Abstract
The global probiotics industry has been undergoing major changes in recent years. Approaches to finding and creating new probiotics, as well as a paradigm of their use in food, medicine, and pharmacology are changing. The catalyst proved to be the increasing popularity and availability of omics technologies, in particular, metagenomic studies of human and animal microbiomes. However, the efficiency and safety of drugs based on probiotic strains, as well as their marketing rates, largely depend on the levels of legal and technical regulation in the field. The present review discusses the aspects of legal regulation in Russia, the European Union and the United States, along with the advantages and disadvantages of probiotics and postbiotics. A consensus is emerging that postbiotics have a number of advantages over classical live probiotic cultures. The review also focuses on the lactobacilli family, which includes the largest number of probiotic strains studied so far and still holds a leading position among probiotics. On the legislative front, Russia is often ahead of its time with adopting such laws as the Federal Law No. 492-FZ on biosecurity, which defined the concept of human and animal microbiota and set forth legislative guidelines for its preservation. The new field of research referred to as microbiome nutrigenomics aims to achieve this goal.
Keywords: probiotics, postbiotics, pharmabiotics, lactobacilli, legal regulation
Probiotic bacteria have long been used by humans as food components and, since the early 20th century, as drug analogues. Their fundamental difference from proper drugs lies in the fact that they have beneficial effects on a macroorganism as a whole, with their mechanisms of action and targets being unknown. Probiotics are intended mainly for healthy people and are used more for prevention than as a cure. In recent years, the interest in probiotics has been steadily increasing with the range of microorganisms constantly expanding [1–5].
Omics technology has made it possible to study both microbiota as a whole and individual bacteria at the level of their genomes. A huge number of microbial species have been identified, primarily the bacteria that inhabit the integuments that are found inside the human body, which made the pharmaceutical industry note the potential of preparations based on living probiotic organisms as means for treating various diseases [8–14]. The research conducted in recent years has demonstrated the enormous potential of probiotics. They are able to affect various processes in the human body, including increasing insulin sensitivity, improving memory and cognitive abilities, reducing anxiety and depression, and reducing the manifestation of allergic reactions. It should be stressed that the manifestation of probiotic properties by bacteria is a strain-specific phenomenon, which therefore requires detailed study of not only certain species, but also specific strains.
However, the use of preparations based on probiotic microorganisms in medicine is hindered by difficulties in their standardisation and development of appropriate protocols that would ensure the beneficial properties of microorganisms throughout the manufacturing process. The large number of terms (eubiotics, prebiotics, probiotics synbiotics, parabiotics, postbiotics) and their arbitrary use also complicates the matter. Nevertheless, these kinds of drugs have been named Live Biotherapeutic Products (LBPs) or pharma-biotics, and the guidelines have been formulated by the Food and Drug Administration (FDA) in the United States and the European Directorate for Drug Quality and Health (EDQM) [6, 7]. A consensus is gradually emerging that it is more promising to invest in postbiotics (inactivated microbial cells or their metabolites), which are easier to control and standardise, than in probiotics, which are based on live bacterial cultures.
According to the state register of drugs, a number of microbiota-normalising drugs have been registered in the Russian Federation. Most of them contain Bifidobacteria and Lactobacilli [8]. In the Russian Federation, the registered microbiota-normalising medicines are mainly used as a follow-up after antibiotic therapy as well as to treat allergic diseases, respiratory infections, and gastrointestinal disorders. This review discusses the legal regulation of probiotics, postbiotics, and pharmabiotics in Russia and abroad. Special attention is paid to lactobacilli as the bacteria most commonly used as probiotics.
PROBIOTICS: USE AS DIETARY SUPPLEMENTS AND MEDICINES IN RUSSIA AND ABROAD
The term probiotic was coined in 1954 by Ferdinand Vergin [9]. Currently, the International Scientific Association for Probiotics and Prebiotics (ISAPP) defines probiotics as living organisms that when consumed in sufficient quantities are beneficial to the health of their host [10].
Probiotics can be used as dietary supplements, as components of functional foods and as medicines, referred to as pharmabiotics or LBP. The term pharmabiotics was first used in 2002 by Colin Hill when he studied probiotics under the supervision of Fergus Shanahan [11]. As dietary supplements and medicines, probiotics are used to prevent and treat gastro-intestinal (GI) diseases (irritable bowel syndrome, gastrointestinal disorders, Helicobacter pilori elimination, and inflammatory bowel diseases), allergies, obesity, type 2 diabetes, nonalcoholic fatty liver disease, cancer side-effects, and nervous system disorders and to modulate the immune system. Probiotics help to maintain the normal microbiota composition, strengthen the intestinal barrier, inhibit the growth of pathogenic bacteria, stimulate proliferation and activity of innate and adaptive immunity components and the synthesis of host enzymes involved in stress protection [12–15].
Probiotics act on the macroorganism by both intact cells and cellular components and individual metabolites. Their targets are diverse and include microbiota bacteria, cellular receptors (including Toll-like receptors), components of signal transduction systems, intestinal epithelium and enteric nervous system cells [16]. It has been suggested that probiotics may affect biochemical and signalling pathways through epigenetic modifications such as DNA methylation, phosphorylation, biotinylation, histone acetylation, and RNA interference [17]. These mechanisms are involved in the epigenetic control of host cell responses, thereby regulating various biochemical processes such as immunomodulation, competitive exclusion, and epithelial cell barrier function. In addition, these biochemical modifications may contribute to prevention of such diseases as cancer and autoimmune disorders.
Probiotic efficiency is determined by the specific bacterial strain used and the disease for the prevention and treatment of which the probiotic is used rather than by the general type of bacteria [18]. For maximum efficiency, probiotic strains should be selected from the same geographical region where they are intended to be used.
The widely used terms prebiotic and synbiotic were introduced in 1995 [19]. ISAPP currently defines prebiotics as nonliving food ingredients that support health by modulating microbiota [20]. The term synbiotic refers to a mixture containing live microorganisms and substrate(s) used selectively by microorganisms, which has beneficial effects on the host [21]. Prebiotics can be used as an alternative to probiotics or together with them to enhance the effect of the latter as a component of synbiotics. The term autoprobiotics is also used. These are produced by in vitro cultivation of bacterial strains from individual human microbiota to be further used as personalised drugs or as food products [22].
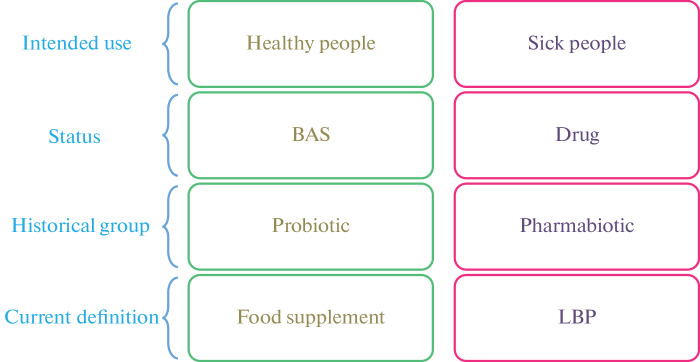
The Status of Probiotics Abroad. In the United States, probiotics can be given the status of a biologically active supplement (BAS), a food ingredient or a live biotherapeutic products (LBP), which is a medicine, depending on whether they are intended for use by healthy or sick people, respectively (Fig. 1).
Fig. 1.
The status of probiotics abroad.
Most probiotics used as food ingredients in the United States have not gone through the approval process before being marketed because they fall under the GRAS (Generally Regarded as Safe) program and are recognised as completely harmless for human. GRAS status is granted automatically to the substances that have been used historically as food constituents before January 1, 1958. The International Dairy Federation (IDF) has established a list of organisms with a documented history of safe use as the components of food products in their Bulletin No. 377 The Inventory of Microorganisms with a Documented History of Use in Food. [23].
Regarding the registration of food supplements in the United States, under the Dietary Supplement Act (DSHEA), food additives used before October 15, 1994, are automatically approved for production. The Council for Responsible Nutrition (CRN) has compiled a list of approved supplements which were in use before October 15, 1994. However, the list unfortunately mentions only probiotic biological species without naming specific strains. Strains isolated later than 15 October 1994 need to be registered as a new diet. The strains isolated after October 15, 1994 should be registered as a new dietary ingredient (NDI) [24]. The registration of probiotics in the United States is regulated by the Federal Food, Drug and Cosmetics Act (abbreviated as FFDCA, FDCA or FD&C) [25].
The EFSA approved a list of bacterial species used in food to which the principle of Qualified Presumption of Safety (QPS) applies [26, 27]. This decision is based on the research which showed that the safety of bacterial strains as food components is primarily dependent on the biological species they belong to.
It is worth noting that in most EU countries, Regulation No. 1924/2006 forbids probiotic labeling to protect consumer rights. The reason for this is that the use of the term probiotic on the label tells the consumer that the product contains components that are beneficial, which is not always scientifically proven. Out of 400 applications for the registration of the beneficial properties of probiotics filed with the EFSA, only one was supported. According to the Article No. 13.1, yogurts containing at least 108 colony-forming units of L. delbrueckii subsp. bulgaricus and Streptococcus thermophilus can be advertised as a product that improves lactose absorption [28].
Registration of Probiotics as LBPs Abroad. In 2010, the United States Food and Drug Administration (FDA) was the first competent authority to propose to consider the status of medicinal products containing live microorganisms used to prevent or treat diseases in humans. In 2016, FDA published guidelines for LBP production. According to the FDA, a product may be classified as an LBP if it contains living organisms, may be used for prevention and treatment of a specific disease or disorder in humans, and is not a vaccine [29].
In 2019, the European Directorate of Drug Quality and Health (EDQM) published a monograph on LBPs, thereby officially recognising them as a new category of medicinal products for use in Europe [6]. Applications for drug registration as an LBP are often discussed with competent authorities such as the EDQM or FDA to clarify which tests should be carried out for a particular product. All requirements for development and use of live culture-based preparations have been detailed in an article describing the preparation based on the Christensenella minuta strain DSM 33407 suggested for the treatment of obesity and metabolic syndrome [30].
The United States National Institutes of Health (NIH) currently approve LBPs for the treatment of gastrointestinal diseases and allergies, dental disorders, diseases related to the gut-brain axis, and others (see Table 1) [31].
Table 1.
A list of medical applications of LBPs according to the United States National Institutes of Health (NIH) [31]
| Gastrointestinal diseases | Antibiotic-associated diarrhoea, Clostridioides difficile infection, constipation, diarrhoea caused by anticancer therapy, diverticular disease, inflammatory bowel disease, irritable bowel syndrome, traveller’s diarrhoea |
|---|---|
| Childhood illnesses | Infant colic, necrotising enterocolitis, neonatal sepsis |
| Dental diseases | Tooth decay, gum disease |
| Allergic diseases | Allergic rhinitis, asthma, atopic dermatitis, allergy prevention |
| The gut-brain axis and related diseases | Anxiety and stress, cognitive functions, depression, autism spectrum disorder, schizophrenia, Parkinson’s disease, Alzheimer’s disease |
| Other illnesses | Inflammation of sebaceous glands, hepatic encephalopathy, upper respiratory tract infections, urinary tract infections, genital tract diseases |
In brief, to register a new LBP, a positive balance of benefits and risks should be demonstrated (Fig. 2). Proof of the positive benefit/risk balance should be derived from reliable and validated preclinical and clinical trials. The benefit/risk ratio is the basis of a product registration application [32]. Clinical trials are studies conducted in humans that aim aim to evaluated the safety and efficiency of medical, surgical, or behavioural interventions. It is the key commonly accepted methodology to determine whether a new treatment, in the form of a new medicine, diet, or medical device, is safe and efficient when used for humans. Clinical trials are carried out in four stages. The ultimate goal of a clinical trial is to test the new treatment, find the right dosage, and identify side-effects. If the medicine or any other kind of intervention is demonstrated to be safe and efficient in the first three phases of clinical trials, it may be approved for the post-marketing phase IV clinical trials. Generally, conducting the first three phases is sufficient to bring a medicine/treatment onto the market.
Fig. 2.
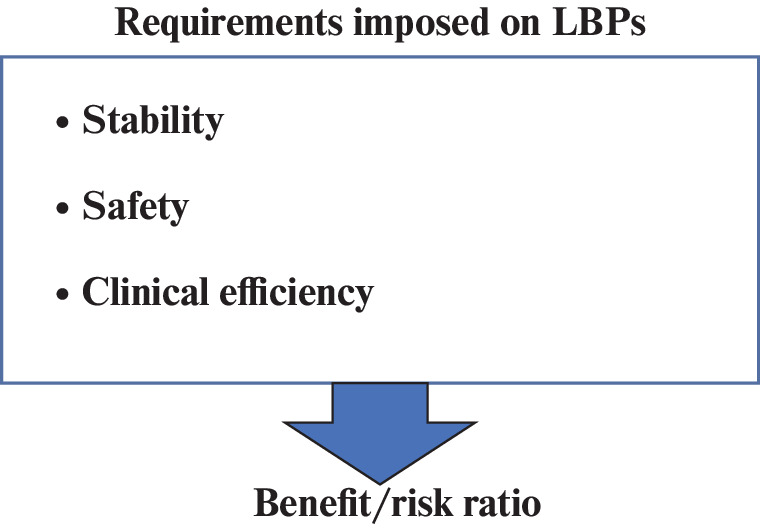
Requirements imposed on LBPs.
There are currently no registered LBPs in the United States. The first such drug may be SER-109, which is based on the spores of Firmicutes bacteria (lat. phylum Firmicutes) for the treatment of antibiotic-associated diarrhoea caused by C. difficile [33]. There are a number of other LBPs in various stages of clinical trials (Table 2) [34].
Table 2.
A list of LBPs that are currently undergoing clinical trials
| Medicinal product | Target | Clinical trial phase |
Product description |
|---|---|---|---|
| SER-109 |
Clostridium difficile infection |
III | SER-109 is an aggregate of bacterial spores obtained from the stool of healthy tested donors. |
| VE303 |
Clostridium difficile infection |
II | VE303 is a live biotherapeutic product containing 8 strains of human commensal bacteria produced in accordance with the GMP requirements. |
| Blautix (MRx1234) |
Irritable bowel syndrome |
a II | Lyophilised product of the patented Blautia hydrogenotrophica bacterium strain |
| LACTIN-V | Bacterial vaginosis | II | LACTIN-V is a live biotherapeutic product for intravaginal administration, containing the Lactobacillus crispatus strain CTV-05 |
| Xla1 |
Obesity and metabolic syndrome |
I | Xla1 is LBP containing a Christensenella minuta bacterium strain |
Regarding LBP registration in the European Union (EU), the Directive No. 2001/83/EC issued by the European Parliament and the EU Council named On the Community Code on Medicinal Products for Human Use defines any product designed to prevent or treat diseases as a medicinal product and therefore requires marketing authorisation from the competent authorities before it can be commercialised. However, even if the pharmaceutical regulatory framework is established at the EU level, obtaining marketing authorisation for LBPs remains a very challenging task. Compared to the other medicinal products currently on the market, assessing the safety of LBPs represents a substantial problem due to their inherent specific properties as living organisms and their complex mechanism of action. For example, LBPs themselves should not enter the bloodstream like most drugs that target distant organs, tissues, or receptors, but rather exert their action through the direct interaction with the host microbiota, which indirectly leads to distant biological effects in the host.
In order to assess the safety of an LBP, it is not sufficient to refer to the historical safe use of a certain strain as a food product component. In the EU, post-marketing surveillance is generally not required for food products and food supplements. Hence, possible side effects in sick people may not be reported. In addition, in their 2011 report, the US Agency for Healthcare Research and Quality concluded that although the clinical trials for probiotics have not yet shown any increased risks, the question of the safety of interventional studies has no confident answer in the modern literature. The issue is that safety and toxicology data are mandatory for drugs, which is not the case for food supplements.
The EMA has not yet developed any requirements for LBP registration since this organisation has not yet dealt with live microorganisms and the issues associated with their standardisation. Among the requirements to be possibly introduced for LBP registration could be counting live, dead, and active bacteria by flow cytometry. Such a standard has already been developed and certified by the ISO and included in the European Pharmacopoeia. It is therefore hoped that in the coming years, the EMA will continue developing, evaluating, and improving the existing procedures for coordination of requirements imposed on LBPs. In the absence of any formal regulation, national initiatives such as the one recently published in the Netherlands on the use of the term probiotic may be expected. According to the ISAPP, the label of a probiotic product should include the genus, species, and strain for all the strains contained in the product; ingredients/allergens; effect/recommended use; daily dose; storage information; shelf life, and company name/contact information [35].
The Status of Probiotics in the Russian Federation. Probiotics are regulated by different legislation in the Russian Federation, depending on whether they will be registered as biologically active supplements (BAS) or as a drug. However, it is important to understand here that only a drug can be prescribed by a doctor as a pharmacological treatment. Probiotics, BAS, can be used for disease prevention. To take them as drugs is prohibited by the Federal Law of the Russian Federation On the Supervision of Dietary Supplements issued July 10, 2009. There is also a third group of probiotic-containing preparations, which are functional foods that contain live probiotic bacteria as their components.
Registration of Probiotics as a BAS in Russia. According to the Federal Service for Consumer Rights Protection and Human Welfare Surveillance (Rospotrebnadzor), biologically active supplements are natural and/or identical to natural biologically active substances and probiotic microorganisms intended for consumption with food or for inclusion in food products [36]. In order to register a probiotic as a BAS in the Russian Federation, it is required to test the product, undergo expert examination of documentation, and issue a certificate of state registration. The testing stage is primarily aimed at proving the product safety and confirming compliance with the properties declared in the associated documentation. Probiotics, as dietary supplements, are also regulated by the requirements for hygienic safety of food products, which are formulated in the technical guidelines of the Customs Union On food safety [37], which is a critical requirement if the product is intended for sale in the Russian Federation. There are microbiological standards that a BAS should meet to ensure product safety. These standards are described in detail in the Uniform Sanitary Epidemiological and Hygienic Requirements to Goods Subject to Sanitary and Epidemiological Surveillance under the supervision of Rospotrebnadzor [37]. The final step is the state registration of a dietary supplement, which implies listing the product in the Unified Catalogue of State Registration Certificates. This catalogue is supervised by Rospotrebnadzor. Prebiotics can only be registered as dietary supplements.
Registration of Probiotics as Drugs in Russia. To register a probiotic as a drug, it is necessary that the drug meet the requirements of the Federal Law of the Russian Federation No. 61-FZ On Medicine Circulation [38]. The circulation of these medicinal products is regulated by the Order of the Ministry of Healthcare of the Russian Federation No. 403n issued July 11, 2017 On Approval of the Guidelines on Dispensing Drugs for Medical Use, Including Immunobiological Drugs, by Pharmacies and Private Entrepreneurs with a License for Pharmaceutical Activity [39].
Today, probiotic-based drugs are considered as immunobiological drugs. Usually they contain live beneficial microorganisms for which the activity, for example antagonistic activity against pathogenic and opportunistic bacteria, must be proven. Such medicinal products should comply with the pharmacopoeial requirements imposed on standard drug production and the quality requirements for a particular pharmaceutical form [40]. There are several pharmacotherapeutic groups that a probiotic drug may be included in; the drug may be described as a probiotic, eubiotic, antidiarrhoeal, or medical immunobiological product and classified according to the Anatomotherapeutic Chemical Classification (ATCC). Probiotic-based drugs may have the following codes: Boulardii saccharomycetes (A07FA02), antidiarrhoeal microorganisms (A07FA), Lactobacilli (G01AX14), lactic acid-producing microorganisms (A07FA01), and lactic acid-producing microorganisms in combination with other drugs (A07FA51). However, it should be noted that the term eubiotic, which refers to a preparation containing one or more probiotic strains, is considered to be obsolete [40].
In terms of legal regulation, probiotics in the Russian Federation are mainly used to treat gastroenterological diseases and cannot be registered as drugs that can be used to treat most of the diseases listed in Table 1 and recognised by NIH [41]. Regarding the levels of evidence, given that dietary supplements and drugs can be registered without specifying the bacterial strain they contain, there is no guarantee that the strain included in the formulation will always exhibit the properties declared by the manufacturer. Hence, it is rather obvious that stricter legal control of probiotics as drugs and dietary supplements is needed. The results of the analysis of legal control of probiotics are summarised in Table 3.
Table 3.
Analysis of legal regulation of probiotics in the Russian Federation (RF), the European Union (EU) and the United States (US)
| Indicator | RF | EU | US | |||
|---|---|---|---|---|---|---|
| BAS | Drug | BAS | Drug | BAS | Drug | |
| Controlling authority | Rospotrebnadzor |
Ministry of Healthcare |
EFSA | EMA | FDA | FDA |
|
Identification of the strain included in the product is required |
No | No | No | Yes | No | Yes |
| Documents regulating product registration |
Food hygiene safety requirements as formulated in the Customs Union Technical Regulation On food safety (TR TS 021/2011) [35] |
The requirements formulated in the Federal Law of the Russian Federation No. 61-FZ On Medicine Circulation General pharmacopoeia article OFS.1.7.1.0008.15 [38] |
The European Parliament and the European Union Council Regulation No. 1924/2006 on Nutritional and Health Claims (NHCR) issued December 20, 2006 [27] |
3053E General monograph on living biotherapeutic products (2019) Directive No. 2001/83/EC [6] |
US Federal Food, Drug and Cosmetic Act (abbreviated as FFDCA, FDCA or FD&C) [25] |
FDA. Early clinical trials of living biotherapeutic products: information on chemical composition, manfacture and control (2012, 2016) |
| Safety requirements |
Uniform Sanitary, epidemiological and hygienic requirements imposed on goods subject to sanitary and epidemiological supervision [35] |
General pharmacopoeia article OFS.1.7.1.0008.15 [38] |
Qualified Presumption of Security (QPS) [25, 26] | Guidelines for human cell-based medicinal products (EMA, 2008) [35] | GRAS or DSHEA [23] |
FDA. Early clinical trials of living biotherapeutic products: information on chemical composition, manufacture and control (2012, 2016) |
Functional food that contains prebiotics, probiotics, and other active ingredients and is intended for consumption by healthy people and is aimed at preventing diseases and maintaining human health is also worth mentioning here. General requirements imposed on probiotics, including dietary supplements, drugs, and functional foods, are formulated by the Federal Law on The Biological Safety in the Russian Federation [42]. The law was adopted by the State Duma on December 24, 2020 and approved by the Federation Council on December 25, 2020; the Law aims at protecting the population of the Russian Federation from hazardous biological factors and environmental protection, as well as at prevention of biological threats and risk monitoring. The formulation of the Law implies that it should be comprehensive. Here, we will focus on the clauses concerning human microbiota. Article 1 defines microbiota as all microorganism communities (symbiotic, opportunistic, and/or pathogenic) that inhabit the different parts of a living organism with homogeneous living conditions. Article 8 specifies that biological threats include disruption of the normal microbiota in humans, farm animals, and plants, leading to the development and spread of related diseases. Article 9 further states that preservation and restoration of normal microbiota in humans, farm animals, and rare and endangered animal and plant species is among the measures aimed at protecting the population of the Russian Federation. Article 10 mentions that the design and production of food, feed, and animal feed additives that are able to normalise microbiota is one of the measures aimed at controlling the spread of infectious and parasitic diseases and for prevention and treatment of diseases related to disruption of normal microbiota of humans, farm animals, and plants; measures are to be implemented to preserve or restore normal microbiota. Article 10, paragraph 7 of the Law states that the aim of preserving the biodiversity of human microbiota, with the ultimate goal of protecting the population from infectious diseases, is to: (1) provide for scientific research in order to understand the role of microbiota in maintaining human health; (2) develop approaches for the diagnosis, prevention, and treatment of certain diseases associated with microbiota disorders; and (3) use the potential of human, animal, and plant microbiota to develop new tools and biotechnologies, including personalised food and drugs. The adoption of the Federal Law No. 492-FZ On Biological Safety in the Russian Federation highlights the importance of studying human microbiota and maintaining its stability and gives hope that the research in this field will be further expanded.
POSTBIOTICS AND PARABIOTICS
Probiotics are live cultures, and for this reason they can in some cases, for example, in immunocompromised patients or in children, trigger pathological inflammatory processes. When live microbial cells are used, it is almost impossible to describe the precise summarised mechanism of action for all the bacterial cell components, and it is thus impossible to exactly predict the response of the macroorganism. In addition, standardisation of live cultures is challenging. These issues have led to the idea of using postbiotics, which are killed bacteria or their individual metabolites [43]. The International Scientific Association for Probiotics and Prebiotics (ISAPP) has defined a postbiotic as a preparation of nonliving microorganisms and/or their components that are beneficial to the host [44].
However, this definition was not found to be satisfactory by many researchers, as it refers both to killed bacterial cells, which are a conglomerate of different structures and metabolites, and to individual compounds. It was proposed to use the term postbiotic to refer to inactivated bacterial cells and their components, and to use the term parabiotic to refer to the individual compounds in probiotic bacteria [45].
Postbiotics are inactivated microbial cells with or without metabolites or cellular components (peptidoglycans, teichoic acids, surface proteins, extracellular vesicles, etc.) proven as safe and beneficial for the host. Inactivation of live bacteria can be achieved by different methods: thermal treatment, treatment with chemicals (e.g., formalin), gamma or ultraviolet irradiation, and ultrasound treatment. Thermal treatment remains the most popular method and involves a wide range of time and temperature combinations to ensure complete suppression of bacterial viability in the suspension. Inactivation can also be achieved by using a combination of tyndallization and freezing [46]. Injections, purified microbial metabolites, and vaccines go beyond the concept of postbiotics.
It is important to note that postbiotics, despite being nonviable, are a promising alternative to probiotics and offer a number of pharmaceutical advantages [47]. Heat-inactivated probiotics have demonstrated the ability to counteract the adhesion of various enteropathogens in the experimental model using Caco-2 cells [48]. These data suggest that postbiotics can be used to control diarrhoeal and foodborne pathogens. In addition, heat-inactivated Lactobacilli strains and cell-free cultural fluids were shown to exert immunomodulatory, antioxidant (the ability to neutralise free radicals) and anti-inflammatory effects in experimental models [49–53].
Parabiotics have advantages over postbiotics: their chemical structure is easily identifiable and a highly purified product can therefore be obtained. It is also easier to determine the dosage and shelf life time and to standardise the manufacture process. Symbiotic (probiotic) microbial strains produce various bioactive molecules that are candidates for parabiotics. These include enzymes, organic acids, glycoproteins, peptides (obtained by hydrolysis of food proteins and proper bacterial proteins), tryptophan and bile acid metabolites, polyamines, secreted proteins, exopolysaccharides, amino acids (including gamma-aminobutyric acid), short-chain fatty acids, polyphosphates, vitamins, antioxidants, bacteriocins, tocopherols, carotenoids, and many others [54–60]. The examples of commercial postbiotic and parabiotic preparations can be found in Table 4.
Table 4.
Post-biotic and parabiotic medicinal products
| Medicinal product | Composition | Product status |
|---|---|---|
| Hilac forte | Aqueous substrate of metabolic products of Escherichia coli, Enterococcus faecalis, Lactobacillus rhamnosus and Lactobacillus helveticus | Drug |
| Zacofalc | Inulin and butyric acid | BAS |
| Bactistatin | Biologically active metabolites from the cell-free cultural liquid of the Bacillus subtilis bacterium | BAS |
| Aktoflor-C | A complex of amino acids and organic acids, the analogues of probiotic bacteria metabolites | BAS |
| Pro-symbioflor | E. coli and E. faecalis cell autolysate | Drug |
| Helinorm | Inactivated Lactobacillus reuteri bacteria | BAS |
Postbiotics and parabiotics do not differ fundamentally in their mechanism of action from live probiotics. They modulate the intestinal microbiome, the immune system, the nervous system, and also strengthen the intestinal barrier and modulate metabolism. Unlike live probiotics, they don’t carry any risk of bacterial translocation from the intestinal lumen into the bloodstream in chronic and immunocompromised patients, the possibility of acquisition and transfer of antibiotic resistance genes, and the risks of viability loss due to cell lysis [61].
To date, there is no regulatory frameworks either for postbiotics as dietary supplements and, correspondingly, for food products containing them, or for postbiotics as drugs. Hence, postbiotics are controlled in the same way as any other substance depending on their scope of application. If it is a food product, appropriate safety tests are required. If it is a drug, clinical trials and proof of specific activity are required. However, it is clear that postbiotics are easier to register than LBPs. In Russia, postbiotics fall under the definition of a probiotic; a probiotic registered as a drug is identified as an immunobiological medicinal product that contains live or inactivated apathogenic microorganisms with antagonistic activity towards pathogenic and opportunistic bacteria [40].
Considering the difficulties in creating drugs based on living organisms, the shift towards prebiotics, postbiotics, and parabiotics is real and inevitable [62, 63].
LACTOBACILLI-BASED PROBIOTICS
Lactobacilli have long been used to produce fermented food products (FFP), according to some estimates, since the 7th century B.C., which is the time when the expansion of a sedentary life style and agrarian culture took place [64]. FFPs appealed to our ancestors as a way of preserving food and sometimes also as a way of eliminating toxicity [65, 66]. Today, a wide variety of FPPs can be found in different countries, ranging from kimchi in Korea, sauerkraut in the central Europe, chutney in India, natto in Japan, to various local fermented dairy products, etc. Lactobacilli have been regarded as a beneficial food supplement since the discovery of Lactobacillus bulgaricus by Dr. Stamen Grigorov [67] and the studies on its role in human health and longevity carried out by the Russian scientist I. Mechnikov [68].
Lactobacilli are now widely used to develop probiotics [69, 70]. Discoveries in the field of human microbiome in the last 2 decades have led to a deeper and more focused study of lactobacilli as probiotics, which has forced the pharmaceutical industry to note to the potential of using drugs based on living probiotic organisms to treat various diseases [71–77]. Omics technologies have made it possible to study both microbiota on the whole and individual bacteria at the level of their genomes. DNA sequencing can be used to determine the presence of drug resistance and pathogenicity genes in microbes and to select strains with useful genes for further study [78, 79].
The vast majority of probiotics contain Lactobacilli strains. This can be accounted for by several facts. Lactobacilli are permanent components of the human microbiota and exhibit properties that are beneficial and important for the macroorganism [80]. Lactobacilli are part of many food products and have long been used to produce fermented food [81]. Thirty-six Lactobacilli species are approved as safe by the EFSA (European Food Safety Authority) and 12 species were given the status of GRAS (Generally Recognized as Safe) by the FDA, which means they can be used as both food and feed additives. In addition, lactobacilli account for 43% of the total number of bacterial species with proven beneficial properties [82].
Lactobacilli-based probiotics have anti-inflammatory, immunomodulatory and antioxidant effects, facilitate dental and periodontal health, reduce cholesterol levels, and are used to combat obesity, cancer (antiproliferative and pro-apoptotic effects), diarrhoea, and mental and neurodegenerative diseases [70, 77]. A large number of Lactobacillus strains have completed phase IV clinical trials. Table 5 presents the examples of such strains.
Table 5.
Examples of live Lactobacillus strains that have completed Phase IV clinical trials
| Medicinal product | Disease under testing/ microbiota under study | Place of study |
|---|---|---|
| Lactobacillus reuteri DSM 17938 | Antibiotic-associated diarrhoea | Istanbul, Turkey |
| Lactobacillus rhamnosus GG | Infectious disease of the digestive tract | St Louis, Missouri, United States |
|
Lactobacillus acidophilus Rossel-52 and Lactobacillus rhamnosus Rosell-11 |
Flu-like disease | Jakarta, Indonesia |
| Lactobacillus reuteri DSM 17938 | Antibiotic-associated diarrhoea | Warsaw, Poland |
| Lactobacillus plantarum 299v | Irritable bowel syndrome | Eastern Cape, South Africa |
|
Lactobacillus paracasei subspecies paracasei F19 |
Gastrointestinal symptoms | Naples, Italy |
| Lactobacillus casei rhamnosus Lcr35 | Constipation | Warsaw, Poland |
| Lactobacillus reuteri DSM 17938 | Functional constipation | Castellana Grotte, Bari, Italy |
| Lactobacillus rhamnosus GG | Gut microbiota, skin microbiota, humoral immune responses, atopic dermatitis | Turku, Finland |
| Lactobacillus casei Shirota | Respiratory tract infections | Antwerp, Belgium |
Lactobacilli are included in preparations registered both as dietary supplements and as drugs. Examples of such preparations are given in Table 6. The species names of lactobacilli are given as they were used by the authors of the cited articles and in the names of dietary supplements and drugs, i.e., according to the old nomenclature. In 2020, the genus Lactobacillus was divided into 23 new genera with changed generic names and unchanged species names [83].
Table 6.
Lactobacillus-based medicinal products
| Name | Product composition | Product status |
|---|---|---|
| Russian medicinal products | ||
| Acipol | Lactobacillus acidophilus | Drug |
| Acylact | L. acidophilus | Drug |
| Lactobacillus | L. acidophilus | Drug |
| Florin forte | Bifidobacterium bifidum and Lactobacillus | Drug |
| Normoflorin D | Lactobacillus casei, B. bifidum, Bifidobacterium longum | BAS |
| Hepafor | B. bifidum, Lactobacillus fermentum | Drug |
| Imported medicinal products | ||
| Linex | Bifidobacterium infantis, L. acidophilius and Enterococcus faecium | Drug |
| Linex forte | L. acidophilus and Bifidobacterium animalis subsp. lactis | Drug |
| Maxilac |
L. helveticus, L. rhamnosus, L. casei, Lactobacillus plantarum, B. longum, Bifidobacterium breve, B. bifidum, Lactococcus lactis, Streptococcus thermophilus and oligofructose |
BAS |
| Buck-Set Forte |
L. casei, L. plantarum, L. rhamnosus, B. bifidum, B. breve, B. longum, L. acidophilus, L. lactis, S. thermophiles, B. infantis, Lactobacillus delbrueckii ssp. bulgaricus, L. helveticus, Lactobacillus salivarius and L. fermentum |
BAS |
| Normobakt | L. rhamnosus and fructooligosaccharides | BAS |
| RioFlora Immuno | Bifidobacterium, Lactobacillus, L. lactis and S. thermophilus | BAS |
| Fluvir | L. plantarum, L. rhamnosus, B. lactis and fructooligosaccharides | BAS |
***
Today, we are well aware of the detrimental effects of antibiotics on human microbiota and human health. Over the past decade, the global scientific community has been searching for and developing alternative approaches and antibacterial agents that could replace antibiotics [84–86]. Classical probiotics were among them. The research carried out in recent years has demonstrated that the consumption of probiotics and postbiotics leads to significant changes in the composition of intestinal microbiota at the species, genus, and larger taxonomic unit levels and its metabolic activity [87]. However, we often know nothing or very little about the ability of a particular strain to produce certain substances with antioxidant or immunomodulatory activity, as well as about the mechanisms of their action. For most bacteria used as probiotics, there is no data on the presence of drug resistance or pathogenicity genes in their genomes and on their specific effects on the structure (colonisation) of healthy human microbiota.
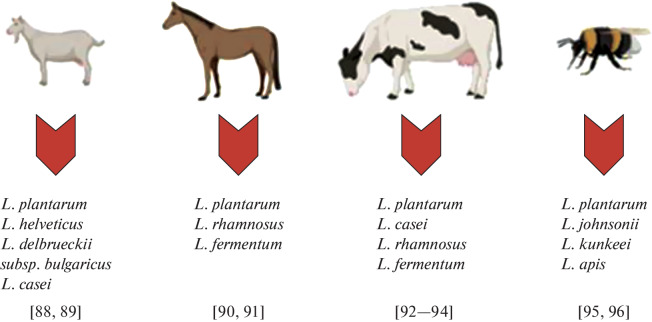
Postbiotics and parabiotics, bacterial metabolites and bacterial cell components are safer and more efficient compared to probiotics. While the strains that originate from the human body or from traditional food (due to their potential safety for humans) are used [66] to obtain live probiotic bacteria with postbiotics and parabiotics, the range of bacteria used to obtain them can be expanded. Considering that fermented dairy products of goat, cow, horse and bee origins are beneficial for human health, these animals may be a promising source in the search for unique bacterial strains with antioxidant and neuromodulatory activities for subsequent use as postbiotics and parabiotics to obtain pharmaceutical substances and functional foods (Fig. 3).
Fig. 3.
Nonconventional and promising sources of isolated lactobacilli strains for future use as postbiotics and ingredients for pharmaceutical substances and functional foods.
The COVID-19 pandemic and its consequences that affected hundreds of millions of people require urgent measures to rehabilitate the affected population. Modern lactobacilli-based products with antioxidant and immunomodulatory properties are essential for rehabilitation with their uses including as ingredients in functional foods.
An interdisciplinary consortium, the Nutrigenomics of the Microbiome, has been established to develop this field in Russia at the initiative of the Vavilov Institute of General Genetics of the Russian Academy of Sciences. Nutrigenomics, which emerged 15–20 years ago, is the branch of science that studies the impacts of biologically active food supplements on human gene expression and health. Intensive research on the human microbiome and its role in food utilisation today allows the concept of microbiome nutrigenomics to take shape [95].
The aim of the consortium is to develop pharmacological ingredients that are beneficial for specific groups of people, as well as the technologies that enable their production by taking advantage of such technologies as metagenomics, comparative genomics, transcriptomics, proteomics, and bioinformatics. The bases for the creation and subsequent development of Microbiome Nutrigenomics were the following results:
– biologically active components of human microbiota were described as the objects of research with the goal of developing personalised products: including prebiotics, postbiotics, and autobiotics;
– a significant role for postbiotics, prebiotics, and autobiotics in correcting gut microbiota composition was demonstrated;
– the functional properties of microbiota components were described including the immunomodulatory, antioxidant, and neuromodulatory properties;
- the global regulatory genes and cascade regulatory systems in the human body that respond to the immunomodulatory, neuromodulatory, and antioxidant activities of specific bacterial products were identified;
– human gut microbiota signatures of some diseases have been identified [78, 97].
What is now needed is for the consortium members to combine their efforts to carry out detailed research in the framework of specific tasks and to translate them into practical applications.
The post-COVID syndrome has affected hundreds of million people worldwide. It has been clearly demonstrated so far that many disease survivors show changes in the composition of their gut microbiome [dysbiosis], which correlate with various neurological, cardiological, and immunological diseases [98–100]. It turns out that classical probiotics (dietary supplements) are ineffective in correcting these disorders. The set goal is to develop pharmabiotics with selective properties that would be able to treat certain post-COVID disorders. The gut microbiota now is a material rather than a virtual organ that requires detailed evidence-based study.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare no conflicts of interest. No experimentation involving animals or human was performed by any of the authors.
Footnotes
Translated by E. Martynova
REFERENCES
- 1.Danilenko V., Devyatkin A., Marsova M., Shibilova M., Ilyasov R., Shmyrev V. J. Inflamm. Res. 2021;14:6349. doi: 10.2147/JIR.S333887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toole P.W., Marchesi J.R., Hill C. Nat. Microbiol. 2017;2:1–6. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- 3.Cordaillat-Simmons M., Rouanet A., Pot B. Exp. Mol. Med. 2020;52:1397–1406. doi: 10.1038/s12276-020-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson E., Cryan J.F., Fitzgerald G.F., Ross R.P., Dinan T.G., Stanton C. Proceedings of the Nutrition Society. 2014;73:477–489. doi: 10.1017/S0029665114001426. [DOI] [PubMed] [Google Scholar]
- 5.Hill C. Hum. Vaccines. 2008;4:271–374. doi: 10.4161/hv.4.4.6315. [DOI] [PubMed] [Google Scholar]
- 6.EDQM (European Pharmacopoeia) 3053E General Monograph on Live Biotherapeutic Products Published. 2019. [Google Scholar]
- 7.FDA . Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. 2012. [Google Scholar]
- 8.https://minzdrav.gov.ru/opendata/7707778246-grls.
- 9.Vergin F. Hipokrates. 1954;25:116–119. [PubMed] [Google Scholar]
- 10.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 11.Hill C. Bioeng. Bugs. 2010;1:79–84. doi: 10.4161/bbug.1.2.10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oleskin A.V., Shenderov B.A. Probiotics Antimicrob. Proteins. 2019;11:1071–1085. doi: 10.1007/s12602-019-09583-0. [DOI] [PubMed] [Google Scholar]
- 13.Yan F., Polk D.B. Front. Immunol. 2020;11:1428. doi: 10.3389/fimmu.2020.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stavropoulou E., Bezirtzoglou E. Front. Immunol. 2020;11:2192. doi: 10.3389/fimmu.2020.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markowiak P., Śliżewska K. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Adv. Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhat M.I., Kumari A., Kapila S., Kapil R. Ann. Microbiol. 2019;69:603–612. doi: 10.1007/s13213-019-01451-0. [DOI] [Google Scholar]
- 18.McFarland L.V., Evans C.T., Goldstein E.J.C. Front. Med. 2018;5:124. doi: 10.3389/fmed.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson G.R., Roberfroid M.B. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 20.Gibson, G.R., Hutkins, R., Sanders, M.E., Prescott, S.L., Reimer, R.A., Salminen, S.J., et al., Nat. Rev. Gastroenterol. Hepatol., 2017, vol. 14 P, pp. 491–502. [DOI] [PubMed]
- 21.Swanson K.S., Glenn R.G., Robert H., Raylene A.R., Gregor R., Kristin V., Karen P.S. Nat. Rev. Gastroenterol. Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suvorov A., Karaseva A., Kotyleva M., Kondratenko Y., Lavrenova N., Korobeynikov A. Front. Microbiol. 2018;9:1869. doi: 10.3389/fmicb.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mogensen G. Bull. Int. Dairy Fed. 2002;377:1–10. [Google Scholar]
- 24.Dickinson A. Fitoterapia. 2011;82:5–10. doi: 10.1016/j.fitote.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Gilsenan M.B. Nutrition and health claims in the European Union: a regulatory overview. Trends Food Sci. Technol. 2011;22:536–542. doi: 10.1016/j.tifs.2011.03.004. [DOI] [Google Scholar]
- 26.Ricci A., Allende A., Bolton D., Chemaly M., Davies R., Fernández Escámez P.S. EFSA J. 2018;16:e05315. doi: 10.2903/j.efsa.2018.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsoumanis, K., Allende, A., Álvarez-Ordóñez, A., Bolton, D., Bover-Cid, S., Chemaly, M., Davies, R., Hilbert, F., Lindqvist, R., and Nauta, M., EFSA J., vol. 17, no. 1, article ID e05555.
- 28.Gilsenan M.B. Trends Food Sci. Technol. 2011;22:536–542. doi: 10.1016/j.tifs.2011.03.004. [DOI] [Google Scholar]
- 29.FDA . Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information. 2016. [Google Scholar]
- 30.Paquet, J.C., Claus, S.P., Cordaillat-Simmons, M., Mazier, W., Rawadi, G., Rinaldi, L., et al., Front. Med., 2021, p. 1289. 10.3389/fmed.2021.716266 [DOI] [PMC free article] [PubMed]
- 31.NCCIH, Probiotics: What You Need to Know, NCCIH, 2011. https://nccih.nih.gov/health/probiotics/introduction.htm.
- 32.FDA . Benefit-Risk Assessment in Drug Regulatory Decision-Making. 2018. [Google Scholar]
- 33.Feuerstadt P., Louie T.J., Lashner B., Wang E.E., Diao L., Bryant J.A. N. Engl. J. Med. 2022;386:220–229. doi: 10.1056/NEJMoa2106516. [DOI] [PubMed] [Google Scholar]
- 34.www.clinicaltrials.gov/.
- 35.Committee for Medicinal Product for Human Use (CHMP), Guideline on Human Cell-Based Medicinal Products–EMEA/CHMP/410869/2006, 2008.
- 36.Rospotrebnadzor, What You Need to Know About Dietary Supplements. www.rospotrebnadzor.ru/about/info/news/news_details.php?ELEMENT_ID=11900.
- 37.Technical Regulation of the Customs Union “On Food Safety” dated December 9, 2011, no. 880 (TR TS 021/2011).
- 38.www.kremlin.ru/acts/bank/30941.
- 39.https://minzdrav.orb.ru/documents/active/7066/.
- 40.https://pharmacopoeia.ru/ofs-1-7-1-0008-15-probiotiki/.
- 41.Ivashkin V.T., Maev I.V., Abdulganieva D.I., Alekseenko S.A., Ivashkina N.Yu., Korochanskaya N.V. Ross. Zh. Gastroenterol., Gepatol. . Koloproktol. 2020;30:76–89. [Google Scholar]
- 42.www.kremlin.ru/acts/bank/46353.
- 43.Shenderov B.A. Microb. Ecol. Health. Dis. 2013;24:20399. doi: 10.3402/mehd.v24i0.20399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salminen S., Collado M.C., Endo A., Hill C., Lebee S., Quigley E.M. Nat. Rev. Gastroenterol. Hepatol. 2021;18:1–19. doi: 10.1038/s41575-020-00369-2. [DOI] [PubMed] [Google Scholar]
- 45.Aguilar-Toalá, J.E., Arioli, S., Behare, P., Belzer, C., Berni, CananiR., Chatel, J.M., et al., Nat. Rev. Gastroenterol. Hepatol., 2021. 10.1038/s41575-021-00521-6 [DOI] [PubMed]
- 46.Taverniti V., Guglielmetti S. Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caimari A., del Bas J.M., Boqué N., Crescenti A., Puiggròs F., Chenoll E., Martorell P., Ramón D., Genovés S., Arola L. J. Funct. Foods A. 2017;38:251–263. doi: 10.1016/j.jff.2017.09.029. [DOI] [Google Scholar]
- 48.Singh T.P., Kaur G., Kapila S., Malik R.K. Front. Microbiol. 2017;8:486. doi: 10.3389/fmicb.2017.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang H.J., Song M.W., Lee N.K., Paik H.D. J. Food Sci. Technol. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsova M., Abilev S., Poluektova E., Danilenko V. World. J. Microbiol. Biotechnol. 2018;34:27. doi: 10.1007/s11274-018-2410-2. [DOI] [PubMed] [Google Scholar]
- 51.Rocha-Ramírez L.M., Hernández-Ochoa B., Gómez-Manzo S., Marcial-Quino J., Cárdenas-Rodríguez N., Centeno-Leija S., García-Garibay M. Microorganisms. 2020;8:79. doi: 10.3390/microorganisms8010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zendeboodi F., Khorshidian N., Mortazavian A.M., Cruz A.G. Curr. Opin. Food Sci. 2020;32:103–123. doi: 10.1016/j.cofs.2020.03.009. [DOI] [Google Scholar]
- 53.Warda A.K. de Almeida, BettioP.H., Hueston, C.M., Di Benedetto, G., Clooney, A.G., and Hill, C. Front. Microbiol. 2020;11:69. doi: 10.3389/fmicb.2020.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh A., Vishwakarma V., Singhal B. Adv. Biosci. Biotechnol. 2018;9:147. doi: 10.4236/abb.2018.94012. [DOI] [Google Scholar]
- 55.Teame T., Wang A., Xie M., Zhang Z., Yang Y., Ding Q., Gao C., Olsen R.E., Ran C., Zhou Z. Front. Nutr. 2020;7:570344. doi: 10.3389/fnut.2020.570344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh S., Whitley C.S., Haribabu B., Jala V.R. Cell. Mol. Gastroenterol. Hepatol. 2021;11:1463–1482. doi: 10.1016/j.jcmgh.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novik G., Savich V. Microbes Infect. 2020;22:8–18. doi: 10.1016/j.micinf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Averina O.V., Zorkina Y.A., Yunes R.A., Kovtun A.S., Ushakova V.M., Morozova A.Y., Kostyuk G.P., Danilenko V.N., Chekhonin V.P. Int. J. Mol. Sci. 2020;21:9234. doi: 10.3390/ijms21239234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Averina O.V., Poluektova E.U., Marsova M.V., Danilenko V.N. Biomedicines. 2021;9:1340. doi: 10.3390/biomedicines9101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rani A., Saini K.C., Bast F., Mehariya S., Bhatia S.K., Lavecchia R., Zuorro A. Molecules. 2021;26:1142. doi: 10.3390/molecules26041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piqué N., Berlanga M., Miñana-Galbis D. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai Y.L., Lin T.L., Chang C.J., Wu T.R., Lai W.F., Lu C.C., Lai H.C. J. Biomed. Sci. 2019;26:3. doi: 10.1186/s12929-018-0493-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vallianou N., Stratigou T., Christodoulatos G.S., Tsigalou C., Dalamaga M. Curr. Obes. Rep. 2020;9:179–192. doi: 10.1007/s13679-020-00379-w. [DOI] [PubMed] [Google Scholar]
- 64.McGovern P.E., Zhang J., Tang J., Zhang Z., Hall G.R., Moreau R.A., Nuñez A., Butrym E.D., Richards M.P., Wang C.S., Cheng G. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17593–17598. doi: 10.1073/pnas.0407921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sokari T.G., Karibo P.S. Food. Add. Contam. 1992;9:379–384. doi: 10.1080/02652039209374084. [DOI] [PubMed] [Google Scholar]
- 66.Peivasteh-Roudsari L., Pirhadi M., Karami H., Tajdar-Oranj B., Molaee-Aghaee E., Sadighara P. J. Food Safe Hyg. 2019;5:1–9. [Google Scholar]
- 67.Fisberg M., Machado R. Nutr. Rev. 2015;73:4–7. doi: 10.1093/nutrit/nuv020. [DOI] [PubMed] [Google Scholar]
- 68.Mackowiak P.A. Front. Public Health. 2013;1:52. doi: 10.3389/fpubh.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arshad F.A., Mehmood R., Hussain S., Khan M.A., Khan M.S. Br. J. Res. 2018;5:43. doi: 10.21767/2394-3718.100043. [DOI] [Google Scholar]
- 70.Zhang Z., Lv J., Pan L., Zhang Y. Appl. Microbiol. Biotechnol. 2018;102:8135–8143. doi: 10.1007/s00253-018-9217-9. [DOI] [PubMed] [Google Scholar]
- 71.Chervinets, Y., Chervinets, V., Shenderov, B., Belyaeva, E., Troshin, A., Lebedev, S., and Danilenko, V., Probiotics Antimicrob. Proteins, 2018, vol. 10, no. 1, pp. 22–33. [DOI] [PubMed]
- 72.Lee E.S., Song E.J., Nam Y.D., Lee S.Y. J. Microbiol. 2018;56:773–782. doi: 10.1007/s12275-018-8293-y. [DOI] [PubMed] [Google Scholar]
- 73.Kim S.K., Guevarra R.B., Kim Y.T., Kwon J., Kim H., Cho J.H., Kim H.B., Lee J.H. J. Microbiol. Biotechnol. 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 74.Marsova M.V., Odorskaya M.V., Novichkova M., Polyakova V., Abilev S.K., Kalinina E., Shtil A., Poluektova E.U., Danilenko V.N. Microorganisms. 2020;8:876. doi: 10.3390/microorganisms8060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yunes R.A., Poluektova E.U., Vasileva E.V., Odorskaya M.V., Marsova M.V., Kovalev G.I., Danilenko V.N. Probiotics Antimicrob. Proteins. 2020;12:973–979. doi: 10.1007/s12602-019-09601-1. [DOI] [PubMed] [Google Scholar]
- 76.Nezametdinova V.Z., Yunes R.A., Dukhinova M.S., Alekseeva M.G., Danilenko V.N. Int. J. Mol. Sci. 2021;22:9219. doi: 10.3390/ijms22179219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poluektova E.U., Yunes R.A., Danilenko V.N. Nutrients. 2021;13:1591. doi: 10.3390/nu13051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Averina O.V., Kovtun A.S., Polyakova S.I., Savilova A.M., Rebrikov D.V., Danilenko V.N. J. Med. Microbiol. 2020;69:558–571. doi: 10.1099/jmm.0.001178. [DOI] [PubMed] [Google Scholar]
- 79.Kovtun A.S., Averina O.V., Alekseeva M.G., Danilenko V.N. Microb. Drug Resist. 2020;26:1307–1320. doi: 10.1089/mdr.2019.0325. [DOI] [PubMed] [Google Scholar]
- 80.George F., Daniel C., Thomas M., Singer E., Guilbaud A., Tessier F.J. Front. Microbiol. 2018;9:2899. doi: 10.3389/fmicb.2018.02899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernardeau M., Guguen M., Vernoux J.P. FEMS Microbiol. Rev. 2006;30:487–513. doi: 10.1111/j.1574-6976.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 82.Salvetti E., O’Toole P.W. Trends Food Sci. Technol. 2017;66:187–194. doi: 10.1016/j.tifs.2017.05.009. [DOI] [Google Scholar]
- 83.Zheng J., Wittouck S., Salvetti E., Franz C.M., Harris H., Mattarelli P. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 84.Kamaruzzaman N.F., Tan L.P., Hamdan R.H., Choong S.S., Wong W.K., Gibson A.J., Chivu A., Pina M.D. Int. J. Mol. Sci. 2019;20:2747. doi: 10.3390/ijms20112747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vivas R., Barbosa A.A., Dolabela S.S., Jain S. Microb. Drug Resist. 2019;25:890–908. doi: 10.1089/mdr.2018.0319. [DOI] [PubMed] [Google Scholar]
- 86.Wieërs G., Belkhir L., Enaud R., Leclercq S., Philippart de Foy J.M. Front. Cell. Infect. Microbiol. 2020;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valles-Colomer M., Falony G., Darzi Y., Tigchelaar E.F., Wang J., Tito R.Y. Nat. Microbiol. 2019;4:623–632. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 88.Barua R., Al Masud H.A., Mahmud N., Hakim M.A. IOSR J. Pharm. Biol. Sci. 2015;10:9–15. [Google Scholar]
- 89.Mulyawati, A.I., Jatmiko, Y.D., Mustafa, I., and Ardyati, T., InIOP Conf. Ser.: Earth Environ. Sci., 2019, vol. 230, no. 1, article ID 012104.
- 90.Kusdianawati Mustopa A.Z., Fatimah Budiarto B.R. Biodiversitas. 2020;21:3225–3233. [Google Scholar]
- 91.Wang D., Liu W., Ren Y., De L., Zhang D., Yang Y., Bao Q., Zhang H., Menghe B. Korean J. Food Sci. Anim. Res. 2016;36:499. doi: 10.5851/kosfa.2016.36.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alnakip M.E., Mohamed A.S., Kamal R.M., Elbadry S. Jpn. J. Vet. Res. 2016;64:23–30. [Google Scholar]
- 93.Azhari A.A. Int. J. Dairy Sci. 2011;6:66–71. doi: 10.3923/ijds.2011.66.71. [DOI] [Google Scholar]
- 94.Audisio M.C., Torres M.J., Sabaté D.C., Ibarguren C., Apella M.C. Microbiol. Res. 2011;166:1–13. doi: 10.1016/j.micres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Parichehreh S., Tahmasbi G., Sarafrazi A., Imani S., Tajabadi N. Apidologie. 2018;49:430–438. doi: 10.1007/s13592-018-0569-z. [DOI] [Google Scholar]
- 96.Dhar D., Mohanty A. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Badis A., Guetarni D., Moussa-Boudjemaa B., Henni D.E., Tornadijo M.E., Kihal M. Food Microbiol. 2004;21:343–349. doi: 10.1016/S0740-0020(03)00072-8. [DOI] [Google Scholar]
- 98.Zuo T., Zhang F., Lui G.C., Yeoh Y.K., Li A.Y., Zhan H., Wan Y., Chung A.C., Cheung C.P., Chen N., Lai C.K. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aktas B., Aslim B. Turk. J. Biol. 2021;45:390–403. doi: 10.3906/biy-2105-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jabczyk M., Nowak J., Hudzik B., Zubelewicz-Szkodzińska B. J. Clin. Med. 2021;10:4537. doi: 10.3390/jcm10194537. [DOI] [PMC free article] [PubMed] [Google Scholar]