Abstract
The suspected endocrine disruptor nonylphenol (NP) is closely associated with anthropogenic activities; therefore, studies on this compound have been clustered in urban areas. This study investigated the NP concentrations in drinking water sources (n = 8), terminal tap water (n = 36), and human urine samples (n = 127) collected from urban and rural areas in Wuhan, China. The mean concentrations of NP measured in drinking water sources in urban and rural areas were 92.3 ± 7.5 and 11.0 ± 0.8 ng/L (mean ± SD), respectively, whereas the mean levels in urban and rural tap waters were 5.0 ± 0.7 and 44.2 ± 2.6 ng/L (mean ± SD), respectively. Nevertheless, NP was detected in 74.1% and 75.4% of the human urine samples from urban and rural participants, with geometric mean concentrations of 0.19 ng/mL (0.26 µg/g creat) and 0.27 ng/mL (0.46 µg/g creat), respectively. Although the NP concentrations measured in the drinking water sources of urban areas were significantly higher than those in rural areas (P < 0.05), the tap water and urine NP concentrations measured in urban areas were unexpectedly lower than those of rural areas (P < 0.05). Additionally, this investigation showed that the materials comprising household water supply pipelines and drinking water treatment processes in the two areas were also different. Our results indicated that the levels of exposure to NP in drinking water and human urine in rural areas were not necessarily lower than those in urban areas. Thus, particular attention should be paid to rural areas in future studies of NP.
Keywords: Nonylphenol (NP), Water, Urine, Rural, Urban
Introduction
In recent years, the environmental endocrine disruptor nonylphenol (NP) has been a growing concern for the environmental and scientific community because of its weak estrogenic activities (Jie et al. 2013, 2017). NP, a major degradation product of alkylphenol ethoxylates (Ruczyńska et al. 2020), is widely used in industrial production and is present in many daily necessities, such as detergents and plastics (Jie et al. 2016; Wang et al. 2019). Although some countries and regions, such as the United States of America (USA) and Europe (Pirard et al. 2012), have taken measures to limit the use of NP, in many countries of Asia, NP is still widely produced and used. According to the 2011 annual report of the China Petroleum and Chemical Industry Federation (CPCIF), the production of NP reached as high as 31,434 tons, accounting for 10% of the world’s total production (Jie et al. 2017), which has led to the environment being under great pressure from NP pollution.
As estrogen–mimetic compounds, the main mechanisms by which NP causes damage are by interacting with estrogen receptors and disrupting normal signaling pathways (Laws et al. 2000). Experiments in fish (Shirdel et al. 2020), rats (Kim et al. 2020), Caenorhabditis elegans (Cao et al. 2020), and other animals have confirmed the involvement of NP in the disruption of the reproductive system. A population survey also indicated that NP may induce male infertility by exerting a negative impact on sperm quality (Noorimotlagh et al. 2017). Furthermore, many studies have also evaluated the effects of NP on exposed individuals’ offspring, pregnancy, and lactation, which are key periods for the development of the nervous system (Qiu et al. 2019). Therefore, environments and populations exposed to NP have been an area of continuing research interest.
Numerous studies have shown an association between NP concentrations in environmental and anthropogenic activities (Berge et al. 2012; Sharma et al. 2009) demonstrating that NP preferentially enters the environment through urban sources. Thus, rural areas are thought to be of less concern. However, the detection of NP in various environmental media, such as water (Bhandari et al. 2021), soil (Hu et al. 2021), sediment (Careghini et al. 2015), and even in air (Bodziach et al. 2021; Xie et al. 2006), reveals the wide diffusion pathways of NP in the environment. Such a wide diffusion pathway may lead a large proportion of the population to the risk of exposure, which indicates that rural areas are likely to be exposed to this compound as well. However, studies of NP have clustered primarily on urban areas, and NP exposure levels in rural areas are rarely considered.
NP that enters the environment will eventually be incorporated into water matrices in various ways (Bhandari et al. 2021), leading to its widespread presence in aquatic environments, including drinking water sources. Drinking water is considered to be one of the main routes for human exposure (Guenther et al. 2002), as water exposure was demonstrated to be ubiquitous but persistent at low doses (Pirard et al. 2012; Yu et al. 2020). Therefore investigating drinking water is particularly important for evaluating the exposure levels of an area. While mounting research has suggested that NP contamination in environmental water occurs globally, there is still little information about the distribution of NP in drinking water.
Human biomonitoring surveys of compounds are important for determining the exposure level of a population and describing geographical differences, and urine is considered to be an appropriate matrix for NP biomonitoring (Dekant and Volkel 2008). Several countries, such as the USA (Calafat et al. 2005), Belgium (Pirard et al. 2012), Japan (Inoue et al. 2003), Korea (Park and Kim 2017), and China (Li et al. 2013; Peng et al. 2016), conducted surveys on the levels of NP in urine. Those studies showed that urinary NP levels vary significantly according to the population studied, suggesting that the differences in exposure levels may depend on geographical location. In addition, studies of urinary NP levels in different areas, especially between urban and rural areas, are still relatively scarce. Therefore, this study focused on the evaluation of NP exposure in environments and humans between geographical residence areas with distinctly different levels of economic development by examining NP levels in drinking water and human urine in urban and rural areas. The results of this study may provide helpful implications for pollution control and NP health risk management.
Materials and methods
Reagents and materials
Standard NP (CAS 25154–52-3, 99.9% purity) and the internal standard 4–n–nonylphenol (4–n–NP) (CAS 104–40-5, > 99% purity) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). HPLC grade acetone, acetonitrile, and ethyl acetate were obtained from Fisher Scientific (Fair Lawn, NJ, USA). β–Glucuronidase and sulfatase were acquired from Sigma–Aldrich (St. Louis, USA). Analytical grade ammonia, ammonium fluoride, acetic acid, sodium acetate hydrochloric acid, and other auxiliary reagents were all obtained from the National Pharmaceutical Group Chemical Reagent Co. Ltd. (Shanghai, China). Sample extraction and purification were completed with an Oasis HLB cartridge (Milford, MA, USA). In addition, ultrapure water was used throughout the study.
Preparation of standard solutions
NP and 4–n–NP (1 mg/mL) standard stock solutions were prepared in methanol, and then the stock standard solutions were diluted with methanol (MeOH) to obtain intermediate standard solutions of NP and 4–n–NP (10 μg/mL), which were further diluted and mixed using methanol to prepare 100 ng/mL mixed intermediate standard solutions. A series of mixed standard working solutions were prepared by diluting the mixed intermediate standard solutions with MeOH before use. All solutions described above were stored at 4 °C.
Study site and population
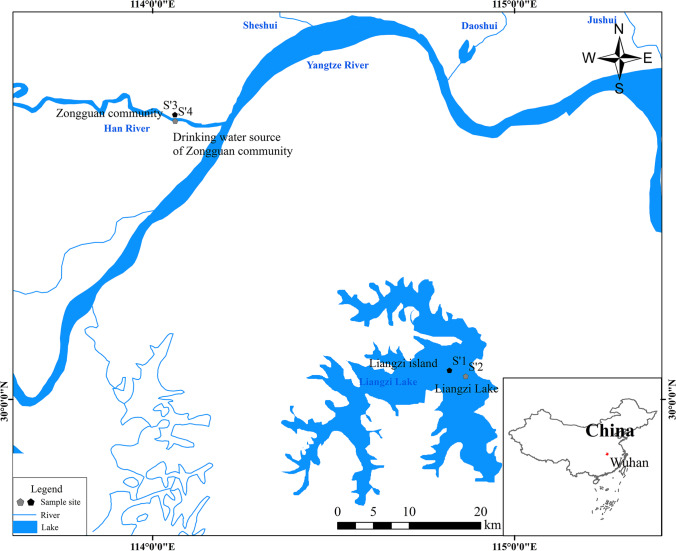
Two communities were chosen as study sites. These communities were representative of an urban and a rural area in Wuhan, China. One (the Zongguan community, S’3) located in the metropolitan downtown center of Wuhan with well-developed industry and commerce in the surrounding areas, industrial, and domestic sources are all the sources of NP in there. And another (the Liangzi Island, S’1) located on the periphery of Wuhan is an isolated island surrounded by Liangzi Lake without any industrial activities; the permanent residents are only 800, and no industrial source of NP exist in there. The corresponding drinking water sources of these two community were Han River water (S’4)and Liangzi lake water (S’2), respectively. The specific sites of this study are shown in Fig. 1.
Fig. 1.
Distribution of sampling locations within the Wuhan metropolitan region, China. S’1 is Liangzi Island, which remains a rural area and tourist destination. S’2 represents the rural drinking water source, Liangzi Lake, a large shallow lake on the outskirts of Wuhan. S’3 represents the Zongguan community, which is located in the middle of the city. S’4 is the urban drinking water
source located in the Han River, the largest tributary to the Yangtze River
Besides, to further study the possible differences between the two areas, an investigation on the materials of the household water supply pipelines and drinking water treatment processes in two areas was further conducted. As shown in Table 1, the water supply pipelines in urban areas were made of stainless-steel pipes, while in rural areas, hard polyvinylchloride (PVC) pipes were used. The urban drinking water treatment process can be briefly described as coagulation-sedimentation-filtration-disinfection, while in rural areas, it was a sedimentation-filtration process.
Table 1.
Information about material of household water supply pipelines and drinking water treatment process in two areas
| Areas | Material of household water supply pipelines | Drinking water treatment process |
|---|---|---|
| Liangzi island | Hard polyvinylchloride (PVC) | Sedimentation–Filtration |
| Zongguan community | Stainless-steel | Sedimentation–Filtration–Coagulation–Sedimentation |
A total of 127 participants aged between 18 and 90 years were selected: 58 from the Zongguan communities and 69 from the Liangzi Island. The age range (18–88) was select based on the age distribution of the population in the two areas, besides 3–17 year olds spend most of their day in school where basically not located in study area. Participants were chosen based on the inclusion that they had lived in the area for more than one year and no household water purification facilities had been installed in their home. Informed consent and a brief questionnaire (including data on age, sex, body size, and weight to obtain BMI status) were obtained from all participants before sampling.
Sample collection
Water samples were taken from 11 locations within the study area. In Zongguan community, 1 location was in drinking water source, and 6 were household taps within participant’s home; in Liangzi island, the specific number were 1 and 3, respectively. Water samples from the drinking water source of the urban and rural areas were collected 100 m upstream from the drinking water intake. The sampling locations of household tap waters were determined as follows. Because the residential building of Zongguan Community was a seven-floor building, which involved a secondary water supply, we randomly selected three household from the first to third floors and three household from the 4th to 7th floors for sampling. The Liangzi Island residential building was a low-level house, and three house was randomly selected for sampling. Of course, all households were selected from participants who had provided urine samples. Two liters of water sample was collected in a 2.5 L brown glass bottle from each location and stored at 4 °C until pretreatment. This process was repeated four times at an interval of 6 h each time, and a total of 44 water samplings were collected.
Urine samples in the two areas was completed on the same day in July 2019 with water sample. All participants were asked to provide first-morning urine voids in a 100 mL polypropylene container. And urine samples were stored at – 80 °C before analysis. The study was reviewed and approved by the Ethics Committee of Hubei Center for Disease Control and Prevention (Wuhan, China).
Sample preparation
The water samples were prepared in two steps as follows. Filtration: water samples (500–1000 mL) were weighed accurately and passed through a 0.45 μm glass fiber microporous filter membrane to remove suspended solids. Extraction: enrichment was performed on an OASIS HLB column (200 mg, 6 mL) previously conditioned with 5 mL of methanol and 5 mL of water. The analytes were eluted from the SPE cartridges with 10 mL of methanol, which were reconstituted in methanol to a final volume of 0.5 mL and passed through a 0.22 μm polytetrafluoroethylene (PTFE) syringe filter before ultra-performance liquid chromatography tandem mass spectrometry (UPLC–MS/MS) analysis.
The urine samples were prepared briefly as follows: 50 ng of 4–n–NP was added to 2 mL of urine, followed by buffering with 100 µL of sodium acetate (pH 5.5). After the addition of 10 µL of β‐glucuronidase/sulfatase, the mixture was incubated at 37 °C for 3 h. After cooling to room temperature, the dispersed sample solution was centrifuged at 3000 rpm. The supernatant was acidified with hydrochloric acid to a pH of 2–3 and then transferred to OASIS HLB cartridges (60 mg, 3 mL), which were previously conditioned with 5 mL of dichloromethane–methanol (9:1 v/v), 3 mL of methanol, and 3 mL of water (pH 3.0–3.5, adjusted with HCl). Initially, 3 mL of 5% methanol was used for cartridge washing, and subsequently, 5 mL of dichloromethane-methanol (9:1 v/v) was used for elution. Finally, the eluate was evaporated to dryness under a stream of nitrogen and reconstituted in 0.5 mL of methanol for UPLC analysis.
UPLC–MS/MS conditions
UPLC analyses on water samples were carried out using an Agilent 1290 liquid chromatograph (Agilent, CA, USA) with an Agilent Eclipse Plus C18 column (50 mm*2.1 mm, 1.8 μm). Mobile phases A and B were 1 mmol/L ammonium fluoride aqueous solution and 100% methanol, respectively. The system was run with a gradient program: 85% A held for 0.0–1.0 min, 85% A linear reduction to 5% A from 1.0 to 5.0 min, 5% A held from 5.0 to 8.0 min, and 5% A linear increase to 85% A from 8.0 to 8.1 min. The flow rate was 0.3 mL/min, the column temperature was 35 °C, and the injection volume was 5 µL. Mass spectrometry was carried out on a Triple Quad™ 3500 mass spectrometer (AB SCIEX, MA, USA). The selected parameters were as follows: ion source temperature: 550 °C, spray voltage: 5500 V, curtain gas: 35 psi, impact gas: 7 psi, and atomizing gas and auxiliary heating gas: 55 psi.
UPLC analyses on urine samples were carried out using a Waters Alliance Acquity-E2695 high-performance liquid chromatography system (Waters, MA, USA), and the analytical column was a ZORBAX 300SB–C18 (4.6 mm*150 mm, 5 μm). Mobile phases A and B were methanol and water, respectively. The gradient elution program used was as follows: 35% A–90% A (2 min), 90% A–100% A (3 min) and held for 2 min, and 100% A–35% A (0.5 min) and held for 2.5 min. The flow rate was 0.3 mL/min, the column temperature was 20 °C, and the injection volume was 10 µl. Mass spectrometry was carried out with a Waters Xevo TQD Triple quadrupole mass spectrometer (Waters, MA, USA) using electrospray ionization (ESI) in MRM scan mode. The selected parameters were as follows: capillary voltage: 3.0 kV, desolvation temperature: 350 °C, and desolvation gas: 800 L/h.
The optimum cone voltage, collision energy, and the characteristic ions for analytes and the internal standards are presented in Table 2.
Table 2.
Selected MRM transitions and optimized potentials of the target compounds
| Sample | Analytes | Precursor (m/z) | Product (m/z) | Cone (V) | CE (eV) |
|---|---|---|---|---|---|
| Water | NP | 219 | 133 | 110 | 45 |
| 4-n-NP | 106 | ||||
| Urine | NP | 219 | 133 | 50 | 20 |
| 4-n-NP | 106 |
Urinary creatinine adjustment
To normalize individual variation due to the differing hydration states of each participant at the time of sampling (Wang et al. 2016), NP concentrations were adjusted by creatinine levels. Therefore, urinary NP concentrations were presented in the following two forms: (a) as an uncorrected concentration (nanograms per liter, ng/L) and (b) as a concentration corrected according to creatinine levels (micrograms per gram creatinine, µg/g creat).
Validation study
The analytical method was validated to illustrate the limit of quantitation (LOQ), accuracy, precision, and recovery of the measurements. Standard solutions of NP and 4–n–NP at low, medium, and high concentrations were added to the samples, and 6 parallel samples were set at each concentration to calculate the spiked recovery rate and relative standard deviation (RSD). The limits of detection (LODs) and LOQs of the method were determined by three times and ten times the signal–to–noise ratios, respectively. As shown in Table 3, the precision and accuracy of this method met the requirements of detection.
Table 3.
Linearity, limit of detection (LOD), method limit of quantitation (LOQ), and the method recoveries and precision for target compounds
| Sample | Analytes | Linear range (ng/ml) | R2 | LOD (ng/ml) | LOQ (ng/ml) | Spiked concentrations (ng/ml) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|---|
| Water | NP | 0.1–100 | > 0.999 | 0.002 | 0.006 | 4, 10 | 65.3–105 | 1.3–1.4 |
| 4-n-NP | 71.9–87.8 | 1.9–2.0 | ||||||
| Urine | NP | 0.1–100 | > 0.999 | 0.03 | 0.1 | 1, 5, 10 | 96.8–101.2 | 6.58–8.8 |
| 4-n-NP | 95.6–102.3 | 8.29–9.2 |
Statistical analysis
Concentrations below the LOD were counted as half the LOD. All statistical analyses were performed with SPSS software, version 16.0 for Windows (SPSS Inc., USA). An independent-samples t test was employed to test the difference in NP concentration in drinking water between the two areas. A nonparametric Mann–Whitney U test was used to analyze the differences in urine NP concentration between two areas. Analysis of variance (one-way ANOVA) was used to compare the baseline characteristics of the participants in two areas. Statistical significance was accepted at P < 0.05 for all comparisons.
Results
NP in drinking water sources and tap waters
The concentration of NP in different water samples across the study area are shown in Table 4. NP was detectable in all 44 water samples with an LOD of 2.0 ng/L. The mean (SD) concentration of NP measured in drinking water sources in urban and rural areas were 92.3 ng/L (7.5) and 11.0 ng/L (0.8), with a range of 82.1–98.9 ng/L and 10.0–11.9 ng/L, respectively. While, in terminal tap water, the average concentration in urban and rural areas were 5.0 ng/L (0.7) and 44.2 ng/L (2.6), with a range of 4.0–6.0 ng/L and 41.7–47.8 ng/L, respectively. In urban areas, the NP concentration in drinking water sources was markedly higher than that of terminal tap water (P < 0.01), while in rural areas, the NP concentration in drinking water sources was unexpectedly lower than that of terminal tap water (P < 0.01). A comparison of the NP concentrations in the tap water and drinking water sources between the two areas is presented in Fig. 2. The concentration of NP in terminal tap water in urban areas was statistical lower (P < 0.05) than that in rural areas, although the NP concentration of drinking water sources in urban areas was significantly higher (P < 0.05) than that in rural areas.
Table 4.
Comparison of NP concentration in drinking water samples in two areas
| Sample site | n | Range of detected levels of water NP (ng/L) | Water NP concentration (ng/L) Mean (SD) |
P value |
|---|---|---|---|---|
| Liangzi island | < 0.01 | |||
| Drinking water source | 4 | 10.0–11.9 | 11.0 ± 0.8 | |
| Tap water | 12 | 41.7– 47.8 | 44.2 ± 2.6 | |
| Zongguan community | < 0.01 | |||
| Drinking water source | 4 | 82.1–98.9 | 92.3 ± 7.5 | |
| Tap water | 24 | 4.0–6.0 | 5.0 ± 0.7 |
Fig. 2.
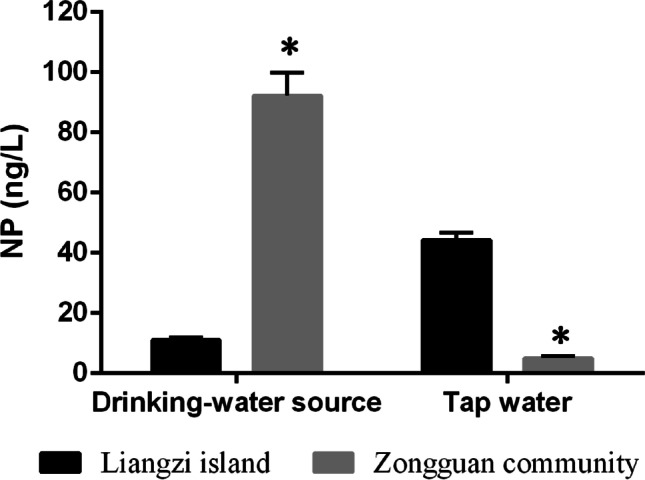
Comparison of the NP concentration among the drinking water
source and terminal tap water of the two areas (ng/L). Quantitative analyses of the NP concentrations between the drinking water source and terminal tap water of the two areas. Each bar denotes the mean ± SD. *P < 0.05 as compared to Liangzi Island
Urine NP levels in urban and rural inhabitants
A total of 127 urine samples (n = 58 and 69 for the urban and rural areas, respectively) from urban and rural areas of Wuhan, China, of nonoccupationally exposed mixed populations were collected. All participants were categorized in three age groups (≤ 40, 40–60, ≥ 60). Participants were 67.2% and 66.7% female for urban and rural areas, respectively, with the mean (SD) ages of 54.1 (13.5) and 54.1 (10.6) years. The baseline characteristics of the participants in these two areas are presented in Table 5. There were no significant baseline differences between the urban and rural participants in terms of sex distribution, age range, or BMI. The frequency of detection, geometric mean (GM), percentile, and range concentrations for urine NP in the two areas are detailed in Table 6. The detection rates of NP in urban and rural areas were 74.1% and 75.4%, respectively, with an LOD of 0.03 ng/mL. This result suggested that NP exposure to the Wuhan population was quite prevalent. The GM concentration for NP in urban areas was determined to be 0.19 ng/mL (0.26 µg/g creat), ranging from ND (not detected) to 1.69 ng/mL (from ND to 13.59 µg/g creat), while the concentration in rural areas was 0.27 ng/mL (0.46 µg/g creat), with a range of ND to 4.24 ng/mL (from ND to 20.32 µg/g creat). Significant differences (P = 0.01) were found between the two geographical areas in terms of urine NP levels. Participants from rural areas exhibited higher urinary NP concentrations than their urban counterparts.
Table 5.
Baseline characteristics of participants in two areas
| Zongguan community | Liangzi island | P value | |
|---|---|---|---|
| N | 58 | 69 | |
| Age, n (%) | 0.49 | ||
| ≤ 40 | 8 (13.8) | 8 (11.6) | |
| 40–60 | 31 (53.4) | 44 (63.8) | |
| ≥ 60 | 19 (32.8) | 17 (24.6) | |
| Gender, n= (%) | 0.95 | ||
| Female | 39 (67.2) | 46 (66.7) | |
| Male | 19 (32.8) | 23 (33.3) | |
| Height, mean (SD), cm | 160.9 (7.2) | 162.5 (8.3) | 0.26 |
| Weight, mean (SD), kg | 62.4 (13.0) | 61.4 (10.3) | 0.63 |
| Body mass index, mean (SD) | 23.6 (5.3) | 23.2 (3.0) | 0.58 |
Table 6.
Number of individuals (N), frequency of detection, geometric mean, range levels, and percentile for urinary NP in two areas
| Zongguan community | Liangzi island | |
|---|---|---|
| N (Positive Samples %) | 58 (74.1%) | 69 (75.4%) |
| Geometric mean μg/ml (μg/g creat) | 0.19 (0.26) | 0.27 (0.46) |
| Range μg/ml (μg/g creat) | 0.02–1.69 (0.02–13.59) | 0.02–4.24 (0.02–20.32) |
| 10th μg/ml (μg/g creat) | 0.02 (0.02) | 0.02 (0.02) |
| 50th μg/ml (μg/g creat) | 0.21 (0.29) | 0.34 (0.48) |
| 90th μg/ml (μg/g creat) | 0.79 (1.38) | 1.03 (2.40) |
| Mann–Whitney U | 1479 | |
| P value | 0.01 | |
Discussion
To fully assess the difference in NP exposure levels between urban and rural areas, we selected both drinking water NP and urinary NP as external and internal exposure indicators, and the levels of both indicators between the two areas were compared. Comparison of the results of the levels of NP in drinking water between the urban and rural areas were noteworthy. In drinking water sources, the NP concentration measured in urban areas was significantly higher than that of rural areas (P < 0.05), while the measured NP concentration in terminal tap water of the urban area was unexpectedly lower than that of rural areas (P < 0.05). The literature has shown that pollutants from discharged sewage effluent are the main factor that causes NP pollution in drinking water sources (Jie et al. 2017). With regard to the sampling locations in this study, the urban drinking water source was located in the Han River, which is one of the main tributaries to the Yangtze River, with well-developed industries and densely populated neighborhoods in the surrounding area. The Han River has become a main recipient water body for sewage treatment plants. On the other hand, the rural drinking water source was located in Liangzi Lake, where the wetland nature reserve of Hubei Province, where sewage discharge has been prohibited. The significant differences in NP concentration in drinking water sources between the two areas may be due to the fact that the Han River receives more effluents from sewage treatment plants and domestic households, leading to higher NP contamination than its rural counterpart, Liangzi Lake.
As described above, the materials of household water supply pipelines and drinking water treatment processes between the two areas were also different. Many studies have revealed that NP is usually used as a heat stabilizer for PVC and can be released into drinking water from household facilities (Cheng et al. 2015; Loyo-Rosales et al. 2004). Our results showed that the NP concentration in PVC drinking water pipes was higher than that of stainless steel pipes, which is consistent with previous studies (Cheng et al. 2015), verifying that the water could absorb NP from PVC pipes. Furthermore, the urban drinking water treatment process is more sophisticated and can be briefly described as coagulation-sedimentation-filtration-disinfection, while in rural areas, it is simply a sedimentation-filtration process. It has been previously shown that different water treatment processes exhibit different elimination efficiencies for endocrine disruptors (Chen et al. 2013; Gilca et al. 2020). The differences in the drinking water treatment processes between these two areas may be another factor that caused the higher NP concentration in terminal tap water samples in rural areas.
Literature data concerning the levels of NP in drinking water are scarce in comparison with those of environmental waters. The presence of NP in tap water and drinking water sources in both urban and rural areas implied that there was NP contamination in the drinking water in Wuhan. A comparison between other cities and our survey in Wuhan was conducted for the NP concentrations in tap water and drinking water sources. We found that the concentrations measured in the present study, ranging from 4.0 to 96.2 ng/L, were markedly lower than those in other cities in China, such as Shanghai (Ma et al. 2006), Shenyang (Tang et al. 2005), Chongqing (Shao et al. 2002), Zunyi (Jie et al. 2017), Suzhou (Chen 2013), and Taiwan (Dai et al. 2019), but were higher than those in Europe, including Spain (Valcárcel et al. 2018) and Portugal (Carvalho et al. 2015). As NP mainly originates from industrial discharge (Fürhacker et al. 2000; Soares et al. 2008), the relatively slight pollution from NP in Wuhan compared to other reported cities in China might have benefited from the restrictions on industrial production that started in June 2019 and lasted for 5 months, for the sake of the 7th CISM Military World Games taking place later during that period. It was not surprising that the concentration of NP in drinking water in Wuhan was higher than that in other cities of Europe given that NP has been restricted for several years in those districts as a hazard to human and environmental safety, while in Asia, NP is still being used.
The concentrations of NP in urine differs widely in various countries worldwide. For example, Park and Kim (2017) found urinary NP in 83.2% of 1865 Koreans aged 18–69 years with a GM concentration of 3.70 ng/mL; Li et al. (2013) positively detected urinary NP in 100% of 287 children and students aged 3 to 24 years in Guangzhou, China, and the GM concentration was as high as 17.40 ng/mL; and Pirard et al. (2012) found that NP was not detected in any sample in a general Belgian population. The concentration of NP detected in urban (0.19 ng/mL) and rural (0.27 ng/mL) areas of Wuhan was lower than that found in Guangzhou, China, but was much higher than that found in Belgium. The concentrations of NP obtained from different populations can be influenced by many factors, such as the age range studied, geographical differences, and study design (Peng et al. 2016). In terms of age, it can be reasonably believed that teenagers normally use more plastic products than the older population and therefore could be more frequently exposed to NP (Pirard et al. 2012). The concentration of NP detected in Wuhan, which was lower than that found in Korea and Guangzhou, China, may be related to the fact that the survey population in this study was mainly middle-aged, while in the studies from Guangzhou and Korea, (especially Guangzhou) contained mostly teenagers.
Differences in urinary NP levels related to sex, age, BMI, and urban or rural areas are still controversial. The ages of the participants in this study in the two areas were divided into three categories: ≤ 40, 40–60, and ≥ 60 years old. The sex distribution between the populations in the two areas showed no significant difference, as did the age and BMI distribution. The results of the urine samples collected in this study showed that the concentration of NP in rural areas was higher than that in urban areas, and the difference was found to be statistically significant (P = 0.01). Literature data of comparative studies regarding urinary NP in areas of different urbanization are scarce, but there are studies on other endocrine factors. Karalius et al. (2014) found that although the release of BPA into the environment has been associated with more urban, industrialized areas, the levels of BPA detected in the urine from rural areas were comparable to those of more urbanized cities, which is consistent with our finding on NP in this study.
Our study has three strengths. First, we included rural areas in our survey and comprehensively assessed the difference in exposure levels between urban and rural areas, even though research on NP is currently concentrated in urban areas. Second, our study first combined both drinking water NP and urinary NP as external and internal exposure indicators to assess the exposure differences between the two areas. Finally, our research has provided more information on the influence of household water supply pipeline materials and drinking water treatment processes on NP levels in drinking water.
On the other hand, our study also has some limitations. The sample size of our survey was small, and the influencing factor of NP in the human body was complex. For instance, more diet and environmental monitoring in the two areas are required to determine whether there are other, different sources of exposure between rural and urban areas, which may account for urinary NP level differences.
Conclusion
Even if literature on NP body burden and drinking water contamination levels has increased over the past few years, date on NP levels of urinary and drinking water in different areas, especially with different levels of economic development, are still relatively scarce. This work provides the first description of NP found in human urine and drinking waters in two geographic regions of distinctly different economic levels in Wuhan, China. As already described above, despite their high concentration in drinking water source in more industrialized area, the levels of NP detected in the urine and terminal tap waters from rural areas in Wuhan were unexpectedly higher to those from the urban area. The different materials used for household water supply pipelines and the drinking water treatment processes in the two areas may account for the results described above. Results of this study suggest that even in rural areas of a less industrialization levels, NP is common and that future studies of this compound should include areas less associated with NP exposure and pollution.
Acknowledgements
We sincerely thank all individuals who volunteered to participate in this study. Our deepest gratitude also goes to Professor Dongru Qiu for his thoughtful suggestions and contributions that helped improve this paper substantially. This work was supported by the Basic Research Plan of Hubei Province (2018HB05), China.
Abbreviations
- NP
Nonylphenol
Author contribution
Chunyan Xu: conceptualization, methodology, writing—original draft. Chuangang Fan: formal analysis, resources. Luojing Xiang: supervision, project administration. Shu Zhang: formal analysis, data curation. Weiwei Li: investigation. Chuan Yi: writing—review and editing. Haibo Ling: funding acquisition, validation.
Funding
This work was supported by the Basic Research Plan of Hubei Province (2018HB05), China.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
The study was reviewed and approved by the Ethics Committee of Hubei Center for Disease Control and Prevention (Wuhan, China).
Consent to participate
All of the authors participated in the study work.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunyan Xu and Haibo Ling contributed equally to this study.
References
- Berge A, Cladiere M, Gasperi J, Coursimault A, Tassin B, Moilleron R. Meta-analysis of environmental contamination by alkylphenols. Environ Sci Pollut Res Int. 2012;19:3798–3819. doi: 10.1007/s11356-012-1094-7. [DOI] [PubMed] [Google Scholar]
- Bhandari G, Bagheri AR, Bhatt P, Bilal M. Occurrence, potential ecological risks, and degradation of endocrine disrupter, nonylphenol, from the aqueous environment. Chemosphere. 2021;275:130013. doi: 10.1016/j.chemosphere.2021.130013. [DOI] [PubMed] [Google Scholar]
- Bodziach K, Staniszewska M, Falkowska L, Nehring I, Ożarowska A, Zaniewicz G, Meissner W. Gastrointestinal and respiratory exposure of water birds to endocrine disrupting phenolic compounds. Sci Total Environ. 2021;754:142435. doi: 10.1016/j.scitotenv.2020.142435. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Yan C, Wu X, Zhou L, Xiu G. Nonylphenol induced individual and population fluctuation of Caenorhabditis elegans: disturbances on developmental and reproductive system. Environ Res. 2020;186:109486. doi: 10.1016/j.envres.2020.109486. [DOI] [PubMed] [Google Scholar]
- Careghini A, Mastorgio AF, Saponaro S, Sezenna E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: a review. Environ Sci Pollut Res. 2015;22:5711–5741. doi: 10.1007/s11356-014-3974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AM, Cardoso V, Rodrigues A, Ferreira E, Benoliel M, Duarte E. Occurrence and analysis of endocrine-disrupting compounds in a water supply system. Environ Monit Assess. 2015;187:1–8. doi: 10.1007/s10661-014-4167-x. [DOI] [PubMed] [Google Scholar]
- Chen HW, Liang CH, Wu ZM, Chang EE, Lin TF, Chiang PC, Wang GS. Occurrence and assessment of treatment efficiency of nonylphenol, octylphenol and bisphenol-A in drinking water in Taiwan. Sci Total Environ. 2013;449:20–28. doi: 10.1016/j.scitotenv.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Chen Y (2013) Investigation on pollution of nonylphenol in major water bodies, drinking water and dining utensils in Suzhou and the removal efficiency of some water treatment techniques. Dissertation, Suzhou University (in Chinese).
- Cheng YC, Chen HW, Chen WL, Chen CY, Wang GS. Occurrence of nonylphenol and bisphenol A in household water pipes made of different materials. Environ Monit Assess. 2015;188:562. doi: 10.1007/s10661-016-5556-0. [DOI] [PubMed] [Google Scholar]
- Dai Y-D, Chao H-R, Chiang P-C. Detection, occurrence, and treatment of nonylphenol and bisphenol-A in Taiwanese drinking water sources. J Hazard Toxic Radioact Waste. 2019;23:04018039. doi: 10.1061/(ASCE)HZ.2153-5515.0000430. [DOI] [Google Scholar]
- Dekant W, Volkel W. Human exposure to bisphenol A by biomonitoring: methods, results and assessment of environmental exposures. Toxicol Appl Pharmacol. 2008;228:114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Fürhacker M, Scharf S, Weber H. Bisphenol A: emissions from point sources. Chemosphere. 2000;41:751–756. doi: 10.1016/S0045-6535(99)00466-X. [DOI] [PubMed] [Google Scholar]
- Gilca AF, Teodosiu C, Fiore S, Musteret CP (2020) Emerging disinfection byproducts: a review on their occurrence and control in drinking water treatment processes. Chemosphere, 127476
- Guenther K, Heinke V, Thiele B, Kleist E, Prast H, Raecker T. Endocrine disrupting nonylphenols are ubiquitous in food. Environ Sci Technol. 2002;36:1676–1680. doi: 10.1021/es010199v. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wu W, Xu D, Guan X, Wang S. Occurrence, uptake, and health risk assessment of nonylphenol in soil-celery system simulating long-term reclaimed water irrigation. J Hazard Mater. 2021;406:124773. doi: 10.1016/j.jhazmat.2020.124773. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kawaguchi M, Okada F, Takai N, Yoshimura Y, Horie M, Izumi S-I, Makino T, Nakazawa H. Measurement of 4-nonylphenol and 4-tert-octylphenol in human urine by column-switching liquid chromatography–mass spectrometry. Anal Chim Acta. 2003;486:41–50. doi: 10.1016/s0003-2670(03)00464-1. [DOI] [Google Scholar]
- Jie Y, Fan QY, Binli H, Biao Z, Zheng F, Jianmei L, Jie X. Joint neurodevelopmental and behavioral effects of nonylphenol and estradiol on F1 male rats. Int J Environ Health Res. 2013;23:321–330. doi: 10.1080/09603123.2012.733936. [DOI] [PubMed] [Google Scholar]
- Jie Y, Xuefeng Y, Mengxue Y, Xuesong Y, Jing Y, Yin T, Jie X. Mechanism of nonylphenol-induced neurotoxicity in F1 rats during sexual maturity. Wien Klin Wochenschr. 2016;128:426–434. doi: 10.1007/s00508-016-0960-6. [DOI] [PubMed] [Google Scholar]
- Jie Y, Jie Z, Ya L, Xuesong Y, Jing Y, Yu Y, Jiaqi Y, Jie X. Pollution by Nonylphenol in river, tap water, and aquatic in an acid rain-plagued city in southwest China. Int J Environ Health Res. 2017;27:179–190. doi: 10.1080/09603123.2017.1332345. [DOI] [PubMed] [Google Scholar]
- Karalius VP, Harbison JE, Plange-Rhule J, van Breemen RB, Li G, Huang K, Durazo-Arvizu RA, Mora N, Dugas LR, Vail L, Tuchman NC, Forrester T, Luke A. Bisphenol A (BPA) found in humans and water in three geographic regions with distinctly different levels of economic development. Environ Health Insights. 2014;8:1–3. doi: 10.4137/EHI.S13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, Cheon YP, Choi D, Lee SH. Histological analysis of reproductive system in low-dose nonylphenol-treated F1 female mice. Dev Reprod. 2020;24:159–165. doi: 10.12717/DR.2020.24.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Li X, Ying GG, Zhao JL, Chen ZF, Lai HJ, Su HC. 4-Nonylphenol, bisphenol-A and triclosan levels in human urine of children and students in China, and the effects of drinking these bottled materials on the levels. Environ Int. 2013;52:81–86. doi: 10.1016/j.envint.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Loyo-Rosales JE, Rosales-Rivera GC, Lynch AM, Rice CP, Torrents A. Migration of nonylphenol from plastic containers to water and a milk surrogate. J Agric Food Chem. 2004;52:2016–2020. doi: 10.1021/jf0345696. [DOI] [PubMed] [Google Scholar]
- Ma X, Gao N, Li Q, Xu B, Le L, J, W (2006) Investigation of several endocrine disrupting chemicals in Huangpu River and water treatment units of a waterworks. China Water Wastewater 22, 1-4
- Noorimotlagh Z, Haghighi NJ, Ahmadimoghadam M, Rahim F. An updated systematic review on the possible effect of nonylphenol on male fertility. Environ Sci Pollut Res Int. 2017;24:3298–3314. doi: 10.1007/s11356-016-7960-y. [DOI] [PubMed] [Google Scholar]
- Park H, Kim K (2017) Urinary levels of 4-Nonylphenol and 4-t-Octylphenol in a representative sample of the Korean Adult Population. Int J Environ Res Public Health 14. 10.3390/ijerph14080932 [DOI] [PMC free article] [PubMed]
- Peng F, Ji W, Zhu F, Peng D, Yang M, Liu R, Pu Y, Yin L. A study on phthalate metabolites, bisphenol A and nonylphenol in the urine of Chinese women with unexplained recurrent spontaneous abortion. Environ Res. 2016;150:622–628. doi: 10.1016/j.envres.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Pirard C, Sagot C, Deville M, Dubois N, Charlier C. Urinary levels of bisphenol A, triclosan and 4-nonylphenol in a general Belgian population. Environ Int. 2012;48:78–83. doi: 10.1016/j.envint.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Wang Y, Chen J. Perinatal exposure to nonylphenol induces microglia-mediated nitric oxide and prostaglandin E2 production in offspring hippocampus. Toxicol Lett. 2019;301:114–124. doi: 10.1016/j.toxlet.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Ruczyńska W, Szlinder-Richert J, Nermer T. The occurrence and distribution of nonylphenols and nonylphenol ethoxylates in different species of fish. Environ Sci Process Impacts. 2020;22:1057–1070. doi: 10.1039/C9EM00584F. [DOI] [PubMed] [Google Scholar]
- Shao B, Hu J, Yang M. A survey of nonylphnol in aquatic environment of Chongqing valley. Acta Sci Circum. 2002;22:12–16. [Google Scholar]
- Sharma VK, Anquandah GAK, Yngard RA, Kim H, Fekete J, Bouzek K, Ray AK, Golovko D. Nonylphenol, octylphenol, and bisphenol-A in the aquatic environment: a review on occurrence, fate, and treatment. J Environ Sci Health, Part A. 2009;44:423–442. doi: 10.1080/10934520902719704. [DOI] [PubMed] [Google Scholar]
- Shirdel I, Kalbassi MR, Esmaeilbeigi M, Tinoush B. Disruptive effects of nonylphenol on reproductive hormones, antioxidant enzymes, and histology of liver, kidney and gonads in Caspian trout smolts. Comp Biochem Physiol C Toxicol Pharmacol. 2020;232:108756. doi: 10.1016/j.cbpc.2020.108756. [DOI] [PubMed] [Google Scholar]
- Soares A, Guieysse B, Jefferson B, Cartmell E, Lester JN. Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int. 2008;34:1033–1049. doi: 10.1016/j.envint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Tang X, Jin Y, Zhang Y, Chi T. Alkyl Pheols in Tap Water in Shenyang. J Environ Health. 2005;22:190–191. [Google Scholar]
- Valcárcel Y, Valdehíta A, Becerra E, de Alda ML, Gil A, Gorga M, Petrovic M, Barceló D, Navas J. Determining the presence of chemicals with suspected endocrine activity in drinking water from the Madrid region (Spain) and assessment of their estrogenic, androgenic and thyroidal activities. Chemosphere. 2018;201:388–398. doi: 10.1016/j.chemosphere.2018.02.099. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, Feng W, Zeng Q, Sun Y, Wang P, You L, Yang P, Huang Z, Yu S-L, Lu W-Q. Variability of metal levels in spot, first morning, and 24-hour urine samples over a 3-month period in healthy adult Chinese men. Environ Health Perspect. 2016;124:468–476. doi: 10.1289/ehp.1409551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xiao H, He N, Sun D, Duan S. Biosorption and biodegradation of the environmental hormone nonylphenol by four marine microalgae. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lakaschus S, Ebinghaus R, Caba A, Ruck W. Atmospheric concentrations and air-sea exchanges of nonylphenol, tertiary octylphenol and nonylphenol monoethoxylate in the North Sea. Environ Pollut. 2006;142:170–180. doi: 10.1016/j.envpol.2005.08.073. [DOI] [PubMed] [Google Scholar]
- Yu J, Tuo F, Luo Y, Yang Y, Xu J. Toxic effects of perinatal maternal exposure to nonylphenol on lung inflammation in male offspring rats. Sci Total Environ. 2020;737:139238. doi: 10.1016/j.scitotenv.2020.139238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.