Abstract
Background and objective
Clinical prediction models determine the pre-test probability of pulmonary embolism (PE) and assess the need for tests for these patients. Coronavirus infection is associated with a greater risk of PE, increasing its severity and conferring a worse prognosis. The pathogenesis of PE appears to be different in patients with and without SARS-CoV-2 infection. This systematic review aims to discover the utility of probability models developed for PE in patients with COVID-19 by reviewing the available literature.
Methods
A literature search on the PubMed, Scopus, and EMBASE databases was carried out. All studies that reported data on the use of clinical prediction models for PE in patients with COVID-19 were included. Study quality was assessed using the Newcastle–Ottawa scale for non-randomized studies.
Results
Thirteen studies that evaluated five prediction models (Wells score, Geneva score, YEARS algorithm, and PERC and PEGeD clinical decision rules) were included. The different scales were used in 1,187 patients with COVID-19. Overall, the models showed limited predictive ability. The two-level Wells score with low (or unlikely) clinical probability in combination with a D-dimer level <3000 ng/mL or a normal bedside lung ultrasound showed an adequate correlation for ruling out PE.
Conclusions
Our systematic review suggests that the clinical prediction models available for PE that were developed in the general population are not applicable to patients with COVID-19. Therefore, their use is in clinical practice as the only diagnostic screening tool is not recommended. New clinical probability models for PE that are validated in these patients are needed.
Keywords: COVID-19, Pulmonary embolism, Diagnostic prediction model, Hypercoagulable state, Computed tomography pulmonary angiography, Thromboinflammation
Abstract
Antecedentes y objetivo
Las escalas de predicción clínica para embolia de pulmón (EP) determinan la probabilidad pretest y valoran la necesidad de las pruebas para estos pacientes. La infección por coronavirus se asocia a un mayor riesgo de EP aumentando su gravedad y confiriendo un peor pronóstico. La patogénesis de la EP parece ser diferente en pacientes con y sin infección por SARS-CoV-2. Esta revisión sistemática pretende conocer, revisando la bibliografía disponible, la utilidad de los modelos predictivos desarrollados para EP en pacientes con COVID-19.
Métodos
Se realizó una búsqueda bibliográfica en las bases de datos de PubMed, Scopus y EMBASE, incluyendo todos los estudios que comunican datos relacionados con la aplicación de escalas de predicción clínica para EP en pacientes con COVID-19. La calidad de los estudios se evaluó con la escala Newcastle–Ottawa para estudios no aleatorizados.
Resultados
Se incluyeron 13 estudios de cohortes que evaluaron cinco modelos predictivos (escala de Wells, puntuación de Ginebra, algoritmo YEARS y las reglas de decisión clínica PERC y PEGeD). Las diversas escalas se aplicaron en 1.187 pacientes con COVID-19. En general, los modelos tuvieron una capacidad predictiva limitada. La escala de Wells de dos categorías con probabilidad clínica baja (o improbable) en combinación con un dímero D < 3000 ng/mL o con una ecografía pulmonar a pie de cama normal mostraron una adecuada correlación para excluir la EP.
Conclusión
Nuestra revisión sistemática sugiere que las escalas de predicción disponibles para EP desarrolladas en población general no son aplicables a los pacientes con COVID-19 por lo que, de momento, no se recomienda su uso en la práctica clínica como única herramienta de cribado diagnóstico. Se necesitan nuevas escalas de probabilidad clínica para EP validadas en estos pacientes.
Palabras clave: COVID-19, Embolia pulmonar, Escala de predicción diagnóstica, Estado hipercoagulable, Tomografía computarizada de arterias pulmonares, Tromboinflamación
Introduction
Coronavirus disease 2019 (COVID-19) predisposes patients to the onset of arterial and venous thrombotic complications1, 2, 3, 4, 5. Numerous studies support the capacity of SARS-CoV-2 coronavirus to invade vascular endothelium cells through angiotensin-converting enzyme 2 (ACE-2) expressed on the cell surface6. This phenomenon induces endothelial inflammation, an increase in proinflammatory cytokine concentrations, activation of the complement, thrombin generation, and recruitment of platelets and neutrophils. It has been postulated that excessive immune system activation provokes a state of hypercoagulability that predisposes patients to thrombi formation7. Therefore, the concept of immunothrombosis (or thromboinflammation) has been proposed as a pathophysiological hypothesis underlying thrombosis in this population8.
Pulmonary embolism (PE) is the thrombotic event most frequently associated with COVID-19. Current evidence shows that this additional complication worsens the disease’s prognosis9, 10. In this regard, it is a priority to take action to diagnose it from the time the patient is admitted. Although there are certain parameters that allow for evaluating PE risk, such as the presence of high levels of D-dimer, C-reactive protein (CRP), lactate dehydrogenase (LDH), and myocardial damage markers, early detection of PE in patients with COVID-19 represents a challenge.
The diagnostic difficulty lies in the overlap of signs and symptoms that appear in acute respiratory distress syndrome (ARDS) associated with SARS-CoV-2 infection. This fact has caused a significant increase in the number of computed tomography pulmonary angiograms (CTPA), with the consequent increase in patients’ exposure to radiation and iodinated contrast. At the same time, it could increase the risk of nosocomial disease transmission due to the in-hospital transfer of the patient to the radiology department11. In addition, the cost of performing the diagnostic test must be noted.
For years, clinical practice guidelines on venous thromboembolism have recommended the use of scales developed for determining pretest probability for the diagnostic approach to patients suspected of having PE (Table 1 ). The Wells score12 and the Geneva score13 have been the most widely validated. Later, the YEARS algorithm14 and the PERC15 and PEGeD16 clinical decision rules were incorporated. These scales, together with a plasma D-dimer determination, can rule out PE in low-risk groups and thus, no more examinations are needed to rule out the diagnosis.
Table 1.
Predictive models for pulmonary embolism diagnosis.
| Diagnostic prediction scale | Wells score | Simplified Wells score | Revised Geneva Score, original version | Revised Geneva Score, simplified version | YEARS algorithm | PERC | PEGeD |
|---|---|---|---|---|---|---|---|
| Items | Clinical symptoms of DVT: 3 points Alternative diagnosis less probable than PE: 3 points Prior VTE: 1.5 points HR >100 bpm: 1.5 points Immobilization (≥ 3 days) or surgery in the previous 4 weeks: 1.5 points Hemoptysis: 1 point Active cancer: 1 point |
Clinical symptoms of DVT: 1 point Alternative diagnosis less probable than PE: 1 point Prior VTE: 1 point HR >100 bpm: 1 point Immobilization (≥ 3 days) or surgery in the previous 4 weeks: 1 point Hemoptysis: 1 point Active cancer: 1 point |
HR ≥95 bpm: 5 points Pain upon pressure in the palpable vein of the lower limb and unilateral edema: 4 points Prior VTE: 3 points Unilateral pain in lower limb: 3 points HR 75-94 bpm: 3 points Surgery under general anesthesia or lower limb fracture in the previous 4 weeks: 2 points Cancer (solid or hematological tumor that is active or cured in the last year): 2 points Hemoptysis: 2 points Age >65 years: 1 point |
HR ≥95 bpm: 2 points Pain upon pressure in the palpable vein of the lower limb and unilateral edema: 1 point Prior VTE: 1 point Unilateral pain in lower limb: 1 point HR 75–94 bpm: 1 point Surgery under general anesthesia or lower limb fracture in the previous 4 weeks: 1 point Cancer (solid or hematological tumor that is active or cured in the last year): 1 point Hemoptysis: 1 point Age >65 years: 1 point |
Clinical signs of DVT Hemoptysis PE as the most probable diagnosis D-dimer |
Age <50 years HR <100 bpm SaO2 >94% at room air No prior history of VTE No trauma or surgery that required hospitalization in the previous 4 weeks No hemoptysis No estrogen treatment No unilateral edema in the lower limbs |
High clinical probability scalea + D-dimer |
| Clinical probability result | High: >6 points Intermediate: 2–6 points Low: <2 points |
Probable: ≥2 points Improbable: ≤1 point |
High: ≥11 points Intermediate: 4–10 points Low: <4 points |
Probable: ≥3 points Improbable: ≤2 points |
High: • Presence of at least one of the three items + D-dimer ≥500 ng/mL • D-dimer ≥1,000 ng/mL in absence of any items |
High: Presence of at least one of the eight items and D-dimer ≥500 ng/mL Low: absence of items or presence of any and D-dimer <500 ng/mL |
Low: scale with low probability + D-dimer <1,000 ng/mL |
PE: pulmonary embolism; VTE: venous thromboembolism; HR: heart rate; DVT: deep vein thrombosis.
The three-category Wells score was used in the original sample.
The phenotype of PE in patients with COVID-19 seems to differ from that of patients without COVID-19. In patients with COVID-19, distal PE and a lower thrombus load are more common17 and the incidence of concomitant deep vein thrombosis is lower18. These differences are at least in part due to the fact that physiopathologically, a high percentage of events associated with infection by this virus is secondary to the development of in situ thrombosis.
The aim of this systematic review is to know the diagnostic yield of the available prediction scales (CPS) for patients with SARS-CoV-2 infection when PE is suspected.
Methods
According to the recommendations of the 2020 PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) declaration19, the following research question was formulated: If the physiopathology of COVID-19-associated PE is mainly attributed to a mechanism of immunothrombosis due to excessive release of inflammatory mediators, are the CPS developed for the general population applicable in these patients?
Search strategy and selection criteria
A bibliographic search was conducted on the PubMed, Scopus, and EMBASE databases aimed at locating studies that evaluated the diagnostic performance of clinical prediction models for the diagnosis of PE in adult patients hospitalized with COVID-19 from January 1, 2020 to March 31, 2022 with no restrictions on language.
The following search strategy was used, combining all terms of interest: coronavirus [Title/Abstract], COVID-19 [Title/Abstract], SARS-CoV-2 infection [Title/Abstract]), pulmonary embolism [Title/Abstract], prognostic models [Title/Abstract], predictive scores [Title/Abstract], and the terms Wells and Geneva [Title/Abstract].
All studies that presented data on the validation of at least one CPS were included. During the selection process, three of the review’s authors independently evaluated all documents obtained via the search strategy. After examining the titles and abstracts to eliminate unrelated articles, the full text of all remaining records was retrieved and verified based on eligibility criteria.
The quality of the studies was evaluated using the Newcastle–Ottawa scale for non-randomized studies. The following information was analyzed for each study selected: first author; data source; study period; median age; sex; hospital area; CPS used; and evaluation of the model's diagnostic yield in terms of sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and area under the ROC curve (AUROC), if available.
In accordance with the Swets classification20, the discriminative capacity of the score was considered adequate when AUROC values are between 0.8 and 1.
Results
Selection of studies
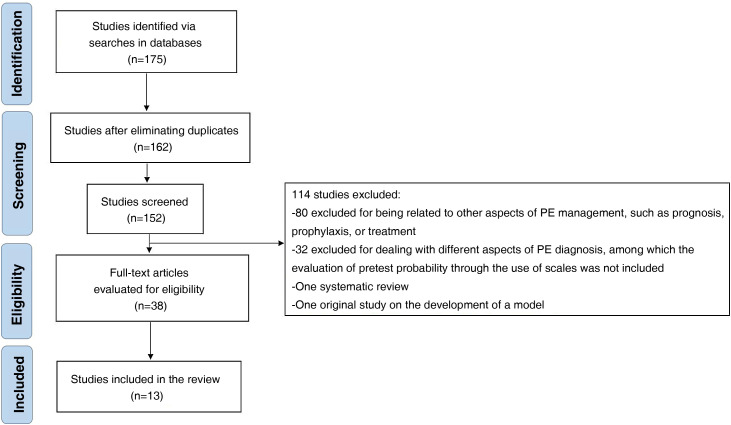
A total of 175 records were identified on the databases explored. After excluding duplicates, the title and abstract of 152 records were reviewed and 38 articles were selected for an extensive analysis. Finally, 13 studies were included in this review (Fig. 1 ). The methodological quality was high in 11 of them (Table 2 ).
Figure 1.
Study selection flowchart.
Table 2.
Quality of the studies included with the Newcastle–Ottawa scale evaluation.
| Study | Selection | Comparability | Outcome | Total score (risk of bias) |
|---|---|---|---|---|
| Whyte et al.21 | **** | * | *** | 8 (low) |
| Kirsch et al.22 | **** | * | *** | 8 (low) |
| Fang et al.23 | **** | * | *** | 8 (low) |
| Monfardini et al.24 | **** | * | *** | 8 (low) |
| Raj et al.25 | **** | * | *** | 8 (low) |
| Polo Friz et al.26 | **** | * | *** | 8 (low) |
| Kampouri et al.27 | **** | * | *** | 8 (low) |
| Zotzmann et al.28 | **** | * | *** | 8 (low) |
| Bagırtan et al.29 | ** | * | *** | 6 (high) |
| Jevnikar et al.30 | ** | * | ** | 5 (high) |
| Scardapane et al.31 | **** | * | ** | 7 (low) |
| Silva et al.32 | **** | * | *** | 8 (low) |
| Porfidia et al.33 | **** | * | ** | 7 (low) |
Characteristics of the studies included
Table 3 shows the characteristics of the studies included. Thirteen studies were included, all of which were retrospective cohort studies21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33.
Table 3.
Characteristics of the studies included.
| Author | Study period | Data source | Study type | Men, % | Median age, years | Patients hospitalized during the study period | Patients who underwent a radiological diagnosis | Patients with confirmed PE | Hospital area |
|---|---|---|---|---|---|---|---|---|---|
| Whyte et al.21 | March 3 to May 7, 2020 | United Kingdom | Retrospective | 60.2 | 61.0 | 1,477 | 214 | 80 (36 ICU patients) | Emergency department, hospitalization ward, and ICU |
| Kirsch et al.22 | February 1 to July 15, 2020 | United States of America | Retrospective | 54.7 | 54.9 | 459 | 64 | 12 | Emergency department, hospitalization ward, and ICU |
| Fang et al.23 | March 23 to April 19, 2020 | United Kingdom | Retrospective | 64.5 | 59.2 | 2,157 | 93 | 41 (12 emergency department patients, 16 hospitalization ward patients, and 13 ICU patients) | Emergency department, hospitalization ward, and ICU |
| Monfardini et al.24 | March 1 to 31, 2020 | Italy | Retrospective | With PE: 77.0 Without PE: 23.0 |
With PE: 61.0 Without PE: NA |
1,207 | 34 | 26 (8 ICU patients) | Emergency department, hospitalization ward, and ICU |
| Raj et al.25 | March 1 to December 1, 2020 | United States of America | Retrospective | NA | With PE: 63.0 Without PE: 55.0 |
1,300 | 109 | 26 | NA |
| Polo Friz et al.26 | April 1 to 30, 2020 | Italy | Retrospective | 27.0 | 71.7 | NA | 41 | 8 | Hospitalization ward and ICU |
| Kampouri et al.27 | February 28 to May 7, 2020 | Switzerland | Retrospective | 57.7 | 68.6 | 443 | 135 | 27 | Emergency department, hospitalization ward, and ICU |
| Zotzmann et al.28 | March 8 to May 31, 2020 | Germany | Retrospective | 70.0 | 61.6 | 113 | 20 | 12 | ICU |
| Bagırtan et al.29 | March 2020 to June 2021 | Turkey | Retrospective | 73.2 | 53.92 | NA | NA | 41 | Hospitalization ward and ICU |
| Jevnikar et al.30 | NA | France | Retrospective | NA | NA | NA | 106 | 15 | Emergency Department |
| Scardapane et al.31 | March 1 to April 30, 2020 | Italy | Retrospective | 51.1 | 65.0 | NA | 43 | 15 | Hospitalization ward and ICU |
| Silva et al.32 | April 1, 2020 to January 31, 2021 | Portugal | Retrospective | With PE: 47.8 Without PE: 60.6 |
With PE: 76.0 Without PE: 71.0 |
NA | 300 | 46 | Emergency Department |
| Porfidia et al.33 | • March 15 to April 10, 2020 • October 11 to November 27, 2020 |
Italy | Retrospective | 77.4 | 68.8 | 93 | 28 | 10 | Emergency department and hospitalization ward |
SD: standard deviation; PE: pulmonary embolism; NA: not available; ICU: intensive care unit.
The first study was published on July 31, 202022 and the last on March 4, 202229. The data originated in Italy, the United Kingdom, Germany, the United States, Switzerland, Portugal, France, and Turkey. The age of patients ranged from 37 to 84 years, with a greater proportion of men than women in most studies. In ten of the 13 studies, patients had a COVID-19 diagnosis confirmed with a laboratory microbiological test. The diagnosis of PE was considered present after confirmation with a computed tomography pulmonary angiogram (CTPA) or ventilation/perfusion lung gammagraphy.
The pretest probability of the various CPS was analyzed in 1,187 patients with COVID-19. The prevalence of PE was 27.8% (292/1,078), excluding the work by Bagırtan et al.29, which did not report the total number of patients with suspected PE. Among the 13 studies selected, five models were evaluated (Wells score, Geneva score, YEARS algorithm, and the PERC and PEGeD rules). The diagnostic yield of the Wells score was analyzed in six studies21, 22, 23, 24, 25, 26. A Swiss work analyzed the dichotomized Wells score together with D-dimer27 and a German study analyzed the Wells score together with a point-of-care lung ultrasound28. Bagırtan et al. used the Geneva score29, Jevnikar et al. used the YEARS algorithm30, and three other studies compared more than one clinical decision model in the same sample31, 32, 33.
Predictive capacity of the various scales
The results of the evaluation of the various CPS are shown in Table 4 .
Table 4.
Predictive capacity of the prediction scales analyzed in the studies included.
| Author | Predictive scale | Sensitivity, % | Specificity, % | NPV, % | PPV, % | AUROC | p (univariate analysis between the scale and presence of PE) | Authors’ conclusions |
|---|---|---|---|---|---|---|---|---|
| Whyte et al.21 | Wells ≥4 | NA | NA | NA | NA | NA | 0.951 | The Wells score did not show predictive capacity |
| Kirsch et al.22 | Wells ≥4 | NA | NA | NA | NA | 0.54 | 0.04 | The Wells score did not show predictive capacity |
| Fang et al.23 | Wells ≥4 | NA | NA | NA | NA | NA | 0.801 | The Wells score did not show predictive capacity |
| Monfardini et al.24 | Wells ≥4 | NA | NA | NA | NA | NA | NA | Of the 34 patients with Wells ≥4, 76% had PE and 24% did not |
| Raj et al.25 | Wells ≥4 + D-dimer ≥500 ng/mL | 96.1 | NA | NA | NA | NA | NA | The Wells score together with a D-dimer ≥500 ng/mL may be a strategy with predictive capacity |
| Polo Friz et al.26 | Wells ≥2 | 13 | 85.0 | 80.0 | 17.0 | NA | 0.851 | The Wells score did not show predictive capacity |
| Kampouri et al.27 | Wells ≥2 | 71.4 | 77.4 | 98.8 | 9.3 | 0.772 | NA | The Wells score together with a D-dimer cut-off point may be a strategy with predictive capacity |
| Wells ≥2 + D-dimer ≥3,000 ng/mL | 57.1 | 91.6 | 98.5 | 18.2 | 0.905 | |||
| Zotzmann et al.28 | Wells ≥2 | 90.0 | 70.0 | 87.0 | 75.0 | 0.813 | NA | The Wells score together with a pulmonary ultrasound showed excellent predictive capacity |
| Wells ≥2 + pulmonary ultrasound | 100 | 80.0 | 100 | 88.0 | 0.944 | 0.042 | ||
| Bagırtan et al.29 | Geneva | NA | NA | NA | NA | NA | NA | 92.7% of patients with PE were classified as low or intermediate risk |
| Jevnikar et al.30 | YEARS | NA | NA | NA | NA | NA | 0.08 | The YEARS algorithm could have avoided a CTPA in 39.7% of patients (39/98) |
| Scardapane et al.31 | Wells ≥4 | NA | NA | NA | NA | NA | 0.170 | The Geneva score showed better predictive capacity than the Wells score |
| Geneva ≥4 | 0.727 | 0.013 | ||||||
| Silva et al.32 | Wells ≥4 | 95.6 | 8.2 | 91.3 | 15.8 | 0.520 | 0.533 | None of the scales showed predictive capacity |
| Geneva ≥4 | 95.6 | 8.2 | 91.3 | 15.8 | 0.520 | 0.784 | ||
| YEARS | 86.9 | 31.1 | 92.9 | 18.6 | 0.589 | 0.150 | ||
| PEGeD | 84.7 | 31.2 | 91.8 | 18.3 | 0.580 | 0.063 | ||
| Wells ≥4 + age-adjusted D-dimer | 89.1 | 15.3 | 88.6 | 16.0 | 0.521 | NA | ||
| Geneva ≥4 + age-adjusted D-dimer | 89.1 | 15.3 | 88.6 | 16.0 | 0.521 | NA | ||
| Porfidia et al.33 | Wells ≥4 | NA | NA | NA | NA | NA | 0.27 | None of the scales showed predictive capacity |
| Geneva ≥4 | 0.27 | |||||||
| PERC | 0.27 | |||||||
| YEARS | 0.03 |
AUROC: area under the ROC curve; PE: pulmonary embolism; NA: not available; CTPA: computed tomography pulmonary angiogram; NPV: negative predictive value; PPV: positive predictive value.
Whyte et al. analyzed the original Wells score in 214 COVID-19 patients with suspected PE21. A total of 80 patients (37.38%) had PE. The proportion of patients with a high clinical probability was similar in subjects with and without PE (20/80 (25.0%) vs. 33/134 (22.3%), respectively; p = 0.951).
In the work by Kirsch et al., the Wells scale did not show capacity for discriminating between patients with and without PE (AUROC 0.54), though it had a smaller sample size and PE incidence22. Similar results were reported in the article by Fang et al.23 In another study conducted on a retrospective cohort of 34 patients, 76% of subjects with a Wells score ≥2 had PE24.
In the study by Raj et al., a Wells score ≥4 in combination with a D-dimer ≥500 ng/mL showed a sensitivity of 96.1%25. Finally, Polo Friz et al. analyzed the dichotomized Wells score in 40 COVID-19 patients with suspected PE26. The diagnosis was confirmed in eight patients (19.51%; 95% confidence interval (95% CI): 8.82–34.87). The sensitivity, specificity, PPV, and NPV were 13%, 85%, 17%, and 80%, respectively.
The two-category Wells score in combination with a D-dimer of ≥3,000 mg/dl was evaluated in 41 patients with COVID-19 with a PE incidence of 65.85%27. This strategy showed a sensitivity of 57.1%, a specificity of 91.6%, a NPV of 98.5%, a PPV of 18.2%, and an AUROC of 0.905. The validity of the scale together with a point-of-care lung ultrasound was used in 20 critical patients, of which 12 (60%) had PE28. The pathological findings on a chest ultrasound included B lines, irregular or fragmented pleura, subpleural consolidations, and pleural effusion. This method showed a sensitivity, specificity, NPV, and PPV of 100%, 80%, 100%, and 88%, respectively, as well as a good discriminatory capacity (AUROC 0.944).
The Geneva score analyzed in 41 patients with PE showed a low diagnostic yield29. Jevnikar et al. used the YEARS algorithm in 98 patients with COVID-19 attended to in the emergency department30. PE was diagnosed in 13 patients (13.2%). The use of the YEARS algorithm would have avoided 39 CTPA (39.79%) at the expense of not diagnosing one patient with PE. Three studies analyzed more than one CPS in the same sample.
Scardapane et al. used the original Wells model and the revised Geneva model in 43 patients with COVID-1931. The incidence of PE in the population studied was 34.88% (15/43). The predictive capacity of the Wells score was not considered adequate. On the contrary, the revised Geneva score showed better performance, with an AUROC of 0.727 (95% CI: 0.52–0.92). The study by Silva et al. analyzed the Wells, Geneva, YEARS, and PEGeD scores in 300 patients with COVID-19 in the emergency department with suspected PE32. The incidence was 15.55% (46/300). The models did not have discriminatory capacity, with an AUROC lower than 0.6 for all.
No differences were observed when analyzing the Wells and Geneva scores together with age-adjusted D-dimer. Similar results to these were obtained with the original Wells, Geneva, PERC, and YEARS scores in a retrospective cohort with a smaller number of patients (n = 28)33.
Discussion
In response to the research question, the available evidence analyzed in this work does not show, in general, predictive capacity in the pretest diagnostic yield of the various scales developed for PE in patients with COVID-19.
At present, it is recognized that a standardized diagnostic study by means of models to determine the pretest clinical probability and D-dimer may rule out the presence of PE with a fair degree of reliability, avoiding performing imaging tests that are not indicated. If the clinical probability is low and the D-dimer value is normal, no further studies are required, given that the NPV is 99%34. This recommendation makes even more sense in patients with COVID-19 due to risk of in-hospital transmission of infection during the transfer and the need to clean and disinfect the radiology ward.
The diagnostic difficulty in following this strategy in patients with COVID-19 lies in the fact that the clinical symptoms overlap with the characteristic symptoms of ARDS that patients with severe COVID-19 develop. In addition, high D-dimer levels are a common finding in these patients35.
In this systematic review, 13 retrospective cohort studies that evaluated five predictive models for PE in patients with COVID-19 were identified. In general, the original Wells score did not show discriminative capacity. In the works by Kampouri et al.27 and Zotzmann et al.28, the predictive capacity of the simplified version can be considered acceptable for ruling out the diagnosis of PE due to its high NPV. The discriminative power of this model improved significantly when it was combined with plasma D-dimer ≥3,000 ng/mL27.
Patients with COVID-19 have high levels of D-dimer in absence of thrombosis; thus, a lower specificity as a predictor of thrombotic events is to be expected and it is necessary to search for a higher cut-off value. As in the work by Kampouri et al.27, the data published in the SEMI-COVID-19 Registry reported that a cut-off point of >3,000 ng/mL was useful for predicting venous thromboembolism in these patients36.
In concordance with a combined proposal, similar results were reported in the work by Zotzmann when the score was associated with a pathological bedside chest ultrasound28. Both strategies were safe as they obtained an AUROC greater than 90%. The evidence found on the performance of the Geneva score, the YEARS algorithm, and the PERC and PEGeD clinical decision rules limit their use. The scores’ limited predictive capacity could be related to the physiopathological mechanism of PE in this population.
The available evidence highlights the role of thromboinflammation in patients with SARS-CoV-2 infection. Thus, viral endotheliitis and a hyperinflammatory state would activate the hemostatic system, triggering in situ vascular thrombosis37. According to this theory, the clinical probability models developed for PE in the general population would not have a good diagnostic yield in patients with COVID-19, mainly because they consider that PE generally originates in the context of deep vein thrombosis and not from a local lung phenomenon.
Another limitation lies in the item “diagnostic alternative less probable than PE” on the Wells score and the YEARS algorithm. Physicians may assume that ARDS is the cause of respiratory failure in these patients, except in the absence of pneumonia on the chest X-ray.
This review has limitations. The main limitation is that all studies included were retrospective, although 11 of the 13 studies had a low risk of bias. Second, the studies selected were not designed to analyze the predictive capacity of the scores evaluated. In this regard, the scales were used after confirming a diagnosis of PE, introducing a selective bias. In addition, some authors did not report the pretest capacity of the model in all clinical probability groups or the reliability of the scale through sensitivity, specificity, and AUROC values. Finally, the heterogeneity of the population studied, with the inclusion of patients from the emergency department, the hospitalization ward, and the ICU limited discovering the utility of the models in critical patients.
In conclusion, this study has aimed to clarify the applicability of various predictive models currently used for the diagnosis of PE in patients with COVID-19.
The data presented demonstrate a limited discriminatory capacity. An improbable pretest probability (≤1) in the two-category Wells score combined with a D-dimer <3,000 ng/mL or with a normal lung ultrasound conducted at the patient’s bedside could be sure strategies for ruling out PE and reducing the performance of unnecessary CTPA in patients with COVID-19 and suspected PE.
The benefit of these tools needs prospective validation. Developing and validating new predictive models for PE in patients with COVID-19 that allow for determining the probability of this complication is a priority.
Funding
This work has not received any type of funding.
Conflicts of interest
The authors declare that they do not have any conflicts of interest.
References
- 1.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.3949/ccjm.87a.ccc024. [DOI] [PubMed] [Google Scholar]
- 2.Mucha S.R., Dugar S., McCrae K., Joseph D., Bartholomew J., Sacha G.L. Coagulopathy in COVID-19: manifestations and management. Cleve Clin J Med. 2020;87:461–468. doi: 10.3949/ccjm.87a.ccc024. [DOI] [PubMed] [Google Scholar]
- 3.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez-Arbelaez D., Ibarra-Sanchez G., Garcia-Gutierrez A., Comanges-Yeboles A., Ansuategui-Vicente M., Gonzalez-Fajardo J.A. COVID-19-related aortic thrombosis: a report of four cases. Ann Vasc Surg. 2020;67:10–13. doi: 10.1016/j.avsg.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nascimento Conde J., Schutt W.R., Gorbunova E.E., Mackow E.R. Recombinant ACE2 expression is required for SARS-CoV-2 to infect primary human endothelial cells and induce inflammatory and procoagulative responses. mBio. 2020;11 doi: 10.1128/mBio.03185-20. e3185-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., et al. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22:95–97. doi: 10.51893/2020.2.pov2. PMID: 32294809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poor H.D. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. 2021;160:1471–1480. doi: 10.1016/j.chest.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez-Fajardo J.A., Ansuategui M., Romero C., Comanges A., Gómez-Arbeláez D., Ibarra G., et al. Mortality of COVID-19 patients with vascular thrombotic complications. Med Clin (Engl Ed) 2021;156:112–117. doi: 10.1016/j.medcle.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabre O., Rebet O., Carjaliu I., Radutoiu M., Gautier L., Hysi I. Severe acute proximal pulmonary embolism and COVID-19: a word of caution. Ann Thorac Surg. 2020;110:e409–e411. doi: 10.1016/j.athoracsur.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J., Ding N., Chen H., Liu X.J., He W.J., Dai W.C., et al. Infection control against COVID-19 in Departments of Radiology. Acad Radiol. 2020;27:614–617. doi: 10.1016/j.acra.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells P.S., Ginsberg J.S., Anderson D.R., Kearon C., Gent M., Turpie A.G., et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998;129:997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Wicki J., Perneger T.V., Junod A.F., Bounameaux H., Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: simple score. Arch Intern Med. 2001;161:92–97. doi: 10.1001/archinte.161.1.92. [DOI] [PubMed] [Google Scholar]
- 14.Van der Hulle T., Fogteloo A.J., Kooij S., Beenen L.F.M., van Bemmel T., van EsJ, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289–297. doi: 10.1016/S0140-6736(17)30885-1. [DOI] [PubMed] [Google Scholar]
- 15.Kline J.A., Mitchell A.M., Kabrhel C., Richman P.B., Courtney D.M. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2:1247–1255. doi: 10.1111/j.1538-7836.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- 16.Kearon C., de Wit K., Parpia S., Schulman S., Afilalo M., Hirsch A., et al. PEGeD Study Investigators. Diagnosis of pulmonary embolism with d-Dimer adjusted to clinical probability. N Engl J Med. 2019;381:2125–2134. doi: 10.1056/NEJMoa1909159. [DOI] [PubMed] [Google Scholar]
- 17.Van Dam L.F., Kroft L.J.M., van der Wal L.I., Cannegieter S.C., Eikenboom J., de Jonge E., et al. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: a different phenotype of thromboticdisease? Thromb Res. 2020;193:86–89. doi: 10.1016/j.thromres.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suh Y.J., Hong H., Ohana M., Bompard F., Revel M.P., Valle C., et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology. 2021;298:E70–80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 21.Whyte M.B., Kelly P.A., Gonzalez E., Arya R., Roberts L.N. Pulmonary embolism in hospitalised patients with COVID-19. Thromb Res. 2020;195:95–99. doi: 10.1016/j.thromres.2020.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch B., Aziz M., Kumar S., Burke M., Webster T., Immadi A., et al. Wells score to predict pulmonary embolism in patients with coronavirus disease 2019. Am J Med. 2021;134:688–690. doi: 10.1016/j.amjmed.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang C., Garzillo G., Batohi B., Teo J.T.H., Berovic M., Sidhu P.S., et al. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: relationship with pulmonary parenchymal disease. Clin Radiol. 2020;75:780–788. doi: 10.1016/j.crad.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monfardini L., Morassi M., Botti P., Stellini R., Bettari L., Pezzotti S., et al. Pulmonary thromboembolism in hospitalised COVID-19 patients at moderate to high risk by Wells score: a report from Lombardy, Italy. Br J Radiol. 2020;93 doi: 10.1259/bjr.20200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj K., Chandna S., Doukas S.G., Watts A., Jyotheeswara Pillai K., Anandam A., et al. Combined use of wells scores and D-dimer levels for the diagnosis of deep vein thrombosis and pulmonary embolism in COVID-19: a retrospective cohort study. Cureus. 2021;13 doi: 10.7759/cureus.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polo Friz H., Gelfi E., Orenti A., Motto E., Primitz L., Donzelli T., et al. Acute pulmonary embolism in patients presenting pulmonary deterioration after hospitalisation for non-critical COVID-19. Intern Med J. 2021;51:1236–1242. doi: 10.21203/rs.3.rs-73601/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kampouri E., Filippidis P., Viala B., Méan M., Pantet O., Desgranges F., et al. Predicting venous thromboembolic events in patients with coronavirus disease 2019 requiring hospitalization: an observational retrospective study by the COVIDIC Initiative in a Swiss university hospital. Biomed Res Int. 2020;2020 doi: 10.1155/2020/9126148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zotzmann V., Lang C.N., Wengenmayer T., Bemtgen X., Schmid B., Mueller-Peltzer K., et al. Combining lung ultrasound and Wells score for diagnosing pulmonary embolism in critically ill COVID-19 patients. J Thromb Thrombolysis. 2021;52:76–84. doi: 10.1007/s11239-020-02323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagırtan B., Altuntas E., Yasar S., Karabay K.O. Changing face of pulmonary embolism with COVID-19. Cardiovasc J Afr. 2022;33:1–5. doi: 10.5830/CVJA-2022-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jevnikar M., Sanchez O., Humbert M., Parent F. Prevalence of pulmonary embolism in patients with COVID-19 at the time of hospital admission and role for pre-test probability scores and home treatment. Eur Respir J. 2021;58 doi: 10.1183/13993003.01033-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scardapane A., Villani L., Bavaro D.F., Passerini F., Ianora A.A.S., Lucarelli N.M., et al. A pulmonary artery filling defects in COVID-19 patients revealed using CT pulmonary angiography: a predictable complication? Biomed Res Int. 2021;2021 doi: 10.1155/2021/8851736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva B.V., Jorge C., Plácido R., Mendonça C., Urbano M.L., Rodrigues T., et al. Pulmonary embolism and COVID-19: a comparative analysis of different diagnostic models performance. Am J Emerg Med. 2021;50:526–531. doi: 10.1016/j.ajem.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porfidia A., Mosoni C., Talerico R., Porceddu E., Lupascu A., Tondi P., et al. Pulmonary embolism in COVID-19 patients: which diagnostic algorithm should we use? Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.714003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douma R.A., Mos I.C., Erkens P.M., Nizet T.A., Durian M.F., Hovens M.M., et al. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism: a prospective cohort study. Ann Intern Med. 2011;154:709–718. doi: 10.7326/0003-4819-154-11-201106070-00002. [DOI] [PubMed] [Google Scholar]
- 35.Rostami M., Mansouritorghabeh H. D-dimer level in COVID-19 infection: a systematic review. Expert Rev Hematol. 2020;13:1265–1275. doi: 10.1080/17474086.2020.1831383. [DOI] [PubMed] [Google Scholar]
- 36.García-Cervera C., Giner-Galvañ V., Wikman-Jorgensen P., Laureiro J., Rubio-Rivas M., Gurjian Arena A., et al. SEMI-COVID-19 Network. Estimation of admission D-dimer cut-off value to predict venous thrombotic events in hospitalized COVID-19 patients: analysis of the SEMI-COVID-19 registry. J Gen Intern Med. 2021;36:3478–3486. doi: 10.1007/s11606-021-07017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Samkari H., Karp Leaf R.S., Dzik W.H., Carlson J.C.T., Fogerty A.E., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]