Abstract
Vasoconstriction is a known effect associated with ergot alkaloid consumption. The vascular contractile responses are often sustained for an extended period after exposure. Ergot alkaloids exist in two molecular configurations, the C-8-(R)-isomer (R-epimer) and the C-8-(S)-isomer (S-epimer). The sustained vascular contractile response to the R-epimers has been studied previously, unlike the S-epimers which are thought to be biologically inactive. Additionally, antagonists have been utilized to attenuate the vascular contraction associated with the R-epimers of ergot alkaloids utilizing ex vivo techniques. This study utilized an arterial tissue bath to examine and compare the sustained vascular contractile response attributed to ergocristine (R) and ergocristinine (S) using dissected bovine metatarsal arteries. The contractile blocking effect of a noncompetitive alpha-adrenergic antagonist, phenoxybenzamine (POB), was also investigated in precontracted arteries. Arteries (n = 6/epimer) were exposed to a single dose of ergocristine or ergocristinine (1 × 10−6 M in buffer). Each of the epimer doses was followed by a POB (1 × 10−3 M) or methanol (control) treatment at 90 min and the response was observed for another 90 min. Both epimers produced a sustained contractile response over the 180-min incubation period in the control groups. The R-epimer caused a greater sustained contractile response from 60 to 180 min post epimer exposure, compared to the S-epimer (P < 0.05, generalized estimating equations, independent t-test). Phenoxybenzamine caused a decrease in the contractile response induced by ergocristine and ergocristinine from 105 to 180 min, compared to the control (P < 0.05, generalized estimating equations, paired t-test). Overall, these results demonstrate the presence of a sustained vascular contractile response attributed to the R- and S-epimer of an ergot alkaloid with differences in contractile response between the epimers, suggesting differences in receptor binding mechanisms. Furthermore, this study demonstrated that a noncompetitive antagonist could attenuate the sustained arterial contractile effects of both ergot configurations ex vivo. Additional investigation into S-epimers of ergot alkaloids is needed. This research contributes to the understanding of the ergot epimer-vascular receptor binding mechanisms, which may support the investigation of different approaches of minimizing ergot toxicity in livestock.
Keywords: artery, Claviceps purpurea, ergocristinine, phenoxybenzamine
The R- and S-epimer of an ergot alkaloid, ergocristine, and ergocristinine, produced a sustained vascular contractile response. A noncompetitive antagonist, phenoxybenzamine, alleviated vascular contraction during the sustained contractile response.
Introduction
Secondary metabolites produced by the fungus Claviceps purpurea pose a health risk to humans and animals. These metabolites, known as ergot alkaloids, have two configurations known as the C-8-(R)-isomer (R-epimer) and C-8-(S)-isomer (S-epimer), which are defined by a rotation at the carbon 8 of the chemical structure (Crews, 2015). Currently, feed consumed by animals may contain ergot alkaloids (Schummer et al., 2020). The ergot alkaloids ergocristine (R) and ergocristinine (S) are found in high concentrations within ergot sclerotia (Tittlemier et al., 2015; Kodisch et al., 2020; Cherewyk et al., 2022). Standards are set for ergot alkaloid concentrations in feed for animal health and safety (Debegnach et al., 2019). When consumed, ergot alkaloids can cause a wide range of negative health effects in livestock, some of which are attributed to the binding of ergot alkaloids to vascular receptors resulting in blood vessel contraction and reduced blood flow (Klotz, 2015). Ergot alkaloids have a similar chemical structure to endogenous ligands, allowing the ergot alkaloid-receptor binding. The vascular receptor targets of ergot alkaloids are dopamine, serotonin, and alpha-adrenergic receptors. Alpha-adrenergic (Muller-Schweinitzer and Sturmer, 1974; Oliver et al., 1993) and serotonin (Dyer, 1993; Schöning et al., 2001; Trotta et al., 2018) receptors mediate vasoconstriction. Alpha-adrenergic receptors are more abundant in the peripheral vasculature (Liu et al., 2020). The mechanism of vascular contraction caused by ergot alkaloids has been studied ex vivo utilizing an aortic tissue bath or myograph approach.
Ergot alkaloid research utilizing ex vivo techniques has provided robust evidence that ergot alkaloids cause a sustained vascular contractile response. Dyer et al. (1993) observed that a single low dose of ergovaline added to suspended bovine uterine and umbilical arteries caused a delayed contractile response, which peaked after 120 min. Furthermore, Klotz et al. (2007) demonstrated that a single addition of ergovaline (1 × 10−4 M) to a bovine lateral saphenous vein caused a sustained vascular contractile response over an incubation period of 105 min. Similarly, Pesqueira et al. (2014) revealed that a single dose of ergocornine, ergocryptine, ergotamine, or ergocristine, at a concentration of 1 × 10−4 M, resulted in a sustained contractile response over an incubation period of 120 min. The sustained vascular contractile response was speculated to be caused by multiple factors such as bioaccumulation of ergot alkaloids in the vascular tissue, high affinity of the alkaloids to the receptor, slow dissociation of the alkaloids from the receptor, and/or nonspecific binding to multiple receptors within the vascular tissue (Schöning et al., 2001; Klotz et al., 2009; Pesqueira et al., 2014). The above ex vivo studies, assessing the sustained vascular contractile response, utilized R-epimers while S-epimers were not examined.
The S-epimers of ergot alkaloids have been denoted as non or less bioactive compared to their corresponding R-epimers (Smith and Shappell, 2002; Klotz, 2015). Recently, the S-epimers demonstrated biological activity, causing vascular contractility in isolated bovine vascular rings (Cherewyk et al., 2020). Investigating the potential sustained contractile response of S-epimers could further evaluate their biological activity (Mulac et al., 2012).
The addition of specific receptor antagonists can be used to attenuate the vascular contractile response related to ergot alkaloid exposure. For example, Dyer et al. (1993) demonstrated that prazosin and phentolamine, an alpha adrenergic selective competitive antagonist, did not inhibit vascular contraction before ergovaline exposure using bovine arteries. However, phenoxybenzamine (POB), a nonselective noncompetitive alpha adrenergic receptor antagonist, successfully antagonized the contraction. The authors denoted the success of POB to the theorized serotonin receptor antagonist properties of POB, and not that POB bound to alpha adrenergic receptors noncompetitively. Schöning et al. (2001) noted that ketanserin, a serotonin (5-HT) competitive receptor antagonist, could antagonize the contractile effect of ergovaline before, but not after, the addition of ergovaline. To the authors’ knowledge, attenuation of precontracted arteries from ergot alkaloid exposure ex vivo using a noncompetitive antagonist has not been studied.
The objective of this study was to examine and compare the sustained vascular contractile response of the ergot epimers ergocristine (R) and ergocristinine (S), over an extended period after a single dose utilizing an ex vivo aortic tissue bath. Ergocristine and ergocristinine were chosen as representative ergot epimers since they are found in high concentrations in certain contaminated commodities worldwide (Poapolathep et al., 2021; Cherewyk et al., 2022). A second objective was to analyze the effect of a noncompetitive alpha-adrenergic antagonist on contracted arteries. The authors hypothesized that the S-epimer would cause a vascular contractile response, and the contractile repose would be different from the R-epimer. Additionally, the authors hypothesized that a noncompetitive antagonist would antagonize the vascular contractile effects due to ergocristine and ergocristinine exposure, during the sustained vascular contractile response.
Materials and Methods
Animal ethics
No live animals were utilized in this study. Bovine arteries were collected from a local abattoir. No animal ethics approval was required; however, required paperwork was submitted to the University of Saskatchewan, including how many samples would be collected.
Artery collection
Arteries were collected, as described previously (Cherewyk et al., 2020). Twelve healthy steers (15–30 mo old) of mixed breed were euthanized at a local abattoir with a single animal being killed during each visit. Immediately after euthanasia, the dorsal metatarsal artery (approximately 15 cm) was carefully dissected from the right foot and placed into a small sealable plastic container containing approximately 50 mL of the oxygenated (95%/5%, oxygen/carbon dioxide) Krebs–Henseleit buffer (13.68 g NaCl, 0.7 g KCL, 0.28 g MgSO4, 0.32 g KH2PO4, 3.6 g NaHCO3 g, 1.8 g glucose, and 0.74 g CaCl2 in two liters of deionized water, made in laboratory). The container containing the buffer and artery was placed on ice within an insulated container for transport to the laboratory. Transport was approximately 20 min. Once at the laboratory, excess fat, muscle, and fascia were removed from the artery. The artery was cut into approximately 3-mm cross sections, ensuring no vascular branches or damaged segments of the artery were included. The artery cross sections were placed in a Petri dish containing 4 °C Krebs buffer until they were mounted onto the aortic tissue bath which was soon after.
Aortic tissue bath
From each animal, four cross sections from the dorsal metatarsal artery were suspended horizontally within a four-channel tissue bath system (Radnoti [159906], Monrovia, CA). Each chamber contained 15 mL of Krebs–Henseleit buffer that was continuously oxygenated (95%/5%, oxygen/carbon dioxide) and maintained at a temperature of 37 °C (pH = 7.4). The holding tank on the apparatus containing buffer was also continuously oxygenated. Each arterial cross section was allowed to equilibrate for 1 h under a resting tension of 1 g to establish a baseline tension with fresh buffer replacement occurring every 15 min. Once the equilibration period was completed, phenylephrine (Fisher Scientific, Fair Lawn, NJ), an alpha-adrenergic agonist, was administered to each of the four arterial cross sections at a final concentration of 1 × 10−4 M within the buffer. This was required to ensure artery viability and to normalize the contractile response of ergot-exposed arterial cross sections to their respective maximum phenylephrine contractile response. Once phenylephrine was administered, arteries were allowed 14 min to achieve maximal contractile response. Buffer replacement to remove phenylephrine was conducted every 15 min until the arterial cross sections relaxed and reached baseline tension.
Sustained vascular contractile response and effect of noncompetitive antagonist
Ergocristine (R-epimer) and ergocristinine (S-epimer) were purchased from Romer Labs (Vancouver, Canada). Ergocristine and ergocristinine were reconstituted in 4 and 1 mL of methanol, respectively. An aliquot of 73.4 µL from ergocristine and ergocristinine obtained a concentration of 1 × 10−6 M within each tissue bath chamber containing 15 mL of buffer. A dose of 1 × 10−6 M was chosen based on the highest concentration the authors could obtain from the ergocristinine standard vial. The same concentration was used for both epimers to compare them. Solvent was below 0.5% of the buffer solution (Klotz et al., 2010).
A single dose of ergocristine (R) or ergocristinine (S) was added to the four arterial cross sections collected from each animal (n = 6 animals/epimer). Ninety minutes after the ergocristine or ergocristinine dose, phenoxybenzamine hydrochloride (PHR1402, Pharmaceutical secondary standard, Sigma Aldrich, Milwakee, USA) dissolved in methanol was added to the buffer of two arterial cross sections (duplicates) at a concentration of 1 × 10−3 M. The same volume of methanol was added to the two other arterial cross sections to serve as a control for the POB treatment. Buffer replacements were made every 15 min for a total incubation period of 180 min. At the end of all the incubation periods, phenylephrine (1 × 10−4 M in buffer) was administered to confirm arterial cross section viability.
Data collection and statistical analysis
Grams of tension from each arterial cross section were recorded utilizing Biopac Student Lab PRO (professional version 3.7.1, Goleta, CA). Grams of tension were recorded every 15 min of the total 180-min incubation period for all arterial cross sections. The tension measurements for each arterial cross section were corrected to their corresponding baseline values, normalized to the first addition of phenylephrine, and averaged for the duplicate arterial cross sections. An average of each of the six independent arteries (n = 6 animal/epimer) was presented as the percent normalized mean contractile response for ergocristine or ergocristinine before and after POB treatment or methanol control.
Statistical analyses and figures were conducted utilizing SPSS 23 (IBM SPSS Statistics for Windows, version 23, IBM Corp., Armonk, NY). Normality was tested for all groups utilizing a one-sample Kolmogorov–Smirnov test, with a normal distribution considered at P > 0.05. Equal variance was investigated utilizing Levene’s test for equality of variances. Equal variance was considered at P > 0.05. These assumptions were tested relative to performed t-tests; normality and equal variance are not an assumption of generalized estimating equations (GEE). Generalized estimating equations with an unstructured matrix, and robust errors, to account for repeated measures over time, was utilized as the statistical analysis, followed by the appropriate post hoc test. Statistical significance was considered at P < 0.05.
The normalized mean contractile response attributed to ergocristine (R) or ergocristinine (S) was compared over time in the methanol control group. The addition of methanol did not affect the vascular contractile response; therefore, the methanol control groups will be referred to as the control groups. A significant effect of time lead to a multiple pairwise comparison with a sequential Sidak correction to assess differences in the normalized mean contractile response over time for each epimer.
Epimer type (R- or S-epimer), time and the interaction between the effect of epimer type and time on the normalized mean contractile response in the control groups was assessed. A significant interaction led to an independent t-test to assess differences in the normalized mean contractile response between ergocristine (R) and ergocristinine (S) at each time measurement.
The normalized mean contractile response between the POB treatment and control groups, for ergocristine (R) and ergocristinine (S) separately, over time was assessed. The interaction between the effects of treatment type (POB treatment vs. control) and time for each epimer was investigated. If a significant interaction occurred, a paired t-test was utilized at each time point to investigate the differences between the POB treatment and control groups for ergocristine or ergocristinine at each time point after the POB dose.
Results
The POB treatment and control groups at all time points were found to be normally distributed (one-sample Kolmogorov–Smirnov test, P > 0.05) except the ergocristine treatment group at 15 and 180 min (one-sample Kolmogorov–Smirnov test, P < 0.05). The ergocristine and ergocristinine control groups had equal variance (Levene’s test for equality of variances, P > 0.05).
Ergocristine and ergocristinine produced a gradual increase in arterial contractile response over the 180-min incubation period in the control groups. The vascular contraction at the end of the 180-min incubation period was greater than the beginning 15-min time point (GEE, pairwise comparison, Sequential Sidak correction, P < 0.05), for each epimer.
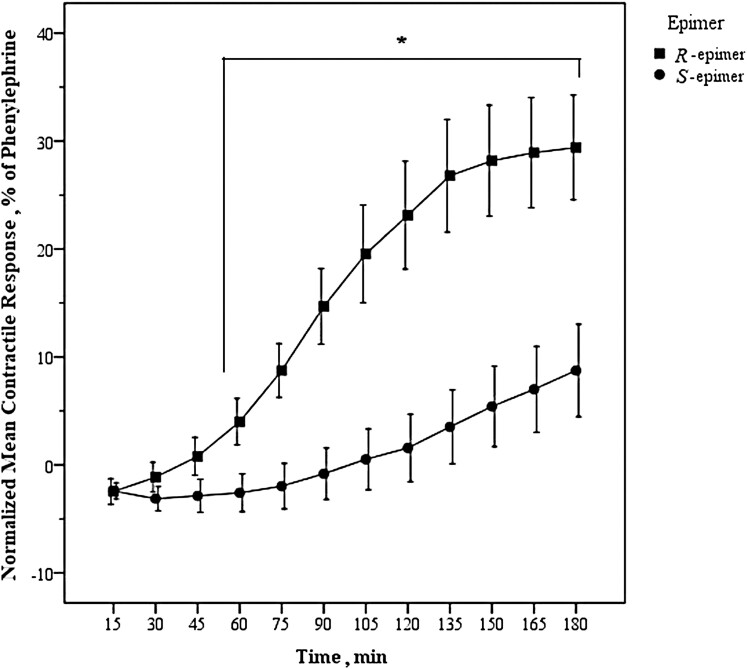
There was an interaction between effect of epimer type (R- and S-epimer) and time on the normalized mean contractile response in the control groups (GEE, Wald Chi Square = 137.4, df = 10, and P < 0.001). The normalized mean contractile response attributed to ergocristine (R) or ergocristinine (S) was not different from one another at the 15-, 30-, 45-min time points (independent t-test P > 0.05). The normalized mean contractile response at the 60-min time point, and each 15-min time point following up to 180 min, was different between the two epimers (independent t-test, P < 0.05), with the R-epimer demonstrating a greater contractile response (Figure 1). Data for the comparison between the ergocristine and ergocristinine control groups are available in Supplementary Table 1.
Figure 1.
Sustained arterial contractile response attributed to R- and S-epimers of an ergot alkaloid. Mean (n = 6) arterial contractile response of bovine dorsal metatarsal artery exposed to ergocristine (R) or ergocristinine (S) (1 × 10−6 M each) normalized to phenylephrine observed over an incubation period of 180 min in the control groups. Error bars are standard error of the mean. Time points at which the normalized mean contractile response was significantly different between the R- and S-epimers are denoted with an * (asterisk) (P < 0.05). The normalized mean contractile response of ergocristine was greater than that of ergocristinine at 60–180 min (generalized estimating equations, independent t-test).
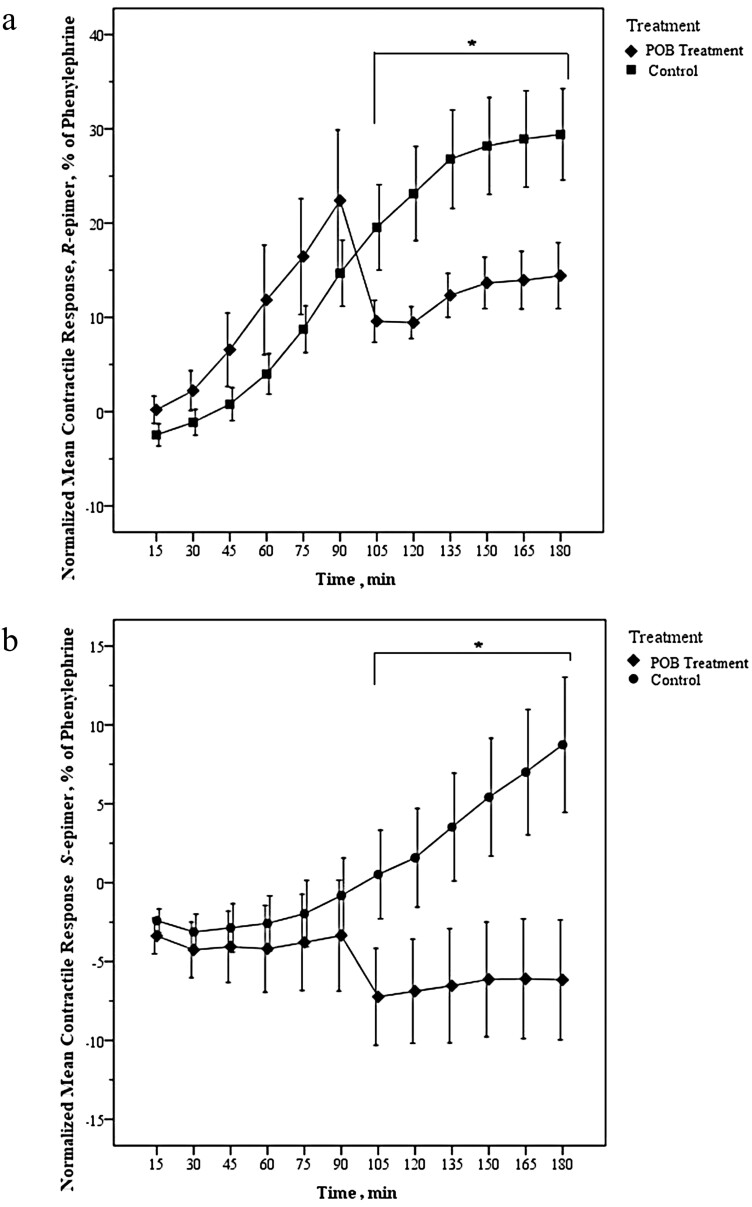
When assessing the effect of the POB on the normalized mean contractile response of ergocristine or ergocristinine, an interaction between the effect of treatment type (POB treatment vs. control) and time and was observed (GEE, Wald Chi Square = 186.33, 275.72, for ergocristine and ergocristinine, respectively, df =5, and P < 0.001 for both epimers). There was a decrease in the normalized mean contractile response for the POB treatment group compared to the control group, from 105 to 180 min for ergocristine (Figure 2a) and ergocristinine (Figure 2b) (paired t-test, P < 0.05 for each epimer). The POB treatment and control group comparison data for ergocristine (R-epimer) and ergocristinine (S-epimer) are available in Supplementary Table 2.
Figure 2.
(a and b) Normalized mean (n = 6) arterial contractile response of a single (a) ergocristine (R-epimer) (1 × 10−6 M) or (b) ergocristinine (S-epimer) (1 × 10−6 M), dose observed before and after the addition of phenoxybenzamine (POB) (1 × 10−3 M) or a volume matched methanol control (control) at 90 min. Bovine dorsal metatarsal arteries were used. Error bars are standard error of the mean. The time at which a significant difference occurred between the normalized mean contractile response of the POB treatment and control groups are represented by * (P < 0.05). There were significant differences in the normalized mean percent contractile response between POB treatment and control at 105–180 min (generalized estimating equations, paired t-test).
Discussion
Vasoconstriction is a well-known adverse effect of ergot alkaloid exposure. Multiple ex vivo studies have demonstrated the vascular contractile effects of ergot alkaloids in arterial rings of different animal species (Schöning et al., 2001; Foote et al., 2011; Klotz and McDowell 2017). A common method of assessing vascular contraction from ergot alkaloid exposure is a cumulative dose-response approach (Klotz et al., 2010). Furthermore, sustained vascular contraction has been demonstrated after a single dose of ergot alkaloids (Solomons et al., 1989; Dyer, 1993; Klotz et al., 2007; Pesqueira et al., 2014). Sustained vascular contractile responses have been assessed utilizing the R-epimers of ergot alkaloids. Specifically, a single dose of 1 × 10−4 M ergocristine to bovine arteries caused a sustained contractile response which appeared to plateau after a 120-min incubation period (Pesqueira et al., 2014). Similarly, in the present study, ergocristine caused sustained vascular contractile response in bovine arteries at a single dose of 1 × 10−6 M over 180-min incubation period. The vascular contractile response also appeared to be approaching a plateau. While the R-epimers of ergot alkaloids have been assessed for sustained vascular contraction, the S-epimers have not.
This study is the first to assess sustained vascular contraction after exposure to a S-epimer of ergot alkaloids. Ergocristinine (S) has been noted to be a stable S-epimer in terms of epimerization (Mulac et al., 2012). Utilizing a bovine artery, ergocristinine caused a sustained vascular contractile response over a 180-min incubation period, which further supports the evidence of S-epimers bioactivity as previously reported (Cherewyk et al., 2020). Ergocristinine is found at high concentrations within ergot contaminated grain (Cherewyk et al., 2022) and feed samples (Poapolathep et al., 2021). It is therefore important to assess the adverse effects of S-epimers.
The sustained vascular contractile response between ergocristine (R) and ergocristinine (S) was different. Ergocristine had a greater sustained contractile response that appeared to reach a plateau, compared to ergocristinine. The later, however, did not appear to approach a plateau even after 180 min of incubation. Ergot alkaloids cause vasocontraction by binding to vascular receptors and have been shown to possess slow association and dissociation rates (Schöning et al., 2001; Saper and Silberstein, 2006; Unett et al. 2013). In this study, the observed differences in sustained vascular contractile response between ergocristine and ergocristinine suggest differences in receptor association and dissociation rates between the R- and S-epimers. Association and dissociation rates are related to affinity. Suggested differences in receptor binding kinetics between ergocristine and ergocristinine may be due to differences in affinity.
Ergot alkaloids have demonstrated high affinity to vascular receptors (MacLennan and Martin, 1990; Hanoun et al., 2003). Particularly, ergot alkaloids have high affinity to alpha adrenergic receptors (Görnemann et al., 2008). The contractile effects of ergot alkaloids cannot be reversed if signs of contractility are present (Schöning et al., 2001), which may relate to the high affinity of ergot alkaloid to the vascular receptors. To reduce the vascular contractile effects of ergot alkaloids ex vivo, antagonists and agonist have been utilized. A noncompetitive antagonist binding to an allosteric, instead of an orthosteric, site of the receptor may, therefore, be more useful in antagonizing ergot alkaloids.
Phenoxybenzamine is a known nonselective, irreversible, noncompetitive antagonist of alpha-adrenergic receptors (Bodenstein et al., 2005). In the present study, POB attenuated the vascular contractile response in already contracted arteries exposed to ergocristine and ergocristinine. Precipitate occurred after POB was administered into the buffer. The attenuation of ergot-induced sustained contractile effects by noncompetitive antagonists may prove useful in alleviating some of the clinical effects of ergotism. The use of such drugs is currently not feasible due to cost.
Ergot alkaloids bind nonspecifically to vascular receptors (Saper and Silberstein, 2006) such as the serotonin receptor, mediating peripheral vascular contracture (Valente et al., 2022). However, POB is foremost an alpha-adrenergic antagonist (Yoham, 2022); therefore, the present study supports the involvement of alpha-adrenergic receptors mediating arterial contraction after exposure to R- or S-epimers of ergot alkaloids. The involvement of alpha-adrenergic receptors with ergot alkaloid exposure has been reported previously (Klotz et al., 2016).
Comparison of the antagonist effects of POB on the R- and S-epimers after the 90 min was not conducted. The vascular contractile response attributed to the S-epimer at 90 min was minimal but increased during the following time points. Therefore, it was not possible to compare the antagonist effect of POB on each epimer after the POB treatment. The 90-min time point was chosen based on previous findings which reported substantial contractile response of R-epimers at this period (Klotz et al., 2007; Pesqueira et al., 2014).
In conclusion, ergocristine (R) and ergocristinine (S) caused sustained vascular contraction of bovine arteries. The sustained vascular contraction differed between the two epimers, suggesting differences in the kinetics and affinity of each epimer to vascular receptors. Further investigation into the binding mechanism for the R- and S-epimer, and S-epimer bioactivity, is needed. The noncompetitive antagonist, POB, attenuated the contractile response associated with the R- and S-epimer, supporting use of noncompetitive antagonists to reduce ergot alkaloid effects ex vivo. Future studies may include a longer incubation period to further assess if the S-epimer reaches a plateau and if the R- and S-epimer can be analytically detected within the arterial segments. It is possible that the contractile response is related to the conversion of the epimers. Limited studied have assessed epimerization of the S to the R-epimers. The S-epimers may convert to the R-epimers in acidic solutions (Komarova and Tolkachev, 2001); however, the present system was maintained at a pH of 7.4 at 37 °C. Based on the literature, the extent of epimerization should be minimal within the experimental time frame and the results likely reflect the effect of the specific epimer (Smith and Shappell, 2002; Mulac and Humpf, 2011). Overall, this research contributes to the understanding of the ergot epimer-receptor binding mechanisms which are crucial for further understanding of new treatments of ergot toxicity.
Supplementary Material
Acknowledgments
We would like to thank Igor Moshynskyy for all assistance, and Friesen’s Meat Processing Inc. for the animal tissues. We acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC). Funding was graciously provided by the NSERC Canadian Graduate Scholarships-Doctoral (CGS-D), Western College of Veterinary Medicine internal ergot grant, and The Saskatchewan Ministry of Agriculture-Agriculture Development Fund (ADF), ADF grant number: 20180361.
Glossary
Abbreviations
- GEE
generalized estimating equations
- POB
phenoxybenzamine
- 5-HT
serotonin
Contributor Information
Jensen E Cherewyk, Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B4, Canada.
Sarah E Parker, Centre for Applied Epidemiology, Large Animal Clinical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B4, Canada.
Barry R Blakley, Department of Veterinary Biomedical Sciences, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B4, Canada.
Ahmad N Al-Dissi, Department of Veterinary Pathology, Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, S7N 5B4, Canada.
Conflict of Interest Statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Bodenstein, J., Venter D. P., and Brink C. B.. . 2005. Phenoxybenzamine and benextramine, but not 4-diphenylacetoxy-N-[2-chloroethyl]piperidine hydrochloride, display irreversible noncompetitive antagonism at G protein-coupled receptors. J. Pharmacol. Exp. Ther. 314:891–905. doi: 10.1124/JPET.105.083568. [DOI] [PubMed] [Google Scholar]
- Cherewyk, J., Grusie-Ogilvie T., Blakley B., and Al-Dissi A.. . 2022. Validation of a new sensitive method for the detection and quantification of R and S-epimers of ergot alkaloids in Canadian spring wheat utilizing deuterated lysergic acid diethylamide as an internal standard. Toxins 14:22. doi: 10.3390/toxins14010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherewyk, J. E., Parker S. E., Blakley B. R., and Al-Dissi A. N.. . 2020. Assessment of the vasoactive effects of the (S)-epimers of ergot alkaloids in vitro. J. Anim. Sci. 98:1–6. doi: 10.1093/jas/skaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews, C. 2015. Analysis of ergot alkaloids. Toxins 7:2024–2050. doi: 10.3390/toxins7062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debegnach, F., Patriarca S., Brera C., Gregori E., Sonego E., Moracci G., and De Santis B.. . 2019. Ergot alkaloids in wheat and rye derived products in Italy. Foods 8:150. doi: 10.3390/foods8050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer, D. C. 1993. Evidence that ergovaline acts on serotonin receptors. Life Sci. 53:223–228. doi: 10.1016/0024-3205(93)90555-H. [DOI] [PubMed] [Google Scholar]
- Foote, A. P., Harmon D. L., Strickland J. R., Bush L. P., and Klotz J. L.. . 2011. Effect of ergot alkaloids on contractility of bovine right ruminal artery and vein. J. Anim. Sci. 89:2944–2949. doi: 10.2527/jas.2010-3626. [DOI] [PubMed] [Google Scholar]
- Görnemann, T., Jähnichen S., Schurad B., Latté K. P., Horowski R., Tack J., Flieger M., and Pertz H. H.. . 2008. Pharmacological properties of a wide array of ergolines at functional alpha1-adrenoceptor subtypes. Naunyn Schmiedebergs Arch. Pharmacol. 376:321–330. doi: 10.1007/s00210-007-0221-3. [DOI] [PubMed] [Google Scholar]
- Hanoun, N., Saurini F., Lanfumey L., Hamon M., and Bourgoin S.. . 2003. Dihydroergotamine and its metabolite, 8ʹ-hydroxy-dihydroergotamine, as 5-HT 1A receptor agonists in the rat brain. Br. J. Pharmacol. 139:424–434. doi: 10.1038/sj.bjp.0705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, J. 2015. Activities and effects of ergot alkaloids on livestock physiology and production. Toxins 7:2801–2821. doi: 10.3390/toxins7082801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, J. L., and McDowell K. J.. . 2017. Tall fescue ergot alkaloids are vasoactive in equine vasculature. J. Anim. Sci. 95:5151–5160. doi: 10.2527/jas2017.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, J. L., Aiken G. E., Bussard J. R., Foote A. P., Harmon D. L., Goff B. M., Schrick F. N., and Strickland J. R.. . 2016. Vasoactivity and vasoconstriction changes in cattle related to time off toxic endophyte-infected tall fescue. Toxins 8:271. doi: 10.3390/toxins8100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, J. L., Bush L. P., Smith D. L., Shafer W. D., Smith L. L., Arlington B. C., and Strickland J. R.. . 2007. Ergovaline-induced vasoconstriction in an isolated bovine lateral saphenous vein bioassay. J. Anim. Sci. 85:2330–2336. doi: 10.2527/jas.2006-803. [DOI] [PubMed] [Google Scholar]
- Klotz, J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. . 2009. Bioaccumulation of ergovaline in bovine lateral saphenous veins in vitro. J. Anim. Sci. 87:2437–2447. doi: 10.2527/jas.2008-1692. [DOI] [PubMed] [Google Scholar]
- Klotz, J. L., Kirch B. H., Aiken G. E., Bush L. P., and Strickland J. R.. . 2010. Contractile response of fescue-naïve bovine lateral saphenous veins to increasing concentrations of tall fescue alkaloids. J. Anim. Sci. 88:408–415. doi: 10.2527/jas.2009-2243. [DOI] [PubMed] [Google Scholar]
- Kodisch, A., Oberforster M., Raditschnig A., Rodemann B., Tratwal A., Danielewicz J., Korbas M., Schmiedchen B., Eifler J., Gordillo A., . et al. 2020. Covariation of ergot severity and alkaloid content measured by HPLC and One ELISA Method in inoculated winter rye across three isolates and three European countries. Toxins 12:676. doi: 10.3390/toxins12110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova, E. L., and Tolkachev O. N.. . 2001. The chemistry of peptide ergot alkaloids. Part 1. Classification and chemistry of ergot peptides. Pharm. Chem. J. 35:504–513. doi: 10.1023/A:1014050926916. [DOI] [Google Scholar]
- Liu, X., Luo D., Zhang J., and Du L.. . 2020. Distribution and relative expression of vasoactive receptors on arteries. Sci. Rep. 10:1–9. doi: 10.1038/s41598-020-72352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan, S. J., and Martin G. R.. . 1990. Actions of non-peptide ergot alkaloids at 5-HT1-like and 5-HT2 receptors mediating vascular smooth muscle contraction. Naunyn Schmiedebergs Arch. Pharmacol. 342:120–129. doi: 10.1007/BF00166953. [DOI] [PubMed] [Google Scholar]
- Mulac, D., and Humpf H.-U.. . 2011. Cytotoxicity and accumulation of ergot alkaloids in human primary cells. Toxicology. 282:112–121. doi: 10.1016/J.TOX.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Mulac, D., Hüwel S., Galla H. J., and Humpf H. U.. . 2012. Permeability of ergot alkaloids across the blood-brain barrier in vitro and influence on the barrier integrity. Mol. Nutr. Food Res. 56:475–485. doi: 10.1002/mnfr.201100431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Schweinitzer, E., and Sturmer E.. . 1974. Investigations on the mode of action of ergotamine in the isolated femoral vein of the dog. Br. J. Pharmac. 51:441–446. doi: 10.1111/j.1476-5381.1974.tb10680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. W., Abney L. K., Strickland J. R., and Linnabary R. D.. . 1993. Vasoconstriction in bovine vasculature induced by the tall fescue alkaloid lysergamide. J. Anim. Sci. 71:2708–2713. doi: 10.2527/1993.71102708x. [DOI] [PubMed] [Google Scholar]
- Pesqueira, A., Harmon D. L., Branco A. F., and Klotz J. L.. . 2014. Bovine lateral saphenous veins exposed to ergopeptine alkaloids do not relax. J. Anim. Sci. 92:1213–1218. doi: 10.2527/jas.2013-7142. [DOI] [PubMed] [Google Scholar]
- Poapolathep, S., Klangkaew N., Zhang Z., Giorgi M., Logrieco A. F., and Poapolathep A.. . 2021. Simultaneous determination of ergot alkaloids in swine and dairy feeds using ultra high-performance liquid chromatography-tandem mass spectrometry. Toxins 13:724. doi: 10.3390/toxins13100724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper, J. R., and Silberstein S.. . 2006. Pharmacology of dihydroergotamine and evidence for efficacy and safety in migraine. Headache J. Head Face Pain. 46:171–181. doi: 10.1111/J.1526-4610.2006.00601.X. [DOI] [PubMed] [Google Scholar]
- Schöning, C., Flieger M., and Pertz H. H.. . 2001. Complex interaction of ergovaline with 5-HT2A, 5-HT1B/1D, and alpha1 receptors in isolated arteries of rat and guinea pig. J. Anim. Sci. 79:2202. doi: 10.2527/2001.7982202x. [DOI] [PubMed] [Google Scholar]
- Schummer, C., Zandonella I., van Nieuwenhuyse A., and Moris G.. . 2020. Epimerization of ergot alkaloids in feed. Heliyon 6:e04336. doi: 10.1016/j.heliyon.2020.e04336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. J., and Shappell N. W.. . 2002. Technical note: epimerization of ergopeptine alkaloids in organic and aqueous solvents. J. Anim. Sci. 80:1616–1622. doi: 10.2527/2002.8061616x. [DOI] [PubMed] [Google Scholar]
- Solomons, R. N., Oliver J. W., and Linnabary R. D.. . 1989. Reactivity of dorasal pedal vein of cattel to selected alkaloids associated with Acremonium coenophialum-infected fescue greass. Am. J. Vet. Res. 50:235–238. PMID: 2719386. [PubMed] [Google Scholar]
- Tittlemier, S. A., Drul D., Roscoe M., and Mckendry T.. . 2015. Occurrence of ergot and ergot alkaloids in western Canadian wheat and other cereals. J. Agric. Food Chem. 63:6644–6650. doi: 10.1021/acs.jafc.5b02977. [DOI] [PubMed] [Google Scholar]
- Trotta, R. J., Harmon D. L., and Klotz J. L.. . 2018. Interaction of ergovaline with serotonin receptor 5-HT2A in bovine ruminal and mesenteric vasculature. J. Anim. Sci. 96:4912–4922. doi: 10.1093/jas/sky346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unett, D. J., Gatlin J., Anthony T. L., Buzard D. J., Chang S., Chen C., Chen X., Dang H. T. M., Frazer J., Le M. K., . et al. 2013. Kinetics of 5-HT2B receptor signaling: profound agonist-dependent effects on signaling onset and duration. J. Pharmacol. Exp. Ther. 347:645–659. doi: 10.1124/JPET.113.207670. [DOI] [PubMed] [Google Scholar]
- Valente, E. E. L., Harmon D. L., and Klotz J. L.. . 2022. Influence of prolonged serotonin and ergovaline pre-exposure on vasoconstriction ex vivo. Toxins 14:9. doi: 10.3390/TOXINS14010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoham, A.L., and Casadesus, D.. Phenoxybenzamine. [Updated 2021 Jul 23]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. —[accessed March 14, 2022]. https://www.ncbi.nlm.nih.gov/books/NBK560667/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.