Abstract
Objective
One of the causes of hypertension is a genetic factor. The purpose of this study was to look at the relationship between apolipoprotein E (APOE) and methylenetetrahydrofolate reductase (MTHFR) polymorphisms and essential hypertension in the Hakka population.
Methods
The study included 2,850 patients with hypertension and 2,034 controls. APOE rs429358, rs7412, and MTHFR rs1801133 were genotyped by polymerase chain reaction (PCR)-microarray. The differences in these polymorphisms between the two groups were analyzed.
Results
The genotype and allele frequency of APOE and MTHFR polymorphisms did not differ significantly between hypertensive patients and controls. Patients with hypertension who were APOE rs429358C/C homozygous had higher TG, TC, LDL-C, and Apo-B levels, whereas patients with the T/T genotype had higher HDL-C levels. Patients with hypertension who were APOE rs7412T/T homozygous had higher TG and TC levels and lower LDL-C and Apo-B levels. Homocysteine (Hcy) levels in patients with MTHFR CC, CT, and TT genotypes were increased, while patients with the TT genotype and T allele had higher Hcy levels than those of patients with other genotypes and the C allele. The APOE rs7412T/T genotype in the co-dominant model (APOE rs7412T/T vs. C/C) (gender-, age-, smoking-, and drinking-adjusted OR 2.682, 95% CI, 1.072–6.710, P=0.035) was a significant risk factor for hypertension. The APOE rs429358 and MTHFR rs1801133 genotypes in co-dominant, dominant, and recessive models were not significant risk factors for hypertension.
Conclusions
It supports that APOE polymorphisms are related to hypertension in the Hakka population. Specifically, the APOE rs7412T/T genotype may be a risk factor for hypertension.
1. Introduction
Hypertension is one of the leading preventable risk factors for certain diseases [1, 2]. Hypertension is a chronic disease characterized by elevated blood pressure in the arteries of the systemic circulation [3–5]. Hypertension is the most prevalent risk factor for some diseases worldwide, affecting 1.39 billion people worldwide [6]. It is predicted that, by 2025, there will be 1.56 billion hypertensive patients all over the world [7]. Between October 2012 and December 2015, 23.2% (about 244.5 million) of the Chinese adults had hypertension and another 41.3% (about 435.3 million) had prehypertension [8]. Although awareness, control, and treatment rates in China have significantly improved, the prevalence of hypertension continues to rise [8]. Hypertension has become a major disease burden in China.
The pathogenesis of hypertension remains unclear. In recent years, scholars have carried out in-depth studies on the regulatory mechanisms underlying the occurrence and development of hypertension, and there are many possible mechanisms, including macrophage polarization [9], gene regulation [10, 11], renin-angiotensin-aldosterone system and sympathetic nervous system activation [12], central nervous system dysfunction [13], and renal damage [14]. The etiology of hypertension involves a complex interplay of environmental and genetic factors [15]. There is a significant genetic predisposition to hypertension, with genetic factors accounting for 30% to 50% of hypertension risks [16]. With the development of molecular biology techniques, the genetic predisposition to hypertension has been studied, but it still needs to be further elucidated.
Lipid levels have been linked to the risk of hypertension. A study has shown that serum triglyceride levels are significantly related to the development of hypertension [17]. High levels of TC and LDL-C are related to hypertension [18]. In addition, apolipoprotein E (ApoE) polymorphisms are associated with plasma lipoproteins [19, 20]. ApoE is one of the important apolipoproteins in plasma that binds to lipids and ApoE receptors (including LDLR and VLDLR), participates in lipid metabolism, and regulates cholesterol balance [21, 22]. ApoE is encoded by the APOE gene. There are two common polymorphisms in the APOE gene: rs429358 (388T>C, Cys112Arg) and rs7412 (526C>T, Arg158Cys), which result in three major alleles (ɛ2(388T–526T), ɛ3(388T–526C), and ɛ4(388C–526C)) [22, 23].
The level of serum homocysteine (Hcy) is related to the incidence of hypertension [24]. Hcy is a sulfur-containing amino acid that can damage blood vessels [25]. Hyperhomocysteinemia is associated with the incidence of hypertension and significantly increases the risk of vascular disease [26, 27]. MTHFR is a key enzyme in Hcy metabolism [28]. MTHFR is encoded by the MTHFR gene, and MTHFR activity is closely related to MTHFR gene polymorphisms. The most important mutation of the MTHFR gene is C677T (SNP rs1801133 and Ala222Val), which can reduce MTHFR activity and produce heat intolerance [29]. The relationship between the MTHFR gene polymorphism and hypertension remains controversial [30]. In the present study, the relationship between APOE and MTHFR polymorphisms and hypertension was analyzed in a Hakka population.
2. Materials and Methods
2.1. Subjects
Between April 2016 and December 2020, 2,850 consecutive inpatients with clinically diagnosed hypertension and 2,034 non-hypertensive controls were retrospectively recruited from Meizhou People's Hospital in China. Inclusive criteria for hypertensive patients were the following: (1) Clinically diagnosed with hypertension. (2) Age ≥16 years old. Age, sex, smoking history, alcoholism history, medical history, and serum lipid levels of each subject were recorded. The control group consisted of healthy people who did not have hypertension.
2.2. DNA Extraction and Genotyping
The genomic DNA was extracted from blood samples using a DNA Blood Mini Kit (Qiagen GmbH, Germany). APOE- and MTHFR-related polymorphisms were amplified by polymerase chain reaction (PCR). The APOE gene was amplified using PCR at 50°C for 2 minutes, 95°C for 15 minutes, and 45 thermal cycles (94°C for 30 s and 65°C for 45 s) (Sinochips Bioscience Co., Ltd., Zhuhai, Guangdong, China). The MTHFR gene was amplified using PCR at 94°C for 5 minutes, followed by 35 thermal cycles (94°C for 25 s, 56°C for 25 s, and 72°C for 25 s) (BaiO Technology Co, Ltd, Shanghai, China). The PCR products were hybridized with wild-type or mutant probes fixed on the chip, and the genotypes of the samples were determined by the hybridization reaction.
2.3. Serum Lipid and Plasma Catecholamine Measurements
Serum lipid levels of the samples were evaluated by an Olympus AU5400 system (Olympus Corporation, Tokyo, Japan). Total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoprotein A1 (Apo-A1), and apolipoprotein B (Apo-B) analyses were performed. Enzyme-linked immunosorbent assay (ELISA) kits (Elabscience Biotechnology Co., Ltd, Wuhan, China) were used for evaluating the concentrations of epinephrine, norepinephrine, and dopamine.
2.4. Statistical Analysis
The SPSS statistical software version 21.0 (IBM Inc., USA) was used for data analysis. The measurement data were compared using either Student's t-test or the Mann–Whitney U test. Genotype composition ratios and allele frequencies between groups were analyzed by the Chi-square test. Logistic regression analysis was applied to examine the relationship between gene polymorphisms and hypertension.
3. Results
3.1. Characteristics of Subjects
This study included 4,884 participants. They were included in this study. 2,850 hypertensive patients (1,761 are men and 1,089 are women) and 2,034 controls (1,399 are men and 635 are women) were enrolled. The average age was 67.62 ± 11.76 years and 65.39 ± 13.04 years in hypertensive patients and controls, respectively. There were statistically significant differences in the percentage of subjects with a history of smoking (P < 0.001) and the percentage of subjects with a history of alcoholism (P < 0.001). The Hcy (P < 0.001), TG (P=0.008), TC (P < 0.001), LDL-C (P=0.001), Apo-A1 (P < 0.001), and Apo-B (P < 0.001) levels in the hypertensive subjects were higher than those in the controls (Table 1).
Table 1.
Clinical characteristics of hypertensive patients and control participants.
| Total (n = 4,884) | Hypertensive patients (n = 2,850) | Controls (n = 2,034) | P values | |
|---|---|---|---|---|
| Age, years | 66.69 ± 12.36 | 67.62 ± 11.76 | 65.39 ± 13.04 | <0.001 |
|
| ||||
| Gender | ||||
| Male, n(%) | 3160 (64.70) | 1761 (61.79) | 1399 (68.78) | <0.001 |
| Female, n(%) | 1724 (35.30) | 1089 (38.21) | 635 (31.22) | |
| History of smoking, n(%) | 1361 (27.87) | 694 (24.35) | 667 (32.79) | <0.001 |
| History of alcoholism, n(%) | 309 (6.33) | 132 (4.63) | 177 (8.70) | <0.001 |
| SBP, mmHg | 141.78 ± 27.02 | 150.96 ± 25.67 | 128.92 ± 23.38 | <0.001 |
| DBP, mmHg | 82.55 ± 15.64 | 86.27 ± 15.56 | 77.35 ± 14.22 | <0.001 |
| Hcy, μmol/L | 16.57 ± 7.97 | 16.95 ± 7.96 | 16.04 ± 7.97 | <0.001 |
| TG, mmol/L | 1.77 ± 1.73 | 1.82 ± 1.65 | 1.69 ± 1.82 | 0.008 |
| TC, mmol/L | 4.88 ± 1.41 | 4.95 ± 1.34 | 4.78 ± 1.50 | <0.001 |
| HDL-C, mmol/L | 1.27 ± 0.39 | 1.28 ± 0.38 | 1.25 ± 0.41 | 0.015 |
| LDL-C, mmol/L | 2.72 ± 0.98 | 2.77 ± 0.95 | 2.67 ± 1.03 | 0.001 |
| Apo-A1, g/L | 1.10 ± 0.33 | 1.13 ± 0.32 | 1.07 ± 0.34 | <0.001 |
| Apo-B, g/L | 0.85 ± 0.29 | 0.86 ± 0.28 | 0.82 ± 0.30 | <0.001 |
3.2. Frequencies of APOE rs429358, rs7412, and MTHFR rs1801133 Genotypes and Alleles in Hypertensive Patients and Controls
The frequencies of APOE rs429358, rs7412, and MTHFR rs1801133 genotypes and alleles were compared between hypertensive patients and nonhypertensive controls. The genotype distributions of APOE rs429358, rs7412, and MTHFR rs1801133 in hypertensive patients (χ2 = 0.389, P=0.533; χ2 = 3.340, P=0.068 and χ2 = 0.030, P=0.863) and controls (χ2 = 0.412, P=0.521; χ2 = 1.683, P=0.195 and χ2 = 0.0002, P=0.988) were consistent with Hardy–Weinberg equilibrium, respectively. It was found that there was no significant difference in the distribution of genotypes and alleles of APOE rs429358 and rs7412 between hypertensive patients and controls (P > 0.05). The same result was observed in the MTHFR rs1801133 gene (Table 2).
Table 2.
Frequencies of APOE rs429358, rs7412, and MTHFR rs1801133 genotypes and alleles in hypertensive patients and controls.
| Genotype/allele | Hypertensive patients (n = 2,850) | Controls (n = 2,034) | χ 2 | P value | |
|---|---|---|---|---|---|
| APOE rs429358 | |||||
| T/T | 2320 (81.40%) | 1633 (80.29%) | 0.985 | 0.609 | |
| T/C | 506 (17.75%) | 382 (18.78%) | |||
| C/C | 24 (0.84%) | 19 (0.93%) | |||
| T | 5146 (90.28%) | 3648 (89.68%) | 0.968 | 0.338 | |
| C | 554 (9.72%) | 420 (10.32%) | |||
| HWE (χ2, P) | χ 2 = 0.389, P=0.533 | χ 2 = 0.412, P=0.521 | |||
|
| |||||
| APOE rs7412 | |||||
| C/C | 2464 (86.46%) | 1758 (86.43%) | 4.391 | 0.111 | |
| C/T | 365 (12.81%) | 270 (13.27%) | |||
| T/T | 21 (0.74%) | 6 (0.29%) | |||
| C | 5293 (92.86%) | 3786 (93.07%) | 0.157 | 0.718 | |
| T | 407 (7.14%) | 282 (6.93%) | |||
| HWE (χ2, P) | χ 2 = 3.340, P = 0.068 | χ 2 = 1.683, P=0.195 | |||
|
| |||||
| MTHFR rs1801133 | |||||
| C/C | 1573 (55.19%) | 1145 (56.29%) | 0.615 | 0.736 | |
| C/T | 1091 (38.28%) | 762 (37.46%) | |||
| T/T | 186 (6.53%) | 127 (6.24%) | |||
| C | 4237 (74.33%) | 3052 (75.02%) | 0.599 | 0.450 | |
| T | 1463 (25.67%) | 1016 (24.98%) | |||
| HWE (χ2, P) | χ 2 = 0.030, P=0.863 | χ 2 = 0.0002, P=0.988 | |||
HWE, Hardy Weinberg equilibrium.
3.3. Characteristics of Hypertensive Patients Stratified by APOE rs429358 and rs7412 Genotypes, APOE ɛ2, ɛ3, and ɛ4 Alleles, and MTHFR Variants
The differences in characteristics in hypertensive patients stratified by APOE and MTHFR genotypes and alleles were analyzed. Patients with hypertension who were APOE rs429358 C/C homozygous had higher TG levels (2.09 ± 2.12 mmol/L vs. 1.71 ± 1.60 mmol/L in T/T and 1.99 ± 2.18 mmol/L in T/C, P < 0.001), higher TC levels (5.20 ± 1.38 mmol/L vs. 4.85 ± 1.40 mmol/L in T/T and 5.01 ± 1.44 mmol/L in T/C, P=0.003), higher LDL-C levels (3.01 ± 1.02 mmol/L vs. 2.69 ± 0.97 mmol/L in T/T and 2.88 ± 1.01 mmol/L in T/C, P < 0.001), and higher Apo-B levels (0.94 ± 0.34 g/L vs. 0.84 ± 0.28 g/L in T/T and 0.89 ± 0.31 g/L in T/C, P < 0.001), while individuals with the APOE rs429358T/T genotype had higher HDL-C levels (1.28 ± 0.39 mmol/L vs. 1.21 ± 0.39 mmol/L in T/C and 1.23 ± 0.32 mmol/L in C/C, P < 0.001) (Table 3).
Table 3.
Clinical characteristics of subjects stratified by APOE rs429358 and rs7412 genotypes and ɛ2, ɛ3, and ɛ4 alleles.
| Clinical characteristics | rs429358 | rs7412 | APOE alleles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T/T (n = 3,953) | T/C (n = 888) | C/C (n = 43) | T/C+ C/C (n = 931) | C/C (n = 4,222) | C/T (n = 635) | T/T (n = 27) | C/T+ T/T (n = 662) | ɛ2 (n = 587) | ɛ3 (n = 3,366) | ɛ4 (n = 856) | Pvalues | |
| Age, years | 66.92 ± 12.39 | 65.80 ± 12.20∗ | 64.37 ± 12.04 | 65.73 ± 12.19∗∗ | 66.67 ± 12.27 | 66.72 ± 12.83 | 68.67 ± 14.47 | 66.80 ± 12.89 | 67.11 ± 12.58 | 66.88 ± 12.35 | 65.85 ± 11.91 | 0.067 |
| Gender | ||||||||||||
| Male, n(%) | 2547 (64.43) | 583 (65.65) | 30 (69.77) | 613 (65.84) | 2738 (64.85) | 400 (62.99) | 22 (81.48) | 422 (63.75) | 367 (62.52) | 2180 (64.77) | 558 (65.19) | 0.529 |
| Female, n(%) | 1406 (35.57) | 305 (34.35) | 13 (30.23) | 318 (34.16) | 1484 (35.15) | 235 (37.01) | 5 (18.52) | 240 (36.25) | 220 (37.48) | 1186 (35.23) | 298 (34.81) | |
| History of smoking, n(%) | 1101 (27.85) | 247 (27.82) | 13 (30.23) | 260 (27.93) | 1198 (28.38) | 153 (24.09)∗ | 10 (37.04) | 163 (24.62)∗ | 146 (24.87) | 955 (28.37) | 243 (28.39) | 0.209 |
| History of alcoholism, n(%) | 256 (6.48) | 52 (5.86) | 1 (2.33) | 53 (5.69) | 267 (6.32) | 40 (6.30) | 2 (7.41) | 42 (6.34) | 39 (6.64) | 217 (6.45) | 50 (5.84) | 0.771 |
| SBP, mmHg | 142.09 ± 26.98 | 140.61 ± 27.38 | 138.23 ± 22.86 | 140.50 ± 27.18 | 141.81 ± 26.79 | 141.54 ± 28.53 | 142.96 ± 27.36 | 141.60 ± 28.47 | 141.53 ± 28.44 | 142.18 ± 26.72 | 140.36 ± 27.05 | 0.204 |
| DBP, mmHg | 82.69 ± 15.58 | 81.90 ± 15.85 | 83.51 ± 17.02 | 81.98 ± 15.90 | 82.62 ± 15.54 | 82.08 ± 16.35 | 83.81 ± 14.60 | 82.15 ± 16.27 | 82.18 ± 16.40 | 82.78 ± 15.43 | 81.98 ± 15.95 | 0.337 |
| Hcy, μmol/L | 16.64 ± 8.08 | 16.33 ± 7.57 | 15.27 ± 5.16 | 16.28 ± 7.48 | 16.59 ± 7.87 | 16.44 ± 8.72 | 17.56 ± 4.88 | 16.48 ± 8.60 | 16.31 ± 7.62 | 16.70 ± 8.16 | 16.15 ± 6.58 | 0.133 |
| TG, mmol/L | 1.71 ± 1.60 | 1.99 ± 2.18∗∗ | 2.09 ± 2.12 | 2.00 ± 2.18∗∗ | 1.76 ± 1.73 | 1.74 ± 1.64 | 2.74 ± 2.36∗ | 1.79 ± 1.69 | 1.75 ± 1.70 | 1.71 ± 1.58 | 2.00 ± 2.22 | <0.001 |
| TC, mmol/L | 4.85 ± 1.40 | 5.01 ± 1.44∗∗ | 5.20 ± 1.38 | 5.02 ± 1.44∗∗ | 4.93 ± 1.41 | 4.54 ± 1.35∗∗ | 5.24 ± 2.07 | 4.57 ± 1.39∗∗ | 4.57 ± 1.43 | 4.90 ± 1.39 | 5.06 ± 1.46 | <0.001 |
| HDL-C, mmol/L | 1.28 ± 0.39 | 1.21 ± 0.39∗∗ | 1.23 ± 0.32 | 1.21 ± 0.38∗∗ | 1.26 ± 0.39 | 1.28 ± 0.39 | 1.27 ± 0.42 | 1.28 ± 0.39 | 1.29 ± 0.39 | 1.28 ± 0.40 | 1.21 ± 0.38 | <0.001 |
| LDL-C, mmol/L | 2.69 ± 0.97 | 2.88 ± 1.01∗∗ | 3.01 ± 1.02∗ | 2.88 ± 1.01∗∗ | 2.78 ± 0.99 | 2.38 ± 0.89∗∗ | 2.11 ± 0.79∗∗ | 2.37 ± 0.89∗∗ | 2.35 ± 0.90 | 2.75 ± 0.97 | 2.92 ± 1.03 | <0.001 |
| Apo-A1, g/L | 1.12 ± 0.33 | 1.06 ± 0.33∗∗ | 1.07 ± 0.28 | 1.06 ± 0.33∗∗ | 1.10 ± 0.33 | 1.12 ± 0.33 | 1.12 ± 0.26 | 1.12 ± 0.32 | 1.13 ± 0.32 | 1.11 ± 0.33 | 1.06 ± 0.33 | <0.001 |
| Apo-B, g/L | 0.84 ± 0.28 | 0.89 ± 0.31∗∗ | 0.94 ± 0.34∗ | 0.89 ± 0.31∗∗ | 0.86 ± 0.29 | 0.76 ± 0.27∗∗ | 0.67 ± 0.25∗∗ | 0.76 ± 0.27∗∗ | 0.75 ± 0.27 | 0.85 ± 0.28 | 0.90 ± 0.31 | <0.001 |
Compared to the patients with the wild-type genotype, ∗P < 0.05 and ∗∗P < 0.01 for rs429358 and rs7412.
Patients with hypertension who were APOE rs7412T/T homozygous had higher TG levels (2.74 ± 2.36 mmol/L vs. 1.76 ± 1.73 mmol/L in C/C and 1.74 ± 1.64 mmol/L in C/T, P=0.013), higher TC levels (5.24 ± 2.07 mmol/L vs. 4.93 ± 1.41 mmol/L in C/C and 4.54 ± 1.35 mmol/L in C/T, P < 0.001), lower LDL-C levels (2.11 ± 0.79 mmol/L vs. 2.78 ± 0.99 mmol/L in C/C and 2.38 ± 0.89 mmol/L in C/T, P < 0.001), and lower Apo-B levels (0.67 ± 0.25 g/L vs. 0.86 ± 0.29 g/L in C/C and 0.76 ± 0.27 g/L in C/T, P < 0.001) (Table 3).
Subjects with the ε2/ε4 genotype (n = 75, 45 patients and 30 controls) were excluded from the analysis of the relationship between APOE alleles and lipid levels because of the opposite effects of the ε2 and ε4 alleles. Hypertensive patients with the APOE ɛ4 allele had higher TG, TC, LDL-C, and Apo-B levels, and lower HDL-C and Apo-A1 levels (Table 3).
Hcy levels in patients with MTHFR CC, CT, and TT genotypes were increased (15.85 ± 6.59 μmol/L, 16.76 ± 8.18 μmol/L, and 21.74 ± 13.71 μmol/L) (P < 0.001), while hypertensive patients with the TT genotype and T allele (21.74 ± 13.71 μmol/L and 17.48 ± 9.35 μmol/L) had higher Hcy levels than patients with other genotypes and the C allele (15.85 ± 6.59, 16.76 ± 8.18 μmol/L, and 16.22 ± 7.29 μmol/L) (P < 0.001) (Table 4).
Table 4.
Clinical characteristics of subjects stratified by MTHFR rs1801133 genotypes and alleles.
| Clinical characteristics | C/C (n = 2,718) | C/T (n = 1,853) | T/T (n = 313) | P values | C allele (C/C + C/T) (n = 4,571) | T allele (C/T + T/T) (n = 2,166) | P values |
|---|---|---|---|---|---|---|---|
| Age, years | 66.81 ± 12.38 | 66.66 ± 12.31 | 65.81 ± 12.40 | 0.394 | 66.75 ± 12.35 | 66.54 ± 12.32 | 0.505 |
|
| |||||||
| Gender | |||||||
| Male, n(%) | 1753 (64.50) | 1193 (64.38) | 214 (68.37) | 0.377 | 2946 (64.45) | 1407 (64.96) | 0.703 |
| Female, n(%) | 965 (35.50) | 660 (35.62) | 99 (31.63) | 1625 (35.55) | 759 (35.04) | ||
| History of smoking, n(%) | 752 (27.67) | 520 (28.06) | 89 (28.43) | 0.933 | 1272 (27.83) | 609 (28.12) | 0.816 |
| History of alcoholism, n(%) | 170 (6.25) | 119 (6.42) | 20 (6.39) | 0.977 | 289 (6.32) | 139 (6.42) | 0.915 |
| SBP, mmHg | 141.80 ± 27.63 | 141.73 ± 26.68 | 141.99 ± 23.52 | 0.987 | 141.77 ± 27.25 | 141.77 ± 26.25 | 0.999 |
| DBP, mmHg | 82.23 ± 15.69 | 82.83 ± 15.73 | 83.67 ± 14.62 | 0.190 | 82.48 ± 15.71 | 82.95 ± 15.57 | 0.243 |
| Hcy, μmol/L | 15.85 ± 6.59 | 16.76 ± 8.18 | 21.74 ± 13.71 | <0.001 | 16.22 ± 7.29 | 17.48 ± 9.35 | <0.001 |
| TG, mmol/L | 1.77 ± 1.85 | 1.76 ± 1.58 | 1.73 ± 1.51 | 0.904 | 1.77 ± 1.74 | 1.76 ± 1.57 | 0.781 |
| TC, mmol/L | 4.91 ± 1.44 | 4.84 ± 1.38 | 4.89 ± 1.30 | 0.301 | 4.88 ± 1.42 | 4.85 ± 1.37 | 0.366 |
| HDL-C, mmol/L | 1.27 ± 0.40 | 1.26 ± 0.39 | 1.26 ± 0.35 | 0.595 | 1.27 ± 0.40 | 1.26 ± 0.38 | 0.518 |
| LDL-C, mmol/L | 2.74 ± 0.99 | 2.71 ± 0.98 | 2.74 ± 0.95 | 0.536 | 2.72 ± 0.99 | 2.71 ± 0.97 | 0.609 |
| Apo-A1, g/L | 1.11 ± 0.34 | 1.10 ± 0.33 | 1.11 ± 0.29 | 0.719 | 1.10 ± 0.33 | 1.10 ± 0.33 | 0.819 |
| Apo-B, g/L | 0.85 ± 0.29 | 0.84 ± 0.29 | 0.86 ± 0.29 | 0.678 | 0.85 ± 0.29 | 0.85 ± 0.29 | 0.904 |
3.4. Association of APOE rs429358, rs7412, and MTHFR rs1801133 Polymorphisms with Hypertension
The association between APOE rs429358 genotypes and hypertension was studied using three genetic modes: co-dominant mode (T/C vs. T/T, C/C vs. T/T), dominant mode (T/C plus C/C vs. T/T), and recessive mode (C/C vs. T/T plus T/C). The APOE rs429358 polymorphism in these genetic modes (gender-, age-, smoking-, and drinking-adjusted) was not a significant risk factor for hypertension. In the same way, the APOE rs7412T/T genotype in the co-dominant mode (T/T vs. C/C) (adjusted OR 2.682, 95% CI 1.072–6.710, P=0.035) was a significant risk factor for hypertension, while the MTHFR rs1801133 polymorphism in these genetic modes was not a significant risk factor for hypertension (Table 5).
Table 5.
Association of APOE rs429358, rs7412, and MTHFR rs1801133 polymorphisms with hypertension.
| SNP | Model | Genotype | Hypertension (n = 2,850) | Control (n = 2,034) | Univariate OR (95% CI) | P values | Multivariate OR (95% CI) | P values |
|---|---|---|---|---|---|---|---|---|
| APOE rs429358 | ||||||||
| Co-dominant | T/T | 2320 (81.40%) | 1633 (80.29%) | 1.000 (reference) | ||||
| T/C | 506 (17.75%) | 382 (18.78%) | 0.932 (0.805–1.080) | 0.351 | 0.929 (0.801–1.078) | 0.333 | ||
| C/C | 24 (0.84%) | 19 (0.93%) | 0.889 (0.485–1.628) | 0.703 | 0.901 (0.490–1.657) | 0.738 | ||
| Dominant | T/T | 2320 (81.40%) | 1633 (80.29%) | |||||
| T/C + C/C | 530 (18.60%) | 401 (19.71%) | 0.930 (0.805–1.075) | 0.327 | 0.928 (0.802–1.074) | 0.316 | ||
| Recessive | T/T + T/C | 2826 (99.16%) | 2015 (99.07%) | |||||
| C/C | 24 (0.84%) | 19 (0.93%) | 0.901 (0.492–1.649) | 0.734 | 0.918 (0.500–1.689) | 0.784 | ||
|
| ||||||||
| APOE rs7412 | ||||||||
| Co-dominant | C/C | 2464 (86.46%) | 1758 (86.43%) | 1.000 (reference) | ||||
| C/T | 365 (12.81%) | 270 (13.27%) | 0.965 (0.815–1.142) | 0.675 | 0.950 (0.802–1.127) | 0.559 | ||
| T/T | 21 (0.74%) | 6 (0.29%) | 2.497 (1.006–6.200) | 0.049 | 2.682 (1.072–6.710) | 0.035 | ||
| Dominant | C/C | 2464 (86.46%) | 1758 (86.43%) | 1.000 (reference) | ||||
| C/T + T/T | 386 (13.54%) | 276 (13.57%) | 0.998 (0.845–1.178) | 0.980 | 0.987 (0.834–1.167) | 0.874 | ||
| Recessive | C/C + C/T | 2829 (99.26%) | 2028 (99.71%) | 1.000 (reference) | ||||
| T/T | 21 (0.74%) | 6 (0.29%) | 2.509 (1.011–6.227) | 0.047 | 2.689 (1.075–6.729) | 0.034 | ||
|
| ||||||||
| APOE allele | ||||||||
| ɛ2 carriera | 341 (11.96%) | 246 (12.09%) | 0.989 (0.830–1.178) | 0.901 | 0.976 (0.818–1.164) | 0.783 | ||
| ɛ4 carrierb | 485 (17.02%) | 371 (18.24%) | 0.920 (0.793–1.068) | 0.275 | 0.917 (0.789–1.066) | 0.260 | ||
|
| ||||||||
| MTHFR rs1801133 | ||||||||
| Co-dominant | C/C | 1573 (55.19%) | 1145 (56.29%) | 1.000 (reference) | ||||
| C/T | 1091 (38.28%) | 762 (37.46%) | 1.042 (0.924–1.175) | 0.499 | 1.048 (0.929–1.183) | 0.444 | ||
| T/T | 186 (6.53%) | 127 (6.24%) | 1.066 (0.840–1.353) | 0.598 | 1.077 (0.847–1.369) | 0.545 | ||
| Dominant | C/C | 1573 (55.19%) | 1145 (56.29%) | 1.000 (reference) | ||||
| C/T + T/T | 1277 (44.81%) | 889 (43.71%) | 1.046 (0.932–1.173) | 0.446 | 1.052 (0.938–1.181) | 0.386 | ||
| Recessive | C/C + C/T | 2664 (93.47%) | 1907 (93.76%) | 1.000 (reference) | ||||
| T/T | 186 (6.53%) | 127 (6.24%) | 1.048 (0.830–1.324) | 0.691 | 1.060 (0.837–1.341) | 0.630 | ||
a ɛ2/ɛ2 plus ɛ2/ɛ3, reference genotype: ɛ3/ɛ3 plus ɛ3/ɛ4 plus ɛ4/ɛ4. bɛ3/ɛ4 plus ɛ4/ɛ4, reference genotype: ɛ2/ɛ2 plus ɛ2/ɛ3 plus ɛ3/ɛ3.
3.5. Comparison of the Plasma Concentration of Catecholamines in Individuals with C/C, C/T, and T/T Genotypes of APOE rs7412 SNP
The plasma concentrations of catecholamines in individuals with C/C (n = 30), C/T (n = 30), and T/T (n = 20) genotypes of APOE rs7412 SNP were compared. Individuals who were APOE rs7412T/T homozygous had higher dopamine levels (87.89 ± 6.67 pg/ml vs. 83.11 ± 5.50 pg/ml, P=0.011) than those with APOE rs7412C/C. Individuals who were APOE rs7412T/T homozygous had higher epinephrine levels (93.59 ± 7.60 pg/ml vs. 90.69 ± 6.09 pg/ml) and higher norepinephrine levels (532.97 ± 42.55 pg/ml vs. 524.96 ± 38.44 pg/ml) than those with APOE rs7412C/C, but the differences were not statistically significant (Figure 1).
Figure 1.
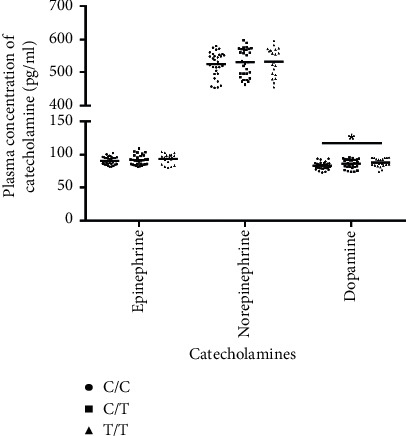
Comparison of plasma concentration of catecholamines in individuals with C/C (n = 30), C/T (n = 30), and T/T (n = 20) genotypes of APOE rs7412 SNP.
4. Discussion
Hypertension is one of the leading causes of the global burden of some diseases [1]. Lipid levels have been linked to the risk of hypertension. Abnormal lipid metabolism caused by genetic factors is closely related to the incidence of cardiovascular and cerebrovascular diseases, with the APOE gene being one of the most important genes affecting lipid metabolism [31]. The serum Hcy level is linked to the incidence of hypertension. MTHFR gene polymorphisms are associated with MTHFR activity and Hcy metabolic disorder and cause hyperhomocysteinemia [29]. In this study, the association of APOE rs429358, rs7412, and MTHFR rs1801133 genetic polymorphisms with hypertension was analyzed in a Hakka population.
Hcy is an intermediate metabolite of the methionine cycle in the body. Hyperhomocysteinemia may increase the risk of some diseases, including hypertension, cardiovascular disorders, pulmonary embolism, and depression [32]. In the present study, hypertensive patients had significantly higher serum Hcy levels than nonhypertensive controls, implying that hyperhomocysteine may be involved in the pathogenesis of hypertension. MTHFR is an enzyme involved in homocysteine metabolism. The gene that encodes this enzyme has many gene polymorphisms, and the most studied polymorphism is MTHFR rs1801133 (C677T). It has been shown that the homozygous (TT) genotype of the MTHFR rs1801133 polymorphism has higher plasma Hcy levels than the heterozygous (CT) and wild (CC) genotypes [33].
So far, there have been many studies on the relationship between MTHFR rs1801133 polymorphism and hypertension. It has been reported that there is no correlation between the MTHFR polymorphism and hypertension in Japanese [34, 35], Chinese [36], Danish [37], and Caucasians [38]. On the contrary, the MTHFR rs1801133C/T genotype was a risk factor for hypertension in a Caucasian population [39]. People who carried the MTHFR rs1801133T allele had a higher risk of hypertension among Chinese in Taiwan [40], a Chinese Han population in Shihezi city [41], Chinese from Jiangxi Province [42], Argentineans from Buenos Aires city [43, 44], and Spaniards [45]. Based on our findings, the MTHFR rs1801133 polymorphism was not associated with an increased risk of hypertension in the Hakka population, but this needs to be confirmed with a larger sample size.
There have been a few studies on the relationship between APOE polymorphisms and hypertension, and these studies have shown inconsistent results. Studies have found that people who carry the ε4 allele have an increased risk of hypertension [46–48]. The APOE gene was not related to the risk of hypertension [49]. In addition to the common APOE polymorphisms, other polymorphisms of APOE have also been reported in this relationship. APOE + 2836G>A polymorphism is associated with the susceptibility to hypertension [50]. An animal study has shown that the pathogenesis of hypertension in ApoE(−/−)/Cyp1b1(+/+) mice fed a high-fat diet is most likely due to oxidative stress caused by CYP1B1, as well as increased plasma lipid levels [51]. The APOE rs7412T/T genotype was found to be a risk factor for hypertension in this study. It has been reported that hypertensive patients have higher catecholamine levels [52, 53]. The results of this study showed that APOE rs7412T/T subjects had higher catecholamine levels than C/C subjects, but there was no statistically significant difference. The pathophysiology of hypertension may be influenced by functional changes in ApoE, which regulates lipoprotein metabolism, as well as sympathetic nervous excitement manifested by elevated plasma catecholamine levels.
The inconsistent results of the above studies may be due to differences in race and sample size. This study is the first to investigate the relationship between both APOE and MTHFR gene polymorphisms and hypertension in the Hakka people. This research has some limitations. First and foremost, information about the subjects' Hakka ethnic characteristics was obtained solely through their own descriptions, with no genetic information analysis. Second, because this is a retrospective study of patients in medical institutions and physical examination subjects, there may be some selection bias because the population is not fully representative. Third, the relationship between only the common SNPs of APOE and MTHFR and hypertension was analyzed, but other polymorphisms of APOE and MTHFR may also influence the development of hypertension. In the future, larger sample sizes, more genes, and polymorphisms will be required to investigate this relationship.
5. Conclusion
In summary, the APOE rs7412T/T genotype may be a risk factor for hypertension in the Chinese Hakka population. It provides evidence that APOE gene polymorphisms are linked to hypertension.
Acknowledgments
This study was supported by the Guangdong Provincial Key Laboratory of Precision Medicine and Clinical Translation Research of Hakka Population (Grant No.: 2018B030322003), the Science and Technology Program of Meizhou (Grant No.: 2019B0202001), and the Scientific Research Cultivation Project of Meizhou People's Hospital (Grant No.: PY-C2020033).
Data Availability
All data reported are included in the manuscript. Any clarification and additional requests can be made to the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Heming Wu designed the study. Heming Wu and Hui Rao collected clinical data. Heming Wu, Zhikang Yu, and Qingyan Huang analyzed the data. Heming Wu and Hui Rao prepared the manuscript. All authors are responsible for critical revisions, and all authors read and approved the final version of this work.
References
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet . 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet . 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah A. S. V., Newby D. E. Less clarity as the fog begins to lift. Heart . 2014;100(14):1073–1074. doi: 10.1136/heartjnl-2014-305877. [DOI] [PubMed] [Google Scholar]
- 4.James P. A., Oparil S., Carter B. L., et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8) JAMA . 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 5.Whelton P. K., Carey R. M., Aronow W. S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension . 2018;71(6):e13–e115. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 6.Ferdinand K. C., Reddy T. K., Vo T. N. Global interventions in hypertension: new and emerging concepts. Current Opinion in Cardiology . 2021;36:436–443. doi: 10.1097/HCO.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 7.Forouzanfar M. H., Liu P., Roth G. A., et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA . 2017;317:165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Chen Z., Zhang L., et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation . 2018;137(22):2344–2356. doi: 10.1161/circulationaha.117.032380. [DOI] [PubMed] [Google Scholar]
- 9.Mouton A. J., Li X., Hall M. E., Hall J. E. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circulation Research . 2020;126:789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu W., Liu D., Jiang S., Zhang K., Zhou H., Lu Q. Polymorphisms in gene MMP-2 modify the association of cadmium exposure with hypertension risk. Environment International . 2019;124:441–447. doi: 10.1016/j.envint.2019.01.041. [DOI] [PubMed] [Google Scholar]
- 11.Stoll S., Wang C., Qiu H. DNA methylation and histone modification in hypertension. International Journal of Molecular Sciences . 2018;19 doi: 10.3390/ijms19041174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirooka Y. Sympathetic activation in hypertension: importance of the central nervous system. American Journal of Hypertension . 2020;33:914–926. doi: 10.1093/ajh/hpaa074. [DOI] [PubMed] [Google Scholar]
- 13.Johnson A. K., Xue B. Central nervous system neuroplasticity and the sensitization of hypertension. Nature Reviews Nephrology . 2018;14:750–766. doi: 10.1038/s41581-018-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Arroyo O., Ortega A., Redon J., Cortes R. Therapeutic potential of extracellular vesicles in hypertension-associated kidney disease. Hypertension . 2021;77:28–38. doi: 10.1161/HYPERTENSIONAHA.120.16064. [DOI] [PubMed] [Google Scholar]
- 15.Oparil S., Acelajado M. C., Bakris G. L., et al. Hypertension. Nature Reviews Disease Primers . 2018;4(1) doi: 10.1038/nrdp.2018.14.18014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miall W. E., Oldham P. D. The hereditary factor in arterial blood-pressure. British Medical Journal . 1963;1(5323):75–80. doi: 10.1136/bmj.1.5323.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tomita Y., Sakata S., Arima H., et al. Relationship between casual serum triglyceride levels and the development of hypertension in Japanese. Journal of Hypertension . 2021;39:677–682. doi: 10.1097/HJH.0000000000002693. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka T., Takada H., Nishiyama Y., et al. Dyslipidemia and the risk of developing hypertension in a working-age male population. Journal of the American Heart Association . 2016;5 doi: 10.1161/JAHA.115.003053.e003053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burman D., Mente A., Hegele R. A., Islam S., Yusuf S., Anand S. S. Relationship of the ApoE polymorphism to plasma lipid traits among South Asians, Chinese, and Europeans living in Canada. Atherosclerosis . 2009;203:192–200. doi: 10.1016/j.atherosclerosis.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Gibas-Dorna M., Piątek J., Kupsz J., et al. Relationship between adipokines and lipid profile in postmenopausal women with different apolipoprotein E genotypes. Women Health . 2017;57:891–904. doi: 10.1080/03630242.2016.1235073. [DOI] [PubMed] [Google Scholar]
- 21.Marais A. D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology . 2019;51:165–176. doi: 10.1016/j.pathol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q., Wu H., Yu Z., Huang Q., Zhong Z. APOE gene ɛ4 allele (388C-526C) effects on serum lipids and risk of coronary artery disease in southern Chinese Hakka population. Journal of Clinical Laboratory Analysis . 2021;35 doi: 10.1002/jcla.23925.e23925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seripa D., D’Onofrio G., Panza F., Cascavilla L., Masullo C., Pilotto A. The genetics of the human APOE polymorphism. Rejuvenation Research . 2011;14:491–500. doi: 10.1089/rej.2011.1169. [DOI] [PubMed] [Google Scholar]
- 24.Yuan X., Wang T., Gao J., et al. Associations of homocysteine status and homocysteine metabolism enzyme polymorphisms with hypertension and dyslipidemia in a Chinese hypertensive population. Clinical and Experimental Hypertension . 2020;42(1):52–60. doi: 10.1080/10641963.2019.1571599. [DOI] [PubMed] [Google Scholar]
- 25.Nam K. W., Kwon H. M., Jeong H. Y., Park J. H., Kwon H., Jeong S. M. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology . 2019;92(4):e317–e325. doi: 10.1212/wnl.0000000000006816. [DOI] [PubMed] [Google Scholar]
- 26.Fu L., Li Y. N., Luo D., Deng S., Wu B., Hu Y. Q. Evidence on the causal link between homocysteine and hypertension from a meta‐analysis of 40 173 individuals implementing Mendelian randomization. Journal of Clinical Hypertension . 2019;21(12):1879–1894. doi: 10.1111/jch.13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balint B., Jepchumba V. K., Guéant J. L., Guéant-Rodriguez R. M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie . 2020;173:100–106. doi: 10.1016/j.biochi.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Ornosa-Martín G., Fernandez-Ballart J. D., Ceruelo S., et al. Homocysteine, the methylenetetrahydrofolate reductase 677C>T polymorphism and hypertension: effect modifiers by lifestyle factors and population subgroups. British Journal of Nutrition . 2020;124(1):1–11. doi: 10.1017/S0007114520000793. [DOI] [PubMed] [Google Scholar]
- 29.Xuan C., Li H., Zhao J. X., et al. Association between MTHFR polymorphisms and congenital heart disease: a meta-analysis based on 9, 329 cases and 15, 076 controls. Scientific Reports . 2014;4(1):p. 7311. doi: 10.1038/srep07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B., Fan S., Zhi X., et al. Associations of MTHFR gene polymorphisms with hypertension and hypertension in pregnancy: a meta-analysis from 114 studies with 15411 cases and 21970 controls. PLoS One . 2014;9 doi: 10.1371/journal.pone.0087497.e87497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil Y. A., Rabès J. P., Boileau C., Varret M. APOE gene variants in primary dyslipidemia. Atherosclerosis . 2021;328:11–22. doi: 10.1016/j.atherosclerosis.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhou S. J., Zhang L. G., Chen H. M. Prevalence and clinical-demographic correlates of hyperhomocysteinemia in inpatients with bipolar disorder in a Han Chinese population. Psychiatry Research . 2018;259:364–369. doi: 10.1016/j.psychres.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 33.Liew S. C., Gupta E. D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. European Journal of Medical Genetics . 2015;58:1–10. doi: 10.1016/j.ejmg.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Nishio H., Lee M. J., Fujii M., et al. A common mutation in methylenetetrahydrofolate reductase gene among the Japanese population. Journal of Human Genetics . 1996;41:247–251. doi: 10.1007/BF01875985. [DOI] [PubMed] [Google Scholar]
- 35.Lwin H., Yokoyama T., Yoshiike N., et al. Polymorphism of methylenetetrahydrofolate reductase gene (C677T MTHFR) is not a confounding factor of the relationship between serum uric acid level and the prevalence of hypertension in Japanese men. Circulation Journal . 2006;70(1):83–87. doi: 10.1253/circj.70.83. [DOI] [PubMed] [Google Scholar]
- 36.Zhan S., Gao Y., Yin X., Huang Y., Hu Y., Li L. A case-control study on the relationship between abnormal homocysteine metabolism and essential hypertension. Chinese Journals in Epidemiology . 2000;21:194–197. [PubMed] [Google Scholar]
- 37.Husemoen L. L. N., Skaaby T., Jørgensen T., et al. MTHFR C677T genotype and cardiovascular risk in a general population without mandatory folic acid fortification. European Journal of Nutrition . 2014;53(7):1549–1559. doi: 10.1007/s00394-014-0659-2. [DOI] [PubMed] [Google Scholar]
- 38.Fowdar J. Y., Lason M. V., Szvetko A. L., Lea R. A., Griffiths L. R. Investigation of homocysteine-pathway-related variants in essential hypertension. International Journal of Hypertension . 2012;2012:9. doi: 10.1155/2012/190923.190923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heux S., Morin F., Lea R. A., Ovcaric M., Tajouri L., Griffiths L. R. The methylentetrahydrofolate reductase gene variant (C677T) as a risk factor for essential hypertension in Caucasians. Hypertension Research . 2004;27:663–667. doi: 10.1291/hypres.27.663. [DOI] [PubMed] [Google Scholar]
- 40.Lin P. T., Cheng C. H., Wei J. C., Huang Y. C. Low plasma pyridoxal 5’-phosphate concentration and MTHFR 677C⟶T genotypes are associated with increased risk of hypertension. International Journal for Vitamin and Nutrition Research . 2008;78(1):33–40. doi: 10.1024/0300-9831.78.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Cai W., Yin L., Yang F., Zhang L., Cheng J. Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomedical Reports . 2014;2:861–868. doi: 10.3892/br.2014.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen C., Lv J. F., Wang L., Zhu W. F., Wan F. S., Wang X. Z. Association of a methylene tetrahydrofolate reductase C677T polymorphism with several blood chemical levels in a Chinese population. Genetic Testing and Molecular Biomarkers . 2015;19(1):24–29. doi: 10.1089/gtmb.2014.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fridman O., Porcile R., Vanasco V., et al. Study on homocysteine levels and methylenetetrahydrofolate reductase gene variant (C677T) in a population of Buenos Aires city. Clinical and Experimental Hypertension . 2008;30:574–584. doi: 10.1080/10641960802251958. [DOI] [PubMed] [Google Scholar]
- 44.Fridman O., Porcile R., Morales A. V., Gariglio L. O., Potenzoni M. A., Turk Noceto P. C. Association of methylenetetrahydrofolate reductase gene 677C>T polymorphism with hypertension in older women in a population of Buenos Aires city. Clinical and Experimental Hypertension . 2013;35:159–166. doi: 10.3109/10641963.2012.690471. [DOI] [PubMed] [Google Scholar]
- 45.Rodríguez-Esparragón F., Hernández-Perera O., Rodríguez-Pérez J. C., et al. The effect of methylenetetrahydrofolate reductase C677T common variant on hypertensive risk is not solely explained by increased plasma homocysteine values. Clinical and Experimental Hypertension . 2003;25:209–220. doi: 10.1081/ceh-120020391. [DOI] [PubMed] [Google Scholar]
- 46.Bhavani A. B., Sastry K. B., Reddy N. K., Padma T. Lipid profile and apolipoprotein E polymorphism in essential hypertension. Indian Heart Journal . 2005;57:151–157. [PubMed] [Google Scholar]
- 47.Niu W., Qi Y., Qian Y., Gao P., Zhu D. The relationship between apolipoprotein E ɛ2/ɛ3/ɛ4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertension Research . 2009;32(12):1060–1066. doi: 10.1038/hr.2009.164. [DOI] [PubMed] [Google Scholar]
- 48.Stoumpos S., Hamodrakas S. J., Anthopoulos P. G., Bagos P. G. The association between apolipoprotein E gene polymorphisms and essential hypertension: a meta-analysis of 45 studies including 13,940 cases and 16,364 controls. Journal of Human Hypertension . 2013;27:245–255. doi: 10.1038/jhh.2012.37. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Zhao H., Zhang J., et al. Gene environment interaction of GALNT2 and APOE gene with hypertension in the Chinese Han population. Bio-Medical Materials and Engineering . 2015;26(S1):S1977–S1983. doi: 10.3233/bme-151501. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Xu J. R., Liu X. M., et al. Polymorphisms of +2836 G>A in the apoE gene are strongly associated with the susceptibility to essential hypertension in the Chinese Hui population. Genetics and Molecular Research . 2014;13(1):1212–1219. doi: 10.4238/2014.february.27.6. [DOI] [PubMed] [Google Scholar]
- 51.Song C. Y., Ghafoor K., Ghafoor H. U., et al. Cytochrome P450 1B1 contributes to the development of atherosclerosis and hypertension in apolipoprotein E-deficient mice. Hypertension . 2016;67:206–213. doi: 10.1161/HYPERTENSIONAHA.115.06427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang C. C., Chung C. M., Leu H. B., Huang P. H. Sex difference in sympathetic nervous system activity and blood pressure in hypertensive patients. Journal of Clinical Hypertension . 2021;23:137–146. doi: 10.1111/jch.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xue X., Chen H., Xia Q., et al. Detection of plasma catecholamines in human pheochromocytoma and primary hypertension based on liquid chromatography tandem mass spectrometry. Annals of Clinical and Laboratory Science . 2019;49:204–211. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reported are included in the manuscript. Any clarification and additional requests can be made to the corresponding author.


