Abstract
Background
Hospitalized lung transplant (LT) recipients (LTRs) have higher post-LT morbidity and mortality than those who are well enough to wait for transplant at home. Outcomes after LT for COVID-19-associated acute respiratory distress syndrome (CARDS) may be even worse; thus, we compared post-LT outcomes between hospitalized LTRs transplanted for CARDS and those transplanted for restrictive lung disease (RLD).
Methods
Between 2014 and 2021, hospitalized LTRs ≥18 years old with CARDS or RLD were included. Primary and secondary outcomes were 1-year post-LT survival and postoperative morbidity. For each patient in the CARDS group, an analysis of 1-to-1 matched patients from the RLD group was performed using logistic regression modeling.
Results
Of 764 LTRs, 163 (21.3%) were hospitalized at the time of LT; 132 met the inclusion criteria: 11 (8.3%) were transplanted for CARDS and 121 (91.7%) for RLD. LTRs with CARDS were younger with longer pre-LT hospitalization stays and higher rates of pretransplant mechanical ventilation, dialysis, and ECMO as a bridge to transplant. A propensity-matched analysis demonstrated comparable rates of intrathoracic adhesions, posttransplant duration of mechanical ventilation, PGD3 at 72 hours, and delayed chest closure. Compared to LTRs with RLD, those with CARDS had significantly longer posttransplant hospital stays and a higher prevalence of ACR ≥A2 and DSA >2000 MFI, but comparable 1-year survival rates.
Conclusion
Even with careful selection, LT for patients with CARDS was associated with significant morbidity; however, 1-year survival of recipients with CARDS was comparable to that of matched hospitalized recipients with RLD.
KEYWORDS: lung transplant; inpatient; COVID-19; SARS-CoV-2; UNOS group D, restrictive lung disease
Lung transplantation (LT) can be lifesaving for patients with end-stage lung diseases. Historically, LT was rarely considered for patients with acute respiratory distress syndrome (ARDS) in the absence of preexisting lung disease due to limitations in transplant evaluation, the presence of critical illness, and significant extrapulmonary complications.1 The ongoing coronavirus disease 2019 (COVID-19) pandemic has led to nearly 6 million deaths worldwide, and the mortality from COVID-19 is driven by severe ARDS, which often requires treatment in an intensive care unit, mechanical ventilation, and, on occasion, extracorporeal membrane oxygenation (ECMO).2 , 3 In addition to respiratory failure, patients with COVID-19–induced ARDS (CARDS) experience other intrathoracic complications such as pneumothorax with bronchopleural fistula, bacterial infections including empyema, pulmonary emboli, and hemothorax. Extrathoracic complications are also common and include delirium, bloodstream infections, renal failure requiring dialysis, malnutrition, secondary sclerosing cholangitis, and debilitating critical illness myopathy and neuropathy. Patients with irreversible lung injury due to COVID-19 depend on LT for survival; however, their LT candidacy is often challenged by critical illness, malnutrition, and extrathoracic complications.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15
In the United States, the lung allocation score (LAS) reflects and prioritizes the urgency and severity of disease for organ allocation.16 Although LT candidates with a high LAS have high healthcare utilization,17 , 18 LT offers a net survival benefit,19 , 20 and these patients are increasingly being accepted for LT.18 Our group has previously demonstrated that candidates with rapid deterioration of preexisting lung disease resulting in acute respiratory failure who are hospitalized at the time of LT have higher post-LT morbidity, but comparable 1-year survival to those who are well enough to remain at home before LT.21 In this study, we compared the clinical outcomes between lung transplant recipients (LTRs) transplanted for CARDS and those transplanted for restrictive lung disease (RLD) who were hospitalized at the time of LT.
Methods
The institutional review board at Norton Thoracic Institute, St. Joseph's Hospital and Medical Center, Phoenix, Arizona approved this retrospective study with waiver of patient consent (PHXU-21-500-137-73-18 dated 3/31/2021), and all patient care was carried out under strict compliance with the International Society for Heart and Lung Transplantation ethics statement. Hospitalized LTRs between January 1, 2014, and August 31, 2021, were included and categorized by the indication for LT: CARDS or RLD. LTRs were excluded for age <18 years and a primary indication for LT other than United Network for Organ Sharing (UNOS) disease group D (restrictive diseases). UNOS group D was chosen due to the physiological similarity of underlying disease to that of CARDS. The primary outcome was 1-year posttransplant survival, and the secondary outcome was postoperative morbidity, including duration of posttransplant mechanical ventilation, primary graft dysfunction grade 3 (PGD3) at 72 hours posttransplant, duration of posttransplant hospital stay, allograft ischemia time, use of intraoperative cardiopulmonary bypass, intraoperative bleeding, ECMO rescue for severe PGD, reintubation before initial hospital discharge, total blood products transfused during the first 2 postoperative weeks, unplanned return to the operating room (OR), and follow-up outcomes including acute cellular rejection and development of de novo donor-specific antibodies (DSAs) with mean fluorescence intensity (MFI) >2000 within 1 year of LT. Unplanned thoracic interventions (decortication/pleurodesis, reexploration for hemothorax evacuation, and sternal hardware repair) and planned surgical interventions during the first posttransplant year were also reviewed in both groups. Due to the large number of subjects in the control group, a propensity-matched analysis was then performed.
All patients were hospitalized at the time of organ offer due to advanced hypoxemia. All LTRs transplanted for CARDS were hospitalized at the time of listing; however, some LTRs transplanted for RLD were listed while outpatient but were subsequently hospitalized before LT. Patients with CARDS were deemed appropriate for LT if they met the following criteria: (1) no evidence of pulmonary recovery despite SARS-CoV-2 clearance, (2) inability to wean from high-flow supplemental oxygen, mechanical ventilation, and/or ECMO for at least 2 months after the onset of ARDS, (3) absence of irreversible nonpulmonary organ dysfunction, and (4) adequate rehabilitation potential.22 None of the patients with CARDS had known lung disease prior to developing COVID-19.
Lung transplant evaluation
Selection of candidates for LT was conducted in compliance with the International Society for Heart and Lung Transplantation guidelines.1 However, LT evaluation was occasionally limited for patients who could not undergo pulmonary function testing due to the presence of a tracheostomy or need for mechanical ventilation, or who were too ill for invasive testing such as endoscopy or colonoscopy.
Statistical analysis
Data were expressed as count (percentage) or median (interquartile range). Nonparametric Kruskal-Wallis test was used to compare continuous variables and Fischer's exact test or chi-square analysis was used to compare categorical variables. Kaplan-Meier method was used to estimate survival and the log-rank test was used to compare survival between the study groups. Although several outcomes of clinical interest were evaluated, we tested each null hypothesis separately rather than using a multi-variate model; therefore, the observed p-value for each hypothesis was reported without adjustment and the prespecified significance level of α was unchanged. All tests were 2-sided with a significance level of 0.05. Analyses were performed with Stata Statistical Software, Release 13 (Stata Corp College Station).
One-to-one (1:1) propensity score matching was performed using a logistic regression model with nearest-neighbor method without replacement. Selection of 1-to-1 matched control patients was processed through maximized execution performance without a caliper. For each hospitalized patient transplanted for CARDS, a matched patient from the control group of hospitalized patients transplanted for RLD was selected based on age, gender, ECMO as a bridge to transplant, and pretransplant hospital stay duration with a match tolerance range of 0.002 to 0.4. Imbalances in matched variables were assessed with standardized mean difference, and a Cohen's d value of 0.2, 0.5, and 0.8 was set as measures of small, medium, and large effect sizes, respectively.23 Propensity-matched analysis was performed using IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. released 2015 and R package 3.1.0 with “psmatching3.03” extension bundle (R Foundation for Statistical Computing).
Results
Of 764 LTs during the study period at our institution, 163 were hospitalized at the time of transplant (21.3%). Patients were excluded for age <18 years (n = 2) and a primary indication for LT other than UNOS disease group D (n = 29: chronic obstructive pulmonary disease, n = 17; cystic fibrosis, n = 8 and pulmonary vascular disease, n = 4). A total of 132 inpatient transplant recipients met the inclusion criteria and were divided into 2 groups: CARDS (8.3%, n = 11) and RLD (91.7%, n = 121).
Baseline characteristics
LTRs transplanted for CARDS or RLD were comparable in terms of gender, body mass index at listing, LAS, pulmonary artery pressure, and pulmonary capillary wedge pressure (Table 1 ). The prevalence of candidates with calculated panel reactive antibodies (cPRA) class I trended higher in the CARDS group than in the RLD group, but the difference was not statistically significant (cPRA class I >1%: 54.5% vs 27.8%, p = 0.065; cPRA class I >50%: 18.2% vs 4.3%, p = 0.056). The prevalence of candidates with cPRA class II was comparable between the CARDS and RLD groups (cPRA class II >1%: 33.3% vs 22.4%, p = 0.457; cPRA class II >50%: 11.1% vs 5.6%, p = 0.505). Of note, 4 LTRs in the RLD group had antibodies against human leucocyte antigens class-II, but cPRA could not be calculated; these 4 patients were excluded from the analysis.
Table 1.
Baseline and Preoperative Characteristics in the Study Groups
| Variable | Hospitalized patients transplanted for CARDS N = 11 | Hospitalized patients transplanted for RLD N = 121 | p-valueb |
|---|---|---|---|
| Age at transplant, yearsa | 47 (42.5, 56.7) | 62.4 (56.4, 68.3) | 0.001 |
| Sex, male | 9 (81.8) | 89 (73.6) | 0.548 |
| Body mass index at listing, kg/m a | 28.9 (25.3, 30.2) | 26.9 (23.6, 30.5) | 0.764 |
| Lung allocation scorea | 84.5 (46.5, 88.5) | 81.3 (57.1, 87.3) | 0.918 |
| Underlying lung disease | |||
| COVID-19 ARDS | 11 (100) | 0 (0) | |
| IPF | 0 (0) | 96 (79.3) | |
| Non-IPF RLD | 0 (0) | 25 (20.7) | |
| Calculated panel reactive antibodies, class I (≥1%), yes | 6 (54.5) | 32 (27.8) | 0.065 |
| Calculated panel reactive antibodies, class II (≥1%), yes | 3 (33.3) | 24 (22.4) | 0.457 |
| Calculated panel reactive antibodies, class I (≥50%), yes | 2 (18.2) | 5 (4.3) | 0.056 |
| Calculated panel reactive antibodies, class II (≥50%), yes | 1 (11.1) | 6 (5.6) | 0.505 |
| Interval between initial hospitalization and transplant, daysa | 100 (85, 121) | 14 (9, 21) | <0.001 |
| Interval between listing and transplant, daysa | 5 (4, 11) | 5 (3, 11) | 0.482 |
| Mean pulmonary artery pressure, mm Hga | 25.5 (22, 36.5) | 25.0 (19, 34) | 0.770 |
| Pulmonary capillary wedge pressure, mm Hga | 11 (8, 16) | 9 (5, 14) | 0.221 |
| Cardiac output, l/mina | 7.35 (6.3, 8.7) | 5.5 (4.8, 6.6) | 0.013 |
| Ventilator support just prior to transplant, yes | 10 (90.9) | 42 (34.7) | <0.001 |
| Vasopressor dependence immediately before transplant, yes | 8 (72.7) | 47 (38.8) | 0.054 |
| ECMO as bridge to transplant | 8 (72.7) | 34 (28.1) | 0.002 |
| Dialysis immediately before transplant, yes | 1 (9.1) | 1 (0.8) | 0.032 |
Abbreviations: CARDS, COVID-19 associated acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; IPF, idiopathic pulmonary fibrosis; LT, lung transplant; RLD, restrictive lung disease.
Bold font indicates statistical significance (p <0.05).
Data expressed as count (percentage) unless otherwise specified.
data expressed as median (interquartile range);
nonparametric Kruskal-Wallis test for equal medians for continuous variables. Fischer's exact test for equal proportions for binary variables. Wald test based on generalized estimating equations for categorical variables with more than 2 levels; the unknown category, if present, was excluded from the testing procedure.
Compared to LTRs with RLD, those with CARDS were significantly younger (47 years vs 62.4 years, p = 0.001), were hospitalized for a longer duration before transplant (100 days vs 14 days, p < 0.001), had a higher cardiac output (7.35 L vs 5.5 L, p = 0.013), and were more likely to require pretransplant mechanical ventilation (90.9% vs 34.7%, p < 0.001), dialysis (9.1% vs 0.8%, p = 0.032), and ECMO as a bridge to transplant (72.7% vs 28.1%, p = 0.002). The time on the waitlist and vasopressor dependence before transplant were comparable in the 2 groups.
Perioperative outcomes
Compared to LTRs transplanted for RLD, those transplanted for CARDS were more likely to have severe adhesions intraoperatively (72.7% vs 28.4%, p = 0.003), to have their chest left open postoperatively (90.9% vs 35.8%, p < 0.001), to require mechanical ventilation >5 days (72.7% vs 41.3%, p = 0.045), to have PGD3 at 72 hours (36.4% vs 9.9%, p = 0.017), and to have a longer post-LT hospital stay (47 days vs 23 days, p = 0.003). However, both groups were comparable in terms of allograft ischemic time, use of intraoperative cardiopulmonary bypass, intraoperative bleeding, ECMO rescue for severe PGD, total blood products transfused during first 2 postoperative weeks, unplanned return to the OR, and prevalence of early complications such as airway dehiscence, sternal dehiscence or osteomyelitis, chest wall complications, stroke, and need for dialysis (Table 2 ). Disposition at discharge was also comparable between the 2 groups; all LTRs with CARDS and 62% with RLD required inpatient rehabilitation (p = 0.093).
Table 2.
Perioperative Outcomes in the Study Groups
| Variable | Hospitalized patients transplanted for CARDS N = 11 | Hospitalized patients transplanted for RLD N = 121 | p-valueb |
|---|---|---|---|
| Right lung ischemia time, minutesa | 250.5 (214, 368) | 286 (214.5, 321.5) | 0.965 |
| Left lung ischemia time, minutesa | 221.5 (170, 297) | 240 (204, 295) | 0.795 |
| Intraoperative cardiopulmonary bypass use | 1 (9.1) | 35 (29.9) | 0.142 |
| Severe intrathoracic adhesions, yes | 8 (72.7) | 31 (28.4) | 0.003 |
| Posttransplant duration of mechanical ventilation | 0.045 | ||
| ≤5 days | 3 (27.3) | 71 (58.7) | |
| >5 days | 8 (72.7) | 50 (41.3) | |
| Posttransplant ECMO rescue, yes | 0 (0) | 4 (3.3) | 0.540 |
| Primary graft dysfunction grade 3, 72 hours | 4 (36.4) | 12 (9.9) | 0.017 |
| Intubated at 72 hours, yes | 9 (81.8) | 56 (46.3) | 0.037 |
| Total blood products transfused during first 2 weeks posttransplanta | 11 (8, 17) | 8 (2, 25) | 0.607 |
| Dialysis between transplant and discharge, yes | 2 (18.2) | 11 (9.1) | 0.333 |
| Stroke between transplant and discharge, yes | 0 (0) | 9 (7.4) | 0.349 |
| Chest left open after LT | 10 (90.9) | 39 (35.8) | <0.001 |
| Unplanned return to OR | 2 (18.2) | 16 (13.2) | 0.646 |
| Interval between transplant and unplanned return to OR, daysa | 2 (1, 2) | 3.5 (2, 10) | 0.101 |
| Airway dehiscence | 0 (0) | 1 (0.8) | 0.762 |
| Sternal dehiscence/osteomyelitis | 0 (0) | 11 (10.1) | 0.269 |
| Chest wall complications | 0 (0) | 3 (2.8) | 0.577 |
| Disposition at discharge | 0.093 | ||
| Home | 0 (0) | 41 (33.9) | |
| Skilled nursing facility | 0 (0) | 2 (1.7) | |
| Inpatient rehabilitation | 11 (100) | 75 (62) | |
| In-hospital mortality | 0 (0) | 3 (2.5) | |
| Length of inpatient hospital stay after transplant, days | 47 (26, 61) | 23 (14, 38.5) | 0.003 |
Abbreviations: CARDS, COVID-19 associated acute respiratory distress syndrome, COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; LT, lung transplant; OR, operating room; RLD, restrictive lung disease.
Data expressed as count (percentage) unless otherwise specified.
Bold font indicates statistical significance (p <0.05).
data expressed as median (interquartile range);
nonparametric Kruskal-Wallis test for equal medians for continuous variables. Fischer's exact test for equal proportions for binary variables. Wald test based on generalized estimating equations for categorical variables with more than 2 levels; the unknown category, if present, was excluded from the testing procedure.
Follow-up outcomes and survival
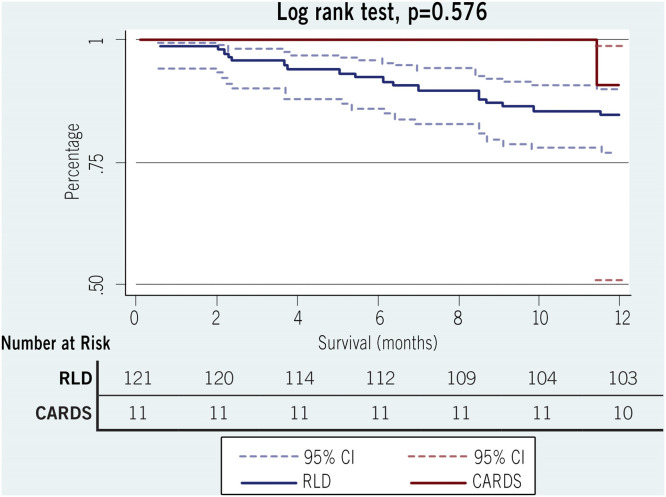
The FEV1 and FVC on the first post-LT pulmonary function test, the FEV1 and FVC at 1-year, and the 30-day readmission rate were comparable between the 2 groups (Table 3 ). Importantly, the number of acute cellular rejection events (≥grade A2) and the prevalence of de novo DSAs (MFI >2000) in the first post-LT year were significantly higher in the CARDS group than in the RLD group (36.4% vs 9.9%, p = 0.015 and 63% vs 23%, 0.017, respectively). However, 30-day mortality, as well as 1-year mortality, was comparable. The probability of survival at 1-year in the CARDS and RLD groups was 90.9% and 85.1%, respectively (p = 0.576; Figure 1 ). Unplanned thoracic interventions after transplant occurred in both groups and included decortication/pleurodesis, operative hemothorax evacuation, sternal hardware repair, thoracic duct ligation, and lobectomy (Table 4 ); chest reexploration for hemothorax was the most common intervention.
Table 3.
Postoperative and 1-year Follow-up Outcomes in the Study Groups
| Variable | Hospitalized patients transplanted for CARDSN = 11 | Hospitalized patients transplanted for RLDN = 121 | p-valueb |
|---|---|---|---|
| % predicted FEV1, first posttransplant PFTa | 53 (40, 60) | 58 (42, 77) | 0.220 |
| % predicted FVC, first posttransplant PFTa | 46 (39, 59) | 53.5 (40, 73) | 0.263 |
| % predicted FEV1, 1-year posttransplanta | 64.5 (55, 78) | 72 (59, 87) | 0.269 |
| % predicted FVC, 1-year posttransplanta | 67.5 (52, 77.5) | 69 (58, 85) | 0.573 |
| ACR within First year posttransplant, yes | 6 (54.5) | 46 (40) | 0.349 |
| ACR events in 1 year, grade A1, yes | 2 (18.2) | 37 (30.6) | 0.312 |
| ACR events in 1 year, ≥ grade A2, yes | 4 (36.4) | 12 (9.9) | 0.015 |
| Number of ACR events in 1 year, totala | 1 (0, 1) | 0 (0, 1) | 0.386 |
| De novo DSA >2000 MFI within 1 year, yes | 5 (62.5) | 16 (22.9) | 0.017 |
| 30-day readmission, yes | 2 (18.2) | 26 (31.3) | 0.461 |
| 30-day mortality, yes | 0 (0) | 1 (0.8) | 0.762 |
| 1-year mortality, yes | 1 (9.1) | 18 (14.9) | 0.601 |
| Survival from transplant with available follow-up, monthsa | 9.3 (7.9, 11.5) | 33.4 (14.5, 60.3) | <0.001 |
Abbreviations: CARDS, COVID-19 associated acute respiratory distress syndrome, ACR, acute cellular rejection; COVID-19, coronavirus disease 2019; DSA, donor-specific antibody; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LT, lung transplant; MFI, mean fluorescence intensity; PFT, pulmonary function test; RLD, restrictive lung disease.
Data expressed as count (percentage) unless otherwise specified.
Bold font indicates statistical significance (p <0.05).
data expressed as median (interquartile range);
nonparametric Kruskal-Wallis test for equal medians for continuous variables. Fischer's exact test for equal proportions for binary variables.
Figure 1.
Kaplan-Meier 1-year survival estimates after inpatient transplant for COVID-19 versus inpatient transplant for restrictive lung disease. Abbreviations: CARDS, COVID-19–associated acute respiratory distress syndrome; RLD, restrictive lung disease; CI, confidence interval.
Table 4.
Thoracic Surgical Interventions in the Study Groups in the First Year After Lung Transplant
| Procedure | Hospitalized patients transplanted for CARDS N = 11 | Hospitalized patients transplanted for RLD N = 121 | p-value |
|---|---|---|---|
| Surgical hemothorax evacuation | 2 (18.2) | 5 (4.1) | 0.046 |
| Decortication and/or pleurodesis | 1 (9.1) | 4 (3.3) | 0.336 |
| Thoracic duct ligation | 0 (0) | 2 (1.6) | 0.667 |
| Anastomotic dehiscence | 0 (0) | 0 (0) | N/A |
| Sternal hardware repair | 0 (0) | 11 (9.1) | 0.296 |
| Lobectomy | 0 (0) | 1 (0.8) | 0.762 |
Abbreviations: CARDS, COVID-19 associated acute respiratory distress syndrome, COVID-19, coronavirus disease 2019; N/A, not applicable; RLD, restrictive lung disease.
Data expressed as count (percentage).
Propensity-matched analysis
A propensity-matched cohort was obtained for 10 of 11 LTRs transplanted for CARDS, which was balanced with those transplanted for RLD. The matched patients in the case and control groups were balanced on age and gender as well as pretransplant hospital stay duration with a small effect size and ECMO as bridge to transplant with a medium effect size (Table 5 ). One patient with CARDS, for whom a match could not be obtained from the control group, was excluded from this analysis.
Table 5.
Variables in the Propensity-Matched Groups
| Variable | Hospitalized LTRs transplanted for CARDS N = 10 | Hospitalized LTRs transplanted forRLD N = 10 | Standardized mean difference (Cohen's d) | p-value |
|---|---|---|---|---|
| Age, years | 49.6 (11.8) | 50.1 (10.8) | 0.04 | 0.992 |
| Gender, male | 8 (80) | 8 (80) | 0.00 | 1.000 |
| ECMO as bridge to transplant, yes | 8 (80) | 6 (60) | 0.44 | 0.329 |
| Pretransplant hospital stay duration, days | 104 (23) | 86.2 (229.9) | 0.11 | 0.811 |
Abbreviations: CARDS, COVID-19 associated acute respiratory distress syndrome, COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; LTR, lung transplant recipient; RLD, restrictive lung disease.
Data expressed as mean (standard deviation) for continuous variables or frequency (proportion) for categorical variables. Effect size measures as small (Cohen's d=0.2), medium (Cohen's d=0.5) and large (Cohen's d=0.8).23
Ten hospitalized LTRs transplanted for CARDS and 10-matched hospitalized LTRs transplanted for RLD had comparable posttransplant outcomes, including prevalence of intrathoracic adhesions, posttransplant duration of mechanical ventilation, primary graft dysfunction at 72 hours, and posttransplant open chest management. The probability of survival at 1-year in the CARDS and RLD groups was also comparable (90% vs 100% respectively, p = 0.317). The propensity-matched analysis confirmed the significantly longer inpatient hospital stay after transplant, higher prevalence of ACR ≥grade A2, and higher prevalence of de novo DSA >2000 MFI in the CARDS group compared to the matched RLD group (Table 6 ).
Table 6.
Posttransplant Outcomes in Propensity-Matched Groups
| Variable | Hospitalized LTRs transplanted for CARDS N = 10 | Hospitalized LTRs transplanted for RLD N = 10 | p-valueb |
|---|---|---|---|
| Right lung ischemia time, minutesa | 250.5 (214, 368) | 276.5 (241, 322) | 0.910 |
| Left lung ischemia time, minutesa | 221.5 (170, 297) | 248 (234, 301) | 0.597 |
| Intraoperative cardiopulmonary bypass use | 0 (0) | 2 (20) | 0.136 |
| Severe intrathoracic adhesions, yes | 7 (70) | 4 (44.4) | 0.260 |
| Posttransplant duration of mechanical ventilation | 0.639 | ||
| ≤5 days | 3 (30) | 4 (40) | |
| >5 days | 7 (70) | 6 (60) | |
| Posttransplant ECMO rescue, yes | 0 (0) | 0 (0) | N/A |
| Primary graft dysfunction grade 3, 72 hours | 4 (40) | 1 (20) | 0.439 |
| Intubated at 72 hours, yes | 9 (90) | 4 (80) | 0.591 |
| Total blood products transfused during first 2 weeks posttransplanta | 11 (8, 17) | 29 (3, 42) | 0.252 |
| Dialysis between transplant and discharge, yes | 2 (20) | 1 (10) | 0.531 |
| Stroke between transplant and discharge, yes | 0 (0) | 0 (0) | N/A |
| Chest left open after LT | 7 (70) | 1 (100) | 0.521 |
| Unplanned return to the OR | 2 (20) | 0 (0) | 0.136 |
| Airway dehiscence | 0 (0) | 0 (0) | N/A |
| Disposition at discharge | 0.171 | ||
| Home | 0 (0) | 2 (20) | |
| Skilled nursing facility | 0 (0) | 1 (10) | |
| Inpatient rehabilitation | 10 (100) | 7 (70) | |
| In-hospital mortality | 0 (0) | 0 (0) | |
| Length of inpatient hospital stay after transplant, daysa | 48 (26, 61) | 23.5 (12, 34) | 0.037 |
| % predicted FEV1, first posttransplant PFTa | 51.5 (40, 60) | 49 (36.5, 72) | 0.870 |
| % predicted FVC, first posttransplant PFTa | 45.5 (39, 59) | 49 (33.5, 72.5) | 0.595 |
| % predicted FEV1, 1-year posttransplanta | 64.5 (55, 78) | 71 (64, 81) | 0.413 |
| % predicted FVC, 1-year posttransplanta | 67.5 (52, 77.5) | 73 (68.5, 86) | 0.162 |
| ACR within first year posttransplant, yes | 6 (60) | 4 (40) | 0.371 |
| ACR events in 1 year, grade A1, yes | 2 (20) | 4 (40) | 0.329 |
| ACR events in 1 year, ≥ grade A2, yes | 4 (40) | 0 (0) | 0.025 |
| De novo DSA >2000 MFI within 1 year, yes | 7 (70) | 0 (0) | 0.018 |
| 30-day readmission, yes | 2 (22.2) | 3 (30) | 0.518 |
| 30-day mortality, yes | 0 (0) | 0 (0) | N/A |
| 1-year mortality, yes | 1 (10) | 0 (0) | 0.305 |
Abbreviations: ACR, acute cellular rejection; CARDS, COVID-19 associated acute respiratory distress syndrome, COVID-19, coronavirus disease 2019; DSA, donor-specific antibodies; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; LT, lung transplant; MFI, mean fluorescence intensity; N/A, not applicable; OR, operating room; PFT, pulmonary function test; RLD, restrictive lung disease.
Data expressed as count (percentage) unless otherwise specified.
Bold font indicates statistical significance (p <0.05).
data expressed as median (interquartile range);
nonparametric Kruskal-Wallis test for equal medians for continuous variables. Fischer's exact test for equal proportions for binary variables. Wald test based on generalized estimating equations for categorical variables with more than 2 levels; the unknown category, if present, was excluded from the testing procedure.
Discussion
Appropriate LT candidate selection is paramount to good organ stewardship and recipient survival. Over time, experienced transplant centers have expanded their candidate selection criteria to include hospitalized and even critically ill patients as short- and long-term posttransplant outcomes for these patients have improved.18, 19, 20 Patients with RLD, most commonly idiopathic pulmonary fibrosis, can experience acute exacerbations characterized by worsening dyspnea, hypoxemia, and radiographic progression of disease with ground-glass opacities overlying areas of fibrosis. These patients are typically afflicted with single organ failure, the lungs, but can require extensive support including mechanical ventilation and ECMO, which are associated with development of additional comorbidities such as critical illness myopathy, infection, and bleeding. Historically, patients with ARDS have been excluded from LT, but the COVID-19 pandemic has changed the face of solid organ transplantation, and patients with CARDS who do not recover from lung injury depend on LT for survival. However, these patients are often more severely ill than even the typical hospitalized LT candidate due to prolonged illness, prolonged hospitalization, multi-organ system dysfunction, and iatrogenic complications.
In this comparison of LTRs with comparable LAS scores (median LAS in CARDS and RLD groups: 84.5 vs 81.3, p = 0.918), we have shown that patients transplanted for CARDS have acceptable 1-year survival that is comparable to that of patients transplanted for RLD. Similarly high survival rates have also been reported by other groups. In a retrospective case series from Northwestern University, Kurihara et al10 reviewed the clinical outcomes of 102 consecutive patients transplanted between January 21, 2020, and September 30, 2021, including 30 patients with CARDS. At follow-up, all 30 LTRs with CARDS were alive (median follow-up, 351 days [IQR, 176-555] after transplant) vs 60 non-CARDS patients (83%) (median follow-up, 488 days [IQR, 368-570] after transplant). In another analysis using the UNOS registry, Roach et al13 included 214 patients transplanted for COVID-19–related respiratory failure (ARDS, n = 140; pulmonary fibrosis, n = 74) between August 1, 2020, and September 30, 2021, in the United States. They reported a 30-day mortality of 2.2% (4 deaths) and 3-month survival of 95.6% (95% CI, 90.1 to 98.1), with a median follow-up of 1.9 months (IQR 1.1-5.8).13 Lastly, our group previously reported comparable posttransplant survival between hospitalized LT candidates and those that were waiting for a transplant at home (hazard ratio of death [95% CI] = 0.32 [0.09, 1.02], p = 0.056).21 Although longer follow-up time is needed, these results are encouraging and suggest that LT offers acceptable short-term and 1-year survival for carefully selected patients with irreversible respiratory failure from COVID-19, and their survival mirrors that of LTRs with RLD hospitalized at the time of transplant.
Despite acceptable 1-year survival, LT candidates with CARDS face a challenging intraoperative course as well as a long and difficult posttransplant recovery. Prior to the pandemic, our group reported that LTRs hospitalized at the time of transplant had higher intraoperative transfusion requirements, a higher incidence of chest reexploration for bleeding, longer posttransplant hospital stays, and higher total, variable, and fixed costs of posttransplant care than LTRs who waited for transplant at home.21 Data from the current study suggest that intraoperative and postoperative care may be even more challenging among hospitalized patients transplanted for CARDS than among those transplanted for RLD in part due to more severe intraoperative adhesions, higher rates of open chest management due to intraoperative hemorrhage, a higher incidence of PGD3 at 72 hours posttransplant, longer durations of mechanical ventilation, and longer posttransplant hospital stays. Similar to our findings, Kurihara et al10 reported that compared to the control group, LTRs with CARDS had a higher prevalence of PGD at 72 hours posttransplant (70% vs 20.8%), longer duration of posttransplant mechanical ventilation (6.5 vs 2 days), longer ICU stay (18 vs 9 days), longer hospital stay (28.5 vs 16 days), and a higher prevalence of extrapulmonary end-organ dysfunction (e.g., permanent hemodialysis [13.3% vs 5.5%]). However, their control group included both LTRs hospitalized at the time of transplant and those that were brought in from home (median LAS: 46.7 [38.9-54.3]; 51.4% had underlying obstructive disease; and very few [<3%] required invasive mechanical ventilation before transplant). Therefore, the higher rates of posttransplant morbidity in the CARDS patients are not surprising given the difference in pretransplant severity of illness. Lastly, in the study by Kurihara et al,10 the development of DSAs and ACR was less common in patients transplanted for CARDS than among those in the control group. This finding was explained by invoking the ‘immune accommodation’ hypothesis, which suggests that lack of DSA development and ACR results from the dilution of antibody titers due to high intraoperative blood loss and increased blood transfusion. In contrast, with 1-year follow-up of the entire cohort as well as that of a propensity-matched cohort, we found a higher incidence of de novo DSA development and ACR (≥grade A2) in patients transplanted for CARDS, which speaks to the need for larger studies, a detailed analysis of posttransplant immunosuppression, and longer posttransplant follow-up.
Although uncommon prior to the COVID-19 pandemic, LT for postviral severe ARDS has also been previously described.24 , 25 Frick et al25 reported outcomes of 13 LTRs from 3 high-volume centers in the Eurotransplant region over a period of 22 years. The majority had postinfluenza ARDS, 11 of 13 (84.6%) were bridged to transplant with ECMO, 3 of 13 (23.1%) had PGD3 at 72 hours posttransplant, and 9 of 13 (69.2%) had perioperative complications including hemorrhage, thrombosis, and sternal dehiscence; the series also reported prolonged posttransplant mechanical ventilation (median 33 days), prolonged posttransplant hospital stay (median 54 days), and reduced 1-year survival (71.6%). These findings suggest that perioperative and postoperative challenges of postviral ARDS are similar irrespective of the underlying viral etiology; however, the posttransplant 1-year survival for CARDS may be higher than that for other viral insults. This difference in survival is unlikely to be related to the underlying viral infection, as the acute infection had resolved at the time of transplant, but much more to do with candidate selection and perioperative and postoperative complications. Furthermore, we found that the technical complexity of the operation may in fact be similar in hospitalized LTRs with CARDS and those with RLD, as evidenced by comparable ischemia times and similar rates of ECMO rescue, blood transfusions, unplanned returns to the OR, and airway and chest wall complications between the 2 groups. Lastly, thoracic surgeons should anticipate a significant pleural adhesive burden in LTRs with CARDS.
Although LT for CARDS can be lifesaving for patients with irreversible lung injury, the operation is challenging, the recovery is arduous, and hospital resource utilization is high. These factors speak to the need for meticulous candidate selection with prioritization of patients without irreversible extrapulmonary organ dysfunction but with good rehabilitation potential, devoted caregiver support, and a steadfast commitment to recovery and long-term medical adherence. In light of the psychiatric and musculoskeletal complications endured by patients with CARDS, our center incorporates a detailed psychosocial assessment into our pretransplant evaluation. This assessment focuses on the patients’ motivation and drive, as well as treatment of an underlying or newly developed psychiatric disorder that may be interfering with his/her ability to participate in rehabilitation. We also, on occasion, attempt to differentiate between critical illness myopathy and neuropathy in the weakest patients via electromyography as the latter tends to have a worse prognosis and confer a more prolonged/incomplete recovery.26 With few exceptions, we now require that eligible transplant candidates with CARDS are able to stand and attempt to march in place prior to transplantation as a marker of musculoskeletal reserve and rehabilitation potential. Lastly, in the current COVID-19 era, CARDS is largely preventable through vaccination; at this time, it is not known whether vaccine hesitancy pretransplant is associated with medication nonadherence posttransplant.
In summary, compared to LTRs with RLD hospitalized at the time of transplant, patients transplanted for CARDS were significantly younger, had longer hospital stays before and after LT, and were more likely to require mechanical ventilation, ECMO support, and hemodialysis before LT. They also had more challenging operations with extensive intrathoracic adhesions, a greater need for open-chest management postoperatively due to a high risk of ongoing bleeding, and higher rates of PGD3 at 72 hours posttransplant. In addition, ACR ≥ grade A2 and de novo DSAs in the first year after transplant were also more common among patients transplanted for CARDS. However, the 2 groups had comparable allograft ischemia times, perioperative blood transfusions, rates of unplanned return to the OR, ECMO rescue rates, reintubation rates, postoperative complications, 30-day readmissions, and 1-year survival. Although this study is limited by its small sample size and retrospective approach, it confirms the experience from another large transplant center that LT is a feasible and lifesaving option for select patients with irreversible respiratory failure due to CARDS. Notably, the challenges in the posttransplant care of these patients highlight the need for an aggressive and focused approach toward musculoskeletal recovery with a close partnership with inpatient physical, occupational, and speech therapy and access to an experienced rehabilitation center.
In conclusion, even among carefully selected patients with CARDS, LT was associated with significant preoperative, intraoperative, and postoperative morbidity. Despite these challenges, LT conferred a good 1-year survival that was comparable to that of LTRs with RLD hospitalized at the time of transplant. Longer follow-up is needed to further understand the long-term prognosis of this novel patient population.
Author contributions
ST, MS, DR, RB and RW made substantial contributions to the conception or design of the work, drafting the work or revising it critically for important intellectual content, and final approval of the version to be published in accordance with ICMJE guidelines. MO, KG, DR and ST worked on the acquisition of the data, DR contributed to analysis, and ST, DR, MO, and MS to the interpretation of data for the work. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
The authors thank Kristine Nally for editorial assistance and Marco Marchionni for graphic design.
Disclosure statement
No outside funding was obtained for this study. None of the authors have conflicts of interest to declare.
Footnotes
Presentation information: These data are accepted for mini-oral presentation at the annual meeting and scientific sessions of the International Society for Heart and Lung Transplantation, April 27-30, Boston, MA, USA.
IRB approval: PHXU-21-500-137-73-18 dated March 31, 2021, with annual renewal.
References
- 1.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2021;40:1349–1379. doi: 10.1016/j.healun.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Tonetti T, Protti A, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrickson KW, Peltan ID, Brown SM. The epidemiology of acute respiratory distress syndrome before and after coronavirus disease 2019. Crit Care Clin. 2021;37:703–716. doi: 10.1016/j.ccc.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharat A, Machuca TN, Querrey M, et al. Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med. 2021;9:487–497. doi: 10.1016/S2213-2600(21)00077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharat A, Querrey M, Markov NS, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abe4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JY, Qiao K, Liu F, et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin Med J (Engl) 2020;133:1390–1396. doi: 10.1097/CM9.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandes LM, Pêgo-Fernandes PM. New challenges for lung transplantation in the era of COVID-19. Sao Paulo Med J. 2022;140:1–4. doi: 10.1590/1516-3180.2022.140126082021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glorion M, De Wolf J, Zuber B, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome: the first French patient. Respir Med Res. 2021;80 doi: 10.1016/j.resmer.2021.100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han W, Zhu M, Chen J, et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann Surg. 2020;272:e33–e34. doi: 10.1097/SLA.0000000000003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurihara C, Manerikar A, Querrey M, et al. Clinical characteristics and outcomes of patients with COVID-19-associated acute respiratory distress syndrome who underwent lung transplant. JAMA. 2022;327:652–661. doi: 10.1001/jama.2022.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang C, Jaksch P, Hoda MA, et al. Lung transplantation for COVID-19-associated acute respiratory distress syndrome in a PCR-positive patient. Lancet Respir Med. 2020;8:1057–1060. doi: 10.1016/S2213-2600(20)30361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh DK, Hong SB, Kim HC, et al. Experience of international air transportation and subsequent lung transplant in a patient with COVID-19-associated acute respiratory distress syndrome: a case report. J Korean Med Sci. 2021;36:e123. doi: 10.3346/jkms.2021.36.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach A, Chikwe J, Catarino P, et al. Lung transplantation for Covid-19-related respiratory failure in the United States. N Engl J Med. 2022;386:1187–1188. doi: 10.1056/NEJMc2117024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford Medicine News Center Double transplant at Stanford saves life of critically ill COVID-19 patient. Standard Medicine News Center. 2021.
- 15.Vanderbilt University Medical Center COVID patient's heart-lung transplant is world's first. VUMC Reporter. 2020.
- 16.Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6(5 Pt 2):1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 17.Arnaoutakis GJ, Allen JG, Merlo CA, et al. Impact of the lung allocation score on resource utilization after lung transplantation in the United States. J Heart Lung Transplant. 2011;30:14–21. doi: 10.1016/j.healun.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller CA, Gonwa TA, White LJ, et al. Utilization and cost analysis of lung transplantation and survival after 10 years of adapting the lung allocation score. Transplantation. 2019;103:638–646. doi: 10.1097/TP.0000000000002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg. 2011;141:1270–1277. doi: 10.1016/j.jtcvs.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2019 annual data report: lung. Am J Transplant. 2021;21(2):441–520. doi: 10.1111/ajt.16495. Suppl. [DOI] [PubMed] [Google Scholar]
- 21.Olson MT, Elnahas S, Roy SB, et al. Inpatient lung transplant evaluation is associated with increased risk of morbidity, mortality, and cost of care after transplant. Prog Transplant. 2021;31:219–227. doi: 10.1177/15269248211024612. [DOI] [PubMed] [Google Scholar]
- 22.Schaheen L, Bremner RM, Walia R, Smith MA. Lung transplantation for coronavirus disease 2019 (COVID-19): the who, what, where, when, and why. J Thorac Cardiovasc Surg. 2022;163:865–868. doi: 10.1016/j.jtcvs.2021.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Y, Lee SO, Shim TS, et al. Lung transplantation as a therapeutic option in acute respiratory distress syndrome. Transplantation. 2018;102:829–837. doi: 10.1097/TP.0000000000002004. [DOI] [PubMed] [Google Scholar]
- 25.Frick AE, Gan CT, Vos R, et al. Lung transplantation for acute respiratory distress syndrome: a multicenter experience. Am J Transplant. 2022;22:144–153. doi: 10.1111/ajt.16759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guarneri B, Bertolini G, Latronico N. Long-term outcome in patients with critical illness myopathy or neuropathy: the Italian multicentre CRIMYNE study. J Neurol Neurosurg Psychiatry. 2008;79:838–841. doi: 10.1136/jnnp.2007.142430. [DOI] [PubMed] [Google Scholar]