Abstract
Purpose of review:
This article discusses the application of neuropsychological evaluation to the workup of individuals with age-related cognitive impairment and suspected dementia. Referral questions, principles of evaluation, and common instruments to detect abnormalities in cognition and behavior in this population are reviewed. The integration of neuropsychological test findings with other clinical and biomarker information enhances early detection, differential diagnosis, and care planning.
Recent findings:
Life expectancy is increasing in the United States, and, accordingly, the prevalence and incidence of dementia associated with age-related neurodegenerative brain disease are rising. Age is the greatest risk factor for the dementia associated with Alzheimer disease, the most common neurodegenerative cause of dementia in people over 65 years of age; other etiologies, such as the class of frontotemporal lobar degenerations, are increasingly recognized in individuals both younger and older than 65 years of age. The clinical dementia diagnosis, unfortunately, is imperfectly related to disease etiology; however, probabilistic relationships can aid in diagnosis. Further, mounting evidence from postmortem brain autopsies points to multiple etiologies. The case examples in this article illustrate how the neuropsychological evaluation increases diagnostic accuracy and, most important, identifies salient cognitive and behavioral symptoms to target for nonpharmacologic intervention and caregiver education and support. Sharing the diagnosis with affected individuals is also discussed with reference to prognosis and severity of illness.
Summary:
The clinical neuropsychological examination facilitates early detection of dementia, characterizes the level of severity, defines salient clinical features, aids in differential diagnosis, and points to a pathway for care planning and disease education.
INTRODUCTION
In 1976, a seminal article by Robert Katzman1 sent a somber message that foreshadowed the current public health crisis posed by the increasing prevalence of cognitive loss and dementia in later adult years. Before that time, Alzheimer disease (AD), as first reported by Alois Alzheimer, was considered a disease of young onset (ie, a “presenile” dementia), whereas cognitive decline in old age to a point of senility was considered inevitable. In 1968, Blessed and colleagues2 published findings relating postmortem plaque counts to scores on a brief mental status questionnaire. This and other evidence linking Alzheimer neuropathology to cognitive loss argued strongly that dementia should be considered a disease and not a natural or obligatory outcome of the brain aging process. Katzman’s prophecy has come to pass; it has been estimated that the clinical syndrome of dementia occurs in 6% to 10% of the population older than 65 years of age, two-thirds due to AD pathophysiology.3 As lifespan in the United States and other developed countries increases, and as age constitutes the greatest risk factor for AD and related disorders, a concomitant increase will be seen in the incidence and prevalence of dementia. AD is not the only neurodegenerative disease that causes dementia, and the past 30 years has seen an increase in our knowledge of these diseases that entail distinct proteinopathies that destroy healthy neurons and their connections. As a result, primary care providers, neurologists, psychiatrists, and other medical specialists will increasingly serve older individuals, many of whom will have mild, moderate, or severe cognitive impairment as the lead symptom or in the background of their primary illness.
So-called normal cognitive aging, although on average portrayed as a steady decline in cognitive test scores can, in fact, be quite heterogeneous, with increased individual variability with advancing age.4 Dementia is the end point of a progressive process during which patients have an initially insidious decline of normal (ie, customary for one’s personal baseline) cognition and/or social-comportmental behavior. The most common cause over age 65 is neurodegenerative brain disease. At postmortem examination, typically one disease (eg, AD) can be identified as a primary finding, but, more often, the primary finding is accompanied by additional types of pathology (eg, limbic Lewy bodies, transactive response DNA-binding protein 43 [TDP-43], or vascular pathology) that are perhaps of lesser magnitude.5 The clinical syndrome of dementia (currently designated as major neurocognitive disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5]6) is defined not only by the presence of cognitive and behavioral decline but also the degree to which this decline interferes with customary functional daily activities. The term neurocognitive was intended to separate neurodegenerative/vascular dementia from other causes of behavioral and cognitive decline that are not specifically linked to a neurologic disease. Before dementia, a patient’s cognition and behavior may be changing for many years but not to the extent that prevents an independent existence. During that time, the individual may remain productive and engaged, with appropriate support (Case 6-1). Many dementia syndromes may begin with so-called atypical symptoms that may be attributed to peripheral organ changes (eg, eye problems) (Case 6-2) and misdiagnosed or go unrecognized until further progression occurs.
CASE 6-1
A 71-year-old woman shared her concerns about memory loss with her primary care physician. She had been a librarian, and her physician, who had known her for 6 years, referred her for neuropsychological evaluation to assess baseline functioning given the patient’s past level of cognitive achievement and the absence of symptoms in routine screening. The patient had recently lost her spouse and was very tearful when describing her loss and the resulting void in her life. She had been prescribed an antidepressant, which she found helpful. She had no past history of depression, and her medical history was relatively benign except for elevated cholesterol, for which she took medication.
She presented to the appointment well-groomed and interacted in a socially engaging and friendly manner. Speech was fluent without evidence of word-finding pauses or errors, and her hearing and vision were adequate to support test performance. Based on her academic and career achievement, her past level of ability was estimated in the average to high average range (estimated premorbid IQ between 110 and 120). This set the standard for comparing scores obtained on testing. Thus, if she had little or no loss, test scores were expected to fall at her premorbid levels when corrected for age. Refer to the grid for her test scores and interpretation of the level of performance on tests of different domains by normative standards.
Performance in many neurocognitive domains fell at the expected premorbid level, with some notable exceptions. First, although the forward digit span of 7 indicated normal immediate span of attention, the backward span, a test of working memory, was only 2, suggesting difficulty maintaining information online even over a short duration of time. This also was noted in the difficulty she had reciting the months in reverse sequence. Her score on the Boston Naming Test, a widely used measure of object naming, was significantly lower than expected, particularly given her profession. In addition, her ability to generate lists of words from a specific category (semantic memory) was also reduced. On standard episodic memory tests of a moderate level of difficulty and complexity, she demonstrated mild to moderate impairment of the ability to remember a story and a complex design she had copied even after 20 minutes. The neuropsychologist questioned whether these memory tests may have been too difficult for her to demonstrate areas of relative impairment and sparing and administered a simple test of retentive memory, the Three Words–Three Shapes test8; this test reduces the executive load required by more difficult tasks, which can confound performance. These accommodations are not ordinarily conducted in the course of a brief examination. On the Three Words–Three Shapes test, with the limited amount of information that she was able to learn after several trials, she lost information after a delay of 5 minutes. Despite the marked limitation in her short-term retentive memory, scores on tests of reasoning were preserved and she did not report a significant level of depression or anxiety, despite mourning the loss of her spouse. A survey of activities of daily living9 completed by a relative disclosed very minimal changes in activities of daily living.
Her cognitive profile was marked by significant decline from expected levels in retentive memory and additional difficulties on tests of attention and semantic memory (ie, amnestic, multidomain profile). The clinical diagnosis was mild cognitive impairment,10 not dementia, because, by informant report, she was still independent in most activities of daily living.
She continued to be followed, and in 4 years, her symptoms had progressed to the point of severely limiting independence, and she required assistance in most complex activities of daily living. This represented both a decline in cognitive test performance and the emergence of difficulties in performing routine tasks. Based on worsening test scores and increased difficulty in activities of daily living, which now were in the severely impaired range, she was given a clinical diagnosis of dementia (major neurocognitive disorder). Because of the prominence of memory loss in the clinical profile, Alzheimer disease neuropathology was high on the list for differential diagnosis of etiology.4
| Neuropsychological tests (total score)a | Initial raw score | Initial normative comparisonb,c | 4-Year follow-up raw scoreb |
|---|---|---|---|
| General cognitive/screening | |||
| Wechsler Test of Adult Reading (WTAR) (predicted Full-Scale IQ) | 43/50 | High average | NA |
| Wechsler Adult Scale of Intelligence 2nd Edition (WASI-II) (administered only at the initial visit as an estimate of premorbid ability) | |||
| Estimated Full-Scale IQ | (SS = 106) | Average | NA |
| Verbal Comprehension Index | (SS = 107) | Average | NA |
| Perceptual Reasoning Index | (SS = 103) | Average | NA |
| Montreal Cognitive Assessment (MoCA) | 27/30 | Normal performance for age based on clinical impression | 23/30 ↓ |
| Activities of Daily Living Questionnaire | 17% | Minimal | 44% (moderate) |
| Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Update Total Index Score | (SS = 105) | Average | SS = 94 ↓ |
| Immediate Memory Index | (SS = 100) | Average | SS = 81 ↓ |
| Visuospatial/Constructions Index | (SS = 131) | Very superior | SS = 131 |
| Language Index | (SS = 96) | Average | SS = 96 |
| Attention Index | (SS = 100) | Average | SS = 103 |
| Delayed Memory Index | (SS = 95) | Average | SS = 71 ↓ |
| Attention | |||
| Digit Span (forward, backward) | 7; 2 | Average; moderately impairedc | 7; 2 |
| Trail Making Test Part A | 39 seconds | Mildly impairedc | 42 seconds |
| Trail Making Test Part B | 121 seconds | Low average | 96 seconds |
| RBANS Coding (number of items) | 40 | Average | 33 |
| Months of Year Forward | 5 seconds | Normal performance for age based on clinical impression | 5 seconds |
| Months of Year Backward | 20 seconds | Mildly impairedc | 23 seconds ↓ |
| Language | |||
| Boston Naming Test | 41/60 | Moderately impairedc | 42/60 |
| Lexical fluency total (F-A-S) | 56 | High average | 52 |
| WASI-II Vocabulary | 37/59 | Average | NA |
| Semantic fluency (animals) | 14 | Mildly impairedc | 15 |
| RBANS Picture Naming | 10/10 | Average | 8/10 ↓ |
| RBANS Semantic Fluency | 18 | Average | 10 ↓ |
| Visuospatial | |||
| Target cancellation time (errors left/right) | 151 seconds (2/0) | Normal performance for age based on clinical impression | 90 seconds (0/1) |
| WASI-II Block Design | 23/71 | Average | NA |
| RBANS Figure Copy | 20/20 | Superior | 19/20 |
| RBANS Line Orientation | 20/20 | High average | 20/20 |
| Memory | |||
| RBANS Update | |||
| List Learning (trial 4) | 8/10 | Average | 8/10 |
| List Delayed Recall | 4/10 | Average | 2/10 ↓ |
| List Recognition (hits; false positives) | 10; 1 | Average | 9; 3 ↓ |
| Story Learning (trial 2) | 9/12 | Average | 7/12 |
| Story Delayed Recall | 10/12 | High average | 6/12 ↓ |
| Story Recognition | 11/11 | Normal performance for age based on clinical impression | 8/11 ↓ |
| Figure Delayed Recall | 5/20 | Mildly impairedc | 6/20 |
| Figure Recognition | 1/1 | Normal performance for age based on clinical impression | 1/1 |
| Wechsler Memory Scale 4th Edition (WMS-IV) | |||
| Logical Memory I | 27/53 | Average | 30/53 |
| Logical Memory II | 5/39 | Moderately impairedc | 4/39 |
| Logical Memory Recognition | 13/23 | Mildly impairedc | 11/30 |
| Three Words-Three Shapes | |||
| Copy (words [W]; shapes [S]) | 3W; 3S | Average; average | 3W; 3S |
| Incidental (W;S) | 2W; 1S | Moderately impairedc; average | 2W; 2S |
| Trial 3 (W; S) | 3W; 2S | Average; average | 3W; 3S |
| Delayed Recall (W; S) | 1W; 1S | Severely impaired; mildly impairedc | 1W; 1S |
| Recognition (hits; false positives) | 3; 0 | Average; average | 3; 0 |
| Executive functions | |||
| WASI-II Similarities | 34/45 | High average | NA |
| WASI-II Matrix Reasoning | 19/30 | High average | NA |
| Mood/behavioral symptoms | |||
| Geriatric Depression Scale (GDS) | 6/30 | Normal performance for age based on clinical impression | 3/30 |
| Beck Anxiety Inventory (BAI) | 4/63 | Minimal | 1/63 |
| Beck Depression Inventory 2nd Edition (BDI-II) | 3/63 | Minimal | 0/63 |
↓ = decline; IQ = intelligence quotient; NA = not administered; SS = standard score.
All published tests are described in Lezak and colleagues,7 with the exception of the Three Words-Three Shapes test. Parentheses include abbreviations of test measures, units of measurement, or specific measure within a test with multiple metrics.
Ranking of scores is based on age and/or education-matched normative data (as available) and clinical impression.
Indicates impairment. Levels of impairment: mildly impaired, z = −1.93 to −1.4; moderately impaired, z = −2.5 to −2.0; severely impaired, z = −2.53 or lower.
COMMENT
This patient’s initial neuropsychological findings allowed her family to understand her level of impairment and begin making plans for care needs down the road. At that time, strategies for providing support for activities she eventually could no longer do (such as managing her finances) were built in, and accommodations to allow her to function maximally at her level were initiated in the form of calendars, checklists, telephone reminders, and someone to accompany her to doctors’ appointments. Despite the decline in neuropsychological test scores over time because of worsening disease, she continued to function with in-home assistance until her death 3 years later. A postmortem brain autopsy showed Alzheimer disease as the predominant neuropathologic finding.
CASE 6-2
A 63-year-old man was referred for neuropsychological evaluation of a 4-year history of progressive visuospatial problems and difficulty acquiring new skills. Once when he was driving his car in the rain, he commented to his spouse that all he could see were the raindrops on the windshield. He was evaluated for visual acuity and was found to need mild correction with glasses; however, he continued to report “difficulty seeing.” Over time, his symptoms worsened, and he often bumped into objects in his path, misreached for objects within grasp, and could not “see” clothing in his closet, although it was in full view. A second visit to the ophthalmologist did not show evidence of changes in acuity. He had been having some challenges at his work as an accountant and was told that he might be experiencing excessive stress. His physician referred him for neuropsychological evaluation.
On examination, he had a perfect score on the Mini-Mental State Examination (MMSE). Despite this, his spouse reported that he had a moderate level of difficulty in executing routine daily tasks, including maintaining his personal belongings, and learning to use new devices, such as the new remote control for the television set. He was not able to manipulate the proper sequence of buttons and always held the controller in an awkward manner. In addition, his spouse reported the emergence of some behavioral changes on the Neuropsychiatric Inventory Questionnaire (NPI-Q),11 largely in the form of apathy and irritability.
The profile derived from his test performance showed particular difficulty with visual attention and other visually based tasks, such as visual memory. Refer to the grid for his test scores and interpretation of the level of performance on tests of different domains by normative standards.
On the Trail Making Test, which is a test of executive attention, his performance was severely reduced not because of executive dysfunction but rather because he could not effectively search for the next item in the sequence. He had no difficulty judging angularity or copying a cube, but visual search in the absence of structure was challenging. Simultanagnosia was evident in his tendency to focus on only parts of an object. He was shown two pages, one of which contained a sentence written in 4-inch-high letters and a second with the same sentence in tiny letters. He immediately read aloud the small script but struggled with the large script, craning his neck and stepping back from the page. This indicated that when the visual image required successive eye movements, as with the large script, he could not “see,” but when the information was obtainable in a single fixation, it was easier for him.
| Neuropsychological testsa | Initial test scores | Current levelb |
|---|---|---|
| General cognitive screening | ||
| Mini-Mental State Examination (MMSE) (30 points) | 30 | Within normal limits based on clinical impression |
| Activities of Daily Living Questionnaire | 45% | Moderately impairedc |
| Attention | ||
| Digit span (forward; backward) | 6; 5 | Average |
| Trail Making Test Part A (seconds) | Scaled score = 1,d T score =12d | Severely impairedc |
| Trail Making Test Part B (seconds) | Scaled score = 4,d T score = 22d | Severely impairedc |
| Language | ||
| Boston Naming Test (60 items) | 58, z score = 0.4d | Average |
| Semantic fluency | 18, z score = 0.0d | Average |
| Lexical fluency (average) | 48, z score = 0.5d | Average |
| Visuospatial | ||
| Target Cancellation (omissions 30 left/30 right, 60 items) | 180 seconds, 8/16 | Severely impairedc |
| CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) Neuropsychological Battery Constructions (11 points) | 9, z score = −0.33d | Average |
| Judgment of Line Orientation (30 items) | 23, 40th percentile | Average |
| Memory | ||
| Wechsler Memory Scale-Revised | ||
| Logical Memory Immediate (50 items) | 28, 80th percentile | High average |
| Logical Memory Delay (50 items) | 21, 68th percentile | Average |
| Visual Reproduction Immediate (41 items) | 19, 7th percentile | Mildly impairedc |
| Visual Reproduction Delay (41 items) | 20, 27th percentile | Average |
| CERAD Word List | ||
| Trials 1-3 (30 words) | 23, z score = 0.5d | Average |
| Delayed Recall (10 words) | 7, z score = 0.0d | Average |
| Recognition (10 words) | 10, z score = 0.4d | Average |
| Executive functions | ||
| Visual Verbal Test (20 sorts, to shifts) | 18, 8 | Average |
| Mood/behavior | ||
| Geriatric Depression Scale (30 items) | 8 | Within normal limits based on clinical impression |
| Neuropsychiatric Inventory Questionnaire (12 items) | 4 | Mildly impairedc |
Most standardized tests are described in Lezak and colleagues.7 Numbers in parentheses refer to units of measurement and/or total possible score on the test.
Ranking of scores is based on age and/or education-matched normative data (as available) and clinical impression.
Indicates impairment. Levels of impairment: mildly impaired, z = −1.93 to −1.4; moderately impaired, z = −2.5 to −2.0; severely impaired, z = −2.53 or lower.
Scaled score: mean = 10, standard deviation = 3; T score: mean = 50, standard deviation = 10; z score: mean = 0, standard deviation = 1.
COMMENT
This patient’s profile was one of progressive visuospatial dysfunction. This cognitive profile, also referred to as posterior cortical atrophy, is associated with occipitoparietal dysfunction and can be caused by Alzheimer neuropathology, cortical Lewy body disease, or corticobasal degeneration.12 Recommendations for care following the initial evaluation included home occupational therapy to ensure safety in the home and strategies for reducing the impact of visual limitations. The patient’s condition declined to the point that he was not able to continue caring for himself because of severe visual restrictions. As symptoms progressed, intervention strategies were initiated to help him adapt to the changes.
Although brain autopsy may disclose one predominant neuropathology in an individual with dementia, more often mixed pathologies are detected.5 AD is currently the best studied of the neurodegenerative causes of dementia, likely because of its prevalence; as a result, we now have in vivo biomarkers of its major protein abnormalities (ie, aggregated amyloid and hyperphosphorylated tau) that can be detected in CSF or by specialized positron emission tomography (PET) scans using tau and amyloid ligands. Less invasive tests for plasma-based biomarkers, including neurofilament light chain, amyloid, and phosphorylated tau, are increasingly used predictively13–15 and, when broadly available, will have a significant impact on the diagnostic process. The emergence of amyloid-altering treatments calls for neuropsychological assessment before considering treatment to determine whether an individual has clinical symptoms of AD.
Biomarkers notwithstanding, however, increasing concern for dementia is causing individuals to seek evaluation when they subjectively sense a change from their baseline abilities. Interestingly, as cognitive symptoms increase, personal awareness decreases and observer awareness increases.16 In the prodromal or earliest stages of a dementia, especially in those with high prior levels of cognitive achievement and/or education, the routine screening accomplished with such tests as the Mini-Mental State Examination (MMSE),17 the Montreal Cognitive Assessment (MoCA),18 or the cursory bedside clinical mental status examination may yield no abnormalities. In those cases, it is important not to assume that cognition is normal but rather to escalate to a more rigorous neuropsychological examination. In this case, the neuropsychological examination can detect subtle impairments, identify nonamnestic symptoms that are atypical for dementia onset (eg, simultanagnosia), and, most important, provide a baseline against which longitudinal follow-up examinations can be compared to track progression. If cognitive screening test scores are in the impaired range, neuropsychological testing is also indicated since the examination provides an objective measure of the severity and characteristics of symptoms to use in care planning.19 In the event that the screening score indicates substantial cognitive impairment (eg, 10/30 on the MoCA or 13/30 on the MMSE), neuropsychological examination is likely to be too challenging for the patient, who in this case would fail on every test, making the detection of a profile impossible. Even in severe cases, however, the neuropsychologist may choose to administer a brief set of appropriately tailored tests to pinpoint the primary area of deficit (eg, aphasia versus amnesia). In many instances, individuals may no longer be able to remain independent, and several ways to assess safety and decision-making capacity20 are available and can be added to the evaluation as required.
Referrals should be preceded by the neurologist’s routine mental status examination, which should include a measure of the ability to learn and retain information after a delay. As noted above, in individuals with a level of impairment that has significantly affected activities of daily living or in those with scores of less than 18 on the MoCA or MMSE, especially with a high level of education, the clinician should weigh whether or not the patient would be able to participate in a full neuropsychological battery. A typical battery can take from 2 to 5 hours, depending on the nature of the referral question (eg, a longer evaluation for the question of whether or not a mildly impaired person can continue to work as a financial advisor). Severe cognitive deficits, by screening or as obtained during the interview, would make it difficult to establish the validity/reliability of subsequent extensive testing. Using a brief mental status test can help the neurologist decide whether to refer for further testing, especially in a symptomatic individual, and to narrow the referral question for which additional testing might be useful. A prominent feature of many forms of dementia is a lack of awareness of illness or minimization of symptoms, known as anosognosia. It is important that the patient be accompanied by someone who knows them well enough to provide additional information about changes from past abilities and symptoms. The neuropsychological evaluation will typically obtain information from an informed collateral source, of course, with the patient’s permission. This collateral information may not be available, however, if the patient has no support network or excludes the collateral.
Although preventing or slowing AD has taken center stage in the public health arena, not all dementia is due to AD. Over the past 20 years, evidence has shown that many forms of frontotemporal lobar degeneration (FTLD) can give rise to a dementia syndrome.21 The clinical features of syndromes associated with FTLD can range from motor neuron disease to behavioral variant frontotemporal dementia, primary progressive aphasia, symptoms of corticobasal disease, and progressive supranuclear syndromes.22 Although many clinical syndromes and many neuropathologic entities exist, no one-to-one correspondence between them is demonstrated. For example, although many individuals with a prominent amnestic dementia will have AD neuropathology, up to 25% may have a different etiology.23 Although 60% to 70% of individuals with the primary progressive aphasia profile will have a form of FTLD, 30% to 40% will have AD or another form of neuropathology.24
THE CLINICAL NEUROPSYCHOLOGICAL EVALUATION
The examination typically begins with a thorough history that includes the patient’s medical conditions; family history of dementia or other medical, psychiatric, or neurologic disease; and concurrent medications. The patient’s level and quality of education and early developmental history are factors that influence the interpretation of neuropsychological test scores and help to determine which weaknesses may have been preexisting. The estimated individual prior peak level of cognitive ability serves as an important guide for the neuropsychologist to select a battery that is appropriate for the individual’s personal academic exposure and career achievements. The neuropsychologist appreciates that even normative standards may not be indiscriminately applied in the case of individuals not educated in the United States, who speak another language, or who have limited education. Thus, appropriate instruments, when available, to test underrepresented groups are critical.25 Also, it is desirable that patients be examined in their preferred language, although a shortage of non–English-speaking neuropsychologists exists in the United States. In this event, the neuropsychologist may work with an interpreter but also must be aware the limitations of this approach.
The examination itself consists of objective standardized tests of attention, language, visuospatial perception, episodic memory, and executive functions (Table 6-126). Each of these domains has been well studied in terms of the brain networks underlying them and how they are disrupted by neurologic disease. The scores obtained are compared with normative standards for the patient’s age, level of education, and other relevant demographics so that the level of functioning in each domain and the degree to which it diverges from expectation can be quantified. Social cognition, also referred to as comportment or social-interpersonal skill, is commonly assessed through informant interview and, in some cases, by objective measures.27 In addition to weighing the impact of education and experience on test performance, the neuropsychologist must also consider whether medications or extant medical conditions could be contributing to test performance.
TABLE 6-1.
Selected Neuropsychological Tests by Cognitive Domain
| Domain | Test |
|---|---|
| Attention | |
| Immediate span | Digit Span |
| Vigilance | “A” letter identification item of the Montreal Cognitive Assessment (MoCA); any continuous performance test |
| Response inhibition | Motor Go/No-Go Task |
| Executive attention | Trail Making Test Part B |
| Processing speed | Trail Making Test Part A; coding tests (eg, Repeatable Battery for the Assessment of Neuropsychological Status, Wechsler Adult Intelligence Scale) |
|
| |
| Language | |
| Aphasia surveys (include tests of speech, word and sentence comprehension, naming, repetition, reading, and writing) | Boston Diagnostic Aphasia Examination, Western Aphasia Battery-Revised |
|
| |
| Naming | Boston Naming Test |
|
| |
| Word comprehension | Peabody Picture Vocabulary Test 4th Edition |
|
| |
| Word list generation | Category list generation (animals, vegetables), letter list generation (F-A-S Test) |
|
| |
| Visuospatial perception | |
| Object perception | Facial Recognition Test, Hooper Visual Organization Test |
| Spatial perception | Line orientation tests |
| Spatial representation | Cube, figure copy tests; block designs; puzzle assembly (also susceptible to executive function deficits) |
|
| |
| Episodic memory | |
| Verbal and nonverbal visual memory | Three Words-Three Shapes Test, Brief Cognitive Screening Examination: Memory test26 |
|
| |
| Verbal and nonverbal memory in auditory and visual modalities, orientation, working memory | Wechsler Memory Scale |
|
| |
| Word list learning test | Rey Auditory Verbal Learning Test |
|
| |
| Word list learning test with embedded categories | California Verbal Learning Test |
Some common scenarios for considering referral for neuropsychological evaluation include the following:
The individual reports cognitive symptoms or has received poor job reviews but tests normally in the cursory mental status examination. Despite having had many longitudinal health metrics such as blood pressure and laboratory tests, most older individuals have not had a baseline cognitive evaluation before concerns about or the appearance of symptoms. For individuals educated in the United States, estimates of peak prior level of cognitive ability can be derived from scores on word-reading tests that can serve as a proxy for premorbid cognitive levels. Many older individuals may lack higher levels of education, and word-reading ability may underestimate their true cognitive baseline. In this case, demographic features such as career, social engagement, community engagement, and other measures of ability must be taken into account when estimating past peak level of ability.
The individual’s family or friends have observed uncharacteristic behavior or thinking, although the individual has no concerns. Some individuals may have undergone psychiatric consultation to understand these changes in personality and reasoning before progression clarifies the diagnosis.
An individual younger than age 50 is expressing progressive memory loss or other cognitive and behavioral symptoms.
Neuropsychological scores can be obtained at annual intervals to track the course of symptoms. In the event of a potentially reversible cause of cognitive impairment (eg, sleep apnea, kidney failure, or liver failure), test scores may actually improve over time.
In older adults, a preexisting dementia may increase the probability of delirium in the context of medications, metabolic disorders, and surgical procedures.28 Baseline neuropsychological testing at a time when the individual is asymptomatic can guide medical decision making.
History Taking
The neuropsychologist probes for evidence of a change from a person’s prior level of cognitive ability, character, and/or emotions and behavior. This is typically difficult to obtain because patients often do not recognize the changes. Even those who observe them may not accurately time the onset of symptoms, becoming aware of them only after a crisis. It is important to ask when patients last seemed like themselves in terms of carrying out routine activities, interacting with others, making decisions, and displaying typical emotional reactions (eg, sympathy, empathy). The key to detecting a dementia syndrome is change, not the specific symptom itself. Most individuals report a variety of cognitive symptoms as “memory loss.” Deeper probing can determine the neurocognitive domain within which the symptom really falls. For example, individuals with aphasia may report forgetting words, individuals with visuospatial deficits may report forgetting how to use common tools, and individuals with behavioral changes may report forgetting their manners. Table 6-2 lists some questions to use in history taking to elicit distinctive symptoms in a framework that is neurologically relevant for cognitive-behavioral neuroanatomic networks.
TABLE 6-2.
Sample Questions for Gathering History of Cognitive/Behavioral Change
| Cognitive domain as probed in history | Questions to aska |
|---|---|
| Language | Does the patient: |
| Seem to search for words in conversation while speaking? | |
| Say the wrong words or sounds within a word? | |
| Have trouble understanding single words or conversation despite normal hearing? | |
|
| |
| Episodic memory | Despite normal ability to speak and understand language, does the patient: |
| Forget important events, appointments? | |
| Forget something they did last week? | |
| Repeatedly ask questions or make the same comment unaware that it was just made? | |
|
| |
| Visuospatial functions | Does the patient: |
| Seem to not see objects in full view despite normal visual acuity? | |
| Misplace or misreach for items on a tabletop? | |
| Bump into things frequently? | |
|
| |
| Reasoning/judgment | Does the patient: |
| Have poor decision-making ability or judgment (eg, was scammed)? | |
| Have the ability to respond appropriately in an emergency? | |
| Seem unaware of the social context, such as showing inappropriate behavior in a setting (eg, laughing out loud at a funeral)? | |
Questions may be directed to the patient, an informant, or both. In cases in which a patient is unaware of deficits, differences may be seen in the responses of the patient and the informant.
The cognitive and behavioral functions surveyed in the examination are based on a model that relates complex cognitive functions to their underlying neuroanatomic and functional brain networks. Symptoms in domains such as language, spatial perception, visual object perception, social cognition, and episodic memory lend themselves to neuroanatomic network localization, whereas symptoms such as reduced arousal and attention are not easily localized in the same manner.8 The goal of the neuropsychological examination of an individual with suspected dementia, especially in the early stages, is to identify whether a single domain is involved (eg, episodic memory versus aphasia) or whether the patient has multiple cognitive and behavioral deficits. The early clinical profile (Figure 6-1) reflects the areas of the brain influenced by disease rather than the disease itself. Thus, aphasia as a predominant early symptom implies left-sided cerebral involvement in most right-handers and half of left-handers, whereas amnesia implies damage to medial temporal limbic areas.
FIGURE 6-1. Neuropsychological profiles of early and late dementia.
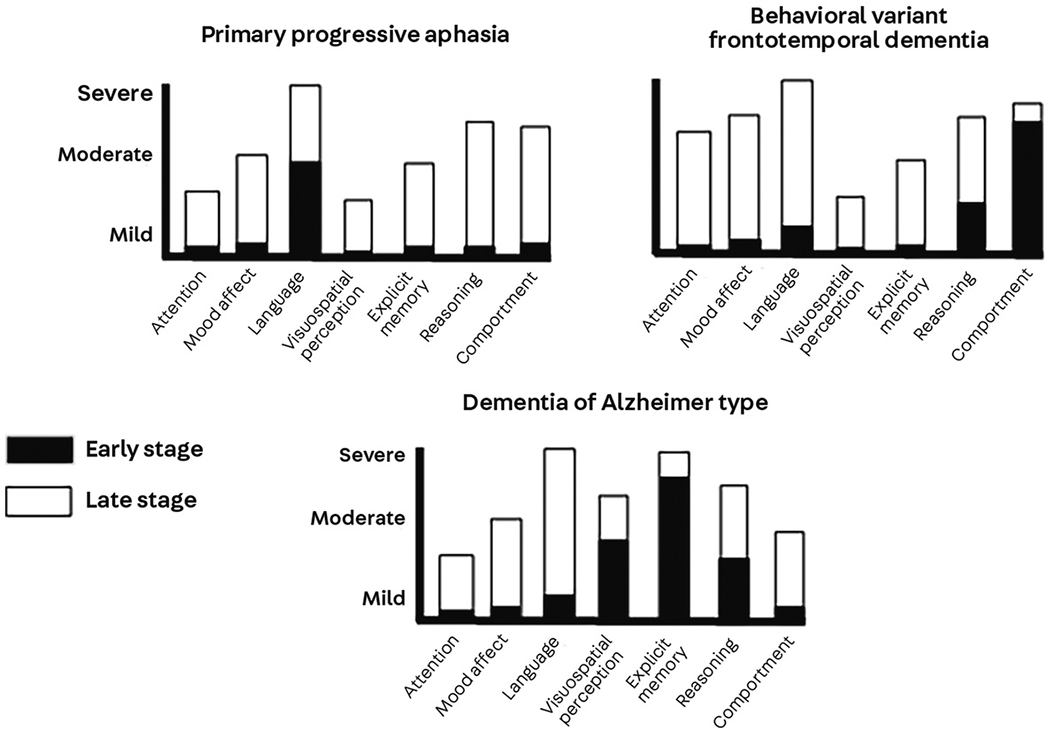
X-axis represents domains tested in the neuropsychological examination. Y-axis represents level of impairment: mild, moderate or severe. Dementia syndromes include primary progressive aphasia, behavioral variant frontotemporal dementia, and the so-called dementia of the Alzheimer type typified by early amnesia. Black bars represent levels of impairment across domains early in the course of illness, and white bars represent levels measured in later stages of illness. It is clear that in the earliest stages, dementia symptoms can remain highly focal. In the case of primary progressive aphasia, prominent language impairment represents left cerebral involvement; in behavioral variant frontotemporal dementia, prominent changes in comportment represent bilateral frontotemporal involvement. In dementia of the Alzheimer type, prominent early amnesia represents medial temporal dysfunction. Reprinted with permission from Weintraub S.4 © 2014 Oxford University Press.
The neuropsychological examination reviews attention, episodic memory, language, visuospatial functions, and executive abilities such as reasoning and set shifting. The interpretation takes into account how deficits in one domain can affect scores on tests in another domain, although that domain itself may be intact. For example, to establish that episodic memory is intact in the patient with word-finding difficulty, tests that will not be affected by the aphasia have to be used. The Three Words–Three Shapes Test was designed to compare verbal and nonverbal memory in the visual modality; the test can distinguish among different clinical dementia syndromes such as amnestic dementia (in which memory loss is paramount) and primary progressive aphasia (in which patterns of performance reflect the integrity of nonverbal episodic memory and recognition of verbal information that may not be retrievable spontaneously).29,30
Biomarkers have clearly been an advance in diagnosis, at least for AD. However, biomarkers do not provide information about the specific cognitive and behavioral symptoms an individual is experiencing, and these symptoms are not homogeneous across individuals with AD or other neurodegenerative diseases. Symptoms can differ vastly and need different interventions.
A BLUEPRINT FOR EDUCATION, SYMPTOM MANAGEMENT, AND MONITORING
The results of the neuropsychological examination are shared with the patient and support network as available, usually in the form of an in-person feedback session. During this session, the neuropsychologist reviews the findings and translates what they mean in terms of the individual’s experience, impact on daily living, and significance for diagnosis. It is important to explain the limits of knowledge based on the examination, that is, that the neuropsychological examination detects whether cognition or behavior is abnormal and specifies which functions are not normal but does not identify the disease causing the abnormality. An explanation of how brain regions implicated in disease are inferred by the test results provides patients with the knowledge that their symptoms reflect disease and are not the product of stress or lack of cooperation. This information also helps caregivers understand that neurologic limitations in the patient’s insight/awareness of illness makes it difficult for them to see reason, understand explanations, or even remember their diagnosis. In the course of feedback, the neuropsychologist can also explain how different diseases can cause dementia and that the biological nature of the disease rests on biomarker tests. It is important to explain that the pathophysiologic diagnosis still rests on biomarkers or postmortem brain autopsy, but the neuropsychological evaluation can give us probabilistic diagnoses.4 This helps caregivers understand why someone with a clinical diagnosis of primary progressive aphasia can be found to have AD or TDP-43 proteinopathy postmortem and that the initial clinical diagnosis is not in error.
CONCLUSION
Cognitive aging is heterogeneous, and not all decline is benign. With increasing recognition that age-related cognitive decline could imply future dementia, it is important for neurologists to obtain an objective measure of a patient’s cognitive function to inform the diagnosis, prognosis, and care recommendations. The clinical neuropsychological examination addresses issues that are not easily assessed in the cursory mental status examination or obtained in routine imaging and laboratory tests:
Does the patient show a significant decline from a prior established level of cognition and behavior? (This is a clue to the integrity of different neuroanatomic networks.)
What are the key domains in which the patient is experiencing decline? (This is another clue to the integrity of different neuroanatomic networks.)
Are unusual nonmemory symptoms (eg, visuospatial dysfunction, social-interpersonal changes) or symptoms in an individual younger than age 50 an indication of a “psychiatric” condition or stress, or are they related to brain disease?
In the absence of available biomarkers and based on the body of postmortem evidence, what is the likelihood of etiology given the neurocognitive profile on the examination?
What resources can the patient and caregiver access to address management needs?
The neuropsychological examination can also document normal mental state in an individual with cognitive symptoms and then serve as a baseline against which future changes can be measured.
KEY POINTS.
The most common neurodegenerative disease that causes dementia in people older than 65 years of age is Alzheimer disease, with or without additional neuropathologic and/or vascular lesions.
Cognitive trajectories vary markedly among individuals as they age, ranging from little/no loss to mild cognitive impairment (an intermediate stage of mild cognitive changes that does not affect daily activities) to progressive impairment leading to incapacitating dementia.
Dementia is a progressive cognitive-behavioral syndrome that interferes with the ability to independently perform routine activities of daily living; earlier stages are detectable by neuropsychological testing before they have an impact on daily functioning.
Dementia syndromes that present with unusual symptoms in the early stages (eg, primary progressive aphasia, behavioral variant frontotemporal dementia, posterior cortical atrophy) are easily missed/misdiagnosed in the cursory mental status examination but can be detected with neuropsychological evaluation.
Cognitive impairment often is accompanied by anosognosia, in which case the patient may be unaware of or deny the symptoms obvious to others. It is essential that the clinician seek information from a relative or close friend about any changes they may have noticed in the patient’s cognition or behavior.
Most individuals report a variety of cognitive symptoms as “memory loss.” Deeper probing can determine the neurocognitive domain within which the symptom really falls.
Neuropsychological reports that are brief and easy to interpret, cover all relevant domains, (neurocognitive systems), and provide education and prescriptive guidance for needed additional workup and/or suitable nonpharmacologic interventions for patients and caregivers are a valuable adjunct to clinical care.
Neuropsychological profiles of early dementia (eg, amnestic, aphasic, visuospatial, social-comportmental) can predict the underlying etiology in a probabilistic manner, and test scores can contribute to staging the level of cognitive, behavioral, and functional impairment.
Footnotes
RELATIONSHIP DISCLOSURE: Dr Weintraub reports no disclosure.
UNLABELED USE OF PRODUCTS/INVESTIGATIONAL USE DISCLOSURE: Dr Weintraub reports no disclosure.
REFERENCES
- 1.Katzman R Editorial: The prevalence and malignancy of Alzheimer disease. A major killer. Arch Neurol 1976;33(4):217–218. doi: 10.1001/archneur.1976.00500040001001 [DOI] [PubMed] [Google Scholar]
- 2.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797 [DOI] [PubMed] [Google Scholar]
- 3.Hendrie HC. Epidemiology of Alzheimer’s disease. Geriatrics 1997;52 suppl 2:S4–S8. [PubMed] [Google Scholar]
- 4.Weintraub S Neuropsychological assessment of dementia: a large-scale neuroanatomical network approach. In: Dickerson BC, Atri A, eds. Dementia: comprehensive principles and practice. Oxford University Press; 2014:487–507. [Google Scholar]
- 5.Yu L, Boyle PA, Leurgans S, et al. Effect of common neuropathologies on progression of late life cognitive impairment. Neurobiol Aging 2015;36(7):2225–2231. doi: 10.1016/j.neurobiolaging.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5). 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 7.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th ed. Oxford University Press; 2004. [Google Scholar]
- 8.Weintraub S Neuropsychological assessment of mental state. In: Mesulam M-M, editor. Principles of Cognitive and Behavioral Neurology. New York: Oxford University Press; 2000. p. 121–173. [Google Scholar]
- 9.Johnson N, Barion A, Rademaker A, Weintraub S. The Activities of Daily Living Questionnaire (ADLQ): a validation study in patients with dementia. Alzheimer Dis Assoc Disord 2004;18:223–230. [PubMed] [Google Scholar]
- 10.Petersen RC, Caracciolo B, Brayne C, et al. Mild cognitive impairment: a concept in evolution. J Intern Med 2014;275(3):214–228. doi: 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- 12.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology 2004;63(7):1168–1174. doi: 10.1212/01.wnl.0000140289.18472.15 [DOI] [PubMed] [Google Scholar]
- 13.Brickman AM, Manly JJ, Honig LS, et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimers Dement 2021;17(8):1353–1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simrén J, Leuzy A, Karikari TK, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer’s disease. Alzheimers Dement 2021;17(7):1145–1156. doi: 10.1002/alz.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 2019;93(17):e1647–e1659. doi: 10.1212/WNL.0000000000008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavett R, Dunn JE, Stoddard A, et al. The Cognitive Change in Women study (CCW): informant ratings of cognitive change but not self-ratings are associated with neuropsychological performance over 3 years. Alzheimer Dis Assoc Disord 2011;25(4):305–311. doi: 10.1097/WAD.0b013e31820d8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. Mini Mental State Examination. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 19.Morhardt D, Weintraub S, Khayum B, et al. The CARE pathway model for dementia: psychosocial and rehabilitative strategies for care in young-onset dementias. Psychiatr Clin North Am 2015;38(2):333–352. doi: 10.1016/j.psc.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moberg PJ, Rick JH. Decision-making capacity and competency in the elderly: a clinical and neuropsychological perspective. NeuroRehabilitation 2008;23(5):403–413. doi: 10.3233/NRE-2008-23504 [DOI] [PubMed] [Google Scholar]
- 21.Mackenzie IR, Neumann M. Molecular neuropathology of frontotemporal dementia: insights into disease mechanisms from postmortem studies. J Neurochem 2016;138 suppl 1:54–70. doi: 10.1111/jnc.13588 [DOI] [PubMed] [Google Scholar]
- 22.Woollacott IO, Rohrer JD. The clinical spectrum of sporadic and familial forms of frontotemporal dementia. J Neurochem 2016;138 suppl 1:6–31. doi: 10.1111/jnc.13654 [DOI] [PubMed] [Google Scholar]
- 23.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer’s disease in a community-based case series. J Am Geriatr Soc 1999;47(5):564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x [DOI] [PubMed] [Google Scholar]
- 24.Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 2014;137(pt 4):1176–1192. doi: 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manly JJ, Byrd DA, Touradji P, Stern Y. Acculturation, reading level, and neuropsychological test performance among African American elders. Appl Neuropsychol 2004;11(1):37–46. doi: 10.1207/s15324826an1101_5 [DOI] [PubMed] [Google Scholar]
- 26.Yassuda MS, da Silva HS, Lima-Silva TB, et al. Normative data for the Brief Cognitive Screening Battery stratified by age and education. Dement Neuropsychol 2017;11(1):48–53. doi: 10.1590/1980-57642016dn11-010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin KP. Measuring behavior and social cognition in FTLD. Adv Exp Med Biol 2021;1281:51–65. doi: 10.1007/978-3-030-51140-1_4 [DOI] [PubMed] [Google Scholar]
- 28.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weintraub S, Peavy GM, O’Connor M, et al. Three words three shapes: a clinical test of memory. J Clin Exp Neuropsychol 2000;22(2):267–278. doi: 10.1076/1380-3395(200004)22:2;1-1;FT267 [DOI] [PubMed] [Google Scholar]
- 30.Weintraub S, Rogalski E, Shaw E, et al. Verbal and nonverbal memory in primary progressive aphasia: the three words-three shapes test. Behav Neurol 2013;26(1-2):67–76. doi: 10.3233/BEN-2012-110239 [DOI] [PMC free article] [PubMed] [Google Scholar]


