Abstract
Osteoid osteoma is a benign bone tumor that usually grows in the long bones of the body and arises from osteoblasts and some components of osteoclasts. It represents the third most frequent type of benign bone tumors, accounting for 11% to 14% of the tumors. The entity usually involves the proximal femur and tibia. It has also been reported in the hand, especially the scaphoid, capitate, and proximal phalanx. The most common symptom is pain, usually during the night, relieved by the use of salicylates and nonsteroidal anti-inflammatory drugs. To date, only 5 cases involving the trapezium have been reported. This article describes a rare case of a large (1.3 cm) osteoid osteoma of the trapezium in a young male patient treated surgically with resection and curettage of the osteoid and provides a review of the existing literature.
Key words: Osteoid Osteoma, Trapezium, Bioactive glass
Osteoid osteoma is the third most frequent type of all benign bone tumors, accounting for 11% to 14% of the tumors.1,2 The most common loci are the femur and tibia.3 Although the involvement of hand bones is more unusual, the most frequently involved bones are scaphoid and capitate.4 The most common clinical presentation is pain, usually during the night, relieved by salicylates and nonsteroidal anti-inflammatory drugs (NSAIDs). The diagnosis is generally made with a computed tomography (CT) scan that detects the topical “nidus” surrounded by a sclerotic reaction. A definitive diagnosis is made with histological examination. The natural history reveals that osteoid osteomas should regress spontaneously within 6–15 years or by conservative treatment with NSAIDs for 30–40 months.5, 6, 7 Conservative treatment must thus be considered for tumors where the osteoma is not easily accessible. The surgical management is the excision of the nidus that must be removed completely for the pain to resolve.4,8,9 When the lesion is big, the excision can be followed by bone grafting or internal fixation.10 Other described techniques are radiofrequency ablation, cryoablation, and laser thermocoagulation.11,12 Among these techniques, CT-guided radiofrequency ablation is often the treatment of choice for osteoid osteoma.13
There have been several published cases of trapezium osteoid osteoma, and all of them were treated by excision and eventually cancellous bone graft. The first osteoid osteoma of the trapezium was described by Hundley14 in 1976 in a 16-year-old boy with long-lasting wrist pain without a precise diagnosis. The patient was treated with surgical excision with instant pain relief after surgery. In this case report, we describe the case of a young male patient with a 1.3-cm osteoid osteoma treated surgically with resection of the osteoid nidus and curettage of the sclerotic rib and bone defect filled with bioactive glass. Bioactive glass is a bone substitute used clinically as a space filler or for regenerative purposes. The filler effect improves when granules are moistened with blood or saline implantation into the defect.15 It has osteoconductive properties, does not increase the risk of infection and avoids the donor morbidity of graft harvest.15, 16, 17, 18, 19 The investigation was performed in compliance with the Declaration of Helsinki and the guidelines for Good Clinical Practice. The patient provided written informed consent for both surgery and follow-up. The follow-up study protocol was approved by the internal ethics committee.
Case Report
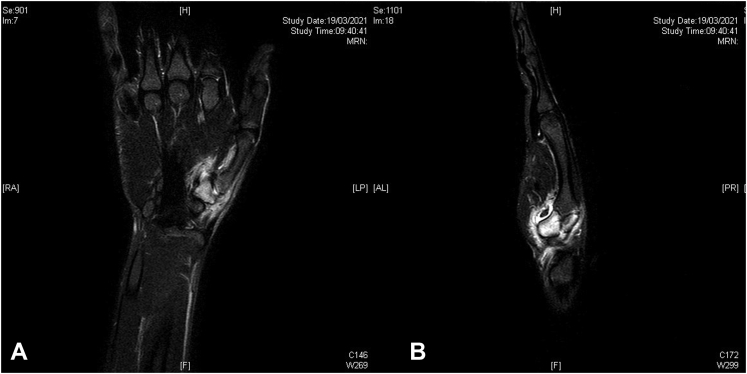
A 19-year-old man was referred to our hospital with a history of intense pain localized at the right thumb basal joint for 1 year. The pain was characterized as dull and persistent and was not relieved with NSAIDs. During the first visit, the patient demonstrated limited thumb opposition with a Kapanji score of 5, a weak pinch test with 5-kg strength (20 kg on the other hand), and difficulty in daily activities.20 His visual analog scale (VAS) was 7, and the Michigan Hand Outcomes Questionnaire showed a value of 31.25%, with a high compromission of daily activities and pain (Table 1, 2).21 X-rays were negative for pathology (Fig. 1). Magnetic resonance imaging (MRI) showed an intense signal corresponding to the trapezium and a diffuse edema of the surrounding tissue (Fig. 2). A subsequent CT scan (Fig. 3) showed the typical image of the osteoid osteoma, with the presence of the sclerotic nidus surrounded by a cortical reaction. At this point, the patient was counseled on surgery for enucleation of the tumor and grafting with bioglass.
Table 1.
Clinical Outcomes Before and After Surgery
| Clinical Examination Test | Before Surgery | T30 After Surgery | T60 After Surgery |
|---|---|---|---|
| Pinch test score | 5 kg | 15 kg | 20 kg |
| Kapanji score | 5 | 9 | 9 |
| Visual analog scale score | 7 | 0 | 0 |
Table 2.
Michigan Hand Outcomes Questionnaire Scores of the Patient
| Brief Michigan Outcomes | Before Surgery | T30 After Surgery | T60 After Surgery |
|---|---|---|---|
| Function (1 very good to 5 very poor) | |||
| Overall, how well did your hand(s) work during the past week? | 5 | 4 | 2 |
| How was the sensation (feeling) in your hand(s) during the past week? | 4 | 4 | 2 |
| Daily activities (1 not at all difficult to 5 very difficult) | |||
| How difficult was it for you to hold a frying pan during the last week? | 4 | 4 | 2 |
| How difficult was it for you to button a shirt or blouse during the past week? | 4 | 4 | 2 |
| Workly activities (1 always to 5 never) | |||
| In the past 4 weeks, how often were you unable to do your work because of problems with your hand(s)/wrist(s) | 3 | 3 | 5 |
| In the past 4 weeks, how often did you take longer to do tasks in your work because of problems with your hand(s)/wrist(s) | 3 | 3 | 5 |
| Pain (1 very mild to 5 very severe) | |||
| How often did the pain in your hands/wrists interfere with your daily activities? | 5 | 3 | 2 |
| Describe the pain in your hand(s)/wrist(s) in the past week? | 5 | 4 | 2 |
| Aesthetics (1 strongly agree to 5 strongly disagree) | |||
| I am satisfied with the look of my hands | 1 | 1 | 1 |
| The appearance of my hands interferes with my normal daily activities | 1 | 1 | 1 |
| Satisfaction (1 very satisfied to 5 very dissatisfied) | |||
| In the past week, how satisfied were you with the motion of your fingers? | 5 | 3 | 2 |
| In the past week, how satisfied were you with the motion of your wrist? | 5 | 4 | 2 |
| Normalization | 31.25% | 37.5% | 70.83% |
Figure 1.
X-ray examinations from months before and after surgery and at 30 days of follow-up. A–C Radiographs before surgery. The trapezium is quite similar in both hands, both in the anteroposterior and lateral view. A Anteroposterior view showing the standard trapezium. B Okay sign view showing the normal trapezium. C Magnified view of the trapezium. D–F Radiographs taken in the operating room at the end of the procedure with the cast including metacarpophalangeal joint. A hyperintense image of the trapezium is observed, which is due to the active bioglass applied in the bone cavity. D Anteroposterior view showing the trapezium filled with bioglass. The trapezium appears hyperintense. E Oblique view showing the trapezium filled with bioglass. F Magnified view of the trapezium. G–I Thirty days after surgery. The trapezium density is similar to that of the normal bone, demonstrating how bioglass is going to integrate. Note that some parts of the active bioglass outside the trapezium will be absorbed in the following months. G Anteroposterior view showing the trapezium filled with bioglass. H Oblique view showing the trapezium filled with bioglass. I Magnified view of the trapezium. J One-year follow-up (front view). The appearance of the trapezius is similar to that of a normal trapezius in terms of density and joint relationships with the other carpals. K One-year follow-up (lateral view). The appearance of the trapezius is similar to that of a normal trapezius in terms of density and joint relationships with the other carpals.
Figure 2.
A Sagittal and B longitudinal views of the MRI examination performed before surgery. The bone aspect is better represented in the CT examination; in fact, the nidus, sclerotic area, and erosion are not visible. The bone edema and flogistic perilesional tissues are visible on the MRI scan.
Figure 3.
Computed tomography performed before surgery. The erosive aspect of the lesion in A longitudinal view and the nidus and sclerotic perilesional area in the B sagittal view are shown. The CT examination is the most accurate imaging examination for suspecting osteoid osteoma.
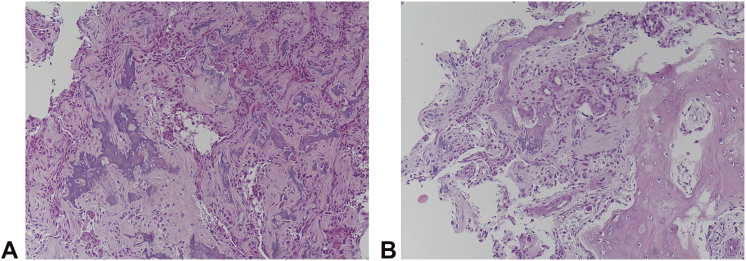
For the surgical procedure, we accessed the tumor with an S incision made on the radial volar side of the first ray. We deroofed the trapezium, isolating the osteoid nidus and the sclerotic bone through a trail of holes made by K-wires (Fig. 4) and taking care not to interrupt the cortex of the trapezium. After enucleation of the nidus with the help of curettes, we filled the bone defect with bioglass mixed with fresh blood (Fig. 5). We reconstructed the capsule, closed the wound leaving a drain, and applied a short arm cast with the thumb included for 4 weeks. The lesion was sent for definitive histological examination, which gave us the diagnosis of osteoid osteoma (Fig. 6).
Figure 4.
Demolitive part of the surgery. A Drawing of surgical access. B Exposure of the tumor. C Deroofing. A trail was made through several drills performed with 1-mm K-wires. D Isolation of the tumor. E Trapezium with bone loss. F Tumor size of 1.3 cm.
Figure 5.
Reconstructive part made with bioactive glass. A Bioglass granules are mixed with fresh blood cells. B Filler of bone loss with bioglass granules. C Coverage of the granules with glass bone putty (45S5 bioglass plus a binder made with polyethylene glycol and glycerol). D Complete application of bioglass. E Hand after surgery sutured with a drainage in situ. F Short arm cast including the thumb.
Figure 6.
The nidus as the well-defined area made of irregular bone trabeculae of different mineralization A, usually surrounded by the osteoblast cells B, is shown.
We followed up with the patient clinically and radiologically monthly for the first 3 months and at 1 year after surgery to rule out exclude recurrence. In the follow-up, we reported the VAS, pinch test, and Kapanji scores (Table 1). At the latest follow-up. the VAS, Pinch test, and Kapanji scores improved (P < .05). For the subjective evaluation of the functional and aesthetic outcomes, the authors administered the brief Michigan Hand Outcomes Questionnaire to the patient (Table 2). The brief Michigan Hand Outcomes Questionnaire global score showed slightly better, but not significant, results at 30 days after surgery (37.5%, P = .633), whereas it significantly showed improvement at 60 days after surgery (70.83%, P = .01).
Discussion
Osteoid osteoma is a benign osteoblastic lesion accompanied by severe pain relieved by salicylates and NSAIDs. It is frequent in individuals aged 10–20 years and in males.3,5 The most common locations are the femur and tibia, followed by the small bones of the hand, feet, and spine.3 It can be found in the bone cortex, subcortical, intracortical, and intraperitoneal, and can rarely occur with more nidi in 1 or more bones.3,22 The reason for remission after the use of NSAIDs is that nidus osteoblasts display a diffusion of cyclooxygenase-2, an enzyme important for prostaglandin production, in particular prostaglandin E2.23 This enzyme is the main cause of pain and explains why the tumor is so responsive to cyclooxygenase-2 inhibitors (NSAIDs).24 This lesion can be confused with osteoblastoma, a similar tumor of bigger size, usually more than 1.5 cm.9,25 This tumor is characterized by irregular sclerosis, and the nidus is not well-defined.26 It does not typically regress and does not respond to NSAIDs.27 Another important condition that can be confused with osteoid osteoma is Brodie’s abscess.22 Plain radiographs are usually the first examinations performed and can show a small radiolucent area, corresponding to the nidus, surrounded by a sclerotic bone area. However, if the tumor is intramedullary, it may not show the surrounding bone sclerosis.28 The diagnosis of osteoid osteoma is usually suspected when CT scans show the nidus. A highly specific and sensitive finding in diagnosis is the presence of fine, low density, linear, vascular channels surrounding the osteoid osteoma.29 Furthermore, CT has better accuracy than plain radiography and MRI.30,31 Magnetic resonance imaging is usually the first examination performed and can show a small radiolucent area, corresponding to the nidus, surrounded by a sclerotic bone area. However, if the tumor is intramedullary located, it may not show that surrounding bone sclerosis.28 Although not as useful, an MRI examination can clearly show bone marrow edema and periarticular fluid. Care must be taken because the reactive soft tissue mass can be misinterpreted as a malignant tumor of the soft tissue or osteomyelitis.32,33 Thus, MRI images should not be assessed without CT and x-ray image references.34 Bone scintigraphy shows a vascular nidus in the arterial phase with a delayed phase within the surrounding reactive bones; the nidus is usually more intense, and the reactive bone is less intense; this is known as the “double density sign,” and it is diagnostic of osteoid osteoma.35,36 In 2010, Bostan et al37 described a case of osteoid osteoma in a 25-year-old patient with a 12-month history of wrist pain, which occurred particularly at night. The patient was initially treated with orthoses and NSAIDs without success. At the clinical examination, swelling was observed over the dorsoradial aspect of the hand. CT examination showed the sclerotic nidus surrounded by a radiolucent osteoid tissue, and MRI examination showed bone marrow edema associated with a focal lesion of the trapezium hypointense. The patient was treated with excisional biopsy, and after surgery, the patient had immediate pain improvement, and no recurrence was observed in follow-up.37 In 2017, Park et al38 described an osteoid osteoma tumor in a 29-year-old patient initially treated for calcification periarthritis with several steroid injections until follow-up, debridement with no effect, until an ulnar deviated x-ray examination showed a sclerotic bone lesion, suspicious for osteoid osteoma and treated with curettage. The patient experienced immediate improvement in clinical pain and no recurrence at a 1-year follow-up. In 2017, Roberts et al39 described a case of a 34-year-old woman with osteoid osteoma initially confused with carpometacarpal arthritis. In this case, an MRI scan showed a hypointense circular lesion along the dorsal aspect of the trapezium, and a CT study was conducted to better characterize the lesion. The lesion was treated with curettage, and the diagnosis was confirmed by histopathology. After excision, the patient experienced complete pain relief and did not have any recurrence.39 In this study, because of the unusually large tumor size of 1.3 cm, we filled the bone loss with bioactive glass to avoid donor morbidity in such a young patient. There are many kinds of bioactive glasses, and we elected to use GlassBONE (Noraker) in 2 different formulations, “granules” to fill the bone defect and “putty,” a formulation with glycol polyethylene and glycerol, which grants ligand property useful for roofing the filled area.40 Bioglass is a bone substitute, first used in hand surgery by Hench et al41 in 1967; it is a bioactive material that can bond to the bone because of a specific chemical reaction. A bioactive material does not cause minimal rejection, is recognized as a biological material, and bonds with the tissue for mechanical interference. It is composed of silicon dioxide, calcium oxide, sodium oxide, and phosphoric anhydride; the equilibrium among these components makes the bioglass active. The gold standard of bioactive glass is the 45S5 (comprising 45% of silicon dioxide, 24.5% of sodium oxide, 24.5% of calcium oxide, and 6% of phosphorus pentoxide).42 When the bioactive glass is in contact with biological fluids, several chemical reactions cause silicon hydrolysis, creating a silica gel layer similar to the bone hydroxyapatite (carbonated hydroxyapatite). The carbonated hydroxyapatite layer absorbs growth factors; these factors attract the M2 macrophages that promote lesion healing and attract staminal mesenchymal cells, which become the osteoblasts.43 This process starts generating and depositing proteins of the extracellular bone matrix (collagen I).40,44 To conclude, bioactive glass causes osteoblasts and osteocytes to spread along the glass surface; this means that the material is mainly osteoconductive.45,46 In this study, after the tumor excision, the bone loss had to be filled, and we chose a bioglass to avoid donor site morbidity.
Conclusion
Analyzing the reports in the literature, we can conclude that osteoid osteoma should be suspected when a patient presents with long-lasting wrist pain with unclear diagnosis, associated with radial side tenderness surrounding the thumb, night pain responsive to NSAIDs, and negative x-rays. The approach has to start with a clinical examination, including the Kapandji test, which shows a reduction of thumb opposition compared with the contralateral hand. Although x-rays can be negative, a CT scan can provide us with the most accurate image of a nidus, whereas an MRI image can show bone edema and surrounding tissue inflammation and exclude other pathologies. A definitive diagnosis is made by histological examination. In our opinion, the best treatment is the curettage of the osteoid osteoma, avoiding trapeziectomy if the carpometacarpal joint is not involved. If the lesion is larger in size, bone grafting, bone substitutes, or bioglass can be useful. The patient typically shows pain relief after surgery and should be followed monthly for 3 months after surgery, and at 6 months to a year with a CT scan to rule out recurrence, then new clinical and radiological control after 3 months and a final control made with CT examination after other 6 months to exclude recurrence.
Footnotes
Declaration of interests: No benefits in any form have been received or will be received by the authors related directly or indirectly to the subject of this article.
References
- 1.Unni K.K. In: Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. Unni K.K., editor. Lippincott Raven Publishers; 1996. Osteoid osteoma; pp. 121–130. [Google Scholar]
- 2.Campanacci M. In: Bone and Soft Tissue Tumors. Campanacci M., editor. Piccin Nuova Libraria S.p.A.; 1999. Osteoid osteoma; pp. 391–414. [Google Scholar]
- 3.Frassica F.J., Waltrip R.L., Sponseller P.D., Ma L.D., McCarthy E.F., Jr. Clinicopathologic features and treatment of osteoid osteoma and osteoblastoma in children and adolescents. Orthop Clin North Am. 1996;27(3):559–574. [PubMed] [Google Scholar]
- 4.Murray P.M., Berger R.A., Inwards C.Y. Primary neoplasms of the carpal bones. J Hand Surg Am. 1999;24(5):1008–1013. doi: 10.1053/jhsu.1999.1008. [DOI] [PubMed] [Google Scholar]
- 5.Moberg E. The natural course of osteoid osteoma. J Bone Joint Surg Am. 1951;33(1):166–170. [PubMed] [Google Scholar]
- 6.Golding J.S. The natural history of osteoid osteoma; with a report of twenty cases. J Bone Joint Surg Br. 1954;36-B(2):218–229. doi: 10.1302/0301-620X.36B2.218. [DOI] [PubMed] [Google Scholar]
- 7.Kneisl J.S., Simon M.A. Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am. 1992;74(2):179–185. [PubMed] [Google Scholar]
- 8.Pettine K.A., Klassen R.A. Osteoid-osteoma and osteoblastoma of the spine. J Bone Joint Surg Am. 1986;68(3):354–361. [PubMed] [Google Scholar]
- 9.Peyser A.B., Makley J.T., Callewart C.C., Brackett B., Carter J.R., Abdul-Karim F.W. Osteoma of the long bones and the spine. A study of eleven patients and a review of the literature. J Bone Joint Surg Am. 1996;78(8):1172–1180. doi: 10.2106/00004623-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Gitelis S., Schajowicz F. Osteoid osteoma and osteoblastoma. Orthop Clin North Am. 1989;20(3):313–325. [PubMed] [Google Scholar]
- 11.Towbin R., Kaye R., Meza M.P., Pollock A.N., Yaw K., Moreland M. Osteoid osteoma: percutaneous excision using a CT-guided coaxial technique. AJR Am J Roentgenol. 1995;164(4):945–949. doi: 10.2214/ajr.164.4.7726054. [DOI] [PubMed] [Google Scholar]
- 12.Lenke L.G., Sutherland C.J., Gilula L.A. Osteoid osteoma of the proximal femur: CT-guided preoperative localization. Orthopedics. 1994;17(3):289–292. doi: 10.3928/0147-7447-19940301-10. [DOI] [PubMed] [Google Scholar]
- 13.De Filippo M., Russo U., Papapietro V.R., et al. Radiofrequency ablation of osteoid osteoma. Acta Biomed. 2018;89(1-S):175–185. doi: 10.23750/abm.v89i1-S.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hundley J.D. Osteoid osteoma of the trapezium. First case report of roentgenographically demonstrated progression in the trapezium. Clin Orthop Relat Res. 1976;(116):170–172. [PubMed] [Google Scholar]
- 15.Heikkilä J.T., Kukkonen J., Aho A.J., Moisander S., Kyyrönen T., Mattila K. Bioactive glass granules: a suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: a prospective randomized 1-year follow-up study. J Mater Sci Mater Med. 2011;22(4):1073–1080. doi: 10.1007/s10856-011-4272-0. [DOI] [PubMed] [Google Scholar]
- 16.Camargo A.F.F., Baptista A.M., Natalino R., de Camargo O.P. Bioactive glass in cavitary bone defects: a comparative experimental study in rabbits. Acta Ortop Bras. 2015;23(4):202–207. doi: 10.1590/1413-785220152304147538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schepers E., de Clercq M., Ducheyne P., Kempeneers R. Bioactive glass particulate material as a filler for bone lesions. J Oral Rehabil. 1991;18(5):439–452. doi: 10.1111/j.1365-2842.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindfors N.C., Heikkilä J.T., Koski I., Mattila K., Aho A.J. Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater. 2009;90(1):131–136. doi: 10.1002/jbm.b.31263. [DOI] [PubMed] [Google Scholar]
- 19.Lindfors N.C., Koski I., Heikkilä J.T., Mattila K., Aho A.J. A prospective randomized 14-year follow-up study of bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B Appl Biomater. 2010;94(1):157–164. doi: 10.1002/jbm.b.31636. [DOI] [PubMed] [Google Scholar]
- 20.Kapandji A. Clinical test of apposition and counter-apposition of the thumb. Ann Chir Main. 1986;5(1):67–73. doi: 10.1016/s0753-9053(86)80053-9. [DOI] [PubMed] [Google Scholar]
- 21.Waljee J.F., Kim H.M., Burns P.B., Chung K.C. Development of a brief, 12-item version of the Michigan Hand Questionnaire. Plast Reconstr Surg. 2011;128:208–220. doi: 10.1097/PRS.0b013e318218fc51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaffe H.L., Lichtenstein L. Osteoid osteoma: further experience with this benign tumor of bone. With particular reference to cases showing the lesion about shaft cortices and commonly misclassified as instances of sclerosing nonsuppurative osteomyelitis or cortical bone abscess. J Bone Joint Surg Am. 1940;22:645–682. [Google Scholar]
- 23.Mungo D.V., Zhang X., O’Keefe R.J., Rosier R.N., Puzas J.E., Schwarz E.M. COX-1 and COX-2 expression in osteoid osteomas. J Orthop Res. 2002;20(1):159–162. doi: 10.1016/S0736-0266(01)00065-1. [DOI] [PubMed] [Google Scholar]
- 24.Carpintero-Benitez P., Aguirre M.A., Serrano J.A., Lluch M. Effect of rofecoxib on pain caused by osteoid osteoma. Orthopedics. 2004;27(11):1188–1191. doi: 10.3928/0147-7447-20041101-17. [DOI] [PubMed] [Google Scholar]
- 25.Sim F.H., Dahlin C.D., Beabout J.W. Osteoid- osteoma: diagnostic problems. J Bone Joint Surg Am. 1975;57(2):154–159. [PubMed] [Google Scholar]
- 26.Gelman B. Radiology of bone tumors. Orthop Clin North Am. 1989;20(3):287–312. [PubMed] [Google Scholar]
- 27.Dorfman H.D., Czerniak B. Bone cancers. Cancer. 1995;75(1 suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Tumeh S.S. Scintigraphy in the evaluation of arthropathy. Radiol Clin North Am. 1996;34(2):215–223. [PubMed] [Google Scholar]
- 29.Liu P.T., Kujak J.L., Roberts C.C., de Chadarevian J.P. The vascular groove sign: a new CT finding associated with osteoid osteomas. AJR Am J Roentgenol. 2011;196(1):168–173. doi: 10.2214/AJR.10.4534. [DOI] [PubMed] [Google Scholar]
- 30.Pikoulas C., Mantzikopoulos G., Thanos L., Passomenos D., Dalamarinis C., Glampedaki-Dagianta K. Unusually located osteoid osteomas. Eur J Radiol. 1995;20(2):120–125. doi: 10.1016/0720-048x(95)00636-5. [DOI] [PubMed] [Google Scholar]
- 31.Thompson G.H., Wong K.M., Konsens R.M., Vibhakar S. Magnetic resonance imaging of an osteoid osteoma of the proximal femur: a potentially confusing appearance. J Pediatr Orthop. 1990;10(6):800–804. doi: 10.1097/01241398-199011000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Woods E.R., Martel W., Mandell S.H., CrabbeJP Reactive soft-tissue mass associated with osteoid osteoma: correlation of MR imaging features with pathologic findings. Radiology. 1993;186(1):221–225. doi: 10.1148/radiology.186.1.8416568. [DOI] [PubMed] [Google Scholar]
- 33.Ehara S., Rosenthal D.I., Aoki J., et al. Peritumoral edema in osteoid osteoma on magnetic resonance imaging. Skeletal Radiol. 1999;28(5):265–270. doi: 10.1007/s002560050513. [DOI] [PubMed] [Google Scholar]
- 34.Assoun J., Railhac J.J., Bonnevialle P., et al. Osteoid osteoma: percutaneous resection with CT guidance. Radiology. 1993;188(2):541–547. doi: 10.1148/radiology.188.2.8327712. [DOI] [PubMed] [Google Scholar]
- 35.Smith F.W., Gilday D.L. Scintigraphic appearances of osteoid osteoma. Radiology. 1980;137(1 Pt 1):191–195. doi: 10.1148/radiology.137.1.6448428. [DOI] [PubMed] [Google Scholar]
- 36.Helms C.A., Hattner R.S., Vogler J.B., III Osteoid osteoma: radionuclide diagnosis. Radiology. 1984;151(3):779–784. doi: 10.1148/radiology.151.3.6232642. [DOI] [PubMed] [Google Scholar]
- 37.Bostan B., Sen C., Gunes T., Erdem M., Koseoglu R.D. Osteoid osteoma of the trapezium: case report. J Hand Surg. 2010;35(4):636–638. doi: 10.1016/j.jhsa.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 38.Park J.H., Kang T.W., Park J.W. Unusual cause of the thumb basal joint pain: osteoid osteoma of the trapezium. Arch Orthop Trauma Surg. 2017;137(6):875–878. doi: 10.1007/s00402-017-2692-0. [DOI] [PubMed] [Google Scholar]
- 39.Roberts S.E., Mirzabeigi M.N., Naik A., Preciado C., Chang B. Osteoid osteoma of the trapezium: case report of an unusual tumor location presenting a diagnostic challenge. Case Rep Orthop. 2017;2017 doi: 10.1155/2017/3683854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cannio M., Bellucci D., Roether J.A., Boccaccini D.N., Cannillo V. Bioactive glass applications: a literature review of human clinical trials. Materials (Basel) 2021;14(18):5440. doi: 10.3390/ma14185440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hench L.L., Splinter R.J., Allen W.C., Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5(6):117–141. [Google Scholar]
- 42.Hench L.L. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74:1487–1510. [Google Scholar]
- 43.Hench L.L., Polak J.M. Third-generation biomedical materials. Science. 2002;295(5557):1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 44.Hench L.L., Ethridge A.C. Academic Press; 1982. Biomaterials: An Interfacial Approach. [Google Scholar]
- 45.Hench L.L., Paschall H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J Biomed Mater Res. 1973;7(3):25–42. doi: 10.1002/jbm.820070304. [DOI] [PubMed] [Google Scholar]
- 46.Ono K., Yamamuro T., Nakamura T., Kokubo T. Quantitative study on osteoconduction of apatite-wollastonite containing glass ceramic granules, hydroxyapatite granules, and alumina granules. Biomaterials. 1990;11(4):265–271. doi: 10.1016/0142-9612(90)90008-e. [DOI] [PubMed] [Google Scholar]