Abstract
Background
Although cervicovaginal microbiome has been associated with cervical human papillomavirus (HPV) infection, little is known regarding the association of oral microbiome with oral HPV, a cause of oropharyngeal cancer.
Methods
A cross-sectional analysis of 495 participants from the Men and Women Offering Understanding of Throat HPV study was conducted. 16S rRNA gene amplicon sequencing was performed on saliva samples. HPV DNA in oral rinse samples was tested. Associations of oral microbiome diversity, taxon abundance, and predicted functional pathways with oral HPV were assessed, adjusting for age, race/ethnicity, education, human immunodeficiency virus, current smoking, and sequencing batch.
Results
Participants with oral HPV (n = 68) compared with those without HPV had similar oral microbiome alpha-diversity yet different beta-diversity (Bray-Curtis distance for bacterial taxa, P = .009; functional pathways, P = .02). Participants with oral HPV had higher abundance of Actinomycetaceae, Prevotellaceae, Veillonellaceae, Campylobacteraceae, Bacteroidetes, and lower abundance of Gemellaceae (false discovery rate <0.10). We also found differential functional potential of oral microbiome by oral HPV status: xenobiotic biodegradation-related pathways were less abundant among participants with oral HPV, suggesting potential xenobiotic-induced toxic effects with implications for HPV susceptibility.
Conclusions
Our findings suggest a shift in oral microbiome community structure, composition, and functional potential between individuals with and without oral HPV.
Keywords: functional pathways, oral HPV, oral microbiome
Oral HPV was associated with differences in beta diversity of oral microbiome and higher abundance of Actinomycetaceae, Prevotellaceae, Veillonellaceae, Campylobacteraceae, Bacteroidetes, and lower abundance of Gemellacea.Predicted functional potential of oral microbiota was shifted among individuals with oral HPV.
Human papillomavirus (HPV) is a common sexually transmitted infection in the United States (US) [1]. Among US adults, approximately 23% have genital high-risk HPV deoxyribonucleic acid (DNA) (ages 18–59 years) and 4% have oral high-risk HPV DNA detectable (ages 18–69 years) [1]. In the past 2 decades, oral HPV has led to a continual increase of oropharyngeal cancer incidence in the United States [2]. Many factors are associated with increased odds of oral HPV, including male sex, higher number of oral sexual partners, current smoking, and immunosuppression [3].
The human oral cavity harbors the second most diverse microbial communities next to the gut [4, 5]. Oral microbiome plays a key role in oral and general health, which contributes to immunological, metabolic, and physiological functions of hosts [5]. However, evidence that links oral microbiome to oral HPV remains inconclusive [6, 7]. A study of 39 pregnant women reported increased oral microbiome richness among those with oral HPV [6]; however, this analysis was limited by the small sample size and a specific study population, and results may not apply to the general population. In comparison, ample evidence shows that cervicovaginal microbiome is associated with cervical HPV infection and disease progression [8–11]. A better understanding of the association of oral microbiome with oral HPV may shed light on the mucosal susceptibility to HPV and the interplay of these factors.
In this study, we leveraged cross-sectional data from a large oral HPV screening study to investigate the association of oral microbiome with prevalent oral HPV. We compared the diversity of oral microbiome by oral HPV status and identified bacterial taxa and functional pathways that were differentially abundant by oral HPV.
METHODS
Study Population
The Men and Women Offering Understanding of Throat HPV (MOUTH) study is a prospective cohort study that screens individuals at risk for oral HPV infection and follows those with oral HPV DNA detected. Participants are enrolled primarily from sites in the Johns Hopkins Medical System (Maryland) and Mount Sinai Medical System (New York). Participant eligibility criteria for the MOUTH study included the following: men with ≥2 lifetime oral sex partners; men and women with a history of anal or genital dysplasia or related cancer; and partners of someone with an HPV-related cancer. Demographics and risk behavior data were collected using computer-assisted self-interview. Saliva samples from the first 497 participants enrolled in the MOUTH study (baseline visit) were sent for 16S V4 rRNA amplicon sequencing and were included in this analysis. The MOUTH protocol was approved by the institutional review boards of all study sites. All study participants provided written informed consent.
Oral Rinse Collection, Processing, and Oral Human Papillomavirus Detection
Oral rinse and gargle samples were collected at baseline visit using 10-mL saline and stored at 4°C until processed [12]. HPV DNA testing was performed on oral rinse samples by DDL Diagnostic Laboratory (https://www.ddl.nl) as previously described [12]. In brief, presence of HPV DNA was tested using the SPF10 primer system and the DNA Enzyme ImmunoAssay (DEIA) detection system. Human papillomavirus type was specified using the SPF10 DEIA/LIPA system. Oncogenic oral HPV types (ie, high-risk oral HPV) include HPV 16/18/31/33/35/39/45/51/52/56/58/59/66, and nononcogenic HPV types (ie, low-risk oral HPV) include HPV 6/11/34/40/42/43/44/53/54/68/73/70/74 [12].
Saliva Sample Collection, Processing, and Oral Microbiome Assay
Saliva samples were collected at baseline visit using Oragene RNA kit (DNA Genotek) and stored at −80°C until processed. Microbiome assay using saliva samples was performed at the Albert Einstein College of Medicine. DNA extraction was conducted using QIAamp DNA Mini Kit (QIAGEN). The V4 region of 16S rRNA gene was amplified using primers 515F (GTGYCAGCMGCCGCGGTA) and 806R (GGACTACHVGGGTWTCTAAT) [13]. High-throughput amplicon sequencing was performed on MiSeq 2 × 300 (Illumina, San Diego, CA) with the KAPA LTP library kit (Kapa Biosystems, Wilmington, MA). Positive community standards of known microbial composition and negative controls were used in each sequencing batch.
Bioinformatics (Microbiome Sequencing Data)
Raw 16S rRNA sequence reads were demultiplexed using sample-specific Golay barcodes [14] with NovoBarcode [15]. Poor-quality bases with Phred score ≤25 were removed using PRINSEQ-lite [16]. Reads that were <50 base pairs after quality trimming were removed. Reads were clustered into operational taxonomic unit (OTU) at 97% similarity (open-reference OTU picking) using VSEARCH 2.8.1 [17]. Taxonomic classification was assigned using Greengenes V12.10 and Human Oral Microbiome Database V15.1 reference sequences. A phylogenetic tree was constructed from aligned reads using FastTree 2.1. Reads that formed singleton OTUs and reads from OTUs that failed to align during the FastTree were filtered out. Metagenomic prediction of Kyoto Encyclopedia of Genes and Genomes (KEGG) orthologous groups (KOs) was performed using PICRUSt 2.1.1-b [18]; KOs were collapsed into KEGG functional pathways. Poorly characterized pathways and pathways within the “human diseases” and “organismal systems” categories were removed.
As part of our quality control process, positive and negative controls were included in the experiments, and results showed high quality of the microbiome sequencing (data not shown). Two samples had sequencing depth <5300 and were removed from downstream analyses. The cutoff was selected by investigating the sequencing depths of all samples and identifying outlier samples whose sequencing depths were much lower than the rest. Microbiome alpha (within-sample) and beta (between-sample) diversities were calculated using rarefied data for bacterial taxa (OTU level) and predicted functional pathways. All samples were rarefied to the lowest sequencing depth for a fair comparison (bacterial sequences, 5300 reads; predicted sequences for KEGG pathways, 1 187 460 reads). Three alpha diversities were calculated, including observed features, inverse Simpson index, and Shannon index (R package microbiome, function alpha). Using bacterial taxa data, 3 beta diversities were calculated including unweighted and weighted UniFrac [19] (R package rbiom, function unifrac) and Bray-Curtis distance (R package ecodist, function bcdist). For functional pathways, only Bray-Curtis distance was calculated for beta diversity.
Statistical Analysis
The primary outcome was prevalent oral HPV DNA of any type. Primary measures of microbiome included alpha and beta diversity of (1a) bacterial taxa and (1b) KEGG pathways and relative abundance of (2a) individual bacterial taxon and (2b) KEGG pathway. Associations of oral microbiome with prevalent oral HPV were assessed.
Baseline characteristics were summarized and compared between participants with and without oral HPV using χ2 tests for categorical variables and analysis of variance (ANOVA) for continuous variables. In the diversity analysis, associations of each alpha diversity with oral HPV were examined using logistic regression. Principal coordinate analysis (PCoA) and permutational multivariate ANOVA [20] ([PERMANOVA] R package vegan, function adonis2, 5000 permutations) were used to visualize and statistically assess the differences in beta diversity between groups. Permutational analysis of multivariate homogeneity of group dispersions was then used to test for differences in dispersion between groups (R package vegan, function permutest.betadisper, 5000 permutations) [21]. Community-level shifts were defined as changes in alpha or beta diversity of oral microbiome. Statistical significance was determined by a 2-sided P < .05 for diversity analysis.
Differential abundance analysis of bacterial taxa and functional pathways was performed using a newly developed beta-binomial model through parametric bootstrapping [22] (R package corncob, function differentialtest, 5000 bootstraps). Difference in log odds of relative abundance of each bacterial taxon/functional pathway (ie, log ) comparing participants with and without oral HPV was calculated. To reduce the number of multiple comparisons, we removed rare taxa/functional pathways that were present in <10% of samples. Differential abundance was considered significant at false discovery rate (FDR) of 0.10 [23].
To control for potential confounders, all models were adjusted for DNA isolation batch and significant predictors for oral HPV DNA, including age, race/ethnicity (non-Hispanic white, non-Hispanic black, and other race/ethnicity), education (high school diploma or less, and some college or more), human immunodeficiency virus (HIV) infection, and current smoking. We conducted several sensitivity analyses for the diversity analysis: (1) additionally adjusting for sex and number of oral sex partners in the last year (0–1, ≥2; called recent oral sex partners hereafter), yielding similar results (data not shown); (2) additionally adjusting for study sites, yielding similar results (data not shown); (3) stratified analyses by HIV status, recent oral sex partners, current smoking, or current alcohol use; and (4) examining microbiome diversities by oral HPV types (no oral HPV, only low-risk, and any high-risk oral HPV). All analyses were conducted using R 4.0.2. OTU table and predictions of KEGG pathways generated from 16s rRNA sequences, and metadata can be accessed at Synapse (syn26529406).
RESULTS
Of the 495 participants in the analysis, 427 (86.3%) had no HPV detected in the oral rinse samples and 68 (13.7%) were HPV DNA positive, including 42 with only low-risk and 26 with 1 or more high-risk HPV types. Participants with oral HPV tended to be older and were more likely to be current smokers, non-Hispanic black, living with HIV, and to have less than high school education (Table 1). There were no significant differences in alcohol use, oral sex behaviors, or oral hygiene by oral HPV status.
Table 1.
Characteristics of 495 Study Participants by Prevalent Oral HPV Status
| N (%) | ||||
|---|---|---|---|---|
| Characteristics | No Oral HPV (N = 427) | Any Oral HPV (N = 68) | Total (N = 495) | P Valuea |
| Sex | .12 | |||
| Male | 374 (87.6%) | 64 (94.1%) | 438 (88.5%) | |
| Female | 53 (12.4%) | 4 (5.9%) | 57 (11.5%) | |
| Age: mean (standard deviation) | 54.0 (10.3) | 57.3 (9.5) | 54.4 (10.2) | .02 |
| Race/ethnicity | <.001 | |||
| Non-Hispanic white | 313 (73.8%) | 36 (54.5%) | 349 (71.2%) | |
| Non-Hispanic black | 61 (14.4%) | 23 (34.8%) | 84 (17.1%) | |
| Other race/ethnicity | 50 (11.8%) | 7 (10.6%) | 57 (11.6%) | |
| Education | .005 | |||
| High school diploma or less | 68 (16.1%) | 20 (30.3%) | 88 (18.0%) | |
| Some college or more | 354 (83.9%) | 46 (69.7%) | 400 (82.0%) | |
| Marital Status | .16 | |||
| Married/living with a partner | 299 (70.5%) | 42 (63.6%) | 341 (69.6%) | |
| Widowed/divorced/separated | 67 (15.8%) | 9 (13.6%) | 76 (15.5%) | |
| Never married | 58 (13.7%) | 15 (22.7%) | 73 (14.9%) | |
| Living with HIV | 35 (8.2%) | 15 (22.7%) | 50 (10.2%) | <.001 |
| Smoking status | .005 | |||
| Never smokers | 250 (59.1%) | 30 (45.5%) | 280 (57.3%) | |
| Former smokers | 129 (30.5%) | 20 (30.3%) | 149 (30.5%) | |
| Current smokers | 44 (10.4%) | 16 (24.2%) | 60 (12.3%) | |
| Pack years, mean (standard deviation) | 7.1 (14.9) | 9.4 (14.4) | 7.4 (14.9) | .24 |
| Current alcohol use | 277 (65.5%) | 39 (59.1%) | 316 (64.6%) | .31 |
| Lifetime number of oral sex partners | .46 | |||
| 0–4b | 142 (33.6%) | 18 (27.3%) | 160 (32.7%) | |
| 5–9 | 106 (25.1%) | 17 (25.8%) | 123 (25.2%) | |
| 10–24 | 104 (24.6%) | 15 (22.7%) | 119 (24.3%) | |
| ≥25 | 71 (16.8%) | 16 (24.2%) | 87 (17.8%) | |
| Number of oral sex partners in past year | .38 | |||
| 0 | 132 (31.2%) | 18 (27.3%) | 150 (30.7%) | |
| 1 | 218 (51.5%) | 32 (48.5%) | 250 (51.1%) | |
| ≥2 | 73 (17.3%) | 16 (24.2%) | 89 (18.2%) | |
| Sexual orientation | .55 | |||
| Heterosexual | 368 (87.8%) | 53 (81.5%) | 421 (87.0%) | |
| Homosexual | 24 (5.7%) | 5 (7.7%) | 29 (6.0%) | |
| Bisexual | 16 (3.8%) | 4 (6.2%) | 20 (4.1%) | |
| Something else/not sure | 11 (2.6%) | 3 (4.6%) | 14 (2.9%) | |
| Have ever had any teeth become loose | 54 (12.8%) | 8 (12.3%) | 62 (12.7%) | .92 |
| Floss teeth usually | 162 (38.2%) | 30 (46.2%) | 192 (39.3%) | .22 |
| Brush teeth ≥1 per day | 399 (94.1%) | 61 (92.4%) | 460 (93.9%) | .60 |
| Study Site | .25 | |||
| Johns Hopkins Medical System (MD) | 391 (91.6%) | 65 (95.6%) | 456 (92.1%) | |
| Mount Sinai Medical System (NY) | 36 (8.4%) | 3 (4.4%) | 39 (7.9%) | |
Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; MD, Maryland; NY, New York.
P value was based on analysis of variance for continuous variables and χ2 test for categorical variables.
Eleven (2.2%) participants reported <2 lifetime oral sex partners, all of whom met eligibility criteria by being a partner of someone with an HPV-related cancer (n = 6) or themselves having a history of anal or genital dysplasia (n = 3) or meeting both criteria (n = 2).
Overall Oral Microbiome Composition
Figure 1 shows the overall oral microbiome composition according to bacterial taxa and KEGG pathways by oral HPV status. In the overall cohort, although oral microbiota was dominated by Firmicutes (35.1%), Bacteroidetes (31.9%), Proteobacteria (18.4%), Actinobacteria (8.2%), and Fusobacteria (5.9%) at the phylum level, there were substantial interindividual variations in the taxonomic composition. In contrast, at the functional level, most KEGG pathways had comparable levels across individuals, consistent with a prior report from the Human Microbiome Project Consortium [24]. This suggests functional pathways are shared across distinct bacterial communities. Patterns of microbial community composition appeared visually similar between participants with and without oral HPV; statistical differences in microbiome diversity and composition by oral HPV status were explored below.
Figure 1.
Taxonomic and functional composition of oral microbiome of 495 Men and Women Offering Understanding of Throat HPV (MOUTH) participants by prevalent oral human papillomavirus (HPV) status. Taxonomic composition of oral microbiome shows relative abundance of the 8 most abundant taxa at the phylum level (A) and the genus level (B), stratified by oral HPV status. Functional composition of oral microbiome shows relative abundance of the 8 most abundant Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (C), stratified by oral HPV status. Sample order is determined by the relative abundance of the most abundant taxon/pathway at each level.
Oral Microbiome Alpha and Beta Diversity
There were no differences in alpha diversity of bacterial taxa by oral HPV (Figure 2A). However, for beta diversity, we observed a shift in microbial community (ie, shifted centroids) between participants with and without oral HPV using Bray-Curtis (Figure 2B) and weighted UniFrac distances (see Supplementary Figure 1B). After adjusting for age, race/ethnicity, education, HIV status, current smoking, and DNA isolation batch, PERMANOVA analysis showed significant differences in beta diversity between participants with and without oral HPV, albeit the magnitude of difference was small (Bray-Curtis P = .009, R2 = 0.5%; weighted UniFrac P = .05, R2 = 0.5%). In contrast, using unweighted UniFrac, the difference in beta diversity between the 2 groups was not significant (P = .25, R2 = 0.2%) (see Supplementary Figure 1A). This suggests that differences in microbial community by oral HPV were mainly explained by different abundance of common taxa. There was no significant difference in group dispersion by oral HPV status (P > .05). Similar microbiome diversity results were observed at the functional level. Although participants with and without oral HPV had similar alpha diversity of KEGG pathways (Figure 3A), they differed significantly in beta diversity (Bray-Curtis P = .02, R2 = 0.7%) (Figure 3B).
Figure 2.
Alpha diversity (A) and beta diversity (B) of oral bacterial taxa by prevalent oral human papillomavirus (HPV) status. Operational taxonomic unit-level bacterial taxa was analyzed. In (A), P value of each alpha diversity index from multivariable logistic regression models for the outcome of having any prevalent oral HPV is shown. In (B), principal coordinate analysis (PCoA) shows beta diversity of bacterial taxa between samples based on Bray-Curtis distance by oral HPV. Each circle represents a participant. Filled triangle indicates the centroid (ie, mean) of participants for each group. The ellipse represents a 95% confidence ellipse for each group. P value and R2 of having any prevalent oral HPV from permutational analysis of variance (PERMANOVA) models are shown. In both analyses, associations were adjusted for age (every 10 years), race/ethnicity (non-Hispanic white, non-Hispanic black, other), education (high school diploma or less, some college or more), human immunodeficiency virus status, current smoking, and DNA isolation batch.
Figure 3.
Alpha diversity (A) and beta diversity (B) of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways by prevalent oral human papillomavirus (HPV) status. In (A), P value of each alpha diversity index from multivariable logistic regression models for the outcome of having any prevalent oral HPV is shown. In (B), principal coordinate analysis (PCoA) shows beta diversity of KEGG pathways between samples based on Bray-Curtis distance by oral HPV. Each circle represents a participant. Filled triangle indicates the centroid (ie, mean) of participants for each group. The ellipse represents a 95% confidence ellipse for each group. P value and R2 of having any prevalent oral HPV from permutational analysis of variance (PERMANOVA) models are shown. In both analyses, associations were adjusted for age (every 10 years), race/ethnicity (non-Hispanic white, non-Hispanic black, other), education (high school diploma or less, some college or more), human immunodeficiency virus status, current smoking, and DNA isolation batch.
Several sensitivity analyses were performed, and results were consistent. When stratified by HIV status, recent oral sex partners, current smoking, or current alcohol use, alpha diversity of bacterial taxa and KEGG pathways remained similar by oral HPV (data not shown). Meanwhile, significant beta diversity differences were observed among most subgroups, including HIV-negative individuals, those with 0–1 recent oral sex partners, current nonsmokers, and current drinkers (see Supplementary Figures 2 and 4). In the remaining subgroups that did not show a significant difference in PERMANOVA analysis, PCoA plot revealed similar shifts in cluster centroids between participants with and without oral HPV, suggesting that lack of statistical significance may be because of the smaller sample size. The only exception was the analysis of beta diversity of KEGG pathways among current smokers where participants with and without oral HPV clustered together on PCoA plot, indicating similar functional community structures (see Supplementary Figure 4C). Finally, we found no difference in alpha and beta diversity between participants with only low-risk versus any high-risk oral HPV, possibly due to the smaller sample size (42 versus 26 individuals) (see Supplementary Figures 3 and 5).
Differential Abundance of Oral Microbiome
We next identified specific oral microbiome features that were differentially abundant by oral HPV (FDR < 0.10). This analysis included 11 phyla, 20 classes, 29 orders, 50 families, 76 genera, and 207 functional pathways. Figure 4 shows 5 taxa with modest but significant increases in abundance levels among participants with oral HPV in the adjusted model, including families Actinomycetaceae, Prevotellaceae, Veillonellaceae, Campylobacteraceae, and phylum Bacteroidetes. Meanwhile, family Gemellaceae was significantly less abundant among participants with oral HPV.
Figure 4.
Differential abundance of oral bacterial taxa by prevalent oral human papillomavirus (HPV) status for taxa at phylum, class, order, family, and genus level. Analyses were restricted to taxa present in ≥10% of samples, which were then collapsed at phylum, class, order, family, and genus level. The horizontal gray dotted line indicates the false discovery rate (FDR) of 0.10. Abundance represents the mean relative abundance of a given taxa within each taxonomic level. Taxa differentially abundant by prevalent oral HPV with FDR <0.10 are depicted by colored circles, and their names at the lowest classified rank are present. Associations were adjusted for age (every 10 years), race/ethnicity (non-Hispanic white, non-Hispanic black, other), education (high school diploma or less, some college or more), human immunodeficiency virus status, current smoking, and DNA isolation batch in Beta-binomial regression models using the Corncob R package. f, family; OTU, operational taxonomic unit; p, phylum.
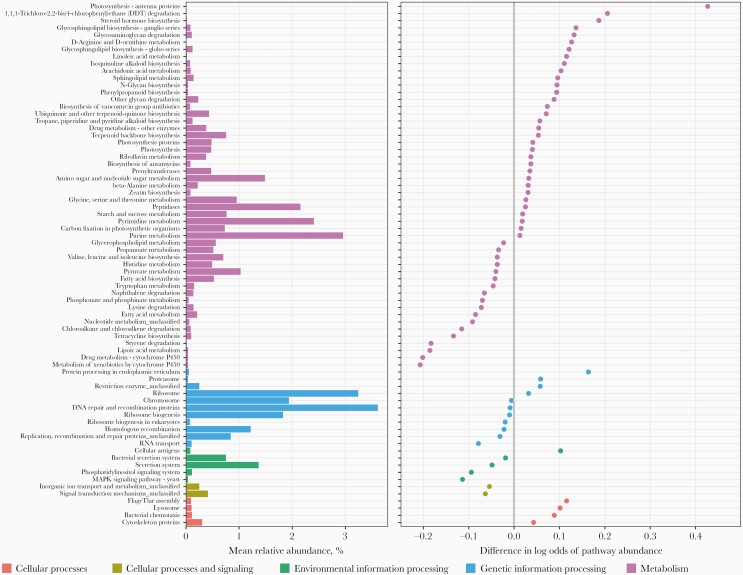
At the functional level, there were 73 KEGG pathways observed to be differentially abundant, including 42 pathways enriched and 31 pathways depleted among those with oral HPV (Figure 5). Most of these pathways were within the overall category of “metabolism”, involved in amino acid metabolism, glycan biosynthesis and metabolism, lipid metabolism, and xenobiotics biodegradation and metabolism. Of note, abundance of naphthalene degradation, chloroalkane and chloroalkene degradation, styrene degradation, and cytochrome P450 metabolism pathways (within xenobiotics biodegradation and metabolism) were significantly lower among those with oral HPV, suggesting decreased microbial potential in degrading these foreign compounds (ie, xenobiotics).
Figure 5.
Differential abundance of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways by prevalent oral human papillomavirus (HPV) status. Analyses were restricted to 207 KEGG pathways within the overall categories of cellular processes, cellular processes and signaling, environmental information processing, genetic information processing, and metabolism that present in ≥10% of samples. Pathways differentially abundant by prevalent oral HPV with false discovery rate <0.10 are depicted (n = 73). Associations were adjusted for age (every 10 years), race/ethnicity (non-Hispanic white, non-Hispanic black, other), education (high school diploma or less, some college or more), human immunodeficiency virus status, current smoking, and DNA isolation batch in Beta-binomial regression models using the Corncob R package.
DISCUSSION
In this study, oral HPV DNA was associated with differences in oral microbiome beta diversity, which represents an overall community-level shift. We also identified differentially abundant bacterial taxa and oral microbiome functional pathways by oral HPV status. To our knowledge, this is the largest study to assess the association between oral microbiome and oral HPV DNA. This study builds on prior research of the effect of microbiome on cervical HPV, suggesting that oral microbiome may also influence or be influenced by oral HPV.
Although the association of oral HPV and HPV-related oropharyngeal cancer is well established [2], it is less clear why some individuals are at higher risk of oral HPV infection and consequent disease progression. Understanding the possible effect of oral microbiota on oral HPV may inform our understanding of HPV-related carcinogenesis. To date, only 2 studies have examined the association of oral microbiome with oral HPV and the results were conflicting. Significant associations between microbiome alpha diversity (higher richness) in mucosal scrapings and oral HPV infection were identified among 39 pregnant women in one study [6], but not in the other study among 35 infants [7]. This differs from the finding in our larger study showing similar microbiome alpha diversity and functional potential between participants with and without oral HPV. Differences in study findings may be due to inclusion of a broader study population in our study in terms of gender, race, health status, and age and methodological differences in microbiome sampling (mucosal scrapings vs saliva samples).
Studies of cervicovaginal microbiome have consistently identified increased alpha diversity associated with prevalent cervical HPV [8, 9]. In particular, bacterial vaginosis, a condition characterized by a shift from Lactobacillus-dominant community to a more diverse community with anaerobic bacteria, has been implicated in cervical HPV natural history [10, 11]. It should be noted that composition of cervicovaginal microbiome is different from oral microbiome [4]; therefore, comparisons of community-level microbiome associations across body sites are difficult.
The current study identified a few bacterial families with higher abundance among participants with oral HPV, including Actinomycetaceae, Prevotellaceae, Veillonellaceae, and Campylobacteraceae (Figure 4). These findings are mostly consistent with previous studies of head and neck squamous cell carcinomas (HNSCC) that showed higher abundance of members of genus Actinomyces (within Actinomycetaceae), Veillonella and Veillonella dispar (within Veillonellaceae), and Campylobacter (within Campylobacteraceae) in oral rinse/saliva samples among HPV-positive HNSCC patients [25–27]. Meanwhile, we observed reduced abundance of Gemellaceae associated with oral HPV, which was in the opposite direction as reported in a study of 42 HNSCC [25]. We did not observe differential abundance for members of genus Fusobacterium, which were found to be enriched in some HNSCC tissues according to a systematic review [28], although their relationship with HPV-related HNSCC remains unclear. The data, taken together, suggest that these microbes may have been involved in different stages of, or potentially throughout the natural history of, HPV-related HNSCC from initial HPV infections to the development of carcinomas.
We also found a positive association of family Prevotellaceae and its parent phylum Bacteroidetes with oral HPV (Figure 4). This is consistent with a prior case-control study that detected a higher count of Prevotella melaninogenica (within Prevotellaceae) in saliva among patients with oral squamous cell carcinoma compared with cancer-free controls [29]; however, the study did not assess the tumor HPV status. Nevertheless, in support of our findings, emerging evidence indicates an association between Prevotella and genital HPV infection among both men and women [8, 30].
Several biological mechanisms for the observed association of oral microbiota with oral HPV are plausible. First, poor oral health, specifically periodontal disease, has been associated with oral HPV infection [31] and HPV-positive HNSCC in some studies [32], although not in others [33]. Members of Prevotella and Actinomyces species, which we identified as differentially abundant (Figure 4), have been associated with periodontal diseases [34, 35]. These microbes with other pathogenic bacteria can induce chronic inflammation [35, 36], mucosal disruption, and epithelial proliferations, which may facilitate HPV infection and transmission [31, 32]. Second, we found bacterial communities with reduced capabilities of xenobiotic biodegradation were associated with oral HPV (Figure 5). Specifically, decreased degradation of xenobiotics (compounds foreign to a living organism) such as naphthalene, styrene, chloroalkane, and chloroalkene may predispose epithelium cells to these toxic chemicals, which have been associated with increased oxidative stress and DNA and tissue damage [37, 38]. In response to double-stranded DNA breaks, ataxia-telangiectasia mutated (ATM) protein kinase is activated, which was shown to be essential for productive HPV replication [39]. Therefore, DNA damage resulting from these toxins may lead to an environment conducive to viral amplification. Alternatively, some bacteria such as Campylobacter species (within Campylobacteracea) produce bacterial genotoxins that can directly induce DNA damage and genomic instability [40]. Therefore, it is plausible that these differentially abundant microbes and shifted functional potential serve as a cofactor to influence the risk of oral HPV infection.
Our study has several limitations. First, in this cross-sectional analysis, we cannot establish temporality between oral microbiome and oral HPV infection. Although it was generally believed that local microbiota influences HPV acquisition and/or clearance, we cannot exclude the possibility that HPV infection changes the inner environment of oral cavity or throat, leading to shifted microbial community [10]. HPV infection and the microbial community may also exert reciprocal impacts on each other. Second, we assessed the oral microbiome through saliva samples and not the oropharynx, where microbial community may have a more direct interplay with HPV-infected cells that can lead to oropharyngeal cancer. However, previous studies reported that microbiota collected from saliva, tongue, tonsils, and throat share similar compositions and diversity [4]. Third, our functional results should be interpreted with caution, considering some limitations of using PICRUSt to predict functional contents of oral microbiome including its dependence on existing reference genomes and gene annotations, differential prediction accuracy by functional categories, and failure in distinguishing strain-specific functions [18, 41, 42]. Fourth, we had unequal sample sizes of those with and without oral HPV. However, even our smaller group has more than 60 subjects, which satisfies the sample size requirement for all methods that we used. For the beta diversity analysis, we did not observe heterogenous dispersions by oral HPV status; in this situation, PERMANOVA is expected to perform reliably for unbalanced designs according to a simulation study [43]. Last, we did not differentiate incident and persistent oral HPV infections that have different risk factors [44]. However, we used number of recent oral sex partners as proxies for the risk of acquiring incident oral HPV, and results were similar when limiting to those without multiple recent oral sex partners, supporting a potential association of oral microbiome with persistent oral HPV. Nevertheless, future studies with longitudinal measurement will expand this work by assessing oral microbiome and viral persistence/clearance among those with prevalent infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr. Cynthia L. Sears (Johns Hopkins University School of Medicine) for providing expertise and feedback during the development of this manuscript.
Financial support. This work was funded by the National Institute of Dental and Craniofacial Research (R35DE026631; to G. D.).
Contributor Information
Yuehan Zhang, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA.
Gypsyamber D’Souza, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA; Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University, Baltimore, Maryland, USA.
Carole Fakhry, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA; Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University, Baltimore, Maryland, USA.
Elaine O Bigelow, Department of Otolaryngology–Head and Neck Surgery, Johns Hopkins University, Baltimore, Maryland, USA.
Mykhaylo Usyk, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Obstetrics and Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Robert D Burk, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Obstetrics and Gynecology and Women's Health, Albert Einstein College of Medicine, Bronx, New York, USA; Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
Ni Zhao, Department of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA.
References
- 1. McQuillan G, Kruszon-Moran D, Markowitz LE, Unger ER, Paulose-Ram R.. Prevalence of HPV in adults aged 18-69: United States, 2011-2014. NCHS Data Brief 2017; 1–8. [PubMed] [Google Scholar]
- 2. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung CH, Bagheri A, D’Souza G.. Epidemiology of oral human papillomavirus infection. Oral Oncol 2014; 50:364–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou Y, Gao H, Mihindukulasuriya KA, et al. Biogeography of the ecosystems of the healthy human body. Genome Biol 2013; 14:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome—An update for oral healthcare professionals. Br Dent J 2016; 221:657–66. [DOI] [PubMed] [Google Scholar]
- 6. Tuominen H, Rautava S, Syrjänen S, Collado MC, Rautava J.. HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci Rep 2018; 8:9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuominen H, Rautava S, Collado MC, Syrjänen S, Rautava J.. HPV infection and bacterial microbiota in breast milk and infant oral mucosa. PLoS One 2018; 13:e02070161–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JE, Lee S, Lee H, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One 2013; 8:e63514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reimers LL, Mehta SD, Massad LS, et al. The cervicovaginal microbiota and its associations with human papillomavirus detection in HIV-infected and HIV-uninfected women. J Infect Dis 2016; 214:1361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H.. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J Obstet Gynecol 2019; 221:9–18. [DOI] [PubMed] [Google Scholar]
- 11. Usyk M, Schlecht N, Pickering S, Nucci-sack A, Diaz A.. The immune landscape of molecular bacterial vaginosis and HPV natural history. Res Square 2021; doi: 10.1038/s41467-021-27628-3. [DOI] [Google Scholar]
- 12. D’Souza G, Clemens G, Troy T, et al. Evaluating the utility and prevalence of HPV biomarkers in oral rinses and serology for HPV-related oropharyngeal cancer. Cancer Prev Res 2019; 12:689–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Qian PY.. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS One 2009; 4:e7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R.. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods 2008; 5:235–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hercus C. NovoCraft short read alignment package. 2009. http://www.novocraft.com/.
- 16. Schmieder R, Edwards R.. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011; 27:863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rognes T, Flouri T, Nichols B, Quince C, Mahé F.. VSEARCH: a versatile open source tool for metagenomics. PeerJ 2016; 4:e2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Langille MGI, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 2013; 31:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R.. UniFrac: an effective distance metric for microbial community comparison. ISME J 2011; 5:169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McArdle BH, Anderson MJ.. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 2001; 82:290–7. [Google Scholar]
- 21. Anderson MJ. Distance-based tests for homogeneity of multivariate dispersions. Biometrics 2006; 62:245–53. [DOI] [PubMed] [Google Scholar]
- 22. Martin BD, Witten D, Willis AD.. Modeling microbial abundances and dysbiosis with beta-binomial regression. Ann Appl Stat 2020; 14:94–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57:289–300. [Google Scholar]
- 24. Huttenhower C, Gevers D, Knight R, et al. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guerrero-Preston R, Godoy-Vitorino F, Jedlicka A, et al. 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection and surgical treatment. Oncotarget 2016; 7:51320–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Börnigen D, Ren B, Pickard R, et al. Alterations in oral bacterial communities are associated with risk factors for oral and oropharyngeal cancer. Sci Rep 2017; 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lim Y, Fukuma N, Totsika M, Kenny L, Morrison M, Punyadeera C.. The performance of an oral microbiome biomarker panel in predicting oral cavity and oropharyngeal cancers. Front Cell Infect Microbiol 2018; 8:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gopinath D, Menon RK, Banerjee M, Su Yuxiong R, Botelho MG, Johnson NW.. Culture-independent studies on bacterial dysbiosis in oral and oropharyngeal squamous cell carcinoma: a systematic review. Crit Rev Oncol Hematol 2019; 139:31–40. [DOI] [PubMed] [Google Scholar]
- 29. Mager DL, Haffajee AD, Delvin PM, Norris CM, Posner MR, Goodson JM.. The salivary microbiota as a diagnostic indicator of oral cancer: a descriptive, non-randomized study of cancer-free and oral squamous cell carcinoma subjects. J Transl Med 2005; 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onywera H, Williamson AL, Cozzuto L, et al. The penile microbiota of Black South African men: relationship with human papillomavirus and HIV infection. BMC Microbiol 2020; 20:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bui TC, Markham CM, Ross MW, Mullen PD.. Examining the association between oral health and oral HPV infection. Cancer Prev Res 2013; 6:917–24. [DOI] [PubMed] [Google Scholar]
- 32. Tezal M, Scannapieco FA, Wactawski-Wende J, et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch Otolaryngol Head Neck Surg 2012; 138:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shewale JB, Pickard RKL, Xiao W, Jiang B, Gillison ML.. Independent association of marijuana use and poor oral hygiene with HPV–negative but not HPV–positive head and neck squamous cell carcinomas. Cancer 2021; 127:2099–110. [DOI] [PubMed] [Google Scholar]
- 34. Vielkind P, Jentsch H, Eschrich K, Rodloff AC, Stingu CS.. Prevalence of Actinomyces spp. in patients with chronic periodontitis. Int J Med Microbiol 2015; 305:682–8. [DOI] [PubMed] [Google Scholar]
- 35. Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017; 151:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cekici A, Kantarci A, Hatice H, Van Dyke TE.. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 2014; 64:57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huff J, Infante PF.. Styrene exposure and risk of cancer. Mutagenesis 2011; 26:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stohs SJ, Ohia S, Bagchi D.. Naphthalene toxicity and antioxidant nutrients. Toxicology 2002; 180:97–105. [DOI] [PubMed] [Google Scholar]
- 39. Moody CA, Laimins LA.. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog 2009; 5:e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gagnaire A, Nadel B, Raoult D, Neefjes J, Gorvel JP.. Collateral damage: Insights into bacterial mechanisms that predispose host cells to cancer. Nat Rev Microbiol 2017; 15:109–28. [DOI] [PubMed] [Google Scholar]
- 41. Sun S, Jones RB, Fodor AA.. Inference-based accuracy of metagenome prediction tools varies across sample types and functional categories. Microbiome 2020; 8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caicedo HH, Hashimoto DA, Caicedo JC, Pentland A, Pisano GP.. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 2020; 38:669–73. [DOI] [PubMed] [Google Scholar]
- 43. Anderson MJ, Walsh DCI.. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: what null hypothesis are you testing? Ecol Monogr 2013; 83:557–74. [Google Scholar]
- 44. Beachler DC, Sugar EA, Margolick JB, et al. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am J Epidemiol 2014; 181:40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.