Abstract
Chemical signature of airborne particulates and deposition dusts is subject of study since decades. Usually, three complementary composition markers are investigated, namely, (i) specific organic compounds; (ii) concentration ratios between congeners, and (iii) percent distributions of homologs. Due to its intrinsic limits (e.g., variability depending on decomposition and gas/particle equilibrium), the identification of pollution sources based on molecular signatures results overall restricted to qualitative purposes. Nevertheless, chemical fingerprints allow drawing preliminary information, suitable for successfully approaching multivariate analysis and valuing the relative importance of sources. Here, the state-of-the-art is presented about the molecular fingerprints of non-polar aliphatic, polyaromatic (PAHs, nitro-PAHs), and polar (fatty acids, organic halides, polysaccharides) compounds in emissions. Special concern was addressed to alkenes and alkanes with carbon numbers ranging from 12 to 23 and ≥ 24, which displayed distinct relative abundances in petrol-derived spills and exhausts, emissions from microorganisms, high vegetation, and sediments. Long-chain alkanes associated with tobacco smoke were characterized by a peculiar iso/anteiso/normal homolog fingerprint and by n-hentriacontane percentages higher than elsewhere. Several concentration ratios of PAHs were identified as diagnostic of the type of emission, and the sources of uncertainty were elucidated. Despite extensive investigations conducted so far, the origin of uncommon molecular fingerprints, e.g., alkane/alkene relationships in deposition dusts and airborne particles, remains quite unclear. Polar organics resulted scarcely investigated for pollution apportioning purposes, though they looked as indicative of the nature of sources. Finally, the role of humans and living organisms as actual emitters of chemicals seems to need concern in the future.
Keywords: Molecular signature of sources, Diagnostic concentration ratios, Particulate organic matter (POM), Air pollution, Toxicants
Introduction
Chemicals released into the atmosphere are known as posing a threat for humans and injuring the environment. Therefore, the knowledge of nature, amount, and land spread of emissions is mandatory whenever legislative or technological actions must be implemented to mitigate the toxicants’ impact (Albaiges et al. 1984; Bascom et al. 1996; Yassaa et al. 2001; Ma and Harrad 2015; Błaszczyk et al. 2017; Sifakis et al. 2017; Cetin et al. 2018; Nieder et al. 2018; Vaz 2018). Chemical and physical characterization of both gaseous and particulate phases plays a key role to picture the behavior of pollutants in the environment (i.e., through valuing the concentrations of selected chemicals in air and exhausts, and comparing them with current legislation). Besides, dedicated studies allow assessing the relative importance of pollution sources that affect the sites or land domains subject of study (Gundel et al. 1993, Hecht 1999; Ventrice et al. 2013, Jedynska et al. 2014a,b, Giulivo et al. 2016, Liu et al. 2017, Praveena et al. 2018, Rabhi et al. 2018, Yury et al. 2018, Brehmer et al. 2020).
The first approaches to identification of the emission sources of organic toxicants by means of molecular signatures were carried out in late twentieth century (Daisey et al. 1986; Harrison et al. 1996). Attention was paid overall to alkanes, PAHs, and nitrated derivatives (NPAHs); however, other groups were taken in account also, including fatty acids, halides (polychlorinated dioxins/furans [PCDD/Fs], polychlorobiphenyls [PCBs], and polybromodiphenyl ethers [PBDEs]), polysaccharides (levoglucosan, mannosan), sterols (cholesterol, stigmasterol, sitosterol), and triterpenols (amyrins). According to these studies, the characterization of organic substances comprised in particulate matters (both airborne particles and dust settled on surfaces and soil) resulted an important tool of investigation but also a challenge for scientists, due to complex nature of these matrices. In fact, organic compounds display a wide variety of chemical and physical properties, e.g., acidity/alkalinity/neutrality, rate of polarity, solubility in water and n-octanol, vapor pressure, resistance to action of oxidants, and light. As for chemical composition, organics include linear and cyclic aliphatic hydrocarbons, polycyclic aromatic hydrocarbons, fatty acids and phenols, amines, carbonyls, organic halides, sulfates, and phosphates.
Many organic compounds have been associated to distinct emission sources and recognized as tracers of living organisms, natural phenomena, and man’s activities. Unfortunately, only in few cases one substance is unequivocally typical of one only emission and allows assessing the impact of that source onto the environment. Usually, chemicals occur in more types of emissions, so that the peculiarity is lost; nevertheless, in this case the composition per groups and the distribution pattern of congeners within each group can aid in identifying the pollution sources. For instance, biofuels are usually richer of esters than fossil fuels, and the reverse occurs with regard to polycyclic aromatic hydrocarbons (PAHs) (Damanik et al. 2018). Besides, the percentages of PAHs associated to ultra-fine, fine, and coarse fractions of suspended particulate are a function of the nature of source (Zielinska et al. 2004). Fresh exhausts undergo the action of light (overall UV) and oxidizing species (O3, NO2, OH, and NO3 radicals) (Arey and Atkinson 2003; Estève et al. 2004; Perraudin et al. 2007; Chu et al. 2010). Therefore, chemicals released primarily into the atmosphere tend to change into degradation products; e.g., alkenes and PAHs into ketones and quinones, polyacids, diols, epoxides, cumulatively defined as secondary pollutants. These transformations alter both chemical signature of the substrate and toxicity of the air parcel impacted by the emissions (Atkinson and Arey 1994; Durant et al. 1999; Bandowe et al. 2014). Reactivity of compounds introduces some rate of uncertainty with regard to identification of emission sources of airborne particulate. On the other hand, due to wide ranges of lifetime characterizing organics, the rank of decomposition can work as an index of aging, i.e., of the importance of processes developing in the atmosphere (Sofowote et al. 2010; Cecinato et al. 2014). With regard to deposition dust, reactivity seems to play a twin role. On the one hand, its high surface extension favors the adsorption of chemicals from the air; the substances are back released when the contour conditions are favorable, the substrate composition changes, and this latter acts as secondary emitter. On the other hand, the collection time of depositions adopted for chemical characterizations is ≥ 15 days, which implies the wide occurrence of decomposition products of primary pollutants.
According to the above considerations, the simple approach of molecular signature of environmental particles suffers some intrinsic constraints; hence, better tools are employed today to trace the emissions, e.g., principal component analysis. Nevertheless, the knowledge of chemical fingerprints remains suitable as a preliminary screening of factors forcing pollution; for instance, chemical profiles of POM (and the resulting numerical parameters) allow excluding or including as real main pollution sources the types of emissions suspected to affect the environment. Molecular signature is easily integrated in statistical approaches based on a number of chemical and physical variables (Kavouras et al. 2001, Mostert et al. 2010, Brown and Brown 2012a, 2012b, Khedidji et al. 2017, Chen et al. 2019, Maechler et al. 2019, Molnar 2019, Sofowote et al. 2020), which look fine for source apportionment studies. Besides, any multivariate analysis approach alone does not add any contribution to knowledge of the nature of sources, whereas no preliminary information is available about the chemical fingerprint of emissions. For instance, multivariate analysis is able to gather or distinguish sets of samples and of chemical species within environmental databases, by putting in the evidence differences and similarities among them. Nevertheless, it is necessary knowing the chemical profiles of emissions and other contour information (e.g., reactivity of compounds, type of locations, size, and chemistry of substrates…) to assign reliably the abovementioned behaviors to specific origins.
With regard to chemicals hosted, interiors as a whole are a space different at all from outdoor environment (Zhao et al. 2007, Guo and Kannan 2013, Sangiorgi et al. 2013, Romagnoli et al. 2014, Hassanvand et al. 2015, Tran et al. 2015, Oliveira et al. 2016, Subedi et al. 2017, Liu et al. 2018, Lu et al. 2018, Lucattini et al. 2018, Steinemann 2018, Wong et al. 2019, Zhu et al. 2019). Three categories of contaminants affect indoor locations, i.e., (i) chemicals released overall outside and driven indoors through building openings and ventilation devices (e.g., hydrocarbons comprised in motor vehicle exhausts); (ii) substances released indoors and outdoors at broadly analogous levels (e.g., nitrogen oxides, psychotropic substances); and (iii) compounds released typically indoors (e.g., cosmetics, plasticizers). Indoors, the substances released meet up reaction chambers with temperatures roughly steady along the whole year, ozone normally much less than outside, and surfaces much larger. These factors deeply influence the chemistry of locations, the lifetime of substances, and the gas/condensed phase equilibria. In particular, in interiors the substance ability to interact with the human body is different with regard to intensity and route, compared with open air. At this regard, it is worth noting that current legislation aimed at preserving health considers only inhalation as primary way of intake; meanwhile, outdoor pollution is viewed as predominant, and the occurrence of toxicants in interiors is linked to intrusion from outside. This is the reason why attention is paid usually to gasses and fine aerosols, as well as to [lung] cancer. Instead, indoors the neat exposure to toxicants is larger, and the amounts of dusts with which humans enter in contact exceed of orders of magnitude those of fine particles inhaled. Therefore, the alternative ways of body intake (i.e., skin contact and ingestion) gain importance (Xing et al. 2011; Hou et al. 2018; Weiss et al. 2018; Settimo et al. 2020a), and the role played by depositions increases, as well as that of health problems other than tumors. That garbles the role of toxicants affecting interiors and promotes the search for their sources.
This paper aims at providing a short review of current knowledge concerning the molecular fingerprints of particulates (both airborne and settled), suitable to elicit information about the sources of pollution. Three major categories of fingerprints are discussed, namely, (i) individual tracers; (ii) diagnostic concentration ratios; and (iii) homolog percent distributions within groups. As for chemicals, non-polar hydrocarbons (i.e., chain- and cyclo-aliphatic compounds) are examined in particular here, and glance is given to need of further investigations aimed at understanding the sources of uncommon alkane/alkene percent distributions. Finally, some insights are provided about the role played by living organisms and humans, as actual emitters of contaminants, with regard to chemistry of their own life places.
The state-of-the-art of research about molecular signatures of pollution sources
General features of emission profiles
Three key factors influence the composition of both anthropogenic and natural emissions, as it results from chemical analysis. They are (i) the operating conditions of source, including the kind of fuel, temperature of exhausts, and the type of abatement devices adopted; (ii) the collection procedure of exhaust (which includes vapors, condensation waters, and particle matters); and (iii) the methodology adopted to process samples and determine chemical composition. These factors hinder to assign thorough emission factors to chemicals released by sources, and precise chemical profiles to groups of substances like alkanes and PAHs (Tobiszewski and Namiesnik 2012; Cecinato et al. 2014). Investigations undertaken with different methodological approaches can lead to results hardly comparable (Kavouras et al. 1999). For instance, the profile of particulate n-alkanes actually identified in emissions depends on the effluent temperature during sampling operation, which influences the loss rates of the most volatile compounds. Analogously, the profile of airborne 3/4-ring PAHs depends on year season as well as on the use of the only filter membrane or also vapor trap to collect samples. Hence, the study of their percent distribution in the emissions and in airborne particulates is preferably restricted to high molecular weight homologs, namely, to hydrocarbons with carbon number ≥ 25 (C25). As for PAHs, compounds with vapor pressures of the same order of magnitude (e.g., fluoranthene/pyrene) or with high molecular weights (e.g., benzo[a]pyrene/benzo[ghi]perylene) are kept in consideration when exploring concentration ratios hypothetically diagnostic for source assessment purposes.
n-Alkanes
Non-polar fraction of particulate organic matters (POM) includes numerous groups. They are alkanes, alkylated mono-aromatics and biphenyls, alkenes, branched and cyclic aliphatic hydrocarbons. Among them, attention has been paid overall to n-alkanes (linear homologs); alicyclic compounds have been investigated as tracers of petrol products, and mono-methyl substituted alkanes as markers of tobacco smoking. As total, n-alkanes are among the most abundant components of particulate organic matter. For a long time, investigations dealing with this group were restricted to chemistry of high plants (Eglinton et al. 1962; Eglinton and Atkinson 1967; Li et al. 2018) and to characterization of vehicle exhausts. Instead, n-alkanes gained concern when the strong dependence of their molecular imprinting on nature of source was ascertained, as well as their toxicity that includes skin inflammation, pulmonary edema, respiratory disfunction, co-carcinogenic and co-tumorogenic properties (Rabovsky and Judy 1989).
The saw-tooth distribution of high-molecular-weight n-alkanes has been associated to terrestrial high plants (Simoneit and Mazurek 1982; Alves et al. 2001; Rabhi et al. 2018). Indeed, biogenic synthesis leads to generation preferably of even C-numbered fatty acids; afterwards, acids tend to loose CO2 through the natural process of decarboxylation, and form odd-C numbered n-alkanes as the final products (or alkenes, in the case of unsaturated acid precursors). Since in the case of high trees this phenomenon is more evident along the range of long-chain homologs, the most used parameter to value the impact of (high) vegetation is carbon preference index starting from normal pentacosane (CPI25) (Alves et al. 2001; Pio et al. 2001; Omar et al. 2007). This parameter is expressed by the following formula (1):
| 1 |
where nCi means the concentration of n-alkane homolog with carbon number equal to i.
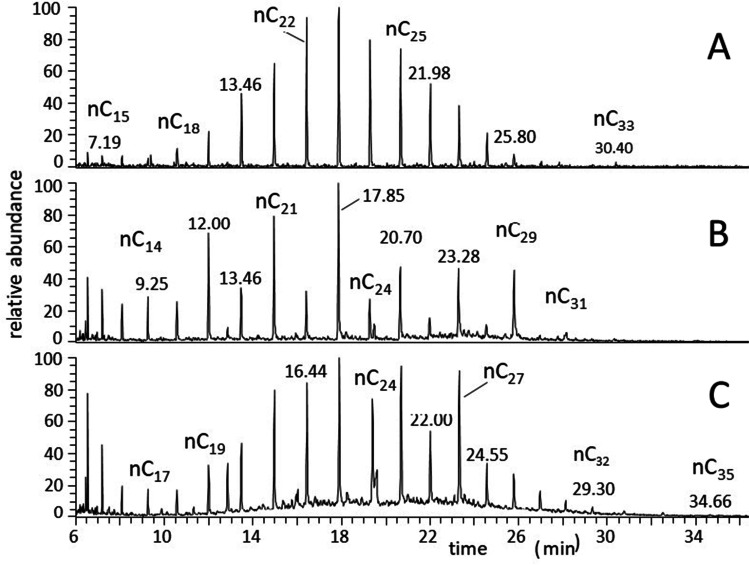
On the other hand, the n-alkane distribution typical of exhausts of fuels derived from petroleum is bell-shaped and mono-modal with the maximum centered between C19 and C26; in this case, CPI25 values range from 0.6 to 1.3 (Simoneit 1984; Perrone et al. 2014). According to that, CPI25 rates equal to ~ 1 were found during an in-field campaigns performed close to a highway in the Algiers metropolitan area (Fig. 1A), while CPI25 values were > 10 in a forest area belonging to Biskra province, Algeria (Fig. 1B). Anyway, usually a mix of the two distributions is observed, e.g., as it occurred in a city garden of Rome, Italy (Fig. 1C).
Fig. 1.
GC–MS profiles of the non-polar fraction (m/z = 85) of airborne particulates collected at four sites variously influenced by emission sources. A Road traffic site; B rural region; C urban location (city garden). Symbols: nCi indicates the n-alkane with carbon number equal to i. [Personal communication. The samples were collected in the frame of a cooperative research project of our institute with INAIL-DIPIA, Rome, Italy, by applying the procedure described in Cecinato A, Marino F, Di Filippo P, Lepore L, Possanzini M (1999). Distribution of n-alkanes, polynuclear aromatic hydrocarbons and nitrated polynuclear aromatic hydrocarbons between the fine and coarse fractions of inhalable atmospheric particulates. J Chromatog A 846, 255–264, 10.1016/S0021-9673(99)00,129–6]
Marine biota behaves some differently. In fact, it retains the predominance of odd n-alkanes typical of biogenic emissions; however, the maximum shifts into the C15–C21 range. Thus, this percent distribution often characterizes the short/medium C-chain non-polar hydrocarbons collected at seaside locations (Romagnoli et al. 2016, Yu et al. 2019, Hernández-Guzmán et al. 2021, Gal et al. 2022); worth of note, this distribution is distinct from that associated to petrogenic sources, where the predominance of odd homologs is not observed.
Further indexes have been examined in order to put in evidence the impact of vegetation (Kumar et al. 2019). Among them, there are (i) the homolog (Cmax) corresponding to the maximum concentration within the n-alkane distribution; (ii) the cumulative percentage attributable to natural waxes (NW%) (Alves et al. 2001, 2011; Rabhi et al. 2018); and (iii) the average chain length of n-alkanes (ACL) (Leider et al. 2013). As for Cmax, motor vehicle exhausts exhibit the prevalence of short and medium chain hydrocarbons (< C24), while the leaf debris of high trees is characterized by the predominance of n-C29 or n-C31. The two distinct behaviors have pictured in Fig. 1A and B, where the maximums correspond to tricosane (C23) and nonacosane (C29), respectively.
The NW% value is provided by the formula (2):
| 2 |
where each term at the numerator is set equal to zero whereas the actual rate results < 0. For instance, NW% values ranging from ~ 10 up to > 70% have been calculated for airborne particulates collected in Athens metropolitan area and in Algeria (Andreou and Rapsomanikis 2009; Rabhi et al. 2018).
The average chain length of n-alkanes (ACL) is calculated through the formula (3):
| 3 |
Carbon preference indexes analogous to CPI25 have been employed also, which consider longer n-alkane ranges (e.g., nC11 ÷ nC36) or the only light homolog segment (< nC25) (Alves et al. 2000, 2001; Aloulou et al. 2010). The use of CPIs computing light hydrocarbons is partly questionable due to volatility of compounds. However, these indexes allow investigating the possible impact of marine biota (algae, plankton) and microorganisms (bacteria, fungi), when molecular signature is extended to include isoprenoids and when nCmax corresponds to nC15/nC17 (Fisher et al. 1972; Ekpo et al. 2005; Andreou and Rapsomanikis 2009; Horikawa et al. 2010; Wang et al. 2010; Caumo et al. 2018). Table 1 provides a synthetic overview of what presented above. The action of marine organisms has been elucidated also as the possible source of squalene (2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-tetracosahexaene) and squalane in offshore and coastal airborne particulates, while abietane (13α-isopropylpodocarpane) and its homologs have been adopted to distinguish the emission of coniferous trees from that of other plants (Simoneit and Mazurek 1982; Fine et al. 2004).
Table 1.
Indexes describing the n-alkane percent distributions, typical of various emission types. References: Simoneit (1984), Alves et al. (2001), Zrafi et al. (2008), Leider et al. (2013), Rabhi et al. (2018), Kumar et al. (2019)
| Index/source | Petroleum | Algae | Microorganisms | High trees | Vegetation | Anthropogenic |
|---|---|---|---|---|---|---|
| Cmax | C16 ~ C21 | C17, C19, C21, C23 | C16, C18, C20 | C29, C31, C33 | C25, C27, C29 | C16 ~ C23 |
| CPI25 | 0.7–1.3 | > 1 | > 10 | > 3 | 0.8–1.3 | |
| CPI16 | 0.8–1.2 | > 1.0 | > 1.0 | |||
| NW% | ~ 0 | > 75 | > 75 | ~ 0–30 |
The bell-shaped and saw-teeth percent profiles are the two most common within medium/long-chain n-alkanes and are used commonly to identify the corresponding principal source. Nevertheless, sometimes a distinct fingerprint is observed in the semi-volatile range, where the even homologs are predominant. This pattern seems typical of sediments (Zrafi-Nouira et al. 2008, Sikes et al. 2009, Aloulou et al. 2010, Jafarabadi et al. 2018, Aghadadashi et al. 2021, Arshinova et al. 2021); nevertheless, it has been observed also in effluents from petroleum-contaminated zones and has been interpreted as a tracer of microorganisms including bacteria, fungi and spores (Stortini et al. 2009; Kuhn et al. 2010; Leider et al. 2013). Hence, the molecular signature over the whole nC14–nC40 range is more complex than as researchers currently believe and is not yet completely understood. For instance, it can exhibit high percentages of even homologs in the short/medium-chain range, and of odd homologs in the long-chain range.
Finally, the R ratio between total low molecular weight (LMW) and high molecular weight (HMW) n-alkanes seems to distinguish petrogenic emission (R > > 1), terrestrial plants (R < < 1), and marine biota (R ≈ 1).
Examples of in-field monitoring aimed at discriminating the sources of organic fraction of airborne particulates, sediments, and waters are Aghadadashi et al. (2021), Aloulou et al. (2010), Alves et al. (2001), Balducci et al. (2014), Bi et al. (2008), Gal et al. (2022), Kang et al. (2018), and Khedidji et al. (2017).
Branched and cyclic hydrocarbons
The important presence of petroleum components (e.g., branched alkanes pristane and phytane) compared with nC17 and nC18, respectively, looks as a track of motor vehicle emission (Hamilton et al. 1984; Alexandrino et al. 2019; Alkhafaji 2021), or petrol spill from contaminated sediments, soils, and waters (Jeng 2006; Stortini et al. 2009; Wang et al. 2011; Shirneshan et al. 2016; Azimi-Yancheshmeh et al. 2017, Hernández-Guzmán et al. 2021). These ratios are adopted also to index the maturity of petroleum and bitumen affecting sediments (Omotoye et al. 2016; Gao et al. 2021).
Complex blends of high molecular weight aliphatic hydrocarbons with branched and/or cyclic structure (e.g., steranes and hopanes) are the bulk of petrol industry products, like fuels and solvents, and affect the exhausts (Xiao et al. 2019; Lu et al. 2021), soils and sediments (Arfaoui 2014). Their identification and quantification, combined with the ratio rates of burdens of subgroups, has revealed that composition depends on oil maturity or on progress of oxidative/biological attack of original blend (Lobodin et al. 2016; Volkman et al. 1997; Simoneit 1999). These compounds trace the environmental pollution associated with fossil fuels (Aboul-Kassim and Simoneit 1995, Fraser et al. 1997; Wang et al. 2006; Jedynska et al. 2014a,b; Iakovides et al. 2021a). Moreover, triterpanes and steranes seem suitable to characterize sedimentary organic matter and contaminated substrates (Arfaoui 2014; Xiao et al. 2019).
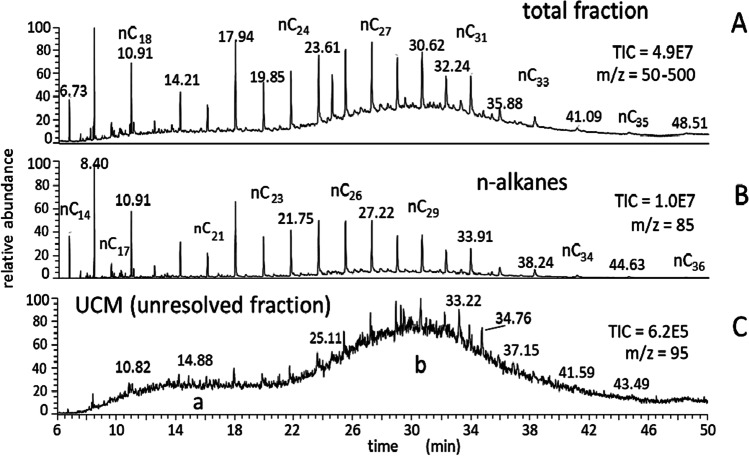
The composition profile of organic matters shows one or two humps of “unresolved mixture” accompanying the n-alkanes sequence (Zheng et al. 2002; Phuleria et al. 2006; White et al. 2013; Jeon et al. 2017). In the case of airborne particulate, a hump comprised of light hydrocarbons originates from gasoline and diesel oil residues. A second hump, including heavy components, is related to lubricating oils (see a and b humps in Fig. 2); the percent profile of vehicle emissions depends on the engine working conditions, and the relative importance of hump(s) raises at unregulated driving regimens, e.g., during cold starts (Zheng et al. 2002, Fang et al. 2020, Iakovides et al. 2021a, Tian et al. 2021).
Fig. 2.
GC–MS profile of the non-polar fraction of diesel exhaust. A Total ion current signal; B Ion trace corresponding to m/z = 85 (labeling n-alkanes); C m/z = 95 ion trace (branched alkanes). Both a and b humps occur in the UCM. Symbols: nCi indicates the n-alkane with carbon number equal to i. [Personal communication. The samples were collected in the frame of a cooperative research project of our institute with Istituto Motori CNR, Naples, Italy, by applying the procedure described in Ciccioli P, Cecinato A, Brancaleoni E, Draisci R, Liberti A (1989). Evaluation of nitrated polycyclic aromatic hydrocarbons in anthropogenic emission and air samples: a possible means of detecting reactions of carbonaceous particles in the atmosphere. Aerosol Sci Technol 10, 296–310, 10.1080/ 02786828908959266]
Though unusual, high percentages of semi-volatile homologs (from C20 to C26) have been detected in airborne particulate coming overall from agricultural areas. This pattern has been found as typical of substrates contaminated with bee waxes (Guenther et al. 1995; Fine et al. 2004).
Among the sources of environmental non-polar hydrocarbons, both tobacco plant leaves and tobacco smoke fumes exhibit a peculiar percent profile with regard to monomethyl-branched alkanes. Indeed, long-chain odd iso-alkanes and even anteiso-alkanes are much abundant compared to normal-alkanes than in other emissions; in particular, anteiso-C30/C32 are more than normal-C30/C32, respectively (Kavouras et al. 1998). Besides, the normal hentriacontane (nC31) is predominant when compared to nC29 and nC33 homologs [nC31/average(n-C29, nC33) > 1.5] (Cecinato et al. 2022). This twin molecular signature was observed in tobacco smoke chambers and in interiors heavily contaminated by smoke and allowed to derive a semi-quantitative index (%ETS) suitable for estimating the percentage contribution of tobacco smoke in airborne particulates and depositions (Cecinato et al. 2022). The %ETS is calculated by applying the formula:
where:
and:
aCj, iCj, and nCj are the anteiso-, iso-, and normal-Cj alkane, respectively.
- A =
1/6 (iC29/nC29 + aC30/nC30 + iC31/nC31 + aC32/nC32 + iC33/nC33 + aC34/nC34),
- B =
1/6 (iC29/aC29 + aC30/iC30 + iC31/aC31 + aC32/iC32 + iC33/aC33 + aC34/iC34),
- C =
1/6 (aC30/nC30 + aC32/nC32 + aC34/nC34 + aC29/nC29 + aC31/nC31 + aC33/nC33),
- ‰(ΣAs)
per thousand content of total alkanes in particulate matter.
(Rem.: For the meaning of 2.58, 1.36, 9.85 and 1.29 at denominators see Cecinato et al. 2022).
Total uncertainty of %ETS is provided by:
Worth of note, this approach does not require searching for minor markers like nicotelline and nitrosamines, nor for nicotine and cotinine, which are ease to decompose and volatilize (see “Fatty acids and alcohols, polar compounds” section).
Alkenes
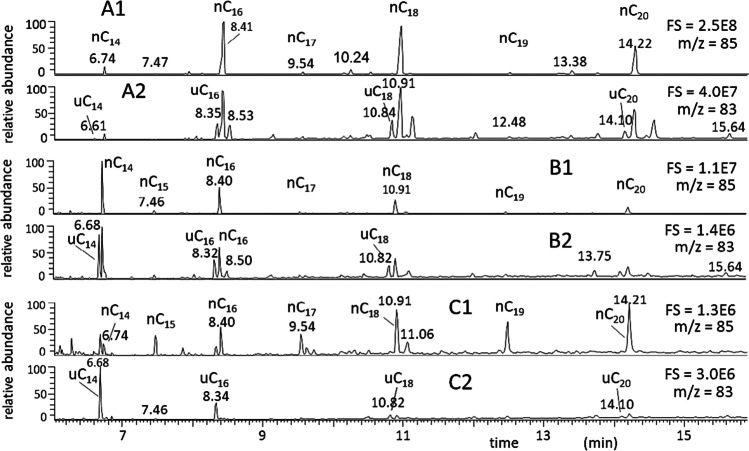
Though scarce attention is paid to unsaturated hydrocarbons occurring in emissions, three distinct molecular signatures of normal alkenes can be distinguished within the light range of non-polar fraction of POM (i.e., mono-unsaturated hydrocarbons with 12 up to 20 carbon atoms in the molecule) (Ekpo et al. 2005). They are (i) the predominant occurrence of n-alkanes, with negligible amounts of n-alkenes; (ii) the prevalence of n-alkenes, displaying high dodecene/dodecane and tetradecene/tetradecane ratios, and low octadecene/octadecane and eicosene/eicosane ratios; and (iii) a merged distribution. The three fingerprints are pictured in Fig. 3. Sample A (deposition dust collected indoors at El Bey, Tunisia) was comprised of much more n-alkanes than alkenes (Fig. 3A1/A2); dusts from Tipaza, Algeria (Fig. 3B1/B2), comprised both alkanes and alkenes; finally, n-alkenes prevailed on n-alkanes in depositions collected in Reggio Calabria, Italy (Fig. 3C1/C2).
Fig. 3.
GC–MS chromatograms of airborne particulate extracts. A1 El Bey, Tunisia, m/z = 85 (n-alkanes); A2 El Bey, Tunisia, m/z = 83 (n-alkenes); B1 Tipaza, Algeria, m/z = 85 (n-alkanes); B2 Tipaza, Algeria, m/z = 83 (n-alkenes); C1 Reggio Calabria, Italy, m/z = 85 (n-alkanes); C2 Reggio Calabria, Italy, m/z = 83 (n-alkenes). [Personal communication. The samples were collected in the frame of a cooperative research project of our institute with Kasdi Merbah University of Ouargla, Dept. Mathematics and Sciences of Matter, Touggourt, Algeria, by applying the procedure described in Romagnoli P, Balducci C, Perilli M, Perreca E, Cecinato A (2016). Particulate PAHs and n-alkanes in the air over Southern and Eastern Mediterranean Sea. Chemosphere 159, 516-525. https://doi.org/10.1016/j.chemosphere.2016.06.024]
It is known that unsaturated hydrocarbons occur as minor components in vegetation emissions; nevertheless, to our knowledge no exhaustive explanation of the three patterns abovementioned has been found until now, nor specific investigations have been undertaken concerning the alkene fingerprints in aerosols and dusts. The fact that in the correspondence of alkene predominance vs. alkanes the even homologs are much more than the odd ones seems to suggest that all compounds enjoy of the same biogenic source; otherwise, alkenes would originate from the twin decarboxylation of unsaturated even α,ω-dicarboxylic acids. Anyway, further investigations are necessary to confirm this hypothesis and search for alternative solutions. For sake of completeness, it is worth to note that the occurrence of ≥ C20 alkenes and polyalkenes in estuarine and coastal sediments, even exceeding the corresponding n-alkanes, has been associated with algae and phytoplankton (Requejo and Quinn 1983; Yongdong et al. 2015).
Polycyclic aromatic hydrocarbons
Though accounting for small fractions of organic matter, PAHs are of big concern, because of their strong toxicity in terms of carcinogenic and mutagenic power as well as of their ability to promote heart morbidity and premature deaths (Collins et al. 1998, European Parliament and Council 2005, IARC 2012). Organic particulates exhibit a plurality of PAH signatures, and many attempts have been made to associate the PAH chemical imprinting with the nature of emission; this was important not only in the perspective of assessing environmental toxicity, but also with regard to forensics sciences and remediation policies (Andersson and Achten 2015; Stout et al. 2015). It is worth to remark that the original percentages of PAHs in emissions do not correspond exactly to those found in PM and dust (Kavouras et al. 1999; Kim et al. 2009; Tobiszewski and Namiesnik 2012; Keyte et al. 2013; Stogiannidis and Laane 2015; Emsbo-Mattingly and Litman 2016). Indeed, most PAHs are released at hot conditions by organic matter that burns (e.g., during forest fires and fuel combustion), and originally exist as vapors; thereafter, PAHs condense onto solid substrates according to vapor pressure of compounds, to temperature and substrate features; besides, fuel spill and evaporation occur from petroleum reservoirs and bitumen/asphalt (Alves et al. 2011). As long as adsorbed, PAHs share with particles the ways of dispersion in air, including long-range transport, and finally settle onto surfaces together with coarse dust (Simoneit 2002; Medeiros and Simoneit 2008; Ravindra et al. 2008; Lammel et al. 2010; Iakovides et al. 2021b). The gas/particle equilibrium developing at the particle surface is dynamic and is influenced by reactivity of congeners, though the principal PAHs (e.g., the sixteen included in the list of priority pollutants (USEPA 1993)) are classified as persistent toxicants (USEPA 1993; MacKay and Callcott 1998; Lodovici et al. 2003; Paolini et al. 2015; Cao et al. 2019), and reactivity changes when the compounds are adsorbed on carbonaceous substrates or silica (Keyte et al. 2013, and references herein). In conclusion, as underlined by several authors, both the collection parameters (e.g., time of sampling, presence of ozone/oxidant traps, fiber membrane material, etc.) and chemical analysis procedure modulate the resulting concentrations of individual PAHs in the particulates (Tobiszewski and Namiesnik 2012; Balducci et al. 2017).
Only a handful of PAHs, individually or as subgroups, have been associated to specific sources. Among them, worth of mention are retene (1-methyl,7-isopropylphenanthrene), methylphenanthrenes, dimethyl/ethyl-phenanthrenes, and benzo[ghi]fluoranthene (Tong and Karasek 1984; Benner et al. 1995; Shen et al. 2012). In fact, retene is typical of wood and is a common tracer of forest fires; dimethyl/ethyl-phenanthrene isomers occur as mixtures that display distinct profiles in the case of vegetation and fossil fuel combustion; and benzo[ghi]fluoranthene is an important component of motor vehicle exhausts. Hence, the dimethyl/ethylphenanthrene molecular fingerprint in atmospheric particulate results depending on the daily and seasonal modulation of sources (Paolini et al. 2015). On the other hand, only very fresh emissions hold benzo[b]anthracene (naphthacene) and anthanthrene, both prone to fast decomposition (Wise et al. 1988; Dominguez et al. 2003; Kim et al. 2009). In fact, the occurrence of the two compounds was documented in exhausts but was not regularly in the atmosphere. The methylphenanthrene mixture displays distinct isomer distributions in accordance with the kind of the emission source. Moreover, analogously to all other alkyl-PAH/parent PAH ratios, the rate of methylphenanthrenes/phenanthrene ratio depends on the predominance of kerosene spill or oil combustion exhausts, and on burning temperature and time. This ratio is also an index of thermal maturity of oil samples (Omotoye et al. 2016).
Normally, numerous PAHs affect particulates, and researchers prefer analyzing concentration ratios between pairs of individual substances to draw information about the nature of emissions (Brandli et al. 2007; Ravindra et al. 2008; Katsoyiannis et al. 2011; Katsoyiannis and Breivik 2014; Famiyeh et al. 2021). Concentration ratios that overall look as diagnostic for this purpose are fluoranthene vs. pyrene (FA/PY), benz[a]anthracene vs. chrysene (BaA/CH), indeno[1.2.3-cd]pyrene vs. benzo[ghi]perylene (IP/BPE), and benzo[a]pyrene vs. benzo[ghi]perylene (BaP/BPE). Other ratios, e.g., phenanthrene vs. anthracene (PHE/AN), total methylphenanthrene vs. phenanthrene (ΣMPHE/PHE), and unsubstituted PAHs vs. total PAHs including alkyl-substituted congeners (parent-PAHs/ΣPAHs), are investigated less frequently. Table 2 provides a list of PAH diagnostic ratios (DRs) and the respective values calculated for several categories of emissions, as described by scientific literature. As shown in Table 2, the DR values associated to various emissions are affected by some variability; therefore, to investigate the sources of particulate PAHs the authors adopted, in field experiments, both short DR ranges and more DRs simultaneously (usually three/four pairs) instead of one only DR and one precise DR rate (Famiyeh et al. 2021). The benzo[a]pyrene/benzo[e]pyrene ratio (BaP/BeP) merits a special comment. Indeed, BeP was neglected for long time, because it is much less carcinogenic than other PAHs; this is the reason why BeP does not appear among the sixteen priority PAHs. On the other hand, BeP occurs in emissions at similar extents as benzofluoranthenes and it is more persistent than benzo[a]pyrene. Thus, the concentration ratio between BaP and BeP is usually ≈1.0 in fresh exhausts but tends to drop slowly to < 0.1, overall in the presence of oxidants. For instance, in two PAH monitoring campaigns carried out in Milan, Italy, during 1991, at a site exposed predominantly to vehicle traffic (Cecinato 1997), the BaP/BeP ratio reached 0.9 in the winter and was as low as 0.1 in the summer. The information obtained through molecular signatures of PAHs alone seems insufficient to quantify the contribution of each emission source to the whole of environmental particulates; however, it helps in recognizing the principal causes of pollution and can be improved through associating other markers like oxy-PAHs, sugars, and alkanes (Zheng et al. 2002; Tian et al. 2021; Shin et al. 2022). Besides, this approach allows highlighting the role of oxidation processes with regard to toxicity, whenever the final products (e.g., PAH quinones and lactones) are more harmful than their parent compounds (Durant et al. 1999).
Table 2.
Rates of the principal PAH concentration ratios currently used as diagnostic tools to draw insights about the nature of source. References: Kavouras et al. (2001), Ravindra et al. (2008), Tobiszewski and Namiesnik (2012), Cecinato et al. (2014), Famiyeh et al. (2021)
| Source | Type | FA/PY | BaA/CH | IP/BPE | BaP/BPE | BaP/BeP |
|---|---|---|---|---|---|---|
| Vehicles | Mixed | 0.60 | 0.55 | |||
| Gasoline | 0.54 | 0.8–1.3 | 0.20–0.35 | 0.35 | 0.95 | |
| Diesel | 0.8–1.1 | 0.38 | 0.65–1.1 | 0.8–1.1 | 0.50 | |
| Domestic heating | Coal | 0.65 | 0.9–1.3 | 1.57 | ||
| Wood, pine | 0.78 | 0.64 | 1.1–1.6 | 1.94 | 2.1 | |
| Wood, oak | 0.75 | 0.70 | 1.2–1.6 | 1.77 | 1.77 | |
| Synthesis fuel | 1.19 | 0.78 | 1.1 | 1.91 | ||
| Heavy oil | 0.83 | 1.01 | 1.6 | 0.81 | 0.52 | |
| Steel plant | Coke | 0.65 | 0.9–1.3 | 1.57 | ||
| Power (coke) | 0.66 | 0.56 | 2.0 | 0.88 | 2.57 | |
| Tobacco smoke | Particulate | 0.96 | 1.3 | 0.18 | 0.23 | 0.38 |
| Waste fumes | Landfill | 1.3 | 0.84 | 0.76 | 0.70 | 0.55 |
| Incinerator | ≈17 | 0.71 | 0.92 | ~ 0.12 | 0.01 |
Nitrated polycyclic aromatic hydrocarbons (NPAHs)
NPAHs begun of big concern when many chemicals belonging to this group were identified in emissions (Hoekman 1992; Zielinska et al. 2004; Liu et al. 2010). In particular, the huge increment of diesel engine vehicles during 1970s and 1980s contributed to the occurrence of NPAHs in the air of cities worldwide. Besides, diesel engines were ascertained as main sources of NPAHs (Bamford and Baker 2003; Bandowe et al. 2014; Bandowe and Meusel 2017); on the other hand, many NPAHs were recognized as direct mutagens and cancer promoters (Gbeddy et al. 2020). The occurrence of NPAHs in the air declined with the updating of normative dealing with vehicle emissions and the consequent renewal of vehicle fleets; hence, NPAHs lost concern and their measurements in the environment dropped since 1990s. Instead, NPAH investigations have started again in recent years, due to their toxic properties (Degrendele et al. 2021) and to occurrence in gasoline-fueled cars (Zhao et al. 2020). Usually, attention is paid to a list of NPAHs affecting airborne particulate; they are nitrated derivatives of naphthalene, fluorene, anthracene, fluoranthene, pyrene, benz[a]anthracene, and chrysene. Nevertheless, NO2-position isomeric PAHs associated to airborne particulates are not the principal ones coming out from emissions. In particular, 2-nitrofluoranthene and 2-nitropyrene are commonly absent in exhausts and exist as products of in situ reactions developing in the atmosphere; the two compounds are sometimes the most abundant NPAHs affecting particulates (Bamford and Baker 2003; Bandowe and Meusel 2017).
The molecular signature of nitrated fluoranthenes and pyrenes has been used to parameterize the relative importance of direct emission and action of oxidants. Taking in account the nitration rate of precursors reacting with OH radicals and NO2, it was suggested that photochemical reactivity is more important than vs. direct emission when the 2-NFA/1-NPY ratio exceeds 5.0 (Pitts et al. 1985). Instead, the formation of 4-NPY can occur only in the presence of NO3 radical or N2O5; thus, this isomer is a tracer of processes started by reaction of O3 with NO2 and developing after sunset. Analogously, distinct nitro-isomers are formed by homogeneous and heterogeneous reactions of other parent PAHs with NO2, OH + NO2, and NO3/N2O5 (Jariyasopit et al. 2014, and references herein).
Fatty acids and alcohols, polar compounds
Medium- and long-chain acids exist overall thanks to living organisms releasing them (Goutx and Saliot 1980; Kawamura and Gagosian 1987; Lindbeck and Puxbaum 1999; Oliveira et al. 2007; Bi et al. 2008; Sangiorgi et al. 2013; Balducci et al. 2014). The percent distribution pattern of fatty acids reveals the clear prevalence of even carbon atom homologs, and the rates of carbon preference indexes (ACPIs), formulated similarly to those of n-alkanes, usually exceed 10 (Alves et al. 2001). Other sources show analogous profiles; e.g., vehicle exhausts hold A12–A22 acids (i.e., linear chain homologs with 12 ÷ 22 carbon atoms), with the maximums corresponding to A16 and A18. Biogenic emissions show also typical percentages of medium- and long-chain fatty acids. Usually, apart from palmitic (A16) and stearic (A18) acids, the profiles display a secondary maximum within the ranges A20 ÷ A24, or > A25; light homologs have been associated with microbiota, small plants, and softwood trees, while heavy acids characterize high plants and hardwood trees (Gelpi et al. 1970). Worth of note, the ACPI rates are lower in the case of microorganisms, due to important percentages of odd-carbon acids from A15 to A21.
Unsaturated and dicarboxylic acids merit a special mention. Unsaturated acids (UAn), e.g., palmitoleic (UA16), oleic (UA18), and linoleic (twin unsaturated A18), are indicative of emission from crops and in interiors of cooking (Schauer et al. 2002; Yu et al. 2021). Dicarboxylic fatty acids (DAn) exist as minor components released by vegetation, whose emissions show the usual even-to-odd carbon prevalence; however, particulate matters are rich of DA2–DA6 homologs, emitted by anthropogenic sources, which influence the molecular signature of the short C-chain range. Besides, air parcels affected by oxidants show the occurrence of azelaic acid (DA9), which is a by-product of oleic acid decomposition (Balducci et al. 2014; Kawamura and Bikkina 2016; Ren et al. 2020).
Linear alcohols, 2-ketones, aldehydes, fatty acid methyl esters, and nitriles were not extensively investigated as tracers of suspended particulate sources, though all of them have been linked to emission from vegetation (Simoneit and Mazurek 1982; Simoneit 2002) and have been found in pyrolysis by-products of sewage sludge treated with aerobic and anaerobic digestion (Dominguez et al. 2003). Linear alcohols exhibit a behavior parallel to that of fatty acids. They display the predominance of even carbon homologs and are among the principal components of organic aerosols in rural regions (Simoneit and Mazurek 1982). Many sterols also (including campesterol, sitosterol, stigmasterol, and amyrins) have been associated to vegetation as components of epicuticular waxes (Guo et al. 2019; Kumar et al. 2019; Gal et al. 2022). The most important exception is cholesterol, which has been recognized as a tracer of meat cooking (Cass 1998; Carreira et al. 2009). Other acid esters (including biopolymers, benzoates, terephthalates, myristates, and glycols) are employed nowadays as surrogates of old components of plastics (Sanchez-Pinero et al. 2021; Evtyugina et al. 2021), because these latter have been classified as emerging contaminants (Cavanagh et al. 2018, Udayakumar et al. 2021). Thus, the occurrence of new esters in soot and dust would be indicative of contamination by new plasticizers and, in interiors, of house cleaning, painting and building commodities as well as of cosmetics and other personal care products. No extensive investigations have undertaken in the environment regarding this topic; however, they should be gain importance in the future, due to the general tendency to replace alkyl phthalates and polyvinyl chloride with eco-friend plasticizers.
Several polar organics, including nitrosamines and nicotelline, have been suggested in the last decade as tracers of mainstream, sidestream, and third-hand tobacco smoke (Apelberg et al. 2013; Blanchard et al. 2014) in addition to, or as substitute for, nicotine, cotinine, fine particulate, and CO (Hecht 1999; Hammond et al. 1987; Daisey 1999). In particular, nicotelline has recognized as fine to perform quantitative assessments of the tobacco smoke contribution to pollution of indoor and outdoor environment, thanks to its low volatility and enough persistence in the air (Aquilina et al. 2021).
Organic halides
Polychlorobiphenyls (PCBs) and polychlorinated dioxins/furans (PCDD/Fs) are probably the most investigated groups of halides affecting the environment (Barbas et al. 2018). PCBs were important industrial products during the twentieth century, since they found a number of applications as mixtures as solvent, in power transformers and heat exchangers, in substrates for pesticides and inks. By contrast, PCDDs and PCDFs exist solely as unwanted by-products of other industrial processes (e.g., paint manufacturing, foundries and steel mill, waste incineration). Despite PCBs and PCDD/Fs have been banned since long time, both groups continue to affect the environment today again (Ngo et al. 2020). Industrial syntheses of PCBs lead to blends characterized by various average chlorine percentages, which sometimes could aid in highlighting the impact of sources suspected as causing environmental pollution. A variety of fingerprints tags the emission sources; for instance, distinct PCDD/Fs patterns have observed for vehicle exhausts, sewage sludge, and steel mill fumes (Mininni et al. 2004; Liu et al. 2015). Besides that, the molecular fingerprints of PCBs and PCDD/Fs change with time owing to ability of congeners to persist to degradation as well as to dissolve in waters and lipids (Di Guardo et al. 2017; Ngo et al. 2018, 2020). Finally, looking to bioavailability of organic halides, we must take in account that these substances are semi-volatile. For instance, 2.3.7.8-tetrachlorodibenzo-p-dioxin exists overall as vapor in the environment, while most dioxin-like congeners occur as adsorbed on particulates (Barbas et al. 2018). Due to key role of dioxin-like compounds regarding to toxicity, the contemporary collection of gaseous and condensed phases of emissions and atmosphere is mandatory to draw information about sources and air quality. Nevertheless, the actual risk for humans depends on the aggregation state of toxicants, because vapors are in part breathed out, while ultra-fine and fine particles are easily retained in lungs with their harmful load.
Other halides have recently gained concern as tracers of water, air, and soil pollution depending on waste spill and contaminated food. In particular, polybromodiphenyl ethers (PBDEs) and phosphoric acid organic esters are present in flame retardant formulas (Lee et al. 2020; Percy et al. 2020), while perfluoroalkyl acids (PFAs) and other perfluorinated chemicals enjoy of many industrial and home care applications as surfactants (Hubbard et al. 2012).
Polysaccharides
The occurrence of numerous organic substances in the environment is associated with biomass burning in general, and specifically with that of specific tree species (Oros and Simoneit 2001a, b, Oros et al. 2002). Many chemicals are carbohydrate molecules (e.g., glucose, xylose, and sucrose) and the respective dehydration-polymerization by-products of them (levoglucosan, galactosan, mannosan, inositols) (Simoneit et al. 2004; Jia and Fraser 2011; Pereira et al. 2017; Bikkina et al. 2019; Lv et al. 2021) and of lignin (e.g., methoxyphenols) (Hawthorne et al. 1988; Hays et al. 2005). Levoglucosan is usually the predominant anhydrosugar; however, other minor polysaccharides allow distinguishing hardwood from softwood burning, thanks to their concentration ratios vs. levoglucosan; in fact, levoglucosan/mannosan ratios ranging from 3 to 10 are typical combustion of softwood, while ratios ranging 15 ÷ 25 of hardwood, and > 40 of crop burning (Kang et al. 2018; Mu et al. 2021). Levoglucosan in particular, typical tracer of wood burning, allowed to demonstrate that even the downtowns of big cities undergo the impact of this kind of emission, due to the generalized use of wood for heating and cooking in the countryside (Fine et al. 2004). Indeed, wide uncertainty remains about the emission rate of these chemicals; nevertheless, according to levoglucosan levels in air, manmade biomass burning looks as the principal source of pollution outside of cities and heavy industry districts (Pomata et al. 2014; Perrino et al. 2019; Ren et al. 2020).
Living organisms as unexpected and unconsidered sources of organic contaminants
All living organisms, including humans, are not only the target, but also the source of a number of contaminants (Settimo et al. 2020b). Microorganisms are exploited to remove organic toxicants through aerobic and anaerobic digestion (Habib et al. 2022; Priya et al. 2022). On the other hand, fungi, spores, bacteria, insects, and indoor plants inhabit our environment, leave everywhere traces of their presence, and often move people to use repellants, pesticides, and disinfectants; meanwhile, pets are the source of allergies, irritations, and breathing problems (Diaz 2016; Zhai et al. 2018; Settimo et al. 2020b; Cui et al. 2022). This phenomenon is much more important in building interiors, where humans contribute in a twin way, i.e., (i) indirectly, through actions related to use of home and personal care products (deodorants, cleaning sprays, soaps, fragrances, plastics), to cooking (foods), wearing (fibers, dyes), and heating/air conditioning (fuels, freezing liquids); and (ii) directly, through emitting vapors, droplets, and particles (e.g., through breath, sweat, hair loss, skin abrasion) (Nazzaro-Porro et al. 1979; Bortz et al. 1989; Camera et al. 2010; Knox and O’Boyle 2021). Chemical composition of man’s skin and hair lipids, sweat, and breath is known since long time; however, the concern about it seems restricted to industry of cosmetics and related production, while at our knowledge no investigations are undertaken concerning their occurrence in our life places. Hence, the search for specific molecular fingerprints that allow indexing their impact on the chemistry of the environments is still at the start point.
Conclusions
The sources of pollution (both direct emissions and atmospheric reactions leading to presence of toxicants in the environment) model the molecular fingerprint of organic contaminants associated to airborne particulates and deposition dusts. This chemical signature is comprised of individual markers and, more often, of distribution patterns within groups of homologs. Both types of signature provide preliminary but useful information about nature of emissions and with regard to their health impact on environment. Though studied since long time, the molecular fingerprints of emissions are not completely elucidated and further investigations seem necessary, due to recent detection of new distribution models of particulate matter components that mess up consolidated behaviors (e.g., alkanes), as well as to the novel concern for emission sources neglected until now (microorganisms). The progress of knowledge about the molecular fingerprints of sources will aid investigators to apply more sophisticated approaches (e.g., providing explanation of the crude results of principal component analysis or source factorization modeling) and assess the relative importance of emissions. Besides, it will favor optimizing the strategies aimed at controlling air pollution and mitigating the impact of toxicants on humans and environment. In that perspective, new studies are advisable to do, aimed at characterizing chemicals released by humans, pets, and living microorganisms, which often prejudice the healthiness of the life places.
Author contribution
This paper originates from specific contributions of the authors. They are:
Dr. Angelo Cecinato: result analysis, conceptualization of molecular signature indexing, original and revised draft preparation, writing and editing.
Prof. Alessandro Bacaloni: supervision and training of doctorate thesis work.
Dr. Paola Romagnoli: chemical analysis of dust and suspended particulate samples.
Tech. Mattia Perilli: in-field campaign preparation, performance and management, paper reviewing.
Dr. Catia Balducci: chemical procedure setup, sample analysis, investigation performance and management, writing (reviewing, revised manuscript editing).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Anyway, those not directly produced by personal investigations of authors are available in the bibliography cited in the paper.
Declarations
Ethics approval and consent to participate
This study did not involve experiments on humans or animals. It consisted of analysis of literature regarding chemical composition of tobacco smoke, as well as of data series about organic matter associated to airborne particulates and dusts, set up in the frame of research programs performed by the authors.
Consent for publication
Not applicable. The manuscript does not contain any individual person’s data, since none was subject of investigation nor personal data treatment.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Angelo Cecinato, Email: angelo.cecinato@iia.cnr.it, Email: angelo.cecinato@uniroma1.it.
Alessandro Bacaloni, Email: alessandro.bacaloni@uniroma1.it.
Paola Romagnoli, Email: romagnoli@iia.cnr.it.
Mattia Perilli, Email: perilli@iia.cnr.it.
Catia Balducci, Email: balducci@iia.cnr.it.
References
- Aboul-Kassim TAT, Simoneit BRT. Aliphatic and aromatic hydrocarbons in particulate fallout of Alexandria, Egypt: sources and implications. Environ Sci Technol. 1995;29(10):2473–2483. doi: 10.1021/es00010a004. [DOI] [PubMed] [Google Scholar]
- Aghadadashi V, Mehdinia A, Molaei S. Normal alkanes in sediments from the Persian Gulf: spatial pattern and implications for autochthonous, allochthonous, and petroleum-originated contaminants. Environ Monit Assess. 2021;193:364. doi: 10.1007/s10661-021-09122-x. [DOI] [PubMed] [Google Scholar]
- Albaiges J, Grimalt J, Bayona JM, Risegrough IR, De Lappe B, Walker W. Dissolved, particulate and sedimentary hydrocarbons in a deltaic environment. Org Geochem. 1984;6:237–248. doi: 10.1016/0146-6380(84)90045-7. [DOI] [Google Scholar]
- Alexandrino GL, Tomasi G, Kienhuis PGM, Augusto F, Christensen JH. Forensic investigations of diesel oil spills in the environment using comprehensive two-dimensional gas chromatography - high resolution mass spectrometry and chemometrics: new perspectives in the absence of recalcitrant biomarkers. Environ Sci Technol. 2019;53:550–559. doi: 10.1021/acs.est.8b05238. [DOI] [PubMed] [Google Scholar]
- Alkhafaji MW. Biomarker assessment of oil biodegradation, water washing, and source rock characteristics of oil seeps from the Foothill Zone along the Tigris River, Northern Iraq. J Petrol Sci Engin. 2021;197:107946. doi: 10.1016/j.petrol.2020.107946. [DOI] [Google Scholar]
- Aloulou F, Kallel M, Dammak M, Elleuch B, Saliot A. Even-numbered n-alkanes/n-alkenes predominance in surface sediments of Gabes Gulf in Tunisia. Environ Earth Sci. 2010;61:1–10. doi: 10.1007/s12665-009-0315-y. [DOI] [Google Scholar]
- Alves C, Pio C, Duarte A. Composition of extractable organic matter of air particles from rural and urban Portuguese areas. Atmos Environ. 2001;35:5485–5496. doi: 10.1016/S1352-2310(01)00243-6. [DOI] [Google Scholar]
- Alves CA, Pio CA, Duarte AC. Particulate size distributed organic compounds in a forest atmosphere. Environ Sci Technol. 2000;34:4287–4293. doi: 10.1021/es000028a. [DOI] [Google Scholar]
- Alves CA, Vicente A, Monteiro C, Gonçalves C, Evtyugina M, Pio C. Emission of trace gases and organic components in smoke particles, from a wildfire in a mixed-evergreen forest in Portugal. Sci Total Environ. 2011;409:1466–1475. doi: 10.1016/j.scitotenv.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Andersson JT, Achten C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycyclic Aromat Compd. 2015;35:330–354. doi: 10.1080/10406638.2014.991042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou G, Rapsomanikis S. Origins of n-alkanes, carbonyl compounds and molecular biomarkers in atmospheric fine and coarse particles of Athens, Greece. Sci Total Environ. 2009;407:5750–5760. doi: 10.1016/j.scitotenv.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Hepp LM, Avila-Tang E, Gundel L, Hammond SK, Hovell MF, Hyland A, Klepeis NE, Madsen CC, Navas-Acie A, Repac J, Same JM, Breyss PN. Environmental monitoring of secondhand smoke exposure. Tob Control. 2013;22:147–155. doi: 10.1136/tobaccocontrol-2011-050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquilina NJ, Havel CM, Cheung P, Harrison RM, Ho KF, Benowitz L, Jacob P., III Ubiquitous atmospheric contamination by tobacco smoke: nicotine and a new marker for tobacco smoke-derived particulate matter, nicotelline. Environ Int. 2021;150:106417. doi: 10.1016/j.envint.2021.106417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey J, Atkinson R. Photochemical reactions of PAHs in the atmosphere. In: Douben PET, editor. PAHs. An ecotoxicological perspective. Chichester England: John Wiley & Sons Ltd; 2003. pp. 47–63. [Google Scholar]
- Arfaoui A. The advantages of using n-alkanes, triterpane, and steranes to determine the characterization of sedimentary organic matter. Arab J Geosci. 2014;7:369–383. doi: 10.1007/s12517-012-0810-4. [DOI] [Google Scholar]
- Arshinova VG, Arshinov MY, Belan BD, Davydov DK, Ivlev GA, Kozlov AV, Kozlov AS, Rasskazchikova TM, Simonenkov DV, Tolmachev GN. Vertical distribution of alkanes in atmospheric aerosol in the Russian Arctic in September 2020. Atmos Ocean Optics. 2021;34:577–585. doi: 10.1134/S1024856021060312. [DOI] [Google Scholar]
- Atkinson R, Arey J. Atmospheric chemistry of gas-phase polycyclic aromatic hydrocarbons: formation of atmospheric mutagens, Environ Health Perspec 102. Suppl. 1994;4:117–126. doi: 10.1289/ehp.94102s4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azimi-Yancheshmeh R, Riyahi-Bakhtiari A, Savabieasfahani M. Oil contamination in surface sediment of Anzali Wetland in Iran is primarily even carbon number n-alkanes. Environ Monit Assess. 2017;189:589. doi: 10.1007/s10661-017-6294-7. [DOI] [PubMed] [Google Scholar]
- Balducci C, Ladji R, Muto V, Romagnoli P, Yassa N, Cecinato A. Biogenic and anthropogenic organic components of Saharan sands. Chemosphere. 2014;107:129–135. doi: 10.1016/j.chemosphere.2014.02.069. [DOI] [PubMed] [Google Scholar]
- Balducci C, Cecinato A, Paolini V, Guerriero E, Perilli M, Romagnoli P, Tortorella C, Nacci RM, Giove A, Febo A. Volatilization and oxidative artifacts of PM bound PAHs at low volume sampling (2): evaluation and comparison of mitigation strategies effects. Chemosphere. 2017;189:330–339. doi: 10.1016/j.chemosphere.2017.09.062. [DOI] [PubMed] [Google Scholar]
- Bamford HA, Baker JE. Nitro-polycyclic aromatic hydrocarbon concentrations and sources in urban and suburban atmospheres of the Mid-Atlantic region. Atmos Environ. 2003;37:2077–2091. doi: 10.1016/S1352-2310(03)00102-X. [DOI] [Google Scholar]
- Bandowe BAM, Meusel H. Nitrated polycyclic aromatic hydrocarbons (nitroPAHs) in the environment - a review. Sci Total Environ. 2017;581–582:237–257. doi: 10.1016/j.scitotenv.2016.12.115. [DOI] [PubMed] [Google Scholar]
- Bandowe BAM, Meusel H, Huang R, Ho K, Cao J, Hoffmann T, Wilcke W. PM2.5-bound oxygenated PAHs, nitro-PAHs and parent-PAHs from the atmosphere of a Chinese megacity: seasonal variation, sources and cancer risk assessment. Sci Total Environ. 2014;473–474:77–87. doi: 10.1016/j.scitotenv.2013.11.108. [DOI] [PubMed] [Google Scholar]
- Barbas B, de la Torre A, Sanz P, Navarro I, Artíñano B, Martínez MA. Gas/particle partitioning and particle size distribution of PCDD/Fs and PCBs in urban ambient air. Sci Total Environ. 2018;624:170–179. doi: 10.1016/j.scitotenv.2017.12.114. [DOI] [PubMed] [Google Scholar]
- Bascom R, Bromberg PA, Costa DA, Devlin R, Dockery DW, Frampton MW, Lambert W, Samet JM, Speizer FE, Utell M. Health effects of outdoor air pollution - part 1. Am J Resp Crit Care Med. 1996;153:3–50. doi: 10.1164/ajrccm.153.2.8564086. [DOI] [PubMed] [Google Scholar]
- Benner BA, Jr, Wise SA, Currie LA, Kloud GA, Klinedinst DB, Zweidinge RB, Stevens RK, Lewis CW. Distinguishing the contributions of residential wood combustion and mobile source emissions using relative concentrations of dimethylphenanthrene isomers. Environ Sci Technol. 1995;29:2382–2389. doi: 10.1021/es00009a034. [DOI] [PubMed] [Google Scholar]
- Bi X, Simoneit BRT, Sheng G, Ma S, Fy J. Composition and major sources of organic compounds in urban aerosols. Atmos Res. 2008;88:256–265. doi: 10.1016/j.atmosres.2007.11.017. [DOI] [Google Scholar]
- Blanchard O, Glorennec P, Mercier F, Bonvallot N, Chevrier C, Ramalho O, Mandin C, Le Bot B. Semivolatile organic compounds in indoor air and settled dust in 30 French dwellings. Environ Sci Technol. 2014;48:3959–3969. doi: 10.1021/es405269q. [DOI] [PubMed] [Google Scholar]
- Bikkina S, Haque MdM, Sarin M, Kawamura K. Tracing the relative significance of primary versus secondary organic aerosols from biomass burning plumes over coastal ocean using sugar compounds and stable carbon isotopes. ACS Earth Space Chem. 2019;3:1471–1484. doi: 10.1021/acsearthspacechem.9b00140. [DOI] [Google Scholar]
- Błaszczyk E, Rogula-Kozłowska W, Klejnowski K, Kubiesa P, Fulara I, Mielżyńska-Švach D. Indoor air quality in urban and rural kindergartens: short-term studies in Silesia, Poland. Air Qual Atmos Health. 2017;10:1207–1220. doi: 10.1007/s11869-017-0505-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortz JT, Wertz PW, Downing DT. The origin of alkanes found in human skin surface lipids. J Investig Dermatol. 1989;93:723–727. doi: 10.1111/1523-1747.ep12284393. [DOI] [PubMed] [Google Scholar]
- Brandli RC, Bucheli TD, Kupper T, Mayer J, Stadelmann FX, Tarradellas J. Fate of PCBs, PAHs and their source characteristic ratios during composting and digestion of source-separated organic waste in full-scale plants. Environ Pollut. 2007;148:520–528. doi: 10.1016/j.envpol.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Brehmer C, Norris C, Barkjohn KK, Bergin MH, Zhang J, Cui X, Teng Y, Zhang Y, Black M, Li Z, Shafer MM. The impact of household air cleaners on the oxidative potential of PM2.5 and the role of metals and sources associated with indoor and outdoor exposure. Environ Res. 2020;181:108919. doi: 10.1016/j.envres.2019.108919. [DOI] [PubMed] [Google Scholar]
- Brown AS, Brown RJC. Correlations in polycyclic aromatic hydrocarbon (PAH) concentrations in UK ambient air and implications for source apportionment. J Environ Monit. 2012;14:2072–2082. doi: 10.1039/C2EM10963H. [DOI] [PubMed] [Google Scholar]
- Brown RJC, Brown AS. Principal component analysis as an outlier detection tool for polycyclic aromatic hydrocarbon concentrations in ambient air. Water Air Soil Poll. 2012;223:3807–3816. doi: 10.1007/s11270-012-1149-x. [DOI] [Google Scholar]
- Camera E, Ludovici M, Galante M, Sinagra JL, Picardo M. Comprehensive analysis of the major lipid classes in sebum by rapid resolution high-performance liquid chromatography and electrospray mass spectrometry. J Lipid Res. 2010;51:3377–3388. doi: 10.1194/jlr.D008391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Wang M, Chen Q, Zhu C, Jie J, Li X, Dong X, Miao Z, Shen M, Bu Q. Spatial, seasonal and particle size dependent variations of PAH contamination in indoor dust and the corresponding human health risk. Sci Total Environ. 2019;653:423–430. doi: 10.1016/j.scitotenv.2018.10.413. [DOI] [PubMed] [Google Scholar]
- Carreira RS, Ribeiro PV, Silva CEM. Hydrocarbons and sterols as indicators of sources and destination of organic matter in sediments of Sepetiba Bay, Rio de Janeiro. Chem Nova. 2009;32:1805–1811. [Google Scholar]
- Cass GR. Organic molecular tracers for particulate air pollution sources. Trends Anal Chem. 1998;17:356–366. doi: 10.1016/S0165-9936(98)00040-5. [DOI] [Google Scholar]
- Caumo S, Vicente A, Custódio D, Alves CA, Vasconcellos P. Organic compounds in particulate and gaseous phase collected in the neighbourhood of an industrial complex in São Paulo (Brazil) Air Quality Atmos Health. 2018;11:271–283. doi: 10.1007/s11869-017-0531-7. [DOI] [Google Scholar]
- Cavanagh JAE, Trought K, Mitchell C, Northcott G, Tremblay LA. Assessment of endocrine disruption and oxidative potential of bisphenol-A, triclosan, nonylphenol, diethylhexyl phthalate, galaxolide, and carbamazepine, common contaminants of municipal biosolids. Toxicol in Vitro. 2018;48:342–349. doi: 10.1016/j.tiv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Cecinato A. Polynuclear aromatic hydrocarbons (PAH), benz(a)pyrene (BaPY) and nitrated-PAH (N-PAH) in suspended particulate matter. Annali di Chimica. 1997;87:483–496. [Google Scholar]
- Cecinato A, Guerriero E, Balducci C, Muto V. Use of the PAH fingerprints for identifying pollution sources. Urban Climate. 2014;10:630–643. doi: 10.1016/j.uclim.2014.04.004. [DOI] [Google Scholar]
- Cecinato A, Bacaloni RP, Perilli M, Balducci C. Indexes of tobacco smoke contribution to environmental particulates based on molecular fingerprints of alkanes. Environ Sci Pollut Res. 2022 doi: 10.1007/s11356-021-16617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin B, Yurdakul S, Gungormus E, Ozturk F, Sofuoglu SC. Source apportionment and carcinogenic risk assessment of passive air sampler-derived PAHs and PCBs in a heavily industrialized region. Sci Total Environ. 2018;633:30–41. doi: 10.1016/j.scitotenv.2018.03.145. [DOI] [PubMed] [Google Scholar]
- Chen YY, Kao TW, Wang CC, Chen YJ, Wu CJ, Lai CH, Chen WL. Exposure to polycyclic aromatic hydrocarbons and risk of disability among an elderly population. Environ Sci Pollut Res. 2019;26:10719–10726. doi: 10.1007/s11356-019-04498-3. [DOI] [PubMed] [Google Scholar]
- Chu SN, Sands S, Tomasik MR, Lee PS, McNeill VF. Ozone oxidation of surface-adsorbed polycyclic aromatic hydrocarbons: role of PAH-surface interaction. J Am Chem Soc. 2010;132:15968–15975. doi: 10.1021/ja1014772. [DOI] [PubMed] [Google Scholar]
- Collins JF, Brown JP, Alexceff GV, Salmon AG. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul Toxicol Pharmacol. 1998;28:45–54. doi: 10.1006/rtph.1998.1235. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zhang H, Zhang J, Lv B, Xie B. The emission of volatile organic compounds during the initial decomposition stage of food waste and its relationship with the bacterial community. Environ Technol Innovat. 2022;27:102443. doi: 10.1016/j.eti.2022.102443. [DOI] [Google Scholar]
- Daisey JM. Tracers for assessing exposure to environmental tobacco smoke: what are they tracing? Environ Health Perspect. 1999;107(Suppl. 2):319–327. doi: 10.1289/ehp.99107s2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisey JM, Cheney JL, Lioy PJ. Profiles of organic particulate emissions from air pollution sources: status and needs for receptor source apportionment modelling. J Air Pollut Control Assoc. 1986;36:17–33. doi: 10.1080/00022470.1986.10466041. [DOI] [PubMed] [Google Scholar]
- Damanik N, Ong HC, Tong CW, Mahlia TMI, Silitonga AS. A review on the engine performance and exhaust emission characteristics of diesel engines fueled with biodiesel blends. Environ Sci Pollut Res. 2018;25:15307–15325. doi: 10.1007/s11356-018-2098-8. [DOI] [PubMed] [Google Scholar]
- Degrendele C, Kanduč T, Kocman D, Lammel G, Cambelová A, Dos Santos SG, Horvat M, Kukučka P, Holubová Šmejkalová A, Mikeš O, Nuñez-Corcuera B, Přibylová P, Prokeš R, Saňka O, Maggos T, Sarigiannis D, Klánová J. NPAHs and OPAHs in the atmosphere of two central European cities: seasonality, urban-to-background gradients, cancer risks and gas-to-particle partitioning. Sci Total Environ. 2021;793:148528. doi: 10.1016/j.scitotenv.2021.148528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wildern Environ Med. 2016;27:153–163. doi: 10.1016/j.wem.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Di Guardo A, Terzaghi E, Raspa G, Borin S, Mapelli F, Chouaia B, Zanardini E, Morosini C, Colombo A, Fattore E, Davoli E, Armiraglio S, Sale VM, Anelli S, Nastasio P. Differentiating current and past PCB and PCDD/F sources: the role of a large contaminated soil site in an industrialized city area. Environ Pollut. 2017;223:367–375. doi: 10.1016/j.envpol.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Dominguez A, Menendez JA, Inguanzo M, Bernad PL, Pis JJ. Gas chromatographic–mass spectrometric study of the oil fractions produced by microwave-assisted pyrolysis of different sewage sludges. J Chromatog A. 2003;1012:193–206. doi: 10.1016/S0021-9673(03)01176-2. [DOI] [PubMed] [Google Scholar]
- Durant JL, Busby WF, Jr, Lafleur AL, Penman BW, Crespi CL. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat Res. 1999;371:123–157. doi: 10.1016/S0165-1218(96)90103-2. [DOI] [PubMed] [Google Scholar]
- Eglinton G, Gonzalez AG, Hamilton RJ, Raphael RA. Hydrocarbon constituents of the wax coatings of plant leaves: a taxonomic survey. Phytochem. 1962;1:89–102. doi: 10.1016/S0031-9422(00)88006-1. [DOI] [PubMed] [Google Scholar]
- Eglinton G, Hamilton RJ. Leaf epicuticular waxes. Science. 1967;156:1322–1335. doi: 10.1126/science.156.3780.1322. [DOI] [PubMed] [Google Scholar]
- Ekpo BO, Oyo-Ita OE, Wehner H. Even-n-alkane/alkene predominances in surface sediments from the Calabar River, SE Niger Delta, Nigeria. Naturwissenschaften. 2005;92:341–346. doi: 10.1007/s00114-005-0639-8. [DOI] [PubMed] [Google Scholar]
- Emsbo-Mattingly SD, Litman E (2016).Polycyclic aromatic hydrocarbon homolog and isomer fingerprinting. In: Standard handbook oil spill environmental forensics, fingerprinting and source identification. Chapter 5, p. 255-312 10.1016/B978-0-12-809659-8.00005-X
- Estève W, Budzinski H, Villenave E. Relative rate constants for the heterogeneous reactions of OH, NO2 and NO3 radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 1: PAHs adsorbed on 1–2 µm calibrated graphite particles. Atmos Environ. 2004;38:6063–6072. doi: 10.1016/j.atmosenv.2004.05.059. [DOI] [Google Scholar]
- Evtyugina M, Vicente ED, Vicente AM, Nunes T, Lucarelli F, Calzolai G, Nava S, Blanco-Alegre C, Calvo AI, Castro A, Fraile R, Oduber F, Cerqueira M, Alves CA. Air quality and particulate matter speciation in a beauty salon and surrounding outdoor environment: exploratory study. Atmos Pollut Res. 2021;12:101174. doi: 10.1016/j.apr.2021.101174. [DOI] [Google Scholar]
- European Parliament and Council (2005). Directive 2004/107/EC of the European parliament and of the council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Official J Europ Union 23, pp 1–16, (26/01/2005).
- Famiyeh L, Chen K, Xu J, Sun Y, Guo Q, Wang CLJ, Tang Y, Snape C, He J. A review on analysis methods, source identification, and cancer risk evaluation of atmospheric polycyclic aromatic hydrocarbons. Sci Total Environ. 2021;789:147741. doi: 10.1016/j.scitotenv.2021.147741. [DOI] [PubMed] [Google Scholar]
- Fang H, Lowther SD, Zhu M, Pei C, Li S, Fang Z, Yu X, Yu O, Wang Y, Zhang Y, Jones KC, WangX, PM2.5-bound unresolved complex mixtures (UCM) in the Pearl River Delta region: abundance, atmospheric processes and sources. Atmos Environ. 2020;226:117407. doi: 10.1016/j.atmosenv.2020.117407. [DOI] [Google Scholar]
- Fine PM, Cass GR, Simoneit BRT. Chemical characterization of fine particle emissions from the wood stove combustion of prevalent United States tree species. Environ Engin Sci. 2004;21:705–721. doi: 10.1089/ees.2004.21.705. [DOI] [Google Scholar]
- Fisher DJ, Holloway PJ, Richmond DV. Fatty acid and hydrocarbon constituents of the surface and wall lipids of some fungal spores. J General Microbiol. 1972;72:71–78. doi: 10.1099/00221287-72-1-71. [DOI] [Google Scholar]
- Fraser MP, Cass GR, Simoneit BRT, Rasmussen RA. Air quality model evaluation data for organics. 4. C2–C36 non-aromatic hydrocarbons. Environ Sci Technol. 1997;31:2356–2367. doi: 10.1021/es960980g. [DOI] [Google Scholar]
- Gal JK, Kim BK, Joo HM, Shim C, Lee B, Kim IN, Jung J, Shin KH, Ha SY. Spatial distribution and origin of organic matters in an Arctic fjord system based on lipid biomarkers (n-alkanes and sterols) Environ Res. 2022;205:112469. doi: 10.1016/j.envres.2021.112469. [DOI] [PubMed] [Google Scholar]
- Gao Y, Han Y, Xia J, Tan J, Wang YP, Wang S. Composition and distribution of aliphatic hydrocarbon compounds and biomarkers in seafloor sediments from offshore of the Leizhou Peninsula (South China) ACS Omega. 2021;6:34286–34293. doi: 10.1021/acsomega.1c03529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbeddy G, Egodawatta P, Goonetilleke A, Ayoko G, Chen L. Application of quantitative structure-activity relationship (QSAR) model in comprehensive human health risk assessment of PAHs, and alkyl-, nitro-, carbonyl-, and hydroxyl-PAHs laden in urban road dust. J Hazard Mater. 2020;383:121154. doi: 10.1016/j.jhazmat.2019.121154. [DOI] [PubMed] [Google Scholar]
- Gelpi E, Schneider H, Mann J, Oro J. Hydrocarbons of geochemical significance in microscopic algae. Phytochem. 1970;9:603–612. doi: 10.1016/S0031-9422(00)85700-3. [DOI] [Google Scholar]
- Giulivo M, Lopez de Alda M, Barceló CE, D, Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A Review Environ Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Goutx M, Saliot A. Relationship between dissolved and particulate fatty acids and hydrocarbons, chlorophyll a and zooplankton biomass in Villefranche Bay, Mediterranean Sea. Marine Chem. 1980;8:299–318. doi: 10.1016/0304-4203(80)90019-5. [DOI] [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman PJ. A global model of natural volatile organic compounds emissions. J Geophys Res Atmosphere. 1995;100:8873–8892. doi: 10.1029/94JD02950. [DOI] [Google Scholar]
- Gundel LA, Daisey JM, de Carvalho RF, Kado NY, Schuetzle D. Polar organic matter in airborne particles. Chemical characterization and mutagenic activity. Environ Sci Technol. 1993;27:2112–2119. doi: 10.1021/es00047a017. [DOI] [Google Scholar]
- Guo Y, Kannan K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ Sci Technol. 2013;47:14442–14449. doi: 10.1021/es4042034. [DOI] [PubMed] [Google Scholar]
- Guo W, Jia G, Ye F, Xiao H, Zhang Z. Lipid biomarkers in suspended particulate matter and surface sediments in the Pearl River Estuary, a subtropical estuary in southern China. Sci Total Environ. 2019;646:416–426. doi: 10.1016/j.scitotenv.2018.07.159. [DOI] [PubMed] [Google Scholar]
- Habib ML, Hasan MM, Biswas S, Hossain MT, Anwaruzzaman M, Kamruzzaman M (2022). Removal of organic micro-pollutants by aerobic and anaerobic microorganisms. In: Biodegradation and detoxification of micropollutants in industrial wastewater. Elsevier, Chapter 4, pp. 55–78. 10.1016/B978-0-323-88507-2.00003
- Hamilton SE, Bates TS, Cline GL. Sources and transport of hydrocarbons in the Green-Duwamish River, Washington. Environ Sci Technol. 1984;18:72–79. doi: 10.1021/es00120a004. [DOI] [PubMed] [Google Scholar]
- Hammond SK, Leaderer BP, Roche AC, Schenker M. Characterization of contaminant emissions from indoor sources collection and analysis of nicotine as a marker for environmental tobacco smoke. Atmos Environ. 1987;21:457–462. doi: 10.1016/0004-6981(87)90027-8. [DOI] [Google Scholar]
- Harrison RM, Smith DJT, Luhanan L. Source apportionment of atmospheric polycyclic aromatic hydrocarbons collected from an urban location in Birmingham, UK. Environ Sci Technol. 1996;30:825–832. doi: 10.1021/es950252d. [DOI] [Google Scholar]
- Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, Gholampour A, Arhami M, Kashani H, Zare A, Niaz S. Characterization of PAHs and metals in indoor/outdoor PM10/PM2.5/PM1 in a retirement home and a school dormitory. Sci Total Environ. 2015;527:100–110. doi: 10.1016/j.scitotenv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Hawthorne SB, Miller DJ, Barkley RM, Krieger MS. Identification of methoxylated phenols as candidate tracers for atmospheric wood pollution. Environ Sci Technol. 1988;22:1191–1196. doi: 10.1021/es00175a011. [DOI] [PubMed] [Google Scholar]
- Hays MD, Fine PM, Geron CD, Kleeman MJ, Gullett BK. Open burning of agricultural biomass: physical and chemical properties of particle-phase emissions. Atmos Environ. 2005;39:6747–6764. doi: 10.1016/j.atmosenv.2005.07.072. [DOI] [Google Scholar]
- Hecht S. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–1210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- Hernández-Guzmán FA, Macías-Zamora JV, Ramírez-Álvarez N, Quezada-Hernández C, Ortiz-López R. Source identification of n-alkanes and isoprenoids using diagnostic ratios and carbon isotopic composition on crude oils and surface waters from the Gulf of Mexico. Environ Monit Assess. 2021;193:633. doi: 10.1007/s10661-021-09440-0. [DOI] [PubMed] [Google Scholar]
- Hoekman SK. Speciated measurements and calculated reactivities of vehicle exhaust emissions from conventional and reformulated gasolines. Environ Sci Technol. 1992;26:1206–1216. doi: 10.1021/es50002a610. [DOI] [Google Scholar]
- Horikawa K, Murayama M, Minagawa M, Kato Y, Sagawa T. Latitudinal and downcore (0–750 ka) changes in n-alkane chain lengths in the eastern equatorial Pacific. Quat Res. 2010;73:573–582. doi: 10.1016/j.yqres.2010.01.001. [DOI] [Google Scholar]
- Hou M, Wang Y, Zhao H, Zhang Q, Xie Q, Zhang X, Chen R, Chen J. Halogenated flame retardants in building and decoration materials in China: implications for human exposure via inhalation and dust ingestion. Chemosphere. 2018;203:291–299. doi: 10.1016/j.chemosphere.2018.03.182. [DOI] [PubMed] [Google Scholar]
- Hubbard H, Guo Z, Liu X, KrebsK RN, Mocka C. Perfluorinated chemicals in consumer articles - monitoring the market trends. Reprod Toxicol. 2012;33:590. doi: 10.1016/j.reprotox.2011.11.014. [DOI] [Google Scholar]
- Iakovides M, Iakovides G, Stephanou EG. Atmospheric particle-bound polycyclic aromatic hydrocarbons, n-alkanes, hopanes, steranes and trace metals: PM2.5 source identification, individual and cumulative multi-pathway lifetime cancer risk assessment in the urban environment. Sci Total Environ. 2021;752:141834. doi: 10.1016/j.scitotenv.2020.141834. [DOI] [PubMed] [Google Scholar]
- Iakovides M, Apostolaki M, Stephanou EG. PAHs, PCBs and organochlorine pesticides in the atmosphere of Eastern Mediterranean: investigation of their occurrence, sources and gas-particle partitioning in relation to air mass transport pathways. Atmos Environ. 2021;244:117931. doi: 10.1016/j.atmosenv.2020.117931. [DOI] [Google Scholar]
- IARC (2012) Robert Baan, Yann Grosse, Kurt Straif, Béatrice Secretan, Fatiha El Ghissassi, Véronique Bouvard, Lamia Benbrahim-Tallaa, Neela Guha, Crystal Freeman, Laurent Galichet, Vincent Cogliano, WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens–Part F: chemical agents and related occupations. IARC monographs on the evaluation of carcinogenic risks to humans. 10.1016/s1470-2045(09)70358-4
- Jafarabadi AR, Bakhtiari AR, Hedouin L, Toosi AS, Cappello T. Spatio-temporal variability, distribution and sources of n-alkanes and polycyclic aromatic hydrocarbons in reef surface sediments of Kharg and Lark coral reefs, Persian Gulf, Iran. Ecotoxicol Environ Saf. 2018;163:307–322. doi: 10.1016/j.ecoenv.2018.07.056. [DOI] [PubMed] [Google Scholar]
- Jariyasopit N, McIntosh M, Zimmermann K, Arey J, Atkinson R, Cheong PHY, Yu TW, Dashwood RH, Simonich SLM. Novel nitro-PAH formation from heterogeneous reactions of PAHs with NO2, NO3/N2O5, and OH radicals: prediction, laboratory studies, and mutagenicity. Environ Sci Technol. 2014;48:412–419. doi: 10.1021/es4043808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedynska A, Hoek G, Eeftens M, Cyrys J, Keuken M, Ampe C, Beelen R, Cesaroni G, Forastiere F, Cirach M, de Hoogh K. Spatial variations of PAH, hopanes/steranes and EC/OC concentrations within and between European study areas. Atmos Environ. 2014;87:239–248. doi: 10.1016/j.atmosenv.2014.01.026. [DOI] [Google Scholar]
- Jedynska A, Hoek G, Wang M, Eeftens M, Cyrys J, Keuken M, Ampe C, Beelen R, Cesaroni G, Forastiere F, Cirach M, de Hoogh K, De Nazelle A, Nystad W, Declercq C, Eriksen KT, Dimakopoulou K, Lanki T, Meliefste K, Nieuwenhuijsen MJ, Yli-Tuomi T, Raaschou-Nielsen O, Brunekreef B, Koote IM. Development of land use regression models for elemental, organic carbon, PAH, and hopanes/steranes in 10 ESCAPE/TRANSPHORM European study areas. Environ Sci Technol. 2014;48:14435–14444. doi: 10.1021/es502568z. [DOI] [PubMed] [Google Scholar]
- Jeng WL. Higher plant n-alkane average chain length as an indicator of petrogenic hydrocarbon contamination in marine sediments. Mar Chem. 2006;102:242–251. doi: 10.1016/j.marchem.2006.05.001. [DOI] [Google Scholar]
- Jeon SK, Kwon D, Lee S. Identification of weathered multiple petroleum products in contaminated soils by characterizing unresolved complex mixture hump in gas chromatograph data. Sci Total Environ. 2017;607–608:42–52. doi: 10.1016/j.scitotenv.2017.06.251. [DOI] [PubMed] [Google Scholar]
- Jia Y, Fraser M. Characterization of saccharides in size-fractionated ambient particulate matter and aerosol sources: the contribution of primary biological aerosol particles (PBAPs) and soil to ambient particulate matter. Environ Sci Technol. 2011;2011(45):930–936. doi: 10.1021/es103104e. [DOI] [PubMed] [Google Scholar]
- Kang M, Ren L, Zhao Y, Kawamura K, Zhang H, Wei L, Sun Y, Wang Z, Fu P. Primary biogenic and anthropogenic sources of organic aerosols in Beijing, China: insights from saccharides and n-alkanes. Environ Pollut. 2018;243(Part B):1579–1587. doi: 10.1016/j.envpol.2018.09.118. [DOI] [PubMed] [Google Scholar]
- Katsoyiannis A, Breivik K. Model-based evaluation of the use of polycyclic aromatic hydrocarbons molecular diagnostic ratios as a source identification tool. Environ Pollut. 2014;184:488–494. doi: 10.1016/j.envpol.2013.09.028. [DOI] [PubMed] [Google Scholar]
- Katsoyiannis A, Sweetman AJ, Jones KC. Diagnostic ratios applied to atmospheric sources: a critical evaluation using two decades of source inventory and air concentration data from the UK. Environ Sci Technol. 2011;45:8897–8906. doi: 10.1021/es202277u. [DOI] [PubMed] [Google Scholar]
- Kavouras IG, Stratigakis N, Stephanou EG. Iso- and anteiso-alkanes: specific tracers of environmental tobacco smoke in indoor and outdoor particle-size distributed urban aerosols. Environ Sci Technol. 1998;32:1369–1377. doi: 10.1021/es970634e. [DOI] [Google Scholar]
- Kavouras IG, Lawrence J, Koutrakis P, Stephanou EG, Oyola P. Measurement of particulate aliphatic and polynuclear aromatic hydrocarbons in Santiago de Chile: source reconciliation and evaluation of sampling artifacts. Atmos Environ. 1999;33:4977–4986. doi: 10.1016/S1352-2310(99)00281-2. [DOI] [Google Scholar]
- Kavouras IG, Koutraki P, Cereceda-Bali F, Oyol P. Source apportionment of urban particulate aliphatics and polynuclear aromatic hydrocarbons (PAHs) using multivariate methods. Environ Sci Technol. 2001;35:2288–2294. doi: 10.1021/es001540z. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Bikkina S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: molecular distributions, sources and transformation. Atmos Res. 2016;170:140–160. doi: 10.1016/j.atmosres.2015.11.018. [DOI] [Google Scholar]
- Kawamura K, Gagosian RB. Implications of ω-oxocarboxylic acids in the remote atmosphere for photo-oxidation of unsaturated fatty acids. Nature. 1987;325:330–332. doi: 10.1038/325330a0. [DOI] [Google Scholar]
- Keyte IJ, Harrison RM, Lammel G. Chemical reactivity and long-range transport potential of polycyclic aromatic hydrocarbons-a review. Chem Soc Rev. 2013;42:9333–9391. doi: 10.1039/C3CS60147A. [DOI] [PubMed] [Google Scholar]
- Khedidji S, Balducci C, Ladji R, Cecinato A, Perilli M, Yassaa N. Chemical composition of particulate organic matter at industrial, university and forest areas located in Bouira province, Algeria. Atmos Pollut Res. 2017;8:474–482. doi: 10.1016/j.apr.2016.12.005. [DOI] [Google Scholar]
- Kim D, Kumfer BM, Anastasio C, Kennedy IM, Young TM. Environmental aging of polycyclic aromatic hydrocarbons on soot and its effect on source identification. Chemosphere. 2009;76:1075–1081. doi: 10.1016/j.chemosphere.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox S, O’Boyle NM. Skin lipids in health and disease: a review. Chem Phys Lipids. 2021;236:105055. doi: 10.1016/j.chemphyslip.2021.105055. [DOI] [PubMed] [Google Scholar]
- Kuhn TK, Krull ES, Bowater A, Grice K, Gleixner G. The occurrence of short chain n-alkanes with an even over odd predominance in higher plants and soils. Org Geochem. 2010;41:88–95. doi: 10.1016/j.orggeochem.2009.08.003. [DOI] [Google Scholar]
- Kumar M, Boski T, Lima-Filho FP, Bezerra FHR, GonzalezVila FJ, Bhuiyan MKA, Gonzalez-Perez JA. Biomarkers as indicators of sedimentary organic matter sources and early diagenetic transformation of pentacyclic triterpenoids in a tropical mangrove ecosystem. Estuarine Coastal Shelf Sci. 2019;229:106403. doi: 10.1016/j.ecss.2019.106403. [DOI] [Google Scholar]
- Lammel G, Klánová J, Ilic P, Kohoutek J, Gasič B, Kovačič I, Skrdlíková L. Polycyclic aromatic hydrocarbons in air on small spatial and temporal scales - II. Mass size distributions and gas-particle partitioning. Atmos Environ. 2010;44:5022–5027. doi: 10.1016/j.atmosenv.2010.08.001. [DOI] [Google Scholar]
- Lee HK, Kang H, Lee S, Kim S, Choi K, Moon HB. Human exposure to legacy and emerging flame retardants in indoor dust: a multiple-exposure assessment of PBDEs. Sci Total Environ. 2020;719:137386. doi: 10.1016/j.scitotenv.2020.137386. [DOI] [PubMed] [Google Scholar]
- Leider A, Hinrichs KU, Schefuss E, Versteegh GJM. Distribution and stable isotopes of plant wax derived n-alkanes in lacustrine, fluvial and marine surface sediments along an Eastern Italian transect and their potential to reconstruct the hydrological cycle. Geochim Cosmochim Acta. 2013;117:16–32. doi: 10.1016/j.gca.2013.04.018. [DOI] [Google Scholar]
- Li X, Anderson BJ, Vogeler I, Schwendenmann L. Long-chain n-alkane and n-fatty acid characteristics in plants and soil - potential to separate plant growth forms, primary and secondary grasslands? Sci Total Environ. 2018;645:1567–1578. doi: 10.1016/j.scitotenv.2018.07.105. [DOI] [PubMed] [Google Scholar]
- Lindbeck A, Puxbaum H. Organic acids in continental background aerosols. Atmos Environ. 1999;33:1847–1852. doi: 10.1016/S1352-2310(98)00347-1. [DOI] [Google Scholar]
- Liu B, Xue Z, Zhu X, Jia C. Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the US. Environ Pollut. 2017;220:1171–1179. doi: 10.1016/j.envpol.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Liu G, Jiang X, Wang M, Dong S, Zheng M. Comparison of PCDD/F levels and profiles in fly ash samples from multiple industrial thermal sources. Chemosphere. 2015;133:68–74. doi: 10.1016/j.chemosphere.2015.03.073. [DOI] [PubMed] [Google Scholar]
- Liu N, Xu L, Cai Y. Methyl siloxanes in barbershops and residence indoor dust, and the implication for human exposures. Sci Total Environ. 2018;618:1324–1330. doi: 10.1016/j.scitotenv.2017.09.250. [DOI] [PubMed] [Google Scholar]
- Liu ZG, Berg DR, Vasys VN, Dettmann MF, Zielinska B, Schauer JJ. Analysis of C1, C2, and C10 through C33 particle-phase and semi-volatile organic compound emissions from heavy-duty diesel engines. Atmos Environ. 2010;44:1108–1115. doi: 10.1016/j.atmosenv.2009.11.036. [DOI] [Google Scholar]
- Lobodin VV, Maksimova EV, Rodgers RP. Gas chromatography/atmospheric pressure chemical ionization tandem mass spectrometry for fingerprinting the Macondo oil spill. Anal Chem. 2016;88:6914–6922. doi: 10.1021/acs.analchem.6b01652. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Venturini M, Marini E, Grechi D, Dolara P. Polycyclic aromatic hydrocarbons air levels in Florence, Italy, and their correlation with other air pollutants. Chemosphere. 2003;5:377–382. doi: 10.1016/S0045-6535(02)00404-6. [DOI] [PubMed] [Google Scholar]
- Lu J, Li H, Luo Z, Lin H, Yang Z. Occurrence, distribution, and environmental risk of four categories of personal care products in the Xiangjiang River, China. Environ Sci Pollut Res. 2018;25:27524–27534. doi: 10.1007/s11356-018-2686-7. [DOI] [PubMed] [Google Scholar]
- Lu X, Li M, Wang X, Wei T, Tang Y, Hong H, Wu C, Yang X, Liu Y. Distribution and geochemical significance of rearranged hopanes in Jurassic source rocks and related oils in the center of the Sichuan Basin, China. ACS Omega. 2021;6:13588–13600. doi: 10.1021/acsomega.1c00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucattini L, Poma G, Covaci A, de Boer J, Lamoree MH, Leonards PEG. A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere. 2018;201:466–482. doi: 10.1016/j.chemosphere.2018.02.161. [DOI] [PubMed] [Google Scholar]
- Lv L, Chen Y, Han Y, Cui M, Wei P, Zheng M, Hu J. High-time-resolution PM2.5 source apportionment based on multi-model with organic tracers in Beijing during haze episode. Sci Total Environ. 2021;772:144766s. doi: 10.1016/j.scitotenv.2020.144766. [DOI] [PubMed] [Google Scholar]
- Ma Y, Harrad S. Spatiotemporal analysis and human exposure assessment on polycyclic aromatic hydrocarbons in indoor air, settled house dust, and diet: a review. Environ Int. 2015;84:7–16. doi: 10.1016/j.envint.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Mackay D, Callcott D. Partitioning and physical chemical properties of PAHs. In: Neilson A, editor. PAH and related compounds chemistry. Berlin: Springer-Verlag; 1998. pp. 325–346. [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2019) Cluster: cluster analysis basics and extensions. R package version 2.1.0, 2019. https://CRAN.R-project.org/package=cluster. Accessed 21 Jan 2022
- Medeiros PM, Simoneit BRT. Source profiles of organic compounds emitted upon combustion of green vegetation from temperate climate forests. Environ Sci Technol. 2008;42:8310–8316. doi: 10.1021/es801533b. [DOI] [PubMed] [Google Scholar]
- Mininni G, Sbrilli A, Guerriero E, Rotatori M. Dioxins and furans formation in pilot incineration tests of sewage sludge spiked with organic chlorine. Chemosphere. 2004;54:1337–1350. doi: 10.1016/S0045-6535(03)00252-2. [DOI] [PubMed] [Google Scholar]
- Molnar C (2019). Interpretable machine learning: a guide for making black box models explainable. URL https://christophm.github.io/interpretable-ml-book. Accessed 15 Sept 2021
- Mostert MMR, Ayoko GA, Kokot S. Application of chemometrics to analysis of soil pollutants. TrAC Trends Anal Chem. 2010;29:430–445. doi: 10.1016/j.trac.2010.02.009. [DOI] [Google Scholar]
- Mu L, Li X, Li Y, Liu X, Bai H, Li X, Liu T. Molecular distribution, seasonal variations, and sources of typical polar organics in PM2.5 from Jinzhong, China. ACS Earth Space Chem. 2021;2021(5):663–675. doi: 10.1021/acsearthspacechem.0c00350. [DOI] [Google Scholar]
- Nazzaro-Porro M, Passi S, Boniforti L, Belsito F. Effects of aging on fatty acids in skin surface lipids. J Invest Dermatol. 1979;73:112–117. doi: 10.1111/1523-1747.ep12532793. [DOI] [PubMed] [Google Scholar]
- Ngo TH, Tsou HH, Chen YF, Chen YW, Chi KH. Sources identification of PCDD/Fs in soil and atmospheric deposition in Taiwan. Chemosphere. 2018;208:374–381. doi: 10.1016/j.chemosphere.2018.05.195. [DOI] [PubMed] [Google Scholar]
- Ngo TH, Yang YH, Chen YC, Pan WC, Chi KH. Continuous nationwide atmospheric PCDD/F monitoring network in Taiwan (2006–2016): variation in concentrations and apportionment of emission sources. Chemosphere. 2020;255:126979. doi: 10.1016/j.chemosphere.2020.126979. [DOI] [PubMed] [Google Scholar]
- Nieder R, Benbi DK, Reichl FX (2018). Soil-borne particles and their impact on environment and human health. In: Soil components and human health, Springer, Dordrecht, Chapter 3, pp. 99–177. 10.1007/978-94-024-1222-2_4
- Oliveira C, Pio C, Alves C, Evtyugina M, Santo P, Goncalves V, Nunes T, Silvestre AJD, Palmgren F, Walin P, Harrad S. Seasonal distribution of polar organic compounds in the urban atmosphere of two large cities from the north and south of Europe. Atmos Environ. 2007;41:5555–5570. doi: 10.1016/j.atmosenv.2007.03.001. [DOI] [Google Scholar]
- Oliveira M, Slezakova K, Delerue-Matos C, de Carmo Pereira M, Morais S. Assessment of polycyclic aromatic hydrocarbons in indoor and outdoor air of preschool environments (3–5 years old children) Environ Pollut. 2016;208:382–394. doi: 10.1016/j.envpol.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Omar NYMJ, Bin Abas MR, Rahman NA, Tahir NM, Rushdi AI, Simoneit BRT. Levels and distributions of organic source tracers in air and roadside dust particles of Kuala Lumpur, Malaysia. Environ Geol. 2007;52:1485–1500. doi: 10.1007/s00254-006-0593-6. [DOI] [Google Scholar]
- Omotoye S, Adekola SA, Adepoju A, Akinlua A. Thermal maturity assessment and characterization of selected oil samples from the Niger Delta, Nigeria. Energy Fuels. 2016;30:104–111. doi: 10.1021/acs.energyfuels.5b01902. [DOI] [Google Scholar]
- Oros DR, Simoneit BRT. Identification and emission factors of molecular tracers in organic aerosols from biomass burning. Part 1. Temperate Climate Conifers. Appl Geochem. 2001;16:1513–1544. doi: 10.1016/S0883-2927(01)00021-X. [DOI] [Google Scholar]
- Oros DR, Simoneit BRT. Identification and emission factors of molecular tracers in organic aerosols from bio-mass burning. Part 2. Deciduous Trees. Appl Geochem. 2001;16:1545–1565. doi: 10.1016/S0883-2927(01)00022-1. [DOI] [Google Scholar]
- Oros DR, Mazurek MA, Baham JE, Simoneit BRT. Organic tracers from wild fire residues in soil and rain/river washout. Water Air Soil Pollut. 2002;137:203–233. doi: 10.1023/A:1015557301467. [DOI] [Google Scholar]
- Paolini V, Guerriero E, Bacaloni A, Rotatori M, Benedetti P, Mosca S. Simultaneous sampling of vapor and particle-phase carcinogenic polycyclic aromatic hydrocarbons on functionalized glass fiber filters. J Aerosol Air Qual Res. 2015;16:175–183. doi: 10.4209/aaqr.2015.07.0476. [DOI] [Google Scholar]
- Percy Z, La Guardia MJ, Xu Y, Hale RC, Dietrich KN, Lanphear BP, Yolton K, Vuong AM, Cecil KM, Braun JM, Xie C, Chen A. Concentrations and loadings of organophosphate and replacement brominated flame retardants in house dust from the home study during the PBDE phase-out. Chemosphere. 2020;239:124701. doi: 10.1016/j.chemosphere.2019.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira GM, Teinilä K, Custódio D, Santos AG, Xian H, Hillamo R, Alves CA, de Andrade JB, da Rocha GO, Kumar P, Balasubramanian R, Andrade MdF, de Castro VP. Particulate pollutants in the Brazilian city of São Paulo: 1-year investigation for the chemical composition and source apportionment. Atmos Chem Phys. 2017;17:11943–11969. doi: 10.5194/acp-17-11943-2017. [DOI] [Google Scholar]
- Perraudin E, Budzinski H, Villenave E. Kinetic study of the reactions of ozone with polycyclic aromatic hydrocarbons adsorbed on atmospheric model particles. J Atmos Chem. 2007;56:57–82. doi: 10.1007/s10874-006-9042-x. [DOI] [Google Scholar]
- Perrino C, Tofful L, Dalla TS, Sargolini T, Canepari S. Biomass burning contribution to PM10 concentration in Rome (Italy): seasonal, daily and two-hourly variations. Chemosphere. 2019;222:839–848. doi: 10.1016/j.chemosphere.2019.02.019. [DOI] [PubMed] [Google Scholar]
- Perrone MG, Carbone C, Faedo D, Ferrero L, Maggioni A, Sangiorgi E, Bolzacchini E (2014). Exhaust emissions of polycyclic aromatic hydrocarbons, n-alkanes and phenols from vehicles coming within different European classes. Atmos Environ 8210.1007/s11356-016-8111-1
- Phuleria HC, Geller MD, Fine PM, Sioutas C. Size-resolved emissions of organic tracers from light- and heavy-duty vehicles measured in a California roadway tunnel. Environ Sci Technol. 2006;40:4109–4118. doi: 10.1021/es052186d. [DOI] [PubMed] [Google Scholar]
- Pio CA, Alves CA, Duarte AC. Identification, abundance and origin of atmospheric organic particulate matter in a Portuguese rural area. Atmos Environ. 2001;35:1365–1375. doi: 10.1016/S1352-2310(00)00391-5. [DOI] [Google Scholar]
- Pitts JN, Jr, Sweetman JA, Zielinska B, Winer AM, Atkinson R. Determination of 2-nitrofluoranthene and 2-nitropyrene in ambient particulate organic matter: evidence for atmospheric reactions. Atmos Environ. 1985;10:1601–1608. doi: 10.1016/0004-6981(85)90212-4. [DOI] [Google Scholar]
- Pomata D, Di Filippo P, Riccardi C, Buiarelli F, Gallo V. Determination of non-certified levoglucosan, sugar polyols and ergosterol in NIST Standard Reference Material 1649a. Atmos Environ. 2014;84:332–338. doi: 10.1016/j.atmosenv.2013.11.069. [DOI] [Google Scholar]
- Praveena SM, Teh SW, Rajendran RK, Kannan N, Lin CC, Abdullah R, Kumar S. Recent updates on phthalate exposure and human health: a special focus on liver toxicity and stem cell regeneration. Environ Sci Pollut Res. 2018;25:11333–11342. doi: 10.1007/s11356-018-1652-8. [DOI] [PubMed] [Google Scholar]
- Priya PS, Kamaraj M, Aravind J, Sudhakar S (2022). Endocrine-disrupting pollutants in domestic and industrial wastewater: occurrence and removal by advanced treatment system for wastewater reuse. In: Biodegradation and detoxification of micropollutants in industrial wastewater. Elsevier, Chapter 9, pp. 177–192. 10.1016/B978-0-323-88507-2.00006-3.
- Rabhi L, Lemou A, Cecinato A, Balducci C, Cherifi N, Ladji R, Yassaa N. Polycyclic aromatic hydrocarbons, phthalates, parabens and other environmental contaminants in dust and suspended particulates of Algiers, Algeria. Environ Sci Pollut Res. 2018;25:24253–24265. doi: 10.1007/s11356-018-2496-y. [DOI] [PubMed] [Google Scholar]
- Rabovsky J, Judy DJ. The in vitro effects of alkanes, alcohols, and ketones on rat lung cytochrome P450-dependent alkoxyphenoxazone dealkylase activities. Arch Toxicol. 1989;63:13–17. doi: 10.1007/BF00334627. [DOI] [PubMed] [Google Scholar]
- Ravindra K, Sokhi R, Van Grieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ. 2008;42:2895–2921. doi: 10.1016/j.atmosenv.2007.12.010. [DOI] [Google Scholar]
- Ren G, Yan X, Ma Y, Qiao L, Chen Z, Xin Y, Zhou M, Shi Y, Zheng K, Zhu S, Huang C, Li L. Characteristics and source apportionment of PM2.5-bound saccharides and carboxylic acids in central Shanghai. China. Atmos Res. 2020;237:104817. doi: 10.1016/j.atmosres.2019.104817. [DOI] [Google Scholar]
- Requejo AG, Quinn JG. Geochemistry of C25 and C30 biogenic alkenes in sediments of the Narragansett Bay estuary. Geochim Cosmochim Acta. 1983;47:1075–1090. doi: 10.1016/0016-7037(83)90238-7. [DOI] [Google Scholar]
- Romagnoli P, Balducci C, Perilli M, Gherardi M, Gordiani A, Gariazzo C, Gatto MP, Cecinato A. Indoor PAHs at schools, homes and offices in Rome, Italy. Atmos Environ. 2014;92:51–59. doi: 10.1016/j.atmosenv.2014.03.063. [DOI] [Google Scholar]
- Romagnoli P, Balducci C, Perilli M, Perreca E, Cecinato A. Particulate PAHs and n-alkanes in the air over Southern and Eastern Mediterranean Sea. Chemosphere. 2016;159:516–525. doi: 10.1016/j.chemosphere.2016.06.024. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pinero J, Moreda-Pineiro J, Moscoso-Perez C, FernandezGonzalez V, Prada-Rodríguez D, Lopez-Mahía P. Development and validation of a multi-pollutant method for the analysis of polycyclic aromatic hydrocarbons, synthetic musk compounds and plasticizers in atmospheric particulate matter (PM2.5) Talanta Open. 2021;4:100057. doi: 10.1016/j.talo.2021.100057. [DOI] [Google Scholar]
- Sangiorgi G, Ferrero L, Ferrini BS, Porto CL, Perrone MG, Zangrando R, Gambaro A, Lazzati Z, Bolzacchini E. Indoor airborne particle sources and semi-volatile partitioning effect of outdoor fine PM in offices. Atmos Environ. 2013;65:205–214. doi: 10.1016/j.atmosenv.2012.10.050. [DOI] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 4. C1–C27 organic compounds from cooking with seed oils. Environ Sci Technol. 2002;36:567–575. doi: 10.1021/es002053m. [DOI] [PubMed] [Google Scholar]
- Settimo G, Manigrasso M, Avino P. Indoor air quality: a focus on the European legislation and state-of-the-art research in Italy. Atmosphere. 2020;11:370. doi: 10.3390/atmos11040370. [DOI] [Google Scholar]
- Settimo G, Bonadonna L, Gherardi M, di Gregorio F, Cecinato A(2020b) (Gruppo di Studio Nazionale Inquinamento Indoor). Strategie di monitoraggio della qualità dell’aria indoor negli ambienti sanitari: strategie di monitoraggio degli agenti chimici e biologici. Rapporti ISTISAN 19/17, VIII. Istituto Superiore di Sanità, Rome, Italy, p 55
- Shen G, Tao S, Wei S, Zhang Y, Wang R, Wang B, Li W, Shen H, Huang Y, Yang Y, Wang W, Wang X, Simonich SLM. Retene emission from residential solid fuels in China and evaluation of retene as a unique marker for soft wood combustion. Environ Sci Technol. 2012;46:4666–4672. doi: 10.1021/es300144m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SM, Lee JY, Shin HJ, Kim YP. Seasonal variation and source apportionment of oxygenated polycyclic aromatic hydrocarbons (OPAHs) and polycyclic aromatic hydrocarbons (PAHs) in PM2.5 in Seoul. Korea. Atmos Environ. 2022;272:118937. doi: 10.1016/j.atmosenv.2022.118937. [DOI] [Google Scholar]
- Shirneshan G, Bakhtiari AR, Memariani M. Distribution and origins of n-alkanes, hopanes, and steranes in rivers and marine sediments from Southwest Caspian coast, Iran: implications for identifying petroleum hydrocarbon inputs. Environ Sci Pollut Res. 2016;23:17484–17495. doi: 10.1007/s11356-016-6825-8. [DOI] [PubMed] [Google Scholar]
- Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70. doi: 10.1016/j.etap.2017.02.024. [DOI] [PubMed] [Google Scholar]
- Sikes EL, Uhle ME, Nodder SD, Howard ME. Sources of organic matter in a coastal marine environment: evidence from n-alkanes and their δ13C distributions in the Hauraki Gulf, New Zealand. Marine Chem. 2009;113:149–163. doi: 10.1016/j.marchem.2008.12.003. [DOI] [Google Scholar]
- Simoneit BR. Organic matter of the troposphere-III - characterization and sources of petroleum and pyrogenic residues in aerosols over the western United States. Atmos Environ. 1984;18:51–67. doi: 10.1016/0004-6981(84)90228-2. [DOI] [Google Scholar]
- Simoneit BRT. A review of biomarker compounds as source indicators and tracers for air pollution. Environ Sci Pollut Res. 1999;6:159–169. doi: 10.1007/BF02987621. [DOI] [PubMed] [Google Scholar]
- Simoneit BRT. Biomass burning - a review of organic tracers for smoke from incomplete combustion. Appl Geochem. 2002;17:129–162. doi: 10.1016/S0883-2927(01)00061-0. [DOI] [Google Scholar]
- Simoneit BRT, Mazurek MA. Organic matter of the troposphere - II - natural background of biogenic lipid matter in aerosol over the rural Western United States. Atmos Environ. 1982;16:2139–2159. doi: 10.1016/0004-6981(82)90284-0. [DOI] [Google Scholar]
- Simoneit BRT, Elias VO, Kobayashi M, Kawamura K, Rushdi AI, Medeiros PM, Rogge WF, Didyk BM. Sugars dominant water-soluble organic compounds in soils and characterization as tracers in atmospheric particulate matter. Environ Sci Technol. 2004;38:5939–5949. doi: 10.1021/es0403099. [DOI] [PubMed] [Google Scholar]
- Sofowote UM, Allan LM, McCarry BE. Evaluation of PAH diagnostic ratios as source apportionment tools for air particulates collected in an urban-industrial environment. J Environ Monit. 2010;12:417–422. doi: 10.1039/B909660D. [DOI] [PubMed] [Google Scholar]
- Sofowote UM, Healy RM, Su Y, Debosz J, Noble M, Munoz S, Jeong CH, Wang JM, Hilker N, Evans JY, Brook JR, Lu G, Hopke PK. Sources, variability and parameterizations of intra-city factors obtained from dispersion-normalized multi-time resolution factor analyses of PM2.5 in an urban environment. Sci Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.143225. [DOI] [PubMed] [Google Scholar]
- Steinemann A. Fragranced consumer products: sources of emissions, exposures, and health effects in the UK. Air Qual Atmos Health. 2018;11:253–258. doi: 10.1007/s11869-019-00693-w. [DOI] [Google Scholar]
- Stogiannidis E, Laane R. (2015). Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: an overview of possibilities. In: Reviews of environmental contamination and toxicology, DM Whitacre (ed.), Springer International Publishing Switzerland, Vol. 234, pp 49-134. 10.1007/978-3-319-10638-0_2 [DOI] [PubMed]
- Stortini AM, Martellini T, Del Bubba M, Lepri L, Capodaglio G, Cincinelli A. n-Alkanes, PAHs and surfactants in the sea surface microlayer and sea water samples of the Gerlache Inlet sea (Antarctica) Microchem J. 2009;92:37–43. doi: 10.1016/j.microc.2008.11.005. [DOI] [Google Scholar]
- Stout SA, Emsbo-Mattingly SD, Douglas GS, Uhler AD, McCarthy KJ. Beyond 16 priority pollutant PAHs: a review of PACs used in environmental forensic chemistry. Polycyclic Aromat Compd. 2015;35:285–315. doi: 10.1080/10406638.2014.891144. [DOI] [Google Scholar]
- Subedi B, Sullivan KD, Dhungan B. Phthalate and non-phthalate plasticizers in indoor dust from childcare facilities, salons, and homes across the USA. Environ Pollut. 2017;230:701–708. doi: 10.1016/j.envpol.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Tian Y, Harrison RM, Feng Y, Shi Z, Liang Y, Li Y, Xue Q, Xu J. Size-resolved source apportionment of particulate matter from a megacity in northern China based on one-year measurement of inorganic and organic components. Environ Pollut. 2021;289:117932. doi: 10.1016/j.envpol.2021.117932. [DOI] [PubMed] [Google Scholar]
- Tobiszewski M, Namiesnik J. PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut. 2012;162:110–119. doi: 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Tong KY, Karasek FW. Quantitation of polycyclic aromatic hydrocarbons in diesel exhaust particulate matter by high-performance liquid chromatography fractionation and high-resolution gas chromatography. Anal Chem. 1984;56:2129–2134. doi: 10.1021/ac00276a034. [DOI] [PubMed] [Google Scholar]
- Tran TM, Kannan K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Arch Environ Contam Toxicol. 2015;68:489–499. doi: 10.1007/s00244-015-0140-0. [DOI] [PubMed] [Google Scholar]
- Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Banat F, Sivamani S, Sivakumar N, Hosseini-Bandegharaei A, Show PL. Biopolymers and composites: properties, characterization and their applications in food, medical and pharmaceutical industries. J Environ Chem Engineer. 2021;9:105332. doi: 10.1016/j.jece.2021.105322. [DOI] [Google Scholar]
- USEPA (1993). Provisional guidance for the quantitative risk assessment of polycyclic aromatic hydrocarbons. Office of Research and Development, Washington, D.C. 1993. EPA/600/R-93/089.
- Vaz S Jr (2018) The main environmental matrices: air, soil, and water. In: Analytical chemistry applied to emerging pollutants, Springer International Publishing AG, part of Springer Nature, Chapter 4, pp 79-10110.1007/978-3-319-74403-2_4
- Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol. 2013;36:88–96. doi: 10.1016/j.etap.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fang X, Zhang T, Li Y, Wu Y, He D, Wang Y. Predominance of even carbon-numbered n-alkanes from lacustrine sediments in Linxia Basin, NE Tibetan Plateau: implications for climate change. Appl Geochem. 2010;25:1478–1486. doi: 10.1016/j.apgeochem.2010.07.002. [DOI] [Google Scholar]
- Volkman JK, AT Revill, AP Murray (1997) Applications of biomarkers for identifying sources of natural and pollutant hydrocarbons in aquatic environments. In: Molecular markers in environmental geochemistry, ACS Symposium Series Vol. 671, Chapter 8, 110–132. 10.1021/bk-1997-0671.ch008, ISBN13: 9780841235182; eISBN: 9780841216358
- Wang H, Kawamura K, Shooter D. Wintertime organic aerosols in Christchurch and Auckland, New Zealand: contributions of residential wood and coal burning and petroleum utilization. Environ Sci Technol. 2006;40:5257–5262. doi: 10.1021/es052523i. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang W, He S, Du J, Sun Z. Sources and distribution of aliphatic and polycyclic aromatic hydrocarbons in Yellow River Delta Nature Reserve, China. Appl Geochem. 2011;26:1330–1336. doi: 10.1016/j.apgeochem.2011.05.006. [DOI] [Google Scholar]
- Weiss JM, Gustafsson A, Gerde P, Bergman A, Lindh CH, Krais AM. Daily intake of phthalates, MEHP, and DINCH by ingestion and inhalation. Chemosphere. 2018;208:40–49. doi: 10.1016/j.chemosphere.2018.05.094. [DOI] [PubMed] [Google Scholar]
- White HK, Xu L, Hartmann P, Quinn JG, Reddy CM. Unresolved complex mixture (UCM) in coastal environments is derived from fossil sources. Environ Sci Technol. 2013;47:726–731. doi: 10.1021/es3042065. [DOI] [PubMed] [Google Scholar]
- Wise SA, Benner BA, Byrd GD, Chesler SN, Rebbert RE, Schantz MM. Determination of polycyclic aromatic hydrocarbons in a coal tar standard reference material. Anal Chem. 1988;60(9):887–894. doi: 10.1021/ac00160a012. [DOI] [Google Scholar]
- Wong F, Robson M, Melymuk L, Shunthirasingham C, Alexandrou N, Shoeib M, Luk E, Helm P, Diamond ML, Hung H. Urban sources of synthetic musk compounds to the environment. Environ Sci Processes Impacts. 2019;21:74–88. doi: 10.1039/C8EM00341F. [DOI] [PubMed] [Google Scholar]
- Xiao H, Li M, Wang W, You B, Liu X, Yang Z, Liu J, Chen Q, Uwiringiyimana M. Identification, distribution and geochemical significance of four rearranged hopane series in crude oil. Org Geochem. 2019;138(102939):103929. doi: 10.1016/j.orggeochem.2019.103929. [DOI] [Google Scholar]
- Xing GH, Liang Y, Chen LX, Wu SC, Wong MH. Exposure to PCBs, through inhalation, dermal contact and dust ingestion at Taizhou, China – a major site for recycling transformers. Chemosphere. 2011;83:605–611. doi: 10.1016/j.chemosphere.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Yassaa N, Meklati BH, Cecinato A, Marino F. Organic aerosols in urban and waste landfill of Algiers metropolitan area: Occurrence and sources. Environ Sci Technol. 2001;35:306–311. doi: 10.1021/es991316d. [DOI] [PubMed] [Google Scholar]
- Yongdong Z, Yaling S, Zhengwen L, Xiangchao C, Jinlei Y, XiaodanD MJ. Long-chain n-alkenes in recent sediment of Lake Lugu (SW China) and their ecological implications. Limnologica. 2015;52:30–40. doi: 10.1016/j.limno.2015.02.004. [DOI] [Google Scholar]
- Yu X, Lü X, Meyers PA, Huang X. Comparison of molecular distributions and carbon and hydrogen isotope compositions of n-alkanes from aquatic plants in shallow freshwater lakes along the middle and lower reaches of the Yangtze River, China. Org Geochem. 2019;158:104270. doi: 10.1016/j.orggeochem.2021.104270. [DOI] [Google Scholar]
- Yu Y, Wang G, Yin X, Ge C, Liao G. Effects of different cooking methods on free fatty acid profile, water-soluble compounds and flavor compounds in Chinese Piao chicken meat. Food Res Intern. 2021;149:110696. doi: 10.1016/j.foodres.2021.110696. [DOI] [PubMed] [Google Scholar]
- Yury B, Zhang Z, Ding Y, Zheng Z, Wu B, Gao P, Jia J, Lin N, Feng Y. Distribution, inhalation and health risk of PM2.5 related PAHs in indoor environments. Ecotoxicol Environ Safety. 2018;164:409–415. doi: 10.1016/j.ecoenv.2018.08.044. [DOI] [PubMed] [Google Scholar]
- Zhai Y, Li x, Wang T, Wang B, Li C, Zeng G, A review on airborne microorganisms in particulate matters: composition, characteristics and influence factors. Environ Int. 2018;113:74–90. doi: 10.1016/j.envint.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hu M, Slanina S, Zhang Y. Chemical compositions of fine particulate organic matter emitted from Chinese cooking. Environ Sci Technol. 2007;41:99–105. doi: 10.1021/es0614518. [DOI] [PubMed] [Google Scholar]
- Zhao T, Yang L, Huang Q, Zhang W, Duan S, Gao H, Wang W. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) and nitrated-PAHs (NPAHs) emitted by gasoline vehicles: characterization and health risk assessment. Sci. Total Environ. 2020;727:138631. doi: 10.1016/j.scitotenv.2020.138631. [DOI] [PubMed] [Google Scholar]
- Zheng M, Cass GR, Schauer JJ, Edgerton ES. Source apportionment of PM2.5 in the Southeastern United States using solvent-extractable organic compounds as tracers. Environ Sci Technol. 2002;36:2361–2371. doi: 10.1021/es011275x. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Jia J, Zhang K, Zhang H, Liao C, Jiang G. Phthalate esters in indoor dust from several regions, China and their implications for human exposure. Sci Total Environ. 2019;2019(652):1187–1194. doi: 10.1016/j.scitotenv.2018.10.326. [DOI] [PubMed] [Google Scholar]
- Zielinska B, Sagebiel J, McDonald JD, Whitney K, Lawson DR. Emission rates and comparative chemical composition from selected in-use diesel and gasoline-fueled vehicles. J Air Waste Manag Assoc. 2004;54:1138–1150. doi: 10.1080/10473289.2004.10470973. [DOI] [PubMed] [Google Scholar]
- Zrafi-Nouira I, Khedir-Ghenim Z, Zrafi F, Bahri R, Cheraeif I, Rouabbia R, Saidane-Mosbahi D. Hydrocarbon pollution in the sediment from the Jarzouna-Bizerte Coastal Area of Tunisia (Mediterranean Sea) Bull Environ Contam Toxicol. 2008;80:566–572. doi: 10.1007/s00128-008-9421-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Anyway, those not directly produced by personal investigations of authors are available in the bibliography cited in the paper.