Abstract
The thymus is a primary lymphoid organ for the T cell development. Increasing evidence found that the thymus is also an important site for development of innate lymphoid cells (ILCs). ILCs generated in thymi acquire unique homing properties that direct their localization into barrier tissues such as the skin and intestine where they help local homeostasis. Mechanisms underlying the developmental programming of unique tissue-homing properties of ILCs are poorly understood. We report herein that thymic stroma-derived Notch signaling is differentially involved in thymic generation of a population of NK1.1+ group 1 ILCs (ILC1s) with the CCR10+ skin-homing property in adult and neonatal mice. We found that thymic generation of CCR10+NK1.1+ ILC1s is increased in T cell-deficient mice at adult but not neonatal stages, supporting the notion that a large number of developing T cells interfere with signals required for generation of CCR10+NK1.1+ ILC1s. In an in vitro differentiation assay, increasing Notch signals promote generation of CCR10+NK1.1+ ILC1s from hematopoietic progenitors. Knockout of the Notch ligand delta-like 4 (DL4) in thymic stroma impairs generation of CCR10+NK1.1+ ILC1s in adult thymi but development of CCR10+NK1.1+ ILC1s in neonatal thymi is less dependent on DL4-derived Notch signals. Mechanistically, the Notch signaling is required for proper expression of the IL-7 receptor CD127 on thymic NK1.1+ ILC1s and deficiency of CD127 also impairs thymic generation of CCR10+NK1.1+ ILC1s at adult but not perinatal stages. Our findings advanced understanding of regulatory mechanisms of thymic innate lymphocyte development.
INTRODUCTION
Innate lymphoid cells (ILCs) are a family of innate lymphocytes. Based on their developmental pathways and functional potentials, ILCs are divided into 3 groups of helper ILCs (ILC1-3) in addition to conventional natural killer (NK) cells and lymphoid tissue-inducing (LTi) cells (1, 2). All ILCs originate from early ILC progenitors (EILPs) that differentiate from lymphoid primed multipotent progenitors (LMPPs) or common lymphoid progenitors (CLP) (3). Under direction of intrinsic transcription factors and local environment-derived signals, EILPs could differentiate to NK progenitor (NKP) cells that give rise to NK cells or common helper-like ILC progenitor (CHILP) cells that generate LTi and the other three groups of helper ILCs (4–6). In general, Eomes is considered as a crucial transcription factor for the NK cell development, while ILC1s depend on T-bet for their development and produce Th1-type cytokines such as interferon (IFN) γ when activated. ILC2s depend on GATA3 and Rorα for their development and are capable of producing Th2-type cytokines such as IL-5 and IL-13; and ILC3s requires Rorγt for the development and produce Th17-type cytokines such as IL-17 and IL-22 (1, 2). ILCs of different functions preferentially reside in barrier tissues such as the skin and mucosa where they play an important role in local homeostatic maintenance (1, 2).
Multiple layers of regulation are involved in the preferential localization of ILCs into various barrier tissues (7, 8). We previously reported that in adult mice, ILCs, mostly ILC2s, could be programmed in skin-draining lymph nodes to acquire a CCR10+ skin-homing property for their preferential localization into the skin (9). Similarly, ILCs could be programmed in mesenteric lymph nodes to express CCR9 for their localization into intestines (10). In addition, during their developmental stages in primary lymphoid organs, ILCs are also preferentially pre-programmed with unique homing properties for their localization into barrier tissues. It was reported that fetal liver-derived ILC progenitors have the ability to migrate into the intestine and accumulate at sites of lymphoid tissue organogenesis to give rise to ILC2s (11, 12). Bone marrow (BM)-derived ILC progenitor cells express homing receptor CXCR6 important for their migration into intestinal lamina propria (13).
While the thymus is the primary lymphoid organ for the T cell generation, increasing evidence suggests that it is also an important site for generation of various subsets of ILCs, particularly at perinatal stages (14–17). Thymus-generated ILCs could acquire homing properties to barrier tissues and might contribute significantly to the immune cell repertoire in the skin, intestine and lung (9, 17, 18). We reported that a population of NK1.1+ ILC1s generated in the thymus, but not the fetal liver or adult BM, acquire the CCR10+ skin-homing property (9). ILC2s generated in the thymus at the fetal stage could acquire a homing property to the intestine (17). A high frequency of ILC2s in the lung have TCRγ chain gene rearrangements, suggesting that they might derive from the thymus (18). The thymic generation of ILCs, including CCR10+NK1.1+ ILC1s, is significantly reduced at adult stages in wild-type (WT) mice, suggesting coordinated regulation of generation of T cells and ILCs in the thymus (9, 15, 16, 18). Consistent with this notion, the reduced thymic generation of CCR10+NK1.1+ILC1s in adult WT mice could be reversed in adult Rag1−/− mice (9).
Molecular mechanisms regulating thymic generation of ILCs of different tissue-homing properties are not well understood. We previously found that the TF PLZF is crucial for thymic generation of CCR10+NK1.1+ ILC1s (9). However, thymus-specific signals regulating the generation of CCR10+NK1.1+ ILC1s are not known. The finding that thymic generation of CCR10+NK1.1+ ILC1s is significantly suppressed in adult thymi of WT but not Rag1−/− mice suggests that competition from a large number of developing T cells might interfere with the signals of thymic environments required for generation of CCR10+NK1.1+ ILC1s. A critical signaling pathway of thymic environments is of the Notch ligand/receptor axis. In mice, there are four Notch ligands and five Notch receptors. One of the Notch ligands, delta-like 4 (DL4), is highly expressed in thymic stromal cells and crucial for the T cell development through interaction on Notch receptors expressed on thymic progenitor cells (19–25). In thymic stroma-specific DL4-knockout mice, the T cell development in the thymus is blocked (24, 25). Notch signals are also reportedly differentially involved in development of ILCs of different groups. An early study reported that Notch signals are not required for generation of thymic NK cells (26). On the other hand, a recent study found that the duration and strength of Notch signals differentially regulate development of ILCs versus T/B cells (27). Furthermore, Notch signals are reported to reduce expression of Nfil3 and Id2 required for generation of NK cells (17). However, Rorα expression overcomes the Notch-induced Nfil3 and Id2 suppression, allowing generation of a population of gut-homing ILC2s from thymic progenitor cells in embryonic thymi (17).
In this report, we analyzed roles of Notch signals in thymic generation of CCR10+NK1.1+ ILC1s in coordination with the T cell development. Our analyses revealed that Notch signals are differentially required for homing molecule expression and development of CCR10+NK1.1+ ILC1s at adult and neonatal stages.
MATERIALS AND METHODS
Mice
CCR10-knockout (KO) /EGFP-knockin (KI) mice were previously described (28). TCRβ−/− (stock # 002118), TCRδ−/− (002120), Rag1−/− (002216), CD127−/− (002295), Foxn1Cre (018448), and C57BL/6 CD45.1 (002014) mice were purchased from Jackson Lab (Bar Harbor, ME). Floxed delta-like 4 (DL4f/f) mice were generously provided by Drs. Freddy Radtke and Ivan P. Maillard (Koch U et al. 2008). All knockout mice were crossed with homozygous CCR10-KO/EGFP-KI (CCR10EGFP/EGFP) mice to introduce one CCR10-knockout/EGFP-knockin allele (CCR10+/EGFP) for purpose to track CCR10 expression using the EGFP reporter. All mice were on the C57BL/6 (CD45.2/2) background unless indicated otherwise. All mice were bred and maintained at a specific pathogen-free (SPF) condition. Age and gender-matched adult littermate mice (8-12 week old) were used in experiments. Gender of newborn mice (postnatal day 0-3) was not identified. All animal experiments were approved by Institutional Animal Care and Use Committees of the Pennsylvania State University and University of Texas Health Science Center at San Antonio.
Antibodies
Anti-mouse CD4 (GK1.5), anti-mouse CD8a (53-6.7), anti-mouse CD103 (2E7), anti-mouse CD127 (A7R34), anti-mouse CD11b (M1/70), anti-mouse granzyme C (SFC1D8), anti-mouse Notch1 (HMN1-12) and anti-mouse Notch2 (HMN2-35), anti-mouse CD117 (c-kit, ACK2), anti-mouse Ly-6A/E (Sca-1, D7), and anti-mouse TCRδ (GL3) antibodies were purchased from Biolegend (San Diego, CA). Anti-mouse Eomes (Dan11mag) and anti-mouse/human T-bet (4B10) were purchased from eBioscience (San Diego, CA). Anti-mouse CD3ε (145-2C11), anti-mouse PLZF (R17-809) and PE-CF594 Streptavidin (RUO) were from BD Biosciences (San Jose, CA). Anti-mouse NK1.1 (PK136) was from Biolegend (San Diego, CA) and eBioscience (San Diego, CA). Mouse lineage cell detection cocktail-biotin (#130-092-613) is purchased from Miltenyi Biotech.
Cell isolation
Isolation of lymphocytes from the skin was performed as we previously described (29). Thymocytes were isolated using Tenbroeck tissue grinder (Kimble). After grinding, thymocytes were filtered with 70 and 40-micron polyester meshes (ELKO Filtering Co.) before used in staining. Bone marrow cells were collected from the femurs and filtered with sterile 40μm cell strainers (BD Falcon).
Cell staining and Flow cytometry
To stain surface markers, cells were incubated with antibodies in staining buffer (PBS supplemented with 3% calf serum and 0.005% sodium azide) for 25 min at 4°C. Intracellular markers were stained overnight in permeabilization buffer (eBioscience) with antibodies after two-step fixation and permeabilization. 4% paraformaldehyde and transcription factor fixation buffer (eBioscience) were used for first and second fixation. BD Fortessa LSRII (BD Biosciences) was used for flow cytometry and the results were analyzed using FlowJo software (FlowJo LLC).
Culture of BM and thymic progenitors on OP9 cells
The procedure was performed similarly as previously reported (30, 31). OP9 and OP9-DL1 cells were kindly provided by Dr. Juan Carlos Zuniga-Pflucker (Sunnybrook Health Sciences Centre, Canada). In brief, 1-2×104 sorter-purified Lin−Sca1+Kit+(LSK) BM progenitor cells or CCR10(EGFP)−CD3−CD4−CD8−NK1.1+ thymocytes were seeded onto 80%-90% confluent OP9 or OP9-DL1 cells in OP9 medium (α-MEM supplemented with 20% FBS and 1% Penicillin-Streptomycin) containing 5ng/mL IL-2, 10ng/mL IL-15, 5ng/mL Flt-3L and 1ng/mL IL-7 and grown at 37°C and 5% CO2.
Fetal thymic organ culture (FTOC)
FTOC was performed similarly as described (32). CCR10(EGFP)−NK1.1+CD3−CD4−CD8−thymocytes from Rag1−/−CCR10+/EGFP mice were sorted by BD FACSAria. BM progenitors from Rag1−/−CCR10+/EGFP mice were enriched using MojoSort™ Mouse Hematopoietic Progenitor Cell Isolation Kit (Biolegend). 3-5000 cells were seeded into one E16-18 fetal thymic lobe that was pre-treated with 2′-deoxyguanosine (2-dG) (Sigma-Aldrich, D0901). After 2 weeks of culture at 37°C and 10% CO2, cells were isolated from the thymic organ culture and analyzed by flow cytometry.
Anti-CD127 antibody treatment of Rag1-KO mice
Adult Rag1−/−CCR10+/EGFP mice were injected intraperitoneally with 0.5mg of anti-mouse IL-7Rα (BE0065, Bio X Cell) (33, 34) or isotype control antibodies (BE0089, Bio X Cell) every other day. Mice were analyzed 12 days after starting the treatment.
Competitive bone marrow transfer
The experiment was performed similarly as previously described (9). Briefly, WT C57BL/6 (CD45.1/1) recipient mice were fed with antibiotics water for 1 week and then irradiated (10G). FACS sorted-BM LSK progenitors from CCR10+/EGFP (CD45.1/2) and CD127−/−CCR10+/EGFP (CD45.2/2) mice were mixed at the 1:1 ratio and injected intravenously into irradiated recipient mice (total 10000 cells/recipient). Recipient mice were analyzed 12-13 days post transfer.
Statistical analyses
Two-tailed Student’s T tests were performed using Prism software (GraphPad Software Inc) for statistical analysis. The data are presented as mean ± SEM. P < 0.05 was considered significant.
RESULTS
A large number of developing T cells suppress generation of CCR10+NK1.1+ ILC1s in adult thymi
We previously reported that skin-homing CCR10+NK1.1+ ILC1s are more preferentially generated in thymi of WT mice at perinatal stages than at adult stages; but thymi of adult Rag1−/− mice have high percentages of CCR10+NK1.1+ ILC1s, suggesting that a large number of developing T cells repress generation of CCR10+NK1.1+ ILC1s in the adult thymus (9). To test this further, we analyzed adult TCRβ−/− and TCRδ−/− mice for their thymic generation of CCR10+NK1.1+ ILC1s. All mice carried a CCR10-KO/EGFP-KI allele (CCR10+/EGFP), allowing for using EGFP (enhanced green fluorescent protein) to report the CCR10 expression (28). As in Rag1−/−CCR10+/EGFP mice, there were increased CD3−NK1.1+ thymocytes in TCRβ−/−CCR10+/EGFP mice compared to WT (CCR10+/EGFP) controls (Supplemental Fig. 1A–B). The percentage of CCR10(EGFP)+ NK1.1+ ILC1s in adult TCRβ−/−CCR10+/EGFP thymi was also increased, although not as high as in adult Rag1−/−CCR10+/EGFP thymi (Fig. 1A–B), suggesting that remaining γδT cells repress the CCR10+NK1.1+ ILC1 generation to some extents. On the other hand, there were similar low percentages of total CD3−NK1.1+ cells and CCR10+NK1.1+ ILC1s in thymi of adult TCRδ−/−CCR10+/EGFP and WT controls (Fig. 1C, Supplemental Fig. 1C), because a large number of developing αβT cells remain in TCRδ−/− mice. Together, these results reveal that presence of αβT and γδT cells in adult thymi suppresses development of CCR10+NK1.1+ ILC1s.
Figure 1. Presence of T cells suppresses generation of CCR10+NK1.1+ ILC1s in adult thymi.
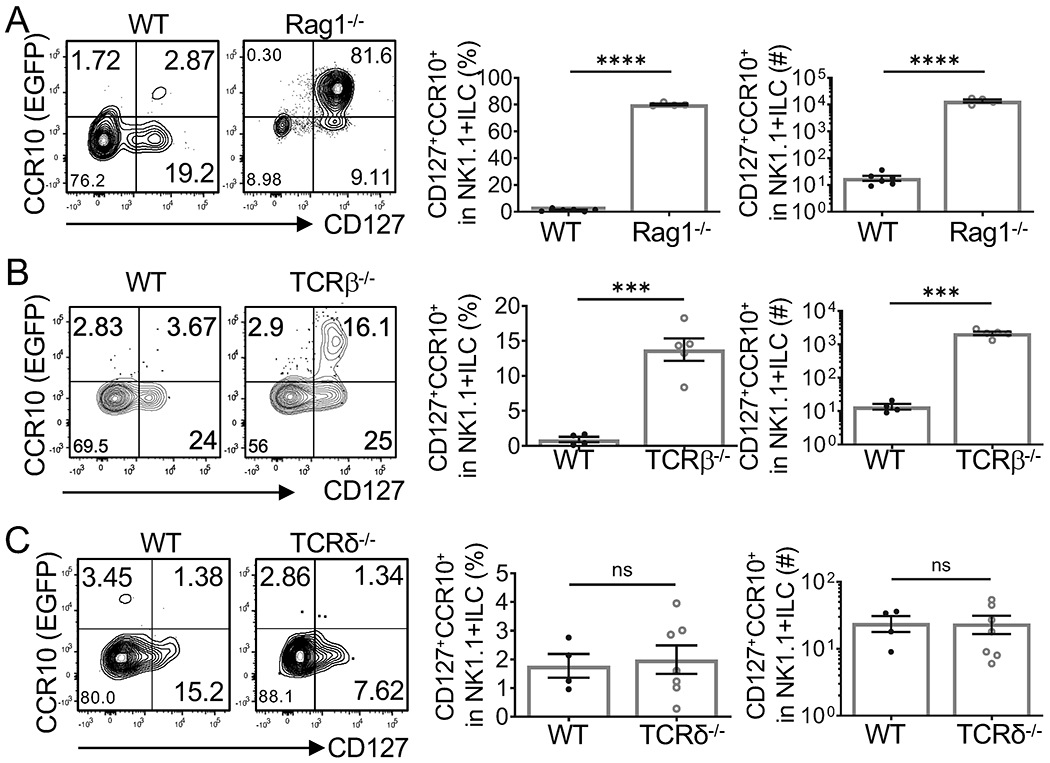
A, B and C) Flow cytometric (FC) analysis of gated CD3−CD4−CD8−NK1.1+ thymocytes for CCR10+CD127+NK1.1+ ILC1s in adult Rag1−/−CCR10+/EGFP (A), TCRβ−/−CCR10+/EGFP (B) and TCRδ−/−CCR10+/EGFP (C) mice. Average percentages and number of the thymic CCR10+CD127+NK1.1+ ILC1s were showed in bar graphs. One dot represents one mouse. Results are representative of two or three independent experiments. ns: not significant, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by two-tailed Student’s t test.
Notch/ligand signals promote generation of CCR10+NK1.1+ ILC1s from hematopoietic stem cells in vitro
Considering that CCR10+NK1.1+ ILC1s are generated in the thymus but not fetal livers or adult BM (9), T cells suppress development of CCR10+NK1.1+ ILC1s in adult thymi likely because they interfere with thymus-specific signals required for generation of CCR10+NK1.1+ ILC1s. A critical signal pathway that distinguishes thymus from other lymphoid organs is of the Notch ligands, which are highly expressed in thymic but not BM stromal cells. The ectopic expression of a Notch ligand Delta-like 1 (DL1) on BM stromal cells is sufficient to render them the ability to support differentiation of hematopoietic progenitor cells into T cells in vitro through engagement of Notch receptors expressed on the progenitor cells (30). CD3−NK1.1+ thymocytes expressed Notch1 and Notch2 (Fig. 2A), suggesting that Notch signals could be involved in promoting generation of CCR10+NK1.1+ ILC1s. To test this, we co-cultured BM progenitor cells isolated of Rag1−/−CCR10+/EGFP mice on BM stroma-derived OP9 cells or OP9 cells ectopically expressing DL1 (OP9-DL1) and analyzed the generation of CCR10+NK1.1+ ILC1s. Using BM progenitor cells of Rag1−/− instead of WT mice was to avoid interference from developing T cells. As reported (35), OP9 cells predominantly supported generation of CD11b+CD127−CD3−NK1.1+ conventional NK cells while OP9-DL1 cells supported generation of CD127+CD11b−CD3−NK1.1+ ILC1s (Figure 2B). A significant fraction of NK1.1+ ILC1s generated in the OP9-DL1 culture expressed CCR10(EGFP) and CD103, an adhesion molecule commonly co-expressed with CCR10 on skin-resident lymphocytes (9, 36), while NK cells generated in the OP9 culture did not either of them (Figure 2B). These results indicate that Notch signals support the CCR10+NK1.1+ ILC1 generation.
Figure 2. Notch/ligand signals promote generation of CCR10+NK1.1+ ILC1s from hematopoietic stem cells in vitro.
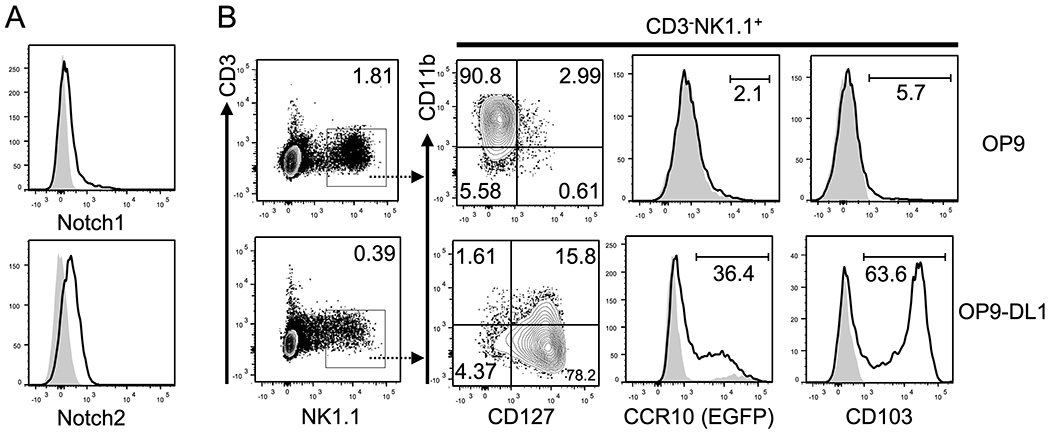
A) FC analysis of the expression of Notch1 (top) and Notch2 (bottom) on thymic CD3−NK1.1+ cells of adult Rag1−/− mice. B) FC analysis of the expression of CD127, CD11b, CCR10(EGFP) and CD103 on gated CD3−NK1.1+ cells (far left) generated from Rag1−/−CCR10+/EGFP BM progenitor cells cultured on OP9 and OP9-DL1 cells for 19 days. Gray areas in the CCR10(EGFP) and CD103 histograms are of WT progenitor cells and isotype control antibody staining respectively, serving as negative controls for EGFP and CD103. Representative of four experiments of cells analyzed on day 18-22 after culture.
The Notch ligand DL4-derived signals are critical for thymic development of CCR10+NK1.1+ ILC1 at adult but not neonatal stages
We then tested whether Notch/ligand-derived signals were critical for thymic generation of CCR10+NK1.1+ ILC1s. Delta-like 4 (DL4) is the dominant Notch ligand expressed in thymic stroma. We thus analyzed thymic stroma-specific DL4-knockout (Foxn1CreDL4f/fCCR10+/EGFP, DL4-KO in short) mice. DL4-KO mice carried a CCR10-KO/EGFP-KI (CCR10+/EGFP) allele to report the CCR10 expression with EGFP (28). DL4-KO mice had almost complete blockage of αβT and γδT cell development in thymi at both adult and neonatal stages (Supplemental Fig. 2) (24, 25, 37). Like adult Rag1−/− mice, adult DL4-KO mice had increased CD3−NK1.1+ thymocytes compared to DL4-sufficient WT (DL4f/fCCR10+/EGFP) littermate controls (Fig. 3A, top row). However, unlike Rag1−/− mice and same as the WT controls, only a very small percentage of CD3−NK1.1+ thymocytes of adult DL4-KO mice expressed CCR10(EGFP) (Fig. 3A, bottom row). These results demonstrate that there is a defect in generation of CCR10+NK1.1+ ILCs in adult DL4-KO thymi even though generation of CD3−NK1.1+ thymocytes is enhanced in absence of the T cell development. Compared to their WT littermate controls, neonatal DL4-KO mice also had increased percentages and numbers of CD3−NK1.1+ thymocytes, even higher than those of Rag1−/− controls (Fig. 3C, top row). However, the percentage of CCR10+NK1.1+ ILC1s in thymi of neonatal DL4-KO mice was slightly lower than that of their WT littermates or newborn Rag1−/− controls while percentages of CCR10+NK1.1+ ILC1s in neonatal WT and Rag1−/− thymi were similar (Fig. 3B, bottom row). Together, these results indicate that thymic generation of CCR10+NK1.1+ ILC1s is dependent on thymic stromal DL4-derived Notch signals and suppressed by presence of T cells, particularly at adult stages.
Fig. 3. The Notch ligand DL4 is critical for thymic development of CCR10+NK1.1+ ILC1s in adult but not newborn mice.
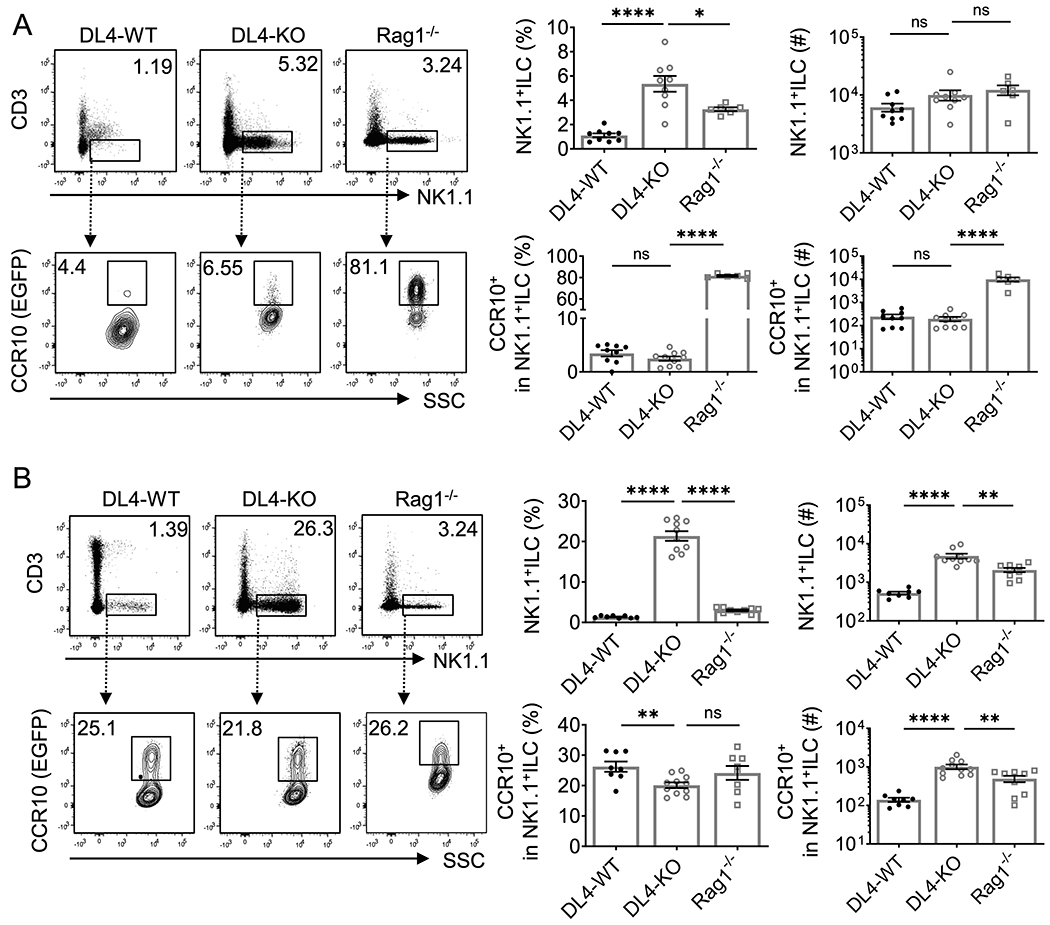
A-B) FC analysis of NK1.1+ ILCs and their expression of CCR10 in adult (A) and day0-3 old neonatal (B) DL4-WT (DL4f/f CCR10+/EGFP), DL4-KO (Foxn1CreDL4f/f CCR10+/EGFP) and Rag1−/−CCR10+/EGFP thymus. Average percentages and numbers of total CD3−NK1.1+ thymocytes and CCR10+NK1.1+ ILCs were shown in bar graphs. NK1.1+ ILC1s are gated from CD3−CD4−CD8− thymocytes. One dot represents one mouse. Results are of four independent experiments. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by two-tailed Student’s t test.
DL4-derived Notch signals are directly involved in promoting differentiation of CCR10+NK1.1+ ILC1s independent of the T cell development
Increased percentages of total CD3−NK1.1+ cells but reduced percentages of their CCR10 expression in thymi of DL4-KO mice led us to hypothesize that Notch signals were required for generation of CCR10+NK1.1+ ILC1s from the CCR10− NK1.1+ precursor cells. To address this assumption, we reconstituted 2-dG-treated WT fetal thymic lobes with CCR10− CD3−NK1.1+ cells purified of thymi of Rag1−/−CCR10+/EGFP mice. The donor cells carried the CCR10+/EGFP reporter while WT fetal thymic lobes did not, allowing us to detect CCR10(EGFP)+ cells of donor origins. Two weeks after culture, the majority of CD3−NK1.1+ cells in the reconstituted fetal thymic lobes expressed CCR10(EGFP+) (Fig. 4A), demonstrating that CCR10−CD3−NK1.1+ thymocytes are precursors of CCR10+NK1.1+ ILC1s. Similarly, purified CCR10− CD3−NK1.1+ thymocytes upregulated CCR10(EGFP) after co-culture with OP9-DL1 cells (Supplemental Fig. 3). Interestingly, CCR10− CD3−NK1.1+ thymocytes also upregulated CCR10(EGFP) after co-culture with OP9 cells (Supplemental Fig. 3). Considering that CCR10− CD3−NK1.1+ thymocytes had received Notch signals before seeding into the culture, this result suggests that they might have committed to differentiation into CCR10+ NK1.1+ ILC1s although exact mechanisms need to be dissected further in vivo.
Figure 4. Reduced generation of CCR10+NK1.1+ ILC1s from hematopoietic progenitor cells in the DL4-KO fetal thymic organ culture.
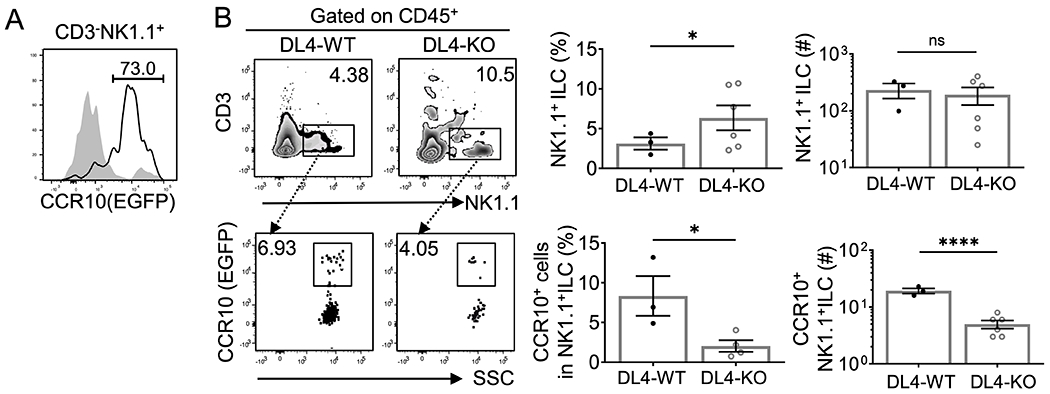
A) FC analysis of CCR10(EGFP) expression on gated CD45+CD3−NK1.1+ cells of the fetal thymic organ culture (FTOC) in which 2-dG-treated WT thymic lobes were reconstituted with CCR10−CD3−NK1.1+ thymocytes purified from adult Rag1−/−CCR10+/EGFP mice. FC analysis of WT thymic lobes without reconstitution (in gray) was used as a negative control. Representative of 12 reconstituted FTOCs and 3 controls. B) FC analysis of gated CD45+ thymocytes of DL4-sufficient WT (DL4f/f) and DL4-KO (Foxn1CreDL4f/f) FTOC reconstituted with Rag1−/−CCR10+/EGFP BM progenitors for CD3−NK1.1+ cells and their expression of CCR10(EGFP). Average percentages and numbers of total CD3− NK1.1+ cells and CCR10+NK1.1+ ILC1s of the FTOC were shown in bar graphs, in which one dot represents results of two combined FTOCs. Results are pooled of three independent experiments. ns = not significant and *p < 0.05, **p < 0.01, ****p < 0.0001 as determined by two-tailed Student’s t test.
Since DL4-KO mice had defective T cell development in thymi, the impaired differentiation of CCR10−NK1.1+ cells into CCR10+NK1.1+ ILCs in DL4-KO thymi could be caused indirectly by the reduced T cell number and/or directly due to deficiency in Notch signals. To clarify this further, we also reconstituted 2-dG-treated WT and DL4-KO fetal thymic lobes with BM progenitor cells of Rag1−/−CCR10+/EGFP mice, which would rid of indirect effects of the different T cell generation in WT and DL4-KO thymi (Fig. 4B). There were higher percentages of CD3−NK1.1+ cells in DL4-KO thymic cultures than in WT thymic cultures although their numbers were similar because total number of cells was lower in DL4-KO thymic cultures (Fig. 4B, top row). However, the percentage and number of CCR10+NK1.1+ ILC1s were significantly lower in DL4-KO thymic cultures than in WT thymic cultures (Fig. 4B, bottom). These results confirm that DL4-derived Notch signals are differentially involved in generation of NK1.1+ ILCs and their CCR10 expression.
Thymic NK1.1+ ILC1s of DL4-KO mice have reduced expression of CD127
To understand potential mechanisms of DL4-derived signals in regulation of thymic CCR10+NK1.1+ ILC1 development, we characterized thymic CCR10+NK1.1+ ILC1s of DL4-KO mice in more details. Expression of CD103 was reduced in both CCR10+ and CCR10−CD3−NK1.1+ ILCs in newborn and adult DL4-KO thymi compared to DL4-sufficient WT (DL4f/f CCR10+/EGFP) littermate and Rag1−/− controls (Fig. 5A), further confirming that DL4-derived signals regulate acquisition of homing properties by thymic NK1.1+ ILCs. PLZF is crucial for thymic development of CCR10+NK1.1+ ILC1s (9). However, PLZF remained normally expressed in thymic CCR10+NK1.1+ ILC1s of DL4-KO mice (Supplemental Fig. 4A), suggesting that PLZF expression is independent of Notch signals. On the other hand, there was notably reduced expression of CD127 in both CCR10− and CCR10+CD3−NK1.1+ cells of neonatal and adult DL4-KO thymi compared to their WT littermate or Rag1−/− controls (Fig. 5B). Like their WT controls, CCR10+CD3−NK1.1+ thymocytes of DL4-KO mice expressed Eomes and T-bet but no CD11b (Supplemental Fig. 4B).
Fig. 5. Reduced expression of CD127 on CCR10+ thymic NK1.1+ ILC1s of DL4-KO mice.
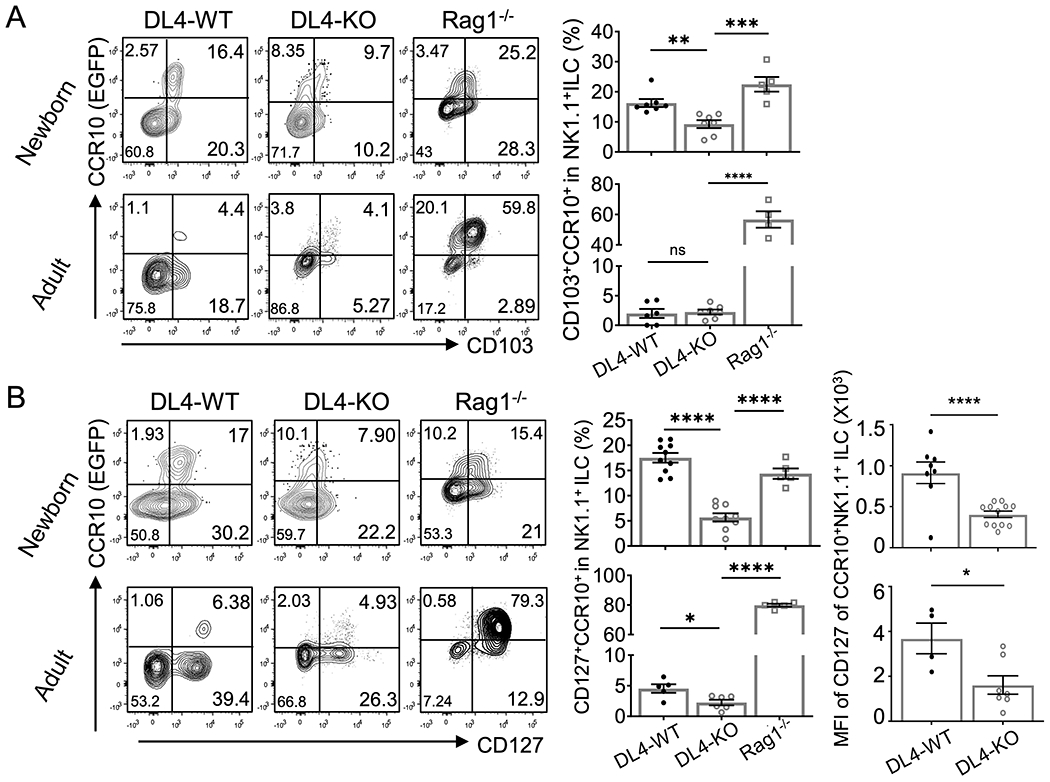
A-B) Comparison of the CD103 (A) and CD127 (B) expression on CCR10− and CCR10+CD3− NK1.1+ thymocytes of adult and newborn DL4-WT (DL4f/f CCR10+/EGFP), DL4-KO (Foxn1CreDL4f/f CCR10+/EGFP) and Rag1−/−CCR10+/EGFP mice. Average expression level or mean fluorescence intensity (MFI) of each marker is on the right. One dot represents one mouse. Results are of three or four independent experiments. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by two-tailed Student’s t test.
CD127 is differentially required for thymic generation of total and CCR10+NK1.1+ ILC1s at adult and neonatal stages
As a component of the IL-7 receptor, CD127 transduced IL-7-derived signals are important in proliferation and survival of lymphocytes and development of T/B cells and ILCs (38–41), and CD127-KO and IL-7-KO mice have severely impaired thymic αβT and γδT cell development (42–44). The reduced CD127 expression on thymic NK1.1+ ILC1s in DL4-KO mice suggests that CD127 might be involved in the Notch-regulated generation of CCR10+NK1.1+ ILC1s in the thymus. We therefore analyzed thymic CCR10+NK1.1+ ILC1s in CD127−/−CCR10+/EGFP mice, which carried a CCR10-KO/EGFP-KI allele for purpose of reporting CCR10 expression with EGFP (28). Same as in adult DL4-KO and Rag1−/− mice, the percentage of CD3−NK1.1+ cells was higher in thymi of adult CD127−/−CCR10+/EGFP mice than WT controls (Fig. 6A, top row), suggesting that their development was not impaired as T cells. However, no CD3−NK1.1+ thymocytes of adult CD127−/−CCR10+/EGFP mice expressed CCR10(EGFP) (Fig. 6A, bottom row), suggesting that IL-7 signals were required for their CCR10 expression. The percentage of CD3−NK1.1+ cells were also increased in thymi of neonatal CD127−/−CCR10+/EGFP mice, same as in neonatal DL4-KO mice (Fig. 6B, top row). Thymic NK1.1+ ILC1s in neonatal CD127−/−CCR10+/EGFP mice expressed CCR10(EGFP) and CD103 at similar levels as their WT littermate controls (Fig. 6B, bottom row), suggesting that CD127-derived signals are not crucial for thymic generation of CCR10+NK1.1+ ILC1s at neonatal stages. These results suggest that CD127 is differentially required for thymic generation of CCR10+NK1.1+ ILCs at adult versus neonatal stages.
Fig. 6. CD127 is required for development of thymic CCR10+NK1.1+ ILC1s at adult but not neonatal stages.
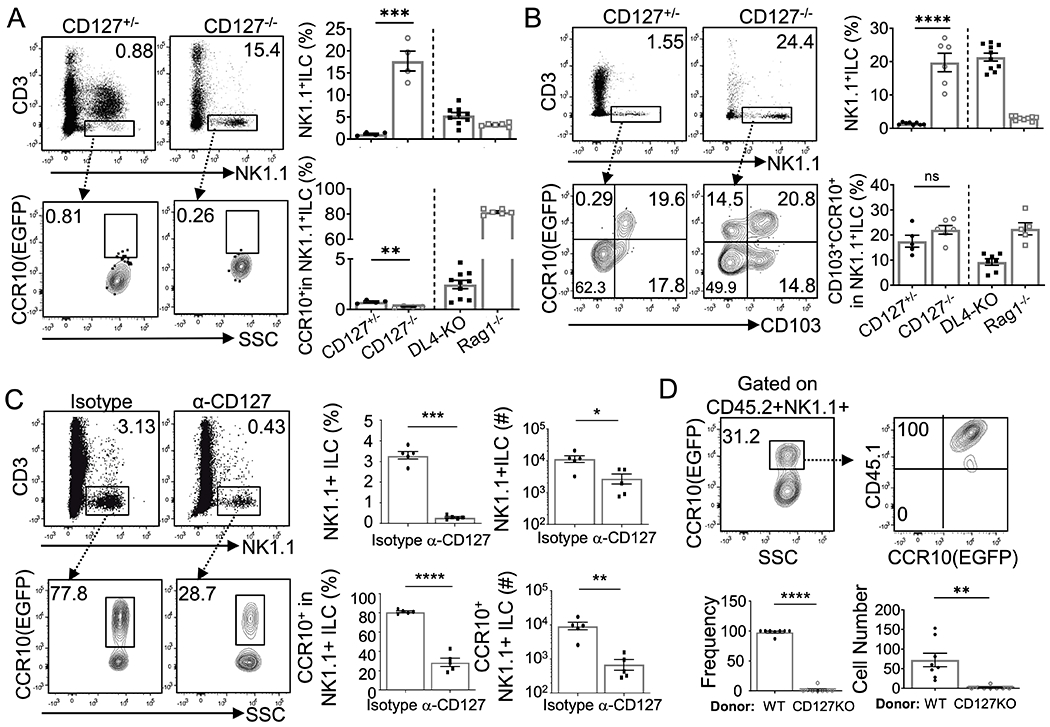
A-B) FC analysis of NK1.1+ ILCs and their expression of CCR10 in adult (A) and neonatal (B) CD127−/−CCR10+/EGFP and CD127+/−CCR10+/EGFP thymi. Average percentages of CD3−NK1.1+ thymocytes and CCR10+NK1.1+ ILC1s were shown in bar graphs. CD3−NK1.1+ cells are gated from CD4−CD8−thymocytes. Results of DL4-KO and Rag1−/− mice are from Fig. 3 and included for comparison. C) FC analysis of total and CCR10(EGFP)+ thymic NK1.1+ ILC1s in Rag1−/−CCR10+/EGFP mice treated with anti-CD127 blocking antibodies or isotype control antibodies. Average percentages and numbers are on the right. D) FC analysis of CCR10(EGFP)+ thymic NK1.1+ ILC1s derived from WT (CD45.1/2) vs. CD127−/− (CD45.2/2) BM donor progenitor cells in WT (CD45.1/1) recipient mice. Gated on CD45.2+ CD3−NK1.1+ thymocytes. Relative percentages and absolute numbers of WT and CD127−/− donor-derived CCR10(EGFP)+ thymic NK1.1+ ILC1s are shown in bar graphs. One dot represents one mouse. Results are of 2-3 independent experiments. ns = not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as determined by two-tailed Student’s t test.
To further assess the requirement of CD127-mediated signals in thymic generation of CCR10+NK1.1+ ILC1s at adult stages, we treated adult Rag1-KO mice with anti-CD127 blocking antibodies (33, 34). Compared to Rag1-KO mice treated with isotype control antibodies, Rag1-KO mice treated with anti-CD127 blocking antibodies had dramatically reduced CCR10+ NK1.1+ ILC1s in thymi (Fig. 6C), indicating that CD127-derived signals are essential for efficient generation of CCR10+ NK1.1+ ILC1s. To assess this notion directly, we also performed the competitive BM reconstitution experiment in which same numbers of WT (CD45.1/2) and CD127−/− (CD45.2/2) BM progenitor cells were co-injected into irradiated WT (CD45.1/1) recipient mice. Nearly all donor-derived CCR10+NK1.1+ ILC1s in thymi of recipients were derived from the WT origin (Fig. 6D). Together, these results demonstrate that intrinsic CD127-transduced signals are required for thymic generation of CCR10+NK1.1+ ILC1s in adult mice.
DISCUSSION
During their developmental stages, ILCs could be pre-programmed with unique homing properties that allow them to migrate directly into barrier tissues where they function to protect local tissue homeostasis. Molecular mechanisms underlying the developmental programming of tissue-homing properties of ILCs are not well understood. Here we assessed roles of DL4-derived Notch signals in regulating thymic development of NK1.1+ ILC1s with the CCR10+ skin-homing property. We found that Notch signals promote acquisition of skin homing properties – indicated by CCR10 and CD103 expression – by NK1.1+ ILC1s. Furthermore, we found that requirements of the DL4-derived Notch signaling in thymic development of CCR10+NK1.1+ ILC1s are different at adult and neonatal stages and are coordinated with the T cell development. In addition, we found that DL4-derived signals regulate expression of CD127 that is involved in thymic generation of CCR10+NK1.1+ ILC1s. These findings advanced our understanding of developmental regulation of generation of ILCs of specific tissue-homing properties.
The thymus is the primary lymphoid organ for the T cell development in a large part because it provides a microenvironment with high levels of Notch signals (24, 25). Increasing evidence suggests that the thymus is also an important site for the ILC development, particularly at perinatal stages, and that the thymus-derived ILCs might contribute significantly to the immune cell repertoire in barrier tissues (9, 14–18, 45). We recently found that a significant fraction of NK1.1+ ILC1s generated in thymi, particularly of perinatal stages, but not adult BM or fetal livers acquire a CCR10+ skin-homing property, suggesting that unique environments of the thymus play an important role in their generation (9). The thymic generation of CCR10+NK1.1+ ILC1s is significantly suppressed at adult stages due to presence of a large number of developing T cells, consistent with the notion that ILCs and T cells derive from same early thymic progenitors and their development is coordinately regulated (9, 17, 27, 45–47). However, absence of T cells has a smaller effect on generation of CCR10+NK1.1+ ILC1s in neonatal thymi, suggesting differentially regulated developmental processes of CCR10+NK1.1+ ILC1s in adult versus newborn thymic environments. In DL4-KO mice in which the thymic T cell development is blocked, there are an increased number of CD3−NK1.1+ thymocytes at both adult and neonatal stages. However, the CD3−NK1.1+ thymocytes could not differentiate into CCR10+ ILC1s in adult DL4-KO mice while the thymic generation of CCR10+ NK1.1+ ILC1s is only slightly reduced in neonatal DL4-KO mice. These findings suggest that acquisition of the CCR10+ skin-homing property by NK1.1+ ILC1s in adult thymi are critically dependent on the DL4-derived Notch signals, which are however suppressed by competition from high numbers of T cells (Fig. 7). At neonatal/fetal stages, a high level of Notch signaling is not crucial for development of CCR10+NK1.1+ ILC1s (Fig. 7). However, CCR10+NK1.1+ ILC1s generated in neonatal DL4-KO thymi have significantly reduced expression of CD103, suggesting that Notch signals are still required for proper expression of other tissue-homing molecules even though they are not crucial for thymic generation of CCR10+NK1.1+ ILC1s at neonatal stages.
Fig. 7. A schematic model of Notch signals in regulating development of CCR10+NK1.1+ ILC1s in adult and perinatal thymi.
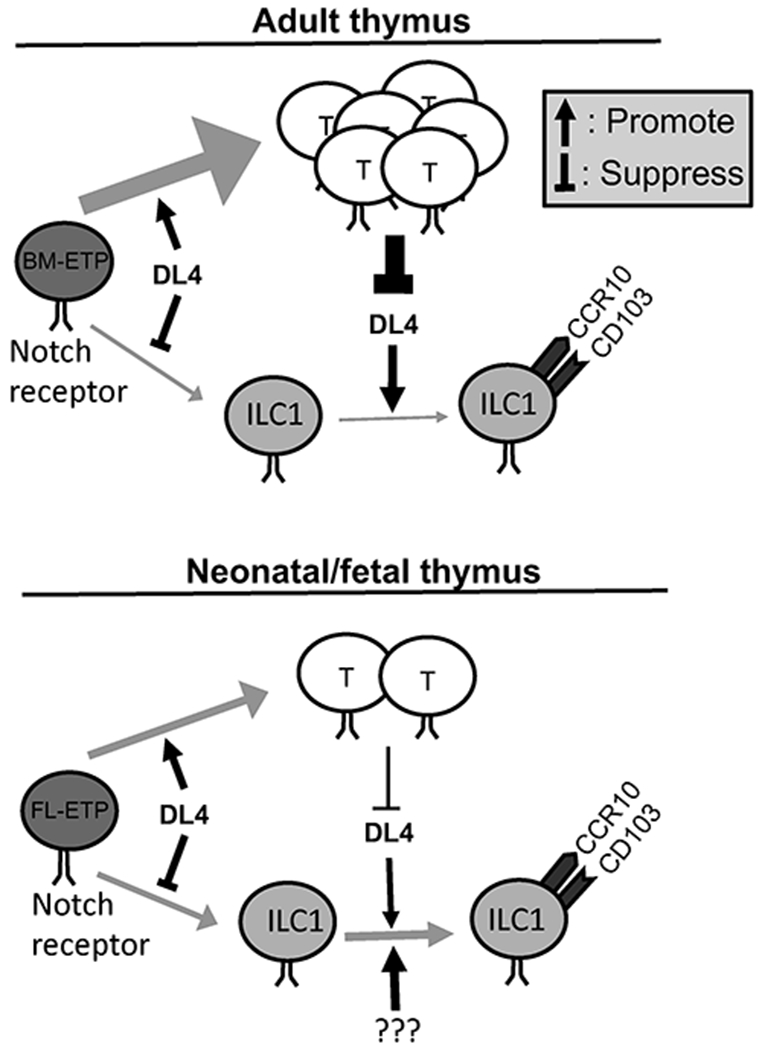
BM-ETP: adult bone marrow-derived early thymic progenitor cells. FL-ETP: fetal liver-derived early thymic progenitor cells.
While DL4-derived signals are required for proper expression of CCR10 and CD103 on thymic NK1.1+ ILC1s, the increased number of total CD3−NK1.1+ cells in both adult and neonatal DL4-KO thymi suggests that DL4-derived Notch signals restricts generation of NK1.1+ ILC1s (Fig. 7). This result is consistent with the notion that a strong Notch signal in the thymus promote the T cell development at expense of the ILC development and a reduced Notch signal preferentially support the ILC development (17, 27). Opposite requirements of Notch signals in thymic generation of total CD3−NK1.1+ innate lymphocytes and their differentiation into CCR10+NK1.1+ ILC1s provide additional evidence in broad roles of the Notch signals in fate decision of various immune cell lineages throughout their developmental steps (9, 17, 27, 48–51). That is, while a strong Notch signal promote differentiation of thymic progenitor cells into T cells and restrict their differentiation into ILCs and B cells, it is required for the expression of homing molecules of CCR10 and/or CD103 on developing NK1.1+ ILC1s (Fig. 7).
Underlying mechanisms for the differential involvement of DL4-derived Notch signals in thymic generation of CCR10+NK1.1+ ILC1s at neonatal and adult stages are yet to be determined. One possible explanation is that thymic microenvironments at neonatal and adult stages differentially express other Notch ligands besides DL4 to mediate thymic lymphocyte development. However, the T cell development is similarly impaired in thymi of neonatal and adult DL4-KO mice, suggesting that DL4 is the major Notch ligand expressed in thymi of both neonatal and adult mice (52). Moreover, we found that thymic stroma-specific knockout of Jag1, another Notch ligand, has no effect on thymic generation of CCR10+NK1.1+ ILC1s at either neonatal or adult stage (data not shown). Alternatively, different sources of early thymic progenitors in neonatal and adult stages – fetal liver vs. adult BM - may create divergent regulatory mechanisms that differentially require the Notch signals for generation of CCR10+NK1.1+ ILC1s. Therefore, fetal liver-derived early thymic progenitor cells (FL-ETP) are less dependent on Notch signals for their differentiation into ILCs and acquisition of the CCR10+ homing property than adult BM-derived early thymic progenitor cells (BM-ETP) are. In the future, it is important to use mice with conditional deletion of different Notch receptors and Notch signal molecules (such as Notch 1, Notch 2 and RBP-J) in lymphoid progenitor cells to further confirm and dissect intrinsic roles of Notch signals in thymic generation of CCR10+NK1.1+ ILC1s at different ontogenic stages. In addition, molecules that are directly involved in regulating expression of CCR10 and other skin-homing molecules in thymic NK1.1+ ILC1s are also worth further investigation. Our previous study suggests that PLZF expressed by NK1.1+ thymic ILC1s is crucial for their CCR10 expression at perinatal stages (9). Notch signals were reported to regulate PLZF expression in thymic iNKT cells (53). However, the PLZF expression on thymic NK1.1+ ILC1s is not altered in DL4-KO mice, suggesting that DL4-derived Notch signals regulate expression of CCR10 and other homing molecules through a PLZF-independent manner.
IL-7 signaling is another vital factor for ILC development (27, 39, 54, 55). Thymic ILC1s of DL4-KO mice had reduced CD127 expression, consistent with the notion that Notch signals regulate CD127 expression (56, 57). In addition, CD127-deficient progenitors are unable to give rise to CCR10+ ILC1s in adult thymi, consistent with the notion that the crosstalk between IL-7 and Notch signaling is crucial for the development of the ILC development (27). However, detailed mechanisms of the IL-7 and Notch interaction require further study. Interestingly, same as DL4-KO mice, CD127-KO mice have a high number of CD3−NK1.1+ thymocytes, indicating that IL-7 signaling is dispensable for their development. However, adult CD127-KO thymi do not generate CCR10+NK1.1+ ILC1s while newborn CD127-KO thymi still efficiently support the generation of CCR10+CD103+ NK1.1+ ILC1s, suggesting that IL-7R and DL4-derived signals have overlapping and distinct roles in generation of skin-homing NK1.1+ ILC1s in the thymus at different ontogenic stages. Mechanisms for the differential involvement of IL-7R- and DL4-derived signals in thymic generation of CCR10+NK1.1+ ILC1s need further investigation.
Finally, how fetal/neonatal thymic CCR10+ NK1.1+ ILC1s contribute to establishment of skin immune cell repertoire requires further investigation. These cells express both T-bet and Eomes, a feature commonly associated with NK cells but not classic ILC1s that express T-bet but not Eomes (1, 2). However, fetal/neonatal thymic CCR10+ NK1.1+ ILC1s express PLZF (9), a transcription factor that is expressed on committed progenitors for ILCs, including ILC1s, but not NK cells (5). In addition, knockout of PLZF severely impaired generation of fetal/neonatal thymic CCR10+ NK1.1+ ILC1s (9). These results suggest that they are a unique population of precursors of skin-resident ILC1s with some features of NK cells. Consistent with this, CCR10+ NK1.1+ ILC1s of the neonatal skin express both T-bet and Eomes (9). Interestingly, CCR10+ NK1.1+ ILC1s of the skin in adult mice do not express Eomes (9). It will be important to test whether CCR10+ NK1.1+ ILC1s of fetal/neonatal thymic origins are related to CCR10+ NK1.1+ ILC1s in the skin of neonatal and adult mice to clarify their developmental relationship.
Supplementary Material
KEY POINTS.
Notch signals promote thymic generation of CCR10+ skin-homing NK1.1+ ILC1s
DL4-KO differently affects thymic CCR10+ ILC1 generation in newborn and adult mice
IL-7 receptor is also involved in thymic generation of CCR10+ NK1.1+ ILC1s.
Acknowledgments
Research reported in this publication was partly supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institute of health (NIH) under the award number U01AI131393 (to N.X). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest
References
- 1.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, and Vivier E. 2013. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 13: 145–149. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, and Spits H. 2018. Innate Lymphoid Cells: 10 Years On. Cell 174: 1054–1066. [DOI] [PubMed] [Google Scholar]
- 3.Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M, Huntington ND, Belz GT, and Carotta S. 2014. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med 211: 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Li F, Harly C, Xing S, Ye L, Xia X, Wang H, Wang X, Yu S, Zhou X, Cam M, Xue HH, and Bhandoola A. 2015. TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat Immunol 16: 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantinides MG, McDonald BD, Verhoef PA, and Bendelac A. 2014. A committed precursor to innate lymphoid cells. Nature 508: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klose CSN, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, Domingues RG, Veiga-Fernandes H, Arnold SJ, Busslinger M, Dunay IR, Tanriver Y, and Diefenbach A. 2014. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157: 340–356. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Zhao L, Xu M, and Xiong N. 2017. Establishment and function of tissue-resident innate lymphoid cells in the skin. Protein Cell 8: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim CH, Hashimoto-Hill S, and Kim M. 2016. Migration and Tissue Tropism of Innate Lymphoid Cells. Trends Immunol 37: 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Restori KH, Xu M, Song EH, Zhao L, Hu S, Lyu P, Wang WB, and Xiong N. 2020. Preferential Perinatal Development of Skin-Homing NK1.1(+) Innate Lymphoid Cells for Regulation of Cutaneous Microbiota Colonization. iScience 23: 101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim MH, Taparowsky EJ, and Kim CH. 2015. Retinoic Acid Differentially Regulates the Migration of Innate Lymphoid Cell Subsets to the Gut. Immunity 43: 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bando JK, Liang HE, and Locksley RM. 2015. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol 16: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider C, Lee J, Koga S, Ricardo-Gonzalez RR, Nussbaum JC, Smith LK, Villeda SA, Liang HE, and Locksley RM. 2019. Tissue-Resident Group 2 Innate Lymphoid Cells Differentiate by Layered Ontogeny and In Situ Perinatal Priming. Immunity 50: 1425–1438 e1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chea S, Possot C, Perchet T, Petit M, Cumano A, and Golub R. 2015. CXCR6 Expression Is Important for Retention and Circulation of ILC Precursors. Mediators Inflamm 2015: 368427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlyle JR, Michie AM, Cho SK, and Zuniga-Pflucker JC. 1998. Natural killer cell development and function precede alpha beta T cell differentiation in mouse fetal thymic ontogeny. J Immunol 160: 744–753. [PubMed] [Google Scholar]
- 15.Vosshenrich CA, Garcia-Ojeda ME, Samson-Villeger SI, Pasqualetto V, Enault L, Richard-Le Goff O, Corcuff E, Guy-Grand D, Rocha B, Cumano A, Rogge L, Ezine S, and Di Santo JP. 2006. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat Immunol 7: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 16.Jones R, Cosway EJ, Willis C, White AJ, Jenkinson WE, Fehling HJ, Anderson G, and Withers DR. 2018. Dynamic changes in intrathymic ILC populations during murine neonatal development. Eur J Immunol 48: 1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira ACF, Szeto ACH, Heycock MWD, Clark PA, Walker JA, Crisp A, Barlow JL, Kitching S, Lim A, Gogoi M, Berks R, Daly M, Jolin HE, and McKenzie ANJ. 2021. RORalpha is a critical checkpoint for T cell and ILC2 commitment in the embryonic thymus. Nat Immunol 22: 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin SB, Lo BC, Ghaedi M, Scott RW, Li Y, Messing M, Hernaez DC, Cait J, Murakami T, Hughes MR, Leslie KB, Underhill TM, Takei F, and McNagny KM. 2020. Abortive gammadeltaTCR rearrangements suggest ILC2s are derived from T-cell precursors. Blood Adv 4: 5362–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsukamoto N, Itoi M, Nishikawa M, and Amagai T. 2005. Lack of Delta like 1 and 4 expressions in nude thymus anlages. Cell Immunol 234: 77–80. [DOI] [PubMed] [Google Scholar]
- 20.Xiao S, Su DM, and Manley NR. 2008. T cell development from kit-negative progenitors in the Foxn1Delta/Delta mutant thymus. J Immunol 180: 914–921. [DOI] [PubMed] [Google Scholar]
- 21.Nowell CS, Bredenkamp N, Tetelin S, Jin X, Tischner C, Vaidya H, Sheridan JM, Stenhouse FH, Heussen R, Smith AJ, and Blackburn CC. 2011. Foxn1 regulates lineage progression in cortical and medullary thymic epithelial cells but is dispensable for medullary sublineage divergence. PLoS Genet 7: e1002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bajoghli B, Aghaallaei N, Hess I, Rode I, Netuschil N, Tay BH, Venkatesh B, Yu JK, Kaltenbach SL, Holland ND, Diekhoff D, Happe C, Schorpp M, and Boehm T. 2009. Evolution of genetic networks underlying the emergence of thymopoiesis in vertebrates. Cell 138: 186–197. [DOI] [PubMed] [Google Scholar]
- 23.Itoi M, Tsukamoto N, Yoshida H, and Amagai T. 2007. Mesenchymal cells are required for functional development of thymic epithelial cells. Int Immunol 19: 953–964. [DOI] [PubMed] [Google Scholar]
- 24.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, and Radtke F. 2008. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 205: 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hozumi K, Mailhos C, Negishi N, Hirano K, Yahata T, Ando K, Zuklys S, Hollander GA, Shima DT, and Habu S. 2008. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med 205: 2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro VS, Hasan M, Wilson A, Boucontet L, Pereira P, Lesjean-Pottier S, Satoh-Takayama N, Di Santo JP, and Vosshenrich CA. 2010. Cutting edge: Thymic NK cells develop independently from T cell precursors. J Immunol 185: 4993–4997. [DOI] [PubMed] [Google Scholar]
- 27.Koga S, Hozumi K, Hirano KI, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, and Moro K. 2018. Peripheral PDGFRalpha(+)gp38(+) mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med 215: 1609–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Xia M, Sun A, Saylor CM, and Xiong N. 2010. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol 185: 5723–5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia M, Hu S, Fu Y, Jin W, Yi Q, Matsui Y, Yang J, McDowell MA, Sarkar S, Kalia V, and Xiong N. 2014. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J Allergy Clin Immunol 134: 634–644 e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt TM, and Zuniga-Pflucker JC. 2002. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17: 749–756. [DOI] [PubMed] [Google Scholar]
- 31.Holmes R, and Zuniga-Pflucker JC. 2009. The OP9-DL1 system: generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harb Protoc 2009: pdb prot5156. [DOI] [PubMed] [Google Scholar]
- 32.Anderson G, and Jenkinson EJ. 2007. Fetal thymus organ culture. CSH Protoc 2007: pdb prot4808. [DOI] [PubMed] [Google Scholar]
- 33.McKinstry KK, Strutt TM, Bautista B, Zhang W, Kuang Y, Cooper AM, and Swain SL. 2014. Effector CD4 T-cell transition to memory requires late cognate interactions that induce autocrine IL-2. Nat Commun 5: 5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, and Rosenblum MD. 2013. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol 190: 4483–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vargas CL, Poursine-Laurent J, Yang L, and Yokoyama WM. 2011. Development of thymic NK cells from double negative 1 thymocyte precursors. Blood 118: 3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J, Hu S, Zhao L, Kaplan DH, Perdew GH, and Xiong N. 2016. Selective programming of CCR10(+) innate lymphoid cells in skin-draining lymph nodes for cutaneous homeostatic regulation. Nat Immunol 17: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, and Owen MJ. 2004. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol 5: 638–644. [DOI] [PubMed] [Google Scholar]
- 38.Bradley LM, Haynes L, and Swain SL. 2005. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol 26: 172–176. [DOI] [PubMed] [Google Scholar]
- 39.Sheikh A, and Abraham N. 2019. Interleukin-7 Receptor Alpha in Innate Lymphoid Cells: More Than a Marker. Front Immunol 10: 2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vonarbourg C, and Diefenbach A. 2012. Multifaceted roles of interleukin-7 signaling for the development and function of innate lymphoid cells. Semin Immunol 24: 165–174. [DOI] [PubMed] [Google Scholar]
- 41.Carrette F, and Surh CD. 2012. IL-7 signaling and CD127 receptor regulation in the control of T cell homeostasis. Semin Immunol 24: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maki K, Sunaga S, Komagata Y, Kodaira Y, Mabuchi A, Karasuyama H, Yokomuro K, Miyazaki JI, and Ikuta K. 1996. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A 93: 7172–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, and Davison BL. 1994. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med 180: 1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shitara S, Hara T, Liang B, Wagatsuma K, Zuklys S, Hollander GA, Nakase H, Chiba T, Tani-ichi S, and Ikuta K. 2013. IL-7 produced by thymic epithelial cells plays a major role in the development of thymocytes and TCRgammadelta+ intraepithelial lymphocytes. J Immunol 190: 6173–6179. [DOI] [PubMed] [Google Scholar]
- 45.Shin SB, and McNagny KM. 2021. ILC-You in the Thymus: A Fresh Look at Innate Lymphoid Cell Development. Front Immunol 12: 681110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elsaid R, Meunier S, Burlen-Defranoux O, Soares-da-Silva F, Perchet T, Iturri L, Freyer L, Vieira P, Pereira P, Golub R, Bandeira A, Perdiguero EG, and Cumano A. 2021. A wave of bipotent T/ILC-restricted progenitors shapes the embryonic thymus microenvironment in a time-dependent manner. Blood 137: 1024–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian L, Bajana S, Georgescu C, Peng V, Wang HC, Adrianto I, Colonna M, Alberola-Ila J, Wren JD, and Sun XH. 2019. Suppression of ILC2 differentiation from committed T cell precursors by E protein transcription factors. J Exp Med 216: 884–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laky K, and Fowlkes BJ. 2008. Notch signaling in CD4 and CD8 T cell development. Curr Opin Immunol 20: 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amsen D, Helbig C, and Backer RA. 2015. Notch in T Cell Differentiation: All Things Considered. Trends Immunol 36: 802–814. [DOI] [PubMed] [Google Scholar]
- 50.Radtke F, Wilson A, and MacDonald HR. 2004. Notch signaling in T- and B-cell development. Curr Opin Immunol 16: 174–179. [DOI] [PubMed] [Google Scholar]
- 51.Wilson A, MacDonald HR, and Radtke F. 2001. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med 194: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Leon MJ, Fuentes P, de la Pompa JL, and Toribio ML. 2018. Dynamic regulation of NOTCH1 activation and Notch ligand expression in human thymus development. Development 145. [DOI] [PubMed] [Google Scholar]
- 53.Oh SJ, Ahn S, Jin YH, Ishifune C, Kim JH, Yasutomo K, and Chung DH. 2015. Notch 1 and Notch 2 synergistically regulate the differentiation and function of invariant NKT cells. J Leukoc Biol 98: 781–789. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, and Coles M. 2012. IL-7: the global builder of the innate lymphoid network and beyond, one niche at a time. Semin Immunol 24: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, Motomura Y, Moreira-Santos L, Bihl F, Braud V, Kee B, Brady H, Coles MC, Vosshenrich C, Kubo M, Di Santo JP, and Veiga-Fernandes H. 2015. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep 10: 2043–2054. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez-Garcia S, Garcia-Peydro M, Martin-Gayo E, Ballestar E, Esteller M, Bornstein R, de la Pompa JL, Ferrando AA, and Toribio ML. 2009. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J Exp Med 206: 779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Zou J, Zhao B, Johannsen E, Ashworth T, Wong H, Pear WS, Schug J, Blacklow SC, Arnett KL, Bernstein BE, Kieff E, and Aster JC. 2011. Genome-wide analysis reveals conserved and divergent features of Notch1/RBPJ binding in human and murine T-lymphoblastic leukemia cells. Proc Natl Acad Sci U S A 108: 14908–14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


