Abstract
A 63-year-old woman who presented for orofacial dystonia showed cortical ribboning, a typical MRI finding in sporadic Creutzfeldt-Jakob disease (sCJD). However, real-time quaking-induced conversion (RT-QuIC), the most sensitive method for an early diagnosis of sCJD, was negative. She developed sCJD six months later, at which time RT-QuIC became positive. The cerebral blood flow showed a decrease in the cerebral cortex (especially in the supramarginal gyrus) consistent with cortical ribboning, but an increase in the basal ganglia, probably involved in orofacial dystonia. Cortical ribboning on MRI might be a better biomarker than RT-QuIC in the prodromal phase of sCJD.
Keywords: Creutzfeldt-Jakob disease, prion, cortical ribboning, orofacial dystonia, prodromal phase
Introduction
Sporadic Creutzfeldt-Jakob disease (sCJD) is a fatal, rapidly progressive neurodegenerative prion disease. Prior to the onset of symptoms, by up to 2.5 to 3 years, diffusion-weighted imaging (DWI) on magnetic resonance imaging (MRI) might identify a ribbon-like signal hyperintensity of the cerebral cortical gyri (cortical ribboning) (1-7), corresponding to the prion pathology (8). This finding suggests that the neuropathological changes of sCJD might start long before appearance of symptoms. Although it has been reported that cortical ribboning gradually expands before the onset of sCJD (1-7), there has been little comparison between the appearance of cortical ribboning and clinical tests, such as the cognitive function, cerebrospinal fluid (CSF) biomarkers, and electroencephalography (EEG) and single-photon emission computed tomography (SPECT) findings.
We found cortical ribboning on MRI DWI of a patient who visited our hospital for orofacial dystonia. This patient had no abnormalities in her cognitive function, CSF biomarkers, or EEG until the onset of sCJD, indicating that cortical ribboning on MRI DWI might be an excellent biomarker for the prodromal phase of sCJD.
Case Report
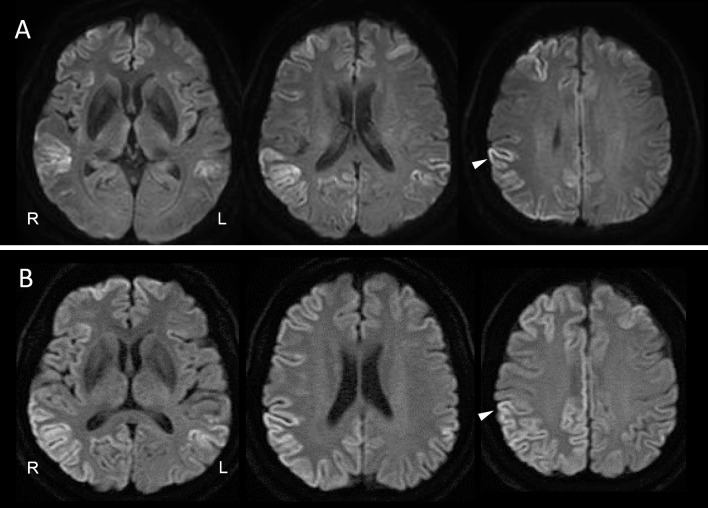
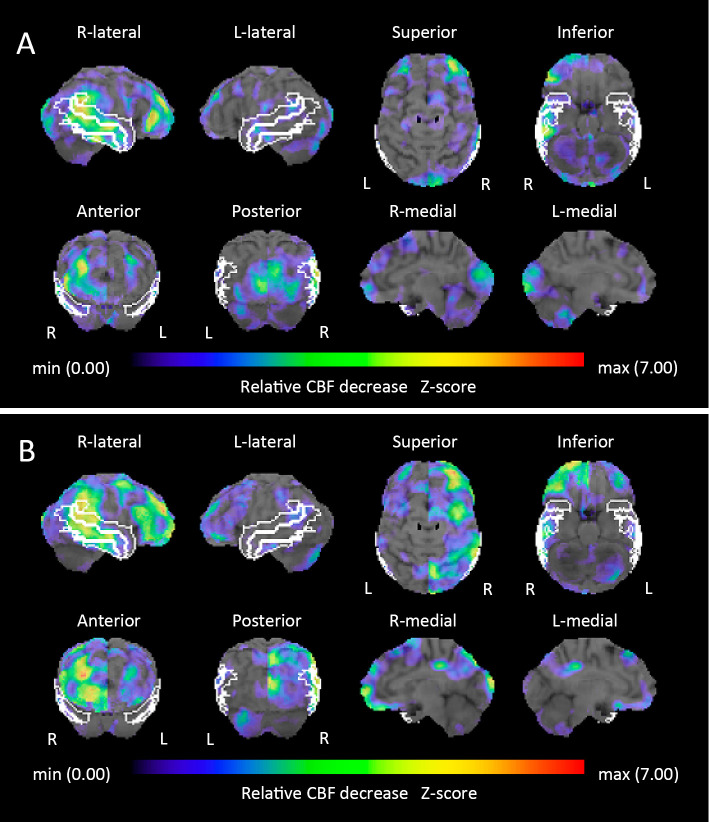
A 63-year-old Japanese woman who had had perioral dystonia for 3 years developed bilateral blepharospasm, which made it difficult for her to open her eyes. Since DWI on brain MRI showed cortical ribboning in the right frontal-temporal-parietal-occipital cortex and left parietal cortex (Fig. 1A), she was referred to our hospital. A neurological examination revealed no abnormalities other than the perioral dystonia and blepharospasm (orofacial dystonia). The patient had a mini-mental state examination (MMSE) score of 30/30. EEG showed no abnormalities. CSF showed mild protein elevation (49 mg/dL) without pleocytosis. Her total CSF tau protein was mildly elevated, but not enough to support a diagnosis of CJD (400 pg/mL; cut-off value for CJD diagnosis >1,300). Her CSF revealed a negative semiquantitative analysis of 14-3-3 protein. A real-time quaking-induced conversion (RT-QuIC) assay was negative. 123I-N-isopropyl-p-iodoamphetamine (123I-IMP) SPECT with three-dimensional stereotactic surface projection (3D-SSP) showed a reduced cerebral blood flow (CBF) in the right supramarginal gyrus, supra-, middle and inferior temporal gyri, inferior occipital gyrus, cuneus and left lingual gyrus (Fig. 2A). In contrast, CBF in the bilateral basal ganglia and left thalamus was mildly increased (Fig. 3A-a, 3A-b). Repeated MRI and the patient's MMSE score remained unchanged four months after her initial visit.
Figure 1.
Brain magnetic resonance imaging (MRI) diffusion-weighted imaging (DWI) at the patient’s first visit (A) and six months later at the clinical onset (B). Arrowheads indicate the right supramarginal gyrus (A, B).
Figure 2.
123I-N-isopropyl-p-iodoamphetamine (123I-IMP) single-photon emission computed tomography (SPECT) with three-dimensional stereotactic surface projection (3D-SSP). The Z-score (threshold 1.64) at the patient’s first visit (A): the right supramarginal gyrus (3.931); supra- (1.96), middle (2.285), and inferior (1.918) temporal gyri; inferior occipital gyrus (1.753); cuneus (1.842); and left lingual gyrus (1.653). The Z-score (threshold 1.64) six months later at the clinical onset (B): the right supramarginal gyrus (4.658); supra- (1.737), middle (2.577), and inferior (1.935) temporal gyri; superior (1.853) and middle (2.371) frontal gyri; orbital gyrus (2.715); and superior (1.935) and inferior (2.806) parietal lobules. The region of interest (ROI) of the supramarginal gyrus and supra-, middle, and inferior temporal gyri is indicated with a white line.
Figure 3.
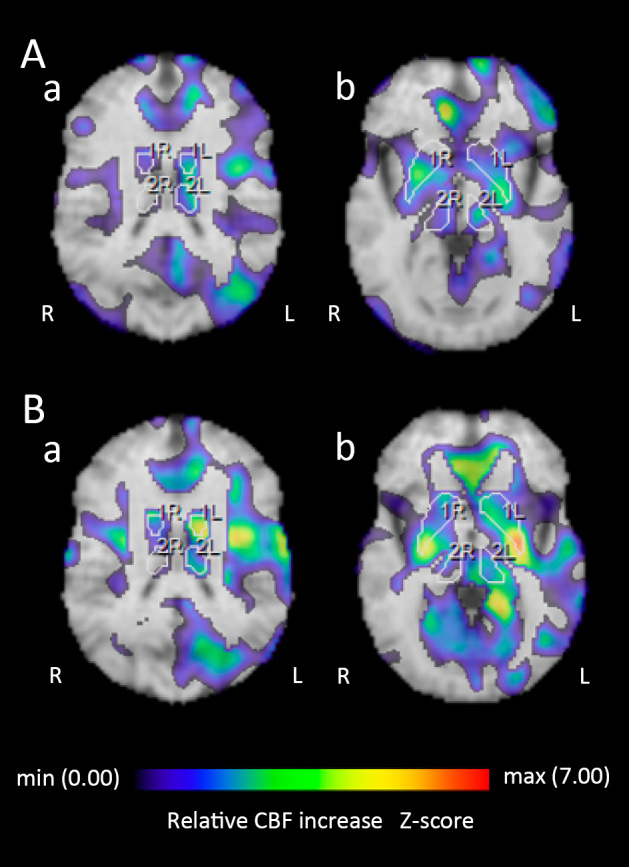
123I-N-isopropyl-p-iodoamphetamine (123I-IMP) single-photon emission computed tomography (SPECT) with three-dimensional stereotactic surface projection (3D-SSP). The Z-score (threshold 1.64) at the patient’s first visit (A-a): the left basal ganglia (1.86) and thalamus (1.71). (A-b): the right basal ganglia (1.92). The Z-score (threshold 1.64) six months later at the clinical onset (B-a): the left basal ganglia (3.90) and thalamus (1.79). (B-b): the left basal ganglia (2.86) and thalamus (1.92), and the right basal ganglia (1.99). The region of interest (ROI) of the basal ganglia and thalamus is indicated with a white line.
Two months later, the patient had difficulty holding a cup with her left hand. Neurological findings revealed limb and truncal ataxia as well as myoclonus in the left upper limb; however, no visual abnormalities, motor weakness, or parkinsonism was observed. The orofacial dystonia persisted as before. Her MMSE score was 21/30, and EEG showed possible periodic synchronous discharge. DWI showed cortical ribboning spreading to the entire cerebral cortex, primarily in the right hemisphere, but not in the basal ganglia (Fig. 1B). 123I-IMP SPECT with 3D-SSP showed a further reduction of the CBF in the right supramarginal gyrus. The reduction of the CBF in the right supra-, middle, and inferior temporal gyri was similar to that of the first analysis. However, the reduction of the CBF in the right inferior occipital gyrus and cuneus and the left lingual gyrus had returned to normal perfusion (Fig. 2B). The CBF in the bilateral basal ganglia and left thalamus was further increased (Fig. 3B-a, 3B-b). The CSF showed mild protein elevation (58 mg/dL) without pleocytosis, but the patient's total tau protein was markedly elevated (>2,200 pg/mL), and 14-3-3 protein was detected. An RT-QuIC assay detected trace amounts of prion protein. A prion protein gene analysis revealed homozygosity for methionine at codon 129, and no pathogenic mutation of the prion gene was detected.
A diagnosis of probable sCJD was made according to the criteria established by Hermann et al. (9).
Discussion
This patient experienced cortical ribboning for at least six months (prior MRI not available) before the clinical onset of sCJD. During the prodromal phase, neither EEG nor CSF biomarkers (total tau protein, 14-3-3 protein and RT-QuIC) showed abnormal findings consistent with the diagnosis of sCJD (9). The cognitive function, EEG findings, and CSF biomarkers in this case were abnormal for the first time at the clinical onset of sCJD, which is consistent with a previous report (7). RT-QuIC is a highly sensitive and specific biomarker that directly detects minute amounts of prions (9,10). Although we were unable to perform a Western blot analysis of proteinase K-resistant prion protein, the clinical features of this case were consistent with those of MM1-type sCJD (11). Although the sensitivity of RT-QuIC is very high in MM1 (93.0-96.6%) (10,12,13), RT-QuIC failed to detect prions in CSF during the prodromal phase when the cortical ribboning was observed. In addition, a reduced sensitivity of CSF surrogate biomarkers (total tau protein and 14-3-3 protein) and EEG has been shown in the early disease stages (9). These results suggest that cortical ribboning on MRI DWI might reveal a burden of pathological prion proteins at a much earlier stage than other tests.
It has been suggested that the basal ganglia play a role as a central network node in dystonia (14). However, the MRI DWI of this case did not show any lesions in the basal ganglia, suggesting that prion pathology was not the direct cause of the orofacial dystonia. In contrast, since overactivity of the basal ganglia and thalamus has been implicated in the development of orofacial dystonia (15-17), the increased CBF in the basal ganglia and thalamus might have been involved in the development of orofacial dystonia in this case. Furthermore, it has been shown that reduced functional connectivity between the left putamen and the right supramarginal gyrus can lead to orofacial dystonia (14). The marked decrease in CBF in the right supramarginal gyrus and the increase in CBF in the left basal ganglia might affect the functional connectivity between them, which might also contribute to the development of orofacial dystonia.
In conclusion, cortical ribboning, a typical MRI finding in sCJD, might appear several months earlier than prion protein detection by RT-QuIC. Therefore, cortical ribboning might be a better biomarker than RT-QuIC in the prodromal phase of sCJD. The orofacial dystonia in this case might have been due to the increased activity of the basal ganglia as well as the impaired functional connectivity between the right supramarginal gyrus and the left putamen.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Satoh K, Nakaoke R, Nishiura Y, et al. Early detection of sporadic CJD by diffusion-weighted MRI before the onset of symptoms. J Neurol Neurosurg Psychiatry 82: 942-943, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Terasawa Y, Fujita K, Izumi Y, Kaji R. Early detection of familial Creutzfeldt-Jakob disease on diffusion-weighted imaging before symptom onset. J Neurol Sci 319: 130-132, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Kawasaki A, Nagashima T, Hirata K. Diffusion-weighted MRI abnormalities antedate the onset of sporadic Creutzfeldt-Jakob disease. Neurology 87: 843-845, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Verde F, Ticozzi N, Messina S, et al. MRI abnormalities found 1 year prior to symptom onset in a case of Creutzfeldt-Jakob disease. J Neurol 263: 597-599, 2016. [DOI] [PubMed] [Google Scholar]
- 5.Zanusso G, Camporese G, Ferrari S, et al. Long-term preclinical magnetic resonance imaging alterations in sporadic Creutzfeldt-Jakob disease. Ann Neurol 80: 629-632, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Novi G, Canosa A, Nobili F, et al. Longitudinal brain magnetic resonance imaging and real-time quaking induced conversion analysis in presymptomatic Creutzfeldt-Jakob disease. Eur J Neurol 25: e127-e128, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Maeda K, Sugihara Y, Shiraishi T, Hirai A, Satoh K. Cortical hyperintensity on diffusion-weighted images as the presymptomatic marker of sporadic Creutzfeldt-Jakob disease. Intern Med 58: 727-729, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenmenger L, Porter MC, Carswell CJ, et al. Evolution of diffusion-weighted magnetic resonance imaging signal abnormality in sporadic Creutzfeldt-Jakob disease, with histopathological correlation. JAMA Neurol 73: 76-84, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermann P, Appleby B, Brandel JP, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol 20: 235-246, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhoads DD, Wrona A, Foutz A, et al. Diagnosis of prion diseases by RT-QuIC results in improved surveillance. Neurology 95: e1017-e1026, 2020. [DOI] [PubMed] [Google Scholar]
- 11.Parchi P, Giese A, Capellari S, et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46: 224-233, 1999. [PubMed] [Google Scholar]
- 12.Foutz A, Appleby BS, Hamlin C, et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol 81: 79-92, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini A, Baiardi S, Hughson AG, et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep 7: 10655, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jochim A, Li Y, Gora-Stahlberg G, et al. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav 8: e00894, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esmaeli-Gutstein B, Nahmias C, Thompson M, Kazdan M, Harvey J. Positron emission tomography in patients with benign essential blepharospasm. Ophthalmic Plast Reconstr Surg 15: 23-27, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Baker RS, Andersen AH, Morecraft RJ, Smith CD. A functional magnetic resonance imaging study in patients with benign essential blepharospasm. J Neuroophthalmol 23: 11-15, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt KE, Linden DE, Goebel R, Zanella FE, Lanfermann H, Zubcov AA. Striatal activation during blepharospasm revealed by fMRI. Neurology 60: 1738-1743, 2003. [DOI] [PubMed] [Google Scholar]