Abstract
The role of the prefrontal cortex in the mechanism of consciousness is a matter of active debate. Most theoretical and empirical investigations have focused on whether the prefrontal cortex is critical for the content of consciousness, i.e., the qualitative aspects of conscious experience. However, there is emerging evidence that, in addition to its well-established roles in cognition, the prefrontal cortex is a key regulator of the level of consciousness, i.e., the global state of arousal. Here we review recent data supporting the hypothesis that the medial prefrontal cortex is a critical node in arousal-promoting networks.
Keywords: consciousness, general anesthesia, sleep, brainstem, thalamus, oscillations
Role of the Prefrontal Cortex in the Content and Level of Consciousness
Identifying the neural correlates of consciousness was formally proposed in the 1990s as an important first step to understand the neurobiological basis of experience. Despite decades of progress, there is still active debate as to whether the neural correlates of consciousness are localized in anterior cortex, posterior cortex, or both as part of a distributed neural network [1, 2]. The role of the prefrontal cortex (PFC) is now at the center of experimental and theoretical controversies in the science of consciousness. Frameworks such as the integrated information theory posit a major network complex in a posterior confluence of association and sensory cortices, the so-called “hot zone” of consciousness, as critical for experience [3]. The focus of the integrated information theory is phenomenal consciousness, which does not include post-perceptual cognitive activity. By contrast, frameworks such as the global neuronal workspace theory posit a critical role for the PFC, not as the source of consciousness per se (as in some higher-order thought theories [4]) but as a key node that activates a reverberant cortical-cortical network that sustains, amplifies, and makes sensory representations accessible to other cognitive processors [5]. The focus of the global neuronal workspace theory is conscious access, which entails phenomenology but also cognitive functions such as working memory and attention. An “adversarial collaboration” is now adjudicating between these two theories—and others—using principles of open science, prespecified analyses, and shared data platforms [6].
It is still undecided whether the PFC is necessary for encoding the content of consciousness, the qualitative aspects of experience, or whether the PFC instead mediates post-perceptual cognitive processes. Although recent data support the hypothesis that the PFC is involved in conscious experience independent of reporting paradigms [7, 8], there is evidence from frontal lesion studies [1], intracranial electrical stimulation in humans [9], and dreaming [10] that favors neural correlates of consciousness in posterior cortex. Beyond the ongoing debate regarding the role of the PFC in the content of consciousness, there is a related and important question as to whether the PFC is critical for regulating the level of consciousness, or global state of arousal. Neuroanatomically, the medial PFC is well positioned to control arousal states because of its reciprocal connections with subcortical wake-promoting nuclei (Figure 1). Because it sits at the apex of a neurocognitive hierarchy, it is arguable that the PFC should be able to exert top-down influence on subcortical wake-promoting areas to influence arousal.
Figure 1: Reciprocal Connectivity of Prefrontal Cortex with Brainstem Arousal Nuclei.
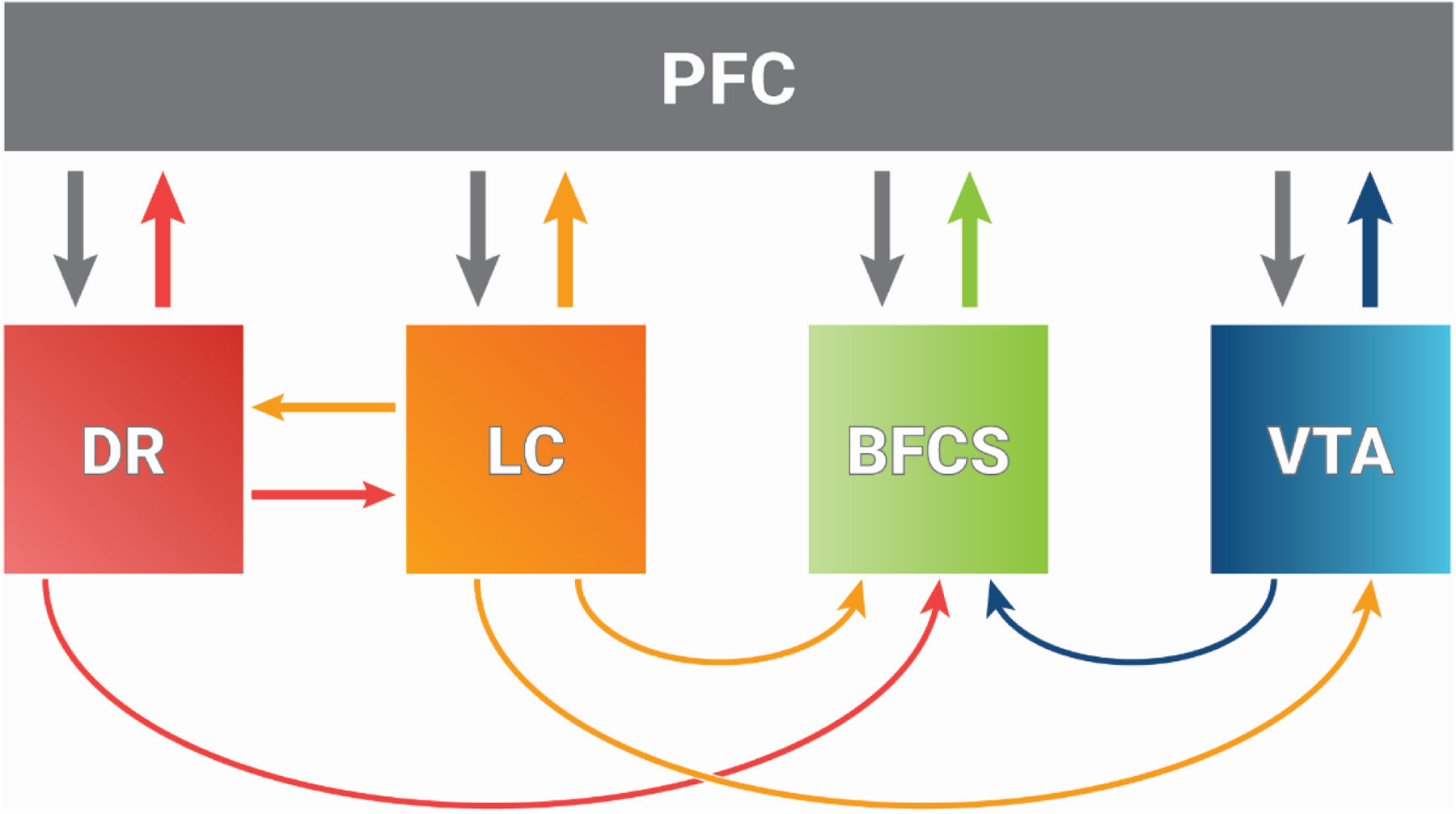
The prefrontal cortex (PFC) is reciprocally connected to numerous subcortical arousal nuclei, including the serotonergic dorsal raphe (DR), noradrenergic locus coeruleus (LC), cholinergic system of the basal forebrain (BFCS), and dopaminergic ventral tegmental area (VTA). Thus, much like the dorsolateral prefrontal cortex is involved in recurrent networks across the cortex that are important for content of consciousness, the medial prefrontal cortex is involved in recurrent networks with subcortical regions that are important for the level of consciousness. Modified from [18].
Here we review evidence supporting the hypothesis that the PFC, long associated with higher cognitive functions, is a critical node in arousal circuitry that regulates level of consciousness. First, we consider the neuroanatomical relationship of the PFC in rodents and primates to aid interpretation of basic research studies. Next, we describe the value of anesthetic state transitions as a model system to study arousal. We then review data from animal studies, involving both stimulation and loss-of-function paradigms, that demonstrate a key role of the medial PFC in emergence from general anesthesia. Unlike investigating the content of consciousness, which is necessarily limited by the need to capture subjective experience through objective measures and is thus largely restricted to humans, the level of consciousness can be studied based on behavioral observations in a range of species, including rodents. Thus, in the animal studies that are described, the level of consciousness is defined in terms of the presence or absence of wakeful behavior, acknowledging that wakefulness is neither sufficient nor necessary for conscious experience. We next complement causal findings in rodents with data from studies in healthy volunteers and clinical investigations, where consciousness is operationally defined as responsiveness to a command, acknowledging that unresponsiveness does not always equate to unconsciousness [11]. Finally, we speculate that the PFC might promote efficient arousal through the activation of recurrent cortical-subcortical networks that control the level of consciousness. Collectively, the studies being reviewed represent an emerging body of evidence arguing for an important role of the PFC in arousal and motivating further research on the topic.
Comparing Prefrontal Cortex in Rodents and Primates
There is a lack of consensus on a unitary cross-species definition of the PFC. Furthermore, the presence or absence of cortical areas in rodents that are homologous or analogous to the primate PFC is a matter of active debate. One of the primary concerns that fuels controversy is that, unlike primates in which the PFC is predominantly granular, the area referred to as the PFC in rodents is completely agranular, i.e., it lacks a distinct input layer IV [12, 13]. Another common defining criterion for the anatomical localization of the PFC—which has been questioned, reconsidered, and revised—is afferent projections from mediodorsal thalamus, which in human and non-human primates can be traced to the dorsolateral PFC [13, 14], but are conspicuously absent from the dorsolateral frontal areas in rodents [13]. As opposed to the dorsolateral PFC, the anterior medial frontal cortex/anterior cingulate cortex (Brodmann Areas 24, 25, 32) in primates is agranular and receives projections from mediodorsal thalamus [12, 13]. The medial frontal cortex in rodents is similarly situated, is agranular, and receives projections from mediodorsal thalamus [12, 13]. Therefore, we would argue that there is a strong rationale to consider the rodent medial PFC—typically divided into prelimbic, infralimbic, and anterior cingulate cortex—as homologous to the primate medial PFC [12, 13]. Caution is warranted, however, when considering the functional implications of this homology. A resting-state magnetic resonance imaging study comparing the rodent, non-human primate, and human medial PFC found that there was consistent intrinsic functional organization across these species but distinct functional connections with other brain regions [15].
Besides the concerns surrounding the generalizability of the experimental findings due to the lack of consensus in defining the PFC across species, equally challenging is the lack of consensus on the terminology used to describe the rodent PFC, which has been referred to by different nomenclature over the years. As has been suggested previously [13, 14], a practical way to navigate the multitude of terminologies allowing precise comparisons between studies is to provide the exact stereotaxic coordinates and the histological evidence of the sites of intervention. Notably, the most recent rat stereotaxic atlas [16, 17] classifies the areas that were formerly labelled as anterior cingulate cortex, prelimbic cortex, and infralimbic cortex as Brodmann areas 32D, 32V, and 25, respectively. A similar scheme has been implemented in the recent mouse atlas [16, 17], which brings much needed clarity for direct comparisons between and within species. However, given the widespread use of the terms anterior cingulate, prelimbic, and infralimbic, we will use the term “medial PFC” when collectivity referring to anterior cingulate, prelimbic, and infralimbic regions or will reference individual regions directly.
Despite the inter-species debate around homology and analogy, there is wide agreement that the prefrontal region across species is involved in higher cognitive and executive functions including learning, memory, and decision making. However, due to the extensive interconnections with arousal-related nuclei—including basal forebrain, locus coeruleus, ventral tegmental area, and dorsal raphae [18] (Figure 1)—the medial PFC in rodents is also positioned as a neural hub that can conceivably regulate levels of consciousness.
Anesthetic State Transitions as a Model System to Study Arousal
Anesthetic state transitions represent a reliable model system that can facilitate studies of the level of consciousness, with the process of emergence from general anesthesia as a particularly powerful tool to study the process of arousal. Unlike sleep, general anesthesia is highly controllable and reproducible. Recovery from general anesthesia also occurs on a timescale amenable to study, unlike the rapid transitions in sleep-wake states that can occur spontaneously or due to external stimuli. Unlike pathologic states of depressed arousal, general anesthesia is fully reversible and largely predictable. Finally, as described in the following paragraphs, the process of arousal from general anesthesia involves widespread neural circuitry, including both subcortical and cortical areas.
Recovery from anesthetic-induced unconsciousness requires restoration of bidirectional communication across the brainstem, thalamus, and cortex. Anesthetics have been shown to activate sleep-promoting neurons in the hypothalamus and inhibit major arousal nuclei in the brainstem [19–22]. Upon discontinuation of the anesthetic, brainstem arousal centers would be predicted to reactivate and stimulate the thalamus, basal forebrain, and cortex [23], contributing to restoration of pyramidal neuron function in the cortex. As anesthetics clear from the thalamus and its reticular nucleus, excitatory thalamocortical projections further activate the cortex. Clinical correlates of these thalamic and cortical effects include return of the oculocephalic and corneal reflexes, dissipation of slow-delta oscillations in the electroencephalogram (EEG) across the entire scalp, and the transition from alpha oscillations to faster beta and gamma oscillations in frontal EEG [24, 25].
However, in addition to reestablishing ascending communication between the brainstem, thalamus, and cortex, recovery of consciousness after general anesthesia also involves restoration of cortical-subcortical communication. As noted, the PFC bidirectionally communicates with a number of arousal-promoting centers in the brainstem and basal forebrain (Figure 1). Furthermore, neurons in the medial PFC and other brain areas project to the nucleus tractus solitarius (NTS) [26–29]. PFC areas projecting to the NTS overlap to a great extent with cortical areas receiving mediodorsal thalamic and dopaminergic input; in rat, these cortical cells are situated in deep layers of the PFC [20]. These descending pathways potentially contribute to the sympathetic responses (such as increased heart rate and increased blood pressure) [30] that are important for arousal and that are commonly observed on recovery from unconsciousness maintained with propofol [31]. The NTS also activates the parabrachial nucleus in the pons, leading to excitatory glutamatergic activation of the thalamus and the basal forebrain. This is one of many examples of how activation of cortical-brainstem pathways might contribute to arousal, which can be studied during anesthetic state transitions.
PFC neurons also send descending projections to the striatum [32]. Among other cortical events during recovery from the anesthetized state, reactivation of cortical pyramidal neurons can lead to direct stimulation of the striatum. The active striatum tonically inhibits the globus pallidus externa; if not inhibited, the globus pallidus externa sends strong GABAA1 receptor-mediated inhibition to the thalamus. Hence, disinhibition of cortical pyramidal neurons leads to disinhibition of the thalamus and provides another example of how activation of descending arousal pathways (cortex to thalamus) can influence ascending arousal pathways (thalamus to cortex). These examples suggest that studying recovery from general anesthesia can potentially elucidate a complex set of events spanning from brainstem to the rostral pole of the cortex, thereby providing an excellent model to probe the subcortical and cortical circuitry controlling arousal.
Basic Research Supporting the Role of Prefrontal Cortex in Arousal
Although the demonstration of arousal state control by stimulation of subcortical nuclei dates back at least to seminal work in the 1940s [33], including in anesthetized animals [34–47], it is only more recently that the role of association cortex in the level of consciousness has been systematically investigated. For example, it has been demonstrated that onset of general anesthesia induced by propofol was associated with synchronization of alpha oscillations between higher-order thalamus and medial (prelimbic) PFC in rats, followed by coherent delta oscillations [48]. The oscillations in these structures differentially decohered in a structured manner during emergence from anesthesia, in line with prior studies demonstrating metastable dynamics en route to the recovery of consciousness after isoflurane anesthesia [49]. The entrainment of neural activity in the PFC, the dynamic range of which would be severely constrained by hypercoherent thalamic oscillations, was posited as a key mediator of the anesthetized state. However, this correlational study design was not able to inform the causal role of the PFC in arousal state transitions and did not include a comparison with posterior cortical sites. Furthermore, the functional equivalent of alpha oscillations in rodents is still under consideration, with some arguing a 3–6 Hz bandwidth as the evolutionary precursor to alpha oscillations in primates [50]. To investigate a causal role of the PFC in arousal, carbachol (a mixed cholinergic agonist) or norepinephrine was reverse dialyzed into the medial (prelimbic) PFC or two areas in the parietal cortex of rats during administration of clinically relevant concentrations of sevoflurane anesthesia [51]. Carbachol-induced stimulation of the PFC, but not sensory (barrel field) or medial-parietal association areas in parietal cortex, was found to restore wakefulness despite ongoing administration of sevoflurane. This study demonstrated a unique, causal role of the PFC in regulating arousal. Evidence from anesthesia is supported by a complementary investigation of sleep-wake states, which showed that carbachol or nicotine microinjection in the medial (prelimbic) PFC of rats during slow-wave sleep reduced the latency to wakefulness; carbachol also increased the overall time spent in wakefulness [52]. The PFC might play a role in arousal changes associated with both sleep and anesthesia. A study in mice showed that administration of an adenosine A2a receptor agonist or adenosine A1 receptor antagonist in the PFC resulted in increased wakefulness and acetylcholine release as well as accelerated recovery from anesthesia [53]. Importantly, evidence was provided that this occurred through a top-down influence on the pontine reticular formation.
Conversely, tetrodotoxin(TTX)-mediated inactivation of the medial PFC in rats both increased sensitivity to general anesthesia and delayed recovery of consciousness after discontinuation of the anesthetic [54]. Importantly, TTX-mediated inactivation of two areas in parietal cortex did not result in a delayed recovery from general anesthesia [54]. This supports the hypothesis that the medial PFC plays a unique and causal role in arousal and recovery of consciousness (Figure 2). It has also been demonstrated that inactivation of prefrontal cortex attenuates the arousal-promoting effects of the basal forebrain [55], thereby suggesting that the PFC serves as a cortical gate for the bottom-up arousal processes, at least those mediated via basal forebrain. The proposed gating function of the PFC for arousal is distinct from the gating function for conscious access, which is thought to be mediated by the anterior insula [56].
Figure 2: Inactivation of Prefrontal Cortex Delays Arousal from General Anesthesia in Rats.
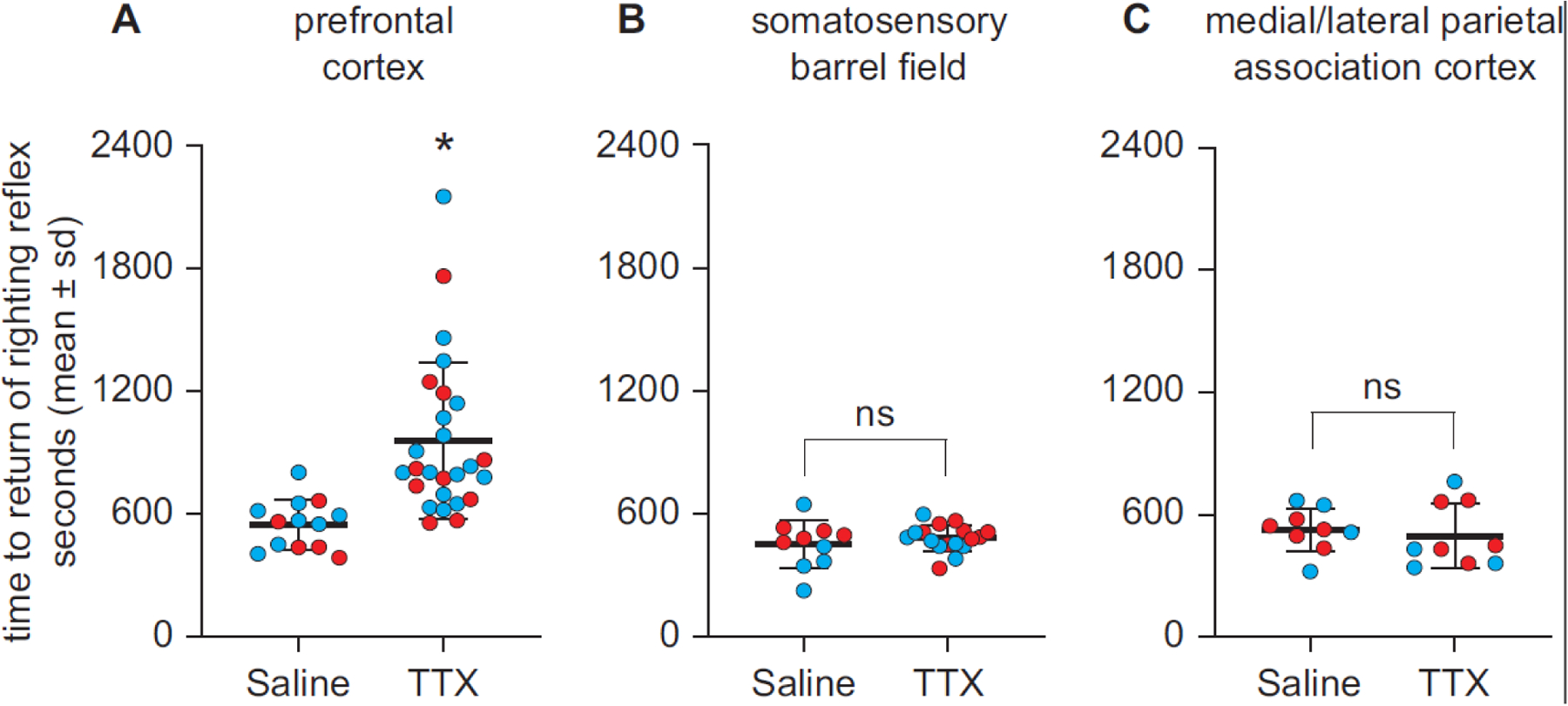
Cholinergic stimulation of the medial prefrontal cortex in rats can reverse the anesthetized state, despite ongoing administration of a general anesthetic, whereas cholinergic or noradrenergic stimulation of parietal cortex cannot. Conversely, as shown here, pharmacological inactivation (using tetrodotoxin, TTX) of medial prefrontal cortex—but not sensory or association parietal cortex—delays recovery from general anesthesia in rats, suggesting that it facilitates efficient arousal. Medial prefrontal cortex has also been shown to be a cortical gate for the arousal-promoting effects of basal forebrain stimulation [55]. Reproduced from [54].
Although one cannot make strict causal inferences regarding the role of the PFC in the recovery of consciousness from general anesthesia in humans, correlational data from rigorous investigations are consistent with animal studies. In a multicenter electroencephalography study of healthy volunteers who were administered deep anesthesia for three hours, permutation entropy values derived from the PFC were comparable to or higher than those observed in posterior cortex just before recovery of consciousness (Figure 3) [57]. Permutation entropy measures the regularity structure of a time series and, when applied to cortical neurophysiology, is often used as a surrogate for information processing. Furthermore, among the six cognitive tests administered sequentially in the post-anesthetic period, performance in abstract matching—known to be mediated by the PFC in humans—was, unexpectedly, the first to recover from the effects of general anesthesia. Thus, both neurophysiologic and neurocognitive indices suggest early engagement of the PFC in arousal mechanisms during recovery from general anesthesia in humans, as defined by response to command. This is further supported by a recent study in humans on disconnected consciousness, i.e., a state of conscious experience in which there is no awareness of the external environment. It was found that, during sleep and sedative-hypnotic administration (dexmedetomidine and propofol), disconnected consciousness is associated with activation of structures in the frontal cortex [58].
Figure 3: Early Engagement of Prefrontal Cortex in the Recovery of Consciousness after Deep Anesthesia in Humans.
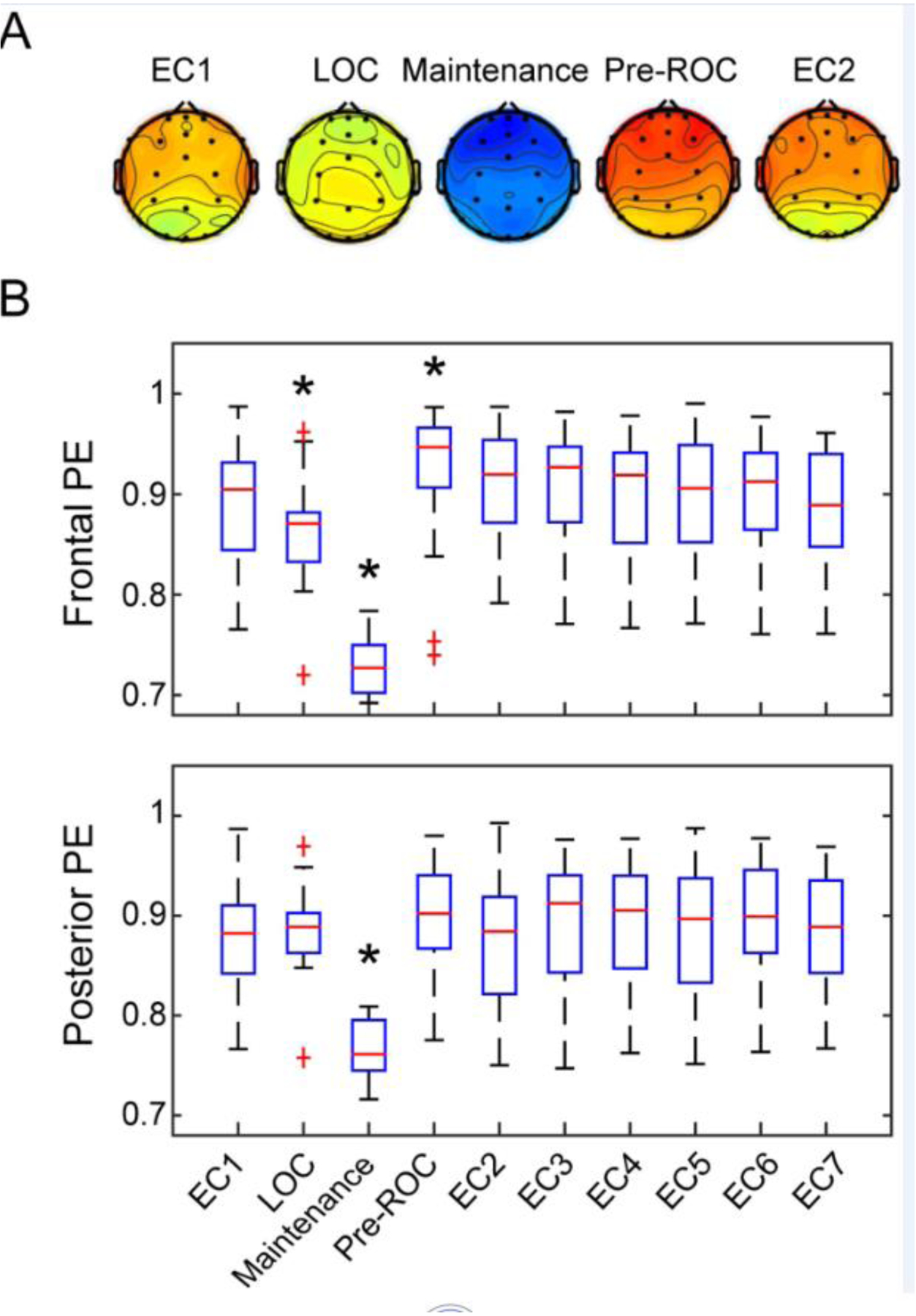
Healthy volunteers were exposed to deep general anesthesia for three hours without surgical intervention and were allowed to recovery spontaneously. Just prior to return of consciousness (ROC), permutation entropy (PE) in prefrontal cortex exceeded that of the baseline condition with eyes closed (EC). After return of consciousness, an executive function mediated by dorsolateral prefrontal cortex was one of the first cognitive domains to recover, further suggesting early engagement of prefrontal cortex in arousal. LOC=loss of consciousness, Maintenance=maintenance of general anesthesia. Reproduced from [57].
Complementing these basic research studies, recent data from patients suffering severe traumatic brain injury provide evidence that connectivity between the thalamus and orbitofrontal PFC, medial PFC, and anterior cingulate (the latter two of relevance to medial PFC in rodents) was associated with early command following during recovery of consciousness [59]. However, to our knowledge, there have been no causal studies (e.g., stimulation) of the medial PFC and arousal states in non-human primates or humans. Furthermore, it is important to note that there is also a body of evidence supporting the role of more posterior cortex in pharmacologic and pathologic alterations of consciousness [60–62].
Clinical Monitoring of Prefrontal Cortex Tracks Arousal States
There is a vast literature on the use of electroencephalography for monitoring arousal states, including specifically in relation to general anesthesia. Commonly used anesthetics such as propofol and sevoflurane induce coherent alpha oscillations (10 Hz) and slow oscillations in the PFC during anesthesia, as recorded on the forehead [63–65]. However, the choice of a frontal montage for commercially available electroencephalography monitors is motivated by convenience rather than neurobiology. Furthermore, electroencephalographic monitoring during surgical anesthesia is not able to distinguish between drug-related (i.e., presence of anesthetic in the brain) and state-related (i.e., conscious vs. unconscious) effects. A recent study in healthy volunteers was able to address both issues using a 64-channel, whole-scalp electroencephalography montage and a protocol that could distinguish arousal states [66]. This investigation demonstrated that functional connectivity (using either coherence or phase lag index measures) in the alpha bandwidth across prefrontal and frontal electrodes could reliably discriminate between conscious and unconscious conditions (i.e., state-related) induced by either propofol or dexmedetomidine, which are mechanistically distinct. By contrast, frontal-parietal connectivity, posited as important for conscious content, could not.
Alpha oscillations are a consistent feature of unconsciousness induced by propofol [64]; a similar phenomenon was first documented in 1937 using barbiturates [67]. Subsequent research has shown that alpha oscillations as well as slow-delta oscillations are characteristic of commonly used GABAergic anesthetics at surgical levels of general anesthesia [24]. Propofol studies across species have revealed two specific properties of these oscillations. First, in a study of non-human primates, the authors documented that alpha oscillations undergo a process of anteriorization [68]. With entry into the anesthetized state, the oscillations seem to move from the occipital area of the scalp to the frontal area. With return of consciousness, there is a movement of the alpha oscillations from the frontal region of the scalp to the occipital region [64, 69]. Second, when a subject is profoundly unconscious, the alpha oscillations are not only concentrated over the frontal cortex, but they are also highly coherent [64, 69], as computed by global coherence or simple pairwise correlations in the alpha band between electrodes.
Computational models have further helped characterize these properties, suggesting that alpha oscillations most likely represent a coupling between the frontal cortex and the thalamus [65]. Computational studies support the hypothesis that GABAergic inhibition in the frontal cortex attenuates local gamma oscillations and produces alpha oscillations [65]. GABAergic inhibition in the thalamus leads to hyperpolarization of the pyramidal cell membranes that then give rise to a hyperpolarizing activating current and, consequently, to alpha oscillations. The structural connections between the thalamus and the PFC couple the two regions together with a coherent alpha oscillation. In contrast, alpha oscillations do not develop in the occipital cortex because the effect of GABAergic inhibition there leads to hyperpolarization of the pyramidal cells, likely due to the lack of activating calcium currents required to produce alpha oscillations [70]. Hence, the apparent movement of alpha oscillations towards the frontal area of the scalp with anteriorization actually represents the alpha oscillations being extinguished posteriorly and then being generated anteriorly. The reverse happens upon recovery of consciousness if the subject is instructed to keep their eyes closed [64].
Experimental research in rodents [48] and more recently in non-human primates [44] has documented that alpha oscillations are indeed a coherent rhythm between the thalamus and the cortex during general anesthesia. Because these constrained thalamocortical dynamics are appreciably different from those observed during consciousness in terms of frequency range and amplitudes, it is neurobiologically plausible they would contribute to a state of unconsciousness. Of note, multiple recent studies have demonstrated a reversal of the traits of general anesthesia after thalamic stimulation, in association with a return of cortical signatures associated with consciousness [43–45].
Prefrontal Cortex as a Choreographer of Arousal Processes
We propose that the PFC might serve as a kind of “choreographer” that integrates various cortical and subcortical networks and promotes efficient arousal from unconscious states. This is supported in principle by the reciprocal structural connectivity between the medial PFC and various wake-promoting nuclei in subcortical regions (Figure 1) as well as computational modeling suggesting that the PFC is important in the recovery of human brain networks from perturbations [71]. Empirically, the fact that cholinergic stimulation of the PFC (but not parietal cortex) can induce wakefulness despite clinically relevant concentrations of general anesthesia provides empirical evidence that the medial PFC can trigger a cascade that promotes arousal [51], which is not associated with a restoration of cortical-cortical connectivity [72]. When the medial PFC (but not parietal cortex) is inactivated, spontaneous arousal after general anesthesia in rats is delayed [54], i.e., recovery is less efficient. In the case of reversal of the anesthetized state by basal forebrain stimulation, inactivation of the medial PFC attenuates or blocks arousal [55].
The role of the PFC as a choreographer of arousal can be considered in an evolutionary framework. First, if the PFC is truly at the apex of a neurocognitive hierarchy of brain organization, it must have control over arousal and cannot be exclusively subservient to, for example, the homeostatic pressures of sleep or circadian rhythms. In evolutionary terms, survival after arousal from sleep would require coordination of sensory representation in the form of access consciousness, rapid categorization of a threatening vs. a non-threatening situation, memory related to the threat, a behavioral action plan, and its motoric execution. The early engagement of the PFC, both in terms of neurophysiology and cognitive function, during recovery from deep unconsciousness in humans provides support for a role of the PFC in coordinating arousal and higher-order functions [57].
Thus, the PFC might play a role in integrating cortical-cortical networks responsible for content of consciousness and cortical-subcortical networks responsible for restoration of level of consciousness, while also maintaining working memory and attentional processes. The PFC is thus well positioned to serve as a kind of master choreographer for efficient arousal processes.
Caveats
Several caveats and competing perspectives merit attention. First, certain lesion studies of the PFC in humans have revealed limited impact on consciousness [reviewed in 1]. However, as was described, controlled lesioning studies in rodents do suggest that the medial PFC has a specific role in mediating efficient arousal from unconscious states (rather than a maintenance role in wakefulness, per se) [54, 55]. Second, we acknowledge that cortical activity, states of consciousness, and behavior can be dissociated under various circumstances [72]. For example, cortical acetylcholine is high in cortex during rapid eye movement sleep in the absence of behavioral arousal, despite an active medial PFC [73]. Similarly, reverse dialysis of carbachol to parietal cortex or reverse dialysis of norepinephrine to either parietal or prefrontal cortices can result in an active cortex without evidence of behavioral arousal [51]. Conversely, in humans, there can be neurophysiological signs of general anesthesia or neuropathology despite evidence of consciousness [74–76]. Finally, arousal in animal models is operationally defined in terms of wakeful behavior and movement, which might be dissociated from conscious experience itself.
Concluding Remarks
Neuroanatomical circuitry and emerging physiological evidence across species support the role of the PFC as a key node in arousal circuitry, especially during the recovery from unconscious states such as general anesthesia. However, various questions remain unanswered (see Outstanding Questions). Studies are needed with more precise neural circuit and PFC manipulation (e.g., using opto- or chemogenetic techniques) in models of physiologic, pharmacologic, and pathologic states of consciousness. Systematically probing various regions within the PFC of rodents and non-human primates will also be important to delineate the individual contributions of specific PFC areas to arousal. Translationally, testing the efficacy of electrical stimulation in the non-human primate medial PFC during states of unconsciousness (e.g., sleep, anesthesia, coma) will be informative in terms of clinical potential for neuropathologic disorders of consciousness in humans.
Outstanding Questions.
What is the circuit-level mechanism of PFC-mediated arousal? Although it has been demonstrated that cholinergic stimulation of PFC can induce arousal, the causal pathways by which this is accomplished remains unknown.
Can stimulation of PFC enhance level of consciousness in patients with disorders of consciousness? Noninvasive stimulation of dorsolateral PFC has been shown to benefit some patients in states of pathologic unconsciousness, but this effect has not been fully characterized, and the medial PFC—which is arguably more important for arousal—has not been sufficiently explored as a therapeutic target.
Are there common neurophysiologic signatures of arousal in PFC that can be used to monitor physiologic, pharmacologic, or pathologic states of unconsciousness? Processed electroencephalogram devices in current use utilize frontal montage but their algorithms are not grounded in principled neurobiology.
Do medial and dorsolateral PFC communicate to coordinate different dimensions of consciousness? There is evidence that lateral PFC mediates the content of consciousness and evidence that medial PFC regulates the level of consciousness, but how these circuits interact to generate conscious experience is unknown.
In conclusion, based on neuroanatomy as well as emerging evidence in animals and humans, further research is warranted on the PFC as a node in an arousal-promoting network, with the recognition that level of consciousness itself might be subject to control by higher-order executive functions at the apex of a neurocognitive hierarchy.
Highlights.
The role of the prefrontal cortex in consciousness is a matter of active debate; although the focus of controversy has been mostly on the content of consciousness, recent studies have also explored the role of the prefrontal cortex in regulating the level of consciousness.
Cholinergic stimulation of medial prefrontal cortex in rodents can reverse the anesthetized state and promote wakefulness during physiologic sleep-wake cycles.
Conversely, inactivation of medial prefrontal cortex in rodents delays recovery from general anesthesia and attenuates the arousal-promoting effects of basal forebrain stimulation.
These and other emerging lines of evidence support the hypothesis that the prefrontal cortex is a key node in the arousal circuitry that controls level of consciousness.
Acknowledgments:
This work was supported by the National Institutes of Health, Bethesda, USA, grant R01GM111293.
Declaration of Interests:
GAM is a paid consultant for TRYP Therapeutics, San Diego, CA. DP receives grant support from TRYP Therapeutics, San Diego, CA. ENB is a co-founder of Pascall Systems, Inc., and owns equity in the company; ENB also serves on the Advisory Board of Trends in Neurosciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boly M et al. (2017) Are the Neural Correlates of Consciousness in the Front or in the Back of the Cerebral Cortex? Clinical and Neuroimaging Evidence. J Neurosci 37, 9603–9613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odegaard B et al. (2017) Should a Few Null Findings Falsify Prefrontal Theories of Conscious Perception? J Neurosci 37, 9593–9602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tononi G et al. (2016) Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci 17, 450–61 [DOI] [PubMed] [Google Scholar]
- 4.Brown R et al. (2019) Understanding the Higher-Order Approach to Consciousness. Trends Cogn Sci 23, 754–768 [DOI] [PubMed] [Google Scholar]
- 5.Mashour GA et al. (2020) Conscious Processing and the Global Neuronal Workspace Hypothesis. Neuron 105, 776–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melloni L et al. (2021) Making the hard problem of consciousness easier. Science 372, 911–912 [DOI] [PubMed] [Google Scholar]
- 7.Bellet J et al. (2022) Decoding rapidly presented visual stimuli from prefrontal ensembles without report nor post-perceptual processing. Neurosci Conscious 2022, niac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor V et al. (2022) Decoding internally generated transitions of conscious contents in the prefrontal cortex without subjective reports. Nat Commun 13, 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raccah O et al. (2021) Does the Prefrontal Cortex Play an Essential Role in Consciousness? Insights from Intracranial Electrical Stimulation of the Human Brain. J Neurosci 41, 2076–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siclari F et al. (2017) The neural correlates of dreaming. Nat Neurosci 20, 872–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders RD et al. (2012) Unresponsiveness ≠ unconsciousness. Anesthesiology 116, 946–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preuss TM and Wise SP (2022) Evolution of prefrontal cortex. Neuropsychopharmacology 47, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlén M (2017) What constitutes the prefrontal cortex? Science 358, 478–482 [DOI] [PubMed] [Google Scholar]
- 14.Laubach M et al. (2018) What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaeffer DJ et al. (2020) Divergence of rodent and primate medial frontal cortex functional connectivity. Proc Natl Acad Sci U S A 117, 21681–21689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G and Watson C eds (2013) The Rat Brain in Stereotaxic Coordinates, 7 th edn., Elsevier; [DOI] [PubMed] [Google Scholar]
- 17.Franklin K and Paxinos G eds (2019) Paxinos and Franklin’s the Mouse Brain in Sterotaxic Coordinates, 5th edn. Elsevier [Google Scholar]
- 18.Briand LA et al. (2007) Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Prog Neurobiol 83, 69–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson LE et al. (2002) The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci 5, 979–84 [DOI] [PubMed] [Google Scholar]
- 20.Franks NP (2008) General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 9, 370–86 [DOI] [PubMed] [Google Scholar]
- 21.Kelz MB and Mashour GA (2019) The Biology of General Anesthesia from Paramecium to Primate. Curr Biol 29, R1199–r1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moody OA et al. (2021) The Neural Circuits Underlying General Anesthesia and Sleep. Anesth Analg 132, 1254–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown EN et al. (2011) General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci 34, 601–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purdon PL et al. (2015) Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology 123, 937–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reshef ER et al. (2019) A Neurologic Examination for Anesthesiologists: Assessing Arousal Level during Induction, Maintenance, and Emergence. Anesthesiology 130, 462–471 [DOI] [PubMed] [Google Scholar]
- 26.van der Kooy D et al. (1984) The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 224, 1–24 [DOI] [PubMed] [Google Scholar]
- 27.Torrealba F and Müller C (1996) Glutamate immunoreactivity of insular cortex afferents to the nucleus tractus solitarius in the rat: a quantitative electron microscopic study. Neuroscience 71, 77–87 [DOI] [PubMed] [Google Scholar]
- 28.Ross CA et al. (1981) Afferent projections to cardiovascular portions of the nucleus of the tractus solitarius in the rat. Brain Res 223, 402–8 [DOI] [PubMed] [Google Scholar]
- 29.Sévoz-Couche C et al. (2006) Cardiac baroreflex facilitation evoked by hypothalamus and prefrontal cortex stimulation: role of the nucleus tractus solitarius 5-HT2A receptors. Am J Physiol Regul Integr Comp Physiol 291, R1007–15 [DOI] [PubMed] [Google Scholar]
- 30.Onai T et al. (1987) Projections to areas of the nucleus tractus solitarii related to circulatory and respiratory responses in cats. J Auton Nerv Syst 18, 163–75 [DOI] [PubMed] [Google Scholar]
- 31.Brown EN et al. (2010) General anesthesia, sleep, and coma. N Engl J Med 363, 2638–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howland JG et al. (2022) The rodent medial prefrontal cortex and associated circuits in orchestrating adaptive behavior under variable demands. Neurosci Biobehav Rev 135, 104569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moruzzi G and Magoun HW (1949) Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1, 455–73 [PubMed] [Google Scholar]
- 34.Alkire MT et al. (2007) Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology 107, 264–72 [DOI] [PubMed] [Google Scholar]
- 35.Alkire MT et al. (2009) Thalamic microinfusion of antibody to a voltage-gated potassium channel restores consciousness during anesthesia. Anesthesiology 110, 766–73 [DOI] [PubMed] [Google Scholar]
- 36.Solt K et al. (2014) Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology 121, 311–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor NE et al. (2016) Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muindi F et al. (2016) Electrical stimulation of the parabrachial nucleus induces reanimation from isoflurane general anesthesia. Behav Brain Res 306, 20–5 [DOI] [PubMed] [Google Scholar]
- 39.Wang TX et al. (2019) Activation of Parabrachial Nucleus Glutamatergic Neurons Accelerates Reanimation from Sevoflurane Anesthesia in Mice. Anesthesiology 130, 106–118 [DOI] [PubMed] [Google Scholar]
- 40.Pillay S et al. (2011) Norepinephrine infusion into nucleus basalis elicits microarousal in desflurane-anesthetized rats. Anesthesiology 115, 733–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo TY et al. (2020) Basal Forebrain Cholinergic Activity Modulates Isoflurane and Propofol Anesthesia. Front Neurosci 14, 559077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao S et al. (2019) Activating an anterior nucleus gigantocellularis subpopulation triggers emergence from pharmacologically-induced coma in rodents. Nat Commun 10, 2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Redinbaugh MJ et al. (2020) Thalamus Modulates Consciousness via Layer-Specific Control of Cortex. Neuron 106, 66–75.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastos AM et al. (2021) Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. Elife 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tasserie J et al. (2022) Deep brain stimulation of the thalamus restores signatures of consciousness in a nonhuman primate model. Sci Adv 8, eabl5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vazey EM and Aston-Jones G (2014) Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 111, 3859–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo T et al. (2018) Parabrachial Neurons Promote Behavior and Electroencephalographic Arousal From General Anesthesia. Front Mol Neurosci 11, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flores FJ et al. (2017) Thalamocortical synchronization during induction and emergence from propofol-induced unconsciousness. Proc Natl Acad Sci U S A 114, E6660–E6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudson AE et al. (2014) Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci U S A 111, 9283–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senzai Y et al. (2019) Layer-Specific Physiological Features and Interlaminar Interactions in the Primary Visual Cortex of the Mouse. Neuron 101, 500–513.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal D et al. (2018) Differential Role of Prefrontal and Parietal Cortices in Controlling Level of Consciousness. Curr Biol 28, 2145–2152.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parkar A et al. (2020) Carbachol and Nicotine in Prefrontal Cortex Have Differential Effects on Sleep-Wake States. Front Neurosci 14, 567849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dort CJ et al. (2009) Adenosine A(1) and A(2A) receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci 29, 871–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huels ER et al. (2021) Inactivation of Prefrontal Cortex Delays Emergence From Sevoflurane Anesthesia. Front Syst Neurosci 15, 690717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dean JG et al. (2022) Inactivation of prefrontal cortex attenuates behavioral arousal induced by stimulation of basal forebrain during sevoflurane anesthesia. Anesth Analg 134, 1140–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Z et al. (2021) Anterior insula regulates brain network transitions that gate conscious access. Cell Rep 35, 109081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mashour GA et al. (2021) Recovery of consciousness and cognition after general anesthesia in humans. Elife 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casey CP et al. (2022) Distinct EEG signatures differentiate unconsciousness and disconnection during anaesthesia and sleep. Br J Anaesth 128, 1006–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cosgrove ME et al. (2022) Thalamo-Prefrontal Connectivity Correlates With Early Command-Following After Severe Traumatic Brain Injury. Front Neurol 13, 826266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanhaudenhuyse A et al. (2010) Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133, 161–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.King JR et al. (2013) Information sharing in the brain indexes consciousness in noncommunicative patients. Curr Biol 23, 1914–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luppi AI et al. (2019) Consciousness-specific dynamic interactions of brain integration and functional diversity. Nat Commun 10, 4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akeju O et al. (2014) Effects of sevoflurane and propofol on frontal electroencephalogram power and coherence. Anesthesiology 121, 990–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Purdon PL et al. (2013) Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A 110, E1142–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ching S et al. (2010) Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A 107, 22665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kallionpää RE et al. (2020) Alpha band frontal connectivity is a state-specific electroencephalographic correlate of unresponsiveness during exposure to dexmedetomidine and propofol. Br J Anaesth 125, 518–528 [DOI] [PubMed] [Google Scholar]
- 67.Gibbs FA et al. (1937) Effect on the electro-encephalogram of certain drugs which influence nervous activity. Arch Intern Med 60, 154–66 [Google Scholar]
- 68.Tinker JH et al. (1977) Anterior shift of the dominant EEG rhytham during anesthesia in the Java monkey: correlation with anesthetic potency. Anesthesiology 46, 252–9 [DOI] [PubMed] [Google Scholar]
- 69.Cimenser A et al. (2011) Tracking brain states under general anesthesia by using global coherence analysis. Proc Natl Acad Sci U S A 108, 8832–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vijayan S et al. (2013) Thalamocortical mechanisms for the anteriorization of α rhythms during propofol-induced unconsciousness. J Neurosci 33, 11070–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim M et al. (2017) Relationship of Topology, Multiscale Phase Synchronization, and State Transitions in Human Brain Networks. Front Comput Neurosci 11, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pal D et al. (2020) Level of Consciousness Is Dissociable from Electroencephalographic Measures of Cortical Connectivity, Slow Oscillations, and Complexity. J Neurosci 40, 605–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muzur A et al. (2002) The prefrontal cortex in sleep. Trends Cogn Sci 6, 475–481 [DOI] [PubMed] [Google Scholar]
- 74.Gaskell AL et al. (2017) Frontal alpha-delta EEG does not preclude volitional response during anaesthesia: prospective cohort study of the isolated forearm technique. Br J Anaesth 119, 664–673 [DOI] [PubMed] [Google Scholar]
- 75.Sanders RD et al. (2018) Is consciousness frontal? Two perioperative case reports that challenge that concept. Br J Anaesth 121, 330–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frohlich J et al. (2021) Consciousness among delta waves: a paradox? Brain 144, 2257–2277 [DOI] [PubMed] [Google Scholar]


