionally regulating sodCI. We have also shown that other genes on Gifsy-1 and Gifsy-2 do not contribute to the regulation of grvA and sodCI expression in laboratory media. Fang et al. (19) reported that expression of a sodCI-lacZ fusion carried on a plasmid was induced in stationary phase. We did not observe stationary-phase induction of our integrated sodCI-lacZ fusion (data not shown). It is possible that the expression of these genes is regulated in the animal and that our conditions do not elicit this regulation. However, an IVET fusion (48, 49) to grvA does not survive in the animal (data not shown). Given the level of expression of grvA, survival in an IVET selection would require an estimated five- to sixfold induction (48). This suggests that grvA is not significantly induced in vivo. Indeed, our results suggest that grvA and sodCI are constitutively expressed at a low level. Although we conclude that GrvA is not a transcriptional regulator of sodCI, we cannot rule out the possibility that GrvA is regulating some other gene or that regulation of SodCI or other gene products takes place at a posttranscriptional level.
A third model suggests that GrvA affects Gifsy-2 phage biology and thereby indirectly affects virulence. We provide direct evidence that this is not the case. Gifsy-2 phage production and virulence are genetically separable. Although all the MudCm insertions abolish phage production, they have different virulence phenotypes. Two MudCm insertions (grvA1::MudCm and zcd-8109::MudCm) increase virulence, while two insertions (zcd-8110::MudCm and gftG1::MudCm) have no effect on virulence (Table 2). Likewise, while neither the sodCI insertion nor the grvA+ plasmid has an effect on Gifsy-2 phage production, they have opposite effects on virulence (Tables 3 and 4). These results suggest that GrvA has no direct effect on phage production. In addition, the data indicate that functional Gifsy-2 phage production is not necessary for virulence of serovar Typhimurium. It should be noted, however, that these insertion mutations in the phage genome most likely affect phage production by interfering with the packaging of phage DNA late in the induction process. Thus, although we can show that phage production is not required for virulence, these results do not address phage induction with a potential increase in virulence gene expression that could be associated with DNA replication of the phage. It has been suggested that, in the macrophage, oxidative stress might induce replication of the Gifsy phages (21). However, since phage induction is presumably lethal to the cell, this type of mechanism would require that the cells that induce phage provide some function that allows noninduced cells to better survive the host immune response. This would be analogous to the proposed mechanism of Shiga toxin production in lysogenic E. coli (42, 55).
What we refer to as antivirulence properties should not be confused with the term “avirulence” used in the plant pathogen field. The products of avirulence genes interact directly with host factors called R factors to elicit a host hypersensitivity response to the pathogen. Avirulence gene products are introduced to the host cell via a type III secretion system (10). We have no evidence to suggest that the GrvA behaves as an avirulence gene in the accepted use of the term.
Why would an organism intentionally decrease virulence? There is a general view that pathogens will naturally evolve toward a less-virulent, symbiotic relationship that does not destroy the host and thereby allows their continued propagation (17, 18, 53). Although this argument is affected by the transmissibility of the pathogen (18), the lower virulence of nontyphoid Salmonella than of typhoid Salmonella is cited as a specific example of evolution towards decreased virulence (17). However, examples of null mutations that increase the virulence of a pathogen are few. Lockman and Curtiss (29, 30) showed that serovar Typhimurium mutants that did not make type 1 fimbriae had a statistically significant 1.8-fold-lower oral LD50 than that of an isogenic Fim+ strain. Subtle effects could also be detected in competition assays with these strains. There was a threefold increase in the ratio of Fim− to Fim+ bacteria in the blood over 5 days. However, the same animals showed a fourfold decrease in the Fim−-to-Fim+ ratio in the spleen. Thus, it appeared that the Fim+ bacteria were cleared more efficiently from the bloodstream. Although these results would imply that type 1 fimbriae are at a disadvantage in the host, when this fim mutation was combined with a mutation (fla) that blocks flagellum production, the double mutant was >200-fold attenuated orally, even though the fla mutation alone conferred no phenotype. Therefore, under certain conditions, type 1 fimbriae confer a clear advantage.
Eriksson et al. (16) recently reported the isolation of serovar Typhimurium mutants that had increased bacterial growth rates in tissue culture macrophages. A number of these mutants apparently decreased NO production by infected macrophages. Interestingly, most of these mutants showed decreased virulence in competition assays in mice. However, one mutant outcompeted the parent strain in the animal analogously to a grvA mutant. Unfortunately, these mutations were isolated in the attenuated LT2 background (see below). The affected gene and the nature of the mutation were not reported in their study.
Other examples of mutations that increase virulence are less satisfying and have to do with proteolytic stability of virulence factors. The response regulator RssB (termed MviA for serovar Typhimurium) controls both the stability of RpoS by affecting access to the ClpX protease (1, 40, 44) and the function of RpoS by acting as an antisigma factor (2). Benjamin et al. (3) showed that, in the serovar Typhimurium LT2 background, mviA strains were more virulent then isogenic mviA+ strains in itys mice. The major virulence defect in LT2 results from decreased expression of RpoS, which regulates a number of virulence-associated genes (27, 52, 58). The mviA mutation apparently stabilizes RpoS, leading to more normal levels in the LT2 background. However, all of the virulent serovar Typhimurium strains examined were mviA+ (3). Moreover, both mutations that increase (3, 57) RpoS levels and those that decrease them (57) attenuate virulent serovar Typhimurium strains. In a related example, Krogfelt et al. (26) showed that rpoS null mutants of E. coli could outcompete an isogenic rpoS+ strain for colonization of the large intestine in streptomycin-treated mice. There was no apparent advantage or defect when mice were infected individually with the two strains. It is difficult to argue that the general stress response of E. coli is not important, but whether the rpoS regulon is more important outside the animal than in the intestine is not clear.
Another example relates to the expression of the Shigella flexneri outer membrane protein IcsA, which is required for polymerization of actin on the bacterial cell surface and hence bacterial movement in the cytoplasm of infected host cells and movement between cells (4). Nakata et al. (41) concluded that the absence of OmpT, an outer membrane protease, in Shigella strains allows proper expression of IcsA. OmpT is carried on the cryptic phage DLP12 in some strains of E. coli. This phage was absent in all examined Shigella strains and E. coli strains that depend on IcsA (41). This differs from our case in that wild-type Shigella normally lacks OmpT. In addition, two groups have shown that Shigella does express an OmpT homolog, termed SopA (14) or IcsP (45), that is required for normal proteolytic cleavage of IcsA. Mutations in sopA/icsP increase IcsA amounts but cause improper localization of the protein on the cell surface. Thus, analogously to the case of RpoS, strains that have either decreased (ompT+) or increased (sopA/icsP) IcsA levels are attenuated. This is in contrast to the situation with GrvA, where both loss and overproduction of GrvA increase the virulence of serovar Typhimurium.
The interaction between Salmonella and the host is complex. The bacteria must invade the host, evade the host's immune defense mechanisms, and utilize the host as a nutrient source. Serovar Typhimurium pathogenesis is the result of a combination of factors that each contributes a small part to virulence. Our data suggest that the Gifsy-2 phage carries a composite of virulence genes that increase pathogenicity and at least one gene that decreases pathogenicity. Although the grvA phenotype is subtle, it is clearly reproducible and statistically significant. We must ultimately understand the subtle aspects of these factors in order to gain a complete understanding of the disease process.
ACKNOWLEDGMENTS
We thank Lionello Bossi, Nara Figueroa-Bossi, and Ferric Fang for their generous gifts of strains and advice; Diane Essex-Sorlie for invaluable help with statistical analysis; Stanley Maloy, James Imlay, Charles Miller, and members of the Slauch lab for helpful advice; and Myung Kim for critically reading the manuscript.
This study was supported by NIH grant AI37530 and ACS junior faculty research award JFRA-633.
REFERENCES
- 1.Bearson S M, Benjamin W H, Jr, Swords W E, Foster J W. Acid shock induction of RpoS is mediated by the mouse virulence gene mviA of Salmonella typhimurium. J Bacteriol. 1996;178:2572–2579. doi: 10.1128/jb.178.9.2572-2579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker G, Klauck E, Hengge-Aronis R. The response regulator RssB, a recognition factor for sigma S proteolysis in Escherichia coli, can act like an anti-sigma S factor. Mol Microbiol. 2000;35:657–666. doi: 10.1046/j.1365-2958.2000.01736.x. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin W H, Jr, Yother J, Hall P, Briles D E. The Salmonella typhimurium locus mviA regulates virulence in Itys but not Ityr mice: functional mviA results in avirulence; mutant (nonfunctional) mviA results in virulence. J Exp Med. 1991;174:1073–1083. doi: 10.1084/jem.174.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardini L M, Mounier J, d'Hauteville H, Coquis-Rondon M, Sansonetti P J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattner F R, Plunkett G, III, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 6.Boyd E F, Wang F S, Beltran P, Plock S A, Nelson K, Selander R K. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J Gen Microbiol. 1993;139:1125–1132. doi: 10.1099/00221287-139-6-1125. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier N A, Lipps C J, So M Y, Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol Microbiol. 1993;7:933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- 8.Campbell A. Genetic structure. In: Hershey A D, editor. Lambda. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1971. pp. 13–44. [Google Scholar]
- 9.Cho E H, Nam C E, Alcaraz R, Jr, Gardner J F. Site-specific recombination of bacteriophage P22 does not require integration host factor. J Bacteriol. 1999;181:4245–4249. doi: 10.1128/jb.181.14.4245-4249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collmer A. Determinants of pathogenicity and avirulence in plant pathogenic bacteria. Curr Opin Plant Biol. 1998;1:329–335. doi: 10.1016/1369-5266(88)80055-4. [DOI] [PubMed] [Google Scholar]
- 11.Datsenko K A, Wanner B L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Groote A M, Ochsner U A, Shiloh M U, Nathan C, McCord J M, Dinauer M C, Libby S J, Vazquez-Torres A, Xu Y, Fang F C. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Groote A M, Testerman T, Xu Y, Stauffer G, Fang F C. Homocysteine antagonism of nitric oxide-related cytostasis in Salmonella typhimurium. Science. 1996;272:414–417. doi: 10.1126/science.272.5260.414. [DOI] [PubMed] [Google Scholar]
- 14.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 15.Elliott T. Transport of 5-aminolevulinic acid by the dipeptide permease in Salmonella typhimurium. J Bacteriol. 1993;175:325–331. doi: 10.1128/jb.175.2.325-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksson S, Bjorkman J, Borg S, Syk A, Pettersson S, Andersson D I, Rhen M. Salmonella typhimurium mutants that downregulate phagocyte nitric oxide production. Cell Microbiol. 2000;2:239–250. doi: 10.1046/j.1462-5822.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- 17.Ewald P W. The evolution of virulence. Sci Am. 1993;268:86–93. doi: 10.1038/scientificamerican0493-86. [DOI] [PubMed] [Google Scholar]
- 18.Ewald P W. Guarding against the most dangerous emerging pathogens. Emerg Infect Dis. 1996;2:245–257. doi: 10.3201/eid0204.960401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang F C, DeGroote M A, Foster J W, Baumler A J, Ochsner U, Testerman T, Bearson S, Giard J C, Xu Y, Campbell G, Laessig T. Virulent Salmonella typhimurium has two periplasmic Cu,Zn-superoxide dismutases. Proc Natl Acad Sci USA. 1999;96:7502–7507. doi: 10.1073/pnas.96.13.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan G, Kroll J S. Bacterial copper- and zinc-cofactored superoxide dismutase contributes to the pathogenesis of systemic salmonellosis. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 21.Figueroa-Bossi N, Bossi L. Inducible prophages contribute to Salmonella virulence in mice. Mol Microbiol. 1999;33:167–176. doi: 10.1046/j.1365-2958.1999.01461.x. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa-Bossi N, Coissac E, Netter P, Bossi L. Unsuspected prophage-like elements in Salmonella typhimurium. Mol Microbiol. 1997;25:161–173. doi: 10.1046/j.1365-2958.1997.4451807.x. [DOI] [PubMed] [Google Scholar]
- 23.Hacker J, Blum-Oehler G, Muhldorfer I, Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 24.Janakiraman A, Slauch J M. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- 25.Juhala R J, Ford M E, Duda R L, Youlton A, Hatfull G F, Hendrix R W. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J Mol Biol. 2000;299:27–51. doi: 10.1006/jmbi.2000.3729. [DOI] [PubMed] [Google Scholar]
- 26.Krogfelt K A, Hjulgaard M, Sorensen K, Cohen P S, Givskov M. rpoS gene function is a disadvantage for Escherichia coli BJ4 during competitive colonization of the mouse large intestine. Infect Immun. 2000;68:2518–2524. doi: 10.1128/iai.68.5.2518-2524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 28.Lissner C R, Swanson R N, O'Brien A D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–3013. [PubMed] [Google Scholar]
- 29.Lockman H A, Curtiss R. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun. 1990;58:137–143. doi: 10.1128/iai.58.1.137-143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockman H A, Curtiss R. Virulence of non-type 1-fimbriated and nonfimbriated nonflagellated Salmonella typhimurium mutants in murine typhoid fever. Infect Immun. 1992;60:491–496. doi: 10.1128/iai.60.2.491-496.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas R L, Lee C A. Unravelling the mysteries of virulence gene regulation in Salmonella typhimurium. Mol Microbiol. 2000;36:1024–1033. doi: 10.1046/j.1365-2958.2000.01961.x. [DOI] [PubMed] [Google Scholar]
- 32.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 33.Maloy S R, Stewart V J, Taylor R K. Genetic analysis of pathogenic bacteria: a laboratory manual. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 34.Mann B A, Slauch J M. Transduction of low-copy number plasmids by bacteriophage P22. Genetics. 1997;146:447–456. doi: 10.1093/genetics/146.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcus S L, Brumell J H, Pfeifer C G, Finlay B B. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect. 2000;2:145–156. doi: 10.1016/s1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 36.Miao E A, Miller S I. Bacteriophages in the evolution of pathogen-host interactions. Proc Natl Acad Sci USA. 1999;96:9452–9454. doi: 10.1073/pnas.96.17.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao E A, Miller S I. A conserved amino acid sequence directing intracellular type III secretion by Salmonella typhimurium. Proc Natl Acad Sci USA. 2000;97:7539–7544. doi: 10.1073/pnas.97.13.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller S I, Pegues D A. Salmonella species, including Salmonella typhi. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 2344–2363. [Google Scholar]
- 39.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muffler A, Fischer D, Altuvia S, Storz G, Hengge-Aronis R. The response regulator RssB controls stability of the ςs subunit of RNA polymerase in Escherichia coli. EMBO J. 1996;15:1333–1339. [PMC free article] [PubMed] [Google Scholar]
- 41.Nakata N, Tobe T, Fukuda I, Suzuki T, Komatsu K, Yoshikawa M, Sasakawa C. The absence of a surface protease, OmpT, determines the intercellular spreading ability of Shigella: the relationship between the ompT and kcpA loci. Mol Microbiol. 1993;9:459–468. doi: 10.1111/j.1365-2958.1993.tb01707.x. [DOI] [PubMed] [Google Scholar]
- 42.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 43.Plant J E, Blackwell J M, O'Brien A D, Bradley D J, Glynn A A. Are the Lsh and Ity disease resistance genes at one locus on mouse chromosome 1? Nature. 1982;297:510–511. doi: 10.1038/297510a0. [DOI] [PubMed] [Google Scholar]
- 44.Pratt L A, Silhavy T J. The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci USA. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shere K D, Sallustio S, Manessis A, D'Aversa T G, Goldberg M B. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol Microbiol. 1997;25:451–462. doi: 10.1046/j.1365-2958.1997.4681827.x. [DOI] [PubMed] [Google Scholar]
- 46.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 47.Slauch J, Taylor R, Maloy S. Survival in a cruel world: how Vibrio cholerae and Salmonella respond to an unwilling host. Genes Dev. 1997;11:1761–1774. doi: 10.1101/gad.11.14.1761. [DOI] [PubMed] [Google Scholar]
- 48.Slauch J M, Camilli A. IVET and RIVET: use of gene fusions to identify bacterial virulence factors specifically induced in host tissues. Methods Enzymol. 2000;326:73–96. doi: 10.1016/s0076-6879(00)26047-3. [DOI] [PubMed] [Google Scholar]
- 49.Slauch J M, Mahan M J, Mekalanos J J. In vivo expression technology for selection of bacterial virulence genes specifically induced in host tissues. In: Clark V L, Bavoil P M, editors. Bacterial pathogenesis. San Diego, Calif: Academic Press; 1997. pp. 309–320. [Google Scholar]
- 50.Slauch J M, Silhavy T J. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stanley T L, Ellermeier C D, Slauch J M. Tissue-specific gene expression identifies a gene in the lysogenic phage Gifsy-1 that affects Salmonella enterica serovar Typhimurium survival in Peyer's patches. J Bacteriol. 2000;182:4406–4413. doi: 10.1128/jb.182.16.4406-4413.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swords W E, Cannon B M, Benjamin W H J. Avirulence of LT2 strains of Salmonella typhimurium results from a defective rpoS gene. Infect Immun. 1997;65:2451–2453. doi: 10.1128/iai.65.6.2451-2453.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas L. Notes of a biology-watcher; germs. N Engl J Med. 1972;287:553–555. doi: 10.1056/NEJM197212142872408. [DOI] [PubMed] [Google Scholar]
- 54.Vidal S, Gros P, Skamene E. Natural resistance to infection with intracellular parasites: molecular genetics identifies Nramp1 as the Bcg/Ity/Lsh locus. J Leukoc Biol. 1995;58:382–390. doi: 10.1002/jlb.58.4.382. [DOI] [PubMed] [Google Scholar]
- 55.Wagner P L, Acheson D W, Waldor M K. Isogenic lysogens of diverse shiga toxin 2-encoding bacteriophages produce markedly different amounts of shiga toxin. Infect Immun. 1999;67:6710–6714. doi: 10.1128/iai.67.12.6710-6714.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waldor M K. Bacteriophage biology and bacterial virulence. Trends Microbiol. 1998;6:295–297. doi: 10.1016/s0966-842x(98)01320-1. [DOI] [PubMed] [Google Scholar]
- 57.Webb C, Moreno M, Wilmes-Riesenberg M, Curtiss III R, Foster J W. Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol Microbiol. 1999;34:112–123. doi: 10.1046/j.1365-2958.1999.01581.x. [DOI] [PubMed] [Google Scholar]
- 58.Wilmes-Riesenberg R M, Foster J W, Curtiss R. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect Immun. 1997;65:203–210. doi: 10.1128/iai.65.1.203-210.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wink D A, Cook J A, Kim S Y, Vodovotz Y, Pacelli R, Krishna M C, Russo A, Mitchell J B, Jourd'heuil D, Miles A M, Grisham M B. Superoxide modulates the oxidation and nitrosation of thiols by nitric oxide-derived reactive intermediates. Chemical aspects involved in the balance between oxidative and nitrosative stress. J Biol Chem. 1997;272:11147–11151. doi: 10.1074/jbc.272.17.11147. [DOI] [PubMed] [Google Scholar]
- 60.Worley J M, Ching K H, Heffron F. Salmonella SsrB activates a global regulon of horizontally acquired genes. Mol Microbiol. 2000;36:749–761. doi: 10.1046/j.1365-2958.2000.01902.x. [DOI] [PubMed] [Google Scholar]
- 61.Youderian P, Sugiono P, Brewer K L, Higgins N P, Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988;118:581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]