Abstract
A 15-year-old female patient with anorexia nervosa presented an unusually prolonged and severe episode of pancytopenia with severe thrombopenia and severe leucopenia. Despite effective refeeding, active specialized interventions were necessary. Upon admission, the patient presented with severe and symptomatic thrombopenia, severe neutropenia and gelatinous marrow transformation. In addition to refeeding, active interventions such as platelet transfusion and granulocyte-colony stimulating factor were successful to manage the patient’s complications. The etiological search for pancytopenia was negative. The patient’s prolonged starvation was probably a key factor. Medical history, clinical presentation, evolution, and biological data including bone marrow aspiration results are presented. Management of cytopenia and of their complications in a context of severe starvation is discussed in regard of existing literature. A simple monitoring attitude may prove insufficient in cases of severe pancytopenia in anorexia nervosa.
Level of evidence V, descriptive study.
Keywords: Anorexia nervosa, Thrombocytopenia, Neutropenia, Pancytopenia, Gelatinous marrow transformation, Medication
Introduction
Hematological abnormalities are well-established complications of anorexia nervosa (AN) due to nutrition deprivation leading to global marrow hypometabolism [1]. Anemia and leucopenia are usually found in one-third of patients [2]. Moderate thrombocytopenia is more seldom found, concerning 5–11% of AN patients [1, 3]. Pancytopenia concerns approximately 3% of AN patients. However, severe thrombocytopenia leading to active bleeding or profound neutropenia with severe infectious complications are rare conditions [1]. The spectrum of bone marrow modifications, classified into four grades of severity, ranges from normal, hypoplastic or aplastic bone marrow with or without gelatinous marrow transformation (GMT) to complete GMT which is the most critical condition [4]. GMT is reported in half of AN patients with changes in the peripheral blood count and in almost all AN patients with pancytopenia [1]. Another rare entity associated with pancytopenia, known as bone marrow necrosis, has also been described in AN patients [5]. In most cytopenia cases, effective nutritional support is sufficient to restore bone marrow function [1, 6, 7]. Active treatments such as granulocytes-colony stimulating factor (G-CSF) or platelet transfusions are not standard practice in these situations. Thresholds for intervention, treatment courses and durations are usually decided by teams on a case-to-case basis with no established guidelines to date. Here is the case of a 15-year-old anorexic adolescent, hospitalized for extreme starvation with severe and prolonged thrombocytopenia, severe neutropenia and moderate anemia, for whom effective refeeding was insufficient, leading to specialized interventions and several active treatments.
Case presentation
A 15-year-old female patient with AN was hospitalized in a multidisciplinary adolescents’ unit [8]. Upon admission, she was in a state of terminal cachexia with major severity criteria such as bradycardia (41 bpm), hypothermia (35.7 °C), low blood-pressure (90/55 mmHg). She weighed 27.5 kg with a height of 159 cm, resulting in a body mass index (BMI) of 10.9 kg/m2.
The patient presented in a state of intense exhaustion and bradyphemia. Main physical signs were severe cachexia, acrocyanosis, lower limb oedema. Petechial purpura was found upon admission in friction areas (dorsal region, one petechial stain on the inner cheek lining). An unhealed skin abrasion on the left knee and several lower lib hematomas were also observed. She reported a 2-year long secondary amenorrhea.
Admission labs showed a white blood cell count (WBC) of 3,72 G/L (4–11), absolute neutrophil count (ANC) of 2,43 G/L (1.7–7.1), hemoglobin of 14,2 g/dL (12–15) (with anisocytosis, poikilocytosis) and a platelet count of 65 G/L (186–440). Serum chemistry included sodium 126 mmol/L and potassium 4,2 mmol/L. There was liver damage with aspartate aminotransferase (AST) of 94 IU/L (17–33), alanine aminotransferase (ALT) of 81 IU/L (8–24), although without liver failure (Prothrombin time was 64% (70–125), and 83% 1 week later; there was no splenomegaly, no collateral circulation, coagulation factor V was normal). Activated partial thromboplastin time (APTT) was slightly elevated at 126 patient/control ratio (< 1.20).
There was renal insufficiency secondary to starvation and dehydration, serum urea was 8.1 mmol/l and serum creatinine was 55 umol/l, creatinine clearance was 65 ml/minute according to Cockcroft and Gault, which subsequently rapidly improved with refeeding. A brain MRI scan showed no abnormality. Electrocardiogram and cardiac ultrasound did not find any dysfunction.
Her clinical presentation was a typical AN case with no purgative behavior and no physical hyperactivity. There was hyponatremia, due to hyperhydration behavior. There was no somatic comorbid condition and no obvious psychiatric comorbidity.
The patient was of Chinese descent, with no previous medical history. Maximum weight before disease onset had been 46 kg /159 cm (BMI 18.2 kg/m2). The patient’s AN had been evolving for at least 2 years with eight courses of complete hospitalizations in a different general hospital, within an adolescents’ pediatric unit. Admission into the unit was the first referral to specialized care, its delay due to specialized care beds shortage in the Paris region which has been exacerbated since the COVID-19 health crisis. There had been moderate leucopenia and thrombocytopenia for at least 6 months prior to admission, which had not been investigated and had been imputed to her weight loss.
Two days after admission, given the severe undernutrition, thrombopenia and aggravation of liver dysfunction, exclusive enteral nutrition by nasogastric tube was started at 36 kcal/kg/day i.e. 1000 kcal/day, which was incrementally raised until a maximum of 70 kcal/kg/day i.e. 2250 kcal/day. Refeeding was effective resulting in a gain of 10 kg in 10 weeks, leading to a BMI increase from 10.9 kg/m2 to 14.3 kg/m2. Treatment included vitamin therapy (A, B1, B6, D, E, PP), as well as zinc, magnesium, phosphorus, and potassium supplementation.
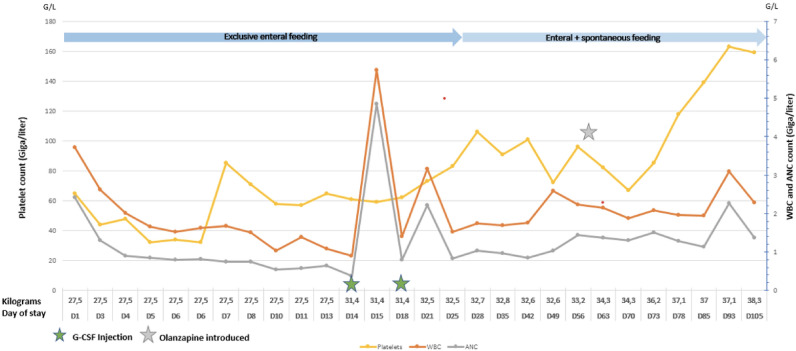
Despite effective refeeding, platelet count declined to a nadir of 32G/L on day 4 of stay (Fig. 1). On day 7, the patient exhibited severe bilateral epistaxis leading to transfer to the local intensive care unit and transfusion of one platelet unit. Recurring light nasal bleeding occurred afterward with need for nasal cauterization by an otorhinolaryngologist.
Fig. 1.
Blood count and weight evolution—ANC Absolute neutrophil count, D Day, G-CSF Granulocyte-colony stimulating factor, WBC White blood count
Regarding the white blood cell line (Fig. 1), the patient’s WBC gradually declined to a nadir of 0.89 G/L (ANC 0.38 G/L) motivating septic isolation and a transfer to the hematology department. After discussion with the hematology team, administration of two doses of G-CSF was performed following which her WBC dramatically improved to 5.89 G/L (ANC 4,85 G/L). Global duration of neutropenia was 91 days.
On day 5, a moderate normochromic, normocytic anemia (hemoglobin 9.4G/L), with acanthocytosis, anisocytosis and poikilocytosis was observed. Anemia was of central origin with low reticulocytes of 38.55 × 10*9/L and an elevated ferritin, a classical finding in AN [2]. Supplementation with iron, vitamins B9 and B12 was provided.
A bone marrow aspiration was performed on day 3 to rule out malignant hemopathy, finding multiple areas of gelatinous marrow transformation (GMT) with hematopoietic hypoplasia, with no evidence of bone marrow necrosis (Fig. 2).
Fig. 2.
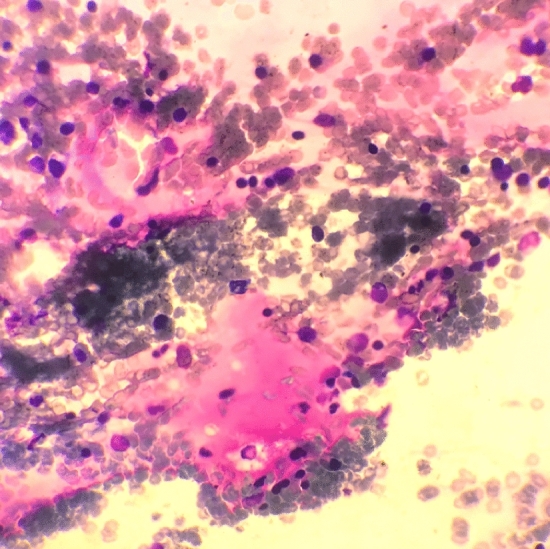
Bone marrow aspirate smear with decreased cellularity and pinkish areas without hematopoiesis corresponding to focal gelatinous marrow transformation (GMT) (May-Grünwald Giemsatain, 50X magnification)
Etiological investigations for this profound cytopenia impacting the three cell lines allowed to rule out differentials such as infection: there was constant apyrexia and no clinical sign of infection, CRP (although seldom elevated in AN patients) was consistently negative, as well as COVID PCR. No argument was found for an autoimmune disease: no anti-platelet antibodies. There was also none for disseminated intravascular coagulation, nor was there any anamnestic argument for iatrogenic myelosuppression. The patient had received two doses of a Moderna (Spikevax) COVID-19 vaccine, the second dose of which occurred more than 6 months before admission.
After 2 months, following pain complaints, a hemi-sacral fracture together with a femoral head fracture were discovered, related to severe osteoporosis. As recommended by a multidisciplinary medical meeting of the rheumatology department, IV bisphosphonates were administrated (one perfusion a week of Pamidronate, 1 mg/kg for 3 weeks), with 6 weeks of non-weight bearing. Pain was managed with Acetaminophen, Nefopam and Tramadol.
Gradually, the patient’s condition improved with introduction of oral meals. Total duration of exclusive feeding tube nutrition was 25 days, while total duration of partial enteral nutrition was 112 days. After 10 weeks of hospitalization, a treatment by Olanzapine was initiated to help treating the patient’s severe anorexic cognitions and anxiety, which was progressively increased up to 10 mg per day. Pursuit of multidisciplinary and psychiatric care led to physical recovery as well as improvement of her depression and anxiety.
Follow-up: Five months after admission the patient’s complete blood count had significantly improved, with WBC 3.2 G/L, ANC 1.76 G/L, hemoglobin 12.2 g/dL, platelet count 153 G/L. AST was 45 IU/L, ALT was 92 IU/L, BMI was 15.2 kg/m2 and there was steady progress in mood as well as anorexic cognitions.
Discussion
The pathophysiology of hematological abnormalities in AN is thought to result from multiple alterations in the bone marrow microenvironment impairing cell differentiation and causing bone marrow hypometabolism [1]. The terminal stage of starvation-linked marrow insufficiency (a condition not specific to AN) is gelatinous bone marrow transformation (GMT) which is a high-risk, albeit generally reversible, condition, in which cytopenia become life-threatening, associated to multiple organ failure in the short term, with a high death rate [9].
In most cases of cytopenia in AN, blood counts usually improve with refeeding across the three blood cell lines, sometimes motivating a simple monitoring attitude. However in some cases especially in profound cytopenia or complications different treatments are required [10]. Moreover, severe comorbidities in AN such as liver damage, but also psychiatric disease severity and patient’s refusal of weight restauration can impact the pace of refeeding [6] and delay cell count restauration. The fact that the patient was treated in a multidisciplinary unit comprising somatic as well as psychiatric intensive support was vital to her acceptance of refeeding as well as of the different lines of treatment.
Although calorie intake increase was initially limited by the patient’s liver dysfunction, weight restauration was efficient. However, the patient’s cytopenia were both severe and prolonged and she presented life-threatening complications with severe thrombocytopenia occurring shortly after initiation of refeeding, active bleeding, and profound neutropenia. These complications, occurring despite effective enteral refeeding, led us to broaden investigations and repeatedly discuss the patient’s case with the hospital’s hematology team.
Severe thrombocytopenia is rather rare in AN, and active bleeding even more so [11]. Besides myelosuppression, additional mechanisms for thrombocytopenia can include decreased thrombopoietin (TPO) production accompanying liver dysfunction [12]. As seen in this case, tendency to bleeding may occur with a slightly higher platelet count in thrombopenic AN patients than in patients with other thrombopenia etiologies. This could be explained by additional risk factors such as vascular fragility [10].
Neutropenia is more frequent in AN but usually improves spontaneously with refeeding and there are no current guidelines for treatment with G-CSF. Use of G-CSF in AN is not standard procedure, although several case reports show it has already been used [13, 14]. The etiology of neutropenia was investigated. As the team had the experience of severe neutropenia in a previous patient of Japanese ancestry [6], ethnic vulnerability was discussed, however, neutropenia is not shown to be more prevalent in Asian patients [15]. These findings thus suggest that treatment with G-CSF can be effective in AN patients with profound neutropenia even in the absence of infection or sepsis.
The severity of platelet and white blood cell impairment was corroborated by the bone marrow findings. When found in AN, GMT is a pejorative sign, however, its global prevalence in AN is not known. Given the unusually severe thrombocytopenia on the initial blood tests, risk of GMT, its aggravation and its reversibility rapidly became major concerns, quickly motivating a bone marrow aspiration. The bone marrow aspirate, showing slightly decreased cellularity and multiple focal GMT, was an object of discussions. The high degree of bone marrow function impairment led us to seek to eliminate marrow necrosis, which is a feared complication of starvation, usually described in terminal states of low blood flow leading to marrow ischemia [5]. In the present case, bone marrow aspiration offered no argument for necrosis. However, bone marrow biopsy would have been a better test to identify necrosis and would have been indicated if the cell count had further deteriorated.
Ultimately, the severity of the patient’s pancytopenia, confirmed by the bone marrow findings, could not be attributed to any other cause than the AN itself. Differential diagnoses were ruled out either by clinical or by biological arguments, and the patient’s characteristics and disease course did not fit the diagnosis of idiopathic aplastic anemia either [7]. The duration and severity of the patient’s starvation previously to hospitalization in the unit (BMI beneath 12 kg/m2 for 1 year) was very likely the key factor in her pancytopenia. Hospital resource shortage especially in the context of the COVID-19 pandemic in France as well as worldwide also severely limited adolescents’ access to specialized care.
Facing a series of successive, unusually prolonged, and profound cytopenia affecting the three cell lines, medical decisions of active treatments such as platelet transfusions and G-CSF were made in an ad hoc manner, in response to life-threatening complications, after multidisciplinary discussions. Guidelines for active treatments in severe and/or prolonged cytopenia in AN patients would be useful to clinicians. Wider case series would be needed to determined thresholds for intervention.
What is already known on this subject?
Mild and transient neutropenia is common in anorexia nervosa, but thrombocytopenia is much less typical. Bone marrow changes range from hypoplasia to complete aplasia which is a critical condition.
What does this study add?
Profound thrombocytopenia is rare but may point to the onset of gelatinous marrow transformation and to life-threatening pancytopenia requiring rescue specialized medication and specific guidelines.
Authors’ contributions
Conceptualization: AL, AC, CB; validation: AL, AC, RB, MRM, IB, CB; drafting: AL, AC, CB; review and editing: AL, AC, RB, MRM, IB, CB.
Funding
None.
Data and material availability
All data and materials are available.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Authors have checked that they are complying with the specific and ethical requirements of their institution.
Informed consent
Written informed consent for publication of the clinical details and images was obtained from the patient' parents. A copy of the consent form is available for review by the Editor of this journal.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hütter G, Ganepola S, Hofmann W-K. The hematology of anorexia nervosa. Int J Eat Disord. 2009;42:293–300. doi: 10.1002/eat.20610. [DOI] [PubMed] [Google Scholar]
- 2.Mamou G, Sider A, Bouscary D, et al. Anemia in anorexia nervosa: the best way to deal with it-an overview of literature. J Hum Nutr Food Sci. 2016;4(1):1081. [Google Scholar]
- 3.De Filippo E, Marra M, Alfinito F, et al. Hematological complications in anorexia nervosa. Eur J Clin Nutr. 2016;70:1305–1308. doi: 10.1038/ejcn.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abella E, Feliu E, Granada I, et al. Bone marrow changes in anorexia nervosa are correlated with the amount of weight loss and not with other clinical findings. Am J Clin Pathol. 2002;118:582–588. doi: 10.1309/2Y7X-YDXK-006B-XLT2. [DOI] [PubMed] [Google Scholar]
- 5.Smith RRL, Spivak JL. Marrow cell necrosis in anorexia nervosa and involuntary starvation. Br J Haematol. 1985;60:525–530. doi: 10.1111/j.1365-2141.1985.tb07449.x. [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim N, Barruchet A, Moro MR, Blanchet C. Severe neutropenia in an anorexic adolescent girl: a stigma of underfeeding syndrome? Eat Weight Disord. 2021;26:1271–1275. doi: 10.1007/s40519-020-01016-0. [DOI] [PubMed] [Google Scholar]
- 7.Takeshima M, Ishikawa H, Kitadate A, et al. Anorexia nervosa-associated pancytopenia mimicking idiopathic aplastic anemia: a case report. BMC Psychiatry. 2018;18:150. doi: 10.1186/s12888-018-1743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit L, Cottin P, Moro MR. What is a “Maison des Adolescents”? A history of integrated youth health care services in France. Early Interv Psychiatry. 2018;12:1000–1005. doi: 10.1111/eip.12680. [DOI] [PubMed] [Google Scholar]
- 9.Hariz A, Hamdi MS, Boukhris I, et al. Gelatinous transformation of bone marrow in a patient with anorexia nervosa: an uncommon but reversible etiology. Am J Case Rep. 2018 doi: 10.12659/AJCR.911287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukudo S, Tanaka A, Muranaka M, et al. Case report: reversal of severe leukopenia by granulocyte colony-stimulating factor in anorexia nervosa. Am J Med Sci. 1993;305:314–317. doi: 10.1097/00000441-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Saito S, Kita K, Morioka CY, Watanabe A. Rapid recovery from anorexia nervosa after a life-threatening episode with severe thrombocytopenia: report of three cases. Int J Eat Disord. 1999;25:113–118. doi: 10.1002/(sici)1098-108x(199901)25:1<113::aid-eat15>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Yoshiuchi K, Takimoto Y, Moriya J, et al. Thrombopoietin and thrombocytopenia in anorexia nervosa with severe liver dysfunction. Int J Eat Disord. 2010;43:675–677. doi: 10.1002/eat.20762. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu H, Hayashi K, Higashiyama F. Treatment with granulocyte colony-stimulating factor in the refeeding phase of anorexia nervosa complicated with severe neutropenia and sepsis: a case report. Eat Weight Disord. 2018;23:897–902. doi: 10.1007/s40519-017-0435-4. [DOI] [PubMed] [Google Scholar]
- 14.Charania RS, Kern WF, Charkrabarty S, Holter J. Successful management of gelatinous transformation of the bone marrow in anorexia nervosa with hematopoietic growth factors. Int J Eat Disord. 2011;44:469–472. doi: 10.1002/eat.20833. [DOI] [PubMed] [Google Scholar]
- 15.Denic S, Narchi H, Al Mekaini LA, et al. Prevalence of neutropenia in children by nationality. BMC Hematol. 2016;16:15. doi: 10.1186/s12878-016-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available.