Abstract
Background
Published data regarding long-lasting immunological rabies memory after pre-exposure prophylaxis (PrEP) are scarce. We tested the hypothesis that rabies booster immunization elicits rapid anamnestic responses.
Methods
For this observational study, we included participants who had received PrEP 10–24 years before inclusion. We measured rabies antibody titers before, and on days 3, 7, and 14 after a single intramuscular booster.
Results
All 28 participants responded adequately regardless of route of administration or 2-dose vs 3-dose PrEP regimen.
Conclusion
Rabies immunological memory is reactivated within 7 days after a single intramuscular booster immunization, even when administered 10–24 years after PrEP.
Keywords: rabies, immunization, boostability, booster immunization, long-term immunogenicity data, post-exposure prophylaxis
Rabies is a lethal zoonotic disease, fully preventable by direct intervention after contact with an infected animal. The type of treatment that needs to be administered after contact depends on whether or not the individual has received a preventive immunization schedule, so called pre-exposure prophylaxis (PrEP) [1, 2]. In any case, post-exposure prophylaxis (PEP) consists of prompt wound care and the administration of anti-rabies vaccinations. In the case of a nonimmunized or immunocompromised individual, this is an elaborate vaccination schedule of 4–5 vaccinations supplemented with the administration of rabies immunoglobulins (RIG) [1, 2]. Unfortunately, access to RIG is often limited or nonexistent in low- and middle-income countries where rabies is endemic [2]. The rationale for the administration of RIG is passive immunization to cover the period between administration of the vaccine and the mounting of an active immune response [3]. If an immune-competent individual has ever received PrEP, there is no need for RIG after exposure because of the presumed existence of rabies memory B cells formed after initial immunization. These memory cells allow an accelerated anamnestic neutralizing antibody response upon booster vaccination, the so-called “boostability” [1–5]. International guidelines suggest lifelong boostability by PEP if preceded by a complete course of PrEP [2].
In a recent systematic review and meta-analysis by Langedijk et al, only 2 studies were available that described booster responses more than 10 years after administration of PrEP or PEP with currently licensed immunizations [6]. The first study reported on 9 exposed individuals who had received PEP 32 years before their booster immunization [7]. Adequate booster responses were measured 30 days after immunization; this was considerably late as current guidelines suggest that the anamnestic rabies antibody response should be measured no later than 1 week after booster immunization [2, 3, 5–7]. The second study described adequate booster responses measured within 7 days after immunization of 53 subjects, who had received either PEP (38 subjects) or only PrEP (15 subjects), 10–21 years before booster immunization [8].
The current World Health Organization (WHO) recommendations state that both PrEP and PEP schedules induce lifelong boostable memory, which is mainly supported by data on booster vaccination within 10 years of primary immunization. Beyond 10 years, however, PrEP boostability with still licensed vaccines are only based on an observation of 15 individuals who had received a 3-dose PrEP schedule. With our study, we aimed to add more evidence to the assumption that PrEP conveys long-term boostable immunologic memory [9, 10].
WHO considers a titer above 0.5 IU/mL as adequate [2]. No specific end points have been described for rabies immunogenicity after booster immunization. In general, a 4-fold increase in antibody titers is considered an adequate booster response, for example in meningococcal polysaccharide vaccines [11]. We tested the hypothesis that all participants who had received PrEP longer than 10 years ago would develop an adequate rabies titer of ≥ 0.5 IU/mL within 1 week after boosting.
METHODS
Study Design and Procedure
This multicenter, prospective, observational study comprised subjects who had undergone different rabies PrEP immunization schedules at least 10 years prior. When the study started in 2016, the officially approved WHO rabies PrEP schedule consisted of a 3-dose immunization sequence, administered on days 0, 7, and 21–28. As of April 2018, the WHO endorsed a 2-dose PrEP schedule for immune-competent individuals.
We intended to include 30 participants in total. Blood samples were taken prior to administration of a single intramuscular (IM) booster immunization, as well as on days 3, 7, and 14 after immunization.
Study End Points
The primary end point was the proportion of participants with an adequate titer (defined as ≥ 0.5 IU/mL) within 1 week after booster immunization [2].
Secondary end points were (1) geometric mean titer (GMT), with a 95% confidence interval (CI), and range of antibody titer [12]; (2) the percentage of titers ≥ 3 and ≥ 10 IU/mL; and (3) the relative increase (fold) of titers compared to day 0.
Study Sites and Study Participants
The 2 Dutch study sites were the sickbay of the Marine Base in Doorn and the Center of Tropical and Travel Medicine of Amsterdam University Medical Centers in Amsterdam. The study was conducted between June 2016 and June 2019. For study participant details see Supplementary Material.
Vaccine
All participants received a single IM booster, that is 1 mL inactivated rabies vaccine, Rabipur (PL16033/0010, GlaxoSmithKline Vaccines)
Rabies Virus Neutralization Antibodies
For determination of rabies virus neutralization antibodies, the rapid fluorescent focus inhibition test (RFFIT) was used, which is considered to be the gold standard by the WHO. All blood samples were shipped to Sciensano, the national reference center for rabies in Brussels, Belgium. RFFIT was performed within 2 weeks after serum collection. Results were expressed in international units per milliliter (IU/mL), with a cut off value of 0.16 IU/mL.
Statistical Analysis
Titers were transformed with a base-10 log for calculation of GMT and 95% CI. Titers between cut off value and adequate titer were not continuous but ordinal measures, and therefore were excluded from calculation of GMT and 95% CI.
Ethical Considerations
The study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice, and national regulations. The study protocol was approved by the local medical research ethics committee of the Academic Medical Center (Amsterdam, the Netherlands under NL57551.018.16/2016_095) and Dutch Ministry of Defense (The Hague, The Netherlands under DGO-310516004). All participants provided written informed consent before enrolment.
RESULTS
In total, 28 participants were included. We excluded 1 subject (see explanation in Supplementary Material 2). Baseline characteristics are shown in Supplementary Table 1.
Participant Groups
We divided study participants into 3 groups based on their initial PrEP schedule; 9 subjects in the 3-dose intramuscular (3-IM) group; 10 in the 3-dose intradermal (3-ID) group; and 9 in the divergent (DIV) group: these participants had received either a 2-dose PrEP regimen (n = 6) or a 3-dose PrEP schedule with intervals differing from guidelines (n = 3).
Primary End Point
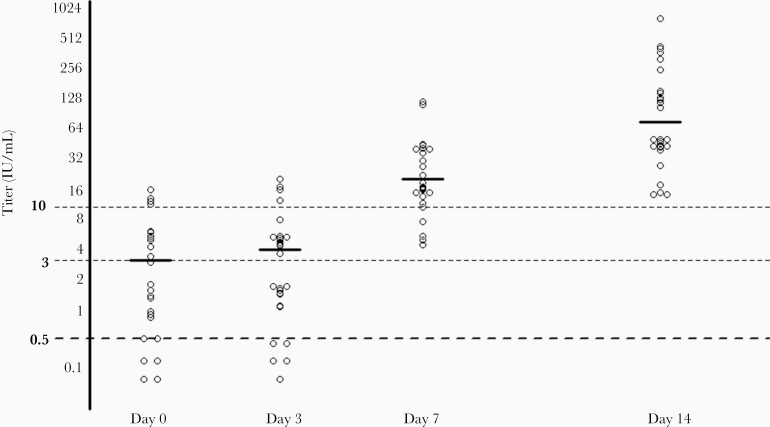
All 28 included participants developed an adequate rabies titer of ≥ 0.5 IU/mL within 1 week after a single IM immunization, regardless of the type of PrEP schedule. Immunogenicity data, including secondary end points, are shown in Table 1 and Figure 1.
Table 1.
Rabies Antibody Response After Boostering a Preexposure Prophylaxis Schedule Administered 10–24 Years Before
| Time Point, d | Immunogenicity per Time Point | |||||
|---|---|---|---|---|---|---|
| Titer ≥ 0.5 IU/mL, % | GMT, IU/mL (95% CI)a | Titer Range, IU/mLa | Titer ≥ 3 IU/mL, % | Titer ≥ 10 IU/mL, % | Fold Increase | |
| Total | ||||||
| 0 | 86 | 3.02 (1.93–4.72) | 0.50–14.92 | 46 | 18 | 1.00 |
| 3 | 82 | 3.79 (2.59–5.55) | 1.04–19.02 | 54 | 14 | 1.25 |
| 7 | 100 | 18.94 (13.68–26.22) | 4.26–110.87 | 100 | 82 | 6.27 |
| 14 | 100 | 70.81 (46.10–108.74) | 13.50–741.03 | 100 | 100 | 23.45 |
| 3-Dose intramuscular (3-IM) group | ||||||
| 0 | 100 | 3.86 (2.03–7.34) | 1.50–14.91 | 55 | 22 | 1.00 |
| 3 | 100 | 3.88 (2.06–7.29) | 1.57–19.02 | 67 | 11 | 1.01 |
| 7 | 100 | 9.50 (5.87–15.37) | 4.26–25.29 | 100 | 56 | 2.46 |
| 14 | 100 | 70.11 (25.04–196.29) | 13.50–741.03 | 100 | 100 | 18.16 |
| 3-Dose intradermal (3-ID) group | ||||||
| 0 | 90 | 3.86 (1.67–8.94) | 0.88–14.92 | 60 | 30 | 1.00 |
| 3 | 90 | 5.06 (2.44–10.49) | 1.04–15.92 | 70 | 30 | 1.31 |
| 7 | 100 | 26.14 (14.85–45.99) | 7.21–105.04 | 100 | 90 | 6.77 |
| 14 | 100 | 62.07 (28.81–133.72) | 13.50–339.30 | 100 | 100 | 16.08 |
| Divergent (DIV) group | ||||||
| 0 | 67 | 1.44 (0.44–4.73) | 0.50–5.87 | 22 | 0 | 1.00 |
| 3 | 56 | 2.17 (1.14–5.38) | 1.07–5.08 | 22 | 0 | 1.51 |
| 7 | 100 | 26.42 (15.43–45.25) | 12.89–110.87 | 100 | 100 | 18.35 |
| 14 | 100 | 82.78 (38.44–178.23) | 26.00–391.57 | 100 | 100 | 57.49 |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; PrEP, pre-exposure prophylaxis.
aIn subjects with adequate titers (≥0.5 IU/mL).
Figure 1.
Individual titers of all participants and geometric mean titers in subjects with adequate titer per time point. Geometric mean titers are indicated by solid lines.
Subanalyses With 2-Dose Immunogenicity Data of the DIV Group
In April 2018, the WHO endorsed a 2-dose PrEP schedule [1, 2]. Long-term boostability of this 2-dose schedule thus became of increasing interest. Therefore, we performed subanalyses of long-term immunogenicity of 2-dose PrEP (6 participants from the DIV group; Supplementary Table 2). At baseline, 5/6 (83%) subjects had adequate titers with an interval of 10–11 years between 2-dose PrEP and a single IM booster immunization. GMT on day 0 was 2.33 IU/mL and increased to 30.68 IU/mL on day 7, resulting in a 13.2-fold increase (Supplementary Table 3). All participants had adequate titers on days 7 and 14.
DISCUSSION
We show that rabies booster immunization, administered more than 10 years after primary immunization, leads to accelerated anamnestic rabies antibody responses (boostability) within 7 days after a single intramuscular booster immunization, regardless of the type of PrEP schedule that had been previously administered. This indicates that rabies immunological memory can be reactivated after more than 10 years (and even after 24 years), independent of the route of PrEP administration. Moreover, different schedules, including 2-dose PrEP, yielded similar results. To the best of our knowledge, our findings represent the first 2-dose boostability data beyond 10 years with still-licensed rabies vaccines.
Our results confirm the earlier findings in a slightly larger cohort reported by Suwansrinon et al in 2006 [8]. This study primarily focused on the immune response after PEP (80/118 participants), whereas the immune response after PrEP was measured in 38 participants, of whom the majority (23 participants) had received PrEP 5–10 years previously. Only 15 participants had received PrEP 10–20 years previously and are therefore comparable with the participants in our study. Suwansrinon et al did not report the administration route of the PrEP schedule and they administered ID booster immunizations at days 0 and 3 [8].
Our observation that not all participants (86%, 24/28) had an adequate titer on day 0 is in line with findings of the systematic review and meta-analysis in 2018 by Langedijk et al [6]. The authors described that a rabies antibody titer can drop below adequate levels of 0.5 IU/mL after 1 year, emphasizing the need for PEP after potential exposure to rabies.
From interpretation of these data, first, all investigated schedules yielded adequate titers in all subjects postboosting. Moreover, the 3-dose ID and 2-dose IM or ID PrEP schedule showed similar boostability to the classic 3-dose IM PrEP schedule. Second, we expected to observe an initial rise in antibody response on day 3. Indeed, we observed slight 1.25-fold increase on that day. A significant 5-fold rise in titers was only seen after 1 week. Therefore, we conclude that the peak of the secondary immune response occurs between days 3 and 7 postboosting.
We consider the long interval between rabies PrEP and boosting as the most important strength of this study, with different schedules and routes of administration analyzed separately. We included 6 participants with a 2-dose PrEP schedule, as currently recommended by WHO and the standard in many countries since April 2018. Although the number of these participants was low, the positive outcome suggest a reliable long term memory response to this schedule, which is in line with WHO guidelines [1, 2]. Finally, all participants completed the study without missing any visits.
However, we report several limitations. Overall, there are no specific end points for rabies immunogenicity after booster immunization. This stands in contrast to primary immunization for rabies, which is well documented in a technical guideline [12]. In the available rabies literature, many different end points are reported after booster immunization [5, 6, 13]. Sometimes a relative antibody titer increase is recommended, for example, the 4-fold increase commonly used in meningococcal polysaccharide vaccine research [11]. Due to high titers preboosting, our 3-IM group yielded only a 2.5-fold increase, while all GMTs were close to or above 10 IU/ML, suggesting excellent immunogenicity of the booster vaccination.
Importantly, it was difficult to find participants who met the inclusion criterion of an interval beyond 10 years without any rabies booster immunization. For that reason, it took 3 years to finish this study, falling just 2 enrolments short of target completion.
Lastly, because of the 3-year enrolment period and because all RFFITs were performed within 2 weeks after serum collection, not all RFFITs were from the same batch, which could have induced intertest variability.
In clinical practice, when a patient has encountered a potential rabies exposure, it is important to verify a history of pre-exposure rabies immunization. When this is confirmed, one of the recommended WHO PEP schedules can be administered without RIG, provided the patient is not immune compromised [2]. Our study suggests an adequate booster response to PEP, regardless of the interval between PrEP and PEP, route of administration (IM or ID), and schedule (2 or 3 dose) of the initial PrEP.
The first studies have been published with boostability data up to 28 months after a single-visit PrEP [14, 15]. In this light, a large ongoing multicenter trial in the Netherlands with a single-visit PrEP has almost been completed. Thus, there will soon be more data available on short-term boostability after a single visit.
Future research should test the hypothesis that even after 1 rabies immunization the immunological memory is boostable, with long-term benefits. This strategy may specifically target populations in endemic areas or travelers departing within 1 week.
CONCLUSION
Rabies immunological memory is reactivated within 7 days after booster immunization, even when the first rabies immunization was administered more than 10 years, to even 24 years, earlier. This long-term boostability is important for clinical practice: in a previously immunized, immunocompetent patient who requires PEP treatment, there is no indication for RIG, supporting daily practice in rabies post-exposure treatment. This small cohort study provides long-term data on the accelerated anamnestic rabies antibody response after a 2-dose PrEP schedule, which is the PrEP schedule recommended by WHO since April 2018. Future research results on long-term (more than 10 years) boostability studies, including a large number of participants that were administered a 2-dose PrEP, will logically not be available before 2028.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: 15th Conference of the International Society of Travel Medicine, 14–18 May 2017, Barcelona, Spain; 10th European Congress on Tropical Medicine and International Health, 16–20 October 2017, Antwerp, Belgium; and 16th Conference of the International Society of Travel Medicine, 5–9 June 2019, Washington DC, USA.
Contributor Information
Cornelis A De Pijper, Amsterdam University Medical Centers, University of Amsterdam, Division of Internal Medicine, Department of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Amsterdam Infection and Immunity, Amsterdam Public Health, Amsterdam, Netherlands.
Annefleur C Langedijk, Amsterdam University Medical Centers, University of Amsterdam, Division of Internal Medicine, Department of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Amsterdam Infection and Immunity, Amsterdam Public Health, Amsterdam, Netherlands.
Sanne Terryn, National Reference Center of Rabies, Viral Diseases, Infectious Diseases in Humans, Sciensano, Brussels, Belgium.
Steven Van Gucht, National Reference Center of Rabies, Viral Diseases, Infectious Diseases in Humans, Sciensano, Brussels, Belgium.
Martin P Grobusch, Amsterdam University Medical Centers, University of Amsterdam, Division of Internal Medicine, Department of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Amsterdam Infection and Immunity, Amsterdam Public Health, Amsterdam, Netherlands.
Abraham Goorhuis, Amsterdam University Medical Centers, University of Amsterdam, Division of Internal Medicine, Department of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Amsterdam Infection and Immunity, Amsterdam Public Health, Amsterdam, Netherlands.
Cornelis Stijnis, Amsterdam University Medical Centers, University of Amsterdam, Division of Internal Medicine, Department of Infectious Diseases, Center for Tropical Medicine and Travel Medicine, Amsterdam Infection and Immunity, Amsterdam Public Health, Amsterdam, Netherlands.
References
- 1. World Health Organization . Rabies vaccines: WHO position paper, April 2018—recommendations. Vaccine 2018; 36:5500–3. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . WHO expert consultation on rabies third report. Geneva, Switzerland: WHO, 2018. [Google Scholar]
- 3. Fooks AR, Cliquet F, Finke S, et al. Rabies. Nat Rev Dis Primers 2017; 3:17091. [DOI] [PubMed] [Google Scholar]
- 4. Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 2015; 15:149–59. [DOI] [PubMed] [Google Scholar]
- 5. Overduin LA, van Dongen JJM, Visser LG. The cellular immune response to rabies vaccination: a systematic review. Vaccines (Basel) 2019; 7:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langedijk AC, De Pijper CA, Spijker R, Holman R, Grobusch MP, Stijnis C. Rabies antibody response after booster immunization: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:1932–47. [DOI] [PubMed] [Google Scholar]
- 7. Fayaz A, Simani S, Janani A, et al. Antibody persistence, 32 years after post-exposure prophylaxis with human diploid cell rabies vaccine (HDCV). Vaccine 2011; 29:3742–5. [DOI] [PubMed] [Google Scholar]
- 8. Suwansrinon K, Wilde H, Benjavongkulchai M, et al. Survival of neutralizing antibody in previously rabies vaccinated subjects: a prospective study showing long lasting immunity. Vaccine 2006; 24:3878–80. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO) . Human and dog rabies prevention and control: Report of the WHO/Bill and Melinda Gates Foundation Consultation. Annecy France, 7–9 October 2009. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 10. World Health Organization . Rabies vaccines: WHO position paper--recommendations. Vaccine 2010; 28:7140–2. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO) . The immunological basis for immunization series: module 15: meningococcal disease. Geneva, Switzerland: WHO, 2010. [Google Scholar]
- 12. World Health Organization . WHO Expert Committee on biological standardization. World Health Organ Tech Rep Ser 2007; 941:1–340, back cover. [PubMed] [Google Scholar]
- 13. Kessels JA, Recuenco S, Navarro-Vela AM, et al. Pre-exposure rabies prophylaxis: a systematic review. Bull World Health Organ 2017; 95:210–9C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonker EFF, Visser LG. Single visit rabies pre-exposure priming induces a robust anamnestic antibody response after simulated post-exposure vaccination: result of a dose-finding study. J Travel Med 2017; 24:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Soentjens P, De Koninck K, Tsoumanis A, et al. Comparative immunogenicity and safety trial of 2 different schedules of single-visit intradermal rabies postexposure vaccination. Clin Infect Dis 2019; 69:797–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.