Abstract
OBJECTIVES
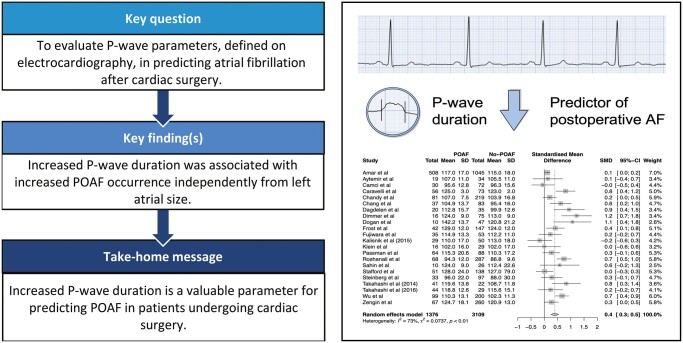
To evaluate the role of P-wave parameters, as defined on preprocedural electrocardiography (ECG), in predicting atrial fibrillation after cardiac surgery [postoperative atrial fibrillation (POAF)].
METHODS
PubMed, Cochrane library and Embase were searched for studies reporting on P-wave parameters and risk of POAF. Meta-analysis of P-wave parameters reported by at least 5 different publications was performed. In case of receiver operator characteristics (ROC-curve) analysis in the original publications, an ROC meta-analysis was performed to summarize the sensitivity and specificity.
RESULTS
Thirty-two publications, with a total of 20 201 patients, contributed to the meta-analysis. Increased P-wave duration, measured on conventional 12-lead ECG (22 studies, Cohen’s d = 0.4, 95% confidence interval: 0.3–0.5, P < 0.0001) and signal-averaged ECG (12 studies, Cohen’s d = 0.8, 95% confidence interval: 0.5–1.2, P < 0.0001), was a predictor of POAF independently from left atrial size. ROC meta-analysis for signal-averaged ECG P-wave duration showed an overall sensitivity of 72% (95% confidence interval: 65–78%) and specificity of 68% (95% confidence interval: 58–77%). Summary ROC curve had a moderate discriminative power with an area under the curve of 0.76. There was substantial heterogeneity in the meta-analyses for P-wave dispersion and PR-interval.
CONCLUSIONS
This meta-analysis shows that increased P-wave duration, measured on conventional 12-lead ECG and signal-averaged ECG, predicted POAF in patients undergoing cardiac surgery.
Keywords: Postoperative atrial fibrillation, Electrocardiography, Cardiac surgery, Receiver operating curve meta-analysis
Postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery and it has been associated with the incidence of early and late postoperative stroke, mortality and prolonged hospitalization [1].
INTRODUCTION
Postoperative atrial fibrillation (POAF) is the most common complication after cardiac surgery and it has been associated with the incidence of early and late postoperative stroke, mortality and prolonged hospitalization [1]. POAF is thought to be an expression of a pre-existing substrate resulting from cardiovascular comorbidities which also predict POAF recurrences after discharge from hospital [1, 2]. P-wave parameters, measured on conventional electrocardiography (ECG), have previously been used to determine the underlying substrate for atrial fibrillation (AF) in the general population and to predict AF in non-surgical patients [3, 4]. However, the clear diagnostic value and the usefulness of these P-wave parameters in POAF prediction are debated. The purpose of this systematic review and meta-analysis is to evaluate the value of preoperative P-wave parameters in predicting POAF.
METHODS
Protocol registration and literature search
The study protocol was registered in PROSPERO international prospective register of systematic reviews (CRD42021261119). For this systematic review, the 2020 Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were followed [5]. In June 2022, a systematic literature search was conducted, after consultation of a trained medical librarian, in PubMed, Cochrane database and Embase (Supplementary Material, Tables S1–S3). Citation tracking was performed to identify additional publications.
Study selection and data extraction
After removal of duplicates, all identified studies were screened on titles and abstracts by 2 (M.J.K. and S.V.D.W.) researchers. Studies in patients not undergoing cardiac surgery, studies not published in English or without description of preoperative ECG-workup and postoperative monitoring for POAF were excluded after screening the titles and abstracts. Full texts of the remaining articles were screened. The inclusion criteria for the systematic review were a preoperative P-wave assessment, cardiac surgery and postoperative monitoring for POAF.
Two authors independently extracted the data (M.J.K. and S.V.D.W.). Data on the following variables were extracted: age, sex, diabetes mellitus, hypertension, left ventricular ejection fraction, chronic obstructive pulmonary disease, right coronary artery occlusion, body mass index, left atrial diameter, definition of POAF and history of AF. Also, the following study characteristics were analysed: study size, type of study (prospective or retrospective) and type of surgery performed. In case of disagreements during the study selection and data extraction process, a third independent author was consulted (E.B.).
Quality assessment
The Downs and Black tool for quality assessment in cohort studies was used to determine the quality of the studies included in the meta-analyses [6]. In addition, the description of the ECG protocol and monitoring for POAF were added to the quality assessment.
Study outcomes
The primary outcome of this study was occurrence of POAF in the early postoperative period after cardiac surgery. POAF had to be registered either by a 12-lead ECG or by telemetric monitoring. No distinction was made between the different definitions of POAF, such as episode duration and monitoring duration. Ultimately, this was corrected for in various subgroup analyses.
The exposure parameters of interest were all P-wave parameters, as determined by ECG, associated with the occurrence of POAF in patients undergoing cardiac surgery.
Statistical analysis
For parameters identified by a minimum of 5 publications, a continuous variables meta-analysis using inverse variance method was performed to assess the overall Cohen’s d, which is a measure of effect size (ES). A minimum of 5 publications was set to obtain the robustness of the models. The following cut-offs for the interpretation of Cohen’s d were selected: 0 < Cohen’s d < 0.2 = no effect, 0.2 ≤ Cohen’s d < 0.5 = small effect, 0.5 ≤ Cohen’s d < 0.8 = intermediate effect, Cohen’s d ≥ 0.8 = large effect. Cochran’s Q-test and I2 statistics were used to assess heterogeneity of the models with a significant cut-off value of P < 0.10 and I2 > 50%, respectively. To explore the patterns of heterogeneity, a leave-one-out analysis or a graphic display of heterogeneity plot was performed [7]. Subgroup analyses and meta-regression were performed to examine the between-study differences and different patterns of ES distribution. The subgroups were predefined based on the suspected contributors to between-study differences (patient characteristics, outcome measures and definitions, or interventions).
To determine pooled diagnostic accuracy of receiver operator characteristics curves (ROC curves) in the original publications, an ROC meta-analysis was performed using a bivariate approach in a linear mixed model [8]. Based on given pairs of sensitivity and specificity for a certain cut-off value, true positives, true negatives, false positives and false negatives were back-calculated and fitted in the ROC meta-analysis. The results were presented in a summary receiver operating curve (SROC) with a range of sensitivity and specificity based on a range of cut-off values. The discriminative power of the model was assessed based on the area under the curve (AUC) with the following cut-off values: AUC < 0.75 = low accuracy, 0.75 ≤ AUC < 0.85 = moderate accuracy, AUC ≥ 0.85 = high accuracy. To assess study differences and their influence on SROC, a meta-regression was performed [8].
The presence of potential publication bias was visually assessed in a funnel plot and by performing the Egger’s test with a P-value <0.05 regarded as statistically significant [9]. In addition, between-study heterogeneity and outliers were considered as potential causes of funnel plot asymmetry. Significant publication bias was explored using a Duval & Tweedie’s trim-and-fill procedure to estimate the actual ES [10]. Trim-and-fill analysis was performed with 3 different estimators (L0, R0 and Q0) to provide more insight into patterns of publication bias [11].
All statistical values were computed with a 95% confidence interval in random-effects models. The 2-tailed P-value cut-off for statistical significance was set at P < 0.05. All statistical models were created in ‘Rstudio Version 1.2.1335’ by using the ‘meta’ (version 4.18–1), ‘metafor’ (version 3.0–2), ‘mada’ (version 0.5.10) and ‘dmetar’ (version 0.0.9000) packages available for performing meta-analyses [12, 13].
RESULTS
Study selection
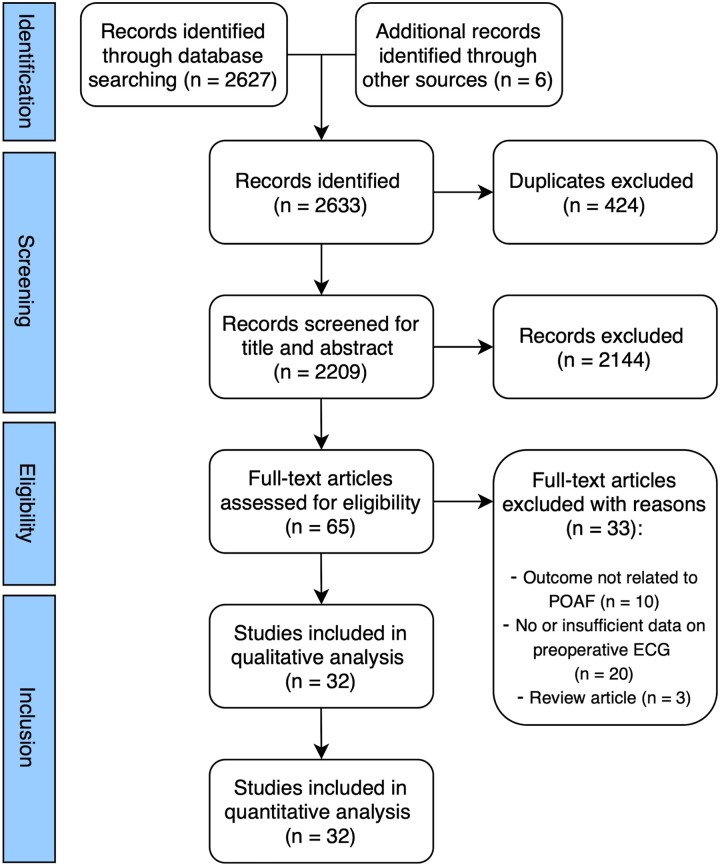
The search generated 2627 results. Additional 6 records were identified by scanning the studies included in a previous meta-analysis [14]. After exclusion of duplicates, 2633 studies were screened on title and abstracts and 65 publications were deemed suitable for full-text evaluation. Eventually, 33 records were excluded with reasons (Supplementary Material, Table S4) and 32 studies were included in the quantitative analysis. An overview of the study selection process is presented in Fig. 1.
Figure 1:
Study selection diagram.
Quality of studies
The overall quality of the studies included in the meta-analyses was high (Supplementary Material, Figs S1 and S2). All studies clearly described the objectives, study outcomes, interventions and their main findings. Also, majority of the studies provided extensive descriptions of ECG protocols, monitoring for POAF and statistical methodology.
Study outcomes
Thirty-two studies (20 201 patients) were included in the current meta-analysis (Table 1). Study subjects were predominantly males (71.2%). The average incidence of POAF was 33.7%. Patients with POAF were significantly older compared to patients without POAF (67.3 vs 61.7 years, P < 0.001, respectively). The studies were conducted between 1993 and 2020 [15–46].
Table 1:
Summary of studies included in the meta-analyses
| Study | Number of participants | Timing of preoperative ECG | Type of ECG | Type of surgery | POAF % | Definition of POAF | Reported parameters |
|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||
| Amar et al. | 1851 | NA | 12-lead | CABG | 33 | >5 min | P-wave duration |
| PR-interval | |||||||
| Aytemir et al. | 59 | 1 day prior | 12-lead/SAECG | CABG | 35.8 | >30 min | P-wave duration |
| Budeus et al. | 101 | Day of surgery | SAECG | CABG | 37 | >10 min | P-wave duration |
| Camci et al. | 102 | 1 day prior | 12-lead | CABG | 29.4 | Any episode. | P-wave duration |
| P-wave dispersion | |||||||
| Caravelli et al. | 129 | 1 day prior | 12-lead/SAECG | CABG | 43 | >30 s | P-wave duration |
| Dagdelen et al. | 148 | 1 day prior | 12-lead | CABG | 38 | Any episode. | P-wave duration |
| P-wave dispersion PR-interval | |||||||
| Dimmer et al. | 91 | NA | 12-lead/SAECG | CABG | 17.5 | Any episode. | P-wave duration |
| Dogan et al. | 57 | NA | -12-lead | CABG | 17 | Any episode. | P-wave duration |
| P-wave dispersion | |||||||
| Frost et al. | 189 | NA | 12-lead/SAECG | CABG | 22 | Any episode. | P-wave duration |
| Fujiwara et al. | 88 | NA | 12-lead | OPCAB | 39.8 | Any episode. | P-wave duration |
| Gang et al. | 205 | 1 day prior | 12-lead/SAECG | CABG | 26 | >10 min | P-wave duration |
| P-wave dispersion PR-interval | |||||||
| Hayashida et al. | 95 | 1-3 days prior | SAECG | CABG/AVR | 29 | >1 h | P-wave duration |
| Kališnik et al. (2019) | 150 | 1 day prior | 12-lead | CABG/AVR | 21 | Any episode. | PR-interval |
| Kališnik et al. (2015) | 79 | 1 day prior | 12-lead | CABG/AVR | 36.7 | >1 min | P-wave duration PR-interval |
| Klein et al. | 45 | 1 week prior | 12-lead/SAECG | CABG | 35.6 | >1 h | P-wave duration |
| Magne et al. | 169 | 1 day prior | 12-lead | CABG | 38 | >10 min | PR-interval |
| Rader et al. | 13356 | NA | 12-lead | CABG/AVR/MV surgery | 35 | Any episode. | PR interval |
| Roshanali et al. | 355 | NA | 12-lead | CABG | 19.2 | >5 min | P-wave duration |
| Sigurdsson et al. | 1227 | 30 days prior | 12-lead | CABG/valve surgery | 31 | Any episode leading to change in treatment. | PR-interval |
| Stafford et al. | 189 | 1 day prior | 12-lead/SAECG | CABG | 27 | >1 h | P-wave duration |
| Steinberg et al. | 130 | 1 day prior | 12-lead/SAECG | CABG/valve surgery | 25 | >30 min | P-wave duration |
| Wu et al. | 299 | 2 days prior | 12-lead | CABG | 33.1 | >5 min | P-wave duration |
| Zaman et al. (1997) | 102 | NA | SAECG | CABG | 26.5 | Any episode. | P-wave duration |
| Zaman et al. (2000) | 326 | NA | SAECG | CABG | 28 | Any episode. | P-wave duration |
| Retrospective studies | |||||||
| Achmad et al. | 42 | NA | 12-lead | CABG | 29 | Any episode identified by ECG | P-wave dispersion |
| Chandy et al. | 300 | 1 week prior | 12-lead | CABG | 27 | >30 min | P-wave duration |
| P-wave dispersion PR-interval | |||||||
| Chang et al. | 120 | NA | 12-lead | CABG | 31 | >30 min | P-wave duration |
| P-wave dispersion | |||||||
| Passman et al. | 152 | NA | 12-lead | CABG | 42.1 | Any episode. | P-wave duration PR-interval |
| Sahin et al. | 36 | 1 day prior | 12-lead | LA myxoma exclusion | 27.8 | Any episode. | P-wave duration |
| P-wave dispersion | |||||||
| Takahashi et al. (2014) | 63 | NA | 12-lead | AVR | 65 | >5 min | P-wave duration |
| Takahashi et al. (2016) | 73 | NA | 12-lead | MV surgery | 60 | >5 min | P-wave duration |
| Zengin et al. | 327 | Within 30 days prior | 12-lead | CABG | 20 | Any episode. | P-wave duration |
| P-wave dispersion PR-interval | |||||||
AF: atrial fibrillation; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; ECG: electrocardiography; LA: left atrium; MV: mitral valve; NA: not available; OPCAB: off-pump coronary artery bypass grafting; POAF: postoperative atrial fibrillation; SAECG: signal-averaged electrocardiography.
P-wave dispersion
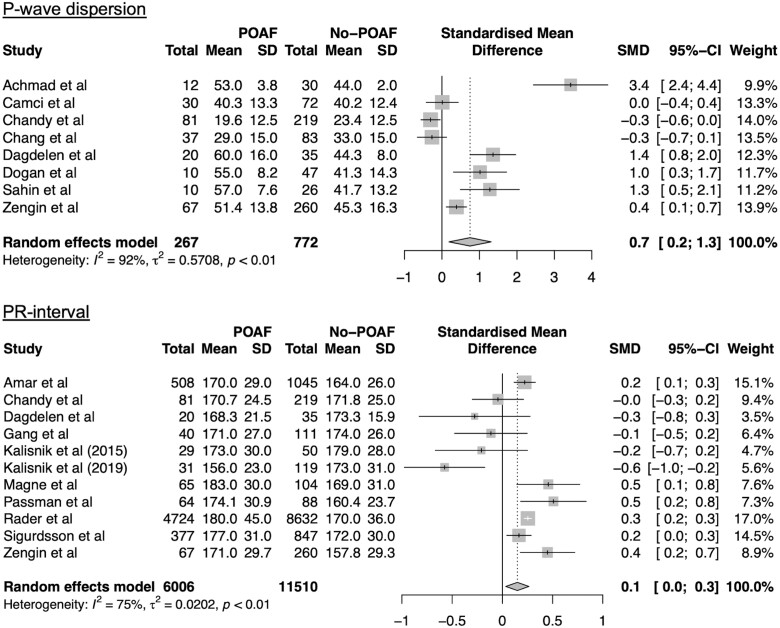
Eight studies were included in a meta-analysis of P-wave dispersion [ES = 0.7, 95% confidence interval (CI): 0.2–1.3; I2 = 92%, P < 0.01; Fig. 2A]. Heterogeneity analysis showed that omitting the study by Achmad et al. reduced overall heterogeneity (I2 = 87%) and ES (ES = 0.1, 95% CI: −0.1 to 0.3) (Supplementary Material, Fig. S3).
Figure 2:
Forest plots for meta-analyses for P-wave dispersion and PR-interval. Studies included in meta-analysis, mean preoperative values for patients with and without POAF, corresponding standard deviations, numbers of subjects, standardized mean differences (SMD), corresponding standard deviations and the weight of the studies are presented. Overall effect size is presented in a diamond shape. CI: confidence interval; POAF: postoperative atrial fibrillation; SD: standard deviation.
PR-interval
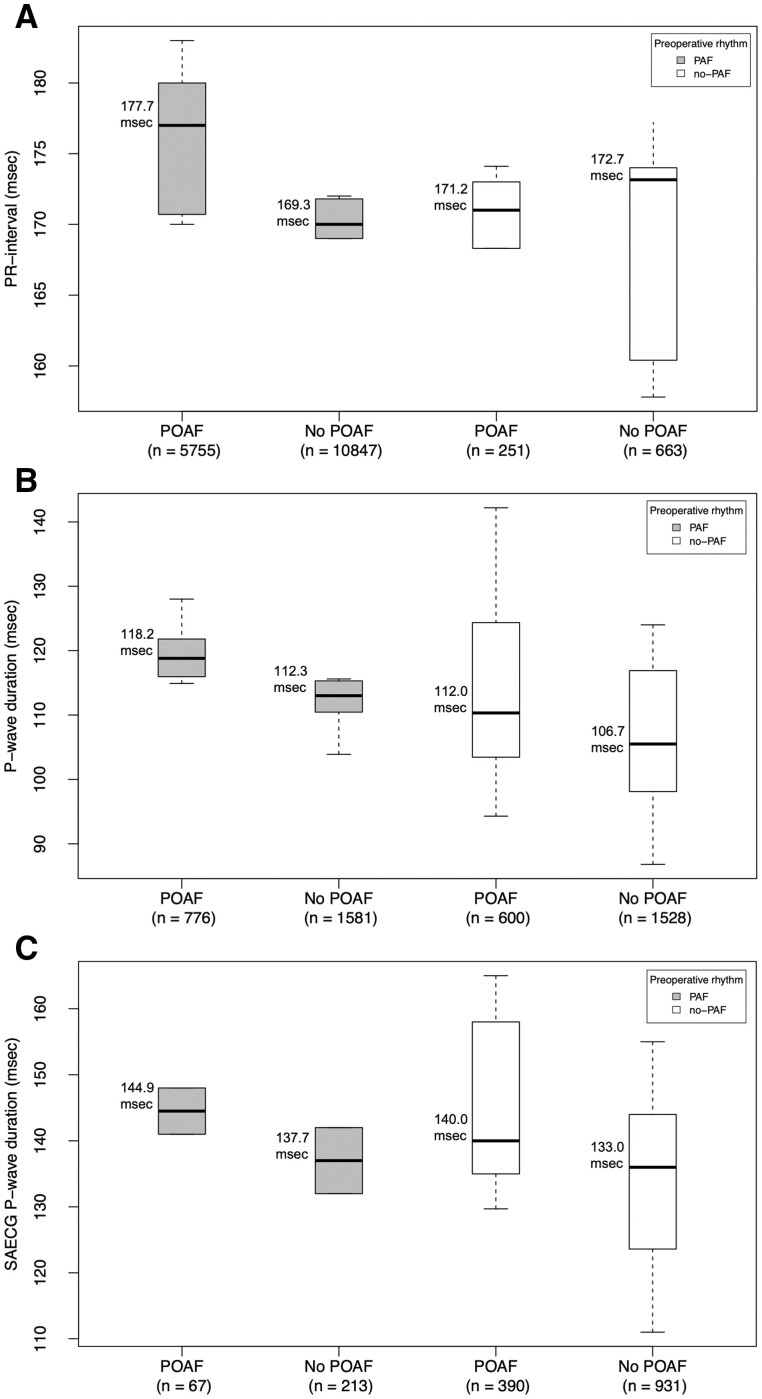
Eleven studies were included in a meta-analysis of PR-interval (ES = 0.1, 95% CI: 0.03–0.3) (Fig. 2B). Differences in PR interval are shown in Fig. 3A. Subgroup analysis showed that larger cohort size was associated with a greater ES (ES = 0.2, 95% CI: 0.1–0.3, P = 0.03) (Table 2). There was no significant difference for ES in the subgroup analysis for PR-interval based on rhythm history, however, there was a greater ES for patients with history of AF (ES = 0.2, 95% CI: 0.1–0.3) (Table 2). Also, higher prevalence of hypertension was associated with a lower ES (Beta = −0.04, P < 0.01), whereas increased average body mass index and increased percentage of patients with diabetes mellitus were associated with increased ES (Beta = 0.82, P = 0.02, and Beta = 0.03, P = 0.01, respectively) (Table 2). Heterogeneity analysis determined study by Kališnik et al. (2019) as the main source of heterogeneity (Supplementary Material, Fig. S4). Sensitivity analysis after omitting this study showed a small ES of 0.2 (95% CI: 0.2–0.3), with still substantial model heterogeneity (I2=63%, Cochran’s Q: p < 0.01) (Supplementary Material, Fig. 4).
Figure 3:
Box plots showing the differences between patients with and without POAF for PR-interval (A), P-wave duration (B), and signal-averaged electrocardiography P-wave duration (C). Patients with history of paroxysmal atrial fibrillation were separated from patients without history of paroxysmal atrial fibrillation. The exact values are provided in milliseconds (msec). POAF: postoperative atrial fibrillation.
Table 2:
Subgroup analysis and meta-regression
| PR-interval |
P-wave duration |
SAECG P-wave duration |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups | Number of studies | SMD 95% CI | P-value | Number of studies | SMD 95% CI | P-value | Number of studies | SMD 95% CI | P-value | |
| Study size (n) | <100 | 2 | −0.24 (−0.59 to 0.12) | 0.03 | 10 | 0.48 (0.18 to 0.78) | 0.50 | 4 | 0.98 (0.64 to 1.31) | 0.47 |
| >100 | 9 | 0.19 (0.07 to 0.30) | 12 | 0.36 (0.20 to 0.53) | 8 | 0.76 (0.29 to 1.23) | ||||
| Type of study | Prospective | 8 | 0.11 (−0.02 to 0.24) | 0.33 | 15 | 0.41 (0.21 to 0.61) | 0.65 | 12 | NA | NA |
| Retrospective | 3 | 0.30 (−0.06 to 0.65) | 7 | 0.35 (0.20 to 0.50) | 0 | NA | ||||
| Type of surgery | CABG | 7 | 0.21 (0.03 to 0.39) | 0.20 | 17 | 0.42 (0.26 to 0.58) | 0.24 | 10 | 0.86 (0.43 to 1.29) | 0.69 |
| AVR | 0 | NA | 1 | 0.23 (−0.24 to 0.71) | 0 | NA | ||||
| Diversea | 4 | 0.02 (−0.19 to 0.24) | 3 | 0.18 (−0.22 to 0.57) | 2 | 0.75 (0.45 to 1.05) | ||||
| Mitral Valve | 0 | NA | 1 | 0.24 (−0.24 to 0.71) | 0 | NA | ||||
| Definition of POAFb | Any | 6 | 0.17 (−0.01 to 0.34) | 0.40 | 9 | 0.47 (0.23 to 0.71) | 0.14 | 4 | 0.44 (0.27 to 0.61) | 0.07 |
| Short episode | 1 | −0.04 (- 0.30 to 0.21) | 7 | 0.46 (0.16 to 0.76) | 3 | 1.25 (−0.24 to 2.75) | ||||
| Long episode | 4 | 0.14 (−0.11 to 0.38) | 6 | 0.22 (0.05 to 0.40) | 5 | 0.84 (0.51 to 1.16) | ||||
| History of AF | No | 6 | −0.01 (−0.38 to 0.35) | 0.24 | 15 | 0.43 (0.27 to 0.60) | 0.44 | 10 | 0.89 (0.46 to 1.33) | 0.16 |
| Yes | 5 | 0.21 (0.13 to 0.30) | 7 | 0.32 (0.09 to 0.55) | 2 | 0.53 (0.24 to 0.80) | ||||
| Meta-regression | Number of studies | Beta 95% CI | P-value | Number of studies | Beta 95% CI | P-value | Number of studies | Beta 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Age, per 1 year | 10 | −0.05 (−0.11 to 0.001) | 0.05 | 22 | −0.003 (−0.04 to 0.03) | 0.85 | 12 | 0.04 (−0.06 to 0.15) | 0.44 |
| DM2, per 1% | 8 | 0.03 (0.006 to 0.05) | 0.01 | 15 | −0.004 (−0.02 to 0.008) | 0.49 | 5 | 0.005 (−0.04 to 0.04) | 0.82 |
| %Male, per 1% | 10 | 0.005 (−0.01 to 0.02) | 0.57 | 20 | −0.007 (−0.02 to 0.009) | 0.41 | 12 | −0.06 (−0.09 to 0.03) | <0.01 |
| Hypertension, per 1% | 8 | −0.04 (−0.06 to 0.01) | <0.01 | 17 | −0.003 (−0.01 to 0.006) | 0.54 | 6 | 0.002 (−0.02 to 0.02) | 0.84 |
| LVEF, per 1% | 4 | −0.009 (−0.07 to 0.05) | 0.77 | 17 | 0.01 (−0.02 to 0.04) | 0.44 | 10 | −0.03 (−0.09 to 0.03) | 0.37 |
| COPD, per 1% | 5 | −0.01 (−0.21 to 0.19) | 0.94 | 10 | 0.01 (−0.01 to 0.02) | 0.23 | 4 | 0.07 (0.01 to 0.13) | 0.01 |
| RCA occlusion, per 1% | 0 | NA | NA | 6 | 0.01 (−0.005 to 0.02) | 0.20 | 7 | −0.01 (−0.02 to 0.01) | 0.22 |
| BMI, per 1 kg/m2 | 3 | 0.82 (0.16 to 1.48) | 0.02 | 10 | −0.03 (−0.13 to 0.08) | 0.62 | 0 | NA | NA |
| LAD, per 1 mm | 0 | NA | NA | 12 | −0.08 (−0.89 to 1.05) | 0.88 | 6 | −0.68 (−4.37 to 3.01) | 0.72 |
AF: atrial fibrillation; AVR: aortic valve replacement; BMI: body mass index; CABG: coronary artery bypass grafting; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DM2 : diabetes mellitus type 2; LAD: left atrial diameter; LVEF: left ventricle ejection fraction; POAF: postoperative atrial fibrillation; RCA: right coronary artery; SAECG: signal-averaged electrocardiography; SMD: standardized mean difference. Bold values are marked as statistically significant with a P-value threshold of 0.05.
Coronary artery bypass grafting, valvular surgery, combined surgery.
Short episodes are defined as POAF duration less than 30 min, whereas long episodes only included POAF duration over 30 min.
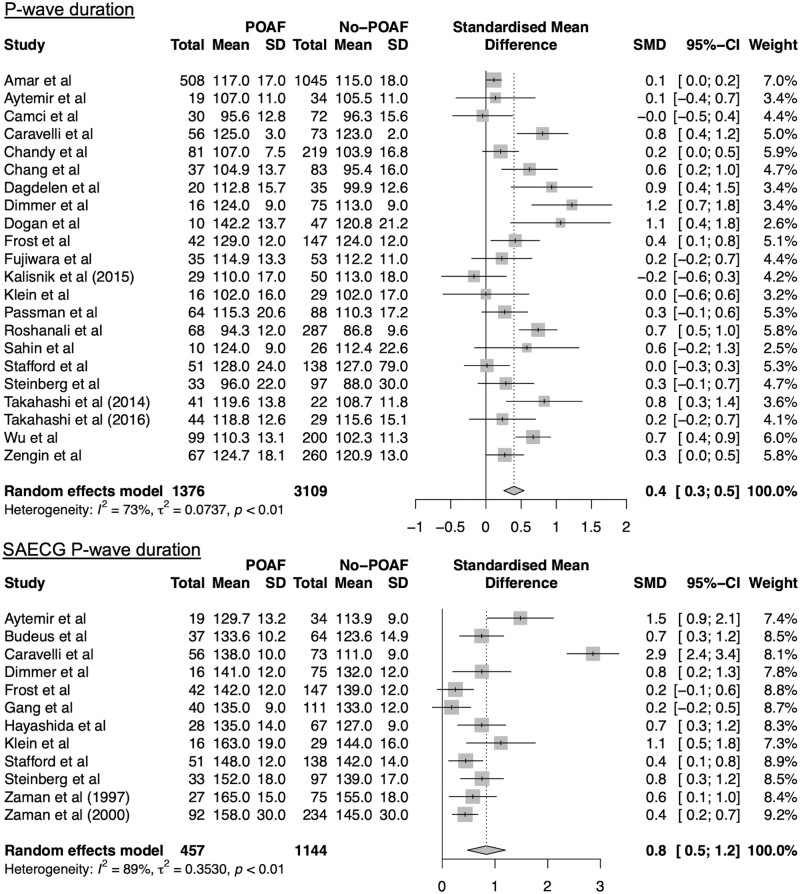
Figure 4:
Forest plots for meta-analyses for P-wave duration (12 leads and signal-averaged electrocardiography). Studies included in meta-analysis, mean preoperative values for patients with and without POAF, corresponding standard deviations, numbers of subjects, standardized mean differences, corresponding standard deviations and the weight of the studies are presented. Overall effect size is presented in a diamond shape. CI: confidence interval; POAF: postoperative atrial fibrillation; SD: standard deviation.
P-wave duration (12-leads electrocardiography)
Twenty-two studies were included in a meta-analysis of P-wave duration measured on 12-lead ECG (ES = 0.4, 95% CI: 0.3–0.5) (Fig. 4). Patients with history of AF had a greater P-wave duration as compared to patients without history of AF (115.2 ms; 95% CI: 112.3–118.2 ms, vs 109.8 ms; 95% CI: 104.5–114.9 ms, p = 0.04, respectively). Also, there was a gradual increase in P-wave duration between no-POAF patients without history of AF, and POAF-patient with history of AF (105.7 ms; 95% CI: 97.9–115.5 ms, vs 118.2 ms; 95% CI: 112.9–123.4 ms, p = 0.03, respectively) (Fig. 3B). Subgroup analysis and meta-regression showed no statistically significant results (Table 2). Heterogeneity analysis revealed studies by Kališnik et al. (2015), Dimmer et al. and Roshanali et al. as main sources of heterogeneity (Supplementary Material, Fig. S5–S7). Sensitivity analysis after omitting these studies showed a small ES (ES = 0.4 95% CI: 0.2–0.5), with still substantial model heterogeneity (I2 = 67%, Cochran’s Q: P < 0.01) (Supplementary Material, Fig. S8).
P-wave duration (signal-averaged electrocardiography)
Twelve studies were included in a meta-analysis of signal-averaged ECG (SAECG) P-wave duration (ES = 0.8, 95% CI: 0.5–1.2) (Fig. 4). Patients with history of AF had a slightly greater P-wave duration as compared to patients without history of AF (140.7 ms; 95% CI: 133.6–147.8 ms, vs 138.8 ms; 95% CI: 133.1–144.6 ms, P = 0.69, respectively) (Fig. 3C). Also, there was a gradual increase in P-wave duration from no-POAF patients without history of AF, to POAF-patient with history of AF (133.0 ms; 95% CI: 124.7–141.3 ms, vs 144.9 ms; 95% CI: 138.1–151.7 ms, P = 0.03, respectively) (Fig. 3C). Meta-regression showed that higher percentage of male subjects was associated with a lower ES (Beta = −0.06, P < 0.01), whereas increased prevalence of chronic obstructive pulmonary disease was associated with increased ES (Beta = 0.07, P = 0.01) (Table 2). Heterogeneity analysis identified study by Caravelli et al. as the main source of heterogeneity (Supplementary Material, Fig. S9). Sensitivity analysis after omitting this publication showed a moderate ES of 0.6 (95% CI: 0.4–0.8), with still moderate model heterogeneity (I2 = 56%, Cochran’s Q: P = 0.01) (Supplementary Material, Fig. S10).
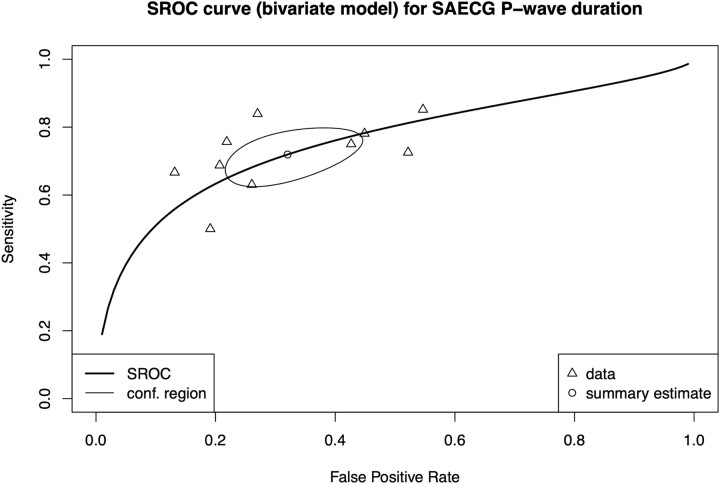
Ten studies were included in an ROC meta-analysis (Table 3). Pooled results of the SROC are presented in Fig. 5. Overall sensitivity was 72% (95% CI: 65–78) and specificity was 68% (95% CI: 58–77) for a range of cut-off values (122.3–155 ms). AUC (0.76) revealed a good discriminative power for the SROC. Meta-regression showed that studies published before year 2000 had lower specificity (Beta = −0.76, P = 0.03) (Table 3).
Table 3:
SAECG P-wave duration diagnostic test accuracy meta-analysis and meta-regression
| Overview of diagnostic accuracy data | ||||||
|---|---|---|---|---|---|---|
| Study | Cut-off value (ms) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
| Aytemir et al. | 122.3 | 68 | 88 | 76 | 83 | 79.6 |
| Budeus et al. | 124 | 75 | 78 | 64 | 86 | 77.2 |
| Caravelli et al. | 135 | 84 | 73 | 70 | 85 | 77.0 |
| Dimmer et al. | 134 | 75 | 57 | 28 | 91 | 60.4 |
| Hayashida et al. | 135 | 50 | 81 | 52 | 79 | 71.9 |
| Klein et al. | 155 | 69 | 79 | 65 | 82 | 75.6 |
| Stafford et al. | 141 | 73 | 48 | 34 | 83 | 54.5 |
| Steinberg et al. | 140 | 77 | 55 | 37 | 87 | 60.9 |
| Zaman et al. (1997) | 155 | 86 | 45 | 37 | 89 | 55.9 |
| Zaman et al. (2000) | 155 | 63 | 74 | 49 | 84 | 70.9 |
| ROC meta-analysis (Reitsma model) | ||||||
|---|---|---|---|---|---|---|
| Cut-off value (ms) | Sensitivity (%) | 95% CI | Specificity (%) | 95% CI | AUC | |
| Summary results | 122.3–155 | 72 | 65–78 | 68 | 58–77 | 0.756 |
| Subgroup analysis and meta-regression | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sensitivity |
Specificity |
|||||||
| Variable | Number of studies | Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| Study size | >100a | 6 | 0.55 | −0.14 to 1.25 | 0.12 | 0.65 | 0.20 to 1.50 | 0.14 |
| <100 | 4 | |||||||
| History of AF | Yesa | 2 | 0.02 | −0.95 to 0.99 | 0.97 | 0.83 | −0.10 to 1.75 | 0.08 |
| No | 8 | |||||||
| Publication year | <2000a | 6 | −0.27 | −0.99 to 0.46 | 0.47 | −0.76 | −1.44 to 0.07 | 0.03 |
| ≥2000 | 4 | |||||||
| Age, per 1 year | 10 | −0.03 | −0.15 to 0.08 | 0.60 | −0.07 | −0.20 to 0.07 | 0.32 | |
| %Male, per 1% | 10 | 0.01 | −0.04 to 0.05 | 0.80 | 0.05 | −0.003 to 0.10 | 0.06 | |
| LVEF, per 1% | 9 | −0.02 | −0.08 to 0.04 | 0.46 | 0.03 | −0.03 to 0.09 | 0.17 | |
| RCA occlusion, per 1% | 6 | 0.01 | −0.02 to 0.04 | 0.41 | 0.01 | −0.03 to 0.04 | 0.64 | |
| LAD, per 1 mm | 6 | 1.31 | −1.15 to 3.77 | 0.30 | 1.56 | −0.45 to 3.57 | 0.13 | |
| Hypertension, per 1% | 4 | 0.01 | −0.03 to 0.05 | 0.67 | −0.01 | −0.07 to 0.07 | 0.99 | |
AF: atrial fibrillation; AUC: area under the curve; CI: confidence interval; LAD: left atrial diameter; LVEF: left ventricle ejection fraction; ms: milliseconds; NPV: negative predictive value; POAF: postoperative atrial fibrillation; PPV: positive predictive value; RCA: right coronary artery. Bold values are marked as statistically significant with a P-value threshold of 0.05.
Reference category.
Figure 5:
Summary receiver operator curve (SROC) for ROC meta-analysis of SAECG P-wave duration. Triangles represent the individual studies, whereas the round shape shows the summary estimate. Ellipse surrounding the summary estimate depicts the confidence region of the summary estimate. SAECG: signal-averaged electrocardiography; SROC: summary receiver operator curve.
Publication bias
Meta-analyses for P-wave dispersion and P-wave duration (12-lead ECG) showed significant publication bias (Egger’s test: P = 0.01 and P = 0.04, respectively) (Supplementary Material, Figs S11–S14). Trim-and-fill analysis was performed for P-wave duration using 3 different model estimators (L0, R0 and Q0) to correct for the presence of publication bias and it showed potentially 2 studies missing on the left side of the funnel plot (Supplementary Material, Fig. S15). Adjustment for these studies showed a lower ES of 0.3 (95% CI: 0.2–0.5) for P-wave duration (12-lead ECG). Trim-and-fill analysis for P-wave dispersion could not be performed due to significant outliers leading to funnel plot asymmetry.
DISCUSSION
In this meta-analysis, we analysed the predictive value of preoperative P-wave parameters for POAF prediction in patients undergoing cardiac surgery. We found that prolonged preoperative P-wave duration, as defined on conventional 12-lead ECG or SAECG, is an important predictor of POAF in the inherently heterogeneous population undergoing cardiac surgery. ROC meta-analysis was only performed for SAECG P-wave duration since other studies did not provide sufficient data for this analysis. This analysis showed that SAECG P-wave duration has an adequate predictive value for POAF (AUC = 0.76), although with a variety of cut-off values across studies (122.3 ms to 155 ms). Differences in cut-off values were mainly caused by different P-wave filtering techniques and distinct study populations, which impeded the selection of a single cut-off value. Nevertheless, our findings are in line with several studies which identified increased P-wave duration as a predictor of AF in the general population further emphasizing its importance as an indicator of AF substrate [3, 4].
In this meta-analysis, we found a significant relationship between preoperative P-wave dispersion and incidence of POAF. However, there was substantial heterogeneity in the model resulting from contradicting evidence in the original articles. Previous studies suggested that P-wave dispersion might be a useful predictor of paroxysmal AF and AF recurrence after catheter ablations, however, its role in predicting POAF is still questionable [47]. Notably, Chandy et al. [20] found a greater increase in postoperative P-wave dispersion in POAF patients as compared to patients without POAF suggesting that intra- and interatrial conduction delays and inhomogeneous wave propagation may intensify due to surgery itself, possibly as a result of atrial ischaemia.
The relationship between atrioventricular conduction, defined as PR-interval, and risk of POAF is still not fully understood, and our meta-analysis showed contradicting results with substantial model heterogeneity even after sensitivity analyses. Since PR-interval is a combination of atrial conduction time and atrioventricular-conduction time, it is influenced by multiple factors which are not directly associated with AF, such as atrioventricular-node dysfunction. Interestingly, subgroup analysis and meta-regression revealed that hypertension was associated with a lower ES of PR-interval, while PR-interval was significantly longer in patients with pre-existing AF developing POAF compared to no-AF patients without POAF. This suggests that PR-interval reflects the underlying substrate for AF development.
On the other hand, P-wave duration was a strong predictor of POAF, independently from the left atrial diameter (Table 2). In fact, P-wave duration seems to provide information on the different phases of electrical remodelling increasing from lowest duration of 106.7 ms in patients without a history of AF and no-POAF to highest duration up to 118.2 ms in patients with a history of AF and POAF development (Fig. 4B and C). From the electrophysiological point of view, prolonged P-wave duration is most likely caused by disturbances in atrial electrical conduction and in lesser degree by atrial dilatation [48]. Previous post-mortem studies have reported that fatty infiltration and fibrosis in major atrial conduction routes, such as Bachmann’s bundle and the crista terminalis, are associated with prolonged P-wave duration [49]. These processes might lead to local areas with conduction blocks which are able to facilitate re-entry wavelets and eventually induction of AF [20]. Accordingly, previous electroanatomical mapping studies reported the relationship between lower atrial conduction velocities, especially in the Bachmann’s bundle, and AF incidence [50]. Furthermore, epicardial mapping of patients without a history of AF undergoing cardiac surgery showed more complex propagation patterns in patients developing POAF as compared to patients without POAF [2]. Also, previous studies have demonstrated the predictive value of total atrial activation time, which is a parameter quantifying intra- and interatrial conduction disturbances, in predicting POAF in a variety of cardiac surgical patients [14].
Clinical implications
Currently, there is accumulating evidence suggesting that POAF is not only limited to the early postoperative phase but that it is associated with long-term AF recurrences [2]. Also, POAF is associated with the incidence of early- and late postoperative stroke, long-term mortality and prolonged hospitalization [51]. Therefore, preoperative prediction of new-onset POAF after cardiac surgery might be of tremendous interest to prevent these complications. Moreover, considering POAF as a surrogate marker for an AF substrate, the identification of patients at risk for POAF during the hospitalization period might provide indications for long-term rhythm follow-up in this select group of patients. This approach might help to identify patients which show progression of the arrhythmia and therefore potentially facilitate timely therapeutic interventions to prevent further progression to sustained AF. Whereas current prediction scores for POAF mostly focus on clinical parameters, we believe that additional parameters, which focus on quantification of an AF-substrate, might contribute to POAF prediction. This meta-analysis shows that P-wave duration, measured on standard 12-lead ECG and SAECG, might be a helpful tool to identify patients at risk for POAF. Additionally, previous studies described the potential of clinical parameters and preoperative transthoracic echocardiography in predicting POAF [1, 14]. However, even though these non-invasive diagnostic modalities are standard of care in the preoperative setting, they are yet to be implemented as a standardized predictive tool for POAF. Future studies should be performed to develop POAF prediction tools consisting of a combination of clinical, echocardiographic and electrocardiographic parameters to improve POAF prediction accuracy.
Limitations
The quality of studies included in a meta-analysis is always a limiting factor for the overall results. As the quality of the studies included in our meta-analyses was high, we believe our results are robust. The first limitation of our study was the substantial heterogeneity of the meta-analyses even after extensive sensitivity analysis and several subgroup analyses. The second limitation was significant publication bias in the meta-analyses for P-wave dispersion and P-wave duration. Trim-and-fill analysis suggested that the results for P-wave duration might be slightly overestimated. The third limitation was the variation in cut-off values of the studies included in the ROC meta-analysis for SAECG P-wave duration what impeded the selection of a single optimal cut-off value. However, we could establish the study-differences that attributed to variance in diagnostic accuracy by performing a meta-regression. The fourth limitation was the broad range of publication dates of the articles included (1993–2020). Nevertheless, we believe that P-wave analysis has remained consistent over the years despite changes in risk factor modifications, clinical practice, surgical techniques and perioperative managements. The fifth and last limitation was the lack of consistency in POAF definition among studies included in the meta-analyses. To explore this bias, we have performed a subgroup-analysis which showed no significant differences for the different definitions of POAF. Despite all these limitations, our results provide a thorough insight into the ECG parameters associated with a higher risk of POAF.
CONCLUSION
In this meta-analysis including 20 201 patients, we found that increased preoperative P-wave duration, measured on conventional 12-lead ECG or SAECG, is a useful tool for POAF prediction after cardiac surgery. Implementation of P-wave duration in substrate-based risk scores for POAF will provide valuable information on the presence and severity of intra- and interatrial conduction disturbances, independently from left atrium size. Future studies should combine these easily accessible and standardized risk prediction models to identify patients at risk of developing POAF and find potential associations with long-term outcomes such as late POAF and stroke.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Michal J Kawczynski, Department of Cardiothoracic Surgery, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands; Department of Physiology, Maastricht University, Maastricht, Netherlands.
Sophie Van De Walle, Department of Cardiothoracic Surgery, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands.
Bart Maesen, Department of Cardiothoracic Surgery, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands; Department of Physiology, Maastricht University, Maastricht, Netherlands.
Aaron Isaacs, Department of Physiology, Maastricht University, Maastricht, Netherlands.
Stef Zeemering, Department of Physiology, Maastricht University, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht, Netherlands.
Ben Hermans, Department of Physiology, Maastricht University, Maastricht, Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht, Netherlands.
Kevin Vernooy, Cardiovascular Research Institute Maastricht (CARIM), Maastricht, Netherlands; Department of Cardiology, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands.
Jos G Maessen, Department of Cardiothoracic Surgery, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands; Department of Physiology, Maastricht University, Maastricht, Netherlands.
Ulrich Schotten, Department of Physiology, Maastricht University, Maastricht, Netherlands.
Elham Bidar, Department of Cardiothoracic Surgery, Heart and Vascular Centre, Maastricht University Medical Centre, Maastricht, Netherlands; Department of Physiology, Maastricht University, Maastricht, Netherlands.
Data Availability
Data are available upon reasonable request.
Author contributions
Michal J. Kawczynski: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Validation; Visualization; Writing—original draft; Writing—review & editing. Sophie Van De Walle: Conceptualization; Data curation; Methodology. Bart Maesen: Data curation; Methodology; Supervision; Writing—review & editing. Aaron Isaacs: Conceptualization; Methodology; Writing—review & editing. Stef Zeemering: Conceptualization; Writing—review & editing. Ben Hermans: Conceptualization; Methodology; Writing—original draft; Writing—review & editing. Kevin Vernooy: Conceptualization; Supervision; Validation; Writing—review & editing. Jos Maessen: Conceptualization; Methodology; Validation; Writing—review & editing. Ulrich Schotten: Conceptualization; Methodology; Supervision; Writing—review & editing. Elham Bidar: Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Writing—original draft; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Benjamin Bidstrup, Tomislav Kopjar and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Woldendorp K, Farag J, Khadra S, Black D, Robinson B, Bannon P.. Postoperative atrial fibrillation after cardiac surgery: a meta-analysis. Ann Thorac Surg 2021;112:2084–93. [DOI] [PubMed] [Google Scholar]
- 2. Bidar E, Zeemering S, Gilbers M, Isaacs A, Verheule S, Zink MD. et al. Clinical and electrophysiological predictors of device-detected new-onset atrial fibrillation during 3 years after cardiac surgery. Europace 2021;23:1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nielsen JB, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MS. et al. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG Study. Heart Rhythm 2013;10:1249–56. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen JB, Kühl JT, Pietersen A, Graff C, Lind B, Struijk JJ. et al. P-wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG Study. Heart Rhythm 2015;12:1887–95. [DOI] [PubMed] [Google Scholar]
- 5. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Downs S, Black N.. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomized studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olkin I, Dahabreh IJ, Trikalinos TA.. GOSH—a graphical display of study heterogeneity. Res Synth Methods 2012;3:214–23. [DOI] [PubMed] [Google Scholar]
- 8. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH.. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–90. [DOI] [PubMed] [Google Scholar]
- 9. Egger M, Smith GD, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duval S, Tweedie R.. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- 11. Shi L, Lin L.. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore) 2019;98:e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2014. www.R-project.org (18 August 2022, date last accessed). [Google Scholar]
- 13. Schwarzer G, Carpenter JR, Rücker G.. Meta-Analysis with R (Use-R!). Cham, Switzerland: Springer International Publishing, 2015. [Google Scholar]
- 14. Kawczynski MJ, Gilbers M, Van De Walle S, Schalla S, Crijns HJ, Maessen JG. et al. Role of pre-operative transthoracic echocardiography in predicting post-operative atrial fibrillation after cardiac surgery: a systematic review of the literature and meta-analysis. Europace 2021;23:1731–43. [DOI] [PubMed] [Google Scholar]
- 15. Amar D, Shi W, Hogue CW Jr, Zhang H, Passman RS, Thomas B. et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol 2004;44:1248–53. [DOI] [PubMed] [Google Scholar]
- 16. Aytemir K, Aksoyek S, Ozer N, Aslamaci S, Oto A.. Atrial fibrillation after coronary artery bypass surgery: P wave signal averaged ECG, clinical and angiographic variables in risk assessment. Int J Cardiol 1999;69:49–56. [DOI] [PubMed] [Google Scholar]
- 17. Budeus M, Hennersdorf M, Rohlen S, Schnitzler S, Felix O, Reimert K. et al. Prediction of atrial fibrillation after coronary artery bypass grafting: the role of chemoreflexsensitivity and P wave signal averaged ECG. Int J Cardiol 2006;106:67–74. [DOI] [PubMed] [Google Scholar]
- 18. Camci S, Ari S, Karakus A, Ari H, Taner T.. The predictive value of the combined systolic-diastolic index for atrial fibrillation after coronary artery bypass surgery. Echocardiography 2020;37:1177–83. [DOI] [PubMed] [Google Scholar]
- 19. Caravelli P, Carlo M, Musumeci G, Tartarini G, Gherarducci G, Bortolotti U. et al. P-wave signal-averaged electrocardiogram predicts atrial fibrillation after coronary artery bypass grafting. Ann Noninv Electrocard 2002;7:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chandy J, Nakai T, Lee RJ, Bellows WH, Dzankic S, Leung JM.. Increases in P-wave dispersion predict postoperative atrial fibrillation after coronary artery bypass graft surgery. Anesth Analg 2004;98:303–10. [DOI] [PubMed] [Google Scholar]
- 21. Chang CM, Lee SH, Lu MJ, Lin CH, Chao HH, Cheng JJ. et al. The role of P wave in prediction of atrial fibrillation after coronary artery surgery. Int J Cardiol 1999;68:303–8. [DOI] [PubMed] [Google Scholar]
- 22. Dagdelen S, Yuce M, Toraman F, Karabulut H, Alhan C.. The value of P dispersion on predicting atrial fibrillation after coronary artery bypass surgery; effect of magnesium on P dispersion. Card Electrophysiol Rev 2003;7:162–4. [DOI] [PubMed] [Google Scholar]
- 23. Dimmer C, Jordaens L, Gorgov N, Peene I, Francois K, Van Nooten G. et al. Analysis of the p wave with signal averaging to assess the risk of atrial fibrillation after coronary artery bypass surgery. Cardiology 1998;89:19–24. [DOI] [PubMed] [Google Scholar]
- 24. Dogan SM, Buyukates M, emir O, Aydin M, Gursurer M, Acikgoz S. et al. Predictors of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis 2007;18:327–31. [DOI] [PubMed] [Google Scholar]
- 25. Frost L, Lund B, Pilegaard H, Christiansen EH.. Re-evaluation of the role of P-wave duration and morphology as predictors of atrial fibrillation and flutter after coronary artery bypass surgery. Eur Heart J 1996;17:1065–71. [DOI] [PubMed] [Google Scholar]
- 26. Fujiwara M, Nakano Y, Hidaka T, Oda N, Uchimura Y, Sairaku A. et al. Prediction of atrial fibrillation after off-pump coronary artery bypass grafting using preoperative total atrial conduction time determined on tissue Doppler imaging. Circ J 2014;78:345–52. [DOI] [PubMed] [Google Scholar]
- 27. Gang Y, Hnatkova K, Mandal K, Ghuran A, Malik M.. Preoperative electrocardiographic risk assessment of atrial fibrillation after coronary artery bypass grafting. J Cardiovasc Electrophysiol 2004;15:1379–86. [DOI] [PubMed] [Google Scholar]
- 28. Hayashida N, Shojima T, Yokokura Y, Hori H, Yoshikawa K, Tomoeda H. et al. P-wave signal-averaged electrocardiogram for predicting atrial arrhythmia after cardiac surgery. Ann Thorac Surg 2005;79:859–64. [DOI] [PubMed] [Google Scholar]
- 29. Kališnik JM, Hrovat E, Hrastovec A, Avbelj V, Žibert J, Geršak B.. Severe cardiac autonomic derangement and altered ventricular repolarization pave the way to postoperative atrial fibrillation. Innovations (Phila) 2015;10:398–405. [DOI] [PubMed] [Google Scholar]
- 30. Kališnik JM, Avbelj V, Vratanar J, Santarpino G, Geršak B, Fischlein T. et al. Cardiac autonomic regulation and PR interval determination for enhanced atrial fibrillation risk prediction after cardiac surgery. Int J Cardiol 2019;289:24–9. [DOI] [PubMed] [Google Scholar]
- 31. Klein M, Evans SJL, Blumberg S, Cataldo L, Bodenheimer MM.. Use of P-wave-triggered, P-wave signal-averaged electrocardiogram to predict atrial fibrillation after coronary artery bypass surgery. Am Heart J 1995;129:895–901. [DOI] [PubMed] [Google Scholar]
- 32. Magne J, Salerno B, Mohty D, Serena C, Rolle F, Piccardo A. et al. Echocardiography is useful to predict postoperative atrial fibrillation in patients undergoing isolated coronary bypass surgery: a prospective study. Eur Heart J Acute Cardiovasc Care 2019;8:104–13. [DOI] [PubMed] [Google Scholar]
- 33. Passman R, Beshai J, Pavri B, Kimmel S.. Predicting post-coronary bypass surgery atrial arrhythmias from the preoperative electrocardiogram. Am Heart J 2001;142:806–10. [DOI] [PubMed] [Google Scholar]
- 34. Rader F, Costantini O, Jarrett C, Gorodeski EZ, Lauer MS, Blackstone EH.. Quantitative electrocardiography for predicting postoperative atrial fibrillation after cardiac surgery. J Electrocardiol 2011;44:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roshanali F, egar MH, Yousefnia MA, Rayatzadeh H, Alaeddini F, Amouzadeh F.. Prediction of atrial fibrillation via atrial electromechanical interval after coronary artery bypass grafting. Circulation 2007;116:2012–7. [DOI] [PubMed] [Google Scholar]
- 36. Sahin M, Tigen K, Dundar C, Ozben B, Alici G, Demir S. et al. Postoperative atrial fibrillation in patients with left atrial myxoma. Cardiovasc J Afr 2015;26:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sigurdsson MI, Muehlschlegel JD, Fox AA, Heydarpour M, Lichtner P, Meitinger T. et al. Genetic variants associated with atrial fibrillation and PR interval following cardiac surgery. J Cardiothorac Vasc Anesth 2015;29:605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stafford PJ, Kolvekar S, Cooper J, Fothergill J, Schlindwein F, deBono DP. et al. Signal averaged P wave compared with standard electrocardiography or echocardiography for prediction of atrial fibrillation after coronary bypass grafting. Heart 1997;77:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E.. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993;88:2618–22. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi S, Fujiwara M, Watadani K, Taguchi T, Katayama K, Takasaki T. et al. Preoperative tissue Doppler imaging-derived atrial conduction time can predict postoperative atrial fibrillation in patients undergoing aortic valve replacement for aortic valve stenosis. Circ J 2014;78:2173–81. [DOI] [PubMed] [Google Scholar]
- 41. Takahashi S, Katayama K, Watanabe M, Kodama H, Taguchi T, Kurosaki T. et al. Preoperative tissue Doppler imaging-derived atrial conduction time predicts postoperative atrial fibrillation in patients undergoing mitral valve surgery for mitral valve regurgitation. Circ J 2016;80:101–9. [DOI] [PubMed] [Google Scholar]
- 42. Wu F, Wu Y, Tao W, Zhao H, Shen D.. Preoperative P-wave duration as a predictor of atrial fibrillation after coronary artery bypass grafting: a prospective cohort study with meta-analysis. Int J Nurs Sci 2018;5:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaman AG, Alamgir F, Richens T, Williams R, Rothman MT, Mills PG.. The role of signal averaged P wave duration and serum magnesium as a combined predictor of atrial fibrillation after elective coronary artery bypass surgery. Heart 1997;77:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG.. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation 2000;101:1403–8. [DOI] [PubMed] [Google Scholar]
- 45. Achmad C, Tiksnadi BB, Akbar MR, Karwiky G, Sihite TA, Pramudya A. et al. Left volume atrial index and P-wave dispersion as predictors of postoperative atrial fibrillation after coronary artery bypass graft: a retrospective cohort study. Curr Probl Cardiol 2021:101031. Article in press. [DOI] [PubMed] [Google Scholar]
- 46. Zengin A, Karataş MB, Çanga Y, Pay L, Eren S, Çalık AN. et al. A novel electrocardiographic parameter for the prediction of atrial fibrillation after coronary artery bypass graft surgery "P wave peak time”. Ir J Med Sci 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 47. Okutucu S, Aytemir K, Oto A.. P-wave dispersion: what we know till now? JRSM Cardiovasc Dis 2016;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schotten U, Verheule S, Kirchhof P, Goette A.. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 49. Huo Y, Mitrofanova L, Orshanskaya V, Holmberg P, Holmqvist F, Platonov PG.. P-wave characteristics and histological atrial abnormality. J Electrocardiol 2014;47:275–80. [DOI] [PubMed] [Google Scholar]
- 50. Walters TE, Teh AW, Spence S, Kistler PM, Morton JB, Kalman JM.. Relationship between the electrocardiographic atrial fibrillation cycle length and left atrial remodeling: a detailed electroanatomic mapping study. Heart Rhythm 2014;11:670–6. [DOI] [PubMed] [Google Scholar]
- 51. Lin MH, Kamel H, Singer DE, Wu YL, Lee M, Ovbiagele B.. Perioperative/postoperative atrial fibrillation and risk of subsequent stroke and/or mortality. Stroke 2019;50:1364–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.